Abstract
Objective
Interstitial lung disease (ILD) has been recognized as an important co-morbidity in rheumatoid arthritis (RA). We aimed to assess incidence, risk factors and mortality of RA associated ILD.
Methods
We examined a population-based incidence cohort of patients with RA and a matched cohort of individuals without RA. All subjects were followed longitudinally until death, migration or January 1, 2006. The lifetime risk of ILD was estimated and Cox models were used to compare the incidence of ILD between cohorts, to investigate possible risk factors and to explore the impact of ILD on patient survival.
Results
582 patients with RA and 603 subjects without RA were followed for a mean of 16.4 and 19.3 years, respectively. The lifetime risk of developing ILD was 7.7% for RA patients and 0.9% for subjects without RA. This difference translated into a hazard ratio of 8.96 (95% CI 4.02, 19.94). The risk of developing ILD was higher in patients with older age at RA onset, among male patients and for individuals with parameters that indicate more severe RA.
Survival of RA patients diagnosed with ILD was worse compared to RA patients without ILD (HR 2.86, 95% CI 1.98, 4.12). ILD contributed approximately 13% to the excess mortality of patients with RA patients when compared to the general population.
Conclusion
Our results emphasize the increased risk of ILD in patients with RA. The impact of ILD on patient survival provides evidence that development of better strategies for the treatment of ILD could significantly lower the excess mortality of individuals with RA.
Keywords: Interstitial lung disease, rheumatoid arthritis, incidence, risk factors
Is there a true association between rheumatoid arthritis (RA) and interstitial lung disease (ILD) – and if yes, what impact does it have on the survival of affected patients? Data suggesting a connection between the most common inflammatory joint disease and this rare pulmonary condition is primarily based on case series and referral center-based patient cohorts. There have been no population-based studies investigating the full spectrum of patients with RA for the presence of ILD and comparing the incidence between individuals with and without RA.
The first description of pulmonary involvement in RA was provided in 1948 by Ellman and Ball [1], who reported three cases in which the classic manifestations of RA and extensive pulmonary disease appeared to be associated with the same underlying process. In two cases, autopsy was available and the lungs in both instances showed a chronic fibrosing type of pneumonitis.
Since the first reports, several studies based on referral cohorts have delivered estimates of prevalent ILD in patients with RA ranging between 1.0 to 58% ([2–7]). The great variation in estimates of occurrence does not come as a surprise, given the plethora of definitions and means of detection employed to diagnose ILD and its analogues.
RA-associated “interstitial lung disease” has served as a collection bin for a variety of different conditions affecting predominantly the pulmonary parenchyma, based on radiologic and/or histologic assessment. Importantly, this term has been used interchangeably with terms such as “RA-lung”, “RA-fibrosing alveolitis”, “RA-diffuse parenchymal interstitial lung disease” and “RA-pulmonary fibrosis” or “connective tissue disease-associated ILD” (CTD-ILD). As a result, studies of ILD in patients with RA have been hampered by the lack of acknowledged terminology and validated classification criteria. Furthermore, incidence or prevalence data based on referral samples may overestimate the incidence of ILD, since extraarticular disease is thought to be more frequent in patients with more severe RA [7–9].
Given these limitations of the current evidence, we aimed to better define the incidence, risk factors and mortality of ILD in patients with RA in a population-based setting, using a strict and reproducible classification criterion for ILD.
PATIENTS AND METHODS
A medical records linkage system in Rochester, MN, the Rochester Epidemiology Project, allows access to the complete (inpatient and outpatient) records from all health care providers for the local population. This data system ensures virtually complete access to clinical and vital status information of all clinically recognized cases of RA among Rochester residents [10].
Cohort of patients with rheumatoid arthritis
Using this data resource, a population-based incidence cohort of all cases of RA, first diagnosed between January 1, 1955 and January 1, 1994, among Rochester, Minnesota residents ≥ 18 years of age was assembled by Gabriel and colleagues, as previously described [11–13]. All cases fulfilled the 1987 American College of Rheumatology (ACR) classification criteria for RA [14]. Incidence date was defined as the first date of fulfillment of four out of the seven ACR diagnostic criteria. This RA incidence cohort consists of 603 subjects.
Matched control cohort of patients without rheumatoid arthritis
For each of the 603 subjects with RA, a birth year (± 3 years) and sex-matched individual without RA has been randomly selected from the same source population. Subjects without RA were also matched on the prior duration of their medical records history. Each subject in this cohort was assigned an index date corresponding to the date of RA diagnosis of their matched RA patient.
Data collection
The data abstraction process has been described in detail [11–13]. Briefly, all subjects (RA and non-RA) were followed up longitudinally through their complete medical records beginning at age 18 years (or date of migration to Rochester, MN for those who became residents after age 18) and continuing until death, migration from Rochester, or January 1, 2006. Demographic and clinical characteristics were abstracted by four trained nurse abstractors blinded to the study hypothesis. These included smoking status (categorized as current, former, or never at RA incidence date), rheumatoid factor (RF) seropositivity (≥40 IU/ml), erythrocyte sedimentation rate (ESR), tender and/or swollen joint counts, erosions, periarticular osteoporosis, and/or destructive changes on radiographs, rheumatoid nodules (absent/present), RA complications, disease duration, the use of disease-modifying antirheumatic drugs (DMARDs) and/or corticosteroids, functional capacity (Steinbrocker index at RA incidence/index date) and sustained elevation of the ESR (3 recorded ESR values of 60 mm/hour, with a minimum interval of 30 days between 2 measurements).
Definition and ascertainment of interstitial lung disease
The criteria employed for classification as ILD were the result of consensus forming discussions among 2 pulmonologist (JHR, RV) and 2 rheumatologists (ELM, TB). For the purpose of this study, ILD was divided into two levels of diagnostic certainty: “probable” and “definite” ILD. Criteria were based on clinical data, pulmonary function test results, radiological studies, and lung biopsy (table 1).
Table 1.
Intersitial lung disease (ILD): classification criteria
| Pulmonary Disease | Criteria for diagnosis |
|---|---|
| Probable ILD | The following two criteria need to be met:
|
| Definite ILD | Diagnosis of ILD by a pulmonologist plus at least 2 of the following three criteria:
|
CT= computed tomography; RA = rheumatoid arthritis; PFT = pulmonary function test; TLC = total lung capacity; DLCO= diffusing capacity of the lung for carbon monoxide
The medical records of all 1206 individuals in the RA and matched control cohort were re- reviewed for parameters of pulmonary disease by 2 physician scientists and 1 trained nurse abstractor. All pulmonary diagnoses, results of pulmonary function tests, chest radiography data, computed tomography (CT) results and pulmonary biopsy results (including autopsy reports) were abstracted into number/identifier codes. After completion of the abstraction process, a computer-based algorithm was applied in order to identify patients who met our ILD classification criteria.
Exploration of accuracy of ILD classification criterion
To investigate the validity of our classification criteria for probable and definite ILD, the medical records for all RA patients who met criteria for probable or definite ILD as well as 30 randomly selected patients from the RA cohort not meeting these criteria were retrieved. The medical records and radiologic images (if available) were reviewed by a pulmonologist (JHR) who was blinded to the criteria based classification of the subjects. Based on this expert review, a reference diagnosis of “no ILD”, “probable ILD” or definite ILD” was assigned to each individual.
Kappa statistics were used to quantify agreement between criteria and expert based diagnosis. In addition, we calculated the test characteristics of our classification criterion using “chart review based expert opinion” as a gold-standard.
Statistical analysis
Descriptive statistics were used to summarize the data. Demographics were compared using two-sample t-tests and chi-square tests (Statistical program: SAS (SAS Institute Inc., Cary, NC) and Splus (Insightful Corp., Seattle, WA). Cumulative incidence of ILD was estimated adjusting for the competing risk of death in patients with RA and subjects without RA. Cox-proportional hazards models adjusting for age, sex and smoking status were used to compare estimates between cohorts and to investigate possible associations of demographic and clinical variables with ILD. Time-dependent co-variates were used to represent risk factors that developed over time.
The survival of RA patients with ILD was compared to the survival of RA patients in the population. Expected survival rates for RA patients were obtained by multiplying the survival rates for the Minnesota White population by the standardized mortality ratio (SMR) for RA survival of 1.3. Sensitivity analyses were performed wherein age group - and sex-specific SMR values were used to modify the population rate table and similar results were obtained. Overall survival following ILD was estimated by obtaining the age-, sex-, and calendar-year-specific survival rates for the RA patients with ILD from this modified rate table. A one-sample log-rank test was used to examine whether observed mortality of the RA patients with ILD differed from the expected mortality for RA patients. Multivariable Cox models were used to examine the effect of interstitial lung disease on survival of patients with RA after adjusting for smoking status, age, gender and presence of comorbidities.
We also estimated the risk of mortality attributable to ILD in the RA cohort. The attributable risk (or etiologic fraction) is the proportion of disease in a population that could be prevented by elimination of an exposure, or risk factor. Attributable risk is commonly calculated as AR= [P(D) − P(D | no F)]/P(D) where AR is the attributable risk, P(D) is the probability of disease (i.e., death), and P(D| no F) is the conditional probability of disease among individuals without the risk factor. The cumulative incidence of death was used to estimate the probability of death, and these estimates were obtained from Cox models to allow for adjustment for age and sex. The conditional probability of death for those without ILD was estimated from the same Cox models, but with a target cohort that matched the observed cohort except that it lacked ILD.
RESULTS
Population-based cohort of subjects with rheumatoid arthritis and matched control cohort of patients without RA
The population-based RA incidence and the matched control cohort comprised 603 patients each. After exclusion of 21 patients with ILD diagnosed prior to their RA diagnosis/index date, 582 patients with incident RA and 603 control subjects were used in the analysis. The mean age at RA incidence/index date was 58 years. The mean duration of follow-up was 16.4 years for RA patients and 19.3 years for subjects without RA. 73% of patients were female. Smoking was more common among RA patients: 28.2% of RA patients were current smokers and 24.7% were former smokers compared to 23.9% current and 19.6% former smokers in the non-RA comparator group (p<0.01). Chest radiographs were available for 230 RA and 352 non-RA patients. “Fibrotic changes” were mentioned in a similar number of RA- and non-RA patients: 27.0% and 25.5%, respectively. A minority of patients underwent pulmonary function testing: 19.1% of patients in the RA and 17.2% of patients in the non-RA cohort. A detailed description of both cohorts is provided in table 2.
Table 2.
Demographic and clinical characteristics of rheumatoid arthritis (RA) and control cohorts*
| Patients with RA (N=582) | Subjects without RA (N=603) | p value | |
|---|---|---|---|
| Age at RA diagnosis/index date, mean ± SD in years | 57.7 ± 15.1 | 58.2 ± 15.2 | 0.58 |
| Years of follow up, mean ± SD | 16.4 ± 10.5 | 19.3 ± 11.1 | -- |
| Female, N (%) | 427 (73.4%) | 441 (73.1%) | 0.93 |
| Smoking status at baseline, N (%) | |||
| Current smoker | 164 (28.2%) | 144 (23.9%) | <0.01 |
| Former smoker | 144 (24.7%) | 118 (19.6%) | |
| Chest X-ray documented, N (%) | 230 (39.5%) | 352 (58.4%) | <0.01 |
| Fibrotic changes on chest X-ray, N (%) | 157 (27.0%) | 154 (25.5%) | 0.57 |
| PFT documented, N (%) | 111 (19.1%) | 104 (17.2%) | 0.42 |
| PFT restrictive pattern (TLC ≤ 80% predicted), N (%) | 27 (4.6%) | 11 (1.8%) | <0.01 |
| CT documented, N (%) | 78 (13.4%) | 85 (14.1%) | 0.73 |
| CT reported presence of parenchymal disease, N (%) | 38 (6.5%) | 24 (4.0%) | 0.05 |
Except where indicated otherwise, values are the number (%) of patients.
RA = rheumatoid arthritis; SD = standard deviation; PFT = pulmonary function test; TLC = total lung capacity; CT = computed tomography
Incidence of ILD in patients with RA
ILD (probable and definite) developed in 46 (7.9%) of 582 patients with RA. Of these, 23 patients (4.0%) met our strict criteria for definite ILD.
The 10-, 20- and 30-year cumulative incidence rates for all ILD were 3.5%, 6.3% and 7.7%, respectively (adjusted for the competing risk of death), conveying a life-time risk of close to 10%. Among control subjects, much lower 10-, 20- and 30-year cumulative incidence rates for probable and definite ILD were detected (0.2%, 0.9% and 0.9%, respectively).
The risk of developing ILD among patients with RA was significantly higher than that of non-RA subjects (hazard ratio [HR]: 8.96; 95% confidence interval [CI]: 4.02 to 19.94 after adjusting for age, sex and smoking status (figure 1).
Figure 1.
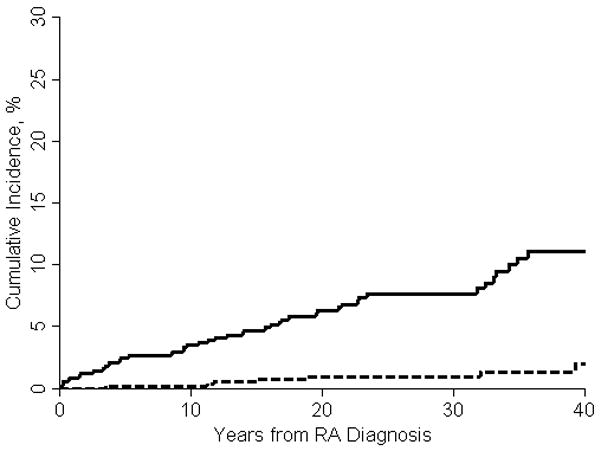
Incidence of ILD in patient with RA (solid line) and control subjects (broken line)
Validation of classification criteria for ILD in patients with RA
All patients identified as having RA associated ILD according to our classification criteria as well as a random sample of 30 patients with RA and no ILD underwent a complete record review by a pulmonologist (JHR) and rheumatologist (TB). Our composite criteria documented excellent agreement with chart review based expert opinion across all 3 categories (no, probable and definite lung disease). Kappa statistics yielded an index of 0.88 (95% CI, 0.79 – 0.97). When considering “chart review based expert opinion” as a gold standard, the sensitivity and specificity of these criteria were 1.0 (95%CI, 0.92–1.0) and 0.91 (95%CI, 0.76 to 0.97), respectively.
Characteristics of ILD in patients with RA and patients without RA
A higher proportion of subjects diagnosed with ILD disease were male in both cohorts. On average, RA patients were 15 years younger when diagnosed with ILD as compared to control subjects.
CT scans of the chest were available for only a limited number of patients with ILD (50.0% of RA subjects and 57.1% of non-RA subjects). Pulmonary biopsy was performed in 14 of 46 patients with RA associated ILD. A summary of ILD characteristics in RA and control subjects is provided in table 3.
Table 3.
Characteristics of interstitial lung disease (ILD) in rheumatoid arthritis (RA) and non-RA patients
| RA patients with ILD (N=46) | Control subjects with ILD (N=7) | |
|---|---|---|
| Probable ILD, N (%) | 23 (50.0%) | 5 (71.4%) |
| Definite ILD, N (%) | 23 (50.0%) | 2 (28.6%) |
| Mean ± SD age at ILD diagnosis in years | 71.3±12.2 | 86.5±10.0 |
| Men, N (%) | 27 (58.7%) | 4 (57.1%) |
| Current smoker, N (%) | 15 (32.6%) | 1 (14.3%) |
| Former smoker, N (%) | 15 (32.6%) | 4 (57.1%) |
| Chest radiograph performed ever, N (%) | 45 (97.8%) | 7 (100%) |
| Chest CT performed ever, N (%) | 23 (50.0%) | 4 (57.1%) |
| Lung biopsy/autopsy performed ever, N (%) | 14 (30.4%) | 0 (0.0%) |
| ILD subtype† based on biopsy and/or CT, N (%) | ||
| UIP typical pattern | 9 (19.6%) | 3 (42.9%) |
| NSIP typical pattern | 1 (2.2%) | 0 (0.0%) |
| OP typical pattern | 3 (6.5%) | 0 (0.0%) |
| Pulmonary fibrosis, not further specified | 15 (32.6%) | 0 (0.0% |
| No CT or biopsy documented | 15 (32.6%) | 3 (42.9%) |
| PFT: TLC < 80%, N (%) | 15 (32.6%) | 2 (28.6%) |
| No TLC documented, N (%) | 21 (45.7%) | 4 (57.1%) |
Except where indicated otherwise, values are the number (%) of patients.
According to the American Thoracic Society consensus classification of idiopathic interstitial pneumonias [15].
RA = rheumatoid arthritis; ILD =interstitial lung disease; SD = standard deviation; UIP = usual interstitial pneumonia; NSIP = non- specific interstitial pneumonia; OP=organizing pneumonia; CT=computed tomogram; PFT = pulmonary function testing; TLC = total lung capacity
Risk factors for ILD in patients with RA
The risk of developing ILD was increased in patients who were older at the time of their RA diagnosis (per 10 year increase in age HR: 1.41 95% CI 1.11, 1.79) and among male patients (HR 4.37 95% CI 2.43, 7.88). Other risk factors which had a statistically significant association with development of ILD were related to markers of disease activity and severity including erosions or destructive changes and rheumatoid nodules, high ESR levels, functional status (3, 4 level versus 1, 2 level) and corticosteroid and methotrexate use (table 4).
Table 4.
Risk factors for interstitial lung disease (ILD) in patients with rheumatoid arthritis (RA)*
| Risk Factor | RA patients with ILD (N=46) | RA patients without ILD (N=536) | Hazard Ratio† | 95% CI† |
|---|---|---|---|---|
| Male gender, N (%) | 27 (58.7%) | 128 (23.9) | 4.37 | 2.43, 7.88 |
| Age at RA onset in years (mean ± SD) | 56.8 ± 14.7 | 57.8 ± 15.2 | 1.41 | 1.11, 1.79 |
| Smoking (ever), N (%) | 30 (65.2) | 278 (51.9) | 1.60 | 0.87, 2.94 |
| Extraarticular disease (ever), N (%) | 6 (13.0) | 80 (14.9) | 1.48 | 0.58, 3.79 |
| Rheumatoid factor (ever), N (%) | 33 (73.3) | 341 (67.0) | 1.84 | 0.91, 3.71 |
| Erosions (ever), N (%) | 10 (25.0) | 83 (17.4) | 1.92 | 0.92, 4.02 |
| Destructive joint changes (ever), N (%) | 27 (67.5) | 271 (56.7) | 2.37 | 1.18, 4.76 |
| ESR - 3 values ≥ 60 (ever), N (%) | 21 (45.7) | 149 (27.8) | 3.52 | 1.94, 6.38 |
| Large joint swelling (ever), N (%) | 39 (84.8) | 448 (83.6) | 1.90 | 0.83, 4.36 |
| Rheumatoid nodules (ever), N (%) | 21 (45.7) | 168 (88.9) | 2.60 | 1.41, 4.79 |
| DMARD use (ever), N (%) | 27 (58.7) | 306 (57.1) | 1.82 | 0.97, 3.41 |
| Methotrexate use (ever), N (%) | 12 (26.1) | 116 (31.3) | 2.31 | 1.15, 4.63 |
| Steroid use (ever), N (%) | 24 (52.2) | 287 (53.5) | 2.01 | 1.12, 3.63 |
| Functional capacity†† = 1+2, N (%) | 33 (71.7) | 425 (79.9) | 2.17 (3,4 versus 1,2) | 1.14, 4.16 |
| Functional capacity†† = 3+4, N (%) | 13 (28.3) | 107 (20.1) |
Except where indicated otherwise, values are the number (%) of patients.
Models in rows 4–14 are adjusted for age at RA, sex, and ever smoker.
According to Steinbrocker index;
ILD = interstitial lung disease; RA = rheumatoid arthritis; CI = confidence interval; ESR = erythrocyte sedimentation rate; DMARD = disease modifying antirheumatic drug
Mortality associated with ILD in patients with RA
The median survival of RA patients after a diagnosis of ILD was 2.6 years, which is significantly lower than the expected median survival of 9.9 years in RA patients of the same age and sex overall (p<0.001). The survival of patients with RA and ILD compared with that of RA patients overall is shown in Figure 2. Survival was significantly worse when comparing patients with RA-associated lung disease to subjects with RA not affected by ILD, after adjusting for age, sex and smoking (HR 2.86, 95% CI 1.98, 4.12)
Figure 2.

Survival of RA patients with ILD (solid line) compared to expected survival of RA patients overall (broken line)
Although the cumulative incidence of ILD was significantly increased in patients with RA, the risk of death associated with ILD was similar among patients with RA and non-RA individuals (HR 1.60; 95% CI 0.63 to 4.09 adjusted for age, sex and smoking).
Excess mortality of patients with RA attributable to interstitial lung disease
During follow-up, 382 patients with RA died, a significant increase over the 347 deaths that were experienced by the non-RA cohort. The cumulative incidence of death was 79.3% in patients with RA at 30 years after RA incidence date, compared to 63.4% in patients without RA at 30 years after index date. Based on this, the RA cohort has an excess mortality of 15.9% at 30 years after RA incidence.
We further explored the question of how much of this excess mortality could be attributed to ILD. When removing the effect of ILD on patient survival, the cumulative mortality of patients with RA dropped by 2.1%. Thus, the excess deaths in RA would be reduced by 13% (2.1% / 15.9%), if the risk of ILD in RA was the same as in non-RA subjects. In other words, if the effect of the increased risk of ILD in RA could be eliminated, approximately 1 in every 8 excess RA deaths could be prevented.
DISCUSSION
According to our estimates, approximately 1 in 10 patients with RA will be diagnosed with ILD over the lifetime of their disease. This risk is significantly higher than in the in the general population.
Because of the inherent methodologic limitations of a retrospective, chart review based diagnosis of diseases, we preferred to establish a range of probabilities for our primary outcome (ILD), rather than a point value which may or may not approach reality. Hence the “true” cumulative incidence for clinically manifest ILD in patients with RA in our population is likely to lie somewhere in between our estimates for “probable” ILD (7.7 %) and definite ILD (3.7 %). Since systematic cross sectional or prospective screening was not performed for this study, it is likely that our estimates are the minimum for ILD incidence in this population. Additionally, patients who developed ILD prior to their RA diagnosis were excluded from our analysis in order to avoid incidence-prevalence bias. Therefore, the overall risk for individuals with RA to be affected by ILD either prior or after their diagnosis of RA will be somewhat higher than our estimate.
Despite our efforts to develop a valid definition for ILD for this study, we acknowledge that our definition of ILD still represents a “collection bin” for many different types of parenchymal lung disease. Because of the changes in availability of diagnostic tools such as CT over time as well as the evolution of definitions used to characterize ILD, reliable assignment of ILD subtypes for every patient according to the most recent ATS consensus classification [15] was not possible.
Other potential limitations of this study include reliance on medical record information that has been accrued in clinical care over several decades, and not through systematic prospective data collection. Thus, the capture of medical information used for our study was entirely dependent on it being documented in a patient’s medical record. Availability and use of technologies such as computer tomography and pulmonary function testing have greatly increased since the starting period for our incidence cohort (1955). As a result, the number of unrecognized cases of ILD may be higher during the earlier years of our follow-up period, resulting in an underestimation of the true incidence.
Differential medical assessment of patients with RA versus individuals without RA is another possible weakness of our approach. In patients with RA, the average frequency of physician visits is likely to be higher than for individuals without RA. This potentially results in a higher likelihood of capturing additional medical conditions such as ILD. However, because all residents included in our 2 cohorts come to medical attention at least once in any 3 year period [10], differential capture of a major medical diagnosis such as ILD appears less likely.
An important advantage of our methodological approach is the avoidance of referral bias, which will inevitably play a role when basing estimates on convenience samples in referral centers. Instead, we aimed to assess every patient diagnosed with RA in a given geographic area, resulting in a better reflection of the full spectrum of disease.
The population of Rochester, MN is mainly white and the socioeconomic characteristics largely resemble those of the US white population in general [10]. Appropriate caution has to be exercised when generalizing our findings to populations with a different demographic composition.
This may be especially true for a disease such as ILD, which is thought to be influenced in its occurrence and severity by gene-environment interaction [16]. The generalization of research results generated in a confined geographic area will be limited by the extent of genetic and environmental dissimilarities to other populations.
How does our data on the incidence of RA-associated ILD compare to information in the existing literature? Turesson et al. [7] found a 30-year cumulative incidence of 6.8% for “pulmonary fibrosis” in the same population-based cohort of Rochester patients (follow-up 5 years less as compared to our study). The classification of pulmonary fibrosis was based on “clinical judgment plus a DLCO of <85% of normal”. This estimate lies well within the range between “probable” and “definite” ILD in our study.
While we were unable to identify additional published data on the incidence of ILD, several reported studies have explored the prevalence of interstitial pulmonary changes and restrictive patterns of pulmonary function in non-population based referral cohorts. In a consecutive sample of 64 patients with longstanding RA and no respiratory symptoms referred to the National Institutes of Health in Bethesda, MD, 21 (33%) were found to have “early ILD” based on “HRCT features of ILD” [5]. The authors noted that changes in most patients were minimal, with an average HRCT score of 0.93 (1=minimal disease). 57% of the patients affected by these pre-clinical changes were judged to have “progressive disease” based on changes in their HRCT scores and/or PFT results over a time period of 2 years. No control data from patients without RA was reported.
Dawson et al. reported that 19% of 150 patients with longstanding RA had “fibrosing alveolitis” in a cohort of hospital outpatients in North-West England, defined as “CT changes suggestive for UIP” [3].
These estimates of prevalence are more than twice as high as the 30-year cumulative incidence for ILD found in our population-based study. Several differences between these studies and our approach offer possible explanations for this discrepancy. First, the aforementioned studies were hospital-based and subject to Berkson’s bias. They were performed using consecutive samples of RA patients, which will likely result in accumulation of more serious cases of RA with a higher likelihood of extraarticular disease [7–9]. Second, our retrospective approach, which had to rely on medical record data, may have underestimated the incidence of ILD in patients with RA as compared to the above mentioned prospective trials with a standardized assessment of every patient. While it is very likely that we did not capture subclinical disease, it is unlikely that we failed to detect a significant number of patients with clinically meaningful disease. A thorough expert review of a sample of RA patients classified as not having ILD according to our composite criteria did not reveal a single case that was missed with our composite criteria based approach. If ascertainment bias has resulted in underestimation of clinically overt ILD, the deviation should be minimal. Third, patients classified as having ILD based on CT and/or pulmonary function changes in the absence of respiratory symptoms may or may not progress to clinically meaningful disease. Our approach of using “physician’s diagnosis of ILD” as an essential classification criterion will condense the definition of ILD to clinically more overt cases and result in lower estimates.
The differences in ILD definition between studies reveal an interesting and important finding: the existence of a significant gap between the large number of asymptomatic individuals with radiographic or functional pulmonary changes, and the small number of patients who will actually develop overt pulmonary disease. Importantly, this gap is not only revealed by contrasting studies which used different definitions of ILD. A within-trial comparison of our data also demonstrates a significant discrepancy between the large number of individuals found to have fibrotic radiographic changes on their CT/chest radiographs - and the relatively small number of patients with clinically meaningful lung disease. In this context, it is important to mention that the number of individuals with a documented finding of “fibrotic changes” on their pulmonary imaging studies was similar when comparing patients with RA to those without RA (27.0% in RA patients and 25.5% in non-RA patients).
These observations converge on a crucial clinical question: where lies the threshold between tolerable subclinical abnormality and progressive disease that may benefit from therapeutic intervention?
Our analysis of risk factors indicated that male gender, higher age at RA onset, high “inflammatory burden”, low functional capacity and use of glucocorticosteroids as well as methotrexate are associated with a diagnosis of RA-ILD. The apparent association of parameters of high disease activity such as persistently elevated ESR, low functional capacity, rheumatoid nodules, glucocorticosteroid and methotrexate use is consistent with earlier findings described by Saag and colleagues [17] as well as the existing evidence of a strong association between RA disease severity and occurrence of extraarticular disease.
It is, however, unclear if these predictors of ILD in patients with RA are also indicators for a high risk of disease progression among patients who have subclinical interstitial pulmonary changes. This information would be crucial for therapeutic decision making in an individual patient. In this context, data published by Dawson et al. [18] provides important information on the likelihood of disease progression in patients with “CT changes suggestive for UIP”: 15 patients with stable RA associated ILD were compared with 10 patients who had progressive disease. Significant predictors for progression were the extent of interstitial changes on high resolution CT, bibasilar crackles and a reduced diffusing capacity for carbon monoxide. Although the low number of patients in the study of Dawson et al. has to be acknowledged, it highlights the importance of integrating clinical, imaging and functional information over time in order to decide whether an individual patient may be a candidate for therapeutic interventions.
The importance of identifying patients with RA who are at risk for progression of ILD becomes evident in the assessment of the significantly higher mortality for these patients in our cohort: the risk of death almost tripled for RA patients affected by ILD, even after adjusting for age, smoking and gender. Importantly, our data did not confirm previous publications [19, 20] which suggested a lower mortality of RA associated ILD compared with idiopathic ILD. This discrepancy may be explained by our population based approach, which resulted in a more complete capture of the full spectrum of disease.
Our findings extends prior observations about the increased risk of respiratory death in patients with RA [21, 22]as well as the significant impact of extraarticular disease on excess RA mortality [23]
It is well acknowledged that patients with RA have premature mortality compared to individuals from the general population [12, 24, 25]. Research over the recent years has focussed on the question why this is the case and how it may be prevented. In this context, most attention has been given to the increased risk of cardiovascular disease in patients with RA and its contribution to premature mortality [26–28]. A recent publication based on the same patient cohort we used for our study, identified congestive heart failure, but not ischemic heart disease as an important contributor to the excess overall mortality in patients with RA [29].
Interestingly, the excess mortality in RA attributable to ILD appears to be very similar to the relative contribution of CHF. Approximately 1 of every 8 excess deaths in patients with RA could hypothetically be prevented if the risk of ILD (or CHF) was the same for individuals with RA compared to patients without RA. Our findings suggest that prevention and treatment of RA associated ILD could significantly improve the survival of patients with RA. Unfortunately, no controlled trials have evaluated the effectiveness of DMARD therapies for treatment of ILD associated with RA.
In summary, we demonstrate that this risk of ILD is significantly increased in patients with RA as compared to the general population. Several risk factors for ILD in RA patients could be identified, but the pathophysiologic links between these factors and pulmonary parenchymal changes remains unclear. Our data provides evidence for the significant mortality associated with ILD, and we delineate the prominent contribution of ILD to premature mortality in patients with RA.
Challenges for future research in RA-associated ILD include the identification of predictors which indicate progressive disease and a need for treatment. The large gap between the number of patients with functional and radiographic abnormalities and the relatively small number of individuals with symptomatic disease highlights the importance of individualized clinical decisions in balancing the risks and benefits of treatment.
References
- 1.Ellman P, Ball RE. Rheumatoid disease with joint and pulmonary manifestations. British medical journal. 1948 Nov 6;2(4583):816–20. doi: 10.1136/bmj.2.4583.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmona L, Gonzalez-Alvaro I, Balsa A, Angel Belmonte M, Tena X, Sanmarti R. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Annals of the rheumatic diseases. 2003 Sep;62(9):897–900. doi: 10.1136/ard.62.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001 Aug;56(8):622–7. doi: 10.1136/thorax.56.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. American journal of respiratory and critical care medicine. 1997 Aug;156(2 Pt 1):528–35. doi: 10.1164/ajrccm.156.2.9609016. [DOI] [PubMed] [Google Scholar]
- 5.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Archives of internal medicine. 2008 Jan 28;168(2):159–66. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 6.Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. The Journal of rheumatology. 2008 Aug;35(8):1513–21. [PubMed] [Google Scholar]
- 7.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Annals of the rheumatic diseases. 2003 Aug;62(8):722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon DA, Stein JL, Broder I. The extra-articular features of rheumatoid arthritis. A systematic analysis of 127 cases. The American journal of medicine. 1973 Apr;54(4):445–52. doi: 10.1016/0002-9343(73)90040-5. [DOI] [PubMed] [Google Scholar]
- 9.Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology (Oxford, England) 1999 Jul;38(7):668–74. doi: 10.1093/rheumatology/38.7.668. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996 Mar;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 11.Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis and rheumatism. 2002 Mar;46(3):625–31. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O’Fallon WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis and rheumatism. 2003 Jan;48(1):54–8. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 13.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis and rheumatism. 1999 Mar;42(3):415–20. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988 Mar;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. American journal of respiratory and critical care medicine. 2002 Jan 15;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 16.Nannini C, Ryu JH, Matteson EL. Lung disease in rheumatoid arthritis. Current opinion in rheumatology. 2008 May;20(3):340–6. doi: 10.1097/BOR.0b013e3282f798ed. [DOI] [PubMed] [Google Scholar]
- 17.Saag KG, Kolluri S, Koehnke RK, Georgou TA, Rachow JW, Hunninghake GW, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis and rheumatism. 1996 Oct;39(10):1711–9. doi: 10.1002/art.1780391014. [DOI] [PubMed] [Google Scholar]
- 18.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2002 Jun;61(6):517–21. doi: 10.1136/ard.61.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. American journal of respiratory and critical care medicine. 2007 Apr 1;175(7):705–11. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 20.Rajasekaran A, Shovlin D, Saravanan V, Lord P, Kelly C. Interstitial lung disease in patients with rheumatoid arthritis: comparison with cryptogenic fibrosing alveolitis over 5 years. The Journal of rheumatology. 2006 Jul;33(7):1250–3. [PubMed] [Google Scholar]
- 21.Minaur NJ, Jacoby RK, Cosh JA, Taylor G, Rasker JJ. Outcome after 40 years with rheumatoid arthritis: a prospective study of function, disease activity, and mortality. J Rheumatol Suppl. 2004 Mar;69:3–8. [PubMed] [Google Scholar]
- 22.Symmons DP, Jones MA, Scott DL, Prior P. Longterm mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. The Journal of rheumatology. 1998 Jun;25(6):1072–7. [PubMed] [Google Scholar]
- 23.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. The Journal of rheumatology. 2002 Jan;29(1):62–7. [PubMed] [Google Scholar]
- 24.Bjornadal L, Baecklund E, Yin L, Granath F, Klareskog L, Ekbom A. Decreasing mortality in patients with rheumatoid arthritis: results from a large population based cohort in Sweden, 1964–95. The Journal of rheumatology. 2002 May;29(5):906–12. [PubMed] [Google Scholar]
- 25.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis and rheumatism. 1994 Apr;37(4):481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 26.Goodson N, Marks J, Lunt M, Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Annals of the rheumatic diseases. 2005 Nov;64(11):1595–601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis and rheumatism. 2005 Mar;52(3):722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 28.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. The Journal of rheumatology. 1997 Mar;24(3):445–51. [PubMed] [Google Scholar]
- 29.Nicola PJ, Crowson CS, Maradit-Kremers H, Ballman KV, Roger VL, Jacobsen SJ, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis and rheumatism. 2006 Jan;54(1):60–7. doi: 10.1002/art.21560. [DOI] [PubMed] [Google Scholar]


