Abstract
BACKGROUND
Improved diagnostic tests for tuberculosis in children are needed. We hypothesized that transcriptional signatures of host blood could be used to distinguish tuberculosis from other diseases in African children who either were or were not infected with the human immunodeficiency virus (HIV).
METHODS
The study population comprised prospective cohorts of children who were undergoing evaluation for suspected tuberculosis in South Africa (655 children), Malawi (701 children), and Kenya (1599 children). Patients were assigned to groups according to whether the diagnosis was culture-confirmed tuberculosis, culture-negative tuberculosis, diseases other than tuberculosis, or latent tuberculosis infection. Diagnostic signatures distinguishing tuberculosis from other diseases and from latent tuberculosis infection were identified from genomewide analysis of RNA expression in host blood.
RESULTS
We identified a 51-transcript signature distinguishing tuberculosis from other diseases in the South African and Malawian children (the discovery cohort). In the Kenyan children (the validation cohort), a risk score based on the signature for tuberculosis and for diseases other than tuberculosis showed a sensitivity of 82.9% (95% confidence interval [CI], 68.6 to 94.3) and a specificity of 83.6% (95% CI, 74.6 to 92.7) for the diagnosis of culture-confirmed tuberculosis. Among patients with cultures negative for Mycobacterium tuberculosis who were treated for tuberculosis (those with highly probable, probable, or possible cases of tuberculosis), the estimated sensitivity was 62.5 to 82.3%, 42.1 to 80.8%, and 35.3 to 79.6%, respectively, for different estimates of actual tuberculosis in the groups. In comparison, the sensitivity of the Xpert MTB/RIF assay for molecular detection of M. tuberculosis DNA in cases of culture-confirmed tuberculosis was 54.3% (95% CI, 37.1 to 68.6), and the sensitivity in highly probable, probable, or possible cases was an estimated 25.0 to 35.7%, 5.3 to 13.3%, and 0%, respectively; the specificity of the assay was 100%.
CONCLUSIONS
RNA expression signatures provided data that helped distinguish tuberculosis from other diseases in African children with and those without HIV infection. (Funded by the European Union Action for Diseases of Poverty Program and others).
Between 500,000 and 1 million new cases of childhood tuberculosis are diagnosed annually, but the true global burden of childhood tuberculosis is unknown because it is often difficult to confirm the diagnosis microbiologically.1-3 Although most cases of tuberculosis in adults are diagnosed through detection of acid-fast bacilli on microscopic examination of a sputum specimen, in the majority of childhood cases, smears and cultures are negative for Mycobacterium tuberculosis, and the diagnosis is made solely on clinical grounds.1,3 Since the symptoms and signs of childhood tuberculosis are seen in a range of other conditions, clinical diagnosis is unreliable.4 Clinical scoring systems designed to aid diagnosis have not been validated against the standard of culture-confirmed diagnosis, and the diagnostic accuracy of these systems varies markedly.5-7 Over diagnosis and thus inappropriate treatment of childhood tuberculosis is common.8 Conversely, underdiagnosis contributes to a poor outcome,9 and tuberculosis is often identified only when patients are critically ill or at postmortem investigations.10
Microbiologic diagnosis of childhood tuberculosis usually requires hospital admission to obtain gastric-lavage fluids or saline-induced sputum.11 Even then, microbiologic confirmation is achieved in only a small proportion of treated cases because of the paucibacillary nature of childhood tuberculosis and the characteristic extrapulmonary presentation.1-3 Radiographic findings in childhood tuberculosis are nonspecific,12 and the tuberculin skin test and interferon-γ-release assay (IGRA) cannot differentiate active disease from latent infection.13 Furthermore, children with tuberculosis, particularly those who are infected with the human immunodeficiency virus (HIV) or are malnourished, may have nonreactive results for both the tuberculin skin test and the IGRA.14-17 Improved methods for diagnosing childhood tuberculosis are thus urgently needed, particularly in countries of sub-Saharan Africa where the burden of tuberculosis and HIV coinfection is highest.1,2,18-20 We investigated the use of genomewide RNA expression in host blood to distinguish tuberculosis from other diseases that are prevalent among African children with and those without HIV infection and explored the use of a score for disease risk derived from the transcriptional signature as the basis for a possible diagnostic test.
METHODS
STUDY CONDUCT AND OVERSIGHT
We recruited patients between February 17, 2008, and January 27, 2011. Clinical data were anonymized, and patient samples identified according to study number. Assignments to diagnostic groups were made independently by two experienced clinicians, and any discrepancies in these assignments were resolved by a third clinician. Statistical analysis was conducted after the database on RNA expression and the clinical database had been locked (on February 4, 2011). An analysis plan was approved and analysis commenced after an amendment was made to use the South African and Malawi cohorts for discovery of the RNA signature and the Kenyan cohort for validation. This decision was necessitated by the lower-than-expected recruitment rate for patients with culture-confirmed tuberculosis. All the authors confirm that the analysis plan was followed and accept responsibility for the conduct of the study and the accuracy of the data.
The study was approved by the research ethics committees of the University of Cape Town, South Africa; the University of Malawi, College of Medicine; the Liverpool School of Tropical Medicine; Imperial College London; and the Kenya Medical Research Institute. Trained health workers obtained written or oral informed consent from the patients’ parents or guardians in their vernacular language. Neither the authors nor the sponsors have commercial interests in the outcomes. Patent applications for the pediatric RNA signatures have been submitted on behalf of the partner institutions, with the aim of the future development of a test for childhood tuberculosis in Africa on a nonprofit basis.
STUDY DESIGN
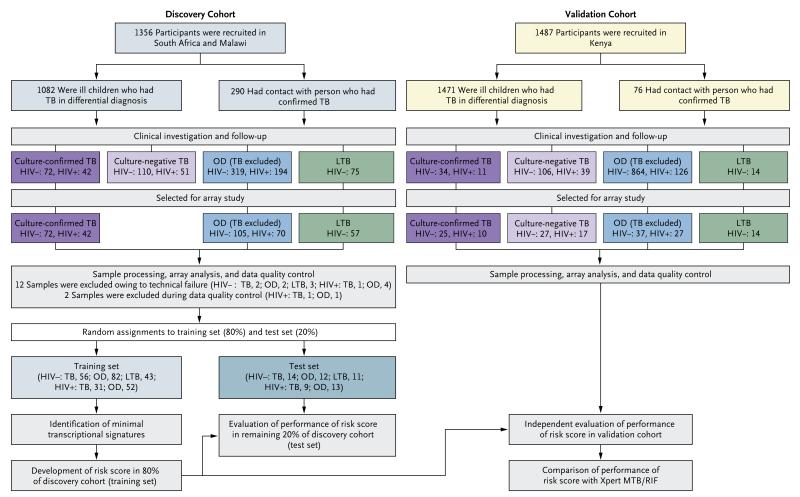
We recruited children from three African countries with a high burden of tuberculosis. To identify RNA-transcript signatures associated with active tuberculosis, we used a discovery cohort comprising children evaluated for suspected tuberculosis in hospitals in South Africa and Malawi. We then assessed the performance of these signatures in an independent validation cohort of children evaluated for suspected tuberculosis in hospitals in Kenya. The overall study design is shown in Figure 1. Further details on the study design and study sites are provided in the protocol and in the Methods section and Figure S1 in the Supplementary Appendix. Gene-expression data are available at the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and can be accessed through GEO Series accession number GSE39941 at www.ncbi.nlm.nih.gov/geo.
Figure 1. Overall Study Design and Numbers of Children Included in the Discovery and Validation Cohorts.
Patients in the discovery cohort were assigned to either the training set (80%) or the test set (20%); the signatures found in the discovery cohort were tested in the test set and then applied in the independent validation cohort. In the discovery cohort, 16 patients were excluded because of withdrawal of consent or inadequate sample collection; during clinical investigation and follow-up, samples were excluded because of inconclusive diagnoses; and during selection for array, samples were randomly selected. In the validation cohort, the patients with tuberculosis in differential diagnosis included 60 patients with features of tuberculosis on screening who had contact with a person with tuberculosis. HIV denotes human immunodeficiency virus, LTB latent tuberculosis (TB), and OD other diseases.
DIAGNOSTIC PROCESS
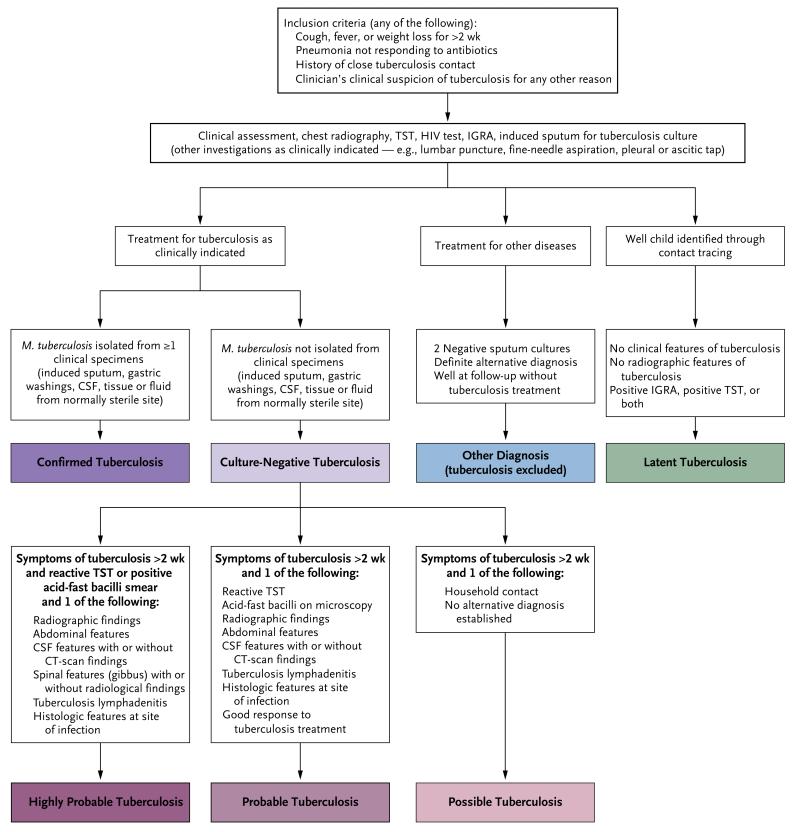
A systematic diagnostic evaluation was performed in children younger than 15 years of age who had a cough, fever, or weight loss of more than 2 weeks’ duration; pneumonia that was unresponsive to antibiotics; any other clinical findings that were suggestive of tuberculosis; or a history of close contact with an adult who had tuberculosis (Fig. 2). The investigation included chest radiography, measurement of the C-reactive protein level, a serologic test or polymerase-chain-reaction (PCR) assay for HIV, and a tuberculin skin test, with or without an IGRA. Two spontaneous or induced sputum samples and a specimen of tissue or cerebrospinal fluid (if clinically indicated) were examined for acid-fast bacilli and cultured for mycobacteria. The Xpert MTB/RIF21 real-time PCR assay (a test for M. tuberculosis and resistance to rifampin) was performed on respiratory samples in the Kenyan cohort. Bacterial cultures, histologic examination of tissue-biopsy specimens, and analysis of blood films for the presence of malaria were performed as clinically indicated. Clinical follow-up was undertaken at 3 months to confirm that children with latent tuberculosis infection remained free of active tuberculosis and other diseases and to determine whether there had been a response to treatment in children with confirmed or suspected tuberculosis.
Figure 2. Diagnostic Algorithm.
With regard to the inclusion criteria, patients with failure to thrive for more than 4 weeks were included in the Kenyan cohort. In the group receiving treatment for other diseases, patients with IGRA-positive results were excluded from the South Africa and Malawi cohorts but included in the Kenyan cohort. In the culture-negative group, the IGRA was repeated for patients in whom tuberculosis was suspected and the initial IGRA was negative; findings on radiography included effusion, extensive consolidation, cavitation, lymphadenopathy, miliary disease, and lobar pneumonia that was not responding to antibiotics, and abdominal features included ascites and lymphadenopathy. CSF denotes cerebrospinal fluid, CT computed tomography, IGRA interferon-γ-release assay, and TST tuberculin skin test.
CASE DEFINITIONS
Culture-confirmed tuberculosis was defined as the isolation of M. tuberculosis from a child with clinical features of tuberculosis, and culture-negative tuberculosis was defined as a negative mycobacterial culture in a child with clinical and radiologic features that prompted empirical treatment for tuberculosis. Culture-negative tuberculosis was further categorized as a case in which tuberculosis was highly probable, probable, or possible on the basis of a priori study definitions (Fig. 2). Children were classified as having latent tuberculosis infection if they had contact with a person who had a positive smear for tuberculosis, were healthy on presentation and follow-up, and had positive results on both the tuberculin skin test and the IGRA if in the discovery cohort and had positive results on either the tuberculin skin test or the IGRA if in the validation cohort. Children were classified as having diseases other than tuberculosis if they received a definitive alternative diagnosis or had no clinical deterioration on follow-up in the absence of tuberculosis therapy (Fig. 2). Since a positive result on an IGRA in the group of patients with diseases other than tuberculosis might indicate either latent tuberculosis infection or primary tuberculosis that had resolved without treatment, we excluded patients in the discovery cohort who had a positive result on an IGRA. In the Kenyan validation cohort, patients who had diseases other than tuberculosis were included in the study regardless of whether an IGRA result was positive or negative.
Microarray Analysis of Blood RNA Expression
Whole blood was collected in PAXgene Blood RNA Tubes (PreAnalytiX) at the time of study recruitment, frozen within 6 hours after collection, and later extracted with the use of PAXgene Blood RNA Kits. RNA was shipped to the Genome Institute of Singapore for analysis on HumanHT-12 v.4 Expression BeadChip arrays (Illumina). Information on microarray methods, quality control, and analysis is provided in the Methods section and Figure S2 in the Supplementary Appendix.
STATISTICAL ANALYSIS
Gene-expression data were analyzed with the use of R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing). Patients in the discovery cohort were assigned to training and test sets (80% and 20% of the cohort, respectively). We used the training set to identify transcripts that were differentially expressed between tuberculosis and other diseases and also between active tuberculosis and latent infection, irrespective of HIV status or geographic location; differential expression was defined as an absolute log2 intensity ratio of more than 0.5. To identify the smallest number of transcripts distinguishing tuberculosis from the comparator groups, we subjected these transcripts to variable selection using elastic net22 (see the Methods section in the Supplementary Appendix).
Array-based technologies are not appropriate for use in resource-poor regions because of their cost and the complex technology required. We therefore developed a method for translating multitranscript RNA signatures into a single score for disease risk that could form the basis of a simple diagnostic test. Transcripts from the minimal signatures were classified as up-regulated or down-regulated on the basis of their expression relative to each comparator group in the training data set. The risk score for disease was derived by adding the total intensity of the up-regulated transcripts and subtracting the total intensity of the down-regulated transcripts (see Equation 1 in the Methods section in the Supplementary Appendix). For each patient, we calculated the risk score using the minimal transcript sets for tuberculosis as compared with other diseases and as compared with latent tuberculosis infection.
In the Kenyan validation cohort, we compared the performance of the disease risk score with that of Xpert MTB/RIF assay within each of four groups: the group with culture-confirmed tuberculosis and the culture-negative groups with highly probable, probable, or possible tuberculosis. In each evaluation, the same tuberculosis-negative comparator group (i.e., children with diseases other than tuberculosis) was used to calculate test specificity (Table 1). We used a range of estimates of the true rate of positive test results for tuberculosis in the groups with highly probable, probable, or possible tuberculosis in order to model the performance of the risk score and the Xpert MTB/RIF assay and to provide an estimate of the sensitivity of each test (effective sensitivity) (see the Methods section in the Supplementary Appendix).
Table 1. Clinical Characteristics of Children in the Kenyan Validation Cohort*.
| Characteristic | Culture-Confirmed TB, HIV-Negative (N =25) |
Culture-Confirmed TB, HIV-Positive (N =10)† |
Culture-Negative TB, HIV-Negative (N = 27) |
Culture-Negative TB, HIV-Positive (N = 17)† |
Latent Infection, HIV-Negative (N = 14) |
Other Diseases, HIV-Negative (N = 37) |
Other Diseases, HIV-Positive (N =27)† |
|---|---|---|---|---|---|---|---|
| Age — mo | |||||||
| Median | 37 | 101 | 22 | 32 | 33 | 20 | 38 |
| Interquartile range | 12 to 106 | 53 to 127 | 12 to 40 | 18 to 60 | 18 to 43 | 11 to 89 | 16 to 78 |
| Male sex — no. (%) | 13 (52) | 6 (60) | 15 (56) | 10 (59) | 7(50) | 24 (65) | 19 (70) |
| Weight-for-age z score‡ | |||||||
| Median | −2.4 | −3.6 | −2.5 | −3.5 | −1.6 | −2.7 | −3.3 |
| Interquartile range | −3.4 to−1.0 | −4.3 to −2.7 | −3.6 to−1.6 | −4.5 to−3.0 | −2.1 to−1.2 | −3.8 to −2.2 | −4.4 to−1.7 |
| BCG vaccination — no. (%) | 24 (96) | 9 (90) | 23 (85) | 16 (94) | 11 (79) | 33 (89) | 23/26 (88) |
| History of close TB contact — no. (%)§ | 14 (56) | 8 (80) | 14 (52) | 7(41) | 14 (100) | 2(5) | 4(15) |
| Positive tuberculin skin test — no. (%)¶ | 17 (68) | 3 (30) | 13 (48) | 2(12) | 14 (100) | 1(3) | 3(11) |
| Positive IGRA—no/total no. (%)1 | 7/10 (70) | 2/5 (40) | 9/14 (64) | 3/10 (30) | 14 (100) | 8/32 (25) | 1/22 (5) |
| Cough for >2 wk — no. (%) | 15 (60) | 8 (80) | 14 (52) | 14 (82) | 0 | 10 (27) | 13 (48) |
| Fever for >2 wk — no. (%) | 15 (60) | 7(70) | 9 (33) | 9(53) | 0 | 11 (30) | 10 (37) |
| Night sweats for >2 wk — no. (%) | 9(36) | 6 (60) | 10 (37) | 2(12) | 0 | 4(11) | 6(22) |
| Weight loss or failure to thrive — no. (%) | 16 (64) | 9 (90) | 18 (67) | 13 (76) | 1(7) | 30 (81) | 20 (74) |
| Radiographic features ofTB — no. (%) | 19 (76) | 9 (90) | 17 (63) | 12 (71) | 0 | 10 (27) | 11 (41) |
| Culture-positive TB — no. (%) | 25 (100)∥ | 10 (100)I | 0 | 0 | 0 | 0 | 0 |
BCG denotes bacille Calmette–Guerin, HIV human immunodeficiency virus, IGRA interferon-γ–release assay, and TB tuberculosis. Where data on a particular feature were not available for all patients in a group, the denominator for which data were available is indicated.
Among the children who were HIV-positive, 16 were receiving antiretroviral therapy.
The weight-for-age z score is based on the number of standard deviations ofthe actual weight ofa child from the median weight of children ofthe same age and sex. The weight-for-age standards were derived with the use ofthe National Center for Health Statistics–World Health Organization international reference population.
A positive result on the tuberculin skin test was defined according to guidelines from the World Health Organization as an induration of 10 mm or more in children without HIV infection or severe malnutrition and as 5 mm or more in children with HIV infection or severe malnutrition.
The total number is the number of children who were tested for whom valid results were available.
Among the children who had a positive culture for TB, six also had positive results on smears for acid-fast bacilli.
RESULTS
DISCOVERY COHORT
After screening and evaluating 1356 children in South Africa and Malawi for symptoms of tuberculosis, we included 157 patients from South Africa and 189 patients from Malawi in the RNA expression studies. Of these 346 children, 114 had culture-confirmed tuberculosis, 175 had diseases other than tuberculosis, and 57 had latent tuberculosis infection. The discovery cohort included only those children with tuberculosis that was confirmed on culture; children for whom the diagnosis of tuberculosis could not be confidently established or ruled out were excluded. (Details of recruitment are provided in Fig. S1A and S1B and clinical details in Table S1A and S1B in the Supplementary Appendix.)
IDENTIFICATION OF TUBERCULOSIS SIGNATURE
In the training set (comprising 80% of samples from the discovery cohort), we identified 409 transcripts that were differentially expressed between tuberculosis and other diseases and 3434 transcripts that were differentially expressed between tuberculosis and latent infection. Using variable selection to identify the smallest number of transcripts that distinguished each group, we found that 51 transcripts distinguished tuberculosis from other diseases and 42 distinguished tuberculosis from latent infection (Table S2A and S2B in the Supplementary Appendix). These minimal transcript sets were used to generate a risk score for each patient in the test set (comprising the remaining 20% of samples from the discovery cohort) that distinguished tuberculosis from other diseases (sensitivity of 78% and specificity of 74%) and that distinguished tuberculosis from latent infection (sensitivity of 96% and specificity of 91%) (Table 2, and Table S3 and Fig. S3 and S4 in the Supplementary Appendix).
ASSESSMENT OF RISK SCORE IN VALIDATION COHORT
A total of 1599 children presenting to hospitals in Kenya met the inclusion criteria for the study, and 1471 were evaluated for tuberculosis.23 We included 157 of these children in a nested case-control study that included the group with culture-confirmed tuberculosis and all subgroups in the group with culture-negative tuberculosis (i.e., those in whom tuberculosis was highly probable, probable, or possible). We included all patients with culture-confirmed tuberculosis or latent infection for whom we had adequate RNA samples (35 and 14 patients, respectively), 44 patients with culture-negative tuberculosis (8 patients in whom tuberculosis was highly probable, 19 in whom it was probable, and 17 in whom it was possible) (Table S4 in the Supplementary Appendix), and 64 randomly selected patients from the group with diseases other than tuberculosis (55 with negative IGRA results and 9 with positive IGRA results). Clinical features of the patients included in the microarray study, which are summarized in Table 1, were similar to the clinical features of patients who were not included, with two exceptions: in the group with probable tuberculosis, a history of close contact with a person who had tuberculosis was more common among patients included in the microarray study (Table S5A in the Supplementary Appendix), and in the group with diseases other than tuberculosis, weight loss and pleural effusion were more common among patients who were included (Table S5B in the Supplementary Appendix).
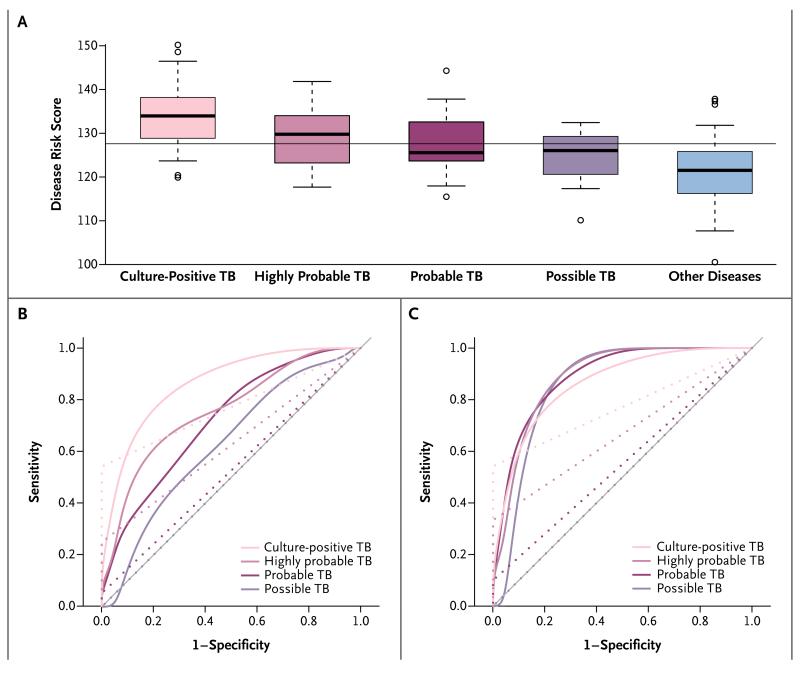
The risk score discriminated culture-confirmed tuberculosis from other diseases in patients with or without HIV infection with a sensitivity of 82.9% and a specificity of 83.6% when patients with other diseases who had a positive IGRA result were excluded. When patients in the group with diseases other than tuberculosis who had a positive IGRA result were included, the specificity and sensitivity of the risk score were not affected (Table 2 and Fig. 3, and Table S6 and Fig. S5 in the Supplementary Appendix). The majority of patients in the group with diseases other than tuberculosis who had a positive IGRA result were classified as not having tuberculosis (7 of 9 patients) (see the Methods section and Fig. S3 in the Supplementary Appendix). Among patients with negative cultures who were treated for tuberculosis, the risk score identified 62.5% of those in whom tuberculosis was highly probable, 42.1% of those in whom it was probable, and 35.3% of those in whom it was possible. Since it was not known what proportion of patients had actual tuberculosis (as opposed to patients who were treated on the basis of suspicion of disease), we adjusted for the estimated prevalence of actual tuberculosis in each of these subgroups to calculate an effective sensitivity (see the Methods section in the Supplementary Appendix); the effective sensitivity of the risk score for highly probable, probable, and possible cases of tuberculosis was 67.6 to 82.3%, 59.3 to 80.8%, and 54.3 to 79.6%, respectively (Table S7 in the Supplementary Appendix). The sensitivity of the risk score was higher than that of the Xpert MTB/RIF assay in all tuberculosis categories, with the Xpert MTB/RIF assay having sensitivities of 27.8 to 35.7% for the subgroup in which tuberculosis was highly probable, 8.8 to 13.3% for that in which it was probable, and 0% for that in which it was possible (Fig. 3, and Table S7 in the Supplementary Appendix). However, the Xpert MTB/RIF assay was highly specific (100%). The risk score also distinguished tuberculosis from latent infection, with a sensitivity of 94% and a specificity of 100% (Table S3 and Fig. S4 in the Supplementary Appendix). Finally, we compared the diagnostic performance of the risk score with that of the IGRA and measurement of the C-reactive protein level (which has been reported as a biomarker of tuberculosis24). The C-reactive protein level proved to be of no value in discriminating childhood tuberculosis from other diseases (Fig. S6 in the Supplementary Appendix), and for this purpose, the risk score was significantly more sensitive than the IGRA (82.9% vs. 60.0%) (Table 2).
Table 2. Diagnostic Performance of the Risk Score in the Discovery and Validation Cohorts and as Compared with the IGRA and the Xpert MTB/RIF Assay in the Validation Cohort*.
| Performance Measure | Risk Score | IGRA | Xpert MTB/RIF Assay† | ||
|---|---|---|---|---|---|
| Test Set in the Discovery Cohort | Validation Cohort, Excluding Children with Positive IGRA in Group with Other Diseases | Validation Cohort, Including Children with Positive IGRA in Group with Other Diseases | Validation Cohort | Validation Cohort | |
| No. of patients with TB | 23 | 35 | 35 | 35 | 35 |
| No. of patients with other diseases | 34 | 55 | 64 | 54 | 55 |
| Area under ROC curve (95% CI) | 86.2 (77.1–94.0) | 89.0 (82.3–94.9) | 89.0 (81.9–95.3) | 71.7 (58.3–84.8) | 77.1 (69.9–85.7) |
| Sensitivity — % (95% CI) | 78.3 (60.9–95.7) | 82.9 (68.6–94.3) | 82.9 (68.6–94.3) | 60.0 (33.3–86.67) | 54.3 (37.1–68.6) |
| Specificity — % (95% CI) | 73.5 (55.9–88.2) | 83.6 (74.6–92.7) | 82.8 (73.4–92.2) | 83.3 (72.2–92.6) | 100.0 (100.0–100.0) |
Patients in the discovery cohort of South African and Malawian children were assigned to training and test sets (80% and 20% of the cohort, respectively). The 51-transcript signature used in the group with diseases other than TB was derived from data on the South African and Malawian patients in the training set and applied to the independent Kenyan validation cohort. All analyses included children with and those without HIV infection. Sensitivity and specificity were calculated with the use of a weighted threshold for classification. ROC denotes receiver-operating-characteristic. CI denotes confidence interval.
The Xpert MTB/RIF assay was positive for 19 of 35 patients with culture-confirmed tuberculosis and none of 55 patients with diseases other than tuberculosis.
Figure 3. Risk Scores and Sensitivity and Specificity in the Kenyan Validation Cohort, According to Diagnostic Group.
Panel A shows the risk scores for tuberculosis according to study group, calculated with the use of a 51-transcript signature applied to the independent Kenyan validation cohort, in which culture-positive tuberculosis was reported in 35 patients, diseases other than tuberculosis were reported in 55 patients, and culture-negative tuberculosis was reported as highly probable in 5 patients, probable in 19 patients, and possible in 17 patients. The bar within each box indicates the median score, the bottom and top of the box indicate the interquartile range, the bars below and above the box are at a distance of 0.8 times the interquartile range from the upper and lower edges of the box, and the circles indicate outliers; the horizontal line across the graph indicates the mean score. Panel B shows smoothed receiver-operating-characteristic (ROC) curves for the sensitivity and specificity of the risk score (solid lines) and the Xpert MTB/RIF assay (dotted lines). Panel C shows ROC curves based on an adjusted analysis in which the actual prevalence of disease was assumed to be 80% among patients in whom the disease was highly probable, 50% among those in whom it was probable, and 40% among those in whom it was possible. Sensitivity and specificity are reported in Table S7 in the Supplementary Appendix.
To explore the ways in which the risk score might contribute to the diagnosis of tuberculosis in clinical practice, we evaluated its positive and negative predictive value assuming different prevalences of tuberculosis, as follows: 10% (the prevalence in the validation cohort), 30% (the prevalence in the discovery cohort), and 50% (the prevalence that might be expected in a non-research setting with greater pretest filtering according to clinical findings). We also included a range of estimates of actual tuberculosis in the study group with culture-negative results (for more information, see the Methods section in the Supplementary Appendix). The negative predictive value was consistently high in all these analyses. As expected, the positive predictive value was higher when more targeted clinical criteria were used to select patients for testing (Table S8 in the Supplementary Appendix).
DISCUSSION
We identified a tuberculosis-specific transcriptome signature in host blood that appears to be valuable in distinguishing tuberculosis from other diseases with similar clinical features in HIV-positive and HIV-negative African children. This prospective study involved the identification of tuberculosis signatures in cohorts from two countries and validation in an independent cohort in a third country. Our findings extend the results of previous studies of transcriptome signatures in adults and children with tuberculosis.25-33
The major challenge in evaluating new biomarkers of childhood tuberculosis is the lack of a reference standard against which to evaluate them,1,2,20 since microbiologic confirmation is achieved in a minority of patients who are treated for tuberculosis. It is generally accepted that clinical diagnostic scores overdiagnose tuberculosis,34 but the extent of overdiagnosis is unknown. Conversely, the diagnosis of tuberculosis is often overlooked in patients who have the disease, and they are often inadvertently treated for other diseases.2 To address this challenge, we first compared the performance of our transcriptome signatures and risk score for disease with the reference standard of culture-confirmed tuberculosis; we then assessed the performance of the signatures in the culture-negative group, for which no reference standard is available. To evaluate biomarkers in patients with culture-negative tuberculosis, we developed an approach in which estimates of the true proportion of patients with tuberculosis in three subgroups (those for whom a diagnosis of tuberculosis was considered highly probable, probable, or possible) were used to calculate an effective sensitivity of the risk score. The gradient in the performance of the risk score in the culture-negative groups was consistent with the different degrees of diagnostic certainty in each group. Our findings suggest that the risk score provides an improved estimate of the actual prevalence of tuberculosis in each diagnostic category and indicate that there is considerable overdiagnosis and overtreatment of childhood tuberculosis, even in research settings where more sophisticated diagnostic tools are available than in most African hospitals.
To define the potential role of our RNA-based approach, we compared our risk score for tuberculosis with other available diagnostic methods, including culture, the Xpert MTB/RIF assay, measurement of the C-reactive protein level, and the IGRA. Although the Xpert MTB/RIF assay is highly specific, our findings confirm the results of other studies35 showing that the sensitivity of this assay for childhood tuberculosis is limited. Our risk score identified higher proportions of culture-confirmed cases of tuberculosis and culture-negative cases than did the Xpert MTB/RIF assay. The risk score was also more sensitive than the IGRA; the C-reactive protein level proved to be a poor biomarker of childhood tuberculosis.
Use of the disease risk score did result in the misclassification of some cases of nontuberculous disease as tuberculosis, which may reflect reduced specificity (perhaps resulting from the wide variation in the other diseases in the study population) or the difficulty of definitively ruling out a diagnosis of tuberculosis among children in populations in which malnutrition, HIV infection, other infections, and primary tuberculosis are common.1 Conversely, some of the patients with culture-negative tuberculosis may in fact have had other diseases that were self-limiting. In areas where there is a high burden of tuberculosis, a considerable proportion of healthy children have latent tuberculosis infection and have positive IGRA results. Since there was no way of establishing whether a child with diseases other than tuberculosis who also had a positive IGRA result had primary tuberculosis or latent infection, we evaluated our risk score with and without the inclusion of children with positive IGRA results. Our finding that the risk score distinguished the majority of children with other diseases from children with tuberculosis, regardless of IGRA positivity, highlights the potential value of the risk score in populations where latent infection is common.
The translation of transcriptional signatures into diagnostic tools in resource-poor communities is challenging. Innovation will be needed to reduce the cost and complexity of the current methods used to detect multiple RNA transcripts.
The best application of a blood test based on our risk score and transcriptional signatures in clinical practice requires further study. The fact that the negative predictive value of the risk score is higher than its positive predictive value suggests that the score may be more useful for ruling out tuberculosis than for confirming the diagnosis. The development of a test based on this risk score for tuberculosis could improve the diagnosis and surveillance of childhood tuberculosis in areas with a high or low burden of disease.
Supplementary Material
Acknowledgments
Supported by a grant from the European Union Action for Diseases of Poverty Program (Sante–2006–105-061), a Malawi–Liverpool–Wellcome Trust Clinical Research Program Core Grant, United Kingdom (084679–Z–08–Z, to Dr. Heyderman), and additional grants from the Wellcome Trust (084323 and 088316, to Dr. Wilkinson; 081835, to Dr. Scott; and 081697, to Dr. Brent); the study also made use of infrastructure and staff at the Wellcome Trust–supported programs in Blantyre, Malawi, and Kilifi, Kenya, the University of Cape Town (084323, 077092), the Imperial College Wellcome Trust Centre for Clinical Tropical Medicine, and the Imperial College Wellcome Trust Centre for Global Health Research.
We thank the patients who participated in the study and Dr. Hajara Elbusaidy (provincial tuberculosis and leprosy coordinator, Coast Province, Kenya) for her support of the study.
Appendix
The authors’ affiliations are as follows: Brighton and Sussex Medical School, University of Sussex, Brighton (S.T.A., C.M.B., F.K.), Section of Paediatrics and Wellcome Trust Centre for Clinical Tropical Medicine, Division of Infectious Diseases, Department of Medicine (M.K., A.J.B., V.J.W., M.S.H., P.R.L., R.J.W., M.L.), and the Department of Genomics of Common Disease, School of Public Health (M.K., L.J.C.), Imperial College London, and the Departments of Infectious Disease Epidemiology (A.C.C., N.F.) and Immunology and Infection (H.M.D.), London School of Hygiene and Tropical Medicine, London, Nuffield Division of Clinical Laboratory Sciences, University of Oxford, Oxford (A.J.B.), and the Institute of Infection and Global Health, University of Liverpool (N.F.), and Liverpool School of Tropical Medicine (R.S.H.), Liverpool — all in the United Kingdom; Malawi–Liverpool–Wellcome Trust Clinical Research Programme, University of Malawi College of Medicine (S.T.A., C.M.B., G.C., R.M., R.S.H.), and Department of Pediatrics, Queen Elizabeth Central Hospital (G.C., R.M.), Blantyre, and the Karonga Prevention Study, Chilumba, Karonga District (A.C.C., N.F.) — all in Malawi; Kenya Medical Research Institute–Wellcome Trust Research Programme, Kilifi (A.J.B., J.A.G.S.), and the Department of Paediatrics, Coast Provincial General Hospital, Mombasa (H.T.) — both in Kenya; Infectious Disease, Genome Institute of Singapore, Singapore (M.L.H., L.L.); the Department of Infectious Diseases, Leiden University Medical Center, Leiden, the Netherlands (T.H.M.O.); Red Cross War Memorial Children’s Hospital (S.P., V.P., B.E.) and Institute of Infectious Disease and Molecular Medicine (R.J.W.), University of Cape Town, Cape Town, South Africa; and the Institute for Molecular Bioscience, University of Queens land, St. Lucia, Australia (L.J.C.).
Footnotes
The authors’ affiliations are listed in the Appendix.
The authors report that some of the work has been submitted as a patent application by Imperial College Innovations. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367:348–61. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 2.Global tuberculosis report 2012. World Health Organization; Geneva: 2012. [Google Scholar]
- 3.Zar HJ, Connell TG, Nicol M. Diagnosis of pulmonary tuberculosis in children: new advances. Expert Rev Anti Infect Ther. 2010;8:277–88. doi: 10.1586/eri.10.9. [DOI] [PubMed] [Google Scholar]
- 4.Marais BJ, Gie RP, Obihara CC, Hesseling AC, Schaaf HS, Beyers N. Well defined symptoms are of value in the diagnosis of childhood pulmonary tuberculosis. Arch Dis Child. 2005;90:1162–5. doi: 10.1136/adc.2004.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatherill M, Hanslo M, Hawkridge T, et al. Structured approaches for the screening and diagnosis of childhood tuberculosis in a high prevalence region of South Africa. Bull World Health Organ. 2010;88:312–20. doi: 10.2471/BLT.09.062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis. 2002;6:1038–45. [PubMed] [Google Scholar]
- 7.Pearce EC, Woodward JF, Nyandiko WM, Vreeman RC, Ayaya SO. A systematic review of clinical diagnostic systems used in the diagnosis of tuberculosis in children. AIDS Res Treat. 2012;2012:401896. doi: 10.1155/2012/401896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuevas LE, Petrucci R, Swaminathan S. Tuberculosis diagnostics for children in high-burden countries: what is available and what is needed. Paediatr Int Child Health. 2012;32(Suppl 2):S30–S37. doi: 10.1179/2046904712Z.00000000076. [DOI] [PubMed] [Google Scholar]
- 9.Drobac PC, Shin SS, Huamani P, et al. Risk factors for in-hospital mortality among children with tuberculosis: the 25-year experience in Peru. Pediatrics. 2012;130(2):e373–e379. doi: 10.1542/peds.2011-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet. 2002;360:985–90. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 11.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365:130–4. doi: 10.1016/S0140-6736(05)17702-2. [Erratum, Lancet 2005;365:1926] [DOI] [PubMed] [Google Scholar]
- 12.Swingler GH, du Toit G, Andronikou S, van der Merwe L, Zar HJ. Diagnostic accuracy of chest radiography in detecting mediastinal lymphadenopathy in suspected pulmonary tuberculosis. Arch Dis Child. 2005;90:1153–6. doi: 10.1136/adc.2004.062315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J. 2011;30:694–700. doi: 10.1097/INF.0b013e318214b915. [DOI] [PubMed] [Google Scholar]
- 14.Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018–32. doi: 10.5588/ijtld.10.0631. [DOI] [PubMed] [Google Scholar]
- 15.Eamranond P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int J Tuberc Lung Dis. 2001;5:594–603. [PubMed] [Google Scholar]
- 16.Graham SM, Marais BJ, Gie RP. Clinical features and index of suspicion of tuberculosis in children. In: Schaaf HS, Zumla A, editors. Tuberculosis: a comprehensive reference. Saunders Elsevier; Philadelphia: 2009. pp. 154–63. [Google Scholar]
- 17.Kampmann B, Whittaker E, Williams A, et al. Interferon-gamma release assays do not identify more children with active tuberculosis than the tuberculin skin test. Eur Respir J. 2009;33:1374–82. doi: 10.1183/09031936.00153408. [DOI] [PubMed] [Google Scholar]
- 18.Brent AJ. Childhood TB surveillance: bridging the knowledge gap to inform policy. J Trop Med. 2012;2012:865436. doi: 10.1155/2012/865436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatherill M, Verver S, Mahomed H. Consensus statement on diagnostic end points for infant tuberculosis vaccine trials. Clin Infect Dis. 2012;54:493–501. doi: 10.1093/cid/cir823. [DOI] [PubMed] [Google Scholar]
- 20.Whittaker E, Zar HJ. Promising directions in the diagnosis of childhood tuberculosis. Expert Rev Respir Med. 2012;6:385–95. doi: 10.1586/ers.12.36. [DOI] [PubMed] [Google Scholar]
- 21.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc B. 2005;67:301–20. [Google Scholar]
- 23.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children. 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease: consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S199–S208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallis RS, Pai M, Menzies D, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–37. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 25.Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen M, Repsilber D, Gutschmidt A, et al. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J Mol Med. 2007;85:613–21. doi: 10.1007/s00109-007-0157-6. [DOI] [PubMed] [Google Scholar]
- 27.Lesho E, Forestiero FJ, Hirata MH, et al. Transcriptional responses of host peripheral blood cells to tuberculosis infection. Tuberculosis (Edinb) 2011;91:390–9. doi: 10.1016/j.tube.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Lu C, Wu J, Wang H, et al. Novel biomarkers distinguishing active tuberculosis from latent infection identified by gene expression profile of peripheral blood mononuclear cells. PLoS One. 2011;6(8):e24290. doi: 10.1371/journal.pone.0024290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maertzdorf J, Weiner J, III, Mollenkopf HJ, et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A. 2012;109:7853–8. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mistry R, Cliff JM, Clayton CL, et al. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J Infect Dis. 2007;195:357–65. doi: 10.1086/510397. [DOI] [PubMed] [Google Scholar]
- 31.Ottenhoff TH, Dass RH, Yang N, et al. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS One. 2012;7(9):e45839. doi: 10.1371/journal.pone.0045839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhagen LM, Zomer A, Maes M, et al. A predictive signature gene set for discriminating active from latent tuberculosis in Warao Amerindian children. BMC Genomics. 2013;14:74. doi: 10.1186/1471-2164-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaforou M, Wright VJ, Oni T, et al. Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS Med. 2013;10(10):e1001538. doi: 10.1371/journal.pmed.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuevas LE, Browning R, Bossuyt P, et al. Evaluation of tuberculosis diagnostics in children. 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children: consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S209–S215. doi: 10.1093/infdis/jir879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicol MP, Workman L, Isaacs W, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis. 2011;11:819–24. doi: 10.1016/S1473-3099(11)70167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.