Abstract
Background
Hyponatremia is the most common electrolyte disorder in clinical practice, and evidence to date indicates that severe hyponatremia is associated with increased morbidity and mortality. The aim of our study was to perform a meta-analysis that included the published studies that compared mortality rates in subjects with or without hyponatremia of any degree.
Methods and Findings
An extensive Medline, Embase and Cochrane search was performed to retrieve the studies published up to October 1st 2012, using the following words: “hyponatremia” and “mortality”. Eighty-one studies satisfied inclusion criteria encompassing a total of 850222 patients, of whom 17.4% were hyponatremic. The identification of relevant abstracts, the selection of studies and the subsequent data extraction were performed independently by two of the authors, and conflicts resolved by a third investigator. Across all 81 studies, hyponatremia was significantly associated with an increased risk of overall mortality (RR = 2.60[2.31–2.93]). Hyponatremia was also associated with an increased risk of mortality in patients with myocardial infarction (RR = 2.83[2.23–3.58]), heart failure (RR = 2.47[2.09–2.92]), cirrhosis (RR = 3.34[1.91–5.83]), pulmonary infections (RR = 2.49[1.44–4.30]), mixed diseases (RR = 2.59[1.97–3.40]), and in hospitalized patients (RR = 2.48[2.09–2.95]). A mean difference of serum [Na+] of 4.8 mmol/L was found in subjects who died compared to survivors (130.1±5.6 vs 134.9±5.1 mmol/L). A meta-regression analysis showed that the hyponatremia-related risk of overall mortality was inversely correlated with serum [Na+]. This association was confirmed in a multiple regression model after adjusting for age, gender, and diabetes mellitus as an associated morbidity.
Conclusions
This meta-analysis shows for the first time that even a moderate serum [Na+] decrease is associated with an increased risk of mortality in commonly observed clinical conditions across large numbers of patients.
Introduction
Hyponatremia, defined as a serum sodium concentration ([Na+]) <136 mmol/L, is the most common electrolyte disorder encountered in clinical practice [1]. The most common cause of hypotonic or dilutional hyponatremia is the syndrome of inappropriate antidiuresis (SIAD). Mild hyponatremia (serum [Na+] 130–135 mmol/L) has been estimated to occur in about 15–30% of hospitalized patients, whereas the prevalence of moderate to severe hyponatremia (serum [Na+] <130) is as high as 7% among in-hospital patients [2].
Hyponatremia represents a serious health problem with significant associated morbidity and mortality. Acute severe hyponatremia is a medical emergency accompanied by severe neurological symptoms due to cerebral edema and can be lethal if not recognized and appropriately treated [3]. The correction of hyponatremia may per se represent a risk and a rare but potentially lethal complication, i.e. the osmotic demyelination syndrome, may be the result of an overly rapid correction [4]. In contrast, mild chronic hyponatremia has traditionally been considered as an asymptomatic or mildly symptomatic condition. However, recent reports indicated that even mild chronic hyponatremia can have long-term adverse effects, such as deficits in gait and attention [5], falls [5], bone loss and fractures [6]–[9], especially in the elderly. More recently, chronic hyponatremia has been shown to exacerbate multiple manifestation of senescence in aged rats, including senile osteoporosis, sarcopenia, cardiac fibrosis, and hypogonadism [10].
The association between hyponatremia and in-hospital mortality has been demonstrated in numerous studies. For instance, a large cohort study, which included all adult hospitalizations (n = 53236) at an academic medical center between 2000–2007, demonstrated that even mild hyponatremia was associated with increased in-hospital mortality, and that the risk of death was increased by 2.3% for each 1 mmol/L decline of serum [Na+] [11].
Hyponatremia has been generally associated with an increased mortality in different conditions such as pneumonia [12], heart failure [13], acute myocardial infarction [14], cirrhosis [15], cancer [14], in the elderly [16], and in intensive care patients [17]. However, whether hyponatremia is an independent risk factor for death or is simply associated with an underlying severe condition that is the cause of death remains to be elucidated [4], [18]. Furthermore, there is the possibility that hyponatremia indirectly contributes to mortality by causing organ dysfunction, such as for example bone loss and fractures which are associated with significant mortality in the elderly. Recently, a meta-analysis that included 22 observational studies and randomized controlled trials published to the end of 2008, that was limited to patients with heart failure, indicated that hyponatremia is a powerful predictor of mortality in these patients regardless of ejection fraction [19]. However, no meta-analysis on the relationship between hyponatremia and mortality has addressed other pathological conditions to date.
The aim of this study was to perform a meta-analysis, which included the studies that compared the mortality rate in subjects with or without hyponatremia, in order to verify whether hyponatremia represents a risk factor for mortality, independently of other confounding factors.
Methods
A meta-analysis was performed including studies comparing mortality rate in subjects with or without hyponatremia. An extensive Medline, Embase, and Cochrane search was performed including the following words: hyponatremia and mortality. The search up to October 1st 2012 was restricted to English-language articles and studies of human participants. The identification of relevant abstracts, the selection of studies based on the criteria described above, and the subsequent data extraction were performed independently by two of the authors (G.P., C.G.), and conflicts resolved by a third investigator (G.C). Full-text articles and meeting abstracts were included. The quality of studies was assessed using the Cochrane criteria [20].
Statistical analysis
Heterogeneity was assessed using the I2 statistics for overall mortality rate. Considering that heterogeneity could not be excluded (I2 = 92.8%), relative risk of mortality between subjects with or without hyponatremia, was calculated using both a random and fixed effect model. For a more conservative approach, results of random effect models were presented. A meta-regression analysis was performed to test the effect of serum [Na+] threshold selected in the different studies on overall mortality rate levels. In addition, a linear regression analysis model, weighing each study for the number of subjects enrolled, was performed to verify the independent effect of hyponatremia on mortality after the adjustment for age, gender and diabetes mellitus as an associated morbidity. It was not possible to include other co-morbidities because there were not enough data to be collected and analyzed from the selected literature. Finally, sensitivity analyses was performed considering only larger studies (including ≥1000 subjects) or those reporting the prevalence of diabetes mellitus. In addition, mean baseline serum [Na+] in subjects who eventually died or not at follow up were meta-analyzed using a random effect model.
Relative risks (RRs) with 95% CIs were calculated using Comprehensive Meta-analysis Version 2, Biostat, (Englewood, NJ, USA). Logistic multivariate analysis was performed on SPSS (Statistical Package for the Social Sciences; Chicago, USA) for Windows 20.1.
Results
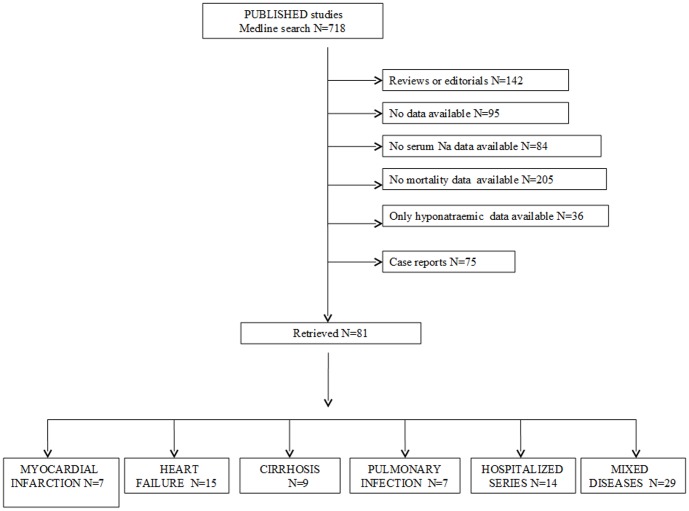
Out of 718 retrieved articles, 637 articles were excluded for different reasons. The flow of the meta-analysis is summarized in Figure 1, and the characteristics of the trials included in the meta-analysis are summarized in Table 1 (see references 3,11–12,16–18, 21–95). Among the 81 selected studies, 7, 13, 8, 5 studies evaluated the effect of hyponatremia on overall mortality rate in subjects with myocardial infarction, heart failure (HF), cirrhosis and pulmonary infections, respectively. In addition, another 26 studies reported data on the effect of hyponatremia on overall mortality for combined mixed diseases, which could not be grouped separately (see Table 1). Finally, 14 studies retrospectively investigated the effect of hyponatremia on overall mortality in hospitalized series of subjects. In these studies, a major diagnosis was not specified.
Figure 1. Trial flow diagram.
Table 1. Studies included in meta-analysis.
| Source | Type of disease | Age | Male | DM | Na+ cut-off (mEq/L) | Patients | H | NH | Deaths H | DeathsNH | Na+ deaths (mEq/L) | Na+ survivors |
| (years) | % | % | (n) | (n) | (n) | (n) | (n) | (mean±SD) | (mEq/L) (mean±SD) | |||
| Flear et al., 1979 [21] | Myocardial infarction | 57.1 | 78.7 | NA | 135 | 235 | 88 | 147 | 19 | 10 | NA | NA |
| Goldberg et al., 2004 [22] | Myocardial infarction | 61 | 78 | 24.2 | 135 | 1047 | 339 | 708 | 61 | 44 | NA | NA |
| Goldberg et al., 2006 [23] | Myocardial infarction | 59.3 | 80.7 | 22.6 | 136 | 978 | 108 | 870 | 26 | 78 | NA | NA |
| Klopotowski et al., 2009 [24] | Myocardial infarction | NA | 72.5 | 8.9 | 135 | 1858 | 96 | 1762 | 13 | 67 | NA | NA |
| Havrànek et al., 2011 [25] | Myocardial infarction | 64 | 66 | 33.9 | 135 | 218 | 72 | 146 | 25 | 30 | NA | NA |
| Tada et al., 2011 [26] | Myocardial infarction | 64.4 | 85 | 41.4 | 136 | 140 | 29 | 111 | 0 | 3 | NA | NA |
| Tang et al., 2011 [27] | Myocardial infarction | 63.8 | 6.8 | 2.9 | 135 | 1620 | 212 | 1408 | 29 | 103 | NA | NA |
| Panciroli et al., 1990 [28] | HF | 67 | 70.2 | 11.8 | 135 | 161 | 64 | 97 | 44 | 39 | NA | NA |
| Adewole et al., 1996 [29] | HF | NA | NA | NA | 125 | 64 | 10 | 54 | 7 | 17 | NA | NA |
| Chen et al., 2003 [30] | HF | 56 | 63.2 | NA | 125 | 234 | 27 | 207 | 20 | 35 | NA | NA |
| Villacorta et al., 2003 [31] | HF | 72.5 | 63 | NA | 135 | 170 | 61 | 109 | 32 | 31 | NA | NA |
| Gheorghiade et al., 2007 [32] | HF | NA | NA | NA | 135 | 40454 | 7882 | 32572 | 473 | 1042 | NA | NA |
| Gheorghiade et al., 2007 [33] | HF | 56.2 | NA | NA | 134 | 430 | 103 | 327 | 31 | 52 | NA | NA |
| Milo-Cotter et al, 2008 [34] | HF | 74.9 | 51 | NA | 135 | 296 | 38 | 258 | 11 | 21 | NA | NA |
| Tribouilloy et al., 2008 [35] | HF | 74 | 53.8 | 25.8 | NA | 662 | NA | NA | NA | NA | 136.7±4.9 | 138.4±3.6 |
| Rusinaru et al., 2009 [36] | HF | 75.8 | 46.6 | 26.2 | 136 | 358 | 91 | 267 | 73 | 159 | NA | NA |
| Barsheshet et al., 2010 [37] | HF | NA | 55.3 | 51.7 | 136 | 2336 | 537 | 1799 | 54 | 74 | NA | NA |
| DeWolfe et al., 2010 [38] | HF | 54.7 | 62.9 | 34.1 | 135 | 364 | 48 | 316 | 8 | 31 | NA | NA |
| Novack et al., 2010 [39] | HF | 75.6 | 52.2 | 38.3 | 136 | 8246 | 1755 | 6491 | NA | NA | 136.4±5.3 | 137.6±4.5 |
| Baldasseroni et al., 2011 [40] | HF | 62 | 74.4 | 11.0 | 135 | 4670 | 463 | 4207 | 123 | 433 | NA | NA |
| Balling et al., 2011 [41] | HF | 68 | 73 | NA | 136 | 3645 | 602 | 2863 | 147 | 429 | NA | NA |
| Shorr et al., 2011 [42] | HF | 74.7 | 46.2 | NA | 135 | 115969 | 24562 | 91407 | 1372 | 2763 | NA | NA |
| Arroyo et al., 1976 [43] | CIRRHOSIS | NA | NA | NA | 130 | 55 | 21 | 34 | 9 | 6 | NA | NA |
| Vila et al., 1999 [44] | CIRRHOSIS | 47.3 | 35.2 | NA | 130 | 45 | 20 | 25 | 7 | 9 | NA | NA |
| Borroni et al., 2000 [45] | CIRRHOSIS | 56.9 | 70.5 | NA | 130 | 191 | 57 | 134 | 15 | 12 | NA | NA |
| Porcel et al,. 2002 [46] | CIRRHOSIS | 62.9 | 62.1 | NA | 130 | 74 | 54 | 20 | 37 | 5 | 123.8±5.6 | 129±7.7 |
| Ruf et al., 2005 [47] | CIRRHOSIS | 49 | 53 | NA | 130 | 194 | 34 | 160 | NA | NA | 130±6.0 | 136±5.0 |
| Hackworth et al., 2009 [48] | CIRRHOSIS | 51 | 78 | NA | 130 | 213 | 90 | 123 | 10 | 10 | NA | NA |
| Radha Krishna et al., 2009 [49] | CIRRHOSIS | 36.3 | 70.2 | NA | NA | 121 | 50 | 71 | 38 | 16 | NA | NA |
| Terg et al., 2009 [50] | CIRRHOSIS | NA | NA | NA | 130 | 81 | 27 | 54 | 12 | 7 | NA | NA |
| Jenq et al., 2010 [51] | CIRRHOSIS | 56 | 76.2 | NA | 135 | 126 | 67 | 59 | 49 | 33 | NA | NA |
| Singhi et al., 1992 [52] | PNEUMOPATHY | 3.14 | NA | NA | 135 | 727 | 371 | 356 | 24 | 17 | NA | NA |
| Sharma et al., 1995 [53] | PNEUMOPATHY | 35 | 51 | NA | 135 | 112 | 42 | 70 | NA | NA | 117.6±5.8 | 132.6±7.7 |
| El-Ebiary et al., 1997 [54] | PNEUMOPATHY | 59.1 | 75 | 17 | 136 | 84 | 9 | 75 | 6 | 19 | NA | NA |
| Hussain et al., 2004 [55] | PNEUMOPATHY | 47 | 42 | 15 | 135 | 110 | 78 | 32 | NA | NA | 127.8±7.4 | 130.6±7.5 |
| Nair et al., 2007 [56] | PNEUMOPATHY | 73.5 | 50 | 20 | 135 | 342 | 95 | 247 | 9 | 8 | NA | NA |
| Song et al., 2008 [57] | PNEUMOPATHY | NA | NA | NA | NA | 929 | 78 | 851 | 16 | 62 | NA | NA |
| Zilberberg et al., 2008 [12] | PNEUMOPATHY | 68.4 | 45.2 | NA | 135 | 7965 | 649 | 7316 | 35 | 293 | NA | NA |
| Sunderam et al., 1983 [58] | AGED | NA | 0 | NA | 130 | 683 | 108 | 575 | 53 | 104 | NA | NA |
| Samadi et al., 1985 [59] | CHRONIC DIARRHEA | <3 | NA | NA | 130 | 1330 | 276 | 1054 | 28 | 38 | NA | NA |
| Cusano et al., 1990 [60] | AIDS | 36.6 | 89 | NA | 130 | 96 | 30 | 66 | 21 | 24 | NA | NA |
| Vitting et., 1990 [61] | AIDS | 39.8 | 98 | NA | 132 | 48 | 34 | 14 | 8 | 2 | 128±2 | 133±1 |
| Erinoso et al., 1993 [62] | MALNUTRITION | <5 | 59.3 | NA | 130 | 120 | 85 | 35 | 40 | 6 | NA | NA |
| Tang et al., 1993 [63] | AIDS | 34.2 | NA | NA | 135 | 210 | 83 | 127 | 30 | 25 | NA | NA |
| Terzian et al., 1994 [16] | AGED | >65 | 43.4 | NA | 130 | 4123 | 145 | 3978 | 23 | 316 | NA | NA |
| Chuah et al., 1996 [64] | AMEBIASIS | NA | 80 | NA | 135 | 60 | 23 | 37 | 15 | 1 | NA | NA |
| Iseki et al., 1996 [65] | DIALYSIS | 50.9 | 56.6 | NA | NA | 1491 | NA | NA | NA | NA | 134.9±7.6 | 136.8±5.8 |
| Srivastava et al., 1998 [66] | FULMINANT HEPATITIS | 5.3 | 68.3 | NA | 125 | 41 | 3 | 38 | 3 | 22 | NA | NA |
| Berghmans et al., 2000 [67] | TUMOURS | NA | NA | NA | 130 | 3306 | 106 | 3200 | 21 | 202 | NA | NA |
| Manary et al., 2000 [68] | MALNUTRITION | 2.7 | 45.3 | NA | NA | 75 | NA | NA | NA | NA | 131 | 132 |
| Oguche et al., 2002 [69] | MALARIA | 3 | 44 | NA | NA | 50 | 8 | 42 | 1 | 10 | NA | NA |
| Agarwal et al., 2004 [70] | ACUTE RENAL FAILURE | <12 | 70 | NA | NA | 54 | 12 | 42 | 9 | 19 | NA | NA |
| Lee et al., 2005 [71] | BONE MARROW TRANSPLANTATION | 32 | 58.2 | NA | 134 | 311 | 185 | 126 | 123 | 43 | NA | NA |
| Sherlock et al., 2006 [72] | SUBARACHNOID HEMORRHAGE | NA | NA | NA | 136 | 316 | 179 | 137 | 19 | 26 | NA | NA |
| Bonney et al., 2008 [73] | LIVER TRANSPLANTATION | 52.8 | 49.1 | NA | NA | 54 | 17 | 37 | 5 | 5 | NA | NA |
| Forfia et al., 2008 [74] | LUNG HYPERTENSION | 55.3 | 17 | NA | 136 | 40 | 13 | 27 | 5 | 11 | NA | NA |
| Olotu et al., 2008 [75] | HEMOLYTIC-UREMIC SYNDROME | NA | 61 | NA | 120 | 31 | 8 | 23 | 7 | 10 | NA | NA |
| Hanson et al., 2009 [76] | MALARIA | 35 | 79.5 | NA | 135 | 168 | 98 | 70 | 31 | 36 | NA | NA |
| Hsu et al., 2009 [77] | TUMOUR LISYS SYNDROME | 55.2 | 66.7 | NA | NA | 12 | NA | NA | NA | NA | 132±6 | 142±3 |
| Kapoor et al., 2010 [78] | PYELONEPHRITIS | 57 | 15.4 | NA | 120 | 39 | 15 | 24 | 5 | 0 | NA | NA |
| Dimopoulos et al., 2010 [79] | CONGENITAL HEART DISEASE | 36.2 | 48.7 | NA | 136 | 1004 | 156 | 848 | 35 | 61 | NA | NA |
| Salvador et al., 2010 [80] | NECROTIZING FASCIITIS | NA | NA | 22 | 135 | 67 | 14 53 | 53 | 6 | 18 | NA | NA |
| Scherz et al., 2010 [81] | PULMONARY EMBOLISM | 67 | 40.2 | NA | 135 | 13728 | 2907 | 10821 | 441 | 866 | NA | NA |
| Stelfox et al., 2010 [82] | HEART SURGERY | 65.4 | 76.4 | 41.9 | 133 | 6727 | 785 | 5942 | 82 | 124 | NA | NA |
| Hoorn et al., 2011 [83] | AGED | 70.3 | 38.5 | 11 | 136 | 5208 | 399 | 4809 | 206 | 1567 | NA | NA |
| Saifudheen et al., 2011 [84] | GUILLAIN BARRE SYNDROME | 42 | 72 | NA | 135 | 50 | 24 | 26 | 4 | 0 | NA | NA |
| Vaa et al., 2011 [85] | ALCOHOLIC HEPATITIS | 51.1 | 85 | NA | NA | 26 | NA | NA | NA | NA | 132 | 136 |
| Tierney et al., 1986 [86] | HOSPITALIZED SERIES | 61.2 | 47 | 19 | 135 | 1514 | 757 | 757 | 165 | 60 | NA | NA |
| Natkunam et al., 1991 [87] | HOSPITALIZED SERIES | NA | NA | NA | 125 | 1217 | 202 | 1015 | 84 | 35 | NA | NA |
| Singhi et al., 1994 [88] | HOSPITALIZED SERIES | NA | 75 | NA | 135 | 264 | 71 | 193 | 6 | 7 | NA | NA |
| Miller et al., 1995 [89] | HOSPITALIZED SERIES | 60–103 | 91.6 | NA | 135 | 119 | 63 | 56 | 11 | 12 | NA | NA |
| Gill et al., 2006 [3] | HOSPITALIZED SERIES | 65 | 47.5 | NA | 125 | 204 | 104 | 100 | 28 | 9 | NA | NA |
| Asadollahi et al., 2007 [90] | HOSPITALIZED SERES | NA | NA | NA | 134 | 1599 | 356 | 1243 | 179 | 377 | NA | NA |
| Stelfox et al., 2008 [17] | HOSPITALIZED SERIES SERIES | 56.1 | 58.9 | NA | 133 | 5985 | 917 | 5068 | 255 | 799 | NA | NA |
| Zilberberg et al, 2008 [91] | HOSPITALIZED SERIES | 61.8 | 45.5 | NA | 135 | 198281 | 10899 | 187382 | 643 | 5621 | NA | NA |
| Hampshire et al., 2009 [92] | HOSPITALIZED SERIES | NA | NA | NA | 130 | 6410 | 285 | 6125 | 208 | 3468 | NA | NA |
| Whelan et al., 2009 [93] | HOSPITALIZED SERIES | 58.5 | 47.5 | NA | 134 | 14039 | 2795 | 11244 | 474 | 893 | NA | NA |
| Whyte et al., 2009 [94] | HOSPITALIZED SERIES | 68.8 | 39.8 | NA | 120 | 226 | 113 | 113 | 24 | 7 | NA | NA |
| Funk et al., 2010 [95] | HOSPITALIZED SERIES | 63.2 | 57.6 | NA | 135 | 140952 | 26782 | 114170 | 4369 | 11074 | NA | NA |
| Wald et al., 2010 [11] | HOSPITALIZED SERIES | 65.3 | 48.2 | 14.9 | 138 | 34761 | 13274 | 21487 | 451 | 430 | NA | NA |
| Chawla et al., 2011 [18] | HOSPITALIZED SERIES | NA | NA | NA | 135 | 209839 | 45693 | 164146 | 2787 | 3775 | NA | NA |
H: patients with hyponatremia; NH: patients without hyponatremia; DM: diabetes mellitus; NA: not available.
The mean±SD serum [Na+] in dead or alive individuals was specified in 3 of the aforementioned studies and in a further 8 studies enrolling patients with HF (n = 2), cirrhosis (n = 1), pulmonary infection (n = 2) or mixed disease (n = 3), respectively (Table 1).
Overall 850222 patients and 147948 hyponatremic subjects were included in the meta-analysis. Hyponatremia was defined according to varying cut-off definitions in the included studies (Table 1). The Begg-adjusted rank correlation test, calculated on the basis of overall mortality rate for hyponatremia, suggested no major publication bias (Kendall tau 0.02; p = 0.82).
When all 81 studies were considered, hyponatremia was significantly associated with an increased risk of overall mortality (RR = 2.60[2.31–2.93]; p<0.0001). Similar results were obtained when patients with specific diseases or series of hospitalized patients were analyzed separately (Figure 2, panels A–E). Similar to what observed for mortality rate, the Begg-adjusted rank correlation test, calculated on the basis of mean serum [Na+] between subjects who eventually died when compared to survivors, suggested no major publication bias (Kendall tau −0.145; p = 0.553). The baseline mean difference of serum [Na+] was significantly lower in subjects who eventually died when compared to survivors (130.1±5.6 vs 134.9±5.1 mmol/L) at follow up (Figure 3). Similar results were observed when studies enrolling less than 100 subjects were excluded from the analysis (mean difference in serum [Na+] between survivors vs dead 3.04[1.81–4.27], p<0.0001). Sub-analysis for mean serum [Na+] in specific diseases was not performed due to insufficient data.
Figure 2. Odds ratio for overall mortality in patients with or without (no) hyponatremia according to the presence of myocardial infarction (A), heart failure (B), cirrhosis (C), pulmonary infection (D), mixed disease (E), or in hospitalized series of subjects (F).
Figure 3. Weighted differences (with 95% CI) of mean serum [Na+] in dead and alive patients.
A meta-regression analysis showed that the hyponatremia-related risk of overall mortality was inversely correlated with the serum [Na+] threshold considered for each report (Figure 4). Hence, the lower threshold considered, the higher the risk of mortality. The latter association was confirmed in a multiple regression model, adjusting for age, gender and diabetes mellitus (adj. r = −0.278; p<0.0001).
Figure 4. Relation between serum [Na+] cut-off definition and overall mortality risk.

Sensitivity analyses performed considering only larger studies (including ≥1000 subjects), those reporting the prevalence of diabetes mellitus or those with severe hyponatremia ([Na+] ≤125 mmol/l), confirmed the association between hyponatremia and mortality (RR = 2.521[2.180–2.916]; p<0.0001 and 2.886[2.228–3.737], 10.036[5.155–19.540]; all p<0.0001, respectively).
Discussion
Hyponatremia has been associated with increased in-hospital mortality [11], but no published comprehensive meta-analysis that analyzed the mortality rate in subjects with or without hyponatremia had been performed to date. Very recently, the Meta-Analysis Global Group in Chronic heart failure (MAGGIC) published a meta-analysis that included 14766 patients from 22 studies that recruited patients with HF and reported death from any cause [19]. Patients with hyponatremia (n = 1618) had an increased risk of death (21%), compared to patients with normal serum [Na+] (16%), and the risk of death appeared to increase linearly with serum [Na+] <140 mmol/L. Hyponatremia was an independent predictor of death either when the patients were considered as a whole, or when they were grouped based on the presence of a reduced (n = 1199) or a preserved (n = 419) ejection fraction. The MAGGIC meta-analysis was limited to patients with HF and considered studies published to the end of 2008.
Our meta-analysis included all of the English-language published studies up until October 1st 2012 that compared the mortality rate in human subjects with or without hyponatremia of any degree. Eighty-one published studies were selected according to specified inclusion criteria for a total of 850222 patients, of whom 17.4% were hyponatremic. This percentage is in general agreement with epidemiological data about the prevalence of hyponatremia among hospitalized patients [2]. Of note, hyponatremia was associated with a significantly increased risk of overall mortality when all studies were considered (RR = 2.60 [2.31–2.93]). A detailed analysis of cause specific mortality was not possible, because this information was not available in several studies, as also was found in the MAGGIC meta-analysis. Nevertheless, we were able to conclude that the risk of mortality was independent of factors including age, gender, and diabetes mellitus as an associated morbidity. Similarly, hyponatremia was found to be associated with an increased risk of death when the patients were analyzed separately based on different disease types or when sensitivity analysis was restricted to larger studies or those reporting the prevalence of diabetes. In particular, we were able to confirm the data of the MAGGIC meta-analysis on hyponatremic patients with HF (RR = 2.47 [2.09–2.92]), analyzing a greater number of patients (168971, of whom 20.4% were hyponatremic). In the MAGGIC meta-analysis, only 11% of patients were hyponatremic, which is below the prevalence of hyponatremia generally reported for hospitalized patients (15–30%) [2]; the authors suggested that this might be due to the fact that all patients in the MAGGIC cohort were outpatients at the time of the baseline data. In contrast with the MAGGIC meta-analysis, patients with hyponatremia in our meta-analysis were neither older, nor more frequently affected by diabetes mellitus. Furthermore, we found an increased risk of mortality in hyponatremic patients with myocardial infarction (total number of patients 6096, of whom 18.3% with hyponatremia), cirrhosis (total number of patients 906, of whom 42.6% were hyponatremic), or pulmonary infections (total number of patients 10047, of whom 12% were hyponatremic). Some studies (n = 26) reported data regarding other mixed diseases or subpopulations (e.g., elderly people), which could not be grouped together. The most represented diseases among these patients (total number of patients 37864, of whom 15.1% were hyponatremic) were AIDS, malaria and malnutrition. Finally, some studies (n = 14, total number of patients 615410, of whom 16.7% were hyponatremic) were considered separately, because the effect of hyponatremia on mortality was investigated retrospectively and the diagnoses were not specified. The meta-analysis of these studies also revealed an increased risk of overall mortality.
The major finding of this meta-analysis is that across all groups of patients the relative risk of mortality in patients with hyponatremia vs patients without hyponatremia ranged between 2.47 and 3.34, thus indicating that this electrolyte disorder strongly predicts prognosis of all hospitalized patients. Another interesting result of our meta-analysis is that a moderate serum [Na+] reduction (i.e., 4.8 mmol/L) was associated with an increased risk of mortality, and a meta-regression analysis showed that the hyponatremia-related risk of overall mortality was inversely correlated with the serum [Na+]. Hence, the lower threshold considered, the higher the risk of mortality. This association was confirmed in a multiple regression model after adjusting for age, gender and diabetes mellitus. The linear increase of risk of death that we showed in our analysis is in agreement with the findings of the MAGGIC meta-analysis, which found a linear increase of mortality starting at serum [Na+] <140 mmol/L. Overall, our findings indicate that even a moderate reduction of serum [Na+] is associated with an increased risk of mortality in patients affected by multiple disease types across large numbers of hospitalized patients.
Although the present meta-analysis both confirms and extends the strong association between hyponatremia and adverse outcomes such as inpatient mortality, it cannot prove a causal relation between these variables. In fact, only diabetes mellitus could be used as a possible confounder in the present study. Perhaps the major outstanding question regarding hyponatremia is whether hyponatremia contributes directly to poor outcomes or is simply a marker for severity of underlying co-morbidities, or possibly for other factors that might influence the progression of underling co-morbidities [96]. Hence, it should be recognized that potential unmeasured confounders such as other chronic diseases, in addition to diabetes mellitus, may have caused residual confounding, but the measured factors that are correlated with such confounders would have mitigated the bias. Few studies to date have attempted to address the issue of a direct effect of hyponatremia on mortality or other adverse outcomes. One oft-cited potential exception is the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) study of patients with congestive heart failure, which failed to show improvements in cardiovascular outcomes in patients with acute heart failure (AHF) treated with the vasopressin type 2 receptor (V2R) antagonist, tolvaptan, versus placebo [97]. However, that study was not powered to examine outcomes in the smaller subgroup of patients enrolled with both heart failure and hyponatremia. More recently, a significant strong positive relationship between an increase in serum sodium and decreased mortality was noted in 322 patients hospitalized for AHF and followed for 1–3 years [98]. In contrast, a multicenter analysis of 2888 patients hospitalized for AHF in Korea confirmed that hyponatremia on admission was associated with a worse prognosis compared with normonatremia, but this relation persisted regardless of whether the hyponatremia improved during the hospitalization [99]. However, this report was a retrospective anaylsis from a registry, not a prospective randomized trial, and the assessment of the change in serum sodium was made only once, prior to or at discharge from the hospital [100]. Thus, whether hyponatremia is merely a marker or also a mediator of adverse patient outcomes is still uncertain in heart failure, and has not been studied in other diseases. The current meta-analysis adds further urgency to the need to answer this question for multiple diseases, not only heart failure.
In conclusion, this study represents the first extensive and updated meta-analysis demonstrating that hyponatremia is significantly associated with an increased risk of overall mortality, and that it is a negative prognostic factor across multiple commonly observed clinical conditions, such as myocardial infarction, HF, cirrhosis and pulmonary infections. These findings might suggest the importance to correct this electrolyte disorder, even when mild, using the most appropriate strategies [101]–[103]. However, our study did not specifically address this issue and this hypothesis at present highlights the need for additional studies of clinical outcomes with effective therapies in all hyponatremic patients.
Supporting Information
PRISMA Checklist.
(DOC)
Acknowledgments
The authors wish to thank Edoardo Mannucci and Matteo Monami for their assistance in analysis of data.
Funding Statement
The authors have no support or funding to report.
References
- 1. Upadhyay A, Jaber BL, Madias NE (2006) Incidence and prevalence of hyponatremia. Am J Med 119: 30–35. [DOI] [PubMed] [Google Scholar]
- 2. Hoorn EJ, Lindemans J, Zietse R (2006) Development of severe hyponatremia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant 28: 70–76. [DOI] [PubMed] [Google Scholar]
- 3. Gill G, Huda B, Boyd A, Skagen K, Wile D, et al. (2006) Characteristics and mortality of severe hyponatremia – a hospital-based study. Clin Endocrinol (Oxf) 65: 246–249. [DOI] [PubMed] [Google Scholar]
- 4. Adrogué HJ (2005) Consequences of inadequate management of hyponatremia. Am J Nephrol 25: 240–249. [DOI] [PubMed] [Google Scholar]
- 5. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G (2006) Mild chronic hyponatremia is associated with falls, unsteadiness and attention deficits. Am J Med 119: 71.e1–8. [DOI] [PubMed] [Google Scholar]
- 6. Gankam KF, Andres C, Sattar L, Melot C, Decaux G (2008) Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 101: 583–588. [DOI] [PubMed] [Google Scholar]
- 7. Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA (2010) Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol 5: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, et al. (2010) Hyponatremia-induced osteoporosis. J Bone Miner Res 25: 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barsony J, Sugimura Y, Verbalis JG (2011) Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem 286: 10864–10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barsony J, Manigrasso MB, Xu Q, Tam H, Verbalis JG (2012) Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age (Dordr) 35: 271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wald R, Jaber BL, Price LL (2010) Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 170: 294–302. [DOI] [PubMed] [Google Scholar]
- 12. Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, et al. (2008) Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study. BMC Pulm Med 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein L, O'Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, et al. (2005) Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Study. Circulation 111: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 14. Waikar SS, Mount DB, Curhan GC (2009) Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 122: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, et al. (2008) Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 359: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Terzian C, Frye EB, Piotrowski ZH (1994) Admission hyponatremia in the elderly: factors influencing prognosis. J Gen Intern Med 9: 89–91. [DOI] [PubMed] [Google Scholar]
- 17. Stelfox HT, Ahmed SB, Khandwala F, Zygun D, Shahpori R, et al. (2008) The epidemiology of intensive care unit-acquired hyponatremia and hypernatraemia in medical-surgical intensive care units. Crit Care 12: R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD (2011) Mortality and the serum sodium: Do patients die with or from hyponatremia? Clin J Am Soc Nephrol 6: 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, et al. (2012) Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta-analysis(†): Meta-Analysis Global Group in Chronic heart failure (MAGGIC). Eur J Heart Fail 14: 1139–1146. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. The Cochrane Collaboration Available from www.cochrane-handbook.org. [Google Scholar]
- 21. Flear CT, Hilton P (1979) Hyponatraemia and severity and outcome of myocardial infarction. Br Med J 1: 1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, et al. (2004) Prognostic importance of hyponatremia in acute ST-elevation myocardial infarction. Am J Med 117: 242–248. [DOI] [PubMed] [Google Scholar]
- 23. Goldberg A, Hammerman H, Petcherski S, Nassar M, Zdorovyak A, et al. (2006) Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch Intern Med 166: 781–786. [DOI] [PubMed] [Google Scholar]
- 24. Klopotowski M, Kruk M, Przyluski J, Kalinczuk L, Pregowski J, et al. (2009) Sodium level on admission and in-hospital outcomes of STEMI patients treated with primary angioplasty: the ANIN Myocardial Infarction Registry. Med Sci Monit 15: CR477–483. [PubMed] [Google Scholar]
- 25. Havránek Š, Bělohlávek J, Škulec R, Kovárník T, Dytrych V, et al. (2011) Long-term prognostic impact of hyponatremia in the ST-elevation myocardial infarction. Scand J Clin Lab Invest 71: 38–44. [DOI] [PubMed] [Google Scholar]
- 26. Tada Y, Nakamura T, Funayama H, Sugawara Y, Ako J, et al. (2011) Early development of hyponatremia implicates short- and long-term outcomes in ST-elevation acute myocardial infarction. Circ J 75: 1927–1933. [DOI] [PubMed] [Google Scholar]
- 27. Tang Q, Hua Q (2011) Relationship between hyponatremia and in-hospital outcomes in Chinese patients with ST-elevation myocardial infarction. Intern Med 50: 969–974. [DOI] [PubMed] [Google Scholar]
- 28. Panciroli C, Galloni G, Oddone A, Marangoni E, Masa A, et al. (1990) Prognostic value of hyponatremia in patients with severe chronic heart failure. Angiology 41: 631–638. [DOI] [PubMed] [Google Scholar]
- 29. Adewole AD, Ikem RT, Adigun AQ, Akintomide AO, Balogun MO, et al. (1996) A three year clinical review of the impact of angiotensin converting enzyme inhibitors on the intra hospital mortality of congestive heart failure in Nigerians. Cent Afr J Med 42: 253–255. [PubMed] [Google Scholar]
- 30. Chen MC, Chang HW, Cheng CI, Chen YH, Chai HT (2003) Risk stratification of in-hospital mortality in patients hospitalized for chronic congestive heart failure secondary to non-ischemic cardiomyopathy. Cardiology 100: 136–142. [DOI] [PubMed] [Google Scholar]
- 31. VillaCorta H, Mesquita ET, Cardoso R, Bonates T, Maia ER, et al. (2003) Emergency department predictors of survival in decompensated heart failure patients. Rev Port Cardiol 22: 495–507. [PubMed] [Google Scholar]
- 32. Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, et al. (2007) Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J 28: 980–988. [DOI] [PubMed] [Google Scholar]
- 33. Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, et al. (2007) Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med 167: 1998–2005. [DOI] [PubMed] [Google Scholar]
- 34. Milo-Cotter O, Cotter G, Weatherley BD, Adams KF, Kaluski E, et al. (2008) Hyponatraemia in acute heart failure is a marker of increased mortality but not when associated with hyperglycaemia. Eur J Heart Fail 10: 196–200. [DOI] [PubMed] [Google Scholar]
- 35. Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, et al. (2008) Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J 29: 339–347. [DOI] [PubMed] [Google Scholar]
- 36. Rusinaru D, Buiciuc O, Leborgne L, Slama M, Massy Z, et al. (2009) Relation of serum sodium level to long-term outcome after a first hospitalization for heart failure with preserved ejection fraction. Am J Cardiol 103: 405–410. [DOI] [PubMed] [Google Scholar]
- 37. Barsheshet A, Shotan A, Cohen E, Garty M, Goldenberg I, et al. (2010) Predictors of long-term (4-year) mortality in elderly and young patients with acute heart failure. Eur J Heart Fail 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 38. DeWolfe A, Lopez B, Arcement LM, Hebert K (2010) Low serum sodium as a poor prognostic indicator for mortality in congestive heart failure patients. Clin Cardiol 33: E13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Novack V, Pencina M, Zahger D, Fuchs L, Nevzorov R, et al. (2010) Routine laboratory results and thirty day and one-year mortality risk following hospitalization with acute decompensated heart failure. PLoS One 5: e12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baldasseroni S, Urso R, Orso F, Bianchini BP, Carbonieri E, et al. (2011) Relation between serum sodium levels and prognosis in outpatients with chronic heart failure: neutral effect of treatment with beta-blockers and angiotensin-converting enzyme inhibitors: data from the Italian Network on Congestive Heart Failure (IN-CHF database). J Cardiovasc Med 12: 723–731. [DOI] [PubMed] [Google Scholar]
- 41. Balling L, Schou M, Videbæk L, Hildebrandt P, Wiggers H, et al. (2011) Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail 13: 968–973. [DOI] [PubMed] [Google Scholar]
- 42. Shorr AF, Tabak YP, Johannes RS, Gupta V, Saltzberg MT, et al. (2011) Burden of sodium abnormalities in patients hospitalized for heart failure. Congest Heart Fail 17: 1–7. [DOI] [PubMed] [Google Scholar]
- 43. Arroyo V, Rodés J, Gutiérrez-Lizárraga MA, Revert L (1976) Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis 21: 249–256. [DOI] [PubMed] [Google Scholar]
- 44. Vila MC, Coll S, Solà R, Andreu M, Gana J, et al. (1999) Total paracentesis in cirrhotic patients with tense ascites and dilutional hyponatremia. Am J Gastroenterol 94: 2219–2223. [DOI] [PubMed] [Google Scholar]
- 45. Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F (2000) Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis 32: 605–610. [DOI] [PubMed] [Google Scholar]
- 46. Porcel A, Díaz F, Rendón P, Macías M, Martín-Herrera L, et al. (2002) Dilutional hyponatremia in patients with cirrhosis and ascites. Arch Intern Med 162: 323–328. [DOI] [PubMed] [Google Scholar]
- 47. Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, et al. (2005) Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl 11: 336–343. [DOI] [PubMed] [Google Scholar]
- 48. Hackworth WA, Heuman DM, Sanyal AJ, Fisher RA, Sterling RK, et al. (2009) Effect of hyponatraemia on outcomes following orthotopic liver transplantation. Liver Int 29: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 49. Radha Krishna Y, Saraswat VA, Das K, Himanshu G, Yachha SK, et al. (2009) Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int 29: 392–398. [DOI] [PubMed] [Google Scholar]
- 50. Terg R, Gadano A, Cartier M, Casciato P, Lucero R, et al. (2009) Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver Int 29: 415–419. [DOI] [PubMed] [Google Scholar]
- 51. Jenq CC, Tsai MH, Tian YC, Chang MY, Lin CY, et al. (2010) Serum sodium predicts prognosis in critically ill cirrhotic patients. J Clin Gastroenterol 44: 220–226. [DOI] [PubMed] [Google Scholar]
- 52. Singhi S, Dhawan A (1992) Frequency and significance of electrolyte abnormalities in pneumonia. Indian Pediatr 29: 735–740. [PubMed] [Google Scholar]
- 53. Sharma SK, Mohan A, Pande JN, Prasad KL, Gupta AK, et al. (1995) Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. QJM 88: 29–37. [PubMed] [Google Scholar]
- 54. El-Ebiary M, Sarmiento X, Torres A, Nogué S, Mesalles E, et al. (1997) Prognostic factors of severe Legionella pneumonia requiring admission to ICU. Am J Respir Crit Care Med 156: 1467–1472. [DOI] [PubMed] [Google Scholar]
- 55. Hussain SF, Irfan M, Abbasi M, Anwer SS, Davidson S, et al. (2004) Clinical characteristics of 110 miliary tuberculosis patients from a low HIV prevalence country. Int J Tuberc Lung Dis 8: 493–499. [PubMed] [Google Scholar]
- 56. Nair V, Niederman MS, Masani N, Fishbane S (2007) Hyponatremia in community-acquired pneumonia. Am J Nephrol 27: 184–190. [DOI] [PubMed] [Google Scholar]
- 57. Song JH, Oh WS, Kang CI, Chung DR, Peck KR, et al. (2008) Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents 31: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sunderam SG, Mankikar GD (1983) Hyponatraemia in the elderly. Age Ageing 12: 77–80. [DOI] [PubMed] [Google Scholar]
- 59. Samadi AR, Chowdhury AI, Huq MI, Shahid NS (1985) Risk factors for death in complicated diarrhoea of children. Br Med J (Clin Res Ed) 290: 1615–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cusano AJ, Thies HL, Siegal FP, Dreisbach AW, Maesaka JK (1990) Hyponatremia in patients with acquired immune deficiency syndrome. J Acquir Immune Defic Syndr 3: 949–953. [PubMed] [Google Scholar]
- 61. Vitting KE, Gardenswartz MH, Zabetakis PM, Tapper ML, Gleim GW, et al. (1990) Frequency of hyponatremia and nonosmolar vasopressin release in the acquired immunodeficiency syndrome. JAMA 263: 973–978. [PubMed] [Google Scholar]
- 62. Erinoso HO, Akinbami FO, Akinyinka OO (1993) Prognostic factors in severely malnourished hospitalized Nigerian children. Anthropometric and biochemical factors. Trop Geogr Med 45: 290–293. [PubMed] [Google Scholar]
- 63. Tang WW, Kaptein EM, Feinstein EI, Massry SG (1993) Hyponatremia in hospitalized patients with the acquired immunodeficiency syndrome (AIDS) and the AIDS-related complex. Am J Med 94: 169–174. [DOI] [PubMed] [Google Scholar]
- 64. Chuah SK, Sheen IS, Changchien CS, Chiu KW, Fan KD (1996) Risk factors associated with fulminant amebic colitis. J Formos Med Assoc 95: 446–451. [PubMed] [Google Scholar]
- 65. Iseki K, Uehara H, Nishime K, Tokuyama K, Yoshihara K, et al. (1996) Impact of the initial levels of laboratory variables on survival in chronic dialysis patients. Am J Kidney Dis 28: 541–548. [DOI] [PubMed] [Google Scholar]
- 66. Srivastava KL, Mittal A, Kumar A, Gupta S, Natu SM, et al. (1998) Predictors of outcome in fulminant hepatic failure in children. Indian J Gastroenterol 17: 43–45. [PubMed] [Google Scholar]
- 67. Berghmans T, Paesmans M, Body JJ (2000) A prospective study on hyponatraemia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer 8: 192–197. [DOI] [PubMed] [Google Scholar]
- 68. Manary MJ, Brewster DR (2000) Intensive nursing care of kwashiorkor in Malawi. Acta Paediatr 89: 203–207. [DOI] [PubMed] [Google Scholar]
- 69. Oguche S, Omokhodion SI, Adeyemo AA, Olumese PE (2002) Low plasma bicarbonate predicts poor outcome of cerebral malaria in Nigerian children. West Afr J Med 21: 276–279. [DOI] [PubMed] [Google Scholar]
- 70. Agarwal I, Kirubakaran C, Markandeyulu V (2004) Clinical profile and outcome of acute renal failure in South Indian children. J Indian Med Assoc 102: 353–356. [PubMed] [Google Scholar]
- 71. Lee JH, Choi SJ, Lee JH, Kim SE, Seol M, et al. (2005) Severe metabolic abnormalities after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 35: 63–69. [DOI] [PubMed] [Google Scholar]
- 72. Sherlock M, O'Sullivan E, Agha A, Behan LA, Rawluk D, et al. (2006) The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf) 64: 250–254. [DOI] [PubMed] [Google Scholar]
- 73. Bonney GK, Aldouri A, Attia M, Lodge PA, Toogood GJ, et al. (2008) Outcomes in right liver lobe transplantation: a matched pair analysis. Transpl Int 21: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 74. Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, et al. (2008) Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 177: 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Olotu AI, Mithwani S, Newton CR (2008) Haemolytic uraemic syndrome in children admitted to a rural district hospital in Kenya. Trop Doct 38: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hanson J, Hossain A, Charunwatthana P, Hassan MU, Davis TM, et al. (2009) Hyponatremia in severe malaria: evidence for an appropriate anti-diuretic hormone response to hypovolemia. Am J Trop Med Hyg 80: 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hsu HH, Chen YC, Tian YC, Chan YL, Kuo MC, et al. (2009) Role of serum sodium in assessing hospital mortality in cancer patients with spontaneous tumour lysis syndrome inducing acute uric acid nephropathy. Int J Clin Pract 63: 751–756. [PubMed] [Google Scholar]
- 78. Kapoor R, Muruganandham K, Gulia AK, Singla M, Agrawal S, et al. (2010) Predictive factors for mortality and need for nephrectomy in patients with emphysematous pyelonephritis. BJU Int 105: 986–989. [DOI] [PubMed] [Google Scholar]
- 79. Dimopoulos K, Diller GP, Petraco R, Koltsida E, Giannakoulas G, et al. (2010) Hyponatraemia: A strong predictor of mortality in adults with congenital heart disease. Eur Heart J 31: 595–601. [DOI] [PubMed] [Google Scholar]
- 80. Salvador VB, San Juan MD, Salisi JA, Consunji RJ (2010) Clinical and microbiological spectrum of necrotizing fasciitis in surgical patients at a Philippine university medical centre. Asian J Surg 33: 51–58. [DOI] [PubMed] [Google Scholar]
- 81. Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, et al. (2010) Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 182: 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stelfox HT, Ahmed SB, Zygun D, Khandwala F, Laupland K (2010) Characterization of intensive care unit acquired hyponatremia and hypernatremia following cardiac surgery. Can J Anaesth 57: 650–658. [DOI] [PubMed] [Google Scholar]
- 83. Hoorn EJ, Rivadeneira F, Van Meurs JB, Ziere G, Stricker BH, et al. (2011) Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res 26: 1822–1828. [DOI] [PubMed] [Google Scholar]
- 84. Saifudheen K, Jose J, Gafoor VA, Musthafa M (2011) Guillain-Barre syndrome and SIADH. Neurology 76: 701–704. [DOI] [PubMed] [Google Scholar]
- 85. Vaa BE, Asrani SK, Dunn W, Kamath PS, Shah VH (2011) Influence of serum sodium on MELD-based survival prediction in alcoholic hepatitis. Mayo Clin Proc 86: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tierney WM, Martin DK, Greenlee MC, Zerbe RL, McDonald CJ (1986) The prognosis of hyponatremia at hospital admission. J Gen Intern Med 1: 380–385. [DOI] [PubMed] [Google Scholar]
- 87. Natkunam A, Shek CC, Swaminathan R (1991) Hyponatremia in a hospital population. J Med 22: 83–96. [PubMed] [Google Scholar]
- 88. Singhi S, Prasad SV, Chugh KS (1994) Hyponatremia in sick children: a marker of serious illness. Indian Pediatr 31: 19–25. [PubMed] [Google Scholar]
- 89. Miller M, Morley JE, Rubenstein LZ (1995) Hyponatremia in a nursing home population. J Am Geriatr Soc 43: 1410–1413. [DOI] [PubMed] [Google Scholar]
- 90. Asadollahi K, Hastings IM, Beeching NJ, Gill GV (2007) Laboratory risk factors for hospital mortality in acutely admitted patients. QJM 100: 501–507. [DOI] [PubMed] [Google Scholar]
- 91. Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, et al. (2008) Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin 24: 1601–1608. [DOI] [PubMed] [Google Scholar]
- 92. Hampshire PA, Welch CA, McCrossan LA, Francis K, Harrison DA (2009) Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care 13: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Whelan B, Bennett K, O'Riordan D, Silke B (2009) Serum sodium as a risk factor for in-hospital mortality in acute unselected general medical patients. QJM 102: 175–182. [DOI] [PubMed] [Google Scholar]
- 94. Whyte M, Down C, Miell J, Crook M (2009) Lack of laboratory assessment of severe hyponatraemia is associated with detrimental clinical outcomes in hospitalised patients. Int J Clin Pract 63: 1451–1455. [DOI] [PubMed] [Google Scholar]
- 95. Funk GC, Lindner G, Druml W, Metnitz B, Schwarz C, et al. (2010) Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med 36: 304–311. [DOI] [PubMed] [Google Scholar]
- 96. Konstam MA, Udelson JE (2009) Hyponatraemia and vasopressin in heart failure: markers or mediators? Eur J Heart Fail 13: 242–244. [DOI] [PubMed] [Google Scholar]
- 97. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, et al. (2007) Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 297: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 98. Madan VD, Novak E, Rich MW (2011) Impact of change in serum sodium concentration on mortality in patients hospitalized with heart failure and hyponatremia. Circ Heart Fail 4: 637–643. [DOI] [PubMed] [Google Scholar]
- 99. Lee SE, Choi DJ, Yoon CH, Oh IY, Jeon ES, et al. (2012) Improvement of hyponatraemia during hospitalisation for acute heart failure is not associated with improvement of prognosis: an analysis from the Korean Heart Failure (KorHF) registry. Heart 98: 1798–1804. [DOI] [PubMed] [Google Scholar]
- 100. Goldsmith SR (2012) Hyponatremia and outcomes in patients with heart failure. Heart 98: 1761–1762. [DOI] [PubMed] [Google Scholar]
- 101. Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH (2007) Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med 120: S1–21. [DOI] [PubMed] [Google Scholar]
- 102. Peri A, Pirozzi N, Parenti G, Festuccia F, Menè P (2010) Hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J Endocrinol Invest 33: 671–682. [DOI] [PubMed] [Google Scholar]
- 103. Peri A (2013) Clinical review: the use of vaptans in clinical endocrinology. J Clin Endocrinol Metab 98: 1321–1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)