Abstract
In patients with leukemia, the portal(s) and reasons for the persistence of an Escherichia coli recurrent bacteremia remain unclear. Adult Hematology Clinic (AHC) databases at the State Clinical Hospital in Gdańsk were reviewed to evaluate the frequency of E. coli bacteremia between 2002 and 2005. Blood and bowel E. coli strains were obtained and the genetic relatedness of the strains was analyzed. The rate of E. coli bacteremia per 1,000 admissions at the AHC was higher (85.0) than in the other clinics of the hospital (2.9), p < 0.001. A higher mortality was observed in patients with a history of E. coli versus non-E. coli bacteremia [30/95 (31 %) vs. 53/430 (12 %), p < 0.001]; 72.8 % of patients with leukemia had an unknown source of bacteremia. In 2005, 6 out of 25 (24 %) patients with leukemia had ≥2 episodes of E. coli-positive blood cultures. These gastrointestinal E. coli isolates were replaced within 3–8 weeks with a new E. coli H genotype. A recurrent episode of bacteremia was usually caused by an infection with a transient E. coli H genotype identical to that found in the subject’s bowel. Consistent with the definition of bowel/blood translocation, the bowel appeared to be a portal for E. coli in these subjects and, hence, a clear source for their recurring bacteremia.
Introduction
While the presence of a hematologic malignancy has been considered a risk for recurrent vancomycin-resistant Enterococcus (VRE) bacteremia, the recurrence of Escherichia coli bacteremia in patients with leukemia has not been well studied [1–3]. Although E. coli is recognized as an important pathogen in recurrent bacteremia, patients who have a bacteremia caused by a Gram-negative organism reportedly have a relatively low risk (6 %) of recurrent episodes [2–9]. In patients with a hematologic malignancy and recurrent bacteremia, VRE colonize the gastrointestinal tract and often persist in the colon for several months [1]. Despite clinical and laboratory examination, in leukemia, the source or portal of infection in those patients with recurrent bacteremia often remains unknown. Further, we lack data on the short- versus long-term E. coli colonization of the bowel and the associated risk of recurrent bacteremia. Several factors may contribute to the risk of recurrent bacteremia. First, E. coli may remain viable in the hospital environment for several months [10–18]. Susceptible patients such as those who have leukemia may be at risk for recurrent infection with a E. coli strain(s) that persists in such a hospital environment. Based on current trends, the risk of recurrent E. coli bacteremia, although low, may also increase due to the increasing prevalence of an “alarm” antibiotic-resistant E. coli [10]. Other factors that may increase the risk of E. coli bacteremia are frequent hospitalizations and the longer survival of patients with leukemia. Because the frequency of E. coli recurrent bacteremia is reportedly low, the risk of mortality in patients with recurrent E. coli versus non-E. coli bacteremia is under-investigated and remains unclear. In this study on E. coli bacteremia, we determined the source/portal of recurrent infection, the mortality incidence in those with a history of E. coli versus non-E. coli bacteremia, and the similarity of E. coli genotypes in blood and bowel over time. This aimed to define whether a recurrence of bacteremia was caused by the same genotype persisting in the bowel as a pathogen or if the recurrence was caused by a transient new colonizer. Here, we report that recurrent E. coli bacteremia was a frequent event in leukemic malignancy and was accompanied by an increased risk of mortality. Recurrent bacteremia was not caused by persistent colonizers: blood re-infections mostly occurred with a new E. coli genotype that was also temporarily present in the bowel, suggesting that the bowel was the source of the translocating pathogen.
Materials and methods
Patients and collection of samples
The analysis was focused on the Independent State Clinical Hospital at Gdańsk University of Medicine (State Hospital), with a total number of 1,265 hospital beds and 34 subspecialty clinics and/or wards, including an Adult Hematology Clinic (AHC). The databases in the State Hospital and the microbiology laboratory were reviewed to identify those patients who had Gram-negative bacteremia between January 1, 2002 and December 31, 2005. All patients with recurrent episodes [at least two episodes that occurred 30 days apart or also occasionally shorter intervals (see results for patient P1) with blood culture results negative in the interim] were identified. In addition, all E. coli cultures obtained from blood and stool samples from 25 patients with leukemia (collected during the period 2004–2005 at the AHC) were saved and stored at −80 °C. A single bacteremic episode was defined by positive blood cultures performed on a single patient within a 7-day period. Polymicrobial bacteremia was considered to be Gram-negative if at least one Gram-negative organism was isolated in the blood culture. Bacteremic episodes with onset >48 h after admission were considered to be nosocomial. A recurrence of bacteremia was defined as a second episode of bacteremia during the study period that was caused by the same Gram-negative species. A relapse was defined as a recurrence of bacteremia caused by the same strain that caused the initial infection. A re-infection was defined as a recurrence of bacteremia caused by a different strain than the one that caused the initial infection [>3 bands difference in pulsed-field gel electrophoresis (PFGE) or coefficient of similarity <90 %] [19]. Bacteremia was considered to be due to a gastrointestinal tract translocation when: (a) an isolate with the same DNA fingerprint as the blood isolate was obtained from a stool specimen (>108 CFU/g of feces); (b) no alternative primary site of infection was detected; (c) urine culture results were negative [<1,000 colony-forming units (CFU)/mL]; and (d) no acquired digestive tract abnormalities were present [20].
Genotyping by PCR MP and REA-PFGE
Frozen isolates were thawed and cultured on trypticase soy agar with 5 % sheep blood agar (Becton Dickinson) and incubated at 37 °C for 24 h. Species identification was done with a Vitek identification card (bioMérieux Vitek). The polymerase chain reaction melting profiles (PCR MP) procedure was carried out according to the method described for E. coli isolates [21]. This method allows specific gradual amplification of genomic DNA in terms of thermal stability starting from less stable DNA fragments amplified at lower Td values to more stable ones amplified at higher Td values. This feature may be used to obtain sets of electrophoretic patterns of DNA fragments amplified during ligation-mediated (LM) PCR performed at various denaturation temperatures. A low Td during LM PCR leads to limited and specific amplification of a small number of DNA fragments characteristic for the bacterial strain. Restriction endonuclease analysis (REA)-PFGE was performed with restriction digestion of the chromosomal DNA with 20 U of XbaI (Sigma) and with the FIGE Mapper Electrophoresis System (Bio-Rad), according to a method described previously [21].
Statistical analysis
Data were statistically analyzed using the Microsoft Excel 2007 program. Categorical variables were compared using Chi-square analysis. p-values less than 0.05 were considered to be significant.
Results
Bacteremia at the Independent State Clinical Hospital, years 2002–2005
The hospital’s and microbiology laboratory’s databases between 2002 and 2005 included records for 152,388 patients admitted, including 2,741 patients admitted to the AHC. While the frequency of bacteremia among all the admitted patients to the State Hospital was 1.6 %, the patient population in the AHC had a significantly higher bacteremia rate of 19.1 % (p < 0.001) (Table 1). The observed difference in the frequency of bacteremia in those subjects in the general population of the State Hospital versus those in the AHC was also calculated as the rates in blood cultures per 1,000 admissions. While, overall, the State Hospital rate was 57.4 per 1,000, it was dramatically higher for the AHC (1,235/1,000) and significantly lower for the remaining State Hospital clinics (35.8/1,000).
Table 1.
State Hospital admissions, number of blood cultures, and Escherichia coli bacteremias in the years 2002–2005
| AHC | Other clinics | Total | p* AHC vs. other | |
|---|---|---|---|---|
| Number of patients hospitalized | 2,741 | 149,647 | 152,388 | – |
| Number (%) of patients with positive blood cultures | 525 (19.1 %) | 1,974 (1.3 %) | 2,499 (1.6 %) | <0.001 |
| Number of positive blood cultures/rate per 1,000 admissions | 3,387/1,235.7 | 5,359/35.8 | 8,746/57.4 | <0.001 |
| Number of Enterobacteriaceae isolates/rate per 1,000 admissions | 531/193.7 | 1,053/7.0 | 1,584/10.4 | <0.001 |
| Number of E. coli strains/rate per 1,000 admissions | 233/85.0 | 431/2.9 | 664/4.4 | <0.001 |
| Number of unique patients/cases of bacteremia with presence of E. coli | 95/223 | 235/429 | 330/652 | – |
| Number (%) of ESBL E. coli | 20 (0.53 %) | 20 (0.33 %) | 40 (0.41 %) | 0.008 |
*p < 0.05 was considered to be significant
In order to understand the increased frequency of bacteremia in the AHC, the analysis was focused on Gram-negative microorganisms. We revealed that Enterobacteriaceae were more frequently isolated in the AHC ward. While the State Hospital rate of Gram-negative bacteremia was low (10.4 per 1,000), the rate was significantly higher for AHC subjects (193.7 per 1,000). Among the Enterobacteriaceae, the most frequently isolated was E. coli; rates of 85.0 and 2.9 per 1,000 for the AHC and the remaining State Hospital clinics, respectively, were noted. The State Hospital records identified a total of 652 episodes of E. coli bacteremia, of which 223 occurred in 95 AHC patients. This suggested the occurrence of multiple bacteremic episodes and/or recurrent E. coli bacteremia in some patients (Table 1).
Recurrence or persistence of bacteremia may be a result of a therapeutic failure due to the presence of so called “alarm” microorganisms that are resistant to multiple antibiotics. Between 2002 and 2005, there were only 40 bacteremias of extended-spectrum beta-lactamase (ESBL) E. coli in the entire State Hospital, and half of these cases were from the AHC. These data suggest that antibiotic resistance was less likely to be a primary factor in the recurrence of E. coli bacteremia.
To understand the underlying cause(s) of the increased frequency of bacteremia at the AHC, we performed an analysis of the identified sources/portals of infection (Table 2). While an extra-intestinal source of bacteremia was found in the majority of clinics, in the AHC, only 27.4 % of cases had this as an identified source. The most frequent portal of E. coli bacteremia was the urogenital tract. However, while a urogenital source of E. coli accounted for 16.8 % of bacteremia for AHC patients, this number was 83.8 % in Internal Medicine and 39.3 % in Surgery. These data are consistent with our hypothesis that, in roughly 72.6 % of AHC patients (Table 2) with an unknown E. coli portal, the bowel was the likely source of their infection.
Table 2.
Frequency (%) of E. coli isolated from the blood of patients, with sources/portals of infection known/unknown
| AHC | Internal Medicine | Surgery | Intensive Care Unit | Pediatric Hematology | Other pediatric clinics | |
|---|---|---|---|---|---|---|
| Patients, n/% | Patients, n/% | Patients, n/% | Patients, n/% | Patients, n/% | Patients, n/% | |
| Total | 95 | 149 | 39 | 30 | 12 | 23 |
| Known | 26/27.4 % | 148/99.3 % | 37/94.9 % | 30/100.0 % | 9/75.0 % | 23/100.0 % |
| Urogenital tract | 16/16.8 % | 125/83.8 % | 13/39.3 % | 7/38.8 % | 3/25.0 % | 4/17.3 % |
| Vascular catheters | 1/1.0 % | 3/2.0 % | 3/9.0 % | 0/0.0 % | 5/41.6 % | 4/17.3 % |
| Respiratory tract | 5/5.2 % | 10/6.7 % | 5/15.1 % | 13/72.2 % | 0/0.0 % | 15/65.2 % |
| Wound | 4/4.2 % | 10/6.7 % | 16/48.4 % | 10/55.5 % | 1/8.3 % | 0/0.0 % |
| Unknown | 69/72.6 % | 1/0.7 % | 2/5.1 % | 0/0.0 % | 3/25.0 % | 0/0.0 % |
As the frequency of E. coli bacteremia was higher in the AHC ward (which serves a patient population with leukemia/lymphoma), we focused our further analyses on these patients (Table 3). The highest rate of E. coli bacteremia was observed in patients with myeloid leukemia, lymphoblastic leukemia, and Hodgkin’s lymphoma (22.2 %, 20.4 %, and 27.3 %, respectively), while lower rates were observed in patients with multiple myeloma and immunocytoma (11.7 % and 14.5 %, respectively). E. coli monobacteremia represented 20.7 to 22.7 % of all E. coli-infected blood cultures in patients with leukemia and lymphoma, and 9.3 % to 12.3 % in multiple myeloma and immunocytoma.
Table 3.
Frequency of E. coli bacteremia and number of episodes in AHC patients with myeloid leukemia, lymphoblastic leukemia, multiple myeloma, immunocytoma, and Hodgkin’s lymphoma in the years 2002–2005
| Myeloid leukemia | Lymphoblastic leukemia | Multiple myeloma | Immunocytoma | Hodgkin’s lymphoma | Other | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P, n | PB, n/% | E, n | P, n | PB, n/% | E, n | P, n | PB, n/% | E, n | P, n | PB, n/% | E, n | P, n | PB, n/% | E, n | P, n | PB, n/% | E, n | |
| Total | 212 | 47/22.2 | 117 | 93 | 19/20.4 | 41 | 77 | 9/11.7 | 17 | 83 | 12/14.5 | 23 | 22 | 6/27.3 | 17 | 38 | 2/5.2 | 8 |
| Recurrent bacteremia | 132a | 10/7.6 | 10 | 56a | 4/7.1 | 4 | 28a | 1/3.6 | 1 | 37a | 2/5.4 | 2 | 16a | 3/18.7 | 3 | 12a | 2/16.6 | 2 |
P number of patients, PB patients with E. coli bacteremia, E episodes with E. coli bacteremia
aAll bacteremia, including bacteremia with E. coli
Because the frequency of E. coli recurrent bacteremia is reportedly low, the risk of mortality in patients with recurrent E. coli versus non-E. coli bacteremia is under-investigated and remains unclear. Table 4 summarizes data on the number of AHC patients who died, relative to their history of E. coli bacteremia. Out of 525 patients with a history of bacteremia, 83 (15.8 %) patients died due to the bacteremia itself. Out of 95 patients with a history of E. coli bacteremia, 30 (31 %) patients died. In contrast, out of 430 patients with a history of bacteremia caused by pathogens other than E. coli, 53 (12 %) patients died. The number of deaths connected to their basic diseases was similar for those with or without a history of E. coli bacteremia (p = 0.134). These results suggest that a history of E. coli bacteremia, per se, was associated with increased risk of mortality (p < 0.001).
Table 4.
Mortality of patients hospitalized in the AHC in the years 2002–2005
| Patients with history of E. coli bacteremia | Patients without history of E. coli bacteremia | Total | p-Value | |
|---|---|---|---|---|
| Number of patients with positive blood cultures | 95 | 430 | 525 | – |
| Number of patients who did not die during hospitalization | 38 | 289 | 327 | 0.002 |
| Number of patients who died due to basic disease | 27 | 88 | 115 | 0.134 |
| Number of patients who died due to bacteremia | 30 | 53 | 83 | <0.001 |
Genetic typing for the characterization of E. coli isolates from patients with leukemia/lymphoma and recurrent bacteremia
Since we postulated that the source/portal of recurrent infection would be the bowel, we focused on the similarity of E. coli genotypes in blood and bowel isolates over time. To define whether a recurrence of bacteremia was caused by the same genotype persisting in the bowel as a pathogen (or if the re-infection was caused by a transient new colonizer), we first characterized E. coli DNA fingerprint patterns produced by PCR MP and REA-PFGE. DNA fingerprinting of 65 E. coli isolates from blood and/or bowel identified 32 genotype patterns, which were named H1 to H32 (Fig. 1, representative results by using the PCR MP method). Among the most frequent genotypes, the H2 pattern was represented in nine E. coli isolates, H17 and H22 were represented in six isolates each, and the remaining H types were restricted to one or two E. coli isolates (Table 5). E. coli genotype H2, which represented a nosocomial strain, was identified in the blood cultures of four patients with leukemia. The other frequent genotype (E. coli H22) was isolated from the blood of two patients (P10 and P18). Among the genotypes that were less frequent, E. coli H9, H11, H13, H15, H16, H20m and H30 were isolated from both the blood and the bowel samples (one patient for each genotype), which is consistent with an E. coli isolate H fingerprint specificity.
Fig. 1.
Polymerase chain reaction melting profiles (PCR MP) fingerprints for representative Escherichia coli isolates from patients of the Adult Hematology Clinic (AHC) ward showing H genotypes from H1 to H20. PCR MP fingerprinting H types are given above each lane. The DNA amplicons were electrophoresed on 6 % polyacrylamide gels
Table 5.
Patients’ clinical histories and results of the genotyping of E. coli isolates
| Clinical recognition | Patient | Isolate | Date of isolation (day.-mo.-yr) | Specimen source | Genotype |
|---|---|---|---|---|---|
| Myeloid leukemia | P1 | 1 | 23-09-2004 | Blood | H1 |
| 2 | 05-02-2005 | Blood | H7 | ||
| 3 | 05-02-2005 | Blood | H8 | ||
| 4 | 05-02-2005 | Blood | H9 | ||
| 5 | 07-02-2005 | Stool | H9 | ||
| 6 | 07-02-2005 | Stool | H10 | ||
| 7 | 10-05-2005 | Blood | H16 | ||
| 8 | 10-05-2005 | Stool | H16 | ||
| 9 | 19-05-2005 | Blood | H16 | ||
| 10 | 19-05-2005 | Blood | H16 | ||
| 11 | 22-05-2005 | Blood | H17 | ||
| 12 | 22-05-2005 | Blood | H17 | ||
| 13 | 25-05-2005 | Blood | H17 | ||
| 14 | 25-05-2005 | Blood | H17 | ||
| 15 | 27-05-2005 | Blood | H17 | ||
| 16 | 08-07-2005 | Blood | H17 | ||
| P2 | 17 | 22-10-2004 | Blood | H4 | |
| P3 | 18 | 22-10-2004 | Blood | H2 | |
| P4 | 19 | 24-11-2004 | Blood | H2 | |
| P5 | 20 | 15-02-2005 | Blood | H11 | |
| 21 | 15-02-2005 | Blood | H11 | ||
| 22 | 18-02-2005 | Stool | H11 | ||
| 23 | 18-02-2005 | Stool | H12 | ||
| 24 | 18-02-2005 | Stool | H11 | ||
| 25 | 18-02-2005 | Stool | H12 | ||
| P6 | 26 | 22-02-2005 | Blood | H13 | |
| 27 | 25-02-2005 | Stool | H13 | ||
| P7 | 28 | 05-03-2005 | Blood | H14 | |
| P8 | 29 | 28-04-2005 | Blood | H15 | |
| 30 | 28-04-2005 | Stool | H15 | ||
| P9 | 31 | 28-07-2005 | Blood | H20 | |
| 32 | 01-08-2005 | Stool | H20 | ||
| 33 | 30-09-2005 | Blood | H23 | ||
| 34 | 30-09-2005 | Blood | H23 | ||
| P10 | 35 | 16-08-2005 | Blood | H22 | |
| 36 | 16-08-2005 | Blood | H22 | ||
| 37 | 16-08-2005 | Stool | H22 | ||
| P11 | 38 | 31-09-2005 | Blood | H25 | |
| 39 | 04-11-2005 | Blood | H28 | ||
| 40 | 04-11-2005 | Blood | H29 | ||
| P12 | 41 | 29-11-2005 | Blood | H31 | |
| P13 | 42 | 05-12-2005 | Blood | H32 | |
| Lymphoblastic leukemia | P14 | 43 | 16-11-2004 | Blood | H5 |
| P15 | 44 | 13-09-2004 | Blood | H2 | |
| 45 | 12-10-2004 | Blood | H2 | ||
| P16 | 46 | 17-06-2005 | Blood | H19 | |
| P17 | 47 | 29-07-2005 | Blood | H21 | |
| 48 | 29-07-2005 | Blood | H21 | ||
| P18 | 49 | 30-08-2005 | Blood | H22 | |
| 50 | 30-08-2005 | Stool | H22 | ||
| 51 | 05-09-2005 | Stool | H22 | ||
| P19 | 52 | 02-11-2005 | Blood | H26 | |
| P20 | 53 | 04-11-2005 | Blood | H27 | |
| P21 | 54 | 06-11-2005 | Blood | H30 | |
| 55 | 06-11-2005 | Stool | H30 | ||
| Hodgkin’s lymphoma | P22 | 56 | 06-10-2004 | Blood | H3 |
| P23 | 57 | 26-11-2004 | Blood | H2 | |
| 58 | 28-11-2004 | Stool | H2 | ||
| 59 | 29-11-2004 | Stool | H2 | ||
| 60 | 15-03-2005 | Stool | H2 | ||
| 61 | 18-03-2005 | Blood | H2 | ||
| P24 | 62 | 27-01-2005 | Blood | H6 | |
| P25 | 63 | 06-07-2005 | Blood | H18 | |
| 64 | 23-10-2005 | Blood | H24 | ||
| 65 | 23-10-2005 | Blood | H24 |
Recurrent E. coli bacteremia was associated with re-infection by different E. coli H genotypes
Among the 25 patients with leukemia/lymphoma hospitalized between 2004 and 2005, six patients (P1, P9, P11 P15, P23, and P25) had two or more episodes of positive E. coli blood cultures. In patient P9, we identified two episodes of bacteremia, each caused by a different E. coli H genotype (H20 and H23, respectively). Patient P11 developed two episodes of E. coli bacteremia including genotypes H28 or H29.
Correlation between the presence E. coli H genotypes in the bowel and blood cultures
Figure 2 presents examples of E. coli H fingerprints showing identical DNA patterns of isolates from the bowel and the blood. For example, patient P5 had a blood and bowel culture positive for E. coli H11. Patient P1 showed several episodes of bacteremia and identical blood and bowel E. coli, suggesting that a different E. coli inhabitant of the bowel translocated to the bloodstream. Patient P1 was hospitalized six times between September 9th 2004 and September 30, 2005, and spent a total of 236 days in the hospital due to a recurrent E. coli bacteremia with multiple E. coli isolates/H fingerprints, with identical E. coli isolates/H fingerprints identified in the bowel and with no evidence of another obvious source of infection (Table 5). Five blood cultures obtained within the above-mentioned period were positive for E. coli. E. coli isolated from blood and stool (cultures done on same day, May 10th) showed an identical H16 genotype. Following episodes of bacteremia (May 22nd, 25th, and 27th, and August 7th) were caused by an ESBL E. coli H17 genotype. This E. coli H17 was isolated from the patient’s urine on June 20th and from his stool on August 7th. Following bone marrow transplant, the patient received ciprofloxacin, and beginning May 9th, he was treated with piperacillin, tazobactam, and amikacin. Once a positive result of ESBL E. coli isolated from blood was obtained, an additional antibiotic (meropenem) was added to the therapeutic strategy and continued until July 6th, 2005. Thus, in our select patient, the E. coli H genotype that caused bacteremia was eliminated from the vascular bed following antibiotic treatment. After an asymptomatic period, the infection was replaced with another rare E. coli H genotype identical to that identified in the bowel at the time. In agreement with the established bacteriologic and DNA fingerprinting criteria for translocation, in the absence of an alternative source of infection in these patients, recurrent E. coli bacteremia resulted from E. coli translocation from the bowel to the bloodstream.
Fig. 2.
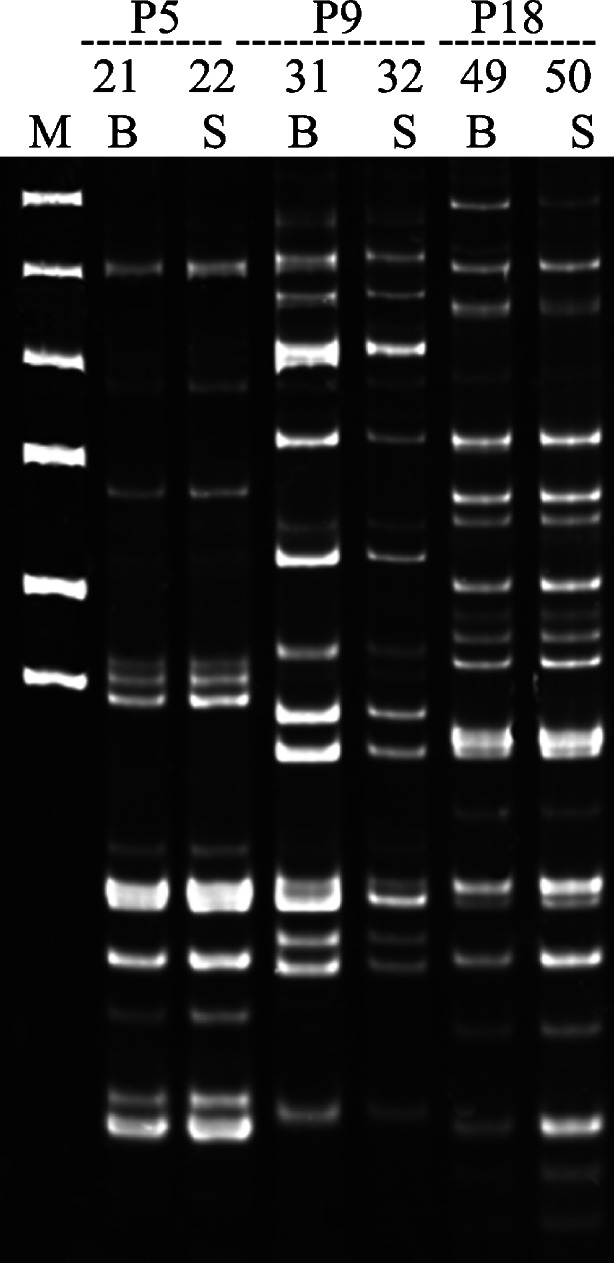
Representative results of monitoring the spread of bacteria within patients by using the PCR MP technique. The isolates shown represent three patients: genotype H11 from patient P5, genotype H20 from patient P9, and genotype H22 from patient P18. Lanes marked by numbers indicate the number of the isolate shown in Table 5. Lanes marked by B and S contain strains isolated from blood and stool, respectively. The DNA amplicons were electrophoresed on 6 % polyacrylamide gels
Discussion
Septicemia and/or bacteremia is the 13th leading cause of death in the United States, and the age-adjusted mortality rate associated with sepsis has increased over the last five decades [2, 3]. In the United States, about 500,000 cases of sepsis occur each year, one-fourth of which are associated with Gram-negative bacteremia [4]. Gram-negative bacteremia is a serious infection, for which the estimated crude mortality rate is 20–50 % [5]. It is estimated that 6–10 % of patients who have an episode of Gram-negative bacteremia will have one recurrent episode [6–9]. E. coli was reported as only the fourth most common cause of nosocomial bacteremias [10–16, 22].
In present study, we report that, unexpectedly, recurrent E. coli bacteremia was a frequent event in leukemic malignancy, and that blood re-infection usually occurred with a new E. coli DNA H genotype present in the bowel. We observed that the recurrent E. coli bacteremia in these leukemic patients was caused either by hospital strains (e.g., E. coli genotype H2) or by multiple, rare H genotypes, each representing a different E. coli strain of unknown origin. On multiple occasions, we found different E. coli H genotypes in blood samples isolated during consecutive episodes of bacteremia, despite an effective therapy following the initial infection. The lack of an identified focus of infection suggested that, after the elimination of E. coli from the bloodstream, another transient bowel inhabitant disseminated to the vascular bed. To our knowledge, the current clinical literature describes only one study including evidence of direct translocations of E. coli from the bowel to the circulation and which involves newborns with urinary tract infections [20].
A study of VRE bacteremia concluded that recurrent VRE bacteremia was uncommon, and that recurrent episodes were separated by 3 months and were caused by identical or related VRE strains [1]. Our study showed that E. coli which were resident in the bowel of a patient with leukemia were much less stable and the dominant genotype changed over a period of a few weeks. Thus, in contrast to an expected persistent several months colonization with the same strain, we observed that episodes of recurrent bacteremia represented independent re-infection events originating from the re-established new bowel E. coli isolates. Re-infection with E. coli is consistent with a study by Johnson et al. [23], who examined E. coli isolates from 35 children with recurrent urinary tract infection (UTI). Five of the nine patients were re-infected with new strains. We hypothesize that antibiotic therapy destabilized the normal intestinal flora and increased the risk for re-colonization with potentially pathogenic transient E. coli colonizers.
The exact mechanism of translocation of indigenous bacteria from the gut is not known but may involve the overgrowth of intestinal bacteria, a deficiency in the host’s immune defenses, and/or damage to the intestinal mucosal barrier [24]. The possibility exists that immunosuppressive therapy resulting in the dysfunction of white cells or the invasive capacity of some E. coli allow the translocation of E. coli to the mesenteric circulation [24–29].
The detection of frequent recurrent E. coli bacteremia suggests the need for a careful patient evaluation, as the risk of mortality is potentially higher compared to those with a history of non-E. coli bacteremia. Our data suggest that standards for the prevention of recurrent bacteremia should be re-evaluated. Clinical trials might include novel regimens for selective decontamination to eradicate the transient E. coli H genotypes from the gut and, therefore, reduce the risk for recurrent bacteremia [30].
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education grant no. N401331239 to A.Ś. and, in part, by U54 RR026140 (NCRR)/U54 MD007593 (NIMHD). The authors express their gratitude to Diana Marver PhD for her editorial comments and suggestions.
Conflict of interest
The authors certify that they have no potential conflicts of interest.
References
- 1.Baran J, Jr, Riederer KM, Ramanathan J, Khatib R. Recurrent vancomycin-resistant Enterococcus bacteremia: prevalence, predisposing factors, and strain relatedness. Clin Infect Dis. 2001;32:1381–1383. doi: 10.1086/319996. [DOI] [PubMed] [Google Scholar]
- 2.Mylotte JM, McDermott C. Recurrent gram-negative bacteremia. Am J Med. 1988;85:159–163. doi: 10.1016/S0002-9343(88)80335-8. [DOI] [PubMed] [Google Scholar]
- 3.Maslow JN, Mulligan ME, Arbeit RD. Recurrent Escherichia coli bacteremia. J Clin Microbiol. 1994;32:710–714. doi: 10.1128/jcm.32.3.710-714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein MP, Reller LB. Clinical importance of “breakthrough” bacteremia. Am J Med. 1984;76:175–180. doi: 10.1016/0002-9343(84)90770-8. [DOI] [PubMed] [Google Scholar]
- 5.Miller PJ, Farr BM. Morbidity and mortality associated with multiple episodes of nosocomial bloodstream infection: a cohort study. Infect Control Hosp Epidemiol. 1989;10:216–219. doi: 10.1086/646005. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Advance report of final mortality statistics, 1990. Monthly Vital Stat Rep. 1993;41(No. 7, Supplement):1–12. [Google Scholar]
- 7.Centers for Disease Control (CDC) Increase in National Hospital Discharge Survey rates for septicemia—United States, 1979–1987. MMWR Morb Mortal Wkly Rep. 1990;39:31–34. [PubMed] [Google Scholar]
- 8.Bone RC. Gram-negative sepsis: a dilemma of modern medicine. Clin Microbiol Rev. 1993;6:57–68. doi: 10.1128/cmr.6.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capdevila JA, Almirante B, Pahissa A, Planes AM, Ribera E, Martínez-Vázquez JM. Incidence and risk factors of recurrent episodes of bacteremia in adults. Arch Intern Med. 1994;154:411–415. doi: 10.1001/archinte.1994.00420040071011. [DOI] [PubMed] [Google Scholar]
- 10.Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002) Diagn Microbiol Infect Dis. 2004;50:59–69. doi: 10.1016/j.diagmicrobio.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41:3655–3660. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javaloyas M, Garcia-Somoza D, Gudiol F. Epidemiology and prognosis of bacteremia: a 10-y study in a community hospital. Scand J Infect Dis. 2002;34:436–441. doi: 10.1080/00365540110080629. [DOI] [PubMed] [Google Scholar]
- 13.Laupland KB, Gregson DB, Flemons WW, Hawkins D, Ross T, Church DL. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol Infect. 2007;135:1037–1042. doi: 10.1017/S0950268806007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyytikäinen O, Lumio J, Sarkkinen H, Kolho E, Kostiala A, Ruutu P, Hospital Infection Surveillance Team Nosocomial bloodstream infections in Finnish hospitals during 1999–2000. Clin Infect Dis. 2002;35:e14–e19. doi: 10.1086/340981. [DOI] [PubMed] [Google Scholar]
- 15.Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR, 3rd, St Sauver JL, Wilson WR, Baddour LM. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 16.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 17.Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/S1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 18.Schwaber MJ, Navon-Venezia S, Kaye KS, Ben-Ami R, Schwartz D, Carmeli Y. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2006;50:1257–1262. doi: 10.1128/AAC.50.4.1257-1262.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wendt C, Messer SA, Hollis RJ, Pfaller MA, Wenzel RP, Herwaldt LA. Molecular epidemiology of gram-negative bacteremia. Clin Infect Dis. 1999;28:605–610. doi: 10.1086/515151. [DOI] [PubMed] [Google Scholar]
- 20.Mahjoub-Messai F, Bidet P, Caro V, Diancourt L, Biran V, Aujard Y, Bingen E, Bonacorsi S. Escherichia coli isolates causing bacteremia via gut translocation and urinary tract infection in young infants exhibit different virulence genotypes. J Infect Dis. 2011;203:1844–1849. doi: 10.1093/infdis/jir189. [DOI] [PubMed] [Google Scholar]
- 21.Krawczyk B, Samet A, Leibner J, Śledzińska A, Kur J. Evaluation of a PCR melting profile technique for bacterial strain differentiation. J Clin Microbiol. 2006;44:2327–2332. doi: 10.1128/JCM.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouza E, Pérez-Molina J, Muñoz P. On behalf of the Cooperative Group of the European Study Group on Nosocomial Infections (ESGNI). Report of ESGNI-001 and ESGNI-002 studies. Bloodstream infections in Europe. Clin Microbial Infect. 1999;5:2S1–2S12. doi: 10.1111/j.1469-0691.1999.tb00536.x. [DOI] [Google Scholar]
- 23.Johnson CE, Maslow JN, Fattlar DC, Adams KS, Arbeit RD. The role of bacterial adhesins in the outcome of childhood urinary tract infections. Am J Dis Child. 1993;147:1090–1093. doi: 10.1001/archpedi.1993.02160340076018. [DOI] [PubMed] [Google Scholar]
- 24.Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–154. doi: 10.1016/S0966-842X(00)88906-4. [DOI] [PubMed] [Google Scholar]
- 25.Nowicki B. In vitro models for the study of uropathogens. In: Warren JW, editor. Urinary tract infections: molecular pathogenesis and clinical management. Washington DC: ASM Press; 1996. pp. 341–369. [Google Scholar]
- 26.Marschall J, Zhang L, Foxman B, Warren DK, Henderson JP, CDC Prevention Epicenters Program Both host and pathogen factors predispose to Escherichia coli urinary-source bacteremia in hospitalized patients. Clin Infect Dis. 2012;54(12):1692–1698. doi: 10.1093/cid/cis252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samel S, Keese M, Kleczka M, Lanig S, Gretz N, Hafner M, Sturm J, Post S. Microscopy of bacterial translocation during small bowel obstruction and ischemia in vivo—a new animal model. BMC Surg. 2002;2:6. doi: 10.1186/1471-2482-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowicki B, Nowicki S. DAF as a therapeutic target for steroid hormones: implications for host–pathogen interactions. Adv Exp Med Biol. 2013;734:83–96. doi: 10.1007/978-1-4614-4118-2_5. [DOI] [PubMed] [Google Scholar]
- 29.Sansonetti PJ, Arondel J, Cantey JR, Prévost MC, Huerre M. Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Meer JW, Vandenbroucke-Grauls CM. Resistance to selective decontamination: the jury is still out. Lancet Infect Dis. 2013;13:282–283. doi: 10.1016/S1473-3099(13)70014-8. [DOI] [PubMed] [Google Scholar]



