Abstract
Musculoskeletal diseases cost the U.S. $849 billion annually. To date, there has been no proof that remote long bone mesenchymal stem cells (BMSC) can home to craniofacial defects for bone regeneration. There has been no report that systemic BMSC injection can increase new bone formation in large animals. The objectives of this study were to use a sex-mismatched canine model for systemic BMSC injection and homing to mandibular defects and to investigate appendicular BMSC migration to craniofacial defects to increase new bone formation. Male beagle dog BMSC were injected into the femoral marrow cavity of female dogs upon which mandibular defects were created. The dogs were sacrificed at 6 weeks. Cells with Y chromosome markers were detected in defects of female dogs with systemic male BMSC injection, indicating the homing of the transplanted BMSC from femoral marrow to the mandibular defect. New bone formation in dogs with systemic BMSC injection was 20–40% higher than control without BMSC injection (p<0.05). Mineralized new bone percentage was increased by 20–40% due to systemic BMSC injection (p<0.05). In conclusion, this study proved that (1) allogeneic BMSC injected into long bone marrow are capable of homing to both appendicular and craniofacial bone in large animals and (2) systemically injected BMSC can significantly increase new bone formation in dog's mandibular defects. These results may help advance the understanding of stem cell homing and present a therapy to enhance bone repair, which may have a wide applicability to the regenerative medicine field.
Introduction
Bone defects are common in a variety of clinical settings, such as congenital malformation, cancer, trauma, and infection.1 Health care costs plus the lost wages for people with musculoskeletal diseases reached approximately $849 billion in 2004 in the United States or 7.7% of the national gross domestic product.1 These numbers are predicted to increase due to an aging population, as the need for bone repair increases with the baby-boomers entering into retirement and having longer life expectancies.2–5 Autografts currently remain the gold standard and are regularly practiced in the treatment of bone defects. However, donor site morbidity and limited available amount are the two main drawbacks that restrict the application of autografts in many patients.6,7 In addition, the bone regenerative capability of autografts may be compromised due to aging8–10 and diseases, such as osteoporosis and arthritis.11,12 Hence, the very patients who are in the most need of bone grafts are likely not able to provide potent autologous stem cells for themselves. Therefore, these patients may need transplantation of allogeneic stem cells for bone tissue engineering treatments.
Recent studies have demonstrated the exciting potential to meet the need for tissue regeneration via tissue engineering approaches.13–19 Stem cells can be guided for osteogenic differentiation and bone regeneration.20–27 Bone marrow-derived mesenchymal stem cells (BMSC) were first isolated in the 1970s and were the progenitors for many mesenchymal tissues, including bone, cartilage, and fat.28–30 BMSC are an important source of osteogenic cells for bone tissue engineering, and many studies have shown evidence that MSC contribute to bone regeneration.24 The mobilization of MSC can be triggered by injury signals, and the MSC can home to the damaged tissues and exert their reparative effects at the site of injury.5 Both laboratory and clinical studies have reported promising results in regenerating bone by local delivery of BMSC.31,32
Besides local delivery of BMSC, several studies have investigated the systemic injection of BMSC, which could subsequently home to the fracture sites in bone and participate in fracture healing and regeneration of bone tissue.33 In animal studies of injured long bones, BMSC were injected into remote bone marrow cavities, and then, the number of BMSC significantly increased at the site of injuries created by osteotomy or irradiation.34 Systemic delivery of BMSC could be a useful therapy for bone healing, for example, in cases of tumor resection and radiotherapy, where the bone defect cannot be immediately treated by local surgery. In addition, for osteoporotic patients with numerous sites of bone defects, the performance of numerous local surgeries may not be feasible, and a systemic BMSC injection therapy may be beneficial. Several studies have shown that systemic injection of BMSC accelerated bone fracture healing35 and that the injected BMSC migrated into the defect site and differentiated into osteoblasts to form new bone tissue.36,37 Previous studies typically used mice and rat models.33,35,37 Using rat bone marrow MSCs, it was shown indirectly that intravenously injected MSCs could contribute significantly to cranial bone formation.38 Another study demonstrated that MSCs could migrate from bone marrow through blood circulation to nonosseous bioceramic implant site and contribute to ectopic bone formation in a canine model.39 However, that study focused on ectopic bone formation and not on bone defects.39 To date, there has been no report on the systemic injection of BMSC and homing to bone defect site in a large animal model, and there has been no evidence that BMSC systemically injected into appendicular bone marrow cavity could home to craniofacial defects to enhance repair and increase new bone formation.
Therefore, the objectives of this study were to (1) use a sex-mismatched beagle dog model to investigate the systemic injection of BMSC and the homing to mandibular defects and (2) investigate whether BMSC injected into appendicular bone of dogs could migrate to craniofacial bone defects and enhance bone regeneration. Y chromosome was used to track cell origin. It was hypothesized that (1) BMSC of male dog injected into the femoral bone marrow cavity of female dog could obtain stable chimerism and home to the female's mandibular defect, where Y chromosomes could be detected in the newly formed bone and (2) there would be significantly more new bone amount in mandibular defects of dogs that received systemic BMSC injection than control dogs without BMSC injection.
Materials and Methods
A sex-mismatched canine BMSC transplantation model was used.40 Briefly, BMSC from a male beagle dog was injected into the remote femoral long bone marrow cavity of a littermate female that had received total body irradiation (TBI) before transplantation. Simultaneously, the surgery was performed in female recipient dog to create a critical-sized mandibular defect.41 Six weeks after the surgery, new bone tissue was harvested at the mandibular defect site. The presence of Y chromosome markers was examined to ascertain the migration of the systemically injected BMSC from the femur to the mandibular defect.
Animal model
Adult male and female mixed-breed littermate beagle dogs (3–4 years old, 8.5–10 kg) were used in the present study. The research protocol and all surgical procedures were approved by the Animal Care and Use Committee of Sichuan University. With the use of highly polymorphic major histocompatibility complex class I and class II microsatellite markers,42 four littermate donor/recipient beagle pairs were matched.
Isolation and culture of canine BMSC
Male dog BMSC were harvested and cultured according to a previous method.43 Briefly, 5 mL of bone marrow aspirate was collected from the iliac crest of the dog under general anesthesia with 3% sodium pentobarbital (1 mL/kg), and gradient centrifugation in Percoll was conducted to separate mononuclear cells, which were later plated in 100-mm dishes at 1×105 cells/cm2. The culture medium consisted of α-minimum essential medium (α-MEM; Gibco), 10% fetal bovine serum (FBS; Gibco), 10−8 M dexamethasone (Sigma), 10 mM β-phosphoglycerol (Sigma), and 50 mM l-2-ascorbic acid (Sigma). The cells were kept at 37°C with 5% CO2, and on the fourth day, the nonadherent cells were removed along with the culture medium. When the cells reached 80–90% confluence, they were detached with 0.25% trypsin/EDTA (Gibco) and re-plated in 100-mm dishes (1×105 cells/cm2). BMSC of the third passage were used in this study.
Sex-mismatched allogeneic BMSC transplantation
Before BMSC transplantation, the recipient female dog received a single dose of 200 cGy TBI.44 While the littermate match reduces immune reaction, the TBI eliminates immune rejection for allogeneic BMSC transplantation. Immediately after irradiation, BMSC of the matching male dog, at a dose of 4×108 cells/kg, were injected into the bone marrow cavity of the matching recipient female dog. Four other female beagle dogs, which did not receive BMSC transplantation but accepted the same irradiation and subsequent mandibular defect treatments, served as control.
Intraosseous injection of BMSC was performed following a method reported earlier.45 The recipients were anesthetized. Two needles were inserted into the bone cavity at both ends of the right femur. A syringe bearing 15 mL of heparin-containing normal saline was connected to one needle, and an empty syringe was connected to the other needle. Normal saline was injected from one syringe to the marrow cavity, while gentle suction was applied on the other syringe to drawn out the bone marrow–saline mixture. This procedure was repeated twice to irrigate the marrow cavity. Twenty milliliters of bone marrow–saline mixture was drawn out to avoid any increase in pressure due to BMSC transplantation. BMSC of the male dog were suspended in 2 mL of phosphate-buffered saline and then injected into the marrow cavity of the matching female dog through one needle, while gentle suction was applied through a syringe attached to the other needle. The needle holes were sealed with bone wax after the surgery.
Mandibular defects
The surgical procedure to create the mandibular defects was performed at the same time of BMSC transplantation in the female dog. After systemic and local anesthesia (2% lidocaine with 1:80,000 epinephrine), the mandible was exposed via an extraoral submandibular approach. A critical-sized rectangular defect of 20 mm in length, 10 mm in width, and 10 mm in depth was made in the buccal cortical plate at the mandibular premolar area on both sides of the dog.41 The periosteum was removed at the same time. The dogs were given standard postgrafting care consisting of cyclosporine (CSP; Zhejiang Ruibang) 15 mg/kg twice daily orally from 1 to 35 days and mycophenolate mofetil (MMF; Hainan Chuntch) 10 mg/kg twice daily subcutaneously from 1 to 27 days.44
Analysis of survival and migration of the transplanted BMSC
To confirm that the male donor BMSC in the bone marrow of female recipient acquired stable chimerism and exerted autospecific function, bone marrow aspirate was collected from the left femur at 7 days after the BMSC infusion (which was injected into the right femur). The cells were harvested and cultured in vitro using the aforementioned methods. DNA was isolated (Universal Genomic DNA Extraction kit Ver.3.0; Takara) from the first generation of the BMSC. Polymerase chain reaction (PCR) was performed using the primers for Y chromosome sex-determining region (Sry)-specific sequences, 5′ - TGG TGT GGT CTC GCG ATC AAA G and 5′ - CTG CGC CTC CTC GAA GAA TGG (Takara Biotechnology Dalian Co, China) and primers for canine Gapdh gene 5′ - GCT CCT TCT GCT GAT GCC CCC A and 5′ - TGG GTG GCA GTG ATG GCA TGG (Takara) as positive control.40 PCR was conducted in a programmed thermal cycles (Esco), including 30 cycles of denaturation for 0.5 min at 94°C, annealing for 1 min at 58°C, and extension for 1 min at 72°C. The PCR products were separated by electrophoresis on a 7.2% polyacrylamide gel and analyzed in parallel with 25 base pair ladder markers on a 2% ethidium bromide-stained agarose gel under UV light.
Fluorescence in situ hybridization (FISH) was conducted to assess the existence of male BMSC. The prepared DNA probes for Y chromosome markers (5′ - GTC TCT ACC GTT TCC TCC GCT TTC ACA, 5′ - GCT GAT CTC TGA GTT TTG CAT TTG GGG A, and 5′ - GGT ATT TCT CTC GGT GCA TGG CCT GTA) were labeled with biotin-16-dUTP. The slices were placed in Tris-buffered saline (pH 8.9) solution, denaturated at 95–100°C for 20 min, and allowed to hybridize overnight at 37°C. The probes were stained with avidin-fluorescein isothiocyanate (FITC), and the nuclei were counterstained with DAPI. The signals were examined with an epifluorescence microscope (Carl Zeiss) coupled to a FISH-2.0 software imaging system.
Histological analysis
Six weeks after the surgery, all female dogs were sacrificed by an overdose of anesthetics. The right part of the mandible was fixed with 4% paraformaldehyde for histological sections, and the left part of the mandible was conserved in liquid nitrogen for detection of male donor BMSC in the newly formed bone tissue.
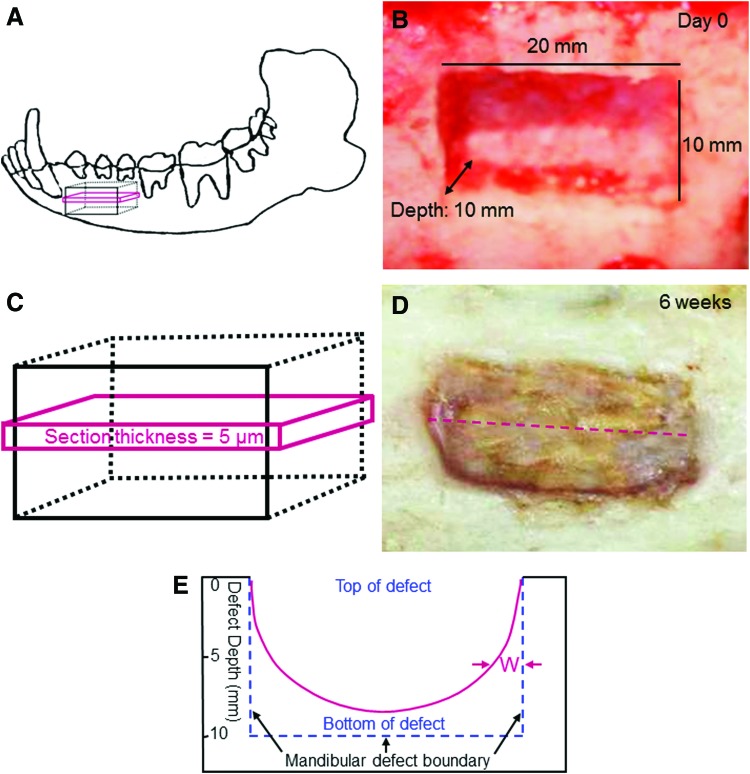
The fixed sample was decalcified and sections with a thickness of 5 μm were prepared for Masson staining. This process was shown in Figure 1. A critical-sized rectangular bone defect was prepared in the mandible (Fig. 1A, B). The rectangular defect had dimensions of approximately 20×10×10 mm. The section was cut along the longest dimension and in the middle of the defect (Fig. 1C, D). The dotted line in (D) indicates the direction of sectioning. Each defect sample was cut into two equal parts from the middle of the defect and then embedded in paraffin. Then, a 5-μm section was obtained from each half. The new bone width “W” was measured as schematically shown in Figure 1E. The width W varied along the depth of the defect and increased when approaching the bottom of the defect. In preliminary study, W was measured from the two sections of the two equal halves of the sample. This was done for several samples at 6 weeks, and there was no significant difference in the value of W between the two halves of each defect. Therefore, in the present study, the section of one of the two halves was randomly chosen from each defect for quantitative analysis. A microscope (DFC 490; Leica) was used to examine the histological images, and the images were captured using the imaging software (Leica). The mineralized new bone area percentage=the area of mineralized new bone (red Mason staining area in the image)/the total area of the image. In addition, the blood vessel density was also measured from these images, with blood vessel density=number of blood vessels in the image/the area of the image.
FIG. 1.
Mandibular defects in female dogs. (A) Schematic representation of bone defect in mandible. (B) Critical-sized mandibular defect in female dog, with length=20 mm, width=10 mm, and depth=10 mm (which removed all cancellous bone in the defect, but the cortical bone at the bottom was left intact). (C) A 5-μm section was cut in the middle of the defect for histological analysis. (D) The section was obtained in the middle of defect and parallel to the longer side of bone defect. The dotted line shows the direction of sectioning. (E) Schematic representation to show the measurement of the new bone width W in the 5-μm section. “W” between the two arrows indicates the width of the new bone region. W was nearly 0 at the top of the defect (when the defect depth was 0 mm on the y-axis) and gradually increased with greater defect depth toward the defect bottom (The original defect depth was 10 mm). Color images available online at www.liebertpub.com/tea
Detection of male donor BMSC in mandibular defects of female dogs
DNA was isolated from 50 mg of the frozen tissue harvested from the mandibular defect of the female dogs and eluted from spin column using 200 μL of Tris-EDTA buffer (1 mM EDTA, 10 Mm Tris-HCl, at pH 8). The PCR experiment followed the aforementioned procedures. The remaining tissue harvested from the mandibular defect of female dogs was prepared as paraffin sections, and the FISH experiment was conducted following the aforementioned procedures. The purpose was to detect the presence of Y chromosomes in the newly formed bone tissue in the mandibular defects of female dogs.
The data were analyzed by a paired t-test with SPSS 10.0 (SPSS). Differences at p<0.05 were statistically significant.
Results
Figure 2 shows the results on the migration of BMSC injected into the right femur of female dogs. PCR results (Fig. 2A) showed donor-derived Y chromosome genes in the bone marrow of the left femur of female recipient at day 7. This was corroborated by the FISH results (Fig. 2B), which detected donor-derived BMSC in the left femur of recipients at day 7, where Y chromosomes in the BMSC were indicated by the arrows. In contrast, no Y chromosomes were detected in control female dogs without BMSC injection (Fig. 2C). These results confirmed the migration of donor BMSC in the recipient 1 week after transplantation by intraosseous injection.
FIG. 2.

Male bone mesenchymal stem cells (BMSC) were injected into the right femur of the female dog, and the donor BMSC migrated into the left femoral marrow at 7 days. (A) Polymerase chain reaction of donor-derived Y chromosome genes in the left femoral marrow of female recipient. Lanes 1 and 2 represent the BMSC drawn from the left femur marrow at 7 days; lanes 3 and 4 represent the original donor BMSC before injection into the right femur. (B) FISH detection of donor-derived BMSC in the left femur of recipients at 7 days. Arrows indicate Y chromosomes probed with fluorescein isothiocyanate (FITC) (small green dots). The nuclei of BMSC were stained blue. (C) No Y chromosomes were detected in the left femoral bone marrow of the control female dogs that did not receive BMSC injection. Color images available online at www.liebertpub.com/tea
Masson staining images of new bone formation were shown in Figure 3 for a BMSC recipient dog. Figure 3A showed a low-magnification histological image of the middle section along the bone defect. There was significantly more new bone near the bottom of the defect than near the top of the defect. In addition, the new bone near the bottom of the defect appeared to be more mature, with a darker red staining, which indicates a relatively higher degree of mineralization (Fig. 3B). The new bone near the top of the defect appeared to be less mature, with a light red/pink staining, which suggests an onset of mineralization (Fig. 3C). This indicates that the new bone near the defect bottom (where the cortical bone was left intact) was formed earlier, and the new bone near defect top was formed later, in the 6-week period. Furthermore, there appeared to be more new bone in the defects of donor BMSC recipient dogs than in the control dogs without systemic BMSC injection.
FIG. 3.
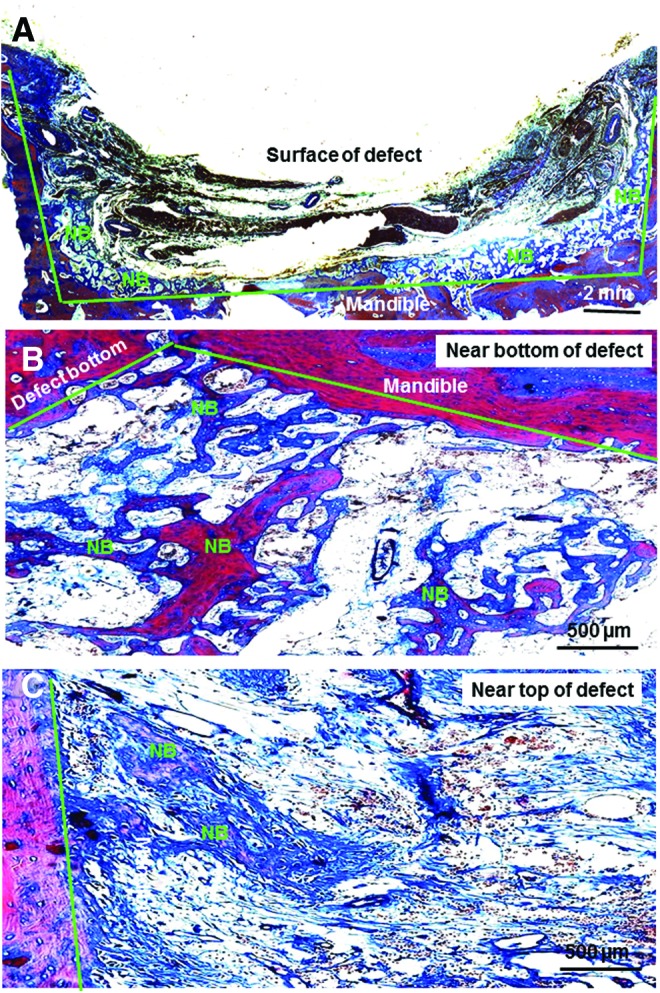
Representative Masson staining images of 5-μm sections for female dogs that had received male BMSC injection. (A) Overview of the middle section of the bone defect at a low magnification. (B) New bone formation near the bottom of the defect. (C) New bone formation near the top of the defect. The green lines indicate the original boundary of the prepared defect. “NB” indicates the areas of mineralized new bone. There was significantly more new bone near the bottom of the defect than near the top of the defect. Color images available online at www.liebertpub.com/tea
The width of the new bone region W was plotted in Figure 4 for the donor BMSC recipient dogs and the control dogs without BMSC injection (mean±SD; n=4). At each defect depth, the width of new bone region for the BMSC recipient dogs was approximately 20–40% more than that of the control group (p<0.05).
FIG. 4.
New bone formation in mandibular defects of beagle dogs. The data show new bone area width, W, for the BMSC recipient dogs along with control dogs without BMSC injection (mean±SD; n=4). Color images available online at www.liebertpub.com/tea
Higher-magnification Mason staining images for the BMSC recipient group at 6 weeks were shown in Figure 5. In Figure 5A, examples of mineralized new bone are indicated by “NB,” and arrows indicate blood vessels. In Figure 5B, arrows indicate osteoblasts. Unmineralized fibers, stained blue in Figure 5B, appeared to be destined to become mineralized bone tissue over time because of the presence of numerous osteoblasts inside these fiber regions.
FIG. 5.

Higher magnification Mason staining images of new bone formation in beagle mandibular defects for the BMSC recipient group at 6 weeks. (A) Mineralized new bone is indicated by “NB.” Arrows indicate blood vessels. (B) Osteoblasts, mineralized new bone “NB,” and unmineralized fibers are shown. Red staining indicates mineralized new bone. The unmineralized fibers are stained blue. Color images available online at www.liebertpub.com/tea
The quantification of the area fraction of mineralized new bone at 6 weeks was plotted in Figure 6A (mean±SD; n=4). The mineralized new bone area percentage steadily increased in locations closer to the defect bottom (with greater defect depth) (p<0.05). Furthermore, at each defect depth, the donor BMSC recipient dogs had a significantly higher percentage of mineralized new bone than the control dogs that did not receive BMSC injection (p<0.05). The blood vessel density was plotted in Figure 6B. There was no significant difference in blood vessel density between the donor BMSC recipient dogs and the control dogs (p>0.1). For both groups, the blood vessel density decreased when the location was closer to the defect bottom (or at greater defect depth) (p<0.05). These results indicate that there are more blood vessels in newly formed bone (near the defect top) than in relatively more mature bone inside the defect (near the defect bottom).
FIG. 6.
(A) In the female dog's mandibular defect at 6 weeks, the mineralized new bone area percentage=area of mineralized new bone (red staining in the Mason image)/total area of the image (mean±SD; n=4). (B) The blood vessel density in the mandibular defect is defined as: blood vessel density=number of blood vessels in the Mason staining image/the area of the image. Color images available online at www.liebertpub.com/tea
The detection of donor BMSC inside the recipient mandibular defect after 6 weeks was shown in Figure 7. The sample was subjected to FISH test, and Y chromosome markers stained with avidin-FITC were observed, as indicated by arrows in Figure 7A. Two representative images were taken for each dog, yielding eight images for the group with systemic BMSC injection. Among these images, the Y-positive cells ranged from 1–2 cells per image to 10–15 cells per image. In contrast, the control samples from dogs without BMSC injection showed no Y chromosomes in the cell nuclei (Fig. 7B). The relatively large areas of a light green color were FITC staining of the cytoplasm.
FIG. 7.

The male donor BMSC that had been systemically injected into the femoral bone marrow of the female dog homed to the mandibular defect. (A) Some of the cells in the mandibular new bone samples inside the defect were Y positive. Y chromosome markers were stained with avidin-FITC (small green dots inside cell nuclei indicated by the arrows). (B) The control group without systemic injection of BMSC showed no Y chromosomes in the mandibular defects. Color images available online at www.liebertpub.com/tea
Discussion
The present study showed that systemically injected BMSC in femur marrow were recruited and homed to the craniofacial bone defect in a beagle dog model and resulted in significantly more new bone formation than control dogs without BMSC injection. The allogeneic BMSC were transplanted via intraosseous injection into the bone marrow cavity of the recipient's right femur, and these cells successfully homed to a remote long bone marrow cavity of the left femur in a short period of time. Furthermore, they were observed to migrate to the defect site in the mandible. Intraosseous injection was chosen rather than an intravenous route to administer the donor BMSC. This was because, first, it was reported that intraosseous transplantation of allogeneic cells induced better donor-specific chimerism and was thus more efficient for donor-cell engraftment than intravenous approach.46,47 Second, a previous in vivo study compared the affinity of BMSC to bone injury sites using different routes through which the cells were administered and reported results that favored the intraosseous route.34
The existence of donor BMSC in both femur and mandible suggests that intraosseously injected allogeneic BMSC may have the ability to repair and regenerate both appendicular and craniofacial bones. As reported previously, intraosseously injected BMSC were able to actively migrate to remote injury sites of long bone.34 Given this premise and considering the results of the present study, it could be concluded that all bone injury sites could trigger the mobilization and recruitment of BMSC from remote bone marrow cavities, which migrate to the injury and participate in bone repair. This is despite the fact that craniofacial bones are repaired via a distinctly different mechanism from appendicular bones.48–50
The regeneration of craniofacial bones as well as their development involve different cellular mechanisms from those of long bones. The development and regeneration of craniofacial bones are largely achieved by intramembranous ossification, whereas those of appendicular skeleton are primarily accomplished by endochondral ossification, although both processes involve rather similar molecular events.48,49 To date, there has been no direct evidence to support the hypothesis that bone injury of the craniofacial region can mobilize the migration and engraftment of remote BMSC from appendicular bone marrow cavities. The present study provided the first direct proof that BMSC were recruited from femur marrow and homed to mandibular defects in a large animal model, yielding significantly more new bone formation in the mandible than the control dogs without BMSC injection into the femur.
It is interesting to compare the results of the present study with previous reports. A previous study performed systemic injection of MSC into mice by tail vein injection to treat tibia fracture, which increased the new bone volume by about 20%, and the mineral content by about 40%, than the group without systemic MSC injection.39 While that study did not use large animals and did not investigate MSC migration from appendicular marrow to craniofacial bone defects, the extent of increase in new bone amount is in agreement with the present study. The present study increased the width of new bone region by approximately 20–40% and increased the mineralized new bone area fraction by 20–40% in the group with systemic BMSC injection, compared to the group without injection. Furthermore, the amount of donor BMSC that migrated to the defect site is also consistent with previous studies. The present study showed evidence of cells in the newly formed bone tissue being Y chromosome positive. The Y-positive cells ranged from 1–2 per image to 10–15 per image, similar to the numbers of transplanted cells that were found in the defect area in images at similar magnifications of previous studies.34,50–52 In addition, in the process of new bone formation, the formation of blood vessels is very important. The present study found that the blood vessel density in the mandibular defect area was significantly lower near the defect bottom and higher near the defect top. Since the cortical bone at the bottom of the defect was left intact, it likely served as the bed for new bone formation, hence new bone formed first on the defect bottom. Then, the formation of new bone gradually moved upward along the side walls of the defect. Over time, while new bone was continuously being formed, the earlier new bone closer to the defect bottom started to mature. At the end of the 6-week period, the new bone near the defect bottom became relatively more mature than the new bone near the defect top. When the earlier new bone started to mineralize and mature in the new bone region near the defect bottom, the density of blood vessels in this region decreased. These results are consistent with previous reports indicating that there are fewer blood vessels in mature bone than in immature bone.53
The present study showed that systemic BMSC injection in large animals could increase new bone formation in craniofacial defects. These results may help provide the theoretical basis for systemic cell therapy of mandibular and other bone defects. Many bone defects, such as those involving malignant tumors and radiotherapy, cannot be treated immediately with local surgery. Instead of leaving the bone defect untreated for an extended period of time, the results of the present study suggested that a systemic BMSC injection could be helpful in enhancing bone defect healing, yielding significantly more new bone regeneration, compared to the group without systemic BMSC injection. A previous study investigated the potential therapeutic effect of MSC infusion in mice following TBI, and the results supported the use of MSC infusion to repair damaged tissues in patients after accidental irradiation and may be used in patients who will undergo controlled radiotherapy for the treatment of solid tumors.51 Radiotherapy can have deleterious effects on the bone metabolism and healing, which can result in infection, atrophy, pathological fractures, and osteoradionecrosis. Previous studies showed that after conventional radiotherapy, the occurrence of osteoradionecrosis ranged from 0.9% to 35%54; when the doses given to the mandible exceeded 60 Gy, the risk became more severe.55 It was indicated that irradiation of the mandible carried severely damaging radiotherapy-induced complications, which could cause the need for surgical resection.56 Therefore, a potential application of the results of the present study is to treat mandibular and other bone defects with systemic BMSC injection to alleviate the damaging effects of radiotherapy and enhance bone repair after tumor resection. In addition, a previous study showed that after systemic BMSC injection, the toughness was doubled and the fracture force was increased by 40% for the new bone tissue.37 Therefore, systemically injected BMSC can home to defect sites to enhance fracture healing and to increase new bone formation, which could strengthen and toughen the bones with lesions at risk for fracture.
Conclusions
The present study proved that (1) allogeneic BMSC intraosseously injected into the long bone marrow cavity were capable of homing to both appendicular and craniofacial bone in a large animal model and (2) systemically injected BMSC participated in bone repair and increased new bone formation. The sex-mismatched allogeneic BMSC transplantation model was shown to be suitable for tracing stem cell migration and cell origin in bone regeneration, and the donor BMSC in the bone marrow of recipient dogs survived and acquired stable chimerism. The new bone amount in mandibular defects of dogs with systemic BMSC injection was 20–40% greater than that without BMSC injection. The results may help advance the understanding of stem cell homing to both appendicular and craniofacial bones, present a route for significantly increasing new bone formation, and provide clinical benefits in formulating novel therapies to treat bone defects.
Acknowledgments
This work was supported by Natural Science Foundation of China (NSFC-81371181 and 81171005), and animal experiments were performed at the West China School of Stomatology. H.H.K.X. contributed to the literature review, discussions, and writing of the article.
Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.United States Bone and Joint Decade (USBJD) 2002–2011. The Burden of Musculoskeletal Diseases in the United States Rosemont, IL: American Academy of Orthopaedic Surgeons, 2008. Foreword. [Google Scholar]
- 2.Mikos A.G., Herring S.W., Ochareon P., Elisseeff J., Lu H.H., Kandel R., Schoen F.J., Toner M., Mooney D., Atala A., Van Dyke M.E., Kaplan D., and Vunjak-Novakovic G.Engineering complex tissues. Tissue Eng 12,3307, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao J.J., Vunjak-Novakovic G., Mikos A.G., and Atala A.Regenerative Medicine: Translational Approaches and Tissue Engineering. Boston, MA: Artech House, 2007 [Google Scholar]
- 4.Yung Y.C., Chae J., Buehler M.J., Hunter C.P., and Mooney D.J.Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci U S A 106,15279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong E.L., Chan C.K., and Goodman S.B.Stem cell homing in musculoskeletal injury. Biomaterials 32,395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodrich J.T., Sandler A.L., and Tepper O.A review of reconstructive materials for use in craniofacial surgery bone fixation materials, bone substitutes, and distractors. Childs Nerv Syst 28,1577, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer A.J., Mesa J., and Buchman S.R.Current and emerging basic science concepts in bone biology: implications in craniofacial surgery. J Craniofac Surg 23,30, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Mueller S.M., and Glowacki J.Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem 82,583, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Mendes S.C., Tibbe J.M., Veenhof M., Bakker K., Both S., Platenburg P.P., Oner F.C., de Bruijn J.D., and Blitterswijk C.A.Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng 8,911, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Stenderup K., Justesen J., Clausen C., and Kassem M.Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33,919, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez J.P., Montecinos L., Rios S., Reyes P., and Martinez J.Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem 79,557, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y., Kim K.J., Kotake S., and Itoh T.Stromal cell activity in bone marrow from the tibia and iliac crest of patients with rheumatoid arthritis. J Bone Miner Metab 19,56, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Lavik E., and Langer R.Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol 65,1, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Shin H., Zygourakis K., Farach-Carson M.C., Yaszemski M.J., and Mikos A.G.Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials 25,895, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Mao J.J., Giannobile W.V., Helms J.A., Hollister S.J., Krebsbach P.H., Longaker M.T., and Shi S.Craniofacial tissue engineering by stem cells. J Dent Res 85,966, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson P.C., Mikos A.G., Fisher J.P., and Jansen J.A.Strategic directions in tissue engineering. Tissue Eng 13,2827, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hwang N.S., Varghese S., Lee H.J., Zhang Z., Ye Z., Bae J., Cheng L., and Elisseeff J.In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A 105,20641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundelacruz S., and Kaplan D.L.Stem-cell and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol 20,646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorsey S.M., Lin-Gibson S., and Simon C.G.X-ray microcomputed tomography for the measurement of cell adhesion and proliferation in polymer scaffolds. Biomaterials 30,2967, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Alsberg E., Anderson K.W., Albeiruti A., Rowley J.A., and Mooney D.J.Engineering growing tissues. Proc Nat Acad Sci U S A 99,12025, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao J.J.Stem cell driven regeneration of synovial joint. Biol Cell 97,289, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Radin S., Reilly G., Bhargave G., Leboy P.S., and Ducheyne P.Osteogenic effects of bioactive glass on bone marrow stromal cells. J Biomed Mater Res A 73,21, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Baksh D., Yao R., and Tuan R.S.Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25,1384, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Moioli E.K., Clark P.A., Sumner D.R., and Mao J.J.Autologous stem cell regeneration in craniosynostosis. Bone 42,332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan A.W., Roskov K.E., Lin-Gibson S., Kaplan D.L., Becker M.L., and Simon C.G.Characterization and optimization of RGD-containing silk blends to support osteoblastic differentiation. Biomaterials 29,2556, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kretlow J.D., Spicer P.P., Jansen J.A., Vacanti C.A., Kasper F.K., and Mikos A.G.Uncultured marrow mononuclear cells delivered within fibrin glue hydrogels to porous scaffolds enhance bone regeneration within critical-sized rat cranial defects. Tissue Eng Part A 16,3555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K., Dean D., Lu A., Mikos A.G., and Fisher J.P.Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in biodegradable nanocomposite scaffolds. Acta Biomater 7,1249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedenstein A.J., Chailakhjan R.K., and Lalykina K.S.The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3,393, 1970 [DOI] [PubMed] [Google Scholar]
- 29.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Derubeis A.R., and Cancedda R.Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng 32,160, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Cancedda R., Giannoni P., and Mastrogiacomo M.A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28,4240, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Kaigler D., Pagni G., Park C.H., Braun T., Holman L.A., Yi E., Tarle S.A., Bartel R.L., and Giannobile W.V.Stem cell therapy for craniofacial bone regeneration: a randomized, controlled, feasibility trial. Cell Transplant 22,767, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devine M.J., Mierisch C.M., Jang E., Anderson P.C., and Balian G.Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res 20,1232, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Shirley D., Marsh D., Jordan G., McQuaid S., and Li G.Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res 23,1013, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Shen F.H., Visger J.M., Balian G., Hurwitz S.R., and Diduch D.R.Systemically administered mesenchymal stromal cells transduced with insulin-like growth factor-I localize to a fracture site and potentiate healing. J Orthop Trauma 16,651, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Taguchi K., Ogawa R., Migita M., Hanawa H., Ito H., and Orimo H.The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun 331,31, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Granero-Molto F., Weis J.A., Miga M.I., Landis B., Myers T.J., O'Rear L., Longobardi L., Jansen E.D., Mortlock D.P., and Spagnoli A.Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 27,1887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji W., Yang F., Ma J., Bouma M.J., Boerman O.C., Chen Z., van den Beucken J.J., and Jansen J.A.Incorporation of stromal cell-derived factor-1a in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials 34,735, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Song G., Habibovic P., Bao C., Hu J., van Blitterswijk C.A., Yuan H., Chen W., and Xu H.H.K.The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials 34,2167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiegler H., Knabel M., Franz M., Kolb H.J., and Just U.Determination of donor-type chimerism using a semi-quantitative PCR-based method in a canine model for bone marrow transplantation. Vet Immunol Immunopathol 84,61, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Huh J.Y., Choi B.H., Kim B.Y., Lee S.H., Zhu S.J., and Jung J.H.Critical size defect in the canine mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100,296, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Wagner J.L., Burnett R.C., DeRose S.A., Francisco L.V., Storb R., and Ostrander E.A.Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation 62,876, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Yuan J., Cui L., Zhang W.J., Liu W., and Cao Y.Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials 28,1005, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Niemeyer G.P., Welch J.A., Tillson M., Brawner W., Rynders P., Goodman S., Dufresne M., Dennis J., and Lothrop C.D.Renal allograft tolerance in DLA-identical and haploidentical dogs after nonmyeloablative conditioning and transient immunosuppression with cyclosporine and mycophenolate mofetil. Transplant Proc 37,4579, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kushida T., Inaba M., Ikebukuro K., Ichioka N., Esumi T., Oyaizu H., Yoshimura T., Nagahama T., Nakamura K., Ito T., Hisha H., Sugiura K., Yasumizu R., Iida H., and Ikehara S.Comparison of bone marrow cells harvested from various bones of cynomolgus monkeys at various ages by perfusion or aspiration methods: a preclinical study for human BMT. Stem Cells 20,155, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Siemionow M., Zielinski M., Ozmen S., and Izycki D.Intraosseus transplantation of donor-derived hematopoietic stem and progenitor cells induces donor-specific chimerism and extends composite tissue allograft survival. Transplant Proc 37,2303, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Klimczak A., Unal S., Jankowska A., Coburn C., and Siemionow M.Donor-origin cell engraftment after intraosseous or intravenous bone marrow transplantation in a rat model. Bone Marrow Transplant 40,373, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Eames B.F., and Helms J.A.Conserved molecular program regulating cranial and appendicular skeletogenesis. Dev Dyn 231,4, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Gersch R.P., Lombardo F., McGovern S.C., and Hadjiargyrou M.Reactivation of Hox gene expression during bone regeneration. J Orthop Res 23,882, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Lee D.J., Cho T.J., Kim J.A., Lee H.R., Yoo W.J., Chung C.Y., and Choi I.H.Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone 42,932, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Francois S., Bensidhoum M., Mouiseddine M., Mazurier C., Allenet B., Semont A., Frick J., Sache A., Bouchet S., Thierry D., Gourmelon P., Gorin N.C., and Chapel A.Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells 24,1020, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Jung D.I., Ha J., Kang B.T., Kim J.W., Quan F.S., Lee J.H., Woo E.J., and Park H.M.A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci 285,67, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Barou O., Mekraldi S., and Vico L.Relationships between trabecular bone remodeling and bone vascularization: a quantitative study. Bone 30,604, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Reuther T., Schuster T., Mende U., and Kubler A.Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients-a report of a thirty year retrospective review. Int J Oral Maxillofac Surg 32,289, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Jereczek-Fossa B.A., and Orecchia R.Radiotherapy-induced mandibular bone complications. Cancer Treat Rev 28,65, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Chrcanovic B.R., Reher P., Sousa A.A., and Harris M.Osteoradionecrosis of the jaws-a current overview-part 1: Physiopathology and risk and predisposing factors. Oral Maxillofac Surg 14,3, 2010 [DOI] [PubMed] [Google Scholar]