Abstract
Hypertension or hypercholesterolemia can induce a proinflammatory and prothrombogenic phenotype in the microcirculation of the brain, however, less is known about how the combination of these risk factors affects the vasculature. We recently reported that a moderate (60%) increase in plasma cholesterol blunts the recruitment of leukocytes and platelets in the cerebral microvessels elicited by hypertension. In this study, we examined whether larger increments in blood cholesterol (4-fold) exerts a similar modulating influence on the vasculature in the presence of hypertension. Apolipoprotein E knockout mice with deoxycorticosterone acetate-salt induced hypertension were placed on a high cholesterol diet and exhibited exaggerated leukocyte and platelet adhesion responses in cerebral microvessels. Intermittent feeding (every 4th day) with the high cholesterol diet yielded similar phenotypic changes in the vasculature. Once mice were placed on high cholesterol diet, 4 days on normal diet was needed to revert to a normal vascular phenotype. Angiotensin II type-1 receptors, and reactive oxygen species appear to contribute to the vascular responses induced by hypercholesterolemia and hypertension. Our findings indicate that the combination of hypertension and large increases in plasma cholesterol concentration results in a severe, but reversible, inflammatory and thrombogenic phenotype in the cerebral microvasculature.
Keywords: Cerebral microvasculature, hypercholesterolemia, high blood pressure, ApoE-KO mice, inflammation
INTRODUCTION
Cardiovascular disease (CVD) continues to represent the major cause of death worldwide, accounting for over 17 million deaths in the past year (1). Extensive research on this problem has led to the identification of a number of factors that increase the risk for development of CVD. These include hypertension (HTN), aging, obesity, diabetes, hypercholesterolemia (HCh), smoking and physical inactivity (2-7). Epidemiological studies have revealed that the risk for CVD increases significantly with the presence of two or more risk factors. For example, the combination of HTN and HCh promotes, at a rate that is greater than with either risk factor alone, the development of atherosclerosis, which can ultimately lead to myocardial infarction and stroke.
While the diverse nature of the risk factors for CVD would suggest different underlying mechanisms for the induction of disease, the similarity of responses of the vasculature to these risk factors suggest otherwise. Inflammation, oxidative stress, diminished nitric oxide bioavailability, and enhanced thrombogenesis are characteristic features shared by most of the CVD risk factors. In the cerebral microcirculation, both HTN and HCh result in an enhanced recruitment of adherent leukocytes and platelets (8-11), impair blood brain barrier (BBB) function (10-12), and alter vasomotor function (13-15). Although the impact of individual risk factors (e.g., HTN vs HCh) on vascular and/or organ function has been extensively studied, less attention has been devoted to defining how combinations of risk factors influence these target tissues. Given the shared actions of risk factors on the vasculature, it would appear likely that a combination of risk factors should produce additive or synergistic responses. However, we have recently demonstrated that diet-induced HCh, with a moderate increase in blood cholesterol concentration (from 70 to 110 mg/dL), blunts, rather than exacerbates, the proinflammatory and prothrombogenic responses of the cerebral microvasculature to HTN (16). Whether higher levels of blood cholesterol would also exert a moderating influence on the proinflammatory and prothrombogenic responses of the cerebral vasculature to HTN remains unclear. A major objective of this study was to address this issue. In addition, we evaluated the effects of angiotensin II type-1 receptor (AT1r) blockade (losartan) and superoxide scavenging (with tempol) on the cerebral microvascular responses to the combination of HCh and HTN.
METHODS
Animals
Male Apolipoprotein E knockout (ApoE-KO) mice (B6.129P2-Apoe (tm1Unc)/J) were obtained from Jackson Laboratories (Bar Harbor, ME). The mice (a total of 110) were housed under specific pathogen-free conditions and fed standard laboratory chow and water prior to entering the study. All of the experimental procedures using animals were reviewed and approved by the Institutional Animal Care and Use Committee of LSU Health Sciences Center and performed according to the criteria outlined by the NIH Guide for the Care and Use of Laboratory Animals.
Control and experimental groups
Following 2 days of acclimatization, under ketamine (150 mg/kg) + xylazine (7.5 mg/kg) intraperitoneal (IP) anesthesia (~ 100 μL/mouse), the left kidney was removed from all mice (6-8 week-old), except one group (intact group) that was not subjected to any surgical or pharmacological intervention. After surgery, the uninephrectomized (Uni) mice were randomly assigned to the following experimental groups: control mice fed normal chow diet (Uni ApoE-KO), mice fed (3-week) a high cholesterol diet (HCD) (Uni ApoE-KO + HCD), deoxycorticosterone acetate (DOCA)-salt hypertensive mice fed normal chow diet (Uni ApoE-KO + DOCA-salt) or HCD (3-week) (Uni ApoE-KO + DOCA-salt + HCD) (n= 6 - 8 per group). A slow release DOCA pellet (50 mg, 21-day-release) (Innovative Research of America, Sarasota, FL, USA) was inserted subcutaneously in the DOCA-salt groups and drinking water was replaced with 1% NaCl / 0.2% KCl solution (9). Non-hypertensive mice received tap water. The HCD (Teklad TD.94059, Indianapolis, IN) contained (in g per kg): casein 75.0; dextrose, monohydrate 30.0; sucrose 16.25; dextrin 16.25; cocoa butter 75.0; cholesterol 12.5; cellulose 12.5; mineral mix, AIN-76 (170915) 8.75; vitamin mix, Teklad (40060) 2.5; choline chloride 1.25. Total body weight was determined before and at the end of the experimental routine. Periepididymal fat pad weight was measured by the end of the experiments. Liquid (consumed over 24h) and food intake (consumed over 24h per 100 g body weight) were measured 10 days after uninephrectomy.
Two Uni ApoE-KO + DOCA-salt + HCD groups were treated with either the ATr1 antagonist losartan (Cozaar, Merck & Co., Whitehouse Station, NJ, USA) or the membrane permeable antioxidant 4-hydroxy-TEMPO (tempol, Sigma-Aldrich, St Louis, MO, USA) in drinking solution beginning just after DOCA pellet implantation (n=5-6 per group), for 21 days (see on line supplemental data for details).
Additional experiments were performed to assess the influence of intermittent feeding the cholesterol-enriched diet on Uni ApoE-KO + DOCA-salt mice. Some mice received a normal diet (ND) and cholesterol-enriched diet on alternate days for 3 weeks (Uni ApoE-KO + DOCA-salt + 1HCD/1ND). In another series of experiments, mice were placed on ND for 3 days followed by one day on the cholesterol-enriched diet over a period of 3 weeks. Experiments were performed either one (Uni ApoE-KO + DOCA-salt + 1HCD/3ND 1st day), two (Uni ApoE-KO + DOCA-salt + 1HCD/3ND 2nd day) or four (Uni ApoE-KO + DOCA-salt + 1HCD/3ND 4th day) days after the last high cholesterol intake (n = 4-7 per group). A comparison of these groups allows for an assessment of the reversibility of the phenotypic changes induced by the HCD.
Blood Pressure Measurement
Blood pressure (BP) was measured in non-anesthetized mice by tail plethysmography using the Hatteras Instruments system (model SC-1000, Cary, NC). Mice were placed on a heated (40°C) platform and a cuff was placed around the tail and inflated for a period of 60 seconds to record systolic BP. The average of five successive measurements was used as the systolic BP for each animal. Animals were previously trained for four consecutive days before final measurements were taken.
Animal Preparation for Microscopy
Mice were anesthetized with IP ketamine (150 mg/kg) and xylazine (7.5 mg/kg) (~ 100 μL/mouse). The left femoral vein was cannulated for intravenous (IV) administration of 6G-rhodamine, labeled platelets and supplemental doses of anesthetics. Body temperature was maintained at 36°C during the experiment and monitored with a rectal temperature probe. After skull fixation, a circular skin incision was made, and a craniotomy was created 3 mm lateral and 2 mm posterior to the bregma. The exposed brain tissue was immersed in an artificial cerebrospinal fluid (17) and covered with a glass slide. Cerebral vessels were observed through the dura mater.
Intravital Videomicroscopy
The procedures used to monitor blood cell–vessel wall interactions in murine cerebral venules are described elsewhere in detail (17). A brief description of this method is available in the online data supplement.
Brain Water Content
Brain was removed, stripped of the dura mater and cerebellum, and divided into 2 hemispheres. Each hemisphere was placed into a 60°C oven for 3 days to achieve complete desiccation. Water content was determined from (wet weight–dry weight)/wet weight and expressed as percent.
Blood–Brain Barrier Dysfunction
BBB permeability was assessed using the Evans blue (EB) extravasation method (18). This procedure is summarized in the online data supplement.
Serum Cholesterol Levels
At the end of the experiments, blood was drawn from the tail vein, centrifuged, and the plasma was frozen for subsequent measurement of cholesterol levels, using a spectrophotometric assay kit (Stanbio Laboratory, Boerne, TX).
Statistical Analysis
All data were expressed as mean ± SE. Statistical difference between the different groups was determined by a one-way analysis of variance with the Tukey post hoc test. All analyses were performed using Prism 5 software (GraphPad Software, Inc.). Statistical significance was set at P<0.05.
RESULTS
Body weight, periepididymal fat pad weight, liquid and food intake, BP, and plasma cholesterol concentration responses to DOCA salt HTN, with or without placement on a HCD
While body weight was similar among all groups before surgery (data not shown), it was increased only in the HTN + HCD, compared to HTN + ND, 3 weeks after nephrectomy (Figure S1, panel A, on line supplemental data). Body weight did not differ statistically between all groups and the Uni group (Figure S1, panel A). However, periepididymal fat pad weight was reduced in HTN vs normotensive mice, and HCD did not modify this response (Figure S1, panel B). While food intake was reduced in HTN vs Uni mice, liquid consumption was increased (Figure S1, panels C & D). HCD partly reversed these changes in HTN mice. Food intake and liquid consumption were similar in normotensive mice fed HCD or ND (Figure S1, panels C & D).
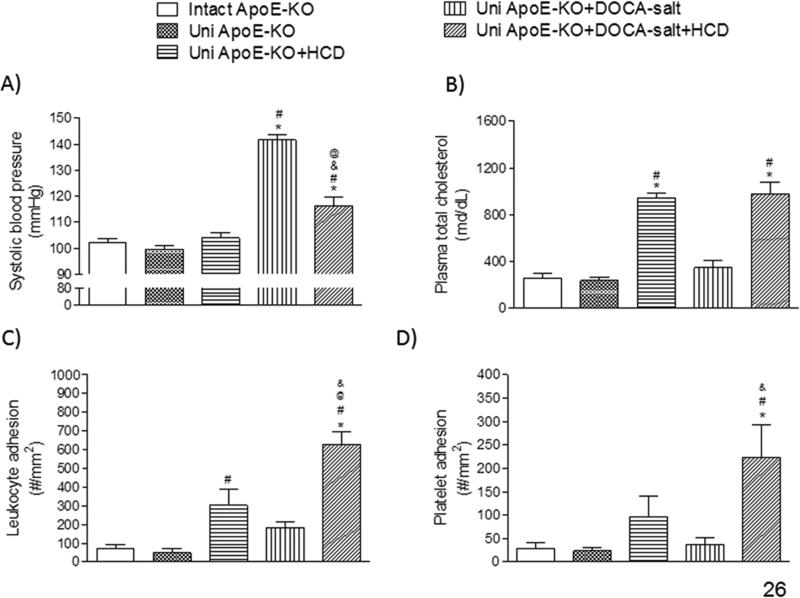
A 40% increase in BP was evidenced in Uni ApoE-KO + DOCA-salt mice, when compared to control groups (intact ApoE-KO or uni ApoE-KO) (Figure 1A). Placement on a HCD did not change the BP of Uni ApoE-KO mice (Figure 1A). However, BP was significantly reduced in Uni ApoE-KO DOCA-salt mice after HCD (Uni ApoE-KO + DOCA-salt + HCD) (Figure 1A). Plasma cholesterol concentration was increased 4-fold in ApoE mice placed on HCD, compared to their ND counterparts (Figure 1B). Most of the increase in plasma cholesterol resulted from an increase in HDL cholesterol (Figure S2, panel A). No change in plasma non-HDL cholesterol was observed in any group tested (Figure S2, panel B). HTN, per se, was not associated with a change plasma cholesterol concentration, and the magnitude of the increase in plasma cholesterol concentration in HCD fed mice was not affected by DOCA-salt HTN (Figure 1B).
Figure 1.
Systolic blood pressure (A), plasma cholesterol concentration (B), and the adhesion of leukocytes (C) and platelets (D) in cerebral venules, measured in the following groups of mice: intact ApoE-KO (n=6), uninephrectomized ApoE-KO (Uni ApoE-KO, n=6), Uni ApoE-KO fed (daily) a cholesterol-enriched diet (Uni ApoE-KO + HCD, n=8), Uni ApoE-KO with DOCA-salt treatment fed normal diet (Uni ApoE-KO + DOCA-salt, n=8) or (daily) cholesterol-enriched diet (Uni ApoE-KO + DOCA-salt + HCD, n=7). * P<0.05 vs. intact ApoE-KO; # P<0.05 vs. Uni ApoE-KO; @ P<0.05 vs. Uni ApoE-KO + HCD; & P<0.05 vs. ApoE-KO + DOCA-salt.
Leukocyte and platelet adhesion responses in cerebral venules of DOCA-salt hypertensive mice ± HCD
Placement of both Uni ApoE-KO and Uni ApoE-KO + DOCA-salt mice on HCD enhanced the recruitment of adherent leukocytes (Figure 1C) and platelets (Figure 1D) in cerebral venules, compared to controls (Intact ApoE-KO, Uni ApoE-KO). When placed on ND, Uni ApoE-KO + DOCA-salt mice did not exhibit an increased blood cell recruitment above controls. The most profound changes in blood cell recruitment were noted in Uni ApoE-KO + DOCA-salt mice placed on HCD (Figures 1C & 1D).
Changes in brain water content and blood-brain barrier permeability
Figure S3 (on line supplemental data) summarizes the changes in brain water content (panel A) and EB extravasation (panel B), a measure of BBB permeability, in the different experimental groups. The results indicate that neither DOCA-salt HTN, HCh nor the combination of the two risk factors alter brain water content and BBB permeability.
Role of AT1 receptors and reactive oxygen species in the BP and cerebral microvascular responses to DOCA-salt HTN and/or a HCD
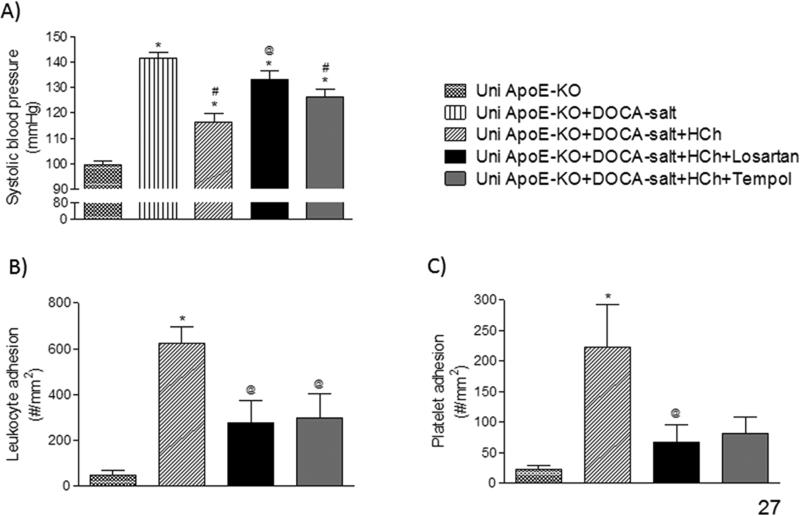
Losartan, but not tempol, prevented the reduction of BP observed in mice placed on HCD. However, both losartan and tempol were effective in blunting the exacerbated recruitment of leukocytes and platelets in cerebral venules (Figures 2B & 2C).
Figure 2.
Effects of treatment with either losartan or tempol on the responses of blood pressure (A) and the adhesion of leukocytes (B) and platelets (C) in cerebral venules of mice in the following groups: uninephrectomized ApoE-KO (Uni ApoE-KO, n=6), Uni ApoE-KO with DOCA-salt treatment (Uni ApoE-KO + DOCA-salt, n=7), Uni ApoE-KO DOCA-salt mice fed (daily) a cholesterol-enriched diet (Uni ApoE-KO + DOCA-salt + HCD, n=7), and treated or not with losartan (Uni ApoE-KO + DOCA-salt + HCD + losartan, n=4) or tempol (Uni ApoE-KO + DOCA-salt + HCD + tempol, n=6). * P<0.05 vs. Uni ApoE-KO; # P<0.05 vs. Uni ApoE-KO + DOCA-salt; @ P<0.05 vs. Uni ApoE-KO + DOCA-salt + HCD.
Responses to intermittent feeding of HCD in ApoE-KO mice with DOCA-salt HTN
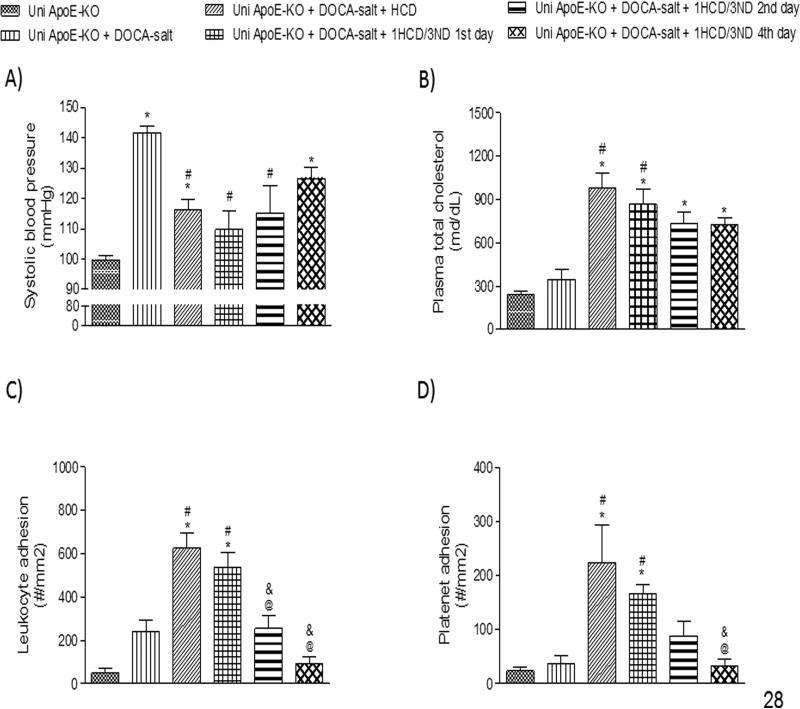
Uni ApoE-KO + DOCA-salt mice placed on ND for 3 days followed by one day on HCD over a period of 3 weeks resulted in changes in BP, plasma cholesterol concentration, and the recruitment of adherent leukocytes and platelets that are comparable (and not significant from) the responses noted with daily HCD over the same time period (Figure 3). Reversal of the phenotypic changes in these variables was noted 2 to 4 days after removing the mice from the cholesterol-enriched diet. Complete reversal of the blood cell adhesion responses was noted on the 4th day of withdrawal from the HCD, i.e., the adhesion responses did not differ from the responses observed in Uni ApoE-KO mice on normal chow. However, BP was significantly reduced compared to Uni ApoE-KO + DOCA-salt, except on the 4th day of withdrawal from HCD, and plasma cholesterol levels remained elevated above the levels detected in Uni ApoEKO mice in all HCD fed groups.
Figure 3.
Effects of intermittent feeding with (and withdrawal from) a cholesterol-enriched diet on the responses of systolic blood pressure (A), plasma cholesterol concentration (B), and the adhesion of leukocytes (C), and platelets (D) in cerebral venules in the following experimental groups: uninephrectomized ApoE-KO (Uni ApoE-KO, n=6), Uni ApoE-KO with DOCA-salt treatment (Uni ApoE-KO + DOCA-salt, n=8), Uni ApoE-KO DOCA-salt with daily intake of a high cholesterol diet (Uni ApoE-KO + DOCA-salt + HCD, n=7), Uni ApoE-KO DOCA-salt mice fed a cholesterol-enriched diet every fourth day, with data collected 1-day after the last high cholesterol intake (Uni ApoE-KO + DOCA-salt + 1HCD/3ND 1st day, n=7), Uni ApoE-KO DOCA-salt fed a cholesterol-enriched diet every fourth day, with data collected 2-days after the last high cholesterol intake (Uni ApoE-KO + DOCA-salt + 1HCD/3ND 2nd day, n=4), and Uni ApoE-KO DOCA-salt fed a cholesterol-enriched diet every fourth day, with data collected 4-days after the last high cholesterol intake (Uni ApoE-KO + DOCA-salt + 1HCD/3ND 4th day, n=5). * P<0.05 vs. Uni ApoE-KO; # P<0.05 vs. Uni ApoE-KO + DOCA-salt; @ P<0.05 vs. Uni ApoE-KO + DOCA-salt + HCD; & P<0.05 vs. Uni ApoE-KO + DOCA-salt + 1HCD/3ND 1st day.
DISCUSSION
While it is well known that CVD is more commonly manifested in individuals with two or more risk factors, relatively few animal studies have addressed the influence of risk factor combinations on the development of vascular dysfunction and tissue injury. We have recently reported that modest increases in plasma cholesterol concentration (from 70 to 110 mg/dL) dampen the proinflammatory and prothrombogenic phenotype that is assumed by the cerebral microvasculature in mice with either angiotensin II- or DOCA-salt induced HTN (16). In this study, we addressed whether the protective effect of HCh in hypertensive mice is also evidenced in the presence of a larger increase in plasma cholesterol concentration (up to 940 mg/dL), achieved by placing ApoE-KO mice on a HCD. Our findings reveal that, in presence of a large increase in plasma cholesterol concentration, the combination of HCh and HTN results in greatly exacerbated inflammatory and thrombogenic responses in the cerebral microvasculature. We also demonstrate that intermittent HCD, i.e., every 4th day, results in a similar level of cerebral microvascular dysfunction as produced by a daily HCD. However, the exaggerated recruitment of leukocytes and platelets noted in these animals subsides within 4 days after returning to a ND. In addition, we have obtained evidence to implicate the AT1r, and reactive oxygen species (ROS) as mediators of the intense proinflammatory and prothrombogenic responses elicited by the combination of HTN and HCh.
HTN and HCh have been previously shown to independently induce a proinflammatory and prothrombogenic phenotype in the cerebral microvasculature (8-11). In this study, we observed that plasma cholesterol levels, mostly HDL cholesterol, increased nearly 4-fold when ApoE-KO mice were placed on HCD (compared to a ND) and this response was accompanied by an increased recruitment of adherent leukocytes and platelets in cerebral venules. Increases in HDL cholesterol following HCD has been previously described in ApoE-KO mice (19-20). However, while plasma cholesterol was increased to a similar extent in both normotensive and hypertensive ApoE-KO mice after placement on HCD, many more leukocytes and platelets were recruited in the microvasculature in the presence of both risk factors. A similar exacerbation of vascular dysfunction has also been described in reports that address the impaired vasomotor function that results from the combination of HTN and HCh, compared to either risk factor alone (15, 21).
The mechanism(s) that underlie the intense leukocyte and platelet recruitment in cerebral microvessels in mice with HTN and severe HCh appears to involve activation of the AT1r and the production of ROS. This assertion is based on our observation that both losartan and tempol were effective in reducing the blood cell recruitment responses elicited by the risk factor combination. Both AT1r and ROS have been previously implicated in the proinflammatory and prothrombogenic responses observed in cerebral microvessels of different animal models of HTN (9-11) and HCh (8). AT1r and ROS have also been implicated in the accelerated atherogenesis that results from the combination of HTN and HCh (22-25) and the vasomotor dysfunction associated with this combination of risk factors (15). The comparable effectiveness of losartan and tempol in blunting the blood cell recruitment elicited by HTN + HCh is consistent with the view that ROS is a critical component of angiotensin II signaling via AT1r (26). Hence, our results suggest that the combination of HTN and severe HCh leads to AT1r activation, the subsequent generation of ROS, and the consequent induction of a proinflammatory and prothrombogenic phenotype in the cerebral microvasculature. However, our observation that neither losartan nor tempol treatment afforded complete protection against the blood cell recruitment response to HTN suggests the involvement of other mechanisms and mediators.
An interesting observation in the present study was the more rapid disappearance of the blood cell adhesion response than the fall in plasma cholesterol level following removal of cholesterol from the diet (Figure 3). This could indicate that the vascular responses (eg, adhesion molecule expression) elicited by HCh (or the mediators released by it) show an off-response that does not parallel the decline of plasma cholesterol concentration. It could also indicate that a threshold level of elevated cholesterol must be achieved to manifest these changes and once the cholesterol level falls below the threshold value then the stimulus for adhesion is dissipated. Finally, it may simply reflect that some other variable related to cholesterol (eg, LDL and or HDL concentrations) is driving the response rather than total cholesterol concentration.
Despite the intense recruitment of leukocytes and platelets that is elicited in normotensive and hypertensive mice by HCD, no alteration of BBB function was detected during either HTN or HCh. We have previously reported that hypercholesterolemic C57Bl/6J mice do not exhibit altered BBB function (16) and now demonstrate the same outcome in ApoE-KO mice. However, cholesterol-induced BBB breakdown may depend on the animal model, composition of the HCh diet, and/or duration of the HCh diet. Indeed, BBB disruption has been detected in New Zealand rabbits fed a HCD (12), in ApoE-KO mice fed a Western diet containing more fat and less cholesterol (27), and in mice placed on HCD for 12 weeks (28). A diet rich in saturated fats has been shown to greatly alter BBB integrity (31). Regarding hypertensive animals, some investigators have described BBB dysfunction in response to HTN, while others have not. Work from our lab (10) and by others (29) has revealed a small but significant leakage of albumin in cerebral microvessels of mice with angiotensin II-induced HTN, compared to normotensive controls. On the other hand, no damage to the BBB was noted in the following hypertensive models: 1) two-kidney one clip or Dahl salt-sensitive rats fed a high salt diet (30-31), and 2) C57Bl mice (16) or Wistar Kyoto rats (31) with DOCA-salt HTN. The reason(s) why an altered BBB integrity is observed in some models of HTN but not in others remain unclear.
Arterial BP is a tightly controlled variable that is regulated by different organs (e.g., brain and kidney) and mediators (e.g., angiotensin II and aldosterone) (32). Dysregulation of these mechanisms can lead to a chronic HTN. In this study, HTN was induced using the DOCA-salt model, a widely used experimental model that results in a high blood concentration of a mineralocorticoid hormone (DOCA), low blood renin levels, and a chronically elevated BP (33). An interesting observation in our study was the significant decline in the DOCA-salt induced elevation in BP when the ApoE-KO mice were placed on a HCD. A reduction of BP caused by placement on a high fat diet has also been reported by others (34). While the precise mechanism underlying the reduction in BP remains unclear, our findings of a reversal of the BP lowering effect of HCh in mice receiving losartan suggests that the AT1r is activated in the presence of cholesterol and releases vasodilators, such as TNF-α. This possibility is consistent with previous reports that demonstrate the ability of TNF-α to acutely lower BP (35-36). Futhermore, TNF-α may be released as consequence of AT1r activation. Indeed, the inhibitory effect of AT1r blockers on TNF-α actions suggests that AT1r activation and TNF release are interdependent processes (37).
In conclusion, the results of this study demonstrate that the combination of HTN and large increases in plasma cholesterol concentration elicits a severe inflammatory and thrombogenic phenotype in the cerebral microvasculature. This response is evident with either persistent or intermittent feeding with HCD, but the response is reversible within 4-days after resuming ND. This combination of risk factors appears to mediate its deleterious effects via a mechanism that involves AT1r activation and ROS generation.
Supplementary Material
PERSPECTIVES.
Targeting AT1r activation and generation of ROS may prove beneficial in reducing the risk of CVD that accompany HTN and HCh.
NOVELTY AND SIGNIFICANCE.
What is new?
Combination of HTN and large increases in circulating cholesterol results in a severe inflammatory and thrombogenic phenotype in the cerebral microvasculature of mice;
These cerebral microvascular responses are reversible;
Intermittent HCD in HTN mice elicits a response similar to daily HCD;
AT1r activation and ROS production underlie these phenotypic changes.
What is relevant?
Risk factor combination yields synergistic deleterious effects on cerebral microvessels.
AT1r blockers and antioxidants may reduce the deleterious impact of the combination of HTN and high cholesterol levels.
Summary.
These findings indicate that this risk factor combination results in a severe, but reversible, inflammatory and thrombogenic phenotype in the cerebral microvasculature that can be mimicked by intermittent high cholesterol intake, and that targeting AT1 receptor activation and the generation of reactive oxygen species may prove beneficial in reducing the risk of cardiovascular diseases that accompany hypertension and hypercholesterolemia.
ACKNOWLEDGMENTS
None.
SOURCE OF FUNDING
National Heart Lung and Blood Institute (HL26441-32).
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
Authors have no conflict of interest to declare.
REFERENCES
- 1.Laslett LJ, Alagona P, Jr, Clark BA, 3rd, Drozda JP, Jr, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1–S49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D. Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv J, Neal B, Ehteshami P, Ninomiya T, Woodward M, Rodgers A, Wang H, MacMahon S, Turnbull F, Hillis G, Chalmers J, Perkovic V. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001293. doi: 10.1371/journal.pmed.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moura FA, Freitas WM, Sposito AC. Emergent cardiovascular risk factors in the very elderly. Expert Rev Cardiovasc Ther. 2012;10:1221–1225. doi: 10.1586/erc.12.98. [DOI] [PubMed] [Google Scholar]
- 5.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rydén L, Mellbin L. Glucose perturbations and cardiovascular risk: challenges and opportunities. Diab Vasc Dis Res. 2012;9:170–176. doi: 10.1177/1479164112451581. [DOI] [PubMed] [Google Scholar]
- 7.Shinkai H. Cholesteryl ester transfer-protein modulator and inhibitors and their potential for the treatment of cardiovascular diseases. Vasc Health Risk Manag. 2012;8:323–331. doi: 10.2147/VHRM.S25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and P-selectin. Circ Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues SF, Granger DN. Cerebral microvascular inflammation in DOCA salt-induced hypertension: role of angiotensin II and mitochondrial superoxide. J Cereb Blood Flow Metab. 2012;32:368–375. doi: 10.1038/jcbfm.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin II-induced hypertension. Microcirculation. 2010;17:641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010;171:852–858. doi: 10.1016/j.neuroscience.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Guo M, Su J, Lu B, Ma D, Zhang R, Yang L, Wang Q, Ma Y, Fan Y. Simvastatin blocks blood-brain barrier disruptions induced by elevated cholesterol both in vivo and in vitro. Int J Alzheimers Dis. 2012;2012:109324. doi: 10.1155/2012/109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon DB, Nogueira A. Increased vascular reactivity in experimental hypertension. Circ Res. 1962;10:269–273. doi: 10.1161/01.res.10.3.269. [DOI] [PubMed] [Google Scholar]
- 14.Heric E, Tackett RL. Altered vascular reactivity in the rabbit during hypercholesterolemia. Pharmacology. 1985;31:72–81. doi: 10.1159/000138101. [DOI] [PubMed] [Google Scholar]
- 15.Kurtel H, Rodrigues SF, Yilmaz CE, Yildirim A, Granger DN. Impaired vasomotor function induced by the combination of hypertension and hypercholesterolemia. J Am Soc Hypertens. 2013;7:14–23. doi: 10.1016/j.jash.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues SF, Vital SA, Granger DN. Mild hypercholesterolemia blunts the proinflammatory and prothrombotic effects of hypertension on the cerebral microcirculation. J Cereb Blood Flow Metab. 2013;33:483–489. doi: 10.1038/jcbfm.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, Granger DN. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- 18.Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 19.Hayek T, Ito Y, Azrolan N, Verdery RB, Aalto-Setälä K, Walsh A, Breslow JL. Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a new animal model and mechanistic studies in human Apo A-I transgenic and control mice. J Clin Invest. 1993;91:1665–1671. doi: 10.1172/JCI116375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian J, Pei H, James JC, Li Y, Matsumoto AH, Helm GA, Shi W. Circulating adhesion molecules in apoE-deficient mouse strains with different atherosclerosis susceptibility. Biochem Biophys Res Commun. 2005;329:1102–1107. doi: 10.1016/j.bbrc.2005.02.090. [DOI] [PubMed] [Google Scholar]
- 21.Arruda RM, Peotta VA, Meyrelles SS, Vasquez EC. Evaluation of vascular function in apolipoprotein E knockout mice with angiotensin-dependent renovascular hypertension. Hypertension. 2005;46:932–936. doi: 10.1161/01.HYP.0000182154.61862.52. [DOI] [PubMed] [Google Scholar]
- 22.Jin SX, Shen LH, Nie P, Yuan W, Hu LH, Li DD, Chen XJ, Zhang XK, He B. Endogenous renovascular hypertension combined with low shear stress induces plaque rupture in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2372–2379. doi: 10.1161/ATVBAHA.111.236158. [DOI] [PubMed] [Google Scholar]
- 23.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrin M, Alonso F, Aubert JF, Bouzourene K, Braunersreuther V, Mach F, Haefliger JA, Hayoz D, Berthelot A, Nussberger J, Laurant P, Mazzolai L. Swimming prevents vulnerable atherosclerotic plaque development in hypertensive 2-kidney, 1-clip mice by modulating angiotensin II type 1 receptor expression independently from hemodynamic changes. Hypertension. 2009;53:782–789. doi: 10.1161/HYPERTENSIONAHA.108.128165. [DOI] [PubMed] [Google Scholar]
- 25.Weiss D, Taylor WR. Deoxycorticosterone acetate salt hypertension in apolipoprotein E-/-mice results in accelerated atherosclerosis: the role of angiotensin II. Hypertension. 2008;51:218–224. doi: 10.1161/HYPERTENSIONAHA.107.095885. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaugh EG, Savalia KK, Zimmerman MC. Antioxidant-Based Therapies for Angiotensin II-Associated Cardiovascular Diseases. Am J Physiol Regul Integr Comp Physiol. 2013;304:R917–R928. doi: 10.1152/ajpregu.00395.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Methia N, André P, Hafezi-Moghadam A, Economopoulos M, Thomas KL, Wagner DD. ApoE deficiency compromises the blood brain barrier especially after injury. Mol Med. 2001;7:810–815. [PMC free article] [PubMed] [Google Scholar]
- 28.Takechi R, Galloway S, Pallebage-Gamarallage MM, Lam V, Dhaliwal SS, Mamo JC. Probucol prevents blood-brain barrier dysfunction in wild-type mice induced by saturated fat or cholesterol feeding. Clin Exp Pharmacol Physiol. 2013;40:45–52. doi: 10.1111/1440-1681.12032. [DOI] [PubMed] [Google Scholar]
- 29.Pelisch N, Hosomi N, Mori H, Masaki T, Nishiyama A. RAS inhibition attenuates cognitive impairment by reducing blood- brain barrier permeability in hypertensive subjects. Curr Hypertens Rev. 2013;9:93–98. doi: 10.2174/15734021113099990003. [DOI] [PubMed] [Google Scholar]
- 30.Sharma HS, Muresanu DF, Patnaik R, Sharma A. Exacerbation of brain pathology after partial restraint in hypertensive rats following SiO2 nanoparticles exposure at high ambient temperature. Mol Neurobiol. 2013;48:368–379. doi: 10.1007/s12035-013-8502-y. [DOI] [PubMed] [Google Scholar]
- 31.Werber AH, Fitch-Burke MC. Effect of chronic hypertension on acute hypertensive disruption of the blood-brain barrier in rats. Hypertension. 1988;12:549–555. doi: 10.1161/01.hyp.12.6.549. [DOI] [PubMed] [Google Scholar]
- 32.Patel BM, Mehta AA. Aldosterone and angiotensin: Role in diabetes and cardiovascular diseases. Eur J Pharmacol. 2012;697:1–12. doi: 10.1016/j.ejphar.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Haack D, Möhring J, Möhring B, Petri M, Hackenthal E. Comparative study on development of corticosterone and DOCA hypertension in rats. Am J Physiol. 1977;233:F403–F411. doi: 10.1152/ajprenal.1977.233.5.F403. [DOI] [PubMed] [Google Scholar]
- 34.Erdos B, Kirichenko N, Whidden M, Basgut B, Woods M, Cudykier I, Tawil R, Scarpace PJ, Tumer N. Effect of age on high-fat diet-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H164–H172. doi: 10.1152/ajpheart.01289.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schäfer T, Sperling J, Kollmar O, Richter S, Schilling MK, Menger MD, Lindemann W. Early effect of hepatic artery TNF-alpha infusion on systemic hemodynamics and inflammation: a dose-response study in pigs. Int J Colorectal Dis. 2010;25:523–532. doi: 10.1007/s00384-009-0827-7. [DOI] [PubMed] [Google Scholar]
- 36.Wilcox PG, Wakai Y, Walley KR, Cooper DJ, Road J. Tumor necrosis factor alpha decreases in vivo diaphragm contractility in dogs. Am J Respir Crit Care Med. 1994;150:1368–1373. doi: 10.1164/ajrccm.150.5.7952566. [DOI] [PubMed] [Google Scholar]
- 37.Kataoka H, Murakami R, Numaguchi Y, Okumura K, Murohara T. Angiotensin II type 1 receptor blockers prevent tumor necrosis factor-alpha-mediated endothelial nitric oxide synthase reduction and superoxide production in human umbilical vein endothelial cells. Eur J Pharmacol. 2010;636:36–41. doi: 10.1016/j.ejphar.2010.03.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.