Summary
Actin cables of budding yeast are bundles of F-actin that extend from the bud tip or neck to the mother cell tip, serve as tracks for bidirectional cargo transport, and undergo continuous movement from buds towards mother cells [1]. This movement, retrograde actin cable flow (RACF), is similar to retrograde actin flow in lamellipodia, growth cones, immunological synapses, dendritic spines and filopodia [2–5]. In all cases, actin flow is driven by the push of actin polymerization and assembly at the cell cortex, and myosin-driven pulling forces deeper within the cell [6–10]. Therefore, for movement and inheritance from mothers to buds, mitochondria must “swim upstream” against the opposing force of RACF [11]. We find that increasing RACF rates results in increased fitness of mitochondria inherited by buds, and that the increase in mitochondrial fitness leads to extended replicative lifespan and increased cellular healthspan. The sirtuin SIR2 is required for normal RACF and mitochondrial fitness, and increasing RACF rates in sir2Δ cells increases mitochondrial fitness and cellular healthspan but does not affect replicative lifespan. These studies support the model that RACF serves as a filter for segregation of fit from less fit mitochondria during inheritance, which controls celllular lifespan and healthspan. They also support a role for Sir2p in these processes.
Results and Discussion
Altering the rate of retrograde actin cable flow affects mitochondrial quality control during inheritance
Microtubules and microfilaments are well-known as tracks for intracellular organelle and cargo movement. In many cases, the tracks are static. However, in the budding yeast, the tracks are moving in the direction that is opposite that of organelles as they move from mother cells to buds during cell division. Here, we tested the hypothesis that retrograde actin cable flow (RACF) can exercise mitochondrial quality control during cell division. If RACF serves as a filter to prevent low-functioning mitochondria from moving from mother cells to buds, then increasing the rate of RACF should result in inheritance of fitter, more motile mitochondria, and slowing the rate of RACF should have the opposite effect.
To test this hypothesis, we measured mitochondrial motility and function in myo1Δ and tpm2Δ yeast, which show altered retrograde cable flow rates. Myo1p, a type II myosin that localizes to the bud neck, generates pulling forces for RACF and for contractile ring closure [9]. Tpm2p is one of two tropomyosins in yeast. The only known function of Tpm2p is to regulate RACF by regulating the binding of Myo1p to actin cables [9].
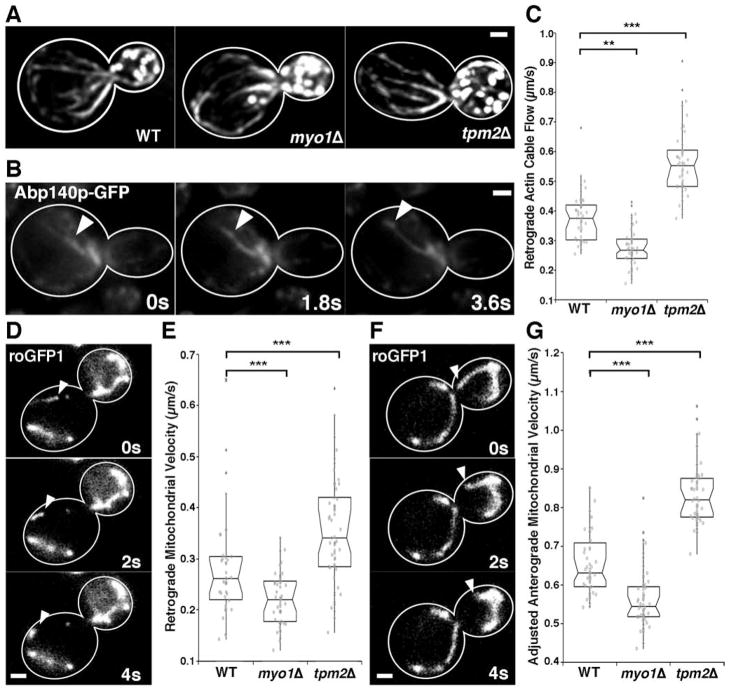
Deletion of MYO1 or TPM2 has no obvious effect on actin cable abundance, polarization of the actin cytoskeleton (as assessed by enrichment of actin patches, endosomes that are invested with a coat of F-actin, in the bud), or the steady state level of the sirtuin Sir2p (Fig. 1A, Fig. S1). Deletion of MYO1 results in 28.5 and 21.4% decreases in the velocity of RACF and retrograde mitochondrial movement, respectively. Conversely, deletion of TPM2 results in 32.3 and 28.1% increases in the velocities of retrograde actin cable and mitochondrial movement, respectively (Fig. 1B-E). Thus, altering the rate of RACF results in a corresponding change in the velocity of retrograde mitochondrial movement.
Fig. 1. RACF affects mitochondrial motility, but does not alter actin cable abundance or polarity.
(A) Images of rhodamine-phalloidin stained actin in wild-type, myo1Δ , and tpm2Δ cells. (B) Still frames of a time-lapse series showing Abp140p-GFP-labeled actin cables undergoing retrograde flow. Arrowheads indicate a fiduciary mark on a motile actin cable. (C) Notched dot box plot of the velocity of RACF in wild-type, myo1Δ , and tpm2Δ yeast. The central band in the box represents the median, boxes indicate the middle quartiles; whiskers extend to the 5th and 95th percentiles, and red points indicate outliers (defined as quartile 1– 1.5x interquartile range and quartile 3 + 1.5x interquartile range). n = 50 from 3 trials. (D) Still frames from a time-lapse series illustrating retrograde mitochondrial movement in wild-type cells expressing mito-roGFP. (E) Notched dot box plot of the velocity of retrograde mitochondrial movement in wild-type, myo1Δ , and tpm2Δ yeast. n = 40-60 from 3 trials. (F) Still frames from a time-lapse series showing anterograde mitochondrial movement in wild-type cells expressing mito-roGFP1. (G) Notched dot box plot of the adjusted velocity of adjusted anterograde mitochondrial movement in WT, myo1Δ , and tpm2Δ yeast. n = 40-60 from 3 trials. ** = p < 0.01, *** = p < 0.001. p values were calculated using Kruskal-Wallis testing. Bars: 1μm. Cell outlines are shown in white.
Next, we measured the velocity of anterograde, bud-directed mitochondrial movement. Since mitochondria undergoing anterograde movement are moving against the opposing force of RACF, the actual velocity of anterograde mitochondrial movement, in the frame of reference of the actin cable, is significantly greater than the apparent velocity of mitochondrial movement, in the frame of reference of the cell. To account for these directional force considerations, we calculated an adjusted velocity of anterograde mitochondrial movement, which better reflects the intrinsic velocity of anterograde movement, by subtracting the effect of RACF (Fig. S1).
The measured velocities of anterograde mitochondrial movement were not different between the mutants. However, taking into account the opposing force of RACF, the adjusted real rates are significantly different. We find that increasing RACF rates by deletion of TPM2 results in a 29.9% increase in the adjusted velocity of anterograde mitochondrial movement. Moreover, decreasing the rate of RACF by deletion of MYO1 reduces the adjusted mitochondrial anterograde velocity by 13.9% (Fig. 1F-G; Fig. S1). Motility reflects the ability to assemble functional motility proteins and provide energy to the motility apparatus, and thus is one indicator of the fitness of an organelle. Our finding that an increase in RACF rates results in increased anterograde mitochondrial motility supports the model that RACF exercises mitochondrial quality control during inheritance.
Consistent with this, we find that mitochondrial redox state, measured using mitochondria-targeted redox-sensing GFP1 (mito-roGFP1), correlates with rates of RACF and anterograde mitochondrial motility. roGFP contains two surface-exposed cysteines. Oxidation or reduction of these cysteines occurs in response to the redox state of the environment and alters the excitation spectrum of roGFP [12]. roGFP has been targeted to yeast mitochondria where it serves as an effective biosensor for mitochondrial redox state in living cells [13]. Indeed, in yeast, mito-roGFP fluorescence ratios show a linear dose-response relationship upon treatment with oxidizing or reducing agents [14]. Moreover, treatment with saturating levels of a membrane permeable reductant or oxidant results in an 40% increase or a 19% decrease, respectively, in mito-roGFP redox ratio, compared to untreated cells (Fig. S2). Thus, a higher ratio indicates a more reducing environment. We find that increasing the rate of RACF by deletion of TPM2 increases mitochondrial redox ratio by 19.7%, and that reduction of the rate of RACF by deletion of MYO1 renders mitochondria 11.7% more oxidizing (Fig. 2A-B). Thus, by two different measures, motility and redox state, cells with increased RACF rates have higher functioning mitochondria.
Fig. 2. Altering RACF changes mitochondrial quality, asymmetric inheritance of mitochondria during division, lifespan and healthspan in rho+ cells, but does not affect mitochondrial quality or lifespan in rho0 cells.
(A) mito-roGFP1 was used to visualize redox state of mitochondria in wild-type, myo1Δ , and tpm2Δ cells. Images are reduced:oxidized mito-roGFP1 ratios overlaid on phase images. Color scale indicates ratio values; higher numbers and warmer colors indicate more reducing mitochondria. Scale bar, 1μm. (B) Notched dot box plot of the average reduced:oxidized mito-roGFP1 ratio in wild-type, myo1Δ , and tpm2Δ cells. n = 97-143 cells per strain. Data is representative of 3 experiments. ** = p-value < 0.01, *** = p-value< 0.001 using the non-parametric Kruskal-Wallis test. (C) Quantitation of asymmetric segregation of fit from less fit mitochondria between mother and daughter cells. The reduced:oxidized mito-roGFP1 ratio was measured in mother cells and buds in cells containing large buds (bud diameter > 50% the diameter of the mother cell). n = 55-65 cells per strain. Data is representative of 2 trials. *** = p < 0.001 calculated by Wilcoxon paired difference test. (D) Replicative lifespans of wild-type, myo1Δ , and tpm2Δ cells were determined as described in Experimental Procedures. Mann-Whitney test was performed to determine p-values: wild-type vs. tpm2Δ : 0.004, wild-type vs. myo1Δ : 0.001. (E) Mean generation time was determined during the replicative lifespan assay by recording the time between emergence of consecutive buds from the same mother. Error bars represent SEM. (D) and (E) n = 40-50 cells per strain. Data shown is representative of 3 trials. (F) rho0 cells were produced by ethidium bromide treatment as described in Experimental Procedures. Images are representative reduced/oxidized roGFP ratios for wild-type (top),TPM2 rho0 (middle), and tpm2Δ rho0 (bottom) cells. (G) Notched dot box plot of the average reduced:oxidized mito-roGFP1 ratio in wild-type, TPM2 rho0, and tpm2Δ rho0 cells. n = 63-83 cells per strain. Data is representative of 3 experiments. *** = p-value< 0.001 using non-parametric Kruskal-Wallis testing. (H) Replicative lifespans of wild-type, TPM2 rho0, and tpm2Δ rho0 cells. n = 30-50 cells per strain. Data is representative of 2 experiments. Mann-Whitney statistical analysis reveals no significant difference between all three groups.
Yet to be determined is why mitochondria in tpm2Δ and myo1Δ cells are more or less reducing and motile, respectively, compared to mitochondria in wild-type cells. Previous studies revealed that mitochondria with higher membrane potential, aconitase activity, redox state and ROS are preferentially inherited by daughter cells during yeast cell division [13, 15, 16]. This segregation of functional from less functional mitochondria is mediated, in part, by anchorage and retention of newly inherited mitochondria in the developing daughter cell. It is also is required for mother-daughter age asymmetry, the process whereby mother cells continue to age and daughter cells are for the most part born young [13].
To determine whether the changes in mitochondrial redox state and motility in tpm2Δ and myo1Δ cells are due to RACF effects on mitochondrial quality control during inheritance, we assessed the effect of modulating RACF rate on mitochondrial redox states in mother and daughter cells. In wild-type cells, there is a small, but statistically significant, asymmetry in mitochondrial redox state between mother and bud. tpm2Δ cells show a 28% larger difference between mother and bud. Conversely, functional segregation of mitochondria is not detectable in myo1Δ cells, which have decreased RACF rates (Fig. 2C). Thus, segregation of more reducing and motile mitochondria from those that are less reducing and motile among mother cells and buds is enhanced by deletion of TPM2 and the associated increase in RACF rate. This is the first direct evidence that RACF serves as a filter to prevent inheritance of less motile and more oxidized mitochondria and to promote inheritance of more motile and reduced mitochondria during yeast cell division.
Altering the rate of RACF affects cellular lifespan and healthspan through effects on mitochondrial function
Recent studies indicate that altering the function of mitochondria in daughter cells can affect yeast lifespan. Specifically, yeast that inherit mitochondria that are more reducing and have lower reactive oxygen species (ROS) have extended lifespan, while yeast that inherit mitochondria that are more oxidized and have more ROS have shortened lifespans [13, 17]. Therefore, we tested the effect of increasing the rate of RACF, and the associated increase in mitochondrial motility and redox state, on yeast lifespan and healthspan.
Aging studies in yeast can model two distinct forms of cellular aging. Chronological lifespan, the survival time of stationary-phase, non-dividing yeast cells, is a model for stress resistance in post-mitotic cells. Replicative lifespan (RLS), the number of times that a cell can divide prior to senescence, is a model for aging of division-competent cells. The mean RLS of wild-type, tpm2Δ and myo1Δ cells are 22.0±1.0, 27.0±1.4 and 17.2±0.9 generations, respectively (Fig. 2D). Thus, deletion of TPM2, which results in an increase in retrograde flow rate, extends RLS by 23% compared to wild-type cells. Conversely, deletion of MYO1 results in a decrease in RLS by 22% compared to wild-type cells. Since Myo1p also functions in contractile ring closure, it is possible that the decreased RLS may be due to Myo1p function in processes other than RACF. However, the only known function of Tpm2p is to control RACF. Therefore, we conclude that increasing RACF can extend lifespan in yeast.
The healthspan of an organism is the period during which it is generally healthy and free of disease or age-related symptoms. The mean generation time of yeast increases as they age [13, 18]. Therefore, maintaining a short generation time is an indicator of cellular healthspan in yeast. tpm2Δ cells, which have increased RACF rates and mitochondria that are more motile and reducing, have a shorter generation time overall, and also maintain a short generation time for more generations than wild-type cells. Decreasing the rate of RACF has the opposite effect (Fig. 2E). Thus, changes in cytoskeletal dynamics can affect mitochondrial quality control during inheritance, and alter replicative lifespan and cellular healthspan in a manner that correlates with mitochondrial fitness.
To determine whether the extended RLS that occurs upon deletion of TPM2 is due to effects on mitochondrial function, we studied the effect of deletion of TPM2 on lifespan in a rho0 strain, which has no mitochondrial DNA (mtDNA). Loss of mtDNA does not affect actin cable abundance or actin patch polarity in cells that contain TPM2 and in tpm2Δ cells (Fig. S2). However, since mtDNA encodes mitochondrial respiratory chain components, rho0 cells cannot grow in media containing a non-fermentable carbon (glycerol) as a sole carbon source (Fig. S2). Consistent with this, our mito-roGFP measurements indicate that mitochondria in rho0 cells are 22% more oxidized compared to mitochondria in rho+ cells, both in cells that contain TPM2 and in tpm2Δ cells. Interestingly, the redox state of mitochondria in rho0 TPM2 cells is not significantly different from that of rho0 tpm2Δ cells (Fig. 2F-G). Thus, the increase in mitochondrial redox state that occurs upon deletion of TPM2 requires mtDNA.
If extension of lifespan in tpm2Δ cells is due to increased mitochondrial function, then the loss of mitochondrial respiratory activity and respiration dependent processes that occurs upon deletion of mtDNA should prevent lifespan extension. Deletion of mtDNA can affect RLS in some genetic backgrounds. We confirmed that deletion of mtDNA does not affect RLS in BY4741 cells: the average RLS of rho+ cells (22.2 ± 1.34) is similar to that of rho0 cells (21.4 ± 1.2). More importantly, deletion of TPM2 in rho0 cells does not affect RLS (21.4 ± 1.2 in rho0 vs. 22.7 ± 1.29 in tpm2Δ rho0 cells) (Fig. 2H). Thus, mitochondrial function is required for the RLS extension that occurs upon deletion of TPM2 and the associated increase in RACF.
Sir2p affects RACF and mitochondrial quality control
Previous studies indicate that deletion of Sir2p alters yeast actin: it results in decreased actin cable abundance, increased sensitivity of yeast to the growth-inhibiting effects of Latrunculin-A (an agent that binds to G-actin and results in rapid actin disassembly) and reduces levels of natively folded actin protein [19]. We confirmed that deletion of SIR2 reduces the number of actin cables and results in in depolarization of actin patches. Deletion of SIR2 also results in a decrease in the velocities of RACF and retrograde mitochondrial movement. Conversely, mild (2.2-fold) overexpression of fully functional, HA-tagged SIR2 has the opposite effect (Fig. 3A-C, Fig. S3). Thus, our studies reveal a novel role for Sir2p in control of RACF. Since the levels of properly folded actin are reduced in sir2Δ cells and SIR2 has genetic interactions with the formin (Bni1p) that nucleates actin for actin cable assembly and movement in the bud tip [19], it is possible that the reduced amount of substrate (native actin) available for actin nucleation and actin cable elongation in sir2Δ cell affects actin cable thickness, abundance, retrograde flow and function in establishment and maintenance of cell polarity.
Fig. 3. Sir2p regulates mitochondrial inheritance, fitness, motility, and RACF.
(A) Images of rhodamine-phalloidin stained actin in wild-type, sir2Δ , and in cells overexpressing SIR2 (SIR2 o/e). (B-D) Quantitation of RACF rates, velocities of retrograde mitochondrial movement, and adjusted velocities of anterograde mitochondrial movement in wild-type, sir2Δ , and SIR2 o/e cells. n = 40-69 cells per strain. Data is pooled from 3 trials. (E) Images of reduced:oxidized mito-roGFP1 ratio images overlaid on phase images in wild-type, sir2Δ , and SIR2 o/e cells. Color scale indicates ratio values, higher numbers and warmer colors indicate more reducing mitochondria. n = 97-127 cells per strain. Data is representative of 3 trials. (F) Quantitation of average reduced:oxidized mito-roGFP1 ratio within individual cells. n = 50-66 cells per strain. Data is representative of 3 trials. Higher values indicate more reducing mitochondria. (G) The average reduced:oxidized mito-roGFP1 ratio was determined in mother cells and buds as for Fig. 2. ** = p < 0.01, *** = p < 0.001. p values were calculated using the non-parametric Kruskal-Wallis test for B-F and using the non-parametric Wilcoxon paired difference test for G. Scale bars, 1μm.
Consistent with the observed effect on RACF, deletion of SIR2 also results in a decrease in the adjusted velocity of anterograde mitochondrial motility, a more oxidizing mitochondrial redox environment, and defects in segregation of more reduced from more oxidized mitochondria between mother and daughter cells. Mild overexpresssion of SIR2 has the opposite effects (Fig. 3D-G; Fig. S3). Deletion of SIR2 has more severe effects on adjusted anterograde mitochondrial movement compared to deletion of MYO1. The simplest explanation for this difference is that deletion of SIR2 has general effects on actin cable abundance, thickness and polarity, in addition to effects on RACF. Overall, these findings support a role for Sir2p in many aspects of the actin cytoskeleton, including RACF, and in mitochondrial quality control.
Increasing the rate of RACF in a sir2Δ cell increases mitochondrial motility and redox state and cellular healthspan
Because SIR2 has many functions, we tested whether changes in mitochondrial movement and redox state could be abrogated by altering retrograde flow rates through deletion of MYO1 or TPM2. Indeed, deletion of MYO1 in a SIR2-overexpressing strain restores the adjusted velocity of anterograde mitochondrial motility and mitochondrial redox state, to wild-type levels (Fig. S4). On the other hand, increasing RACF rate by deletion of TPM2 in sir2Δ cells improves but does not completely restore mitochondrial redox state, cell division-linked segregation of more reduced from more oxidized mitochondria, and anterograde mitochondrial motility (Fig. 4A-F). Increasing RACF in sir2Δ also restores polarity of the actin cytoskeleton, but does not restore actin cable abundance (Fig. S4). These findings provide further support for the concept that Sir2p affects mitochondrial function by RACF-dependent and -independent mechanisms, including general effects on actin cable abundance.
Fig. 4. Modulation of RACF rates promotes mitochondrial motility and cell healthspan in sir2 Δ yeast.
(A-C) Quantitation of RACF rates, velocities of retrograde mitochondrial movement, and adjusted velocities of anterograde mitochondrial movement in wild-type, sir2Δ , and sir2Δ tpm2Δ cells. n = 39-51 cells per strain. Data is pooled from 3 trials. (D) Images showing reduced:oxidized mito-roGFP1 ratio overlaid on phase images in wild-type, sir2Δ , and sir2Δ tpm2Δ cells. Color scale at right indicates ratio values; higher numbers and warmer colors indicate more reducing mitochondria. Scale bar, 1μm. (E) Quantitation of average reduced:oxidized mito-roGFP1 ratio within individual cells. n = 60-100 cells per strain. Data shown is representative of 2 trials. (A-E) * = p < 0.05, ** = p < 0.01, *** = p < 0.001 were calculated using the non-parametric Kruskal Wallis test. (F) Quantification of asymmetric segregation of fit from less fit mitochondria during cell division. The average reduced:oxidized mito-roGFP1 ratio was measured in mother cells and buds. n = 50-53 cells per strain. Data shown is representative of 2 trials. ** = p < 0.01, *** = p < 0.001. p values were calculated using a non-parametric Wilcoxon paired difference test. (G) Replicative lifespans of wild-type, sir2Δ , and sir2Δ tpm2Δ cells. Mann-Whitney test was performed to determine p-values: wild-type vs. sirΔ : <0.0001, wild-type vs. sir2Δ tpm2Δ : <0.0001, sir2Δ vs. sir2Δ tpm2Δ : 0.5. (H) Mean generation time was determined during the replicative lifespan assay as for Fig. 2. Error bars represent SEM. n = 38-52 cells per strain. Data is representative of 2 trials.
Finally, we studied the effect of increasing RACF in a sir2Δ on lifespan and healthspan. The mean RLS of sir2Δ tpm2Δ cells (14.2±0.72) is similar to that of sir2Δ cells (14.3±1.08) (Fig. 4G). Thus, increasing RACF and mitochondrial motility and redox state in sir2Δ cells does not increase RLS. This finding indicates that Sir2p affects lifespan through multiple mechanisms, beyond mitochondria and actin dynamics, which is consistent with previous reports [20]. On the other hand, the mean generation time of sir2Δ tpm2Δ yeast is shorter than that of sir2Δ cells in young cells that have undergone 0–10 replications (Fig. 4H). Thus, increasing RACF rates can promote cellular healthspan in a sir2Δ cell, potentially through effects on mitochondrial redox state and motility and/or effects on actin cable polarity.
Here, we describe novel roles for the actin cytoskeleton in mitochondrial quality control and lifespan regulation. Specifically, our studies support the model that RACF exercises mitochondrial quality control by serving as a filter to prevent less motile and more oxidized mitochondria from leaving the mother cell and select for the inheritance of more motile and reducing mitochondria. Retrograde actin network flow also occurs at lamellipodia, growth cones, immunological synapses, dendritic spines and filopodia of mammalian cells, and can drive organelle and particle movement [2–5, 21]. Therefore, it is possible that similar quality control mechanisms exist in other eukaryotes, perhaps selecting subpopulations of organelles that will be delivered to cellular regions with needs for mitochondrial function.
We also find that modulation of actin cable dynamics can alter lifespan and healthspan in a manner that correlates with and is dependent upon mitochondrial function, and we reveal a new role for Sir2p, a conserved lifespan modulator, in RACF. Mitochondria are aging determinants in yeast and other cell types, and mutations in mitochondrial quality control during inheritance can affect lifespan in yeast [13]. However, mitochondria are not the only aging determinant whose segregation is influenced by retrograde actin flow. Oxidatively damaged proteins are also retained in the mother cell by mechanisms that are not fully understood, but are dependent upon the actin cytoskeleton [19, 22]. Moreover, vacuoles, which are lysosome-like organelles that undergo actin cable-dependent movement in yeast, are also aging determinants: vacuolar acidity declines with replicative age, and preventing the decline in vacuolar acidity suppresses age-associated declines in mitochondrial function and extends lifespan [23]. Thus, it is possible that RACF affects asymmetric distribution of other aging determinants.
Supplementary Material
Highlights.
Retrograde actin cable flow (RACF) prevents inheritance of more oxidized, less motile mitochondria in dividing yeast.
Increasing RACF rates promotes mitochondrial and cellular fitness and extends lifespan.
Sir2p is required for normal RACF and mitochondrial fitness.
Increasing RACF in sir2Δ cells promotes mitochondrial fitness and cellular healthspan.
Acknowledgments
We thank the members of the Pon laboratory for technical assistance and valuable discussion, Wolfgang Pernice for artwork, and Marija Saparauskaite and other members of the Feinstein laboratory (CUNY Hunter College) for plasmid construction and valuable discussion. This work was supported by grants from the National Institutes of Health (NIH) (5 T32 DK7647) to RH, from the HHMI 56006760 to JDV, and from the NIH (GM045735 and GM096445) and the Ellison Medical Foundation (AG-SS-2465-10) to LP. One of the microscopes used for these studies was supported in part through a NIH/NCI grant (5 P30 CA13696).
Abbreviations
- RACF
retrograde actin cable flow
- mito-roGFP
mitochondrial targeted redox sensing GFP
- ROS
reactive oxygen species
- RLS
replicative lifespan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang HC, Pon LA. Actin cable dynamics in budding yeast. Proc Natl Acad Sci U S A. 2002;99:751–756. doi: 10.1073/pnas.022462899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 3.Small JV, Resch GP. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr Opin Cell Biol. 2005;17:517–523. doi: 10.1016/j.ceb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Tatavarty V, Das S, Yu J. Polarization of actin cytoskeleton is reduced in dendritic protrusions during early spine development in hippocampal neuron. Mol Biol Cell. 2012;23:3167–3177. doi: 10.1091/mbc.E12-02-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer C, Faust U, Kirchgessner N, Merkel R, Hoffmann B. The filopodium: a stable structure with highly regulated repetitive cycles of elongation and persistence depending on the actin cross-linker fascin. Cell Adh Migr. 2011;5:431–438. doi: 10.4161/cam.5.5.17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theriot JA, Mitchison TJ. Comparison of actin and cell surface dynamics in motile fibroblasts. J Cell Biol. 1992;119:367–377. doi: 10.1083/jcb.119.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown J, Bridgman PC. Role of myosin II in axon outgrowth. J Histochem Cytochem. 2003;51:421–428. doi: 10.1177/002215540305100403. [DOI] [PubMed] [Google Scholar]
- 8.Jurado C, Haserick JR, Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol Biol Cell. 2005;16:507–518. doi: 10.1091/mbc.E04-10-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huckaba TM, Lipkin T, Pon LA. Roles of type II myosin and a tropomyosin isoform in retrograde actin flow in budding yeast. J Cell Biol. 2006;175:957–969. doi: 10.1083/jcb.200609155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig EM, Van Goor D, Forscher P, Mogilner A. Membrane tension, myosin force, and actin turnover maintain actin treadmill in the nerve growth cone. Biophys J. 2012;102:1503–1513. doi: 10.1016/j.bpj.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehrenbacher KL, Yang HC, Gay AC, Huckaba TM, Pon LA. Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr Biol. 2004;14:1996–2004. doi: 10.1016/j.cub.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 13.McFaline-Figueroa JR, Vevea J, Swayne TC, Zhou C, Liu C, Leung G, Boldogh IR, Pon LA. Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging Cell. 2011;10:885–895. doi: 10.1111/j.1474-9726.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vevea JD, Alessi Wolken DM, Swayne TC, White AB, Pon LA. Ratiometric Biosensors that Measure Mitochondrial Redox State and ATP in Living Yeast Cells. J Vis Exp. 2013 doi: 10.3791/50633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai CY, Jaruga E, Borghouts C, Jazwinski SM. A mutation in the ATP2 gene abrogates the age asymmetry between mother and daughter cells of the yeast Saccharomyces cerevisiae. Genetics. 2002;162:73–87. doi: 10.1093/genetics/162.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klinger H, Rinnerthaler M, Lam YT, Laun P, Heeren G, Klocker A, Simon-Nobbe B, Dickinson JR, Dawes IW, Breitenbach M. Quantitation of (a)symmetric inheritance of functional and of oxidatively damaged mitochondrial aconitase in the cell division of old yeast mother cells. Exp Gerontol. 2010;45:533–542. doi: 10.1016/j.exger.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Swayne TC, Zhou C, Boldogh IR, Charalel JK, McFaline-Figueroa JR, Thoms S, Yang C, Leung G, McInnes J, Erdmann R, et al. Role for cER and Mmr1p in anchorage of mitochondria at sites of polarized surface growth in budding yeast. Curr Biol. 2011;21:1994–1999. doi: 10.1016/j.cub.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer RK, Johnston JR. Life span of individual yeast cells. Nature. 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nystrom T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babich A, Li S, O'Connor RS, Milone MC, Freedman BD, Burkhardt JK. F-actin polymerization and retrograde flow drive sustained PLCgamma1 signaling during T cell activation. J Cell Biol. 2012;197:775–787. doi: 10.1083/jcb.201201018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 23.Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492:261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.