Significance
Multiple system atrophy (MSA) is a neurodegenerative disorder characterized by the accumulation of misfolded α-synuclein protein in glial cells within the brain. Transgenic mice expressing mutant α-synuclein that were inoculated with brain homogenate from MSA patients developed clinical, biochemical, and pathological signs of a neurodegenerative disease, indicating that MSA is transmissible under certain conditions. This transmissibility is reminiscent of the human prion disorders, such as Creutzfeldt–Jakob disease, and suggests that MSA is caused by the accumulation of toxic α-synuclein prions in the brain.
Keywords: neurodegeneration, bioluminescence imaging, seeding, proteinopathies
Abstract
Prions are proteins that adopt alternative conformations, which become self-propagating. Increasing evidence argues that prions feature in the synucleinopathies that include Parkinson’s disease, Lewy body dementia, and multiple system atrophy (MSA). Although TgM83+/+ mice homozygous for a mutant A53T α-synuclein transgene begin developing CNS dysfunction spontaneously at ∼10 mo of age, uninoculated TgM83+/− mice (hemizygous for the transgene) remain healthy. To determine whether MSA brains contain α-synuclein prions, we inoculated the TgM83+/− mice with brain homogenates from two pathologically confirmed MSA cases. Inoculated TgM83+/− mice developed progressive signs of neurologic disease with an incubation period of ∼100 d, whereas the same mice inoculated with brain homogenates from spontaneously ill TgM83+/+ mice developed neurologic dysfunction in ∼210 d. Brains of MSA-inoculated mice exhibited prominent astrocytic gliosis and microglial activation as well as widespread deposits of phosphorylated α-synuclein that were proteinase K sensitive, detergent insoluble, and formic acid extractable. Our results provide compelling evidence that α-synuclein aggregates formed in the brains of MSA patients are transmissible and, as such, are prions. The MSA prion represents a unique human pathogen that is lethal upon transmission to Tg mice and as such, is reminiscent of the prion causing kuru, which was transmitted to chimpanzees nearly 5 decades ago.
Intracellular protein deposits containing aggregated α-synuclein are the predominant neuropathological feature of three human neurodegenerative diseases: Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). MSA includes the diseases previously referred to as Shy–Drager syndrome, olivopontocerebellar atrophy, and striatonigral degeneration (1, 2). Patients with MSA typically develop signs of parkinsonism such as muscle rigidity and tremor, cerebellar dysfunction including ataxia, as well as autonomic nervous system deficits. In MSA brains, argyrophilic protein deposits in oligodendrocytes are often referred to as glial cytoplasmic inclusions (GCIs) (3). Whereas neurons containing Lewy bodies composed of aggregated α-synuclein are the hallmark of PD and DLB, GCIs containing aggregated α-synuclein are pathognomonic of MSA (4–6). Transgenic (Tg) mice that overexpress wild-type (WT) human α-synuclein specifically in oligodendrocytes develop GCI-like α-synuclein deposits and exhibit loss of oligodendrocytes and neurons (7), suggesting a causal role for α-synuclein aggregates in MSA.
It is becoming increasing clear that many human neurodegenerative diseases are caused by the self-propagation of alternative protein conformations that are β-sheet rich and have a propensity to aggregate (8, 9). These characteristics are indistinguishable from those of prions, and like prions, these pathogenic proteins exhibit progressive spread from one region of the CNS to another (10). Many features of prions were first described for the protein that causes scrapie in sheep and Creutzfeldt–Jakob disease (CJD) in humans. In these diseases, misfolded prion protein (PrP) induces the conformational conversion of normal cellular PrP (PrPC) into a disease-causing isoform (PrPSc) (11). The prion nature of Aβ has been described for Alzheimer’s disease (12, 13). Importantly, a different protein causes each distinct group of neurodegenerative diseases after it undergoes transformation into a prion.
Evidence is mounting that α-synuclein aggregates can become prions. In PD, α-synuclein aggregates are first observed in the brainstem and then spread to more cortical regions of the brain (14), indicative of a prion-like spread of protein aggregation. Furthermore, Lewy bodies were found in fetal substantia nigra tissue that had been grafted into the striatum of PD patients ∼10 y earlier, suggesting a host-to-graft spreading of α-synuclein aggregates (15–17). Numerous studies have also demonstrated cell-to-cell transmission of soluble or aggregated α-synuclein both in cultured cells and in mouse brains, resulting in α-synuclein aggregation and neuronal dysfunction in the recipient cells (18–21). Recently, it was demonstrated that intracerebral inoculation of Tg mice expressing human α-synuclein containing the familial PD-associated A53T mutation, denoted TgM83+/+ mice (22), with either brain homogenate from aged, spontaneously ill TgM83+/+ mice or preformed amyloid fibrils composed of recombinant α-synuclein, resulted in clinical and pathological signs of neurologic dysfunction in the inoculated mice (23, 24). These results argued for a prion-mediated induction of disease; however, the transmissions were performed in TgM83+/+ mice, which ultimately developed spontaneous neurologic dysfunction, contending that this phenomenon may be more akin to disease acceleration rather than true de novo induction. Inoculation of WT mice with recombinant α-synuclein fibrils resulted in the induction of Lewy body-like α-synuclein deposition as quickly as 90 d postinoculation (25), whereas inoculation of WT mice with DLB brain extract resulted in only minor deposition of α-synuclein after 450 d (26).
To investigate whether α-synuclein aggregates in the brains of synucleinopathy patients initiate disease when inoculated into Tg mice, we used hemizygous TgM83+/− mice expressing lower levels of α-synuclein and that do not develop signs of spontaneous neurologic illness. Intracerebral inoculation of these mice with brain homogenate from MSA patients resulted in the induction of a rapidly progressive synucleinopathy characterized by clinical signs of neurologic dysfunction and deposition of aggregated α-synuclein in the brain. This de novo induction of disease in inoculated mice provides compelling evidence that α-synuclein prions are present in the brains of MSA patients.
Results
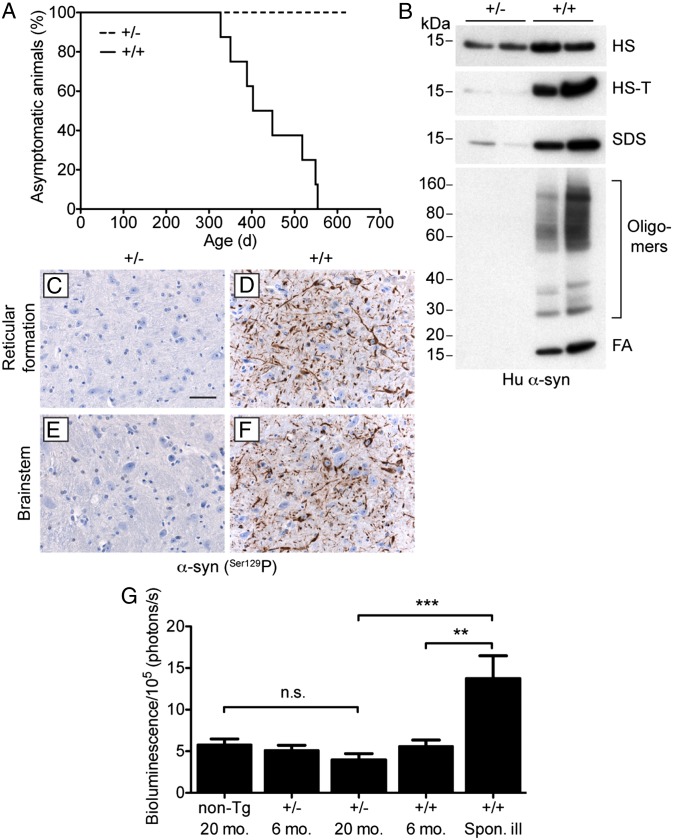
TgM83+/+ mice homozygous for a mutant A53T human α-synuclein transgene exhibit signs of spontaneous neurologic dysfunction beginning at ∼240 d of age (22). We crossed homozygous TgM83+/+ mice with Tg(Gfap-luc) reporter mice (27) to generate bigenic mice that were hemizygous for both transgenes, denoted Tg(M83+/−:Gfap-luc) mice. We also generated bigenic mice in which the M83 transgene is present in a homozygous state, denoted Tg(M83+/+:Gfap-luc) mice. We found that Tg(M83+/−:Gfap-luc) mice express mutant α-synuclein ∼40% lower relative to Tg(M83+/+:Gfap-luc) mice (Fig. S1 A and B). Tg(M83+/−:Gfap-luc) mice did not show any signs of spontaneous neurologic illness for greater than 600 d (Fig. 1A and Table 1). In contrast, Tg(M83+/+:Gfap-luc) mice developed signs of spontaneous neurologic dysfunction, including ataxia, dysmetria, bradykinesia, and circling behavior, beginning at ∼320 d of age (Fig. 1A and Table 1).
Fig. 1.
Absence of spontaneous neurologic disease in hemizygous Tg(M83+/−:Gfap-luc) mice. Tg(M83+/−:Gfap-luc) mice are denoted as “+/−” and Tg(M83+/+:Gfap-luc) mice as “+/+.” (A) Kaplan–Meier curves for the appearance of signs of spontaneous neurologic dysfunction in Tg(M83+/−:Gfap-luc) (dashed line, n = 7) and Tg(M83+/+:Gfap-luc) (solid line, n = 8) mice. Only the Tg(M83+/+:Gfap-luc) mice developed spontaneous disease. (B) Sequential extraction of α-synuclein from the brains of two asymptomatic Tg(M83+/−:Gfap-luc) mice at 629 d of age (lanes 1–2) and two spontaneously ill Tg(M83+/+:Gfap-luc) mice at 549 d (lanes 3) and 389 d (lanes 4) of age. Brain homogenates were sequentially extracted with high salt (HS), high salt containing Triton X-100 (HS-T), SDS, and formic acid (FA) buffers and then analyzed by immunoblotting for human α-synuclein (Syn211 antibody). Aggregated, FA-extractable α-synuclein was only observed in the spontaneously ill Tg(M83+/+:Gfap-luc) mice. (C–F) Immunohistochemical detection of phosphorylated α-synuclein in the brain of an asymptomatic Tg(M83+/−:Gfap-luc) mouse at 629 d of age (C and E) and a spontaneously ill Tg(M83+/+:Gfap-luc) mouse at 549 d of age (D and F). The reticular formation is shown in C and D and the brainstem in E and F. Phosphorylated α-synuclein deposits were only observed in the spontaneously ill Tg(M83+/+:Gfap-luc) mice. [Scale bar (in C): 50 μm (applies to D–F).] (G) Quantification of brain BLI signals (n = 6–14 per group) in asymptomatic Tg(M83+/+:Gfap-luc) and Tg(M83+/−:Gfap-luc) mice as well as Tg(Gfap-luc) mice (“non-Tg”) at the ages indicated, compared with spontaneously ill Tg(M83+/+:Gfap-luc) mice between 308 and 554 d of age (last BLI scans taken at 282–548 d, before the appearance of clinical symptoms). Brain BLI levels were significantly higher in spontaneously ill Tg(M83+/+:Gfap-luc) mice compared with either young Tg(M83+/+:Gfap-luc) mice (**P < 0.01) or aged Tg(M83+/−:Gfap-luc) mice (***P < 0.001), whereas levels were not significantly different (n.s.) between aged Tg(M83+/−:Gfap-luc) and Tg(Gfap-luc) mice.
Table 1.
Transmission of α-synuclein prions to Tg(M83+/−:Gfap-luc) mice
| Inoculum | Line | Mean incubation time ± SEM, d | Mean age of disease onset ± SEM, d | Signs of neurological dysfunction, n/n0* |
| None | Tg(M83+/+:Gfap-luc) | — | 442 ± 32 | 8/8 |
| Tg(M83+/−:Gfap-luc) | — | >620 | 0/7 | |
| Spontaneously ill TgM83+/+ (i) | Tg(M83+/−:Gfap-luc) | 216 ± 18 | 280 ± 18 | 8/8 |
| Spontaneously ill TgM83+/+ (ii) | Tg(M83+/−:Gfap-luc) | 217 ± 13 | 275 ± 13 | 7/7 |
| Human control | Tg(M83+/−:Gfap-luc) | >362 | >412 | 0/8 |
| MSA (case i) | Tg(M83+/−:Gfap-luc) | 143 ± 16 | 206 ± 16 | 7/8 |
| MSA (case ii) | Tg(M83+/−:Gfap-luc) | 106 ± 11 | 169 ± 11 | 8/8 |
n, number of ill mice; n0, number of mice observed/inoculated.
In sequential extraction experiments using a series of buffers with increasing ability to solubilize proteins, brains from aged (389- and 549-d-old), spontaneously ill Tg(M83+/+:Gfap-luc) mice contained large quantities of SDS- and formic acid-extractable human α-synuclein, including oligomeric and aggregated species that exhibited lower electrophoretic mobilities (Fig. 1B). In contrast, no formic acid-extractable and little SDS-extractable human α-synuclein was observed in 629-d-old asymptomatic Tg(M83+/−:Gfap-luc) mice. Because the primary pathologic α-synuclein species is phosphorylated at codon S129 (28), we also examined phosphorylated α-synuclein deposition in the brain. Abundant deposits of phosphorylated α-synuclein were observed in the reticular formation and brainstem in spontaneously ill Tg(M83+/+:Gfap-luc) mice, but not in 629-d-old asymptomatic Tg(M83+/−:Gfap-luc) mice (Fig. 1 C–F). Elevated levels of glial fibrillary acidic protein (GFAP) staining, indicative of astrocytic gliosis, as well as increased staining of Iba1, which is a marker of activated microglia, were also observed in the brainstems of spontaneously ill Tg(M83+/+:Gfap-luc) mice but not in asymptomatic, aged Tg(M83+/−:Gfap-luc) mice (Fig. S2).
Previously, we demonstrated that neurodegeneration can be monitored in living mice by performing bioluminescence imaging (BLI) on the brains of Tg(Gfap-luc) mice (29, 30). In these mice, neurodegeneration triggers reactive astrocytic gliosis, resulting in a concomitant increase in Gfap transcription, which can be quantified using BLI. To determine whether the kinetics of α-synuclein deposition in the brain could be tracked using BLI, we analyzed bigenic Tg(M83:Gfap-luc) mice throughout their life span. Spontaneously ill Tg(M83+/+:Gfap-luc) mice exhibited a significant increase in the brain BLI signal compared with young asymptomatic Tg(M83+/+:Gfap-luc) mice (Fig. 1G). On average, the BLI signal began to increase in Tg(M83+/+:Gfap-luc) mice ∼1 mo before the onset of clinical symptoms, indicating that astrocytic gliosis is a relatively late marker of disease in these animals. In Tg(M83+/−:Gfap-luc) mice, the brain BLI signal remained unchanged throughout the life span of the animals and was not significantly different from Tg(Gfap-luc) mice (Fig. 1G). Because of the lack of spontaneous clinical signs of neurologic illness and the absence of α-synuclein deposits in the brains of aged Tg(M83+/−:Gfap-luc) mice, we conclude that Tg(M83+/−:Gfap-luc) mice do not develop a spontaneous synucleinopathy.
We sought to determine whether the brains of MSA patients contain α-synuclein aggregates that could transmit disease to bigenic Tg(M83+/−:Gfap-luc) mice. Therefore, we inoculated these mice intracerebrally with brain homogenates prepared from two MSA patients (cases i and ii). Both brains contained abundant GCIs within oligodendrocytes that were immunopositive for α-synuclein (Fig. S3). As a negative control, we inoculated the bigenic mice with brain homogenate from an age-matched, nonneurodegenerative disease patient. As a positive control, we inoculated the mice with brain homogenates generated from two TgM83+/+ mice that developed spontaneous illness at 270 and 330 d of age. SDS- and formic acid-extractable, phosphorylated human α-synuclein protein was found in both the MSA and TgM83+/+ inocula but not the control inoculum; notably, the levels of formic acid-extractable, phosphorylated α-synuclein were much higher in the TgM83+/+ samples than in the MSA cases (Fig. 2A).
Fig. 2.
Survival and BLI in Tg(M83+/−:Gfap-luc) mice inoculated with brain homogenate containing α-synuclein aggregates. (A) Sequential extraction of phosphorylated α-synuclein in brain homogenates used as inocula. Samples from human control, MSA (cases i and ii), and spontaneously ill TgM83+/+ (samples i and ii) brains were sequentially extracted with high-salt, high-salt containing Triton X-100, SDS, and formic acid (FA) buffers, and then the SDS and FA fractions were analyzed by immunoblotting. Aggregated, SDS- and FA-extractable phosphorylated α-synuclein was only observed in the MSA and TgM83+/+ samples. S129-phosphorylated α-synuclein was detected using the antibody EP1536Y. (B) Kaplan–Meier curves for the appearance of clinical signs of neurologic dysfunction in Tg(M83+/−:Gfap-luc) mice injected with inocula prepared from a human control brain (green line, n = 8), spontaneously ill TgM83+/+ mice (two independent samples, blue lines, n = 8 and 7), and MSA patients (two independent cases, red lines, n = 8 each). (C) BLI curves showed a significant increase (*P < 0.05) in TgM83+/+-inoculated animals (blue curve, n = 10) compared with uninoculated controls (black curve, n = 16) beginning at ∼150 d postinoculation. (D) MSA-inoculated Tg(M83+/-:Gfap-luc) mice at 84–112 d postinoculation exhibited higher brain BLI signals than control-inoculated mice at 356 d postinoculation.
We monitored injected mice and uninoculated controls by observing clinical signs of neurologic dysfunction as well as by BLI. Bigenic mice inoculated with brain homogenates prepared from spontaneously ill TgM83+/+ mice began to exhibit clinical signs of neurologic dysfunction, including ataxia, circling behavior, weight loss, proprioceptive deficits, dysmetria, and paralysis, beginning at ∼160 d postinoculation (Fig. 2B). All of the inoculated bigenic mice succumbed to disease with a mean incubation period of 216 d (Table 1). The brain BLI signal began to increase at ∼140 d in the bigenic mice inoculated with brain homogenates from ill TgM83+/+ mice but remained low in age-matched, uninoculated animals (Fig. 2C). Remarkably, MSA-inoculated bigenic Tg(M83+/−:Gfap-luc) mice began to exhibit signs of neurologic illness, most commonly ataxia and circling behavior, beginning at ∼90 d postinoculation (Fig. 2B). Despite the absence of the A53T α-synuclein mutation in the MSA brains, the mean incubation periods for the MSA samples in Tg(M83+/−:Gfap-luc) mice were significantly shorter than those for the TgM83+/+ brain samples (P < 0.05; Table 1). MSA case ii exhibited more α-synuclein–containing GCIs and formic acid-extractable, phosphorylated α-synuclein (Fig. S3 and Fig. 2A), potentially explaining the shorter mean incubation period for this sample compared with MSA case i. None of the bigenic mice inoculated with control brain homogenate developed signs of disease for up to 1 y postinoculation (Fig. 2B and Table 1). Additionally, MSA-inoculated mice exhibited higher brain BLI signals compared with control-inoculated animals (Fig. 2D). We conclude that the brains of MSA patients contain α-synuclein prions that were capable of rapidly transmitting disease to Tg(M83+/−:Gfap-luc) mice.
Next, we determined whether α-synuclein in the brain homogenates of clinically ill inoculated mice was insoluble. Levels of detergent-insoluble phosphorylated α-synuclein were ∼12- to 14-fold higher in Tg(M83+/−:Gfap-luc) mice inoculated with TgM83+/+ brain homogenate compared with age-matched, uninoculated controls (Fig. 3 A and B). Similarly, insoluble phosphorylated α-synuclein levels were ∼10-fold higher in the MSA-inoculated Tg(M83+/−:Gfap-luc) mice compared with asymptomatic, control-inoculated animals (Fig. 3 C and D). Detergent-insoluble phosphorylated α-synuclein oligomers were also observed in bigenic mice inoculated with brain homogenates prepared from either TgM83+/+ mice or MSA patients (Fig. 3 A and C). We also performed sequential extractions on brain homogenates from uninoculated and inoculated Tg(M83+/−:Gfap-luc) mice. SDS- and formic acid-extractable phosphorylated α-synuclein, including oligomeric species, were found in the brains of bigenic mice inoculated with TgM83+/+ or MSA brain homogenates, but not in the brains of age-matched, uninoculated mice or in control-inoculated mice (Fig. 3E).
Fig. 3.
Biochemical analysis of α-synuclein in the brains of inoculated Tg(M83+/−:Gfap-luc) mice. (A) Levels of detergent-insoluble phosphorylated α-synuclein were higher in the brains of clinically ill TgM83+/+-inoculated Tg(M83+/−:Gfap-luc) mice (191–250 d postinoculation) compared with age-matched, uninoculated mice. Two different inocula [M83 (i) and M83 (ii)] were prepared from two different spontaneously ill TgM83+/+ mice. Total levels of human α-synuclein are shown as a control. (B) Quantification of detergent-insoluble phosphorylated α-synuclein levels in the brains of clinically ill, TgM83+/+-inoculated Tg(M83+/−:Gfap-luc) mice and age-matched, uninoculated controls (n = 4 each). Phosphorylated α-synuclein levels were significantly higher in the inoculated mice (**P < 0.01, ***P < 0.001). (C) Levels of detergent-insoluble phosphorylated α-synuclein were higher in the brains of clinically ill Tg(M83+/−:Gfap-luc) mice that were inoculated with two different cases of MSA (88–110 d postinoculation) compared with control-inoculated mice (362 d postinoculation). Total levels of human α-synuclein are shown as a control. (D) Quantification of detergent-insoluble phosphorylated α-synuclein levels in the brains of clinically ill, MSA-inoculated Tg(M83+/−:Gfap-luc) mice and asymptomatic, control-inoculated mice (n = 4 each). Phosphorylated α-synuclein levels were significantly higher in the MSA-inoculated mice (**P < 0.01). (E) Sequential extraction of phosphorylated α-synuclein from the brains of Tg(M83+/−:Gfap-luc) mice inoculated with TgM83+/+ samples (clinically ill at 238 and 191 d postinoculation), control brain (asymptomatic at 362 d postinoculation), and MSA samples (clinically ill at 105 and 92 d postinoculation). For comparison, samples from asymptomatic, uninoculated Tg(M83+/−:Gfap-luc) mice at 259 d of age (None) are shown. Aggregated, FA-extractable phosphorylated α-synuclein was only observed in the TgM83+/+- and MSA-inoculated mice. For all immunoblots, S129-phosphorylated α-synuclein was detected using the antibody EP1536Y.
When the brains of ill Tg(M83+/−:Gfap-luc) mice inoculated with TgM83+/+ or MSA brain homogenates were examined neuropathologically, widespread deposits of phosphorylated α-synuclein were found throughout the brain (Fig. 4, center and right columns). Although small numbers of deposits were observed in the cortical layers of the brain, the largest concentration of α-synuclein deposits was located in the subcortical regions of the midbrain as well as in the brainstem. Deposits were found in both the cell bodies of neurons as well as in neuronal processes, and there was no discernible difference between the brains of bigenic mice inoculated with TgM83+/+ or MSA brain homogenates. In contrast, no phosphorylated α-synuclein deposits were found in any of the control-inoculated animals at 362 d postinoculation (Fig. 4, left column). Although phosphorylated α-synuclein deposits in the inoculated Tg(M83+/−:Gfap-luc) mice were found in similar brain regions where deposits were found in spontaneously ill Tg(M83+/+:Gfap-luc) mice (most notably the brainstem and reticular formation), the Tg(M83+/−:Gfap-luc) mice inoculated with TgM83+/+ or MSA brain homogenates also showed phosphorylated deposits in more frontal regions of the brain, including the striatum, motor cortex, and the thalamus. Levels of astrocytic gliosis and activated microglia were also strongly increased in bigenic mice inoculated with TgM83+/+ or MSA brain homogenates compared with control-inoculated animals (Fig. S4).
Fig. 4.
Widespread deposition of phosphorylated α-synuclein in the brains of inoculated Tg(M83+/−:Gfap-luc) mice. Immunohistochemical detection of phosphorylated α-synuclein in the brains of asymptomatic, control-inoculated Tg(M83+/−:Gfap-luc) mice (362 d postinoculation, left column) as well as clinically ill TgM83+/+-inoculated mice (194–209 d postinoculation, center column) or MSA-inoculated mice (88–105 d postinoculation, right column). The cortex is shown in A–C, the thalamus in D–F, the subthalamic nuclei in G–I, the periaqueductal gray in J–L, the reticular formation in M–O, and the brainstem in P–R. Phosphorylated α-synuclein deposits in the cell bodies of neurons and neuronal processes were only observed in the TgM83+/+- and MSA-inoculated mice. [Scale bar (in A): 50 μm (applies to all panels).]
To determine the protease resistance of the induced α-synuclein deposits, we digested brain homogenates from clinically ill inoculated Tg(M83+/−:Gfap-luc) mice with proteinase K (PK). Similar to spontaneously ill Tg(M83+/+:Gfap-luc) mice, bigenic mice inoculated with TgM83+/+ or MSA brain homogenates also showed insoluble, phosphorylated, but PK-sensitive α-synuclein in their brains (Fig. S5). Collectively, these results demonstrate that inoculation of Tg(M83+/−:Gfap-luc) mice with brain homogenates from MSA patients induced a synucleinopathy characterized by clinical signs of neurologic dysfunction as well as deposition of protease-sensitive α-synuclein aggregates in the brain.
Discussion
In the studies reported here, we demonstrate that a fatal synucleinopathy can be initiated in Tg(M83+/−:Gfap-luc) mice that do not spontaneously develop a neurologic illness, by intracerebral inoculation with brain homogenate from MSA patients. These results parallel recent reports describing the induction of α-synuclein deposits and dopaminergic neuron loss, but not overt clinical signs of neurologic dysfunction, in non-Tg mice following inoculation with recombinant α-synuclein fibrils (25, 26). Our study reveals that self-propagating, transmissible α-synuclein aggregates (i.e., α-synuclein prions) are formed not just in Tg mice that overexpress mutant α-synuclein, but also in the brains of individuals with a degenerative synucleinopathy such as MSA.
Despite the predilection for oligodendrocytic deposition of α-synuclein in MSA, we did not observe appreciable levels of phosphorylated α-synuclein deposition in oligodendrocytes within the brains of MSA-inoculated bigenic mice. This observation suggests that additional human brain-specific factors may be responsible for encoding the oligodendrocyte-specific tropism of α-synuclein aggregates in MSA. However, a more simple explanation is that the heterologous Prnp promoter that drives mutant α-synuclein expression in TgM83 mice does not engender a native spatial pattern of α-synuclein expression. This difference may preclude deposition in mature oligodendrocytes, which do not express α-synuclein mRNA (31). Inoculation of Tg mice expressing A53T mutant human α-synuclein under the control of the SNCA promoter or even non-Tg mice with MSA brain homogenate may help to resolve this issue.
Although some investigators prefer to use alternate terms to describe the recently recognized “prion” proteins involved in PD, Alzheimer’s disease, and the tauopathies, the shared features of these protein-mediated degenerative diseases are becoming progressively more apparent. Some terms suggested to distinguish self-propagating Aβ, tau, and α-synuclein aggregates from those composed of PrP include “prion-like protein aggregates,” “transmissible proteins,” “templated proteins,” “prionoids,” “proteopathic seeds,” “misfolded proteins,” and “protein pathogens” (32, 33). However, we believe that α-synuclein aggregates fulfill all of the criteria for being labeled a prion. First, brain homogenates prepared from MSA patients or spontaneously ill TgM83+/+ mice containing abundant α-synuclein deposits induce the deposition of insoluble α-synuclein in the brains of recipient Tg(M83+/−:Gfap-luc) mice following intracerebral inoculation [Figs. 3 and 4 (23, 24)], demonstrating that α-synuclein aggregates, like PrPSc, are self-propagating. Second, intracerebral inoculation with samples containing pathological α-synuclein aggregates causes not only seeding of protein aggregation in the brain, but also the induction of clinical signs of neurologic dysfunction, indicative of true disease transmission [Table 1 (23, 24)]. Third, disease can be initiated by recombinant α-synuclein that had been polymerized into fibrils (24), indicating that α-synuclein aggregates, like PrP (34), are sufficient to induce disease. Fourth, transmission of a degenerative synucleinopathy can occur in animals that do not develop spontaneous illness within their normal lifespan [Figs. 1 and 2 (25, 26)], ruling out disease acceleration as a mechanism of transmission. Together, these data mount a compelling case for α-synuclein aggregates in the brains of MSA patients as being prions.
Although α-synuclein aggregates are clearly capable of behaving like prions at the molecular level, there is currently no evidence to suggest that MSA or the other human synucleinopathies are transmissible between humans, in contrast to CJD, which can be transmitted through the use of PrPSc-infected dura mater grafts or growth hormone preparations (35) as well as the reuse of PrPSc-contaminated neurosurgical instruments (36). It is currently unknown whether α-synuclein prions can attach to surgical instruments and to what extent they may persist following sterilization. Although attempts to transmit PD to monkeys by intracerebral inoculation were unsuccessful (37), our transmission data suggest that caution should be exercised when reusing neurosurgical instruments that have been previously used on suspected cases of MSA or PD to minimize any risk for iatrogenic transmission of the disease. Although deep brain stimulation is not commonly used to treat MSA patients, its increasingly wide use to control dyskinesias often found in many patients with advanced PD requires surgical implantation (38) and, as such, may represent a potential risk for human-to-human transmission of α-synuclein prions.
The rapid transmission of the MSA inocula, compared with TgM83+/+ samples, in Tg(M83+/−:Gfap-luc) mice was surprising for two reasons. First, the MSA samples do not harbor α-synuclein with the A53T mutation, which is present in the TgM83 line. For PrPSc prions, even a single amino acid mismatch between PrPSc in the inoculum and PrPC in the host can dramatically prolong the disease incubation period (39). Thus, there does not appear to be a substantial “transmission barrier” between the WT α-synuclein aggregates present in the MSA inocula and the A53T mutant α-synuclein present in the mice. Second, the levels of insoluble phosphorylated α-synuclein were much lower in the MSA brains than in the brains of the spontaneously ill TgM83+/+ mice used as inocula. This observation could suggest that the most infectious α-synuclein species may consist of smaller, more soluble assemblies, as has been observed for PrPSc and Aβ prions (40, 41). However, a more likely explanation for the rapid transmission of MSA prions is that these α-synuclein aggregates constitute a distinct “strain” of prion from the aggregates found in spontaneously ill TgM83+/+ mice. In prion disease, distinct strains are believed to result from conformational differences in PrPSc (42, 43). Indeed, conformationally distinct “strains” of recombinant α-synuclein aggregates that possess varying ability to initiate tau aggregation have recently been identified (44). Thus, the α-synuclein aggregates found within oligodendrocytes in the brains of MSA patients may be conformationally distinct from those found in the brains of TgM83+/+ mice, engendering distinct transmission properties. The rapid transmissibility of the MSA strain of α-synuclein prions may reflect the fact that the α-synuclein aggregates are not sequestered in Lewy bodies, which may constitute a protective mechanism to limit the spread of a distinct group of strains of α-synuclein prions in PD and DLB.
The successful transmission of MSA prions to Tg(M83+/−:Gfap-luc) mice described herein represents a unique human neurodegenerative disease that demonstrates lethality upon transmission to animals and is reminiscent of the transmission of kuru, CJD, and related diseases to nonhuman primates (45, 46). Although Aβ and tau prions derived from the brains of Alzheimer’s disease or tauopathy patients, respectively, stimulate prion formation as detected by protein aggregation and deposition upon inoculation into susceptible Tg mice, neither induces overt signs of neurologic disease nor lethality in the recipient animals (12, 47). Importantly, MSA-inoculated bigenic mice may comprise a reliable system for assessing the therapeutic efficacy of drugs designed to target the formation of α-synuclein prions.
Materials and Methods
Additional methods are provided in SI Materials and Methods.
Human Tissue Samples.
Frozen brain tissue samples of two neuropathologically confirmed cases of the parkinsonian subtype of MSA (“MSA-P”) were supplied by the Parkinson’s United Kingdom Tissue Bank at Imperial College. Tissue samples were obtained from the basal ganglia. Case i was prepared from an 86-y-old male (disease duration, 8 y) and case ii from a 71-y-old male (disease duration, 5.5 y). Both cases were characterized by the abundant deposition of α-synuclein in oligodendrocytes and neurons in the absence of classical Lewy bodies. No significant deposition of phosphorylated tau or Aβ was observed in either case. Control brain tissue was obtained from the frontal cortex of a 79-y-old male who did not exhibit any clinical or pathological signs of a neurodegenerative disease. The three brains contained equal amounts of soluble human α-synuclein.
Mice.
Homozygous TgM83+/+ mice, which express human α-synuclein with the A53T mutation under the control of the mouse prion protein promoter (22) as well as endogenous mouse α-synuclein, were purchased from The Jackson Laboratory. Tg(Gfap-luc) mice, which express firefly luciferase (luc) under the control of the murine Gfap promoter (27), were a generous gift from Caliper Life Sciences. Hemizygous bigenic Tg(M83+/−:Gfap-luc) mice were generated by crossing TgM83+/+ mice with homozygous Tg(Gfap-luc) mice. Tg(M83+/+:Gfap-luc) mice were generated by backcrossing Tg(M83+/−:Gfap-luc) mice with TgM83+/+ mice. All animal studies were performed in compliance with the guidelines set forth by the University of California, San Francisco, Institutional Animal Care and Use Committee.
Inoculations.
Brains from spontaneously ill TgM83+/+ mice at 270 and 330 d of age were graciously provided by Prof. Virginia Lee. Human brain tissue samples and mouse brains were homogenized to 10% (wt/vol) in calcium- and magnesium-free PBS (Life Technologies) and then diluted to 1% for inoculation using 5% (wt/vol) BSA. Extracts were not sonicated before inoculation. Weanling (∼2-mo-old) Tg(M83+/−:Gfap-luc) mice were anesthetized with isoflurane, and then inoculated in the right parietal lobe with 30 μL of the 1% brain homogenate (∼30 μg of total protein) using a 27-gauge syringe. Mice were assessed daily for routine health and checked three times weekly for the presence of signs of neurologic illness. Mice were euthanized when two or more neurologic signs were apparent, using the standard diagnostic criteria for assessing prion disease in mice. Brains were then removed and bisected along the midsagittal plane. The left (contralateral) hemisphere was snap-frozen on dry ice for biochemical analyses, and the right (ipsilateral) hemisphere was fixed in formalin for neuropathology.
Supplementary Material
Acknowledgments
We thank Warren Olanow for stimulating discussions, the staff at the Hunter’s Point animal facility for their assistance with the animal experiments, Marta Gavidia for mouse genotyping, Sunny Grillo and Joanne Lee for bioluminescence imaging, and Virginia Lee and Kelvin Luk for the aged TgM83+/+ brains. This work was supported by National Institutes of Health Grants AG002132, AG010770, and AG031220, and by gifts from Brinton Family and The Sherman Fairchild Foundation. J.C.W. was supported by National Institute on Aging K99 Grant AG042453. Tissue samples were supplied by the Parkinson’s United Kingdom Tissue Bank at Imperial College, funded by Parkinson’s United Kingdom, a charity registered in England and Wales (948776) and in Scotland (SC037554). A sabbatical for S.B.P. at Imperial College was supported by the Leverhulme Trust.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318268110/-/DCSupplemental.
References
- 1.Shy GM, Drager GA. A neurological syndrome associated with orthostatic hypotension: A clinical-pathologic study. Arch Neurol. 1960;2:511–527. doi: 10.1001/archneur.1960.03840110025004. [DOI] [PubMed] [Google Scholar]
- 2.Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1969;32(1):28–34. doi: 10.1136/jnnp.32.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94(1–3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 4.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. α-Synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249(2–3):180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, et al. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 6.Tu PH, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol. 1998;44(3):415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 7.Yazawa I, et al. Mouse model of multiple system atrophy α-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45(6):847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336(6088):1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 2012;209(5):889–893. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colby DW, Prusiner SB. Prions. Cold Spring Harb Perspect Biol. 2011;3(1):a006833. doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313(5794):1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 13.Stöhr J, et al. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc Natl Acad Sci USA. 2012;109(27):11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 15.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 16.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 17.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA. 2009;106(31):12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luk KC, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen C, et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpicelli-Daley LA, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giasson BI, et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34(4):521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 23.Mougenot A-L, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33(9):2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Luk KC, et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuda-Suzukake M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(Pt 4):1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, et al. Non-invasive imaging of GFAP expression after neuronal damage in mice. Neurosci Lett. 2004;367(2):210–212. doi: 10.1016/j.neulet.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 29.Tamgüney G, et al. Measuring prions by bioluminescence imaging. Proc Natl Acad Sci USA. 2009;106(35):15002–15006. doi: 10.1073/pnas.0907339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts JC, et al. Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci USA. 2011;108(6):2528–2533. doi: 10.1073/pnas.1019034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller DW, et al. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112(12):1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- 32.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64(6):783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Hardy J, Revesz T. The spread of neurodegenerative disease. N Engl J Med. 2012;366(22):2126–2128. doi: 10.1056/NEJMcibr1202401. [DOI] [PubMed] [Google Scholar]
- 34.Colby DW, et al. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci USA. 2009;106(48):20417–20422. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown P, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55(8):1075–1081. doi: 10.1212/wnl.55.8.1075. [DOI] [PubMed] [Google Scholar]
- 36.Bernoulli C, et al. Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet. 1977;1(8009):478–479. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs CJ, Jr, Gajdusek DC. Amyotrophic lateral sclerosis, Parkinson’s disease, and the amyotrophic lateral sclerosis-Parkinsonism-dementia complex on Guam: A review and summary of attempts to demonstrate infection as the aetiology. J Clin Pathol Suppl (R Coll Pathol) 1972;6:132–140. [PMC free article] [PubMed] [Google Scholar]
- 38.Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2012;367(16):1529–1538. doi: 10.1056/NEJMct1208070. [DOI] [PubMed] [Google Scholar]
- 39.Manson JC, et al. A single amino acid alteration (101L) introduced into murine PrP dramatically alters incubation time of transmissible spongiform encephalopathy. EMBO J. 1999;18(23):6855–6864. doi: 10.1093/emboj/18.23.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437(7056):257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langer F, et al. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J Neurosci. 2011;31(41):14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bessen RA, Marsh RF. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol. 1992;66(4):2096–2101. doi: 10.1128/jvi.66.4.2096-2101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telling GC, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274(5295):2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 44.Guo JL, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154(1):103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibbs CJ, Jr, et al. Creutzfeldt-Jakob disease (spongiform encephalopathy): Transmission to the chimpanzee. Science. 1968;161(3839):388–389. doi: 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- 46.Gajdusek DC, Gibbs CJ, Jr, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209(5025):794–796. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- 47.Clavaguera F, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA. 2013;110(23):9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.