Abstract
Background:
Human exposure to fungal elements is inevitable, with normal respiration routinely depositing fungal hyphae within the nose and paranasal sinuses. Fungal species can cause sinonasal disease, with clinical outcomes ranging from mild symptoms to intracranial invasion and death. There has been much debate regarding the precise role fungal species play in sinonasal disease and optimal treatment strategies.
Methods:
A literature review of fungal diseases of the nose and sinuses was conducted.
Results:
Presentation, diagnosis, and current management strategies of each recognized form of fungal rhinosinusitis was reviewed.
Conclusion:
Each form of fungal rhinosinusitis has a characteristic presentation and clinical course, with the immune status of the host playing a critical pathophysiological role. Accurate diagnosis and targeted treatment strategies are necessary to achieve optimal outcomes.
Keywords: Acute, chronic, fungal, invasive, review, rhinosinusitis, sinusitis
An estimated 1.5 million fungal species inhabit Earth, with the vast majority poorly described or undiscovered.1 Because fungi are present throughout the environment, human exposure is inevitable and normal respiration will routinely deposit fungal elements within the nose and paranasal sinuses.2 In most instances, the presence of fungal elements in the nose is of no consequence and will remain unknown to the individual unless elaborate culture techniques are used. In select instances, fungal species can cause sinonasal disease, with clinical outcomes ranging from mild symptoms to intracranial invasion and death. Fungal rhinosinusitis has been categorized primarily based on whether the fungus invades local tissues or not, a characteristic intimately associated with the status of the host's immune system.3 Noninvasive fungal rhinosinusitis includes fungal colonization, fungal ball, and allergic fungal rhinosinusitis (AFRS). Spread of fungus into local tissues characterizes acute invasive, chronic invasive, and chronic granulomatous forms of fungal rhinosinusitis. This article will cover each recognized form of fungal rhinosinusitis, including presentation, diagnosis, and current management strategies.
FUNGAL UBIQUITY ON SINONASAL MUCOSA
Considering how common fungal species are in the environment, it should not be surprising to also find them in the human upper respiratory tract. However, early culture techniques and staining methods were relatively insensitive and failed to identify fungal species in most cases. It was not until the development of enhanced culture techniques and speciation via polymerase chain reaction that the true prevalence of sinonasal fungal elements was appreciated.4,5 Ponikau et al. in 1999 examined 210 consecutive patients with chronic rhinosinusitis (CRS) and were able to culture fungal species in 96%.6 Kim et al. identified fungal elements in 76/82 (92.5%) of CRS patients compared with only 23.2% using standard cultures.7 Fungal elements are not unique to patients with CRS but are also seen in most healthy controls. The Ponikau6 and Kim7 studies cultured fungi in 100 and 97.5% of normal controls, respectively. The ubiquitous presence of fungi in the sinonasal tract suggests that fungal-related sinonasal disease has less to do with the presence or absence of fungi. Instead, the presence of fungal rhinosinusitis and its various forms relates more to the status of the host's immune system and subsequent host–microbe interaction.
IMMUNE STATUS AND FUNGAL RHINOSINUSITIS
Assessing the viability of the host's immune system is central to correctly differentiating and managing fungal rhinosinusitis (Table 1). Immune dysfunction, whether overt or subtle, is the key factor predisposing to fungal invasion of sinonasal tissues and must be considered in all patients with CRS. Presumably, fungi are unable to penetrate the epithelial layer when the immune system is functioning normally.8,9 Suppression of the immune system, such as from diabetes mellitus, chemotherapy, or corticosteroids, creates a condition in which fungi are able to penetrate normal mucosal barriers and invade host tissues. On the other end of the spectrum, AFRS likely represents a hypersensitive response of a competent host immune system to fungal elements.10 In AFRS, chronic mucosal inflammation may be mediated in part through IgE-mediated (type 1) reactions to fungal species trapped in sinonasal mucous. An appreciation of the host's immune status, together with other key clinical characteristics, thus directs the proper diagnosis and classification of fungal sinonasal disorders.
Table 1.
Summary clinical characteristics of fungal rhinosinusitis

*Most common status of immune system.
FRS = fungal rhinosinusitis; ESS = endoscopic sinus surgery; SLIT = sublingual immunotherapy; SCIT = subcutaneous immunotherapy.
NONINVASIVE FUNGAL RHINOSINUSITIS
Localized Fungal Colonization
From time to time nasal crusts can become colonized by macroscopic collections of fungi.11 This saprophytic fungal colonization is most commonly seen in patients with an intact immune system who have had prior sinus surgery. The crust provides a suitable environment for fungal replication and nearby mucosa is usually unaffected. Theoretically, collections could grow over time such that they resemble a fungal ball and could begin to impact surrounding mucosa. However, in most instances the crusts are readily visualized on endoscopic exam and their removal provides full resolution.
Fungal Ball
A dense accumulation of fungal elements within a single sinus is known as a fungal ball. This nomenclature was favored by the International Society for Human and Animal Mycology Working Group over fungal mycetoma or aspergilloma.12 Nicolai et al. reported the maxillary sinus the most commonly involved (84%), followed by the sphenoid sinus (14%) and, rarely, the ethmoid or frontal sinus.13 For unknown reasons, fungal balls are more common in middle-age or older women, usually with a normally functioning immune system. The precise mechanism by which fungal balls form is not known. Many reports suggest that overfilling of maxillary cavities with zinc oxide could provide a nidus for development.14,15 However, some patients with fungal balls have never had dental procedures and this theory would not explain sphenoid fungal balls.16 Perhaps the simplest explanation is that fungal species deposited within the sinus via normal respiration and are inadequately cleared by mucociliary movement. Replication of organisms leads to growth of the fungal ball, irritation of surrounding mucosa, and ostial blockage.
Presentation.
Patients usually present with nonspecific symptoms typical of CRS, such as nasal congestion and facial pressure. The disease has a distinct radiographic appearance on computed tomography (CT) scan, with hyperattenuated materials filling a single sinus, often with associated calcifications (Fig. 1).17 Mucosa of the sinus is typically hypoattenuating and the surrounding bony walls may be expanded and thin or sclerotic and thickened. The fungal ball itself is typically hypointense on T1-weighted and T2-weighted images because of relative dehydration compared with normal mucosa and will fail to enhance with contrast, two features that differentiate it from a neoplastic process.
Figure 1.
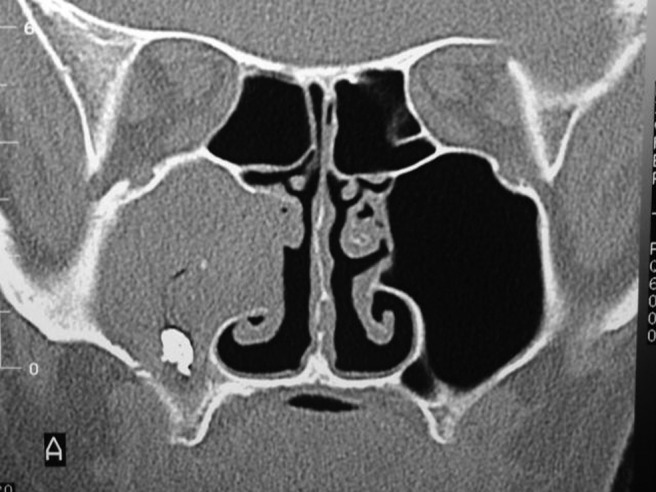
Fungal ball. Computed tomography (CT) scan shows hyperattenuated focus with complete opacification of right maxillary sinus.
Diagnosis.
The diagnosis of a fungal ball is generally confirmed during surgery when chalk-like concretions are found within the sinus. The surrounding sinus mucosa often appears inflamed, and microscopic examination will reveal nonspecific chronic inflammation without evidence of mucosal invasion. Histologically, the concretions are comprised of dense collections of fungal hyphae. Aspergillus fumigatus is the most common fungal species, but in as many as 65% of cases fungal cultures fail to grow and precise speciation is not possible.18
Management.
Surgical opening of the natural sinus ostium with evacuation of fungal debris is the treatment of choice. After removal of fungal hyphae, the sinus mucosa generally returns to a normal state of health and no additional treatment is usually necessary. Very consistent outcomes data are available from retrospective case series (level 4). Pagella et al.,18 Lee et al.,19 and Nicolai et al.13 report surgical cure rates of 95% (77/81), 100% (85/85), and 100% (160/160), respectively. Surgical revision is needed only in instances where the surgically created antrostomy becomes stenosed. Antifungal antibiotics are not indicated in routine cases. Although tissue invasion is atypical of fungal balls, several reports of more aggressive cases exist.20,21 In these rare instances, fungal balls have been associated with local tissue invasion, behaving in a fashion more characteristic of chronic invasive fungal sinusitis. Some controversy exists regarding whether sinus mucosa should be examined for evidence of tissue invasion in cases of obvious fungal ball.12 Data on which to base recommendations are sparse, but status of the host's immune system should factor heavily in this decision.
Allergic Fungal Rhinosinusitis
As a unique clinical entity, AFRS was first described nearly 30 years ago.22 Ten years later, Bent and Kuhn established well-known diagnostic criteria23 and considerable research followed exploring pathophysiological mechanisms and treatment strategies. In many ways, recent research has generated more questions than answers regarding this condition and significant controversy remains. Much of the debate focuses on the precise role fungi play in the disease process, including whether they specifically drive the inflammatory disease process or are simply an associated finding.
Presentation.
Individuals with AFRS experience symptoms typical of CRS, including nasal congestion, facial pain/pressure, nasal discharge, and diminished olfaction. From an epidemiological perspective, AFRS patients are younger, more likely to be male subjects, and more likely to be black American than other patients with CRS and nasal polyps.24,25 An estimated 5–10% of CRS patients requiring surgery have AFRS,10 although a distinct geographic variation exists with cases concentrated in warm, humid climates such as the southeastern United States and India.26 Patients typically have an intact immune system and often have a history of atopy, including allergic rhinitis and/or asthma.
A diagnosis of AFRS is often suspected based on radiographic characteristics.17 Hyperattenuated mucin is often visible within the sinus lumen on CT scan and in many cases there has been progressive expansion and thinning of sinus walls. In select cases, bone can be completely eroded such that sinus mucosa is in direct apposition with underlying dura.27 Multiple sinuses are typically affected in a bilateral fashion, although radiographic findings are often more severe on one side than the other. T1-weighted MRI may show mixed signal intensities, whereas T2-weighted images are frequently hypointense and may also show flow voids from either concentrated metals within fungal hyphae or low free-water content (Fig. 2).
Figure 2.
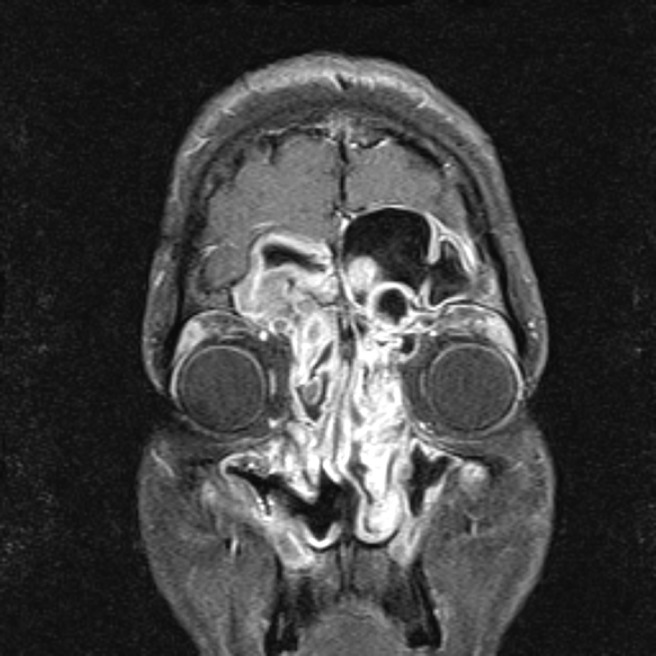
Allergic fungal rhinosinusitis. T1-weighted MRI with contrast shows enhancing mucosa characteristic of polyps in the ethmoid, frontal, and maxillary sinuses.
Diagnosis.
The Bent and Kuhn criteria for AFRS consist of the following: (1) nasal polyposis, (2) fungi on staining, (3) eosinophilic mucin without fungal invasion into sinus tissue, (4) type I hypersensitivity to fungi, and (5) characteristic radiological findings with soft tissue differential densities on CT scanning.23 When using these criteria, several caveats should be considered. Patients who have undergone prior sinus surgery may only show generalized mucosal edema as opposed to frank polyps. The presence of fungi on staining can also be problematic, because fungal elements may be sparse and histopathologists may not routinely use fungal stains. The use of type 1 hypersensitivity, either via skin-prick or radioallergosorbent testing, is a fundamental criterion of the aforementioned classification system. However, studies often report disparities between the fungal species cultured from patients and fungal-specific sensitivities on allergy testing.28
The International Society for Human and Animal Mycology Working Group spent much time considering the pathophysiological basis of AFRS and similar conditions.12 What is clear from prior research is that a subtype of chronic rhinosinusitis with nasal polyps (CRSwNP) patients has eosinophilic mucin evident on histopathology. In those CRSwNP patients with eosinophilic mucin, some will have detectable fungal hyphae within the mucin and some will not (Table 2). Those with fungal hyphae on staining would be classified as AFRS if they also show type 1 hypersensitivity to fungi. Those CRSwNP patients with eosinophilic mucin and detectable fungal hyphae, but absence of type 1 responses, would be classified as eosinophilic fungal rhinosinusitis (EFRS). From a mechanistic standpoint, AFRS and EFRS differ based on whether sinonasal inflammation is associated with an IgE-mediated process or not. From a clinical management standpoint, this distinction only matters insomuch as immunotherapy (subcutaneous or sublingual) could be considered in AFRS whereas it would not be indicated in EFRS.29 Apart from immunotherapy, the clinical management of AFRS and EFRS would be indistinguishable at this point in time.
Table 2.
Clinical features of AFRS and EFRS

AFRS = allergic fungal rhinosinusitis; EFRS = eosinophilic fungal rhinosinusitis; IT = immunotherapy.
Management
Surgical Therapy.
Most clinical series describe surgical therapy to remove polyps, open sinus ostia, and clear eosinophilic fungal mucin, followed by aggressive medical therapies. From the literature it appears that surgery in combination with other medical treatments leads to improved outcomes. A retrospective review reported that incomplete removal of all fungal and eosinophilic mucin contributed to disease recurrence and the need for revision surgery.30 Champagne et al. showed improvements in quality of life and endoscopy scores in all patient groups at 12 months follow-up.31 In this series, all patients were treated postoperatively with saline irrigations, topical nasal steroid spray, oral antibiotics and a 1-month oral steroid taper. Their maintenance treatment consisted of topical nasal steroid spray, nasal saline, montelukast, budesonide irrigations, and month-long bursts of oral steroids for exacerbations. Thus, it is difficult to isolate the impact of surgery alone from the rest of a comprehensive regimen. Overall, recurrence rates after surgery have been reported from 10 to 100%.32
Medical Therapy.
Most reports on treatment options for AFRS are combined into larger series addressing CRSwNP patients and it is therefore difficult to discern if there are varying effects in the AFRS population as opposed to the entire CRSwNP population. In general, medical therapies have been divided into oral and topical steroids, oral and topical antifungals, leukotriene antagonists, and immunotherapy. In all but the mildest cases of AFRS, it is felt that medical therapy alone without surgical intervention is not effective in the long term; thus, most efficacy studies examining medical treatments have been performed postoperatively.
Oral and Topical Anti-Inflammatory Medications.
Oral steroid studies specific to AFRS patients have generally been conducted in the postoperative setting where benefit has been established. In a prospective, randomized double-blinded, placebo-controlled trial in AFRS patients examining the effectiveness of postoperative oral steroids, as well as the side effects of such treatments, patients received oral prednisolone (50 mg daily × 6 weeks, and then additional 6-week taper) or placebo for 2 weeks after surgery.33 All patients received fluticasone nasal spray and oral itraconazole for 12 weeks. At 12-week follow-up, symptoms and endoscopy were improved in the oral steroid group. All 12 patients in the steroid group suffered from weight gain, 5 developed Cushingoid features, 2 developed acne, and 1 developed steroid-induced diabetes mellitus. At 18 months of follow-up, patients who stopped all treatment, including topical steroids, developed recurrent disease. It is unclear if postoperative oral steroids for 12 weeks had an impact at 18 months. A number of other non–placebo-controlled case series have been reported with highly variable dosing protocols and durations but generally reporting a positive effect when using postoperative oral steroids.34–37 It does not appear that prospective studies on the effects of topical steroids alone have been conducted in the AFRS population; however, the majority of studies include topical steroid medications as an integral component of a comprehensive treatment regimen. No controlled studies of leukotriene antagonists have been performed in AFRS patients. Leukotriene antagonists are sometimes included in a comprehensive medical regimen and thus individual efficacy apart from other medications is difficult to discern. One case report of improvement on leukotriene antagonist therapy has been reported.38
Oral or Topical Antifungal Therapy.
Limited non–placebo-controlled case series have reported benefits of oral antifungal therapies in patients with AFRS. Chan et al. reported outcomes of 32 patients with AFRS treated with 100 mg of itraconazole three times daily for 1 month, followed by 100 mg twice daily for 2 months.39 Nasal endoscopy findings improved in 37.5% and symptom scores improved in 56%.
Seiberling and Wormald40 reported a retrospective case series of 23 patients with disease classified as either AFRS or EFRS. Patients refractory to other treatments were dosed with oral itraconazole twice daily for 6 months. After treatment, 69.6% were felt to have a favorable response in clinical symptoms or endoscopy based on chart review, although it is unclear what constituted a favorable response because no objective criteria were described. Elevated liver function studies were noted in 17–19% of patients. Several randomized controlled trials of topical antifungal therapies have failed to show a benefit in CRSwNP patients, although few have specifically evaluated the AFRS population.41–43
Subcutaneous Immunotherapy.
A large, retrospective series reported that compliance with immunotherapy for all fungal and nonfungal antigens was beneficial in preventing recurrence of disease. A 3- to 4-year course of subcutaneous immunotherapy (SCIT) showed benefit 12–26 months after discontinuation44 and prolonged courses of systemic steroids were not used in these patients.45 However, a subsequent study by the same group on a smaller subset of patients with longer-term follow-up ranging from 46 to 138 months failed to indicate any benefit of SCIT, with 60% of SCIT patients having normal mucosa or only mild edema on endoscopy, and 100% of non-SCIT patients had normal mucosa or mild edema.46 This study was not randomized and obviously has the potential for bias in selecting treatment arms. Fortunately, reports of both high-dose and low-dose SCIT have all established safety.47
INVASIVE FUNGAL RHINOSINUSITIS
Acute Invasive Fungal Rhinosinusitis
Defects in the host's immune system may allow fungal species to invade surrounding sinonasal tissues. Patients with poorly controlled diabetes mellitus and those receiving chemotherapy, particularly bone marrow transplant recipients, are most commonly affected. Fungal invasion has also been described in individuals on chronic oral corticosteroids and those with human immunodeficiency virus.
Presentation.
Diagnostic criteria for acute invasive rhinosinusitis requires disease presentation to occur in <4 weeks.12 Symptoms initially are nonspecific and mimic typical cases of CRS, including nasal congestion, drainage, and facial pain/pressure. For this reason, astute diagnosis requires a heightened level of suspicion based on known or potential immunosuppression. As disease progresses, patients may develop fever and epistaxis. Spread of disease from the sinuses to orbit can occur, with proptosis, ophthalmoplegia, and decreased visual acuity. Spread of fungus beyond the maxillary sinus walls can result in palatal and cheek necrosis. Intracranial involvement can occur through direct spread across the skull base or along cranial nerves via skull base foramina. Sinus walls are often necrotic but may also be intact if fungal spread is along vascular channels. Cranial nerve deficits and mental status changes are ominous findings and predict severe disease and a poor prognosis. Noncontrast CT scan will show hypoattenuating mucosal thickening in the affected portion of the nose and sinuses, most often the nasal cavity.48 Radiographic findings may be bilateral but are often worse on a particular side, suggesting a unilateral process (Figs. 3 and 4). With progressive disease, bone erosion may be seen as well as findings external to the sinonasal cavity, such as retroantral soft tissue thickening. MRI is favored for evaluating retroantral, intraorbital, or intracranial spread; but acquisition of images can be time-consuming and could delay definitive diagnosis.
Figure 3.
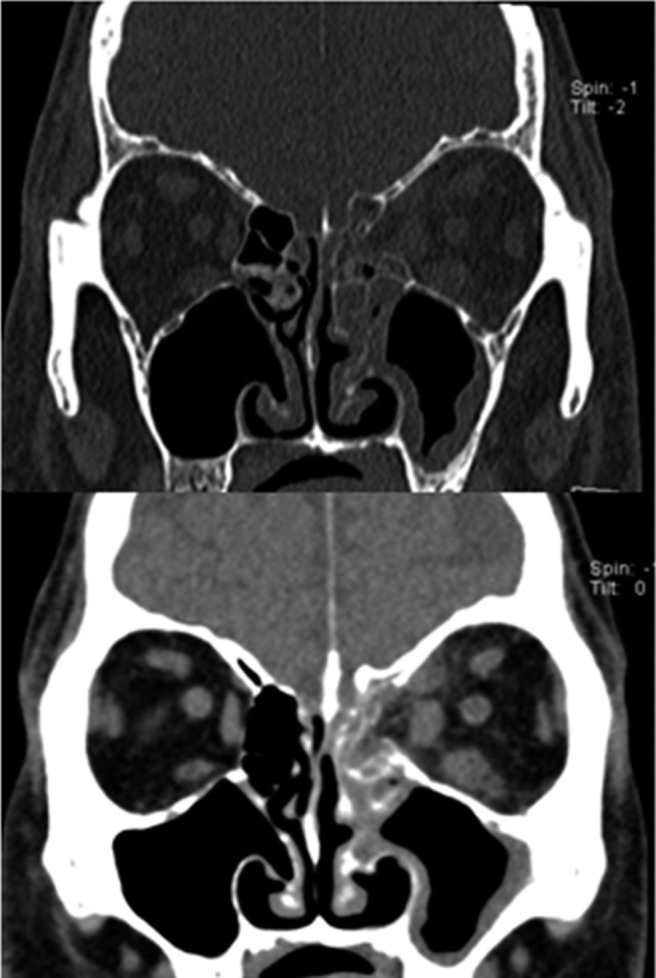
Acute invasive fungal rhinosinusitis. Computed tomography (CT) scan with bone (top) and soft tissue (bottom) windowing showing destruction of left lamina papyracea with inflammation of orbital tissues.
Figure 4.
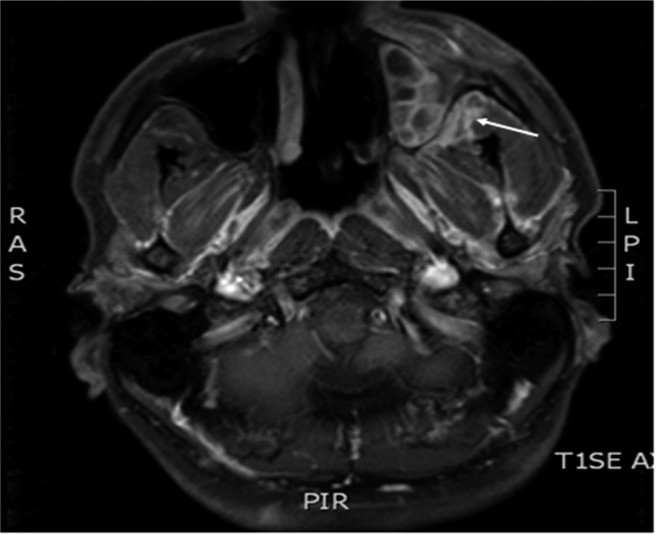
Acute invasive fungal rhinosinusitis. T1-weighted MRI scan with contrast showing enhancement of left retroantral soft tissues.
Diagnosis.
Patients with a clinical presentation suggestive of invasive fungal disease require rapid clinical assessment, including sinonasal endoscopy. Endoscopic findings are variable but classically include pale and/or necrotic nasal mucosa. Reports have variably described hypoesthesia and hyperesthesia on nasal examination but these distinctions are relative and rarely helpful. Gillespie et al. found the most likely site of disease to be the anterior aspect of the middle turbinate (67%) and suggested directing initial biopsies in this location, in addition to any other abnormal area.49 The diagnosis is confirmed on histopathology if fungal elements are visualized invading mucosa, blood vessels, soft tissue, or bone.3 Rapid bedside or intraoperative pathological evaluation is critical and special fungal stains such as the Calcofluor White are used to visualize fungal hyphae.50
Medical comorbidities predispose to certain fungal species, but correlations are not consistent enough to guide empiric therapy. Patients with diabetes mellitus, particularly those in ketoacidosis, are more likely to have species from the order Zygomycetes, including Rhizopus, Rhizomucor, Mucor, and Absidia.51 Patients with hematologic malignancy are more likely to have Aspergillus flavus but may also grow A. fumigatus, Mucor, Fusarium, and Penicillum.52 The appearance of fungal hyphae on histopathology can give some clue as to speciation; however, most clinicians defer to results of fungal cultures before narrowing antifungal coverage. Serum Aspergillus galactomannan antigen assay has also been used to identify invasive fungal disease from Aspergillus species. Chen et al. found elevated Aspergillus antigen in 7/11 cases with invasive Aspergillus and 0/3 cases with Mucor; however, the exact role this assay plays in disease detection and antifungal choice is unclear.52
Management
Surgical Therapy.
Treatment of invasive fungal sinusitis includes surgical resection of necrotic tissues, systemic antifungal antibiotics, and reversal of immune dysfunction. The goal of surgical therapy is to remove necrotic, nonviable tissue.53 The extent of surgery is guided by the macroscopic appearance of tissues, usually debriding until healthy-appearing, bleeding tissue is reached. Theoretically, this reduces a significant portion of the fungal burden, the remainder of which can be reached by systematic antifungal medications. Intra-operative histopathologic analysis can also guide the extent of surgery when tissues are of questionable viability or are vital for form and function.54 The efficacy of surgery is based on numerous case series, usually from single institutions. Most case series are retrospective and involve comprehensive management strategies making it difficult to evaluate the impact of surgery distinct from other strategies, such as medical treatment alone.49,55,56 Despite these shortcomings, the necessity of surgical debridement is well accepted. The extent of surgery is more controversial, particularly with respect to disfiguring procedures such as maxillectomy and orbital exenteration.57 In instances where disease involves only the medial orbital wall, authors have reported acceptable outcomes with resection of lamina papyracea and periorbita, leaving vital orbital contents intact.57 Intracranial extension of frank disease is rarely treated with direct surgical resection, because outcomes are notoriously poor.58,59 Evolution of disease can be followed with clinical and endoscopic bedside examination, with some authors advocating second-look procedures in the operating room. Based on their case series, Otto and Delgaudio recommend following patients at least until remucosalization of the sinuses and resolution of crusting.60
Medical Therapy.
Intravenous antifungal antibiotics are the mainstay of treatment, with liposomal amphotericin B the empiric drug of choice.61 Amphotericin has activity against Zygomycetes, as well as many Aspergillus isolates. If Aspergillus is proven on histopathology or culture, than azoles such as voriconazole are optimal choices.62 The newer azole posaconazole is an appealing secondary choice because it covers both Zygomycetes and Aspergillus and can be delivered orally, making it an excellent choice for prolonged, outpatient regimens.63 Although caspofungin has no activity against Zygomycetes in isolation, there is evidence that combination therapy with amphotericin B may be synergistic.64 Some authors have also used topical amphotericin B irrigations into the postoperative sinonasal cavity, although optimal dosing is unknown and efficacy is difficult to determine distinct from other concurrent therapies. Some case series include hyperbaric oxygen as part of comprehensive regimens; however, this treatment is unproven, has limited availability, and may not be a realistic option for an acutely ill patient.65
Treatments aimed at normalizing the immune system are critical to the success of comprehensive regimens. In diabetic patients, this includes reversal of diabetic ketoacidosis via fluids and aggressive insulin regimens. In those with neutropenia secondary to chemotherapy and/or bone marrow transplantation, granulocyte-macrophage colony-stimulating factor has been used to augment the immune system, although case series are small and efficacy is difficult to determine.66 Chronic corticosteroids should also be discontinued immediately.
Outcomes.
Outcomes data regarding acute invasive fungal rhinosinusitis comes mostly from case reports and retrospective, single-institution case series.49,55,56 These data can be difficult to interpret because of different treatment regimens, variable contributing medical comorbidities, and varied fungal species. In 2004, Parikh et al. reported an 18% overall mortality in 45 cases of invasive fungal disease, with mortality apparently higher in diabetic patients (40%) and those with Mucor (28%), when compared with patients with hematologic malignancy (11%) or those with Aspergillus (11%).67 More recently, Chen et al. reported outcomes of 46 patients with invasive fungal disease, each with underlying hematologic malignancy.52 Overall, 19/46 (41%) died within 6 weeks, and those with acute myeloid leukemia or refractory leukemia had the greatest odds of death. In this series, surgical therapy was positively associated with survival, although there is certainly the potential for selection bias. Although outcomes vary across case series, failure to reverse immunosuppression and the presence of intracranial spread are consistently associated with increased mortality.68
Chronic Invasive Fungal Rhinosinusitis
Invasive fungal infections with a time course of >12 weeks are termed chronic invasive fungal rhinosinusitis.12 Chronic invasive fungal disease often occurs in the context of subtle immunosuppression, as seen with diabetes, corticosteroid use, and human immunodeficiency virus. However, cases have been reported in individuals without obvious immune defects.69 Patients typically have been diagnosed with CRS and symptoms can initially be nonspecific. Fungal invasion into surrounding tissues occurs slowly over time and specific symptoms will reflect the region of invasion. Destruction of surrounding bone can sometimes be seen on imaging, including the zygoma, lamina papyracea, or lateral sphenoid sinus (Fig. 5). At times a hyperattenuating soft tissue mass can be seen filling a sinus, potentially mimicking a neoplasm, but at other times findings are nonspecific and include only mucosal thickening.17
Figure 5.
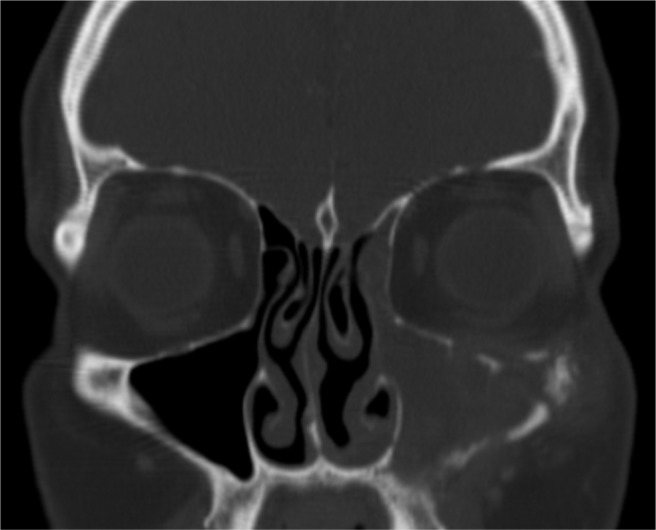
Chronic invasive fungal rhinosinusitis. Computed tomography (CT) scan shows maxillary sinus opacification, erosion of orbital floor, and erosion of zygoma.
The diagnosis of chronic invasive fungal rhinosinusitis is confirmed at surgery when histopathology shows fungal hyphae infiltrating mucosa, blood vessels, or bone. Surrounding tissues often exhibit necrosis and a nonspecific inflammatory infiltrate, which differs from the noncaseating granulomas that characterize chronic granulomatous disease.70 Fungal-specific cultures can grow a variety of species including dematiaceous molds (Bipolaris, Curvularia, and Alternaria), Aspergillus species, and Zygomycetes.68
Treatment of chronic invasive fungal disease includes surgical removal of necrotic tissues and systemic antifungal antibiotics. As in acute invasive disease, amphotericin B is often the initial antifungal choice, with cultures guiding long-term management. Tight glycemic control in diabetic patients and discontinuation of corticosteroids is also critical. Outcomes data are limited to case reports and small case series, but overall morbidity and mortality appears to be better than acute invasive disease.
Granulomatous Invasive Fungal Rhinosinusitis
Granulomatous invasive fungal rhinosinusitis is characterized by a time course of >12 weeks and characteristic histopathological findings.12 This disease entity is most commonly found in countries such as India, Pakistan, and the Sudan but has been reported in the United States.71 Patients often present with proptosis secondary to an enlarging sinonasal mass. Imaging findings can be variable and indistinct from those seen in chronic invasive fungal sinusitis. Diagnosis is confirmed on histopathology by the presence of noncaseating granulomas.70 Fungal hyphae may be found within Langerhans-type giant cells, together with surrounding vasculitis and perivascular fibrosis. This unique pathology differentiates granulomatous invasive fungal disease from the more general chronic invasive fungal rhinosinusitis. A. fumigatus is causative in the majority of cases, allowing one of the azole antifungals to be used empirically while awaiting culture results.68
SUMMARY
Fungal species are ubiquitously present in the environment, including the human sinonasal tract. Fungal rhinosinusitis encompasses a wide spectrum of disease, ranging from simple colonization to acute invasion. Each disease entity has a characteristic presentation and clinical course, with the immune status of the host playing a critical pathophysiological role. Accurate diagnosis and targeted treatment strategies are necessary to achieve optimal outcomes.
Footnotes
Presented at the North American Rhinology and Allergy Conference, Puerto Rico, February 5, 2012
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Hawksworth DL. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol Res 105:1422–1432, 2001. [Google Scholar]
- 2. Green BJ, Sercombe JK, Tovey ER. Fungal fragments and undocumented conidia function as new aeroallergen sources. J Allergy Clin Immunol 115:1043–1048, 2005. [DOI] [PubMed] [Google Scholar]
- 3. deShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med 337:254–259, 1997. [DOI] [PubMed] [Google Scholar]
- 4. Catten MD, Murr AH, Goldstein JA, et al. Detection of fungi in the nasal mucosa using polymerase chain reaction. Laryngoscope 111:399–403, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Polzehl D, Weschta M, Podbielski A, et al. Fungus culture and PCR in nasal lavage samples of patients with chronic rhinosinusitis. J Med Microbiol 54:31–37, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc 74:877–884, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Kim ST, Choi JH, Jeon HG, et al. Comparison between polymerase chain reaction and fungal culture for the detection of fungi in patients with chronic sinusitis and normal controls. Acta Otolaryngol 125:72–75, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun 80:1304–1313, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hontelez S, Sanecka A, Netea MG, et al. Molecular view on PRR cross-talk in antifungal immunity. Cell Microbiol 14:467–474, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Schubert MS. Allergic fungal sinusitis: Pathophysiology, diagnosis, and management. Med Mycol 47:S324–S330, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Ferguson BJ. Definitions of fungal rhinosinusitis. Otolaryngol Clin North Am 33:227–235, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Chakrabarti A, Denning DW, Ferguson BJ, et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope 119:1809–1818, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nicolai P, Lombardi D, Tomenzoli D, et al. Experience in 160 patients treated with endoscopic surgery. Laryngoscope 119:2275–2279, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Park GY, Kim HY, Min J, et al. Endodontic treatment: A significant risk factor for the development of maxillary fungal ball. Clin Exp Otorhinolaryngol 3:136–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mensi M, Piccioni M, Marsili F, et al. Risk of maxillary fungus ball in patients with endodontic treatment on maxillary teeth: A case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:433–436, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Pagella F, Pusateri A, Matti E, et al. Sphenoid sinus fungus ball: Our experience. Am J Rhinol Allergy 25:276–280, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Aribandi M, McCoy VA, Bazan C. Imaging features of invasive and noninvasive fungal sinusitis: A review. Radiographics 27:1283–1296, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Pagella F, Matti E, De Bernardi F, et al. Paranasal sinus fungus ball: Diagnosis and management. Mycoses 50:451–456, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Lee KC. Clincal features of the paranasal sinus fungus ball. J Otolaryngol 36:270–273, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Robey AB, Obrien EK, Richardson BE, et al. The changing face of paranasal sinus fungus balls. Ann Otol Rhinol Laryngol 118:500–505, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Bowman J, Panizza B, Gandhi M. Sphenoid sinus fungal balls. Ann Otol Rhinol Laryngol 116:514–519, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Katzenstein AL, Sale SR, Greenberger PA. Pathologic findings in allergic aspergillus sinusitis. A newly recognized form of sinusitis. Am J Surg Pathol 7:439–443, 1983. [DOI] [PubMed] [Google Scholar]
- 23. Bent JP, Kuhn FA. Diagnosis of allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg 111:580–588, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Wise SK, Ghegan MD, Gorham E, Schlosser RJ. Socioeconomic factors in the diagnosis of allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg 138:38–42, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Thahim K, Jawaid MA, Marfani MS. Presentation and management of allergic fungal sinusitis. J Coll Physians Pak 17:23–27, 2007. [PubMed] [Google Scholar]
- 26. Ferguson BJ, Barnes L, Bernstein JM, et al. Geographic variation in allergic fungal rhinosinusitis. Otolaryngol Clin North Am 33:441–449, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Wise SK, Rogers GA, Ghegan MD, et al. Radiologic staging systems for allergic fungal rhinosinusitis (AFRS). Otolaryngol Head Neck Surg 140:735–740, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Pant H, Schembri MA, Wormald PJ, Macardle PJ. IgE-mediated fungal allergy in allergic fungal sinusitis. Laryngoscope 119:1046–1052, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Mabry RL, Mabry CS. Allergic fungal sinusitis: The role of immunotherapy. Otolaryngol Clin North Am 33:433–440, 2000. [DOI] [PubMed] [Google Scholar]
- 30. Marple BL, Mabry RL. Allergic fungal sinusitis: Learning from our failures. Am J Rhinol 14:223–226, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Champagne JP, Antisdel JL, Woodard TD, Kountakis SE. Epidemiologic factors affect surgical outcomes in allergic fungal sinusitis. Laryngoscope 120:2322–2324, 2010. [DOI] [PubMed] [Google Scholar]
- 32. Marple BF. Allergic fungal rhinosinusitis: Current theories and management strategies. Laryngoscope 111:1006–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 33. Rupa V, Jacob M, Mathews MS, Seshadr MS. A prospective, randomised, placebo-controlled trial of postoperative oral steroid in allergic fungal sinusitis. Eur Arch Otorhinolaryngol 267:233–238, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Kinsella JB, Bradfield JJ, Gourley WK, et al. Allergic fungal sinusitis. Clin Otolaryngol Allied Sci 21:389–392, 1996. [DOI] [PubMed] [Google Scholar]
- 35. Kuhn FA, Javer AR. Allergic fungal rhinosinusitis: Our experience. Arch Otolaryngol Head Neck Surg 124:1179–1180, 1998. [DOI] [PubMed] [Google Scholar]
- 36. Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. II. Treatment and follow-up. J Allergy Clin Immunol 102:395–402, 1998. [DOI] [PubMed] [Google Scholar]
- 37. Kupferberg SB, Bent JP, Kuhn FA. Prognosis for allergic fungal sinusitis. Otolaryngol Head Neck Surg 117:35–41, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Schubert MS. Antileukotriene therapy for allergic fungal sinusitis. J Allergy Clin Immunol 108:466–467, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Chan KO, Genoway KA, Javer AR. Effectiveness of itraconazole in the management of refractory allergic fungal rhinosinusitis. J Otolaryngol Head Heck Surg 37:870–874, 2008. [PubMed] [Google Scholar]
- 40. Sieberling K, Wormald PJ. The role of itraconazole in recalcitrant fungal sinusitis. Am J Rhinol Allergy 23:303–306, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Ebbens FA, Georgalas C, Luiten S, et al. The effect of topical amphotericin B on inflammatory markers in patients with chronic rhinosinusitis: A multicenter randomized controlled study. Laryngoscope 119:401–408, 2009. [DOI] [PubMed] [Google Scholar]
- 42. Gerlinger I, Fittler A, Fónai F, et al. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis, with a review of the antifungal therapy. Eur Arch Otorhinolaryngol 266:847–855, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Liang KL, Su MC, Shiao JY, et al. Amphotericin B irrigation for the treatment of chronic rhinosinusitis without nasal polyps: A randomized, placebo-controlled, double-blind study. Am J Rhinol 22:52–58, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Mabry RL, Marple BF, Folker RJ, Mabry CS. Immunotherapy for allergic fungal sinusitis: Three years' experience. Otolaryngol Head Neck Surg 119:648–651, 1998. [DOI] [PubMed] [Google Scholar]
- 45. Folker RJ, Marple BF, Mabry RL, Mabry CS. Treatment of allergic fungal sinusitis: A comparison trial of postoperative immunotherapy with specific fungal allergens. Laryngoscope 108:1623–1627, 1998. [DOI] [PubMed] [Google Scholar]
- 46. Marple B, Newcomer M, Schwade N, Mabry R. Natural history of allergic fungal rhinosinusitis: A 4- to 10-year follow-up. Otolaryngol Head Neck Surg 127:361–366, 2002. [DOI] [PubMed] [Google Scholar]
- 47. Greenhaw B, deShazo RD, Arnold J, Wright L. Fungal immunotherapy in patients with allergic fungal sinusitis. Ann Allergy Asthma Immunol 107:432–436, 2011. [DOI] [PubMed] [Google Scholar]
- 48. DelGaudio JM, Swain RE, Jr, Kindgdom TT, et al. Computed tomography findings in patients with invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg 129:236–240, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Gillespie MB, O'Malley BW, Jr, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg 124:520–526, 1998. [DOI] [PubMed] [Google Scholar]
- 50. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 54:S55–S60, 2012. [DOI] [PubMed] [Google Scholar]
- 51. Artis WM, Fountain JA, Delcher HK, Jones HE. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: Transferring and iron availability. Diabetes 31:1109–1114, 1982. [DOI] [PubMed] [Google Scholar]
- 52. Chen C-Y, Sheng W-H, Cheng A, et al. Invasive fungal sinusitis in patients with hematological malignancy: 15 Years experience in a single university hospital in Taiwan. BMC Infect Dis 11:250, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Epstein VA, Kern RC. Invasive fungal sinusitis and complications of rhinosinusitis. Otolaryngol Clin North Am 41:497–524, 2008. [DOI] [PubMed] [Google Scholar]
- 54. Taxy JB, El-Zayaty S, Langerman A. Acute fungal sinusitis: Natural history and the role of frozen section. Am J Clin Pathol 132:86–93, 2009. [DOI] [PubMed] [Google Scholar]
- 55. Kasapoglu F, Coskun H, Ozmen OA, et al. Acute invasive fungal rhinosinusitis: Evaluation of 26 patients treated with endonasal or open surgical procedures. Otolaryngol Head Neck Surg 143:614–620, 2010. [DOI] [PubMed] [Google Scholar]
- 56. Suslu AE, Ogretmenoglu O, Suslu N, et al. Acute invasive fungal rhinosinusitis: Our experience with 19 patients. Eur Arch Otorhinolaryngol 266:77–82, 2009. [DOI] [PubMed] [Google Scholar]
- 57. Dhiwakar M, Thakar A, Bahadur S. Invasive sino-orbital aspergillosis: Surgical decisions and dilemmas. J Laryngol Otol 117:280–285, 2003. [DOI] [PubMed] [Google Scholar]
- 58. Jung SH, Kim SW, Park CS, et al. Rhinocerebral mucormycosis: Consideration of prognostic factors and treatment modality. Auris Nasus Larynx 36:274–279, 2009. [DOI] [PubMed] [Google Scholar]
- 59. Peterson KL, Wang M, Canalis RF, Abemayor E. Rhinocerebral mucormycosis: Evolution of the disease and treatment options. Laryngoscope 107:855–862, 1997. [DOI] [PubMed] [Google Scholar]
- 60. Otto KJ, Delgaudio JM. Invasive fungal rhinosinusitis: What is the appropriate follow-up? Am J Rhinol 20:582–585, 2006. [DOI] [PubMed] [Google Scholar]
- 61. Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: A review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs 69:361–392, 2009. [DOI] [PubMed] [Google Scholar]
- 62. Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole verus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415, 2002. [DOI] [PubMed] [Google Scholar]
- 63. Langner S, Staber PB, Neumeister P. Posaconazole in the management of refractory invasive fungal infections. Ther Clin Risk Manag 4:747–758, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spellberg B, Fu Y, Edwards JEJ, Ibrahim AS. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob Agents Chemother 49:830–832, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kauffman CA, Malani AN. Zygomycosis: An emerging fungal infection with new options for management. Curr Infect Dis Rep 9:435–440, 2007. [DOI] [PubMed] [Google Scholar]
- 66. Offner F. Hematopoietic growth factors in cancer patients with invasive fungal infections. Eur J Clin Microbiol Infect Dis 16:56–63, 1997. [DOI] [PubMed] [Google Scholar]
- 67. Parikh SL, Venkatraman G, DelGaudio JM. Invasive fungal sinusitis: A 15-year review from a single institution. Am J Rhinol 18:75–81, 2004. [PubMed] [Google Scholar]
- 68. Thompson GR, Patterson TF. Fungal disease of the nose and paranasal sinuses. J Allergy Clin Immunol 129:321–326, 2012. [DOI] [PubMed] [Google Scholar]
- 69. Alrajhi A, Enani M, Mahasin Z, Al-Omran K. Chronic invasive aspergillosis of the paranasal sinuses in immunocompetent hosts from Saudi Arabia. Am J Trop Med Hyg 65:83–86, 2001. [DOI] [PubMed] [Google Scholar]
- 70. Das A, Bal A, Chakrabarti A, et al. Spectrum of fungal rhinosinusitis; histopathologist's perspective. Histopathology 54:854–859, 2009. [DOI] [PubMed] [Google Scholar]
- 71. Stringer SP, Ryan MW. Chronic invasive fungal rhinosinusitis. Otolaryngol Clin North Am 33:375–387, 2000. [DOI] [PubMed] [Google Scholar]


