Abstract
Study Objectives:
To determine the clinical variables that best predict long- term continuous positive airway pressure (CPAP) adherence among patients with cardiovascular disease who have obstructive sleep apnea (OSA).
Design:
12-mo prospective within-trial observational study.
Setting:
Centers in China, Australia, and New Zealand participating in the Sleep Apnea cardioVascular Endpoints (SAVE) study.
Patients:
There were 275 patients age 45-70 y with cardiovascular disease (i.e., previously documented transient ischemic attack, stroke, or coronary artery disease) and OSA (4% oxygen desaturation index (ODI) > 12) who were randomized into the CPAP arm of the SAVE trial prior to July 1, 2010.
Methods:
Age, sex, country of residence, type of cardiovascular disease, baseline ODI, severity of sleepiness, and Hospital Anxiety and Depression Scale (HADS) scores plus CPAP side effects and adherence at 1 mo were entered in univariate analyses in an attempt to identify factors predictive of CPAP adherence at 12 mo. Variables with P < 0.2 were then included in a multivariate analysis using a linear mixed model with sites as a random effect and 12-mo CPAP use as the dependent outcome variable.
Measurements and Results:
CPAP adherence at 1, 6, and 12 mo was (mean ± standard deviation) 4.4 ± 2.0, 3.8 ± 2.3, and 3.3 ± 2.4 h/night, respectively. CPAP use at 1 mo (effect estimate ± standard error, 0.65 ± 0.07 per h increase, P < 0.001) and side effects at 1 mo (-0.24 ± 0.092 per additional side effect, P = 0.009) were the only independent predictors of 12- mo CPAP adherence.
Conclusion:
Continuous positive airway pressure use in patients with coexisting cardiovascular disease and moderate to severe obstructive sleep apnea decreases significantly over 12 months. This decline can be predicted by early patient experiences with continuous positive airway pressure (i.e., adherence and side effects at 1 month), raising the possibility that intensive early interventions could improve long-term continuous positive airway pressure compliance in this patient population.
Clinical Trials Register:
Clinical Trials, http://www.clinicaltrials.gov, NCT00738179.
Citation:
Chai-Coetzer CL; Luo YM; Antic NA; Zhang XL; Chen BY; He QY; Heeley E; Huang SG; Anderson C; Zhong NS; McEvoy RD. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. SLEEP 2013;36(12):1929-1937.
Keywords: Cardiovascular disease, compliance, continuous positive airway pressure, obstructive sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is a condition characterized by repeated upper airway obstruction during sleep, recurrent oxygen desaturations, and increased arousals from sleep. Untreated OSA is associated with an increased risk of hyper-tension,1 motor vehicle crashes,2 and neurocognitive impairment,3 and increasing evidence has come to light to support a possible causal link between OSA and cardiovascular disease (CVD).4–6 Continuous positive airway pressure (CPAP) therapy has been shown to improve symptoms of excessive sleepiness and quality of life, and reduce the risk of motor vehicle crashes in patients with OSA,7,8 and is considered the primary form of therapy for symptomatic, moderate to severe disease.
The Sleep Apnea cardioVascular Endpoints (SAVE) study (NCT00738179, ClinicalTrials.gov) is a large, international, multicenter, randomized controlled trial that aims to determine whether the addition of CPAP to standard medical treatment will reduce cardiovascular (CV) events in patients with coexisting CVD and moderate to severe OSA9. In this study, patients with a history of coronary artery disease or cerebrovascular disease have been randomized to receive either CPAP and standard care, or standard care alone, for an average 4-y follow-up. Patient adherence to CPAP in the treatment arm will be critically important for the success of this long-term study.
Although highly effective in abolishing sleep disordered breathing events and minimizing symptoms of OSA, CPAP therapy is not always well tolerated by patients and long-term adherence to treatment can be problematic. Previous studies examining CPAP adherence in patients with OSA have reported that the rates of CPAP nonadherence, as defined by a mean use of < 4 h per night, can vary from anywhere between 29% to 83%.10 Increased hours of CPAP use per night has been shown to be associated with greater improvements in OSA symptoms, daytime sleepiness, quality of life, and blood pressure.11–13
It has been proposed that long-term patterns of CPAP adherence are established as early as the first week after commencement of therapy.14,15 However, no individual factor has been shown to consistently predict CPAP use, and adherence to therapy is thought to be influenced by a complex array of, as yet, poorly characterized factors that may vary among individuals.16 Patient characteristics such as age, sex, and marital status have not consistently been shown to be predictors of CPAP adherence. Severity of OSA, as determined by the apnea-hypopnea index (AHI), oxygen desaturation index (ODI), or daytime sleepiness, have been shown to have a weak relationship with CPAP use in some studies,17–19 but this has not been consistently demonstrated.15 CPAP side effects also have not been consistently shown to influence CPAP adherence.20–22
Previous studies of long-term CPAP adherence have predominantly involved patients in whom symptomatic OSA has been diagnosed in a sleep clinic. In contrast, the SAVE study has focused on patients with OSA and CVD who have been recruited predominantly from cardiology and neurology clinics and who might therefore be expected to be less symptomatic than sleep clinic patients. Furthermore, the SAVE study protocol dictates that subjects with very high levels of daytime sleepiness (i.e., Epworth Sleepiness Scale (ESS) score > 15) be excluded. Greater levels of subjective daytime sleepiness as measured by ESS, as well as a patient's perception of symptomatic benefit, have previously been observed to predict CPAP use.17,20,23,24 Thus, CPAP compliance could potentially be problematic in patients, such as those in the SAVE study, who report few OSA symptoms prior to commencement of therapy. Alternatively, because patients with OSA and CVD might believe that they could potentially benefit from CPAP, patients recruited into the SAVE study may be more motivated to comply with treatment, resulting in high levels of compliance.
Recruitment for the SAVE study commenced initially in China, and then in Australia and New Zealand. Although African-American race has been shown to be associated with increased risk of nonadherence compared with Caucasian patients, few studies have compared the patterns of CPAP use among other ethnic groups, including the Chinese population. One study examining the predictors of CPAP use in a group of Chinese patients with OSA revealed a high baseline AHI as the only independent predictor of increased CPAP compliance at 1 and 3 mo.21
The aims of this study were to evaluate the average nightly hours of CPAP use in the first 12 mo for patients randomized into the treatment arm of the SAVE study, and to identify the demographic and clinical variables that predict successful, long-term CPAP adherence.
METHODS
Study Participants
All patients randomized into the CPAP treatment arm of the SAVE study from the commencement of recruitment on December 28, 2008 and prior to July 1, 2010, who survived for at least 12 mo postrandomization and for whom we had at least 1 and 12 mo of CPAP adherence data, were included in the analysis. Participants in the SAVE study were age 45-75 y and had established CVD and coexisting moderate to severe OSA. Participants in the current study were recruited from 37 centers in China, nine in Australia, and four in New Zealand. Established CVD was defined as a history of coronary artery disease (i.e., previous myocardial infarction ≥ 90 days prior; stable or unstable angina with a diagnostic coronary angiography or positive exercise stress test; multivessel percutaneous angioplasty and/or stent ≥ 90 days prior; and/or multivessel coronary artery bypass graft ≥ 1 y prior) or a history of cerebrovascular disease (i.e., previous stroke ≥ 90 days prior or neurologist-diagnosed transient ischemic attack (TIA) of the brain or retina 30 days to 1 y prior). Patients were excluded from participation if they had any of the following: coronary or carotid revascularization procedure planned within the next 6 mo; severe respiratory disease, defined as severe chronic obstructive pulmonary disease (forced expiratory volume in 1 s (FEV1)/forced vital capacity < 70% and FEV1 < 50%) or resting awake oxygen saturation (SaO2) ≤ 90% by ApneaLinkTM (ResMed, Sydney, Australia); New York Heart Association heart failure categories III-IV; stroke due to subarachnoid haemorrhage; other household member enrolled in the study; prior use of CPAP for OSA; increased risk of a sleep related motor vehicle accident and/ or excessive daytime sleepiness (driver occupation; fall asleep or near-miss accident in the previous 12 mo; and/or an ESS score > 15); > 10% overnight recording time with SaO2 ≤ 80%; or Cheyne-Stokes respirations identified during ApneaLink monitoring. A diagnosis of moderate to severe OSA was determined using a ≥ 4% oxygen desaturation index (≥ 4% ODI) ≥ 12/h on overnight monitoring with an ApneaLink device, which has been shown to have a high diagnostic accuracy for moderate to severe OSA (i.e., American Academy of Sleep Medicine 1999 criteria25 AHI ≥ 30/h)26. The SAVE study was approved by the relevant human research and ethics committees for each participating site and all participants provided written informed consent.
CPAP Initiation
Prior to study entry, potentially eligible participants undertook a 1-w run-in period with sham CPAP in order to exclude patients who were unable to comply with CPAP therapy (i.e. average use < 3 h/night). Patients who successfully completed the sham run-in phase were then randomized to receive either CPAP treatment in addition to standard care, or standard care alone, using a web-based randomisation program with stratification of treatment allocation by site, type of CVD, and OSA severity (i.e., ESS). For patients assigned to the CPAP treatment group, autotitrating CPAP (REMstar Auto M Series, Philips Respironics Inc, USA) was commenced for a 1-w period to determine a fixed treatment pressure (based on the 90th percentile pressure as determined by the autotitrating device) for subsequent CPAP therapy during the study. The same CPAP device used during the auto-CPAP trial was used for long-term treatment but was converted to a fixed pressure mode. Participants were provided with on-site training on the use of CPAP, which included viewing of a patient education DVD and a phone call 3 days after starting the sham CPAP trial. Participants randomized to CPAP were reassessed for mask fit, and provided with take-home written materials as well as central review of CPAP data by sleep research staff for troubleshooting when needed. Sleep research staff were advised to consult the “CPAP Troubleshooting Guide for SAVE Investigators/Study Coordinators” (Figure S1), which was contained within the Local Clinical Centre Manual of Procedures provided to all study sites to deal with complaints of CPAP side effects. Heated humidification was recommended if patients complained of nasal congestion or mouth/airway dryness, and added to CPAP therapy if required at the discretion of the sleep research staff. Remote monitoring of overall CPAP adherence at each study site was regularly performed each month by Regional Coordinating Centres and the Core Laboratory located in Adelaide, Australia, to identify study sites with low average CPAP adherence (i.e. < 3 h/night) or a significant proportion of patients with low adherence or who had discontinued CPAP therapy.
Outcome Measures
At baseline, individual demographic and anthropometric data were collected including patient age, sex, type of CVD, and body mass index (BMI). Severity of OSA was determined using the ≥ 4% ODI on ApneaLink monitoring, and patients completed an ESS score and answered questions about the frequency and loudness of snoring and frequency of witnessed apneas. Patients also completed the Hospital Anxiety and Depression Scale (HADS) and Short Form-36 Health Survey (SF-36).
Participants in the CPAP arm of the SAVE study at all study sites were reviewed by direct, in-person consultations with research staff on the day of randomization (baseline, day 0), at 1 w, and then at 1, 3, and 6 mo, and then every 6 mo thereafter. CPAP adherence (i.e., average h of use per night) was objectively monitored on CPAP devices using an inbuilt compliance meter with breathing detection that was downloaded by research staff at each review appointment. The residual AHI and average leak scores were also recorded and downloaded from CPAP devices. Adverse events and/or problems associated with CPAP use were documented and categorized into seven main types (i.e., mouth dryness; nasal symptoms; eye problems; claustrophobia; noise problems; facial soreness or skin irritation from the mask; and mask fit or leak problems [i.e. trouble keeping the mask in place, air leaks from the mask or difficulty putting the mask on]), with a patient receiving a score of one point for the presence of any complaints within each category, producing a total possible side effect score of seven points.
Data Collection and Statistical Analysis
Baseline patient characteristics, including demographic data, BMI, type of CVD, and measures of OSA severity (i.e. ESS and ODI), were calculated according to the region of recruitment, and then for all patients combined. CPAP adherence, in terms of the mean h of daily use, was calculated at each review for the preceding period (i.e., 5 to 10 days before randomization for the sham CPAP trial and randomisation to first mo, mo 4 to 6 and mo 7-12, for 1-, 6-, and 12-mo reviews, respectively). The percentage of patients adherent, as defined by CPAP use ≥ 4 h/ night, was also determined at 1, 6, and 12 mo. Patients who withdrew from the study during the follow-up period were retained for the purposes of this analysis and were deemed to have zero CPAP adherence at the subsequent planned follow-up visit times. If data were missing at 6 or 12 mo (e.g., missed visit or a technical problem with data download) a value was imputed if there were data recorded at the scheduled visits either side of this visit. Average daily CPAP use measured at 12 mo (i.e. the average daily CPAP use for the 7- to 12-mo follow-up period) was the dependent outcome variable of interest. Statistical analyses were conducted in SAS version 9.2 (SAS Institute, Cary, NC, USA).
To determine predictors of long-term CPAP adherence, analyses were conducted using a linear mixed model with sites as a random effect. Potential predictors were predetermined based on the results of previous studies, which have shown that a patient's very early experience with CPAP appears to predict long-term treatment adherence. We focused particular attention on the influence of early (i.e., 1 mo) side effects and levels of CPAP adherence. For the univariate analysis, potential predictors were age, sex, BMI, country of recruitment, socioeconomic status (SES, as determined by the gross domestic product [GDP] of the region of recruitment), type of medical department, CVD type, ≥ 4% ODI, baseline ESS and OSA symptoms, change in ESS from baseline to 1 mo, CPAP adherence during the sham trial and at 1 mo, CPAP side effects at 1 mo, and baseline HADS and SF-36 scores. Age, sex, country of recruitment, and variables found on univariate analysis to have a P value < 0.20 were included in the multivariate analysis to determine the independent predictors of 12-mo CPAP adherence. A P value < 0.05 was considered to be statistically significant.
RESULTS
Baseline Characteristics
A total of 710 patients completed a 1-w trial of sham CPAP, 603 (85%) of whom used CPAP for at least 3 h per night. There were 590 of these patients who were eligible for and were randomized into the SAVE study prior to July 1, 2010, 296 of whom were allocated to the CPAP study arm (Figure 1). Of these, 275 patients (225 patients from China, and 50 patients from Australia or New Zealand) had available CPAP adherence data at 1 and 12 mo and were included in the analysis. Twenty-one patients were excluded for the following reasons: one patient died prior to the 12-mo review; two patients were found to be ineligible for the study after randomization; five patients were removed due to a violation of study protocol at the site of recruitment; data were not available for one patient due to failure of the CPAP download card and results could not be imputed; 10 patients did not attend their 12-mo review appointment, and two patients withdrew from the study and 1-mo CPAP data were unavailable. Patients who were excluded from the analysis tended to have a higher proportion of coronary artery disease than transient ischemic attack (TIA) or stroke, higher ESS scores, reported louder snoring, had fewer CPAP side effects, and had lower mean nightly hours of CPAP use at 1, 3, and 6 mo, compared to patients analyzed.
Figure 1.

Flow diagram of patient recruitment and randomization.
The baseline characteristics of the patients, separated by country of recruitment, are shown in Table 1. Patients enrolled in the SAVE study were predominantly middle-aged, over-weight, or obese males with moderate to severe OSA. Patients recruited in China had a lower mean (± standard deviation [SD]) BMI (27.6 ± 3.7 kg/m2) than those from Australia and New Zealand (31.9 ± 4.3 kg/m2). However, there was a higher percentage of patients with more severe degrees of OSA in the Chinese group (43% versus 28% with a ≥ 4% ODI > 30/h). Despite this, patients from Australia and New Zealand reported higher degrees of sleepiness on the ESS score than the Chinese group (36% versus 23% with ESS ≥ 10). In terms of the CVD type, there were similar proportions of patients with coronary artery disease and cerebrovascular disease in the Chinese group, whereas almost all patients from Australia and New Zealand had coronary artery disease.
Table 1.
Summary of participant characteristics at baseline

CPAP Adherence
The mean nightly hours of CPAP use during the sham trial and at 1, 6, and 12 months are shown in Table 2. Average CPAP use per night was similar in the Chinese and Australia/New Zealand groups at all time points. Over the first 12 mo of the SAVE trial, there appeared to be a progressive decline in mean nightly CPAP use in both the Chinese and Australia/New Zealand groups, which fell from 4.4 ± 2.0 h/night at 1-mo follow-up to 3.3 ± 2.4 h/night at 12 mo in the two groups combined.
Table 2.
Continuous positive airway pressure adherence data - average hours use per night
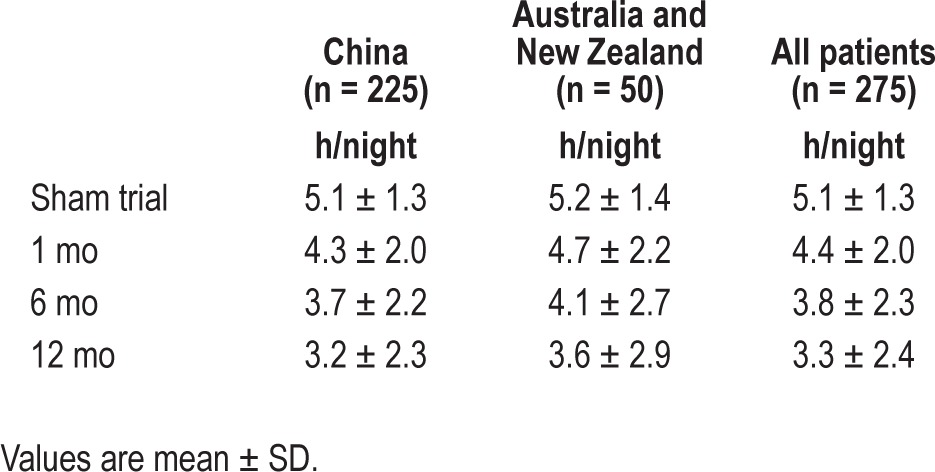
Table 3 shows the percentage of patients adherent to CPAP at 1, 6, and 12 months, as defined by a mean nightly CPAP use ≥ 4 h/night. The percentage of patients adherent to CPAP progressively decreased over the 12-mo follow-up period. At 1 mo, 62% (n = 171) patients in the study were using CPAP ≥ 4 h/night, with a drop to 39% (n = 107) by 12 mo.
Table 3.
Continuous positive airway pressure adherence data - percentage using continuous positive airway pressure ≥ 4 h/night

Predictors of Long-Term CPAP Adherence
On univariate analysis, the variables associated with CPAP adherence at 12 mo were sham and 1-mo CPAP adherence, baseline ESS score, change in ESS score from baseline to 1 mo, and loudness of snoring (Table 4). Increasing hours of daily use of CPAP during the sham trial and increasing CPAP adherence at 1 mo were significantly associated with higher CPAP adherence at 12 mo. Lesser degrees of subjectively recorded sleepiness, as indicated by an ESS score between 0 to 5, relative to a higher ESS score of 11 to 15, was associated with lower CPAP use at 12 mo. Greater reductions in ESS score from baseline to 1 mo were associated with higher levels of CPAP use at 12 mo. Patients who reported their snoring loudness as being “very loud” recorded increased hours of average daily CPAP use compared to those with quieter levels of snoring.
Table 4.
Predictors of continuous positive airway pressure adherence at 12 mo - univariate analysis

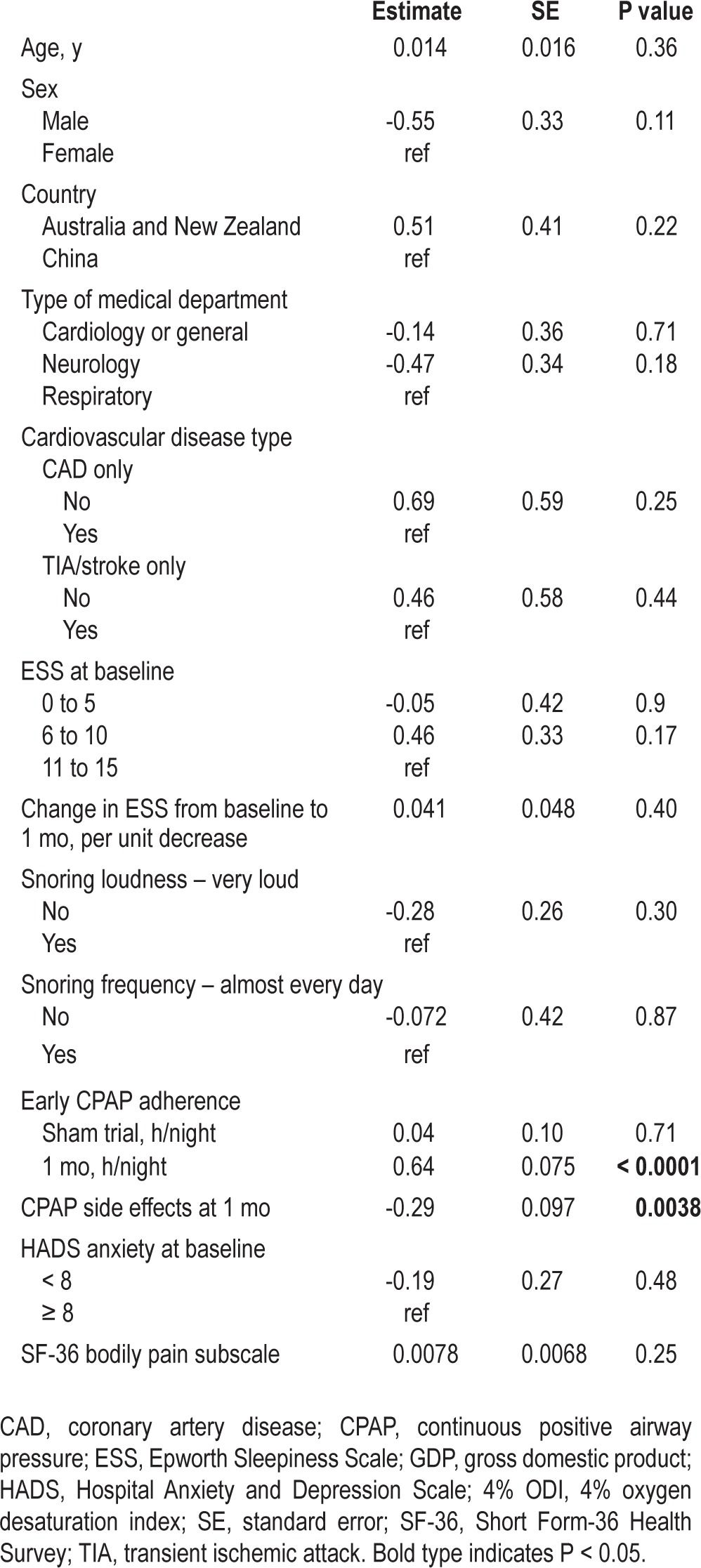
Age, sex, country of recruitment, and variables with P < 0.20 on univariate analysis, namely the type of medical department, baseline ESS score, change in ESS from baseline to 1 mo, sham CPAP adherence, 1-mo CPAP adherence, CPAP side effect score, history of coronary artery disease only, history of TIA or stroke only, snoring loudness, snoring frequency, HADS anxiety score, and SF-36 bodily pain subscale score, were included in the multivariate analysis. The results of the multivariate analysis are shown in Table 5. Independent predictors of CPAP adherence at 12 mo were the mean daily CPAP use at 1 mo (P < 0.0001) and the side effect score at 1 mo (P = 0.0038). The results equate to a 38-min increase in 12-mo CPAP use for every hour increase in CPAP use at 1 mo, and a 17-min reduction in 12-mo CPAP use for every one-point increase in the CPAP side effect score at 1 mo.
Table 5.
Predictors of continuous positive airway pressure adherence at 12 mo - multivariate analysis
The frequency of CPAP side effects by category reported by patients at 1 mo are shown in Table 6. The most commonly reported CPAP side effects at 1 mo were dry mouth, nasal symptoms, and mask fit or leak problems. A significantly higher proportion of patients from Australia/New Zealand reported experiencing CPAP side effects compared to patients from China for six of the seven possible categories.
Table 6.
Frequency of reported continuous positive airway pressure side effects at 1-mo follow-up

DISCUSSION
In patients with CVD and moderate to severe OSA in the treatment arm of the SAVE study, mean CPAP usage at the 12-mo review was 3.3 ± 2.4 h/night. The factors that were found to predict successful, long-term CPAP adherence were the average hours of nightly CPAP use and side effects at 1 mo following initiation of therapy. Twelve-mo CPAP adherence was not found to be associated with nationality, SES, type of CVD, OSA severity (as measured by baseline OSA symptoms, ≥ 4% ODI or ESS score), or SF-36 or HADS scores.
Our finding that early CPAP use predicts long-term adherence is consistent with results from previous studies. McArdle et al. examined the determinants of objective CPAP compliance in 1,211 consecutive patients with symptomatic OSA who were prescribed CPAP with a median duration of follow-up of 22 mo (interquartile range, 12 to 36 mo).17 In this study, average nightly CPAP use at the first clinic visit (conducted within the first 3 mo of treatment initiation) ≥ 2 h/night was found to be strongly predictive of long term CPAP use. Similarly, in a study by Popescu et al.,27 increasing mean hours of CPAP use during an initial 2-w trial was associated with continuing CPAP use at 1 y.
Studies have shown that patterns of CPAP adherence are in fact established very early, possibly within the first week, following treatment initiation. Weaver et al. demonstrated in an analysis of the night-to-night variability between 17 “consistent” (CPAP use > 90% of nights) and 15 “intermittent” users of CPAP that significant differences in the duration of use was evident between groups by the fourth night of therapy.14 This finding was confirmed in a subsequent study by Budhiraja et al.15 involving 100 patients with OSA treated with CPAP, which showed that good adherence to therapy (as defined by ≥ 4 h of CPAP use/night) at 3 days posttreatment initiation was predictive of CPAP adherence measured at 30 days.
Side effects from CPAP are commonly reported, with approximately two-thirds of patients reporting at least some degree of difficulty tolerating therapy.24 In our study, increasing side effect score at 1 mo was found to be independently associated with reduced CPAP adherence at 12 mo. Lewis et al. demonstrated that patients who reported problems during the first night of CPAP use had significantly lower CPAP usage after 1-mo follow-up.28 Engleman et al. also found an increase in CPAP mask time (i.e., time spent at an effective CPAP pressure) at 1-3 mo in patients reporting no side effects from CPAP compared to those reporting at least one side effect (mean 4.1 ± standard error 0.6 versus 2.4 ± 0.4 h/night, P = 0.02).29 However, an association between CPAP side effects and treatment adherence has not been consistently demonstrated in other studies.20–22
Although CPAP adherence in the SAVE study appeared to be lower than ideal, with only 39% of patients using their device ≥ 4 h/night at 12 mo, our results are consistent with previous studies that have assessed CPAP use in patients with CVD. Others who have used CPAP in patients with OSA recruited from stroke services30–33 or patients with central sleep apnea recruited from heart failure clinics34 have shown similar patterns of adherence. For example, Martinez-Garcia et al. conducted a 5-y follow-up study involving 166 patients who underwent polysomnography testing at least 2 mo after suffering from an ischemic stroke.30 Of the 96 patients who were found to have moderate to severe OSA (i.e., AHI ≥ 20/h) and offered CPAP therapy, 43 patients (45%) withdrew therapy within the first 6 mo, and by 5 y follow-up, only 28 patients (29%) were using their device for ≥ 4 h/night on ≥ 70% of nights.
A recently published Spanish study by Barbé et al.35 examined the effect of CPAP therapy on the incidence of CV events in nonsleepy (i.e., ESS ≤ 10) patients with OSA. They showed no effect of CPAP therapy on the incidence of hypertension and CV events among patients overall, but on post hoc analysis a statistically significant reduction in the incidence of hypertension and CV events was found amongst those patients using CPAP ≥ 4 h/night. Barbé et al. reported a higher CPAP adherence rate than in our study, with 230 of 357 patients (64%) in the treatment arm using CPAP for ≥ 4 h/night after a median 4-y follow-up. The reason for the difference in CPAP adherence is unclear, although patients in their study were recruited from sleep clinics with symptoms of snoring and witnessed apneas with no prior history of a CV event. In contrast, participants in the current study were recruited in stroke or cardiac clinics, were on average 10 y older, with established CVD at entry, and most had little to no prior knowledge or concern about OSA. Thus, the differences in CPAP adherence between the two studies may relate to differences in patient selection, average age, and degree of underlying CV morbidity.
No previous studies have directly compared long-term CPAP adherence in Chinese and Caucasian populations. A recently published study by Wang et al. assessed CPAP adherence in a group of 193 patients with OSA in China36 and also found relatively low compliance levels. After a mean (± SD) follow-up period of 59 (± 32) mo, only 100 patients (52%) patients reported still using CPAP, of whom 17 patients did not fulfill adherence criteria (as defined by CPAP use ≥ 4 h/night for ≥ 70% of nights per w). Sixty-four patients (33%) reported that therapy did not commence after the initial CPAP titration study, and 29 patients (15%) discontinued CPAP after an initial period of use. Another study by Hui et al.21 previously evaluated the pattern and determinants of CPAP compliance in a Chinese population residing in Hong Kong. In a group of 112 newly diagnosed patients with OSA (AHI ≥ 10 events/h) plus self-reported sleepiness in a respiratory clinic, objective compliance was 5.4 ± 1.6 h/night at 1 mo and 5.3 ± 1.6 h/night at 3 mo. The percentage of patients using CPAP ≥ 4 h/night for at least 70% of nights was 75 ± 28% at 1 mo and 72 ± 28% at 3 mo, which is higher than in the SAVE study. Unlike our study findings, the only factor found to be predictive of CPAP compliance at 1 and 3 mo was a high baseline AHI, and CPAP side effects were not found to be associated with treatment adherence. Other studies have also found disease severity, as measured by either the AHI or ODI, to be an independent predictor of CPAP adherence17–19,27; however, this was not demonstrated in our study population, nor in several other studies.15,29
It is noteworthy that country or region was not a significant predictor of CPAP adherence. CPAP treatment for OSA was discovered in Australia approximately 30 y ago37 and is now widely deployed as first-line treatment for OSA. All but one of the sites in Australia and New Zealand involved in the SAVE study at this time were led by respiratory investigators experienced with CPAP treatment. By comparison, sleep services in China and CPAP availability in particular were markedly limited. Furthermore, one-third (12 of 37) of the sites in China were led by neurologists or cardiologists with no previous experience in the diagnosis or management of OSA. Thus, it appears that the SAVE study protocols, investigator training, and patient educational materials ensured a reasonably even level of CPAP adherence despite diverse cultural and medical experiences.
The finding in the current study that adherence and side effects at 1 mo were independent predictors of long-term CPAP use has direct implications for the conduct of the SAVE study. Although the finding of a CPAP adherence level of 3.3 h/night at 12 mo is in keeping with our a priori assumption about long-term CPAP adherence levels in this study population, early, more intensive in-trial interventions to optimize CPAP use and minimize side effects may nevertheless improve long-term CPAP compliance and the chances of showing a benefit with respect to CV outcomes. The findings also point to the general challenge of deploying CPAP treatment for OSA in minimally symptomatic, nonsleep center-referred patients who have significant co-morbidities.
It was interesting to note that the baseline characteristics of patients with OSA and CVD were different between those recruited from China and those from Australia and New Zealand, with Chinese patients having an overall lower mean BMI and ESS scores but greater severity of OSA as determined by the ≥ 4% ODI. Previous studies have reported a similar prevalence of OSA in Asian countries compared to Caucasian populations despite a lower prevalence of obesity.38,39 Craniofacial abnormalities that cause structural narrowing of the upper airway are thought to be an important risk factor for OSA in Asian populations.40 It was also interesting to find a higher proportion of patients reporting CPAP side effects at 1 mo from the Australia/New Zealand group compared to those from China. Although it is possible that some of these differences may have resulted from anatomical/physiological variations between the groups, the fact that significant differences between them were noted in all but one side effect category suggests that cultural differences and/or reporting biases were the likely reasons.
Our study has several limitations. Most of the participants in this study were from China, with a smaller number of patients from Australia and New Zealand. It may be difficult to generalize the findings to other cultural or ethnic groups, although the convergence of results between these two remarkably different cultural, ethnic, and medical systems suggests to us that this is not a major limitation of our study. We also acknowledge that the patients in this study had already been given a 1-w trial of sham CPAP prior to study entry and were excluded if they did not use CPAP for at least 3 h during the sham trial. Therefore, patients who did not initially tolerate or accept CPAP therapy were not included in our analysis. However, 85% of patients who undertook the sham CPAP trial used the device for at least 3 h and were potentially eligible for inclusion in the SAVE study. Although exclusion of the 15% of patients in whom the sham trial failed may have resulted in a slight elevation in the adherence rates, we believe this is a reflection of clinical practice whereby patients will trial CPAP prior to committing to long-term therapy.
In summary, this is a report of our early experience with CPAP use in patients at high CVD risk with moderate to severe OSA in the treatment arm of the SAVE study, who have been recruited from China, Australia, and New Zealand. CPAP adherence in the study group overall at 12 mo was similar to previous studies of adherence, and although it appeared to be relatively low with less than 40% of participants using their CPAP device for at least 4 h per night after 12 mo, this was not unexpected given the selection of patients from cardiovascular clinics, with an average age greater than that generally encountered in sleep clinic populations and with high levels of cardiovascular comorbidity. Average nightly CPAP use and the number of side effects at 1 mo were found to be independent predictors of long-term CPAP adherence. Thus, interventions to optimize early CPAP use and minimize side effects may be helpful in improving longer term CPAP adherence in patients with CVD and moderate to severe OSA.
DISCLOSURE STATEMENT
The major sponsor of the project was Philips Respironics (direct funding plus CPAP equipment) with additional sponsorship from ResMed (diagnostic equipment), Fisher & Paykel (direct funding) and the Australian National Health and Medical Research Council (Project grant 1006501). Dr. Antic has received an honorarium for a lecture series and other educational sessions from ResMed. Dr. Heeley has received funding from the National Health and Medical Research Council of Australia. Dr. Anderson has received a speaker fee and travel expenses to attend a Global Stroke Symposium from General Electric. Other than the funding for the project, the other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Qiang Li for his statistical advice and assistance, as well as SAVE study project managers, Aiwu Song (China) and Ruth Martens (Australia).
The authors acknowledge the following medical centers that participated in the study:
China – Fuwai Hospital: Z. Liu; The First Affiliated Hospital of Nanjing Medical University: X. Zhang; Guangdong Provincial People's Hospital: Q. Ou; The First Affiliated Hospital of Guangzhou Medical College: Y. Lou; Central Hospital Baotou: Y. Li; The Second Affiliated Hospital of Soochow University: R. Chen; Beijing Shijitan Hospital (General Railway Hospital), Department of Neurology: M. He; Jiangsu Provincial Hospital of State Organ, Cardiology Department: Z. Pan; Hebei Medical University Second Hospital: L. Tai; General Hospital of Tianjin Medical University: B. Chen; Hejian Hospital in Hebei Province: B. Du; Peking Union Medical College Hospital: Y. Xiao; Shanghai Huadong Hospital: H. Zhu; The Fifth Affiliated Hospital of Zhongshan University (Zhuhai) Sun Tat-Sen University: Z. Li; The Third Hospital of Hebei Medical University: H. Wang; Xinhua Hospital Affiliated to Shanghai Jiatong University School of Medicine: X. Guo; Beijing Haidian Hospital: F. Yu; The Second Affiliated Hospital of Guangzhou Medical College: E. Xu; Beijing Police Hospital: G. Xiao; Zhejiang Hospital: G. Qin; First Affiliated Hospital of Chinese Medical University: W. Wang; Beijing Shijitan Hospital (General Railway Hospital), Sleep Center: L. Pan; Guangxi Provincial People's Hospital: J. Liu; Shanghai Ruijin Hospital: M. Li; Zhejiang Hospital: M. Li; The First People's Hospital of Foshan: G. Zhen; Jiangsu Provincial Hospital of State Organ: G. Lu; Shanghai Pulmonary Hospital: Y. Liang; Zhongshan Hospital Fudan University: S. Li; The First Affiliated Hospital of Shanxi Medical University: S. Ren; Shidong Hospital Yanpu District, Cardiology Department: M. Zhou; Shougang Hospital: W. Gao; 301 Hospital: L. Gai; Sino-Japan Friendship Hospital: J. Lin; The First People's Hospital of Changzhou: C. Li; Beijing Tongren Hospital, Neurology Department: X. Zhang; First Affiliated Hospital of Baotou Medical College, China; L. Wu.
Australia – Western Australian Sleep Disorders Research Institute: N. McArdle; Royal Prince Alfred Hospital: C. Anderson; Repatriation General Hospital: D. McEvoy; Lyell McEwin Hospital: B. Jeffries; Caulfield Clinical Trials Centre: M. Naughton; The Royal Melbourne Hospital: J. Goldin; Flinders Medical Centre: D. Chew; Eastern Clinical Research Unit, Box Hill: A. Young; The Prince Charles Hospital: J. Douglas.
New Zealand – Christchurch Hospital: M. Hlavac; Waikato Hospital: C. Chang; Dunedin Hospital: R. Taylor; Hutt Hospital: K. Ferrier.
SUPPLEMENTAL MATERIAL
CPAP Troubleshooting Guide for SAVE Investigators/Study Coordinators.
REFERENCES
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 3.Engleman HM, Douglas NJ. Sleep 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 7.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEvoy RD, Anderson CS, Antic NA, et al. The sleep apnea cardiovascular endpoints (SAVE) trial: rationale and start-up phase. J Thorac Dis. 2010;2:138–43. doi: 10.3978/j.issn.2072-1439.2010.02.03.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 14.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 15.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–4. [PubMed] [Google Scholar]
- 16.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 18.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–32. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 19.Krieger J, Kurtz D, Petiau C, Sforza E, Trautmann D. Long-term compliance with CPAP therapy in obstructive sleep apnea patients and in snorers. Sleep. 1996;19(9 Suppl):S136–43. doi: 10.1093/sleep/19.suppl_9.s136. [DOI] [PubMed] [Google Scholar]
- 20.Hoffstein V, Viner S, Mateika S, Conway J. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Patient compliance, perception of benefits, and side effects. Am Rev Respir Dis. 1992;145:841–5. doi: 10.1164/ajrccm/145.4_Pt_1.841. [DOI] [PubMed] [Google Scholar]
- 21.Hui DS, Choy DK, Li TS, et al. Determinants of continuous positive airway pressure compliance in a group of Chinese patients with obstructive sleep apnea. Chest. 2001;120:170–6. doi: 10.1378/chest.120.1.170. [DOI] [PubMed] [Google Scholar]
- 22.Engleman HM, Asgari-Jirhandeh N, McLeod AL, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest. 1996;109:1470–6. doi: 10.1378/chest.109.6.1470. [DOI] [PubMed] [Google Scholar]
- 23.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 24.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 26.Gantner D, Ge JY, Li LH, et al. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnoea in a Chinese population at high cardiovascular risk. Respirology. 2010;15:952–60. doi: 10.1111/j.1440-1843.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 27.Popescu G, Latham M, Allgar V, Elliott MW. Continuous positive airway pressure for sleep apnoea/hypopnoea syndrome: usefulness of a 2 week trial to identify factors associated with long term use. Thorax. 2001;56:727–33. doi: 10.1136/thorax.56.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27:134–8. doi: 10.1093/sleep/27.1.134. [DOI] [PubMed] [Google Scholar]
- 29.Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49:263–6. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–9. doi: 10.1136/jnnp.2005.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–7. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 33.Bravata DM, Concato J, Fried T, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34:1271–7. doi: 10.5665/SLEEP.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 35.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Gao W, Sun M, Chen B. Adherence to CPAP in patients with obstructive sleep apnea in a Chinese population. Respir Care. 2012;57:238–43. doi: 10.4187/respcare.01136. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, In K, You S, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 39.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–9. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 40.Lam B, Ip MS, Tench E, Ryan CF. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax. 2005;60:504–10. doi: 10.1136/thx.2004.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CPAP Troubleshooting Guide for SAVE Investigators/Study Coordinators.


