Abstract
Background:
Reports regarding the incidence and antibiotic susceptibility of methicillin-resistant Staphylococcus aureus (MRSA) in rhinosinusitis (RS) are limited. This study was designed to identify epidemiology and trends of MRSA incidence and antimicrobial resistance in the sinonasal cavities.
Methods:
This is a retrospective case series. All intranasal/sinus cultures obtained by otolaryngologists at Stanford over a 20-year period (1990–2010) were retrospectively reviewed by mining the microbiology database. Nested searches were then made for all S. aureus and MRSA cultures. Patterns of incidence and changes in antibiotic susceptibilities were tabulated and statistical analysis was performed.
Results:
Our search retrieved 10,387 positive intranasal culture samples, with S. aureus found in 800 (7.7%), and MRSA comprising 110 (1.06%) of this subset. Between the years of 1990 and 1999, only 2/112 (1.7%) of S. aureus–positive nasal cultures were positive for MRSA, with a sharp rise in incidence to 86/606 (14.2%) from 2000 to 2005, and to 22/82, 26.8% from 2006 to 2010. On a percent basis, using logistic regression modeling, this represents a statistically significant increasing trend (p < 0.0001) for MRSA sinusitis. However, over the 20-year interval studied, the patterns of antibiotic resistance among MRSA remained unaltered, especially with regard to trimethoprim-sulfamethoxazole and vancomycin.
Conclusion:
S. aureus and MRSA isolates from intranasal cultures, which were essentially absent before the year 2000, became significantly more common earlier this decade. These data show the increased role of MRSA in sinusitis. MRSA antibiotic susceptibilities have remained, however, largely stable during this time period.
Keywords: Antibiotic resistance, antibiotic susceptibility, intranasal/sinus culture, methicillin-resistant S. aureus, MRSA, Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus (MRSA) is an increasingly appreciated pathogen that is involved in a wide range of community-acquired and nosocomial infections.1 The first report of this resistant organism was in 1961,2 shortly after the introduction of methicillin, and MRSA outbreaks became quite prevalent soon thereafter in the same decade.3 In the United States, MRSA was first isolated at Boston City hospital in 1968,4 and within 20 years was considered endemic in all 50 states.5
To receive a diagnosis of MRSA, strains of S. aureus typically possess a minimum inhibitory concentration to oxacillin of ≥4 μg/mL using traditional testing methods. Isolates resistant to oxacillin or methicillin are usually found to be resistant to many of the antibiotics containing β-lactam rings, such as nafcillin and cefazolin.6,7 Currently, presence of the mecA gene assists in the final identification of S. aureus as MRSA.8
There has been a steady interval increase in the incidence of MRSA in both the community and the hospital settings. The percentage of MRSA among all S. aureus isolates from hospitals rose from 2.4% in 1975 to 29% in 1991, and to 40% in 1996.9,10 The increasing prevalence and involvement of MRSA in nosocomial infections highlights the importance of understanding the role of this microbe in various diseases.
Rhinosinusitis (RS) afflicts millions of patients annually, with varying degrees of severity and differing contributions of intranasal inflammation versus infection that fuels the disease process.11 Numerous studies have shown that sinusitis can significantly impact overall quality of life, in comparison with chronic debilitating diseases such as diabetes and congestive heart failure.12 Signs and symptoms required to diagnose RS include mucopurulent discharge from the middle meatus or ethmoid region, nasal obstruction, facial pressure, and hyposmia/anosmia.13
Common bacterial strains isolated from patients with RS include aerobic and facultatively anaerobic Gram-positive cocci and Gram-negative species and anaerobes.14,15 Cultures obtained through transnasal, endoscopic swabs of the ethmoid cavity or middle meatus have been shown to be effective for identifying involved bacterial flora, which then leads to more accurate culture-directed therapy.16
Nadel et al. (1998) found a prevalence of 27% of Gram-negative rods and a 16% prevalence of Pseudomonas spp. in patients with chronic RS (CRS).17 Similar findings were reported by Bolger.18 In other reports on the microbiology of recurrent rhinosinusitis after endoscopic sinus surgery, Gram-positive cocci predominated (37.9%), followed by Gram-negative rods (14.8%), and in 30% of the cases, no growth was observed.19 These studies also identified a high incidence of organisms producing β-lactamase enzymes and showing other resistance to other antimicrobials, especially among coagulase-negative staphylococci.
There are limited reports regarding the prevalence of MRSA in the setting of CRS within the nasal cavity. Incidence of MRSA causing CRS was 9.22% in 2001–2003 according to Manarey et al.20 Jiang et al reported a 4.75% incidence of intranasal MRSA infection in the setting of functional endoscopic sinus surgery with CRS.21 Huang et al. analyzed the incidence of MRSA infection in acute RS in pediatric and adult cohorts.22 The incidence of MRSA was found to be 2.7%, with the most important predisposing risk factors being previous antibiotic use in children and sinus procedures in adults. This group also suggested that successful treatment of community-acquired MRSA was readily achieved with oral antibiotics.22
Similarly, when MRSA sinusitis has been encountered, previous reports suggest a 92% rate of resolution when treated with culture-directed oral and topical medications. Oral antibiotic choices are guided by susceptibility data, but typically include trimethoprim/sulfamethoxazole or tetracyclines.23
The current study was undertaken to identify trends of MRSA incidence and antimicrobial resistance in nasal cavity cultures in our hospital database taken by otolaryngologists, largely representing patients with acute or CRS at a single tertiary care institution in a major metropolitan area. Furthermore, we assessed antibiotic resistance patterns for MRSA+ cultures, especially with regard to trimethoprim-sulfamethoxazole and vancomycin.
MATERIALS AND METHODS
After obtaining Stanford Institutional Review Board approval (no. 24947), we retrospectively reviewed the microbiological database of all nasal cavity cultures obtained at Stanford University Medical Center from January 1990 to May 2010. We limited our data mining and analysis to positive intranasal cultures (those positive for growth of an identifiable organism) that were submitted by Stanford otolaryngologists either during clinic or intraoperatively. This latter parameter was used as an indirect means of establishing an increased likelihood that positive intranasal cultures were derived from the middle meatus or other intranasal mucosal sites rather than anterior nares or other superficial nasal skin.
Laboratory Processing of Sinus Samples for Microbiological Culture
Aspirate samples were received in a Lukens trap or in an anaerobic transport vial (Anaerobe Systems, Morgan Hill, CA) if anaerobic cultures were ordered. Per standard microbiology laboratory protocols, purulent material was transferred with a swab for inoculation onto trypticase soy agar 5% sheep blood, MacConkey, and Chocolate agar plates (BD Microbiology Systems, Sparks, MD) for aerobic cultures with the addition of three anaerobic agar plates (Brucella blood with vitamin K and hemin, phenylethyl alcohol blood, and a laked kanamycin-vancomycin and Bacteroides bile esculin biplate) and a chopped meat carbohydrate enrichment broth (all from Anaerobe Systems) for anaerobic cultures. Aspirate material was spread thinly on a glass slide with sterile wooden sticks for the Gram stain. If the sample for anaerobic culture appeared to be tissue, it was ground up in Eugon broth (laboratory prepared) before inoculating to the media described and preparing the slide. Media were incubated in the appropriate atmosphere (5% CO2, anaerobic boxes and chamber, or ambient air) at 35°C according to the laboratory's standard protocols. Only if no growth occurred on anaerobic plates was the chopped meat broth subcultured to media based on organisms seen in a Gram stain from the cloudy broth. Swabs were processed for aerobic culture only by being rolled directly onto the three aerobic agar plates and incubated as described previously.
Identification and Determination of Methicillin-Resistance of Staphylococci
Colonies suggestive of staphylococci on any medium (entire, convex, opaque, color white to yellow, and usually β-hemolytic on blood agar) were examined by Gram stain and 3% H2O2 (catalase reagent). Gram-positive cocci in clusters with a positive catalase reaction were further tested using slide or tube coagulase (rabbit plasma with EDTA). If coagulase positive, they were identified as Staphylococcus aureus. Antimicrobial susceptibility tests were performed using either the MicroScan WalkAway (Siemens, Sacramento, CA), or by disk diffusion. Methicillin resistance was confirmed by cefoxitin disk during part of the study period before November 2008 and later by detection of the mecA gene using a laboratory-derived polymerase chain reaction test.8 Within the past 20 years the introduction of polymerase chain reaction in 2008 has only improved the turnaround time of drug susceptibility testing but it has not changed the sensitivity and specificity of MRSA detection.
Statistical Analysis
For each year, the number of S. aureus samples and the number of MRSA samples was extracted. Yearly proportions were calculated and exact (Clopper-Pearson) confidence intervals were calculated. A secular trend in the proportion of MRSA was evaluated using logistic regression, with year entered linearly (on the logit scale). The possibility of a local maximum was tested using a quadratic term for year. Logistic regression was used to determine the presence of a time trend. A quadratic was also used to allow for a possible decrease from the peak, which was only marginally significant (p < 0.03) using the likelihood ratio test.
RESULTS
Reviewing of the microbiology database using these parameters ultimately identified a total of 10,387 positive intranasal cultures performed at Stanford University; 6278 of the cultures were taken from women and 4109 from men. Average age of patients was 51 years.
Eight hundred (7.7%) positive intranasal cultures grew S. aureus, with MRSA comprising only 110 (1.06%) of this cohort. When compared with more commonly associated respiratory pathogens such as pneumococcus, Haemophhilus influenzae, and Pseudomonas spp., S. aureus and MRSA comprised a distinct minority of cultures, as expected (see Table 1).
Table 1.
Breakdown of all positive nasal cultures from year 1990 to 2010 according to pathogen

MRSA = methicillin-resistant S. aureus; MSSA = methicillin-sensitive S. aureus.
For trend analysis as a function of time, we then clustered our search results into three groups of years: 1990–1999, 2000–2005, and 2006–2010. When grouped into these arbitrary subsets, trends emerged. The earliest positive MRSA intranasal culture at our tertiary care institution occurred in 1998, despite the endemic nature of this pathogen in the United States at earlier time points (see beginning of article). In fact, only two positive MRSA cultures were isolated before the year 2000 (Table 1). The absolute number of MRSA+ cultures peaked between the years 2000 and 2005 and declined in the last cluster of years. This initially suggested a promising decline in MRSA incidence (Fig. 1 A).
Figure 1.
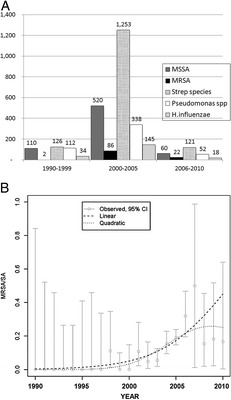
Absolute incidence and logistic regression of methicillin-resistant Staphylococcus aureus (MRSA) incidence over 20 years. (A) Frequency of positive nasal cultures of methicillin-sensitive S. aureus (MSSA), MRSA, Streptococcus spp., Pseudomonas spp., and Haemophilus influenza (y-axis, denoted the number of cases) (B) Trends in positive, intranasal MRSA culture on a percent basis. Error bars denote 95% confidence interval for each year's proportion, the dashed line marks the calculated trend in incidence using logistic regression analysis, and the dotted line marks the quadratic.
However, when these same data were analyzed on a percent basis, and were subjected to logistic regression analysis, there was a statistically significant trend toward increasing incidence of MRSA (p < 0.0001) over time (Fig. 1 B), indicating that the proportion of MRSA in patients having RS is definitively increasing with time.
We next analyzed antimicrobial resistance patterns that have emerged over this 20-year interval to MRSA. We found that MRSA was variably but consistently resistant to clindamycin, erythromycin, and moxifloxacin over each time interval assessed. Conversely, we saw little evidence for emerging resistance of MRSA to trimethoprim-sulfamethoxazole or vancomycin, two of the current mainstay agents for MRSA treatment (Table 2). Of note, susceptibility to trimethoprim-sulfamethoxazole since the year 2000 was preserved in 86–92% of specimens, while 100% susceptibility to vancomycin was maintained throughout the 20-year interval under study.
Table 2.
Antibiotic sensitivity trends of MRSA over time

( ) = Denotes percent of cultures that are sensitive to a given antibiotic; ND = not determined; Abx = antibiotic; MRSA = methicillin-resistant S. aureus.
DISCUSSION
We have been interested in determining the factors that lead to microbial growth, antibiotic resistance, and host defense in various regional subsites. In this report, we began a comprehensive evaluation of MRSA presence, and trends of antibiotic resistance, within the nasal cavity and paranasal sinuses, given the ubiquitous nature of this organism, and concerns over the future advent of “vancomycin-resistant” S. aureus strains.
Our findings from our mining of the microbiology database at Stanford University over a 20-year period indicate that (1) on a percent basis over time, the incidence of sinus-associated MRSA is rising; (2) there is no evidence of new antibiotic resistance patterns for intranasal MRSA in patients served in tertiary care institutions available in the literature. Isolated MRSA strains had preserved susceptibility to both trimethoprim-sulfamethoxazole and vancomycin, despite detection of this organism at our institution since at least 1998.
One of the limitations to our study is lack of data available to us regarding the intranasal subsites of the cultures, the clinical diagnoses of the patients, and the setting in which these cultures were taken. Nonetheless, we have reason to believe that the majority of our data reflect specimens obtained from patients presenting with RS, given that we studied only those intranasal culture specimens submitted by known otolaryngologists at our institution. In this setting, our experience is that cultures are most commonly taken endoscopically from the middle meatus/sinus cavities in cases of RS either in the outpatient setting or during sinus surgeries and thus most probably represent community-acquired infections. It has previously been shown that reliable, endoscopic middle meatal cultures provide a valid and meaningful culture result representing sinus cavity bacterial pathogens.24,25 More so, to further strengthen our assessment, the percent of MRSA in routine nares surveillance cultures at Stanford is 4.88% (NB and EJB, unpublished data, 2012), which is in contrast to the much lower 1% incidence in our subgroup analysis. This data illustrate that our cultures are not mere MRSA contamination from the anterior nares, and that there is a low likelihood that we are incorrectly ascribing a causal relationship of these cultures to RS cultures. We thus believe that our findings show a steadily increasing trend toward MRSA RS. As mentioned previously, data whether these cultures were obtained from patients with chronic versus acute RS were not available to us. Nonetheless, based on our university's typical tertiary practice pattern and referral base, the majority of these cultures were likely to be obtained in the patients with CRS.
The root cause for the rising prevalence of this endemic pathogen is perhaps worth exploring. There is mounting evidence that MRSA colonization is associated with antecedent antibiotic use and smoking.26 A recent meta-analysis suggests a direct correlation between antibiotic exposure and subsequent development of MRSA infection.27 This team estimated MRSA colonization within ∼120 days of prior antibiotic use, with the relative risk of acquiring MRSA elevated 1.8-fold in those patients who had taken antecedent antibiotics. Similarly, other groups report a strong association with use of fluoroquinolones and macrolides and subsequent detection of intranasal MRSA. Whether this is creating a “conducive environment” for MRSA growth, or permitting “primary selection” for resistant organisms is unclear.23,28
In addition, a survey of treatment patterns by United States otolaryngologists for CRS reported use of oral antibiotics (94%) in part of their maximal medical management, with a mean duration of treatment of 5 weeks.29
Finally, our results suggest that vancomycin and trimethoprim-sulfamethoxazole have remained reliable effective agents for treatment of the majority of sinonasal MRSA infections. Although mupirocin irrigation for effective treatment of MRSA sinusitis has been reported by others,30 susceptibility testing to this topical agent is not currently available at our institution.
CONCLUSIONS
Longitudinal prospective studies and regular sampling for MRSA in patients with acute and chronic sinusitis are both recommended based on these data. The results of our study, which show the increased role of MRSA in sinusitis, underline the importance of performing routine endoscopic cultures, especially in patients who fail to respond to empiric antimicrobial therapy. Nonetheless, our results suggest that vancomycin and trimethoprim-sulfamethoxazole have remained reliable effective agents for treatment of the majority of sinonasal MRSA infections. Future studies include prospective stratification of MRSA sinusitis into acute community-acquired MRSA sinusitis versus chronic community-acquired MRSA sinusitis versus nosocomial MRSA sinusitis, which is difficult to accurately determine with our current database search limitations. In addition, assessing partial versus complete clinical responses to treatment with appropriate antimicrobial agents would be invaluable adjunct information to the susceptibilities as we have reported here.
ACKNOWLEDGMENTS
The authors thank Alex McMillan, Ph.D., for his contributions to the statistical elements and analysis of this work.
Footnotes
Presented at the 56th annual meeting of the American Rhinologic Society, Boston, Massachusetts, September 25, 2010
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598, 1998. [DOI] [PubMed] [Google Scholar]
- 2. Barber M. Methicillin-resistant staphylococci. J Clin Pathol 14:385–393, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benner EJ, Kayser FH. Growing clinical significance of methcillin-resistant Staphylococcus aureus. Lancet 2:741–744, 1968. [DOI] [PubMed] [Google Scholar]
- 4. Barrett FF, McGehee RF, Jr, Finland M. Methicillin-resistant Staphylococcus aureus at Boston City Hospital. Bacteriologic and epidemiologic observations. N Engl J Med 279:441–448, 1968. [DOI] [PubMed] [Google Scholar]
- 5. Boyce JM. Increasing prevalence of methicillin-resistant Staphylococcus aureus in the United States. Infect Control Hosp Epidemiol 11:639–642, 1990. [DOI] [PubMed] [Google Scholar]
- 6. Lowy FD. Antimicrobial resistance: The example of Staphylococcus aureus. J Clin Invest 111:1265–1273, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karchmer AW. Staphylococcus aureus and vancomycin: The sequel. Ann Intern Med 115:739–741, 1991. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen DT, Yeh E, Perry S, et al. Real-time PCR testing for mecA reduces vancomycin usage and length of hospitalization for patients infected with methicillin-sensitive staphylococci. J Clin Microbiol 48:785–790, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panlilio AL, Culver DH, Gaynes RP, et al. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975–1991. Infect Control Hosp Epidemiol 13:582–586, 1992. [DOI] [PubMed] [Google Scholar]
- 10. Fluckiger U, Widmer AF. Epidemiology of methicillin-resistant Staphylococcus aureus. Chemotherapy 45:121–134, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Slavin RG. Sinusitis—Present state of the art. Allergy Proc 12:163–165, 1991. [PubMed] [Google Scholar]
- 12. Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 113:104–109, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Thomas M, Yawn BP, Price D, et al. EPOS Primary care guidelines: European Position paper on the primary care diagnosis and management of rhinosinusitis and nasal polyps 2007—A summary. Prim Care Respir J 17:79–89, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Slack CL, Dahn KA, Abzug MJ, Chan KH. Antibiotic-resistant bacteria in pediatric chronic sinusitis. Pediatr Infect Dis J 20:247–250, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Hartog B, Degener JE, Van Benthem PP, Hordijk GJ. Microbiology of chronic maxillary sinusitis in adults: Isolated aerobic and anaerobic bacteria and their susceptibility to twenty antibiotics. Acta Otolaryngol 115:672–677, 1995. [DOI] [PubMed] [Google Scholar]
- 16. Tantilipikorn P, Fritz M, Tanabodee J, et al. A comparison of endoscopic culture techniques for chronic rhinosinusitis. Am J Rhinol 16:255–260, 2002. [PubMed] [Google Scholar]
- 17. Nadel DM, Lanza DC, Kennedy DW. Endoscopically guided cultures in chronic sinusitis. Am J Rhinol 12:233–241, 1998. [DOI] [PubMed] [Google Scholar]
- 18. Vogan JC, Bolger WE, Keyes AS. Endoscopically guided sinonasal cultures: A direct comparison with maxillary sinus aspirate cultures. Otolaryngol Head Neck Surg 122:370–373, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Bhattacharyya N, Kepnes LJ. The microbiology of recurrent rhinosinusitis after endoscopic sinus surgery. Arch Otolaryngol Head Neck Surg 125:1117–1120, 1999. [DOI] [PubMed] [Google Scholar]
- 20. Manarey CR, Anand VK, Huang C. Incidence of methicillin-resistant Staphylococcus aureus causing chronic rhinosinusitis. Laryngoscope 114:939–941, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Jiang RS, Jang JW, Hsu CY. Post-functional endoscopic sinus surgery methicillin-resistant Staphylococcus aureus sinusitis. Am J Rhinol 13:273–277, 1999. [DOI] [PubMed] [Google Scholar]
- 22. Huang WH, Hung PK. Methicillin-resistant Staphylococcus aureus infections in acute rhinosinusitis. Laryngoscope 116:288–291, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Gerencer RZ. Successful outpatient treatment of sinusitis exacerbations caused by community-acquired methicillin-resistant Staphylococcus aureus. Otolaryngol Head Neck Surg 132:828–833, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Dubin MG, Ebert CS, Coffey CS, et al. Concordance of middle meatal swab and maxillary sinus aspirate in acute and chronic sinusitis: A meta-analysis. Am J Rhinol 19:462–470, 2005. [PubMed] [Google Scholar]
- 25. Benninger MS, Payne SC, Ferguson BJ, et al. Endoscopically directed middle meatal cultures versus maxillary sinus taps in acute bacterial maxillary rhinosinusitis: A meta-analysis. Otolaryngol Head Neck Surg 134:3–9, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Brook I, Hausfeld JN. Microbiology of acute and chronic maxillary sinusitis in smokers and nonsmokers. Ann Otol Rhinol Laryngol 120:707–712, 2011. [DOI] [PubMed] [Google Scholar]
- 27. Tacconelli E, De Angelis G, Cataldo MA, et al. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother 61:26–38, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Brook I, Foote PA, Hausfeld JN. Increase in the frequency of recovery of methicillin-resistant Staphylococcus aureus in acute and chronic maxillary sinusitis. J Med Microbiol 57:1015–1017, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Kaszuba SM, Stewart MG. Medical management and diagnosis of chronic rhinosinusitis: A survey of treatment patterns by United States otolaryngologists. Am J Rhinol 20:186–190, 2006. [PubMed] [Google Scholar]
- 30. Solares CA, Batra PS, Hall GS, et al. Treatment of chronic rhinosinusitis exacerbations due to methicillin-resistant Staphylococcus aureus with mupirocin irrigations. Am J Otolaryngol 27:161–165, 2006. [DOI] [PubMed] [Google Scholar]


