Abstract
Study Objectives:
To determine current patterns and predictors of use of prescription medications commonly used for insomnia (MCUFI) in the U.S.
Design:
Cross-sectional study.
Setting:
National Health and Nutrition Examination Survey, 1999-2010.
Participants:
32,328 noninstitutionalized community-dwelling U.S. adults.
Interventions:
N/A.
Measurements and Results:
We defined MCUFI use as use of any of the following medications in the preceding month: benzodiazepine receptor agonists (eszopiclone, zaleplon, zolpidem, estazolam, flurazepam, quazepam, temazepam, triazolam), barbiturates (amobarbital, amobarbitalsecobarbital, chloral hydrate), doxepin, quetiapine, ramelteon, and trazodone. We estimated prevalence of MCUFI use and concurrent use of another sedating medication. We determined predictors of MCUFI use using multivariate logistic regression.
Overall, 3% percent of adults used a MCUFI within the preceding month. Zolpidem and trazodone were used most commonly. Overall MCUFI use increased between 1999-2000 and 2009-2010 (P value for trend < 0.001). Concurrent use of other sedating medications was high, with 55% of MCUFI users taking at least one other sedating medication and 10% taking ≥ 3 other sedating medications. Concurrent use of MCUFIs with opioids (24.6%) and non-MCUFI benzodiazepines (19.5%) were most common. After adjustment, adults seeing a mental health provider (aOR 4.68, 95% C.I. 3.79, 5.77), using other sedating medications (aOR 4.18, 95% C.I. 3.36, 5.19), and age ≥ 80 years (aOR 2.55, 95% C.I. 1.63, 4.01) had highest likelihood of MCUFI use.
Conclusion:
In this nationally representative sample, reported use of prescription medications commonly used for insomnia (MCUFIs) within the preceding month was common, particularly among older adults and those seeing a mental health provider, with high use of sedative polypharmacy among MCUFI users.
Citation:
Bertisch SM; Herzig SJ; Winkelman JW; Buettner C. National use of prescription medications for insomnia: NHANES 1999-2010. SLEEP 2014;37(2):343-349.
Keywords: Insomnia, prescription medication use, NHANES, hypnotics and sedatives, benzodiazepines, sleep disorders
INTRODUCTION
Insomnia affects an estimated 10-15% of American adults.1 Growing awareness of insomnia and other sleep disorders led to a doubling of physician visits for sleeplessness and an over 7-fold increase in visits with insomnia diagnoses from 1993 to 2007.2 Prescriptions for medications for insomnia have increased accordingly, with a striking 30-fold increase in prescriptions for nonbenzodiazepine sedative hypnotics (e.g., zolpidem) during this time.2
Despite these remarkable trends, as well as ongoing concerns regarding safety3,4 and tolerance of prescription medications indicated for insomnia, particularly among older adults,5 few have investigated the current prevalence, patterns, and predictors of use of prescription medications for insomnia. Most prior analyses have described psychotropic prescribing more generally, rather than focusing on medications commonly used for insomnia,6–9 and reflect prescribing patterns from more than a decade ago.6–15 As a result, data on use of newer nonbenzodiazepine sedative hypnotics are sparse. Furthermore, since the majority of patients suffering from sleep difficulties never consult a physician,16 rates of prescription medication use for insomnia derived from office-based studies utilizing the National Ambulatory Medical Care Survey (NAMCS), may underestimate the true rate of use in the community, where patients may share prescription medications.17–19 Additionally, NAMCS does not capture data on visits at hospital based or federally operated outpatient practices, where prescribing patterns and patient characteristics may differ.20–22
To identify current prevalence, patterns, and predictors of use of prescription medications commonly used for insomnia (MCUFI) among U.S. adults, we used the National Health and Nutrition Examination Survey (NHANES) 1999-2010. In contrast to NAMCS, NHANES provides information on patient-reported prescription medication use, with in-home medication reconciliation and verification by prescription medicine containers among community dwelling adults. We additionally sought to define rates of sedating medication poly-pharmacy by investigating concurrent use of other medications with sedating properties.
METHODS
Study Population
The National Health and Nutrition Examination Survey (NHANES) is an in-person survey of the civilian, noninstitutionalized U.S. population, conducted by the National Center for Health Statistics (NCHS). Households are selected for face-to-face interviews in English and/or Spanish using a multistage probability sampling design. Low-income persons, persons ≥ 60 years of age, African Americans, and persons of Mexican origin are oversampled. Demographic information is collected on all household members, and at least one household member is selected for the sample person questionnaire, which elicits information on medical conditions, health habits, and prescription medication use. Since 1999, data have been collected continuously and released at 2-year intervals.
Our study sample included adults ≥ 20 years of age who participated in NHANES 1999-2010 (n = 32,328), and completed home interviews and self-administered questionnaires. Consent was obtained from participants by the NCHS after approval by the NHANES Institutional Review Board/ NCHS Research Ethics Review Board. Our analyses were approved for exemption by our institutional review board based on 45 CFR 46.101(b) (4).
Assessment of Medication Use
During all surveyed years (1999-2010), sampled participants were asked, “In the past 30 days, have you used or taken a medication for which a prescription is needed?” Those reporting prescription medication use were then asked to show all of their medication containers to the interviewer for recording. If participants could not produce containers, interviewers asked for verbal confirmation of medication name.
Outcomes of Interest
We defined our primary outcome as use of at least one prescription medication commonly used for insomnia (MCUFI) within the past month (yes/no). Our definition included medications that are more often prescribed for treatment of insomnia than for other indications, and was consistent with prior epidemiologic studies examining prescription medication use for insomnia.2,10 Prescription medications classified as MCUFIs included nonbenzodiazepine benzodiazepine-receptor agonists or “Z-meds” (eszopiclone, zaleplon, zolpidem); benzodiazepines (estazolam, flurazepam, quazepam, temazepam, triazolam); barbiturates (amobarbital, amobarbital-secobarbital, chloral hydrate); doxepin; quetiapine; ramelteon; and trazodone.
We also separately explored use of categories of MCUFIs and individual MCUFIs (yes/no) within the past month, for which sample sizes were sufficient, and trends in use over the survey periods studied. Data on non-prescription medications used for insomnia, as well as reasons for use of prescription medications, were not available.
Additional Factors of Interest
Non-MCUFI Medications with Sedative Effects
We defined “other sedative medications” as prescription medications that are likely to cause clinically important sedating effects and are more often prescribed for non-sleep related than sleep related indications. Hence, our definition comprised a broad range of prescription medication categories, including antihistamines, antipsychotics, non-MCUFI barbiturates, non-MCUFI benzodiazepines, muscle relaxants, opioids, antiepileptic drugs, sedating antidepressants (e.g., tricyclic anti-depressants), and “sedatives-NOS” (see supplemental material for comprehensive list). For combination medications, only one medication had to satisfy these criteria.
Additional Covariates of Interest
Sociodemographic characteristics included age, sex, race/ ethnicity, marital status, educational attainment, and family income. As a measure of respondents' health habits, we considered smoking status (past, current, or never), physical activity, and alcohol intake. Physical activity was based on NHANES' categorization of physical activity levels during the last 30 days and defined as vigorous if participants reported any “vigorous activity for at least 10 minutes that caused heavy sweating or large increases in breathing or heart rate,” moderate if participants reported “moderate activities for at least 10 minutes that cause only light sweating or a slight to moderate increase in breathing or heart rate,” or sedentary if neither vigorous nor moderate activity was reported. Alcohol intake was classified as abstain/rare (< 12 drinks/year), light (< 1 drink/week), moderate (1-7 drinks/week for women, 1-14 drinks/week for men), and heavy (> 7 drinks/week for women and > 14 drinks/week for men) adapted from definitions used by the National Institute on Alcohol Abuse and Alcoholism. We defined health care access and network using several proxies including insurance status (Private, Medicare, Medicaid or other government insurance, none/unknown), usual source of care (clinic or health center, doctor's office/health maintenance organization, emergency department, other place NOS, no usual place/unknown), and number of visits to health care providers in the past year (0-1, 2-3, 4-9, > 9) categorized to achieve sufficient sample sizes in each group. We also used self-reported health status (excellent/ very good, good, and fair/poor), body mass index (BMI, kg/m2), chronic medical conditions (history of arthritis, cancer, cardiovascular disease, diabetes, liver disease, lung disease), and seen by a mental health professional within past year (yes/no), as a proxy for respondents' medical and psychiatric illness burden.
Statistical Methods
Analyses were performed using SAS (v. 9.2, Cary, NC), SUDAAN (v. 11.0.0, Research Park Triangle, NC), and STATA (v. 12.1, College Station, TX). Estimates were weighted to account for the unequal probabilities of selection resulting from the complex sample design, non-response, and planned oversampling of selected populations. We used chi-square tests to compare characteristics between MCUFI users and non-users. We calculated the prevalence of use of MCUFIs overall, categories of MCUFIs, and concurrent use of other sedative medications.
To determine predictors of MCUFI use, we performed multivariate logistic regression. As there were significant missing data for income (n = 2,403) and alcohol intake (n = 4,445) among participants included in the main model (n = 32,328), we performed multiple imputation by chained equations.23 All analyses were adjusted for the following list of potential confounders that were specified a priori: age, sex, race/ethnicity, educational level, imputed family income, marital status, insurance status, smoking status, imputed alcohol intake, physical activity level, self-reported health status, chronic medical conditions, having seen a mental health professional in past year, and use of other prescription sedative medication. To examine the robustness of our results, in sensitivity analyses, we further adjusted for additional measures of health-care access (number of visits to health-care provider in the last year and usual source of care).
Subpopulation Analyses
Since BMI is associated with sleep disorders, we repeated our models adding in BMI among the subpopulation of adults also participating in the physical examination component of the NHANES (n = 29,082). Lastly, among the subsample participating in NHANES 2005-2008 (n = 10,878) who were also queried specifically about use of pills or medications “to help with sleep,” we quantified prevalence of use of sleep aids (yes/ no) and explored concurrent use of MCUFIs and sleep aids. Of note, specific types of medications used to help with sleep were not elicited. Among the NHANES 2005-2008 subsample, we explored concurrent use of MCUFIs and self-reported use of medications for sleep.
RESULTS
Sample Characteristics
Overall, 906 sampled participants, representing an estimated 3% of the U.S. population or over 6 million adults, reported using a prescription medication commonly used for insomnia (MCUFI) within the preceding month. Table 1 presents the descriptive characteristics of adults by MCUFI status and unadjusted, stratified prevalence of MCUFI use. Compared with adults reporting no use, MCUFI users tended to be older, female, previously married, of non-Hispanic white ethnicity, have a family income of < $20,000/year, and have Medicaid or Medicare insurance. They also tended to have poorer health habits (i.e., current or former smoker; low physical activity levels), reported worse health status, had higher rates of use of other sedative medications in the preceding month, and reported seeing a mental health professional within past year. Unadjusted prevalence of MCUFI use was highest among those with fair/poor health status (6.5%), seeing a mental health provider (14.1%), using other sedative medications (11.9%), having Medicare or Medicaid/governmental insurance (5.9-6.5%), having chronic medical conditions (5.0-7.8% for conditions of interest), and also among older adults (4.0-5.3%).
Table 1.
Characteristics of adults by use of prescription medication commonly used for insomnia (MCUFI), past month (n = 32,328, N = 207,085,151)

Use of Medications Commonly Used for Insomnia
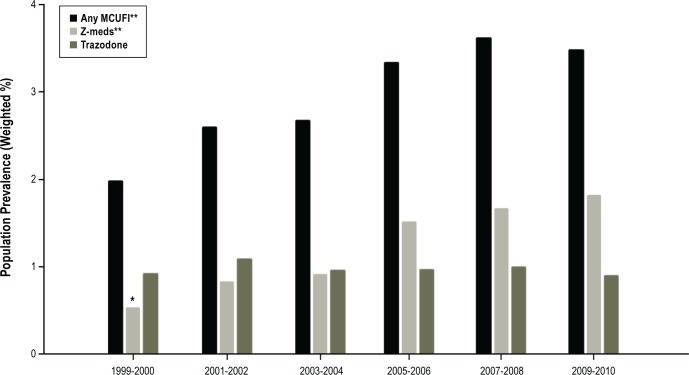
Table 2 lists prevalence of MCUFI use; Z-meds were the most commonly used category, followed by trazodone—each with a prevalence of use of about 1% nationally. Zolpidem accounted for 87.5% of Z-med use. Use of doxepin was uncommon. Overall MCUFI use increased significantly over the years studied, from 2.0% in 1999-2000 to 3.5% in 2009-2010 (P value for trend < 0.001, Figure 1). Use of Z-meds also increased significantly, from 0.5% in 1999-2000 to 1.8% in 2009-2010 (P value for trend < 0.001), while use of trazodone did not change (P value for trend = 0.78) during the same time period. Among adults using MCUFIs, we found that over 55% also reported using other prescription sedative medications during the preceding month. Opioids (24.6%) and non MCUFI benzodiazepines (19.5%) were the most commonly reported sedative medications used concurrently with MCUFIs. Additionally, we found that over 10% of MCUFI users reported taking 3 or more other sedative medications within the past month.
Table 2.
Prevalence of use of prescription medication commonly used for insomnia (MCUFI), past month

Figure 1.
Prescription medication commonly used for insomnia (MCUFI), past month: 1999-2010. *Unreliable estimate (n < 30). **P value for trend < 0.001. Z-meds: eszopiclone, zaleplon, zolpidem.
In our subsample analyses, using data from only NHANES 2005-2008 (n = 10,878) in which participants were queried specifically about use of sleep aids, we found that 19.2% of adults reported using at least one pill or medication to help them sleep during the past month. Among these 19.2% of participants, 19.6% used a MCUFI only and 41.9% reported use of a MCUFI and/or another sedative medication. Thus 58.1% of participants taking a pill or medication specifically for sleep within the preceding month did not report use of a MCUFI or other prescription medication with sedative effects during this same time period.
Correlates of Use of Medications Commonly Used for Insomnia
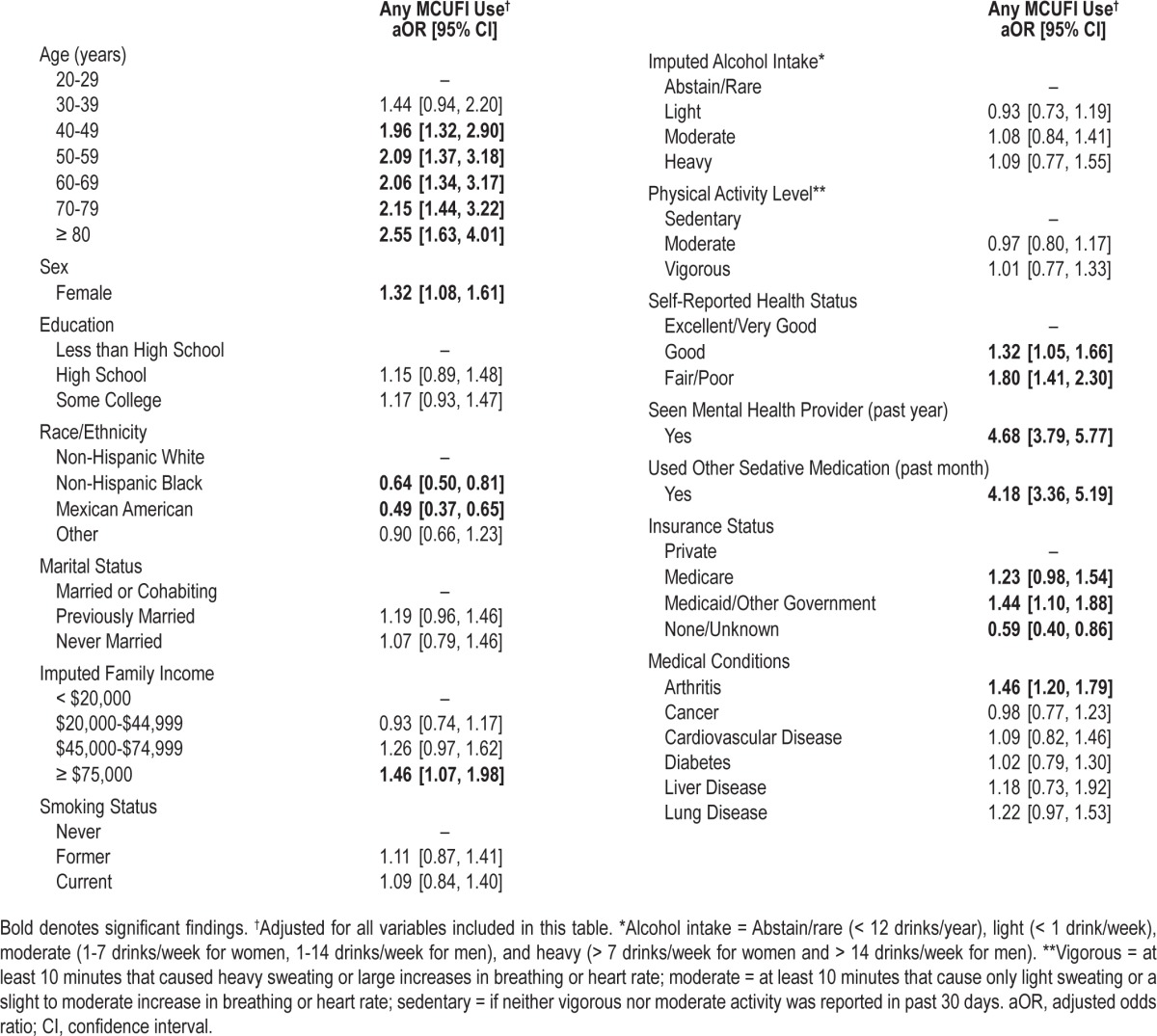
Factors found to be significantly associated with MCUFI use in our primary model (Table 3) included increasing age, female sex, higher income, having Medicaid/or other governmental health insurance, worse self-reported health status, having arthritis, seeing a mental health professional within the past year, and taking other sedative prescription medication. Factors significantly associated with a lower likelihood of MCUFI use included Non-Hispanic black or Mexican American race/ethnicity and having none/unknown insurance status. Further adjustment for health care access did not substantially alter these findings. In secondary analyses, body mass index was not significantly associated with MCUFI use, and did not substantively alter the relationship between MCUFI use and other factors of interest.
Table 3.
Factors associated with of use of prescription medication commonly used for insomnia (MCUFI), past month (n = 32,328)
DISCUSSION
In this large, nationally representative study, we found increasing prevalence of MCUFI use over time, from 2.0% in 1999-2000 to 3.5% in 2009-2010. A majority of MCUFI users reported concurrent use of other prescription medications with sedative properties. Having seen a mental health professional in the past year and use of other sedating medications were the strongest independent predictors of MCUFI use. Among sociodemographic factors, increasing age, female sex, and higher income were associated with higher likelihood of use, while those of Non-Hispanic black and Mexican American race/ethnicity had lower use. Even with increasing rates of use of MCUFIs, between 2005-2008 the vast majority of adults using sleep aids did not report using MCUFIs.
Consistent with prior studies estimating overall sleep medication use on a national level, we found that prescription medication use has continued to increase over time. Using data from NAMCS, Moloney et al. reported that prescriptions for medications indicated for insomnia increased per office visit from 1993 to 2007. Specifically, they found a 50% increase in benzodiazepine prescriptions—from 2.5 million in 1993 to 3.7 million in 2007—and a 30-fold increase in non-benzodiazepine sedative hypnotic prescriptions (i.e., Z-meds and ramelteon), reaching 16.2 million prescriptions in 2007.2 We found a similar, though much less robust, 3- to 4-fold increase in use of Z-meds within the preceding month from 1999-2010. This discrepancy is likely due to the approval of zolpidem in 1992, with its use being more common by the start of our study period (1999-2000). Together these data demonstrate a significant increase in prescriptions for and use of medications indicated for insomnia over the past decade, with much of this rise attributable to Z-meds rather than “older medications” prescribed for sleep (e.g., trazodone). Though current data support use of Z-meds for treatment of insomnia and suggest adverse events from Z-meds may be less frequent than benzodiazepines,3 given the lack of long-term studies assessing efficacy,24 fall-risks, cognitive impairment,25 and other well-described side effects (e.g., complex sleep related behaviors),26,27 further research into adverse effects in relation to prescribing patterns of these medications is warranted.3
In addition to research on overall prevalence of MCUFI use, Balkrinshnan et al. investigated predictors of pharmacotherapy for insomnia using NAMCS data from 1996 to 2001.10 Consistent with our results, they found that older adults (65 years or older), women, white race, and individuals with public, as compared to private insurance, had higher likelihood of receiving pharmacotherapy for insomnia. Similarly, Paulose-Ram et al. studied use of prescription psychotropic medication in NHANES from 1988 to 1994, also identifying female sex, white race, and older age as independent predictors of psychotropic medication use.7 Use of these medications in older adults is of particular concern as they have a nearly five times increased odds of experiencing cognitive side effects compared with placebo,28 with Z-meds having similar rates of psychomotor-type adverse events (reports of dizziness, loss of balance, or falls) and cognitive adverse events (memory loss, confusion, disorientation), compared with benzodiazepines in adults over 60 years of age.28
Our finding that 58.1% of participants who reported taking any pill or medication to aid with sleep within the preceding month did not report concurrent use of a MCUFI or other prescription medication with sedative effects, suggests that use of over-the-counter sleep aids (e.g., diphenhydramine) is highly prevalent and vastly underestimated in studies that rely on prescription data. Therefore our study also undoubtedly underestimates the prevalence of sedating medication poly-pharmacy. These findings highlight the importance that physicians prescribing medications with sedating properties should inquire and counsel about concurrent use of both prescription and over-the-counter medications with sedative properties.
Limitations
A major limitation of our study is that NHANES data collected on indications for prescription medication are not publicly available, and hence not all MCUFI prescriptions may have been for treatment of insomnia, likely leading to some misclassification bias. Additionally, we did not include all medications potentially used for insomnia in our definition of MCUFI, as we chose to focus on medications that are more often prescribed for treatment of insomnia than for other indications to assure specificity of our outcome definition. Thus, the rate of medication use for insomnia is likely higher than the estimate derived in our study owing to our necessarily conservative exposure definition. Although self-reported use of prescription medications is verified with prescription bottles, 20% of prescription medications in NHANES are not confirmed with a dispensed container, which could have led to further misclassification bias. However, 93% of respondents classified as MCUFI users did report use of a medication for sleep difficulties, suggesting high specificity of our outcome definition. Additionally, information was collected on prescription medication use only within the preceding month, limiting our ability to distinguish the characteristics of short-term MCUFI users to those using MCUFIs longer-term, which may be of clinical importance.
CONCLUSION
In the United States, use of MCUFIs within the preceding month, most notably Z-meds, was common and increased from 2.0% in 1999-2010 to 3.5% in 2009-2010, with likely higher rates of use when accounting for use of other prescription medications for insomnia (i.e., non-MCUFIs). High rates of MCUFI use were reported among older individuals and those seeing a mental health provider. Among adults using MCUFIs, there is a high rate of concurrent use of other sedative medications.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bertisch was supported by K23 Career Development Award (K23AT005104). Dr. Winkelman was supported by R01 MH095792. Dr. Buettner was supported by K23 Career Development Award (K23AR055664). Dr. Bertisch has received research support from UCB. Dr. Winkelman has consulting/advisory arrangements with UCB, Zeo Inc., and Xenoport and has received research support from GlaxoSmithKline, Impax Pharmaceuticals, NIH, and UCB. The other authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Other Sedative Medications: Prescription medications likely to cause clinically important sedating effects that are more often prescribed for non-sleep related indications.
A
acetaminophen/butalbital
acetaminophen/butalbital/caffeine
acetaminophen/butalbital/caffeine/codeine
acetaminophen/caffeine/chlorpheniramine maleate/hydrocodone/phenylephrine
acetaminophen/caffeine/dihydrocodeine
acetaminophen/chlorpheniramine/phenylephrine/phenyltoloxamine
acetaminophen/codeine
acetaminophen/dichloralphenazone/isometheptene
acetaminophen/hydrocodone
acetaminophen/oxycodone
acetaminophen/pentazocine
acetaminophen/phenyltoloxamine
acetaminophen/propoxyphene
acetaminophen/tramadol
acetylcarbromal
acrivastine/pseudoephedrine
alprazolam
aminophylline/amobarbital/ephedrine
aminophylline/ephedrine/phenobarbital/potassium iodide
aminophylline/ephedrine/guaifenesin/phenobarbital
amitriptyline/chlordiazepoxide
anhydrous calcium iodide/codeine
aripiprazole
aspirin/butalbital
aspirin/butalbital/caffeine
aspirin/butalbital/caffeine/codeine
aspirin/caffeine/orphenadrine
aspirin/carisoprodol
aspirin/hydrocodone
aspirin/methocarbamol
aspirin/oxycodone
atropine/hyoscyamine/phenobarbital/scopolamine
atropine/phenobarbital
B
baclofen
belladonna
belladonna/butabarbital
belladonna/caffeine/ergotamine/pentobarbital
belladonna/ergotamine/phenobarbital
belladonna/opium
betamethasone/indomethasone/methocarbamol
biperiden
bromazepam
brompheniramine
brompheniramine/carbetapentane/phenylephrine
brompheniramine/codeine/phenylpropanolamine
brompheniramine/dextromethorphan/guaifenesin/phenylephrine
brompheniramine/dextromethorphan/guaifenesin/pseudoephedrine
brompheniramine/dextromethorphan/phenylephrine
brompheniramine/dextromethorphan/phenylpropanolamine
brompheniramine/dextromethorphan/pseudoephedrine
brompheniramine/diphenhydramine/phenylephrine
brompheniramine/hydrocodone/phenylephrine
brompheniramine/hydrocodone/pseudoephedrine
brompheniramine/phenylephrine
brompheniramine/phenylpropanolamine
brompheniramine/pseudoephedrine
buprenorphine/naloxone
buspirone
butabarbital
butabarbital/hyoscyamine/phenazopyridine
butalbital
C
cannabis
carbamazepine
carbetapentane/chlorpheniramine
carbetapentane/chlorpheniramine maleate/ephedrine/phenylephrine
carbetapentane/chlorpheniramine/phenylephrine
carbetapentane/phenylephrine/pyrilamine
carbinoxamine
carbinoxamine/dextromethorphan/pseudoephedrine
carbinoxamine/hydrocodone/pseudoephedrine
carbinoxamine/pseudoephedrine
carisoprodol
chlordiazepoxide
chlordiazepoxide-clidinium
chlordiazepoxide-methscopolamine
chlormezanone
chlorphenesin
chlorpheniramine
chlorpheniramine/codeine/pseudoephedrine
chlorpheniramine/dextromethorphan/guaifenesin/phenylephrine
chlorpheniramine/dextromethorphan/phenylephrine
chlorpheniramine/dextromethorphan/pseudoephedrine
chlorpheniramine/dihydrocodeine/phenylephrine
chlorpheniramine/hydrocodone
chlorpheniramine/hydrocodone/phenylephrine
chlorpheniramine/hydrocodone/pseudoephedrine
chlorpheniramine/methscopolamine/phenylephrine
chlorpheniramine/methscopolamine/pseudoephedrine
chlorpheniramine/phenylephrine
chlorpheniramine/phenylephrine/phenylpropanolamine/phenyltoloxamine
chlorpheniramine/phenylephrine/phenyltoloxamine
chlorpheniramine/phenylephrine/pyrilamine
chlorpheniramine/pseudoephedrine
chlorpromazine
chlorzoxazone
clemastine
clidinium
clonazepam
clorazepate
clozapine
codeine
codeine/guaifenesin
codeine/guaifenesin/pseudoephedrine
codeine/phenylephrine/promethazine
codeine/promethazine
cyclizine
cyclobenzaprine
cyproheptadine
D
dantrolene
dexbrompheniramine/hydrocodone/phenylephrine
dexbrompheniramine/pseudoephedrine
dexchlorpheniramine
dexchlorpheniramine/dextromethorphan/phenylephrine
dexchlorpheniramine/dextromethorphan/pseudoephedrine
dextromethorphan/diphenhydramine/phenylephrine
dextromethorphan/promethazine
diazepam
dichloralphenazone
dihydrocodeine/guaifenesin/pseudoephedrine
dimenhydrinate
diphenhydramine
diphenhydramine/hydrocodone/phenylephrine
diphenhydramine/phenylephrine
diphenhydramine/pseudoephedrine
divalproex sodium
doxacurium
dronabinol
droperidol
dyphylline/ephedrine/guaifenesin/phenobarbital
E
ephedrine/phenobarbital/potassium iodide/theophylline
ephedrine/hydroxyzine/theophylline
ephedrine/phenobarbital/theophylline
ethosuximide
F
fentanyl
fluoxetine/olanzapine
fluphenazine
G
gabapentin
guaifenesin/hydrocodone
guaifenesin/hydrocodone/pheniram/phenylpropanolamine/pyrilamin
guaifenesin/hydrocodone/pheniramine/phenylephrine/phenylpropanolamine
guaifenesin/hydrocodone/phenylephrine
guaifenesin/hydrocodone/pseudoephedrine
guaifenesin/phenylephrine/pyrilamine
H
haloperidol
homatropine/hydrocodone
hydrocodone
hydrocodone/ibuprofen
hydrocodone/pheniramine/phenylephrine/phenylpropanolamine/pyrilamine
hydrocodone/phenylephrine
hydrocodone/phenylephrine/pyrilamine
hydrocodone/phenylephrine/pyrilamine
hydrocodone/phenylpropanolamine
hydrocodone/potassium guaiacolsulfonate
hydrocodone/pseudoephedrine
hydrocodone/pseudoephedrine/triprolidine
hydromorphone
hydroxyzine
hyoscyamine/phenobarbital
L
lamotrigine
levetiracetam
lithium
lorazepam
loxapine
M
meclizine
meperidine
meperidine/promethazine
mephobarbital
mesoridazine
metaxalone
methadone
methdilazine
methocarbamol
methotrimeprazine
methsuximide
metoclopramide
mivacurium
morphine
N
nabilone
O
olanzapine
opium
orphenadrine
oxazepam
oxcarbazepine
oxycodone
oxymorphone
P
pancuronium
pentazocine
perphenazine
phenindamine
pheniramine
pheniramine/phenylephrine nasal
pheniramine/phenylpropanolamine/phenyltoloxamine/pyrilamine
pheniramine/phenylpropanolamine/pyrilamine
pheniramine/phenyltoloxamine/pseudoephedrine/pyrilamine
pheniramine/phenyltoloxamine/pyrilamine
phenobarbital
phenylephrine/promethazine
phenylephrine/pyrilamine
phenyltoloxamine
phenytoin
pramipexole
pregabalin
prochlorperazine
prochlorperazine
promazine
promethazine
propiomazine
propoxyphene
pyrilamine
R
risperidone
ropinirole
S
scopolamine
succinylcholine
T
tapentadol
thiethylperazine
thioridazine
tiagabine
tizanidine
topiramate
tramadol
trifluoperazine
triflupromazine
trimeprazine
trimethobenzamide
tripelennamine
triprolidine
tubocurarine
V
valproic acid
Z
ziprasidone
zonisamide
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Moloney ME, Konrad TR, Zimmer CR. The medicalization of sleeplessness: a public health concern. Am J Public Health. 2011;101:1429–33. doi: 10.2105/AJPH.2010.300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verster JC, Veldhuijzen DS, Patat A, Olivier B, Volkerts ER. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63–71. doi: 10.2174/157488606775252674. [DOI] [PubMed] [Google Scholar]
- 5.Fick D, S T, Beizer J, Brandt N, et al. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aparasu RR, Mort JR, Brandt H. Psychotropic prescription use by community-dwelling elderly in the United States. J Am Geriatr Soc. 2003;51:671–7. doi: 10.1034/j.1600-0579.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- 7.Paulose-Ram R, Jonas BS, Orwig D, Safran MA. Prescription psychotropic medication use among the U.S. adult population: results from the third National Health and Nutrition Examination Survey, 1988-1994. J Clin Epidemiol. 2004;57:309–17. doi: 10.1016/j.jclinepi.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Paulose-Ram R, Safran MA, Jonas BS, Gu Q, Orwig D. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiol Drug Saf. 2007;16:560–70. doi: 10.1002/pds.1367. [DOI] [PubMed] [Google Scholar]
- 9.Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA. 1998;279:526–31. doi: 10.1001/jama.279.7.526. [DOI] [PubMed] [Google Scholar]
- 10.Balkrishnan R, Rasu RS, Rajagopalan R. Physician and patient determinants of pharmacologic treatment of sleep difficulties in outpatient settings in the United States. Sleep. 2005;28:715–9. doi: 10.1093/sleep/28.6.715. [DOI] [PubMed] [Google Scholar]
- 11.Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey data, 1997-2002. Clin Ther. 2006;28:1044–53. doi: 10.1016/j.clinthera.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Radecki SE, Brunton SA. Management of insomnia in office-based practice. National prevalence and therapeutic patterns. Arch Fam Med. 1993;2:1129–34. doi: 10.1001/archfami.2.11.1129. [DOI] [PubMed] [Google Scholar]
- 13.Rasu RS, Shenolikar RA, Nahata MC, Balkrishnan R. Physician and patient factors associated with the prescribing of medications for sleep difficulties that are associated with high abuse potential or are expensive: an analysis of data from the National Ambulatory Medical Care Survey for 1996-2001. Clin Ther. 2005;27:1970–9. doi: 10.1016/j.clinthera.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Roehrs T, Roth T. ‘Hypnotic’ prescription patterns in a large managed-care population. Sleep Med. 2004;5:463–6. doi: 10.1016/j.sleep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Wysowski DK, Baum C. Outpatient use of prescription sedative-hypnotic drugs in the United States, 1970 through 1989. Arch Intern Med. 1991;151:1779–83. [PubMed] [Google Scholar]
- 16.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–53. [PubMed] [Google Scholar]
- 17.Ellis J, Mullan J. Prescription medication borrowing and sharing--risk factors and management. Aust Fam Physician. 2009;38:816–9. [PubMed] [Google Scholar]
- 18.Goldsworthy RC, Schwartz NC, Mayhorn CB. Beyond abuse and exposure: framing the impact of prescription-medication sharing. Am J Public Health. 2008;98:1115–21. doi: 10.2105/AJPH.2007.123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen EE, Rasmussen SA, Daniel KL, Yazdy MM, Honein MA. Prescription medication borrowing and sharing among women of reproductive age. J Womens Health (Larchmt) 2008;17:1073–80. doi: 10.1089/jwh.2007.0769. [DOI] [PubMed] [Google Scholar]
- 20.Burt CW, Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1999--2000. Vital Health Stat. 2004:1–70. [PubMed] [Google Scholar]
- 21.Forrest CB, Whelan EM. Primary care safety-net delivery sites in the United States: A comparison of community health centers, hospital outpatient departments, and physicians' offices. JAMA. 2000;284:2077–83. doi: 10.1001/jama.284.16.2077. [DOI] [PubMed] [Google Scholar]
- 22.Nelson CR, Knapp DE. Medication therapy in ambulatory medical care. National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey, 1992. Adv Data. 1997:1–24. [PubMed] [Google Scholar]
- 23.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 24.Silber MH. Clinical practice. Chronic insomnia. N Engl J Med. 2005;353:803–10. doi: 10.1056/NEJMcp043762. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health & Human Services. U.S. Food and Drug Administration. FDA Drug Safety Communication: Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist) 2013. [Accessed February 5, 2013]. http://www.fda.gov/Drugs/DrugSafety/ucm334033.htm.
- 26.Dolder CR, Nelson MH. Hypnosedative-induced complex behaviours : incidence, mechanisms and management. CNS Drugs. 2008;22:1021–36. doi: 10.2165/0023210-200822120-00005. [DOI] [PubMed] [Google Scholar]
- 27.Hwang TJ, Ni HC, Chen HC, Lin YT, Liao SC. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71:1331–5. doi: 10.4088/JCP.09m05083bro. [DOI] [PubMed] [Google Scholar]
- 28.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]