Abstract
Background
Noneosinophilic asthma is common across asthma severities. However, in patients with moderate-to-severe disease, the absence of sputum eosinophilia cannot distinguish between asthmatic subjects with eosinophilic inflammation controlled by corticosteroids versus those in whom eosinophilic inflammation is not a component of the disease.
Objectives
We sought to develop a method to quantify eosinophil proteins in airway macrophages as a novel biomarker of eosinophilic airway inflammation.
Methods
Eosinophil proteins in airway macrophages were assessed by means of flow cytometry, immunofluorescence, and cytoplasmic hue change after ingestion of apoptotic eosinophils. Airway macrophage median percentage of red-hued area in stained sputum cytospin preparations was assessed by means of image analysis from (1) subjects with mild-to-severe asthma, subjects with nonasthmatic eosinophilic bronchitis, and healthy control subjects; (2) subjects with eosinophilic severe asthma after treatment with prednisolone; and (3) subject with noneosinophilic asthma before corticosteroid withdrawal.
Results
Eosinophil proteins were detected in airway macrophages, and cytoplasmic red hue increased after ingestion of apoptotic eosinophils. Airway macrophage percentage red-hued area was increased in subjects with moderate-to-severe asthma compared with that seen in subjects with mild asthma and healthy control subjects, was similar in those with or without a sputum eosinophilia, and was increased after corticosteroid therapy. In asthmatic subjects without sputum eosinophilia, the airway macrophage percentage red-hued area was increased in subjects who did versus those who did not have sputum eosinophilia after corticosteroid withdrawal.
Conclusions
Eosinophil proteins can be reliably measured in airway macrophages. In combination with sputum eosinophilia, the macrophage eosinophil protein content might further define the asthma phenotype and provide an additional tool to direct therapy.
Keywords: Asthma, macrophage, eosinophil, computer-assisted image analysis, induced sputum
There is increasing recognition that asthma is a heterogeneous condition, particularly in those with severe disease.1 The currently available biomarkers are able to detect recent but not ongoing inflammation, especially in treated subjects. Sputum induction has allowed the analysis of large numbers of asthmatic subjects and has led to the identification of different inflammatory phenotypes defined by their granulocytic composition, namely eosinophilic, neutrophilic, mixed granulocytic, and paucigranulocytic.2
Eosinophilic inflammation is considered a hallmark of asthma, but only about 50% of asthmatic subjects have sputum evidence of eosinophilic inflammation.3 The eosinophilic phenotype is associated with a good response to corticosteroids4 and anti–IL-5 mAb therapy.5,6 Directing corticosteroid therapy to normalize the sputum eosinophil count in subjects with severe asthma has also led to a reduction in exacerbations without an increase in overall corticosteroid therapy.7,8 The success of this approach was due to the appropriate uptitration and downtitration of therapy. However, after corticosteroid withdrawal in subjects with severe asthma, there is often an emergence of eosinophilic inflammation.9 The natural variation in disease, corticosteroid treatment, and resolution can alter the sputum eosinophil differential count.10 Therefore a normal eosinophil count might reflect controlled eosinophilic inflammation as a consequence of corticosteroid treatment or might identify a group of asthmatic subjects in which the eosinophil is not an important factor in the disease’s pathogenesis. Therefore there is a need for noninvasive biomarkers to estimate the eosinophilic load within the airway.
The burden of eosinophilic airway inflammation within a subject is a constant balance between eosinophil influx into the airway wall and clearance by airway macrophages.11 Although eosinophil products are demonstrated in macrophages,11,12 to date, the eosinophil load within the macrophage is not quantified or used to assess past eosinophilic inflammation. This might provide additional insight into the kinetics of eosinophilic inflammation and identify patients with high macrophage eosinophil content that hitherto have been labeled as noneosinophilic.
We hypothesized that airway macrophage eosinophil protein content is increased in subjects with severe asthma independent of sputum eosinophil count and that the combination of a normal sputum eosinophil count and low airway macrophage eosinophil protein content identifies a true noneosinophilic phenotype that predicts success of weaning from corticosteroids. To test our hypothesis, we aimed to (1) develop a method to quantify eosinophil proteins in airway macrophages as a novel biomarker of eosinophilic airway inflammation and (2) compare airway macrophage eosinophil content across asthma severities, in subjects with nonasthmatic eosinophilic bronchitis (NAEB), and in healthy control subject subjects, as well as its response to corticosteroid therapy and withdrawal.
METHODS
Subjects
Subjects with asthma (n = 72) and NAEB (n = 15) and healthy control subjects (n = 10) were recruited from patients attending Glenfield Hospital, Leicester, United Kingdom; hospital staff; and respondents to local advertising. Asthma was defined and classified according to the Global Initiative for Asthma (GINA) guidelines,13 and NAEB was defined as per the American College of Chest Physicians’ guidelines.14 Subjects underwent standardized clinical assessment, including spirometry, skin prick testing to common aeroallergens, methacholine challenge testing,15 and sputum induction.16 All of the subjects had participated in previous studies.5,7,17,18 The study was approved by the Leicestershire Research Committee, and subjects provided written informed consent.
Romanowsky-stained sputum cytospin preparations were obtained from (1) subjects with mild (GINA class I), moderate (GINA class II/III), and severe (GINA class IV/V) asthma, as well as subjects with NAEB and healthy control subjects; (2) subjects with sputum eosinophilia4 and severe asthma before and after prednisolone, 30 mg daily, for 2 weeks; and (3) subjects without baseline sputum eosinophilia before corticosteroid withdrawal in the sputum arm of a randomized, controlled 1-year study of the use of sputum analysis to direct corticosteroid therapy.7 These subjects were dichotomized into those for whom corticosteroids were weaned to maintenance of 0 to 400 μg of beclomethasone equivalent per day without the emergence of sputum eosinophilia (ie, successful weaning) versus those with sputum eosinophilia on corticosteroid withdrawal (ie, unsuccessful weaning).
Eosinophil proteins in macrophages after ingestion of apoptotic eosinophils
Macrophages from lung resection and peripheral blood eosinophils from healthy volunteers were obtained as previously described.19,20 Apoptosis in eosinophils was induced by aging in culture.21 For additional information, see the Methods section in this article’s supplementary material. Lung macrophages or THP-1 cells (a macrophage cell line) were cocultured with apoptotic eosinophils at a ratio of 10:1 to 1:10 for 4 hours.22 Eosinophil cationic protein (ECP) and eosinophil peroxidase (EPO) expression in macrophages and THP-1 cells was analyzed by means of immunofluorescence and intracellular flow cytometry with mAbs for ECP or EPO (Diagnostics Development, Uppsala, Sweden) indirectly labeled with allophycocyanin (APC) and CD68–fluorescein isothiocyanate (FITC) and appropriate isotype controls. Cytospin preparations were obtained and stained by using the Romanowsky method.
Eosinophil proteins in sputum macrophages
Sputum macrophages from subjects with asthma were analyzed for the presence of eosinophil proteins by means of immunolabeling with mAbs for ECP and EPO and indirectly labeling with APC and CD68-FITC and appropriate isotype controls. For additional information, see the Methods section in this article’s supplementary material.
Imaging and color-image analysis of cytospin preparations
From the cytospin preparations, 100 macrophages were imaged by using ×40 objective at ultrahigh resolution (4080 × 3072; Olympus DP70 digital camera; AnalySIS, Soft Imaging System GmbH, Munster, Germany). Image J software (ImageJ 1.40g/java 1.6.0_05, National Institutes of Health Image) was used to analyze the percentage area of cytoplasm with a red hue by means of thresholding (see Fig E1). Median percentage area of cytoplasm with a red hue (primary measure) in 100 macrophages per subject was used as a measure of eosinophil protein content. Imaging and image analysis was performed by a person blinded to clinical characteristics. Intraobserver and interobserver variability was assessed in 50 airway macrophages. Inclusions of apoptotic eosinophils were frequently observed in the macrophages (see Fig E2).
Statistics
Statistical analysis was performed with GraphPad Prism 5 software (GraphPad Software, Inc, San Diego, Calif). Subjects’ characteristics are presented as means (SEs) or medians (ranges or interquartile ranges). Interobserver and intraobserver variation was assessed by using intraclass correlation. Comparisons across groups were analyzed with the Kruskal-Wallis test for nonparametric data, with between-group comparisons using the Mann-Whitney test for unpaired data and the Wilcoxon matched pairs test for paired data as appropriate and proportions using the Fisher exact test. Differences were considered significant at a P value of less than .05.
RESULTS
Eosinophil proteins in macrophages after ingestion of apoptotic eosinophils
Example flow cytometric histograms showing that CD68+ macrophages expressed ECP and EPO after apoptotic eosinophil ingestion are as shown in Fig 1, A. The geometric mean fluorescence intensity of macrophages after ingesting apoptotic eosinophils and stained with EPO antibodies was significantly higher than that for macrophages alone (887 [95% CI, 356-2,209] vs 2 [95% CI, 0-27] P = .016; Fig 1, B). This was confirmed in THP-1 cells fed with eosinophils. The geometric mean fluorescence intensity increased with the increasing proportion of eosinophils to macrophages stained with ECP and EPO (P < .001; Fig 1, C).
FIG 1.
ECP and EPO expression within macrophages and THP-1 cells: A macrophages gated by size, granularity, and CD68 expression (left panel) and protein expression (middle and right panels). Representative histograms of macrophages cultured in the absence (middle panel) or presence (right panel) of apoptotic eosinophils are shown. ECP (blue), EPO (green), and isotype (red) values are shown. B and C, Geometric mean fluorescence above control value of macrophages (Fig 1, B) or THP-1 cells (Fig 1, C; n = 3). *P < .05 compared with control.
Similarly, the expression of ECP and EPO by macrophages after apoptotic eosinophil ingestion was confirmed by means of immunofluorescence. Example immunofluorescent photomicrographs illustrating macrophages expressing ECP and EPO after ingestion of eosinophils are as shown in Fig 2, A. The percentage of positively stained lung macrophages or THP-1 cells was significantly higher after phagocytosis of eosinophils and related to the ratio of eosinophils to macrophages (Fig 2, B and C).
FIG 2.
A, Representative immunofluorescence staining of macrophages cultured alone (i) or with apoptotic eosinophils (ii and iii). Macrophages were stained with CD68-FITC (green) and ECP-APC (red; ii) or EPO-APC (red; iii) and 4′,6-diamidino-2-phenylindole (blue). B and C, Percentage of APC expressing cells above control values for macrophages (Fig 2, B) and THP-1 cells (Fig 2, C). *P < .05 compared with control. D, THP cells cultured alone (i) show blue cytoplasm, and THP cells cultured with apoptotic eosinophils (ii) show color change in Romanowsky stained cytospin. E, Representative immunofluorescence staining: i, isotype control macrophage; ii, macrophage; iii, eosinophil. EPO-APC (red), CD68-FITC (green), and 4′,6-diamidino-2-phenylindole (blue) staining in sputum cytospin preparation is shown.
THP-1 cells had red granules and apoptotic eosinophils in them after phagocytizing apoptotic eosinophils, changing the macrophage cytoplasm color to red-purple (Fig 2, D).
Airway macrophage eosinophil protein staining in sputum cytospin preparations
The presence of eosinophil proteins (ECP and EPO) in sputum macrophages was confirmed by means of immunofluorescence. Example immunofluorescent photomicrographs are shown in Fig 2, E.
The intraclass correlation coefficient of intraobserver and interobserver variation for the image analysis method to calculate the median percentage of red-purple hue per airway macrophage was 0.99 (95% CI, 0.99-0.99) and 0.97 (95% CI, 0.96-0.98), respectively.
Cross-sectional assessment of subjects with asthma, subjects with NAEB, and healthy control subjects
The clinical characteristics of subjects are as shown in Table I. Examples of sputum cytospin preparations showing the differences in the macrophage color from healthy control subjects, subjects with mild asthma, subjects with NAEB, and subjects with severe asthma with and without sputum eosinophilia are shown (Fig 3, A-E). The median airway macrophage percentage red-hued area was significantly higher in subjects with severe asthma compared with that seen in healthy control subjects (P < .0001) and subjects with mild asthma (P = .002), trends toward significance when compared with that seen in subjects with moderate asthma (P = .065), but was similar to that seen in subjects with NAEB (P = .9; Kruskal-Wallis P < .0001; Fig 3, F, and Table I). The subjects in GINA class IV and V appear to be divided into 2 groups (Fig 3, F): one with median airway macrophage red-hued percentage area of less than 50 (n = 29) and one with median airway macrophage red-hued percentage area of greater than 50 (n = 17). A further comparison of these 2 groups did not show any significant differences in age, sex, FEV1, and eosinophil differential count. There was no significant difference in the median airway macrophage percentage red-hued area in those asthmatic subjects with (n = 29; median, 17.5 [interquartile range, 7.3-70.3]) versus those without (n = 43; median, 20.4 [interquartile range, 4.6-60.2] sputum eosinophilia (P = .39, examples are shown in Fig 3, D and E). The subjects with eosinophilic and noneosinophilic asthma are shown in Fig 3, F, as solid and open symbols, respectively.
TABLE I.
Demographic characteristics, induced sputum differential count, median percentage area of red hue/airway macrophage for the healthy, eosinophilic bronchitis, and asthma groups
| Healthy (n = 10) |
NAEB (n = 15) |
GINA class I (n = 8) |
GINA class II-III (n = 18) |
GINA class IV-V (n = 46) |
|
|---|---|---|---|---|---|
| Age (y), mean (SE) | 32 (5) | 48 (3) | 46 (7) | 55 (3) | 50 (2) |
| Male sex, no. (%) | 5 (50) | 8 (53) | 4 (50) | 10 (56) | 24 (52) |
| FEV1 (% predicted), mean (SE) | 106 (6) | 100 (3) | 94 (4) | 87 (5) | 78 (4) |
| Induced sputum (%), median (range) | |||||
| Eosinophils | 0.4 (0-2.5) | 8.3 (3.3-68) | 0.9 (0-33.5) | 1.1 (0.1-45.3) | 1.5 (0-90.5) |
| Neutrophils | 40.7 (8.3-66.8) | 47.3 (8.75-82.7) | 20 (0.4-77.2) | 60.8 (5.0-95.0) | 54.4 (2-93.5) |
| Macrophages | 51.4 (28.8-90) | 27 (06-84) | 76 (12.4-90.6) | 26.5 (3.5-78.5) | 25.9 (5.2-78.50) |
| Lymphocytes | 1.2 (0-9.8) | 0.5 (0-2.2) | 0.2 (0-0.5) | 0.8 (0-2.0) | 0.8 (0-4.5) |
| Percentage area of red hue/airway macrophage, median (IQR) | 2.1 (1.9-3.8) | 36.3 (14.3-40.7) | 2.0 (0.9-9.1) | 10.7 (3.0-38.7) | 28.0 (11.8-68.0) |
IQR, Interquartile range.
FIG 3.
Representative images of sputum macrophages: A, healthy control subjects; B, subjects with NAEB; C, subjects with GINA class I asthma; D, subjects with severe noneosinophilic asthma; E, subjects with severe eosinophilic asthma. F, Scatter plot showing comparison of median percentage area of red-purple hue in sputum macrophages in the healthy, NAEB (EB), and GINA class I, GINA class II and III, and GINA class IV and V asthma groups. Solid circles represent a sputum eosinophil count of greater than 1.9, and open circles represent a sputum eosinophil count of less than 1.9.
Response to corticosteroids in subjects with severe eosinophilic asthma
Clinical characteristics before and after prednisolone are as shown in Table II. Examples of sputum cytospin preparations showing differences in macrophage color before and after prednisolone are shown in Fig 4, A and B. The median airway macrophage percentage red-hued area significantly increased after treatment with prednisolone (P = .02; Fig 4, C, and Table II).
TABLE II.
Lung function, sputum differential count, and median percentage area of red hue before and after prednisolone treatment in subjects with severe asthma (n = 8)
| Before prednisolone | After prednisolone | P value | |
|---|---|---|---|
| FEV1 (L), mean (SD) | 2.47 (0.98) | 2.77 (0.82) | .09 |
| FVC (L), mean (SD) | 3.48 (1.25) | 3.70 (0.88) | .51 |
| FeNO (ppb), mean (SD) | 53 (52) | 34 (16) | .26 |
| Induced sputum (%), median (range) | |||
| Eosinophils | 31.63 (3.5-90.5) | 0.87 (0.0-16.25) | .03 |
| Neutrophils | 29.88 (2-74.25) | 66.65 (4.18-87.0) | .05 |
| Macrophages | 35.39 (6.5-56.75) | 28.50 (9.75-80.23) | .90 |
| Percentage area of red hue/AM, median (IQR) | 17.4 (8.0-57.2) | 56.6 (13.9-79.3) | .02 |
AM, Airway macrophage; FeNO, fraction of exhaled nitric oxide; FVC, forced vital capacity; IQR, interquartile range.
FIG 4.
Representative images of sputum macrophages after treatment with prednisolone, 30 mg/d, for 2 weeks. A and B, High number of eosinophils and macrophages with blue cytoplasm before treatment (Fig 4, A) and reduced eosinophil counts with increase in eosinophilic staining of macrophages in the same patient after treatment (Fig 4, B). C, Graph showing the median percentage of red hue/airway macrophage before and after oral corticosteroids.
Airway macrophage hue and corticosteroid-weaning success
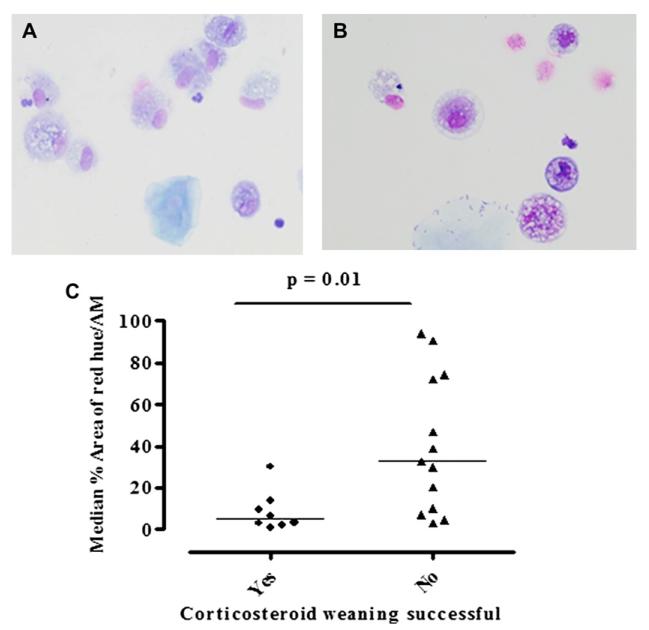
Clinical characteristics of the subjects with a normal sputum eosinophil count who had successful or unsuccessful corticosteroid weaning are as shown in Table III. At baseline, there was no significant difference in age, lung function, and sputum eosinophil differential between those who were successfully weaned and those who were not. Representative sputum cytospin preparations from the 2 groups are as shown in Fig 5, A and B. The median airway macrophage percentage red-hued area at baseline was significantly lower in those who were successfully weaned versus those who were not (P = .01; Fig 5, C, and Table III).
TABLE III.
Demographic, lung function, and sputum differential count in subjects with successful and unsuccessful weaning
| Successful weaning (n = 8) |
Unsuccessful weaning (n = 13) |
P value | |
|---|---|---|---|
| Age (y), mean (SE) | 56 (6) | 52 (4) | .55 |
| Male sex, no. (%) | 5 (63) | 6 (46) | .65 |
| FEV1 (% predicted), mean (SE) | 84 (24) | 80 (8) | .78 |
| Induced sputum (%), median (range) | |||
| Neutrophils | 61 (5-93) | 48 (12-95) | .79 |
| Macrophages | 35 (6-79) | 36 (4-72) | .97 |
| Eosinophils | 0.37 (0.1-1.0) | 0.25 (0.1-1.5) | .63 |
| Percentage area of red hue/AM, median (IQR) | 4.9 (2.3-12.8) | 32.8 (8.6-73.4) | .01 |
AM, Airway macrophage; IQR, interquartile range.
FIG 5.
A and B, Representative images of induced sputum macrophages in subjects with low eosinophil counts (<1.9%) in whom weaning of corticosteroid treatment was successful (Fig 5, A) and unsuccessful (high eosinophilic staining; Fig 5, B). C, Scatter plots comparing median macrophage percentage of red hue in these 2 groups.
DISCUSSION
We describe here that the eosinophil protein content of airway macrophages is a reliable biomarker of ongoing eosinophilic airway inflammation. The quantity of eosinophil protein content in macrophages can be determined by the measurement of the percentage of red hue of macrophages on routinely stained sputum cytospin preparations. Importantly, airway macrophage percentage of red hue was increased in subjects with moderate-to-severe asthma independent of current sputum eosinophil counts, suggesting that the absence of sputum eosinophilia in these subjects was a consequence of corticosteroid therapy rather than a reflection of a true noneosinophilic phenotype. In addition to its potential role in phenotyping asthma, the combination of a normal sputum eosinophil count and macrophage percentage of red hue determined from the same cytospin preparation was a good predictor of successful weaning from corticosteroid treatment.
Macrophage ingestion of apoptotic eosinophils is a well-documented phenomenon resulting in resolution of inflammation.11 Woolley et al11 have reported the presence of eosinophil products in sputum macrophages after treatment with corticosteroids. Similar findings have been confirmed in vitro22 and in electron microscopic images of macrophages.12 However, to date, the quantity of eosinophil proteins in airway macrophages has not been used as a marker of eosinophilic inflammation. We have confirmed with flow cytometry and immunofluorescence that eosinophil proteins (ECP and EPO) are detectable in airway macrophages after ingestion of apoptotic eosinophils. An increase in macrophage eosinophil protein content with a higher eosinophil/macrophage ratio confirmed a positive dose response. Apoptotic eosinophils were observed within macrophages but in some macrophages intact eosinophils were no longer visible. We therefore chose to examine the potential application of the red color on Romanowsky-stained cytospin preparations, which reflects material staining for eosin as a measure of ingested eosinophils and their products. Macrophage color changed from blue to red-purple after ingestion of apoptotic eosinophils, a method that was applied to analyze cytospin preparations. This color change was analyzed by using hue spectrum in image analysis because it corresponds to the color perceived by the human eye and does not depend on light intensity or saturation. Importantly, the described image analysis method had excellent interobserver and intraobserver variation. We are therefore confident that macrophage color reflects eosinophil protein content and has potential as a biomarker of ongoing eosinophilic airway inflammation.
There is increasing recognition that sputum eosinophilia is only present in about 50% of asthmatic subjects across all severities.3,23 This observation has undermined previous dogma that asthma is predominately characterized by TH2-mediated eosinophilic inflammation and has introduced the possibility that other inflammatory pathways are dominant in different asthma subphenotypes.24 This view has enormous implications for asthma treatment and pathogenesis. In corticosteroid-naive asthmatic subjects the absence of sputum eosinophilia (<1.9% nonsquamous cells) on 2 or more occasions has been used to define noneosinophilic asthma.4,23 However, in asthmatic subjects treated with corticosteroid therapy, it is not possible to determine whether the lack of sputum eosinophilia is a consequence of corticosteroid therapy or represents a true noneosinophilic phenotype. Longitudinal sputum assessments and corticosteroid withdrawal studies suggest that the presence of a sputum eosinophil count in asthmatic subjects treated with corticosteroids is variable and changes in response to disease stability and current therapy.8,9,25,26 One of our most important findings in this report is that in patients with moderate-to-severe asthma, the macrophage percentage of red hue was markedly increased independent of the sputum eosinophil count, with no difference between the noneosinophilic and eosinophilic groups (see the Results section and Fig 3, D and E). This suggests that in asthmatic subjects treated with corticosteroids, the absence of sputum eosinophilia reflects response to therapy (ie, adequate clearance by macrophages). This view was further supported by our findings that the macrophage percentage of red hue increased in response to 2 weeks of prednisolone therapy in subjects with severe asthma. Therefore true noneosinophilic asthma is probably less common in subjects with moderate-to-severe asthma than previously considered.
This might provide an explanation for the observation that children with noneosinophilic severe asthma responded to corticosteroids27 and suggests that eosinophil-directed therapy might be applicable to a large group of subjects with severe asthma. This is supported by our recent study of mepolizumab, which demonstrated that this anti–IL-5 mAb therapy led to a marked reduction in asthma exacerbations in subjects with severe asthma, even though one third of these subjects did not have sputum eosinophilia at study entry.5 These subjects did have at least 1 episode of sputum eosinophilia in the last 2 years, which underscores the current need in some patients for analysis of repeated sputum samples to define the asthma phenotype. This therefore highlights the potential added value of using the eosinophil protein content in macrophages together with the differential cell count. Indeed, we found that subjects with severe asthma followed up for 1 year in a study designed to test the application of sputum analysis to direct corticosteroid therapy who had a normal sputum eosinophil count also had a low macrophage percentage of red hue among those who were successfully weaned off or onto maintenance low-dose inhaled corticosteroids, whereas those subjects with normal sputum eosinophil counts but moderate-to-high eosinophil protein content in their macrophages (at the start of weaning) were unsuccessful at corticosteroid withdrawal. The high eosinophil protein content in macrophages suggests ongoing eosinophilia. Therefore the combination of the sputum eosinophil count and macrophage color on a single sputum cytospin preparation might have clinical utility in directing therapy and warrants further testing in prospective studies.
Some subjects had low airway macrophage red hue in combination with high eosinophil counts, which suggests (1) an inadequate amount of corticosteroid reaching the airways or (2) or an inherent defect of phagocytosis. However, an increase in airway macrophage hue after treatment with systemic steroids differentiates between the 2 possible causes. When the airway macrophage red hue and eosinophil count are both high, it suggests a possibility of ongoing eosinophilia beyond the capacity of macrophage clearance mechanisms.
Interestingly, subjects with corticosteroid-naive NAEB had similar content of eosinophilic staining as those with severe asthma. One possible explanation is that there is more spontaneous resolution of eosinophilic airway inflammation in subjects with NAEB compared with that seen in subjects with asthma. This might be due to increased eosinophil apoptosis in subjects with NAEB, which could be due to the decreased IL-13 concentration in the airway in subjects with NAEB, which is known to promote eosinophil survival.28 This important distinction between mild asthma and NAEB requires further study.
One potential limitation of our study is that the macrophage color does not quantify the exact amount of eosinophil proteins, which might reduce its accuracy. However, we have demonstrated that the test is repeatable, responds to corticosteroid therapy, and has the potential to direct therapy. It provides additional and complementary information to the differential cell count. Importantly, it does not require further processing of sputum; moreover, ImageJ is freely downloadable software. The macro speeds up the process significantly by combining the majority of the analysis steps in to a single step (see Fig E1). Critically, data in this study are derived from the analysis of retrospective sputum cytospin preparations collected as part of previous studies and need to be replicated in prospective studies. Whether this new biomarker can be more widely applied to other airway diseases, such as chronic obstructive pulmonary disease or chronic cough, requires further study.
In conclusion, macrophage eosinophil protein content can be reliably assessed from sputum cytospin preparations by measuring macrophage color and reflects ongoing airway eosinophilia. The absence of sputum eosinophilia and normal macrophage eosinophil protein content is unusual in subjects with moderate-to-severe asthma. The combination of macrophage eosinophil protein content and eosinophil count is useful in predicting response to treatment withdrawal. Prospective studies of the clinical utility of sputum macrophage color as a new biomarker of eosinophilic inflammation are warranted.
Supplementary Material
Includes supplementary methods, and figures E1 and E2
Clinical implications.
Sputum macrophage eosinophil content (1) is useful in identifying ongoing eosinophilia, even with normal eosinophil counts, and (2) might be useful, along with eosinophil counts, in predicting response to treatment withdrawal.
Acknowledgments
We thank Katy Roach, Bev Hargadon, Will Monteiro, Carlene Reid, Rubeen Sehra, Michelle Powell, Vijay Mistry, Carol Ighofose, Jamie Green, Caroline Day, Michelle Muessel, Rohini Rattihalli, Stephanie Cooper, and Adesimbo Sogbesan for help in sample collections, processing, and image acquisition.
Supported by the Midland Allergy and Asthma Association, Asthma UK, Trent NHS R&D, Wellcome Senior Fellowship (CB), GlaxoSmithKline (Severe Asthma Study funding), and AstraZeneca (THP-1 Cells).
Abbreviations used
- APC
Allophycocyanin
- ECP
Eosinophil cationic protein
- EPO
Eosinophil peroxidase
- FITC
Fluorescein isothiocyanate
- GINA
Global Initiative for Asthma
- NAEB
Nonasthmatic eosinophilic bronchitis
REFERENCES
- 1.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 3.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–8. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–9. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 7.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 8.Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemiere C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483–94. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 9.Leuppi JD, Salome CM, Jenkins CR, Anderson SD, Xuan W, Marks GB, et al. Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am J Respir Crit Care Med. 2001;163:406–12. doi: 10.1164/ajrccm.163.2.9912091. [DOI] [PubMed] [Google Scholar]
- 10.Pavord ID, Jeffery PK, Qiu Y, Zhu J, Parker D, Carlsheimer A, et al. Airway inflammation in patients with asthma with high-fixed or low-fixed plus as-needed budesonide/formoterol. J Allergy Clin Immunol. 2009;123:1083–9. doi: 10.1016/j.jaci.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Woolley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Woolley MJ. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:237–43. doi: 10.1164/ajrccm.154.1.8680686. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak AM, Weller PF, Monahan-Earley RA, Letourneau L, Ackerman SJ. Ultra-structural localization of Charcot-Leyden crystal protein (lysophospholipase) and peroxidase in macrophages, eosinophils, and extracellular matrix of the skin in the hypereosinophilic syndrome. Lab Invest. 1990;62:590–607. [PubMed] [Google Scholar]
- 13.Global Initiative for Asthma [Accessed September 17, 2009];Global Strategy for Asthma Management and Prevention. 2008 www.ginasthma.com
- 14.Brightling CE. Chronic cough due to nonasthmatic eosinophilic bronchitis. Chest. 2006;129(suppl):116S–21S. doi: 10.1378/chest.129.1_suppl.116S. [DOI] [PubMed] [Google Scholar]
- 15.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing—1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 16.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58:528–32. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ, et al. Qualitative analysis of high resolution CT scans in severe asthma. Chest. 2009;136:1521–8. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day CE, Zhang SD, Riley J, Gant T, Wardlaw AJ, Guillen C. A novel method for isolation of human lung T cells from lung resection tissue reveals increased expression of GAPDH and CXCR6. J Immunol Methods. 2009;342:91–7. doi: 10.1016/j.jim.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood. 2001;98:2239–47. doi: 10.1182/blood.v98.7.2239. [DOI] [PubMed] [Google Scholar]
- 21.Walsh GM, Sexton DW, Blaylock MG, Convery CM. Resting and cytokine-stimulated human small airway epithelial cells recognize and engulf apoptotic eosinophils. Blood. 1999;94:2827–35. [PubMed] [Google Scholar]
- 22.Matsumoto K, Terakawa M, Fukuda S, Saito H. Rapid and strong induction of apoptosis in human eosinophils by anti-CD30 mAb-coated microspheres and phagocytosis by macrophages. Int Arch Allergy Immunol. 2007;143(suppl 1):60–7. doi: 10.1159/000101407. [DOI] [PubMed] [Google Scholar]
- 23.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–9. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6:256–9. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 25.in’t Veen JC, Smits HH, Hiemstra PS, Zwinderman AE, Sterk PJ, Bel EH. Lung function and sputum characteristics of patients with severe asthma during an induced exacerbation by double-blind steroid withdrawal. Am J Respir Crit Care Med. 1999;160:93–9. doi: 10.1164/ajrccm.160.1.9809104. [DOI] [PubMed] [Google Scholar]
- 26.Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- 27.Lex C, Jenkins G, Wilson NM, Zacharasiewicz A, Erin E, Hansel TT, et al. Does sputum eosinophilia predict the response to systemic corticosteroids in children with difficult asthma? Pediatr Pulmonol. 2007;42:298–303. doi: 10.1002/ppul.20570. [DOI] [PubMed] [Google Scholar]
- 28.Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, et al. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114:1106–9. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes supplementary methods, and figures E1 and E2