Abstract
The human immune system is comprised of cellular and molecular components designed to coordinately prevent infection while avoiding potentially harmful inflammation and auto-immunity. Immunity varies with age, reflecting unique age-dependent challenges including fetal gestation, the neonatal phase and infancy. Herein, we review novel mechanistic insights into early life immunity, with emphasis on emerging models of human immune ontogeny, which may inform age-specific translational development of novel anti-infectives, immunomodulators and vaccines.
Keywords: Neonate, Newborn, Infant, Immune Ontogeny, Toll-like receptor (TLR), Dendritic cell, Vaccines
Challenges in newborn and infant immunity
Most of the global mortality under the age of 5 is due to infection and is concentrated among newborns and young infants who are particularly susceptible to microbes [1–4]. In this context, a growing number of biomedical investigators and biomedical funding agencies have focused on a more complete understanding of early life immunity. Such studies may not only uncover important principles of immune ontogeny, but may inform development of novel vaccines and anti-infective strategies. Newborns express a unique immune system rendering them vulnerable to infection, in part due to distinct immune responses specifically adapted for early postnatal life [1]. Although the early life immune system does enable certain microbe-induced responses, its distinct nature, including reduced pro-inflammatory/T helper 1 (Th1) cell-polarizing function, impairs responses to microbes and most vaccines. Technological advances have facilitated modeling of immune ontogeny both in vitro and in vivo providing fresh insights and opening new horizons in this area of biomedical importance. Concurrently, several recent significant policy and funding initiatives have promoted characterization of neonatal and infant health. Among these initiatives are the National Institute of Health funding program focused on The Infant Immune System: Implications for Vaccines and Response to Infections [3] and the Bill & Melinda Gates Foundation’s Decade of Vaccines initiative that focuses on reducing the burden of infectious diseases for people in resource-limited settings [5, 6]. The importance of taking into consideration the distinct nature of early life immunity is a key concept shared by these and other funding agencies.
Key to understanding early life immunity is the concept of ontogeny- the development in an individual of the immune system from fetal life though adulthood (Figure 1). Unique features of human immunity in early life include age-dependent innate responses to danger- or pathogen-associated molecular patterns (DAMPs and PAMPs, respectively) [2, 7] as well as of adaptive immune responses to pathogens and vaccines [8, 9]. Upon challenge with immune stimuli, children under the age of 2 months express an innate Th-2- and Th-17 cell polarization, weak Th1-polarization, and low innate antiviral type 1 interferon (IFN) responses [10]. Of note, the pattern of cytokine induction in early life corresponds to age-dependent susceptibility to infection: (a) Impaired Th17 responses in preterm newborns correspond to increased susceptibility to infections with extracellular pathogens such as E. coli and Candida spp [10], and (b) impaired Th1 responses correspond to increased risk of infection with intracellular pathogens such as Listeria monocytogenes, Mycobacterium tuberculosis and herpes simplex virus. Relatively weak innate Th1-polarizing cytokine production gradually matures during infancy. Moreover, given the limited exposure to antigen (Ag) in early life, both the T cell and B cell lymphocyte compartments exhibit age-dependent maturation, with low numbers of memory-effector T and B cells detectable after birth into early infancy [11]. These distinct neonatal/newborn Th17- and early-life-infant Th2- polarized responses, in combination with fewer memory-effector cells, potentially limit the efficacy of early life immune responses against intracellular infections and diminish Th1 vaccine responses [2, 12]. Nevertheless, certain pathogenic organisms, adjuvants and self-adjuvanted vaccines may induce inflammatory and/or Th1-polarizing responses in early life, possibly by stimulating distinct and/or multiple innate immune receptors/pathways in an additive or synergistic manner.
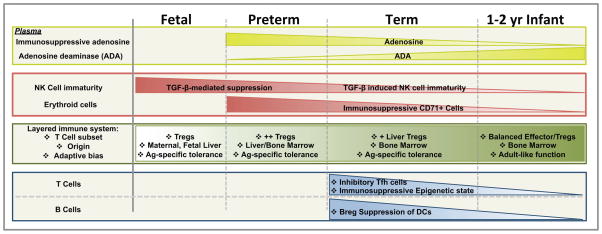
Figure 1. Ontogeny of Early Life Immunomodulation.
Preterm (<37 weeks gestational age, GA) and full-term newborns (37–41 weeks GA). Abbreviations: ADA (adenosine deaminase); Breg (Regulatory B Cell); NK (Natural Killer); Tfh (T follicular helper cells); TGF-β (Transforming growth factor beta); Tregs (Regulatory T Cells)
Modulation of the maternal immune system during fetal gestation and postnatal microbial colonization may play fundamental roles in the induction, training, and function of the host immune system, as has been recently reviewed [13–15]. Herein we review ontogeny of infant immunity including the response to immunization, with a focus on neonatal immunity. We summarize current and emerging methodologies to characterize early life immune ontogeny, many of which are centered on in vitro studies of human cord or infant peripheral blood, or blood-derived leukocytes, and in vivo studies of newborn mice, non-human primates or adult humans. We consider the importance of often under-appreciated soluble immunomodulatory factors in autologous plasma as well as cell-based immunity, focusing on the ontogeny of immunosuppressive erythroid precursors, granulocyte/neutrophil function, pattern recognition receptor (PRR)-based responses of antigen-presenting cells (APCs) such as monocytes and dendritic cell (DCs) and of T and B cells. Lastly, we will emphasize how the distinct nature of early life immune ontogeny may inform treatment of infections diseases and development of age-specific immunomodulators and vaccines [3, 16].
Emerging models to study immune ontogeny
Study of human early life immunity is challenging due to the transient nature of this phase of life, inherent logistical obstacles posed by the smaller size of newborns and infants (and therefore typically smaller amounts of biosamples that can be obtained), and the distinct societal place of children. Limitations in primary sample collection (i.e. volumes and sizes) from both human and other animal sources have led researchers to explore new methods, approaches and models. A variety of animal models are available for the study of immune ontogeny and vaccine development [17]. Due to the extensive immunological toolkits and reagents available in combination with the relatively short gestational period (~20 days), neonatal mice are often employed (see Table 1). For similar reasons, mice are also leveraged to study fetal and preterm immune ontogeny [18]. However, these in utero models often require specific skills and facilities, and there is understandably limited accessibility to anatomically distinct immune compartments. Murine and rat models also are limited by species-specific features of the innate immune system, which is hypervariable between species [19, 20]. For example: a) mice express divergent Toll-like receptors (TLRs) that can respond differently than human cells to certain TLR agonists (TLRAs) and b) murine polymorphonuclear leukocytes (PMNs) do not express defensin peptides which are abundant components of human PMN [21]. Study of pigs and non-human primates (for example, the Indian Rhesus macaque -Macaca mulatta) may overcome some of these issues, more closely modeling human immune responses and allow ready accessibility of immune compartments. For example, it is feasible to obtain relatively large volumes of non-human primate placental cord and newborn and infant peripheral whole blood for in vitro stimulation assays [22]. However, research using these animals is costly, as they require highly specialized housing, care, and personnel training (see Table 1).
Table 1.
Approaches to study early life immune ontogeny.
| Method | Advantages of Approach | Limitations of Approach | Refs. |
|---|---|---|---|
| Animal Models/In vivo | |||
| Fetal/preterm mice |
|
|
[18] |
| Neonatal mouse |
|
|
[55, 65, 102, 147–157] |
| Neonatal rat |
|
|
[158, 159] |
| Neonatal piglet |
|
|
[160–163] |
| Neonatal/infant Non-human primate |
|
|
[97, 164–167] (Dowling et al., unpublished) |
| In vitro assays | |||
| Human fetal tissues |
|
|
[57, 90] |
| Non-human primate neonatal/infant whole blood assay |
|
|
[22] |
| Neonatal mice bone marrow and splenic dendritic cells |
|
|
[101] |
| Human neonatal/infant whole blood assay |
|
|
[25, 27, 28, 30, 33, 35, 39, 73, 121, 168, 169] |
| Isolated Mono-nuclear cells & Monocytes |
|
|
[22, 28, 33, 35, 70, 142, 170, 171] |
| Human neonatal monocyte-derived dendritic cells |
|
|
[22, 39] |
| Human neonatal Tissue Construct dendritic cells |
|
|
[172] (Sanchez-Sanchez et al., unpublished) |
| Human Observational Clinical Trials | |||
| Human Neonatal/infant with immune evaluation |
|
|
[32–34, 84, 173, 174] |
In vitro study of human neonatal immune responses has often employed placenta-derived cord blood [23]. Standardized 96-well methods for whole cord blood assays have been developed, incorporating flow cytometry and multiplexing cytokine assays [24]. Since human whole blood assays are practical and relatively inexpensive, they enable longitudinal studies of geographically diverse populations [25, 26] in resource-poor environments [27, 28]. Such longitudinal studies have characterized the ontogeny of interleukin 12 (IL-12)-producing capacity to bacterial components throughout childhood [29], which is diminished early in life. Conversely, TLR9-mediated cytokine responses to CpG oligonucleotide stimulation of cord blood cells induces significantly greater IL-6, CXCL8 (formerly IL-8), IL-1β and the anti-inflammatory cytokine IL-10 compared to peripheral blood from 3 month-old infants [30]. Collection of relatively low (< 10 mL) volumes of human neonatal and infant peripheral blood allows in vitro immune evaluation, as demonstrated in clinical vaccination trials [31–34].
In addition to whole blood assays, mononuclear cell (MC) cultures are also a popular approach to in vitro immune evaluation [33, 35]. Such assays have highlighted numerous ontological immune differences. For example, lipopolysaccharide (LPS; a TLR4 agonist)-induced MC production of Tumor Necrosis Factor (TNF), C-X-C motif chemokine 10 (CXCL10) and IL-12p70 by MCs reaches adult levels between 6–9 months of life [30]. Of note, early life MC poly-functionality- i.e. the ability of a single cell to produce multiple cytokines simultaneously- is diminished [35]. Human neonatal monocyte-derived dendritic cell (MoDC) assays have also been developed [36, 37], which enable evaluation of age-specific adjuvant and adjuvanted-vaccine activation of DCs, which are key to vaccine responses (see Table 1).
In addition to ontological differences in cellular function, increasing attention has turned to age-specific soluble factors that modulate immune responses. Although traditionally human leukocytes have been cultured in xenologous (e.g., fetal bovine) and/or heat-treated serum, a growing literature indicates that these conditions may not accurately model human responses [38]. Accordingly, an increasing number of studies have employed autologous plasma [22, 39], a rich source of multiple soluble factors that modulate immune responses in an age-specific manner [38] (Box 1). Efforts to more accurately predict human immune function in vitro will continue to incorporate the newest available technologies. For example, the study of MC fractions within three-dimensional matrix-assisted microphysiologic tissue constructs, that facilitate the autonomous differentiation of DC subsets may more accurately model DC development in vivo [40]. These new methodologies have accelerated characterization of the ontogeny of multiple leukocyte populations including neutrophils, monocytes, DCs and Natural killer (NK) cells.
Box 1. Ontogeny of plasma-mediated immune regulation and inflammasome-mediated innate immune responses.
Plasma-mediated immune regulation
Plasma, the fluid phase of blood, modulates pro-inflammatory danger signals that initiate host defense during infection. In addition to the high expression of basal anti-inflammatory cytokines (IL-4, IL-10 IL-13, TGF-β) in cord blood vs. adult plasma, various immuno-suppressive plasma factors are expressed at higher concentrations early in human life [38]. These plasma-derived factors, including proteins, lipids, purines, and sugars, may help maintain in utero feto-maternal tolerance; allow for microbial colonization after birth and serve anti-inflammatory/pro-resolving functions during infection [2]. Relative to adult plasma, neonatal plasma, especially that from preterm infants, demonstrates a gestational age-dependent inadequacy in multiple antimicrobial proteins and peptides (APPs), including bactericidal/permeability increasing protein (BPI) and the cathelicidin LL37 [47]. Indeed, replenishing APPs is an attractive novel approach to prevent and/or treat neonatal sepsis [119].
Age-dependent expression of plasma purine metabolizing enzymes may influence inflammatory responses. Adenosine-5′-triphosphate (ATP) is a danger signal that enhances inflammatory responses, including inflammasome activation in neonatal DCs [22]. Sequential de-phosphorylation of ATP generates adenosine that has anti-inflammatory/pro-resolving properties [120]. Of note, newborns have high concentrations of the enzymes that generate adenosine, such as CD73 and alkaline phosphatase, and have relatively lower concentrations of adenosine deaminase (ADA1), an enzyme that deaminates adenosine thereby rending it immunologically inert, resulting in a higher basal concentration of plasma adenosine at birth [51]. Therefore, higher adenosine generation in newborn blood may promote an anti-inflammatory immunological status.
Additional immunomodulatory soluble plasma factors remain to be identified, including two distinct factors that suppress TLR4-mediated IL-12p70 production or induce IL-10 or production [121, 122]. This phenotype is maintained up to 1 month of age. Overall, the distinct composition and immunomodulatory effects of plasma highlight the importance of conducting in vitro assays in autologous plasma conditions as opposed to xenologous (e.g., fetal bovine serum) or heat-treated conditions which may result in divergent and possibly unphysiologic immune responses. Such microphysiologic modeling may enable identification of agents that are more likely to be active in vivo. For example, certain stimuli such as TLR8 agonists are relatively refractory to adenosine inhibition [22, 123], and may be promising candidates as potential neonatal and infant vaccine adjuvants.
Inflammasome-mediated innate immune responses
Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are a cytosolic PRR family that respond to a range of PAMPs and DAMPs by triggering caspase-dependent IL-1β/IL-18 production [124, 125]. Multiple studies have characterized human TLR- and NLR-agonist stimulated cytokine production by monocytes in neonatal and infant whole blood [126]. Prematurity has been associated with reduced IL-1β production in LPS-treated cord blood [127] and defective mononuclear cell production of IL-18 [128]. In contrast, among term newborns, TLR-and NLR-mediated neonatal whole blood IL-1β production is greater than that in adult peripheral blood [28, 35] and slowly decreases to adult levels over the first years of life [27]. Accordingly, IL-1 receptor antagonist concentrations are elevated in neonates then decline to adult levels within days [129]. TLR7/8- [35] and NOD-mediated [130] IL-1β production by neonatal mononuclear cells in vitro are generally comparable with older individuals, while TLR2/1- or TLR4-mediated IL-1β production only becomes equivalent after 12 months of age [73, 84].
Inflammasome-mediated responses to the adjuvant alum are also age-dependent. A study of infants in Papua New Guinea demonstrated that combined alum and LPS-induced IL-1β production in whole blood significantly declined with age (~ 6–18 months) [25]. In the same study, combined alum and LPS-induced IL-1β was also significantly higher in CBMCs vs. PBMCs. Higher IL-1β production in neonatal whole blood may provide an additional level of redundancy for MyD88-dependent signaling via the IL-1R1 complex [131]. In contrast to greater monocyte-derived IL-1 production, neonatal MoDCs demonstrate diminished caspase-1 activation and IL-1β production to (LPS + ATP)-induced inflammasome activation, that can be overcome by stimulation with TLR7 and/or TLR8-activating imidazoquinolines [22].
Distinct ontogeny instructs immune function
Our understanding of early life mammalian immunology has undergone significant advancement over the past two decades. The following section highlights some recent discoveries characterizing cellular and soluble aspects of early life immune ontogeny.
Neutrophils: quantitative and qualitative differences
Neutrophils, the most abundant of the polymorphonuclear leukocytes, are one of the first cellular lines of host defense, eliminating pathogens by multiple microbicidal mechanisms [41]. Preterm and term newborns demonstrate both quantitative and qualitative differences in neutrophil function as compared to older individuals [2]. Early rodent studies demonstrated that, under stress or activation conditions, neonatal neutrophils demonstrate slower mobilization after bacterial challenge in vivo, due to reduced neutrophil storage pools, and quiescent neutrophil progenitor cell populations in the bone marrow [42]. High neonatal production of IL-6, a Th2-polarizing cytokine with anti-inflammatory properties, including reduction of neutrophil recruitment [43], may also play a role. Moreover, under activated or stress conditions, neonatal neutrophils demonstrate functional differences including impaired recruitment [44], phagocytic activity [45], reduced killing mechanisms such as expression and/or release of antimicrobial proteins and peptides (APPs) and reactive oxygen species (ROS) [46, 47], and impaired formation of DNA-based neutrophil extracellular traps (NETs) that serve as scaffolds for APPs that kill microbes and neutralize their toxins [48, 49]. In general, to the extent that they have been evaluated, functional differences, such as diminished neutrophil rolling and adhesion, are even more severe in the preterm [50]. Interestingly, extracellular plasma concentrations of adenosine, an endogenous purine metabolite that inhibits multiple inflammatory pathways including neutrophil-endothelial adhesion, is elevated in newborns, potentially contributing to these distinct neutrophil responses in early life (see Box 1) [51].
Ontogeny of immunosuppressive mechanisms
NK cells are large granular lymphocytes that play a central role in the control of viral infections [52] and are functionally characterized by cytolytic functions and IFN-γ production. Of note, age-related changes in mature NK cell populations have been noted from childhood through old age [53]. Early in life, NK cell responses such as degranulation and release of lytic factors are reduced as compared to older individuals [54], increasing infant susceptibility to viral infection. These distinct aspects on early life NK cell function had previously been attributed to intrinsic deficiencies in the commitment of precursor NK cells to a fully differentiated mature form. More recently, extrinsic factors have been implicated in that maturation of neonatal NK cells progressed faster in cluster of differentiation (CD)11cdnR mice, whose NK cells lack transforming growth factor-β (TGF-β) receptor signaling, resulting in the formation of a mature NK cell pool early in life [55]. Consistent with these findings, TGF-β inhibits adult NK-cell proliferation and function by efficiently suppressing the transcriptional control factors T-bet and GATA-3, while also promoting differentiation of T regulatory (Treg) cells that robustly produce TGF- β [56]. Human fetal NK cells derived from aborted fetal tissues are highly susceptible to TGF-β-mediated suppression [57]. When cultured with TGF-β for 48 hours, fetal and neonatal NK cells, but not adult cells, demonstrated hypo-responsive degranulation and reduced anti-CD20 induced antibody (Ab)-dependent cell-mediated cytotoxicity against target cells. Thus while the source(s) of TGF-β remain to be identified, these finding suggest that TGF-β contributes to NK cell immaturity and increased susceptibility to infection in early life [55]. Such results highlight the need for additional age-focused studies to understand the mechanisms of how NK cell lineage development and that of other innate lymphoid cells proceeds through early life, and how in turn these differences may affect responses to pathogens [58].
Most recently, immunosuppressive CD71+ erythroid precursor cells (nucleated red blood cells) have been shown to compromise neonatal host defense against infection. Upon adoptive transfer into adult mice, CD71+ erythroid cells derived from the spleens of neonatal mice are immunosuppressive, via the action of the enzyme arginase-2 [59]. Ablation of CD71+ erythroid cells in neonatal mice, or natural postnatal age-related decline in their numbers, correlated closely with the loss of suppression, enhanced stimulus-induced TNF production by phagocytes and APCs, and increased resistance to perinatal pathogens such as Listeria monocytogenes and Escherichia coli [59]. Human cord blood cells were noted to contain an equally enriched proportion of CD71+ cells, the Ab-mediated depletion of which increased heat-killed L. monocytogenes-induced mononuclear cell IL-6 production [59]. Given that reduced gestational age correlates with higher frequency of nucleated red blood cells in cord blood [60], the immunosuppressive effect of CD71+ erythroid precursors may be more broadly linked to gestational age-dependent neonatal susceptibility to infection. Thus, CD71+ cells may play an important role in the temporal dampening of inflammation induced by early life colonization with commensal microorganisms after parturition.
Distinct APC ontogeny may impact early life responses to pathogens and vaccines
APCs are critical for induction of adaptive immunity and tolerance [61, 62]. Ontological related differences within each subset may limit effective immune responses. Cord blood MCs demonstrate markedly reduced LPS-induced TNF production as compared to adult blood MCs [63]. In addition to plasma factors (Box 1), reduced LPS-induced TNF production by neonatal MCs has recently been attributed to functionally lower TLR4 expression on activated or patrolling CD14+ CD16 (FcγRIII)+ monocytes, whose numbers are lower at birth [64]. A key feature of the distinct APC cytokine responses in early life is that the production of cytokines is polarized (e.g. high production of anti-inflammatory IL-6 and IL-10). Similarly, human and murine neonatal macrophage production of immunosuppressive IL-27, a heterodimeric cytokine of the IL-12 family, peaks during infancy [65]. Both human cord blood- and neonatal murine-derived splenic macrophages express IL-27 genes and proteins at elevated levels compared to adult counterparts. Neutralization of IL-27 with soluble IL-27 receptor enhances neonatal macrophage IFN-γ responses to the attenuated pediatric tuberculosis vaccine Bacillus Calmette–Guérin (BCG) in vitro [65]. Such approaches could potential improve vaccine responses early in life, given the fact BCG-attributable increases in IFN-γ post-vaccination correlates with better with levels of protection induced by immunization.
As part of their function as professional APCs, DCs can integrate information from both self (e.g., danger-associated molecular patterns) and foreign stimuli (e.g., components of pathogens or vaccines) and orchestrate these signals into appropriately regulated adaptive immune responses [66]. Both qualitative and quantitative differences in DC maturation and functionality with age have been demonstrated [67]. As the investigation of human fetal and neonatal circulating- and tissue resident- DC cellular development is intrinsically difficult, murine studies dominate the published literature in this field. DCs are present in the lymphoid organs of newborn mice, although in low numbers. Conventional CD11c+ DCs can be detected in the thymus of embryonic mice as early as day 17 of gestation, with adult-like levels of CD8α+ DCs not acquired until 2-weeks post birth [68]. The composition of DC populations in the spleen of young mice differs significantly from those in adult mice. DCs are among the first leukocytes to colonize the spleen, [69], detectable at of day 1 newborn mice [68]. Changes in splenic DC composition occur in distinct waves of colonization with murine CD4-CD8α-CD205+ DCs and plasmacytoid DCs (pDCs, a main source of anti-viral type-I IFNs) present at birth [69], CD4−CD8α+CD205+ DC developing next (~7 days) then CD4+CD8α−CD205− and CD4−CD8α−CD205− DCs (~3 wks). Murine CD4+ DCs become the prevalent subset by ~3 weeks of age, reaching adult levels at ~5 weeks [68]. Conventional DC of young mice (< 5 wks) are also functionally less mature than their adult counterparts, exhibiting reduced stimulus-induced cytokine production (IL-12p70, IFN-γ) and lower Ag-processing/presentation ability [68].
Human term neonate cord blood contains total numbers of pDCs comparable to adult peripheral blood [35], but with variation in phenotype and subset composition. These differences have seemingly few consequences on pDC-directed antiviral responses (e.g. the ability to produce IFN-γ upon TLRA challenge) in term infants [70], but are significantly relevant for preterm neonates, who are especially susceptible to viral and other intracellular infections [71]. Newborn cord blood has fewer conventional DCs (cDCs; MHCII+, CD11c+, CD14−/low, CD123−) than adult peripheral blood [35, 72], followed by a steady increase in the cDC population over the first 2 years of life [73]. Term neonatal cDCs, [35, 74] and in vitro-differentiated neonatal MoDC are generally less responsive to TLR stimulation [22, 37]. Specifically, neonatal cDCs demonstrate impairments in TLR-mediated IL-12p70 production [75, 76], while both cDCs and pDCs demonstrate reduced TLR-mediated polyfunctionality (i.e. have a lower ability to produce multiple cytokines per cell) [35]. In addition, newborn MoDCs displayed impaired (LPS + Adenosine-5′-triphosphate (ATP))-induced caspase-1-mediated IL-1βproduction [22] (Box 1). Neonatal cDCs are also highly susceptible to extrinsic factors such as regulatory B cell suppression [77]. However, among TLR agonist, those that activate TLR8 demonstrate greater efficacy in inducing maturation of neonatal DC maturation. For example, small synthetic imidazoquinolines, antiviral compounds with structural homology to the purine adenosine, can induce adult-like responses [22, 39] in an adenosine/cyclic AMP-refractory manner (Box 1). TLR7/8-activating imidazoquinolines also induce adult-level inflammasome activation in human newborn MoDCs without requiring exogenous ATP. Robust activation of human neonatal DCs can may also be achieved using agonists that activate multiple TLRs that may act synergistically to increase production of inflammatory cytokines [37].
The distinct functional features of neonatal DC function appear to persist into infancy, as seen with the ontogeny of IL-12p70-synthetic capacity throughout the childhood years. IL-12p70 production by peripheral blood mononuclear cells in response to either LPS or heat-killed Staphylococcus aureus was reduced at birth and was still below adult levels in both 5- and 12-year-old children [29]. Because IL-12p70 was predominantly derived from circulating HLA-DR+ cells, the slow maturation of IL-12p70-synthetic capacity in the childhood years can be attributed to reduced numbers and/or functionality of DCs [29, 78]. TLR-mediated up-regulation of DC co-stimulatory molecules such as CD80 and HLA-DR reached adult levels within the first 3 months of life for myeloid DCs (mDCs) stimulated with LPS and at 6–9 months of life for monocytes and pDCs stimulated with the TLR9 agonist CpG [30]. Environmental factors may also influence the ontogeny of DC maturation and functional responses. DCs from neonates born into traditional environmental conditions, including a high microbial burden, such as Papua New Guinea, are functionally more quiescent (i.e. reduced abilities for antigen uptake/processing and induction of T-cell proliferation in vitro) compared with DCs from children born into more modern environments (Australia) [79, 80]. Overall, the distinct ontogeny of DC hematopoiesis and functionality may reduce protective adaptive immunity to pathogens and contribute to reduced vaccine responses early in life [8].
Ontogeny of Adaptive Immunity
Since infants have limited exposure to Ags in utero to prime adaptive immunity, they are heavily dependent on innate immunity for protection against infections. Suboptimal neonatal memory responses contribute to the substantial challenges to effective disease prevention [81] and impede global efforts to achieve early immunization to many infectious diseases [7, 82]. This has been attributed, in part, to the immaturity of DCs in the neonatal and infant periods, as outlined above. Functional differences include: (i) reduced capacity for MHC Class II Ag presentation and subsequent stimulation of Ag-specific T-cell memory [72], (ii) the predisposition for neonatal human DCs to prime the generation of non-polarized CD4 T-cell effectors [36], (iii) the ability of neonatal DCs to induce a tolerogenic state by preventing the differentiation of naive T-helper cells towards Th1, irrespective of their subtype [83] and (iv) distinct inflammatory responses might spur alternative/detrimental T-cell development [84, 85]. MicroRNA-mediated control of neonatal naive CD4+ T Cell activation may also contribute to polarization bias [86].
Distinct age-specific functional lymphocyte programs may also be modulated by early-life developmental, nutritional and environmental factors [79, 87] and hematopoiesis [88] (Box 2). Newborns and infants have relatively fewer effector memory-T cells and effector memory-B cells [11], with large numbers of Tregs present in fetal lymph nodes, and high frequencies of recent thymic emigrant protein tyrosine kinase-7 (PTK7)+ thymus-derived CD4+ T-cells in peripheral tissues [89]. As the source of hematopoiesis switches to the newborn bone marrow, the ratio of effector T cell/Treg becomes more adult-like [90]. Early life B cell responses in humans and mice demonstrate limitations of primary Ab responses to vaccines and infections [91]. These B cell limitations in early life are ascribed to extrinsic factors, such as limited plasma cell differentiation, germinal center responses and lower DC activation signals as well as intrinsic factors such as reduced strength of B cell receptor signaling in naive neonatal B cells. Overall, these studies suggest that extrinsic immunosuppressive factors and intrinsic functional features of key leukocyte populations and signaling pathways combine to instruct distinct early life innate immune ontogeny functions. These factors have significant impact on the distinct, and often reduced, adaptive immune responses to pathogens and vaccines in early life.
Box 2. Ontogeny of T (helper) cell populations in early life.
Newborns and infants have relatively fewer effector memory-T cells (CD45RA−, CD45RO+) and effector memory-B cells (CD27+) [11, 132]. Additionally, neonatal CD4+ T cells are epigenetically biased towards Th2 cytokine production and demonstrate higher susceptibility to apoptosis of Th1 cells due to increased expression of IL-4Rα/IL-13Rα1 hetero-receptors [8]. Infants have relatively high frequencies of protein tyrosine kinase 7+ (PTK7+) recent thymic emigrant (RTE) antigenically naive CD4+ T-cells in secondary lymphoid organs [89], which upon completion of intrathymic maturation emigrate from the thymus to the periphery. PTK7+ RTEs have limited functional Th1 polarization activity, including reduced anti-CD3/anti-CD28-induced proliferation, IL-2-and IFN-γ-production, possibly contributing to infant susceptibility to intracellular pathogens [133].
Fetal hematopoiesis may generate distinct populations of T cells that temporally coexist. During gestation, both maternal [134, 135] and fetal [90] adaptive immune responses are biased towards Ag-specific immune tolerance. Large numbers of CD4+, CD25+, Foxp3+ Regulatory T cells (Tregs) are preferentially present in fetal lymph nodes, originating from 1) fetal liver hematopoietic stem and progenitor cells and 2) migratory maternal Tregs crossing the placenta, possibly residing in secondary lymphoid organs for years after birth. Maternal Foxp3+ CD4+ T-cells with fetal-Ag specificity accumulate (>100-fold through parturition), persist at elevated levels after delivery and sustain beneficial regulatory tolerance through antigen-specific ‘memory’ Tregs [135]. These recently appreciated features suggest a “layered immune system”, with specific functional biases at different stages of immune development [104, 136]. As the source of hematopoiesis transitions to the infant bone marrow, the resulting infant effector T cell/regulatory T cell ratio becomes more adult-like [90]. Consistent with this theory, the cytokine milieu also plays an important role in determining precursor cell differentiation. In newborns, impaired production of pro-inflammatory cytokines allows for the dominance of the FOXP3 transcription factor over the Th17-polarizing RORγT [137], but Th17 differentiation capacity also develops as CD161+ naive CD4+ IL-17-producing T Cells emerge from the postnatal thymus, in response to IL-1β and IL-23 [138, 139], cytokines highly expressed early infancy [10, 140]. Furthermore, as Th17 and Treg cells have reciprocal development pathways, perturbation of the Th17 lymphocyte development may influence development of subpopulations of Tregs [141] as well as early onset atopy [142].
Development of novel age-specific immunomodulators and vaccines
Immunization aims to induce a protective immune response against infection or disease [9]. However, decreased magnitude of immunogenicity and reduced persistence of functional Abs is a major concern for early life immunization strategies. Approaches to this challenge include design of multi-dose immunization schedules, greater length of time between vaccine doses and/or administration of doses later in infancy, all of which increase immunogenicity of pediatric vaccines [92]. Subunit vaccines consisting of purified microbial products often lack the necessary adjuvant activity to induce and optimally shape an immune response. In this context, inclusion of alum adjuvants has been key to the efficacy of these subunit vaccine formulations. Although Alum has some efficacy as an adjuvant, alum-adjuvanted vaccines often require multiple doses for protection that is often not achieved until after 24 months of age given current immunization schedules. Accordingly, increased appreciation of immune ontogeny may inform development of rationally designed age-specific vaccine formulations, which may include adjuvants that more effectively enhance immune responses in early life [9]. For example, there has been some success with mucosally-targeted human pediatric vaccines. The live attenuated influenza nasal-spray vaccine may be more effective than intramuscularly injected trivalent-inactivated vaccine influenza vaccines in children aged 6–71 months [93].
A number of adjuvanted, including live (self)-adjuvanted vaccines induce relatively robust responses in neonates. For example, immunization of neonatal mice with a Sindbis virus replicon-based DNA vaccine encoding measles virus glycoproteins induced adult-like neutralizing Abs and cell-mediated immunity, even in the presence of maternal Abs [94]. A plasmid expressing IL-2 [95, 96] or cationic liposome adjuvants [97] enhanced protection with this measles virus DNA vaccine in infant non-human primates. Adult-like multifunctional anti-mycobacterial T cell responses can be induced through neonatal immunization with either an anti-microbial peptide/immunostimulatory oligodeoxynocleotide based- or liposome-based mycobacterial vaccine, which induced adjuvant-dependent DC activation [98, 99]. Heterologous prime-boost vaccination strategies may also be advantageous in early life. Intranasal priming of newborn mice with S. typhi Ty21a expressing anthrax protective Ag (PA) followed by intramuscularly PA-boosting, induced greather B and T cell-mediated immunity compared to intramuscularly administration alone [100, 101]. Optimally, the most desirably approaches would be single dose immunization strategies that would instruct life long protection to subsequent challenges. For example, immunization of neonatal mice with a live-replicating attenuated strain of Listeria monocytogenes protects immediately and for life regardless of age at vaccination [102]. 6 day old mice immunized with a single dose of attenuated L. monocytogenes induced vaccine-peptide-specific IFN-γ-producing T cells and protected mice by day 7 post-vaccination and for 2 years thereafter when challenged with a virulent L. monocytogenes strain. Robust innate immune activation inherent to the use of a live vaccine may contribute to the rapid kinetics and longevity of protective responses. Of note, the inhibitory milieu and T cell-intrinsic factors that limit the expansion of neonatal follicular T helper (Tfh) cell populations, and subsequently reduced germinal center B cell responses, may be circumvented by vaccine adjuvantation with TLRAs that support Tfh cell differentiation [103].
Concluding remarks
The past decade has seen a substantial increase in our understanding of early life neonatal and infant immune ontogeny [3]. In vitro experiments characterizing the functions of neonatal and infant leukocytes have become increasingly sophisticated with respect to physiologic modeling (e.g., inclusion and characterization of autologous plasma) as well as measurement of multiple immunologic biomarkers (e.g., polychromatic flow cytometry, multi-analyte cytokine analyses, etc). In vivo experiments have concurrently become more commonly employed in an effort to verify in vitro observations. The human adaptive immune system may develop in distinct layers with specific functions at different stages of development [104], with age-specific mechanisms regulated by the major human APC subsets [73]. Remarkably, innate immunity may also share traits of adaptive immunity [105], but in a broader manner than classical immunological memory (Box 3). Since neonates and infants are more dependent on their innate immune system for protection against infections early in life [106], these observations may be particularly relevant for translational studies in this population. Indeed, there is rationale to incorporate into future studies, investigations into how primary challenge with infectious agents early in life may lead to cross-protection to antigenically-unrelated pathogens [107]. Future characterization of immunomodulatory plasma factors [38, 51] will also provide further insight into the ontogeny of innate immune development and may identify novel targets for the prevention and treatment of neonatal infection.
Box 3. Innate immune memory may confer heterologous immunity.
Features of human innate immunity may share characteristics of adaptive immunity [105], exhibiting an immunological memory after stimulation by infection or immunization. This “trained immunity” was initially observed in plants and invertebrate animals [143], and has more recently been demonstrated in mice [123] and humans [144]. Innate memory-based heterologous immunity in mammals is broader than classical immunological memory, is dependent on innate immune cells (i.e. NK cells, APCs) and may function through enhanced expression of PRRs and heightened protective inflammatory responses. Trained innate immunity may mediate the ability of stimuli such as infection [107], TLR agonists [123] and/or self-adjuvanted live vaccines (e.g., BCG) [144–146] to enhance host defense against a broad range of unrelated pathogens independent of T/B cell adaptive responses. Much remains to be learned about the scope, mechanisms and characteristics of innate immune memory and heterologous immunity, concepts with intriguing implications for optimization of immunization schedules and future vaccine development.
Distinct early life immune ontogeny has intriguing implications for current and future pediatric vaccine development [7]. Indeed, pediatric vaccine adjuvants such as alum and TLR agonists have age-dependent immune activity [25]. Of note, both the nature of a given adjuvanted vaccine and the timing of its administration may polarize innate immune cytokine production in later life to unrelated stimuli. For example, a birth dose of alum-adjuvanted pneumococcal conjugate vaccine resulted in altered TLR-mediated cytokine responses at 9 months of age [31]. Therefore, future development of novel age-specific vaccine formulations and delivery systems is likely warranted [108]. Given that ~11% of the global population is born preterm [109], the high burden of preterm infection [110], and that preterms demonstrate distinct immunity [111] which is incompletely characterized, there is a particular need to accurately model preterm immune responses to adjuvants and vaccines. The study of infant immunity in the developing world, where the greatest burden of infectious death occurs, presents its own unique challenges [112, 113]. Innate immune ontogeny may vary between geographically diverse populations [25, 27, 28] due to environmental factors [114], infant nutritional statues [115], maternal nutritional statues [116], birth season [117] and in utero exposure [118] to pathogen-associated Ags. Given the complexity of relevant factors, studies that correlate in vitro and in vivo responses will be crucial for refining our in vitro models and accelerating development of safe and effective pediatric vaccines [7]. Caution will also be required when interpreting results characterizing innate and adaptive immune responses of circulating human blood cells as these may not be indicative of local responses at other anatomic sites relevant to vaccinology such as mucosal surfaces and muscle (Box 4).
Box 4. Topics for future research.
Development of micro-physiologic in-vitro systems to more accurately model age-specific human immune responses and immune ontogeny, particularly at sites of immune perturbation/immunization, such as mucosa, muscle tissue and blood.
Characterize molecular mechanisms that regulate immune ontogeny.
Characterize linear vs. layered models of immune ontogeny, including potential role of newly generated lymphocyte populations.
Define ontogeny of antigen-presenting cell function.
Assess whether targeted and selective modulation of immune ontogeny may represent a novel approach to developing age-specific anti-infectives.
Determine whether immune ontogeny may inform development of novel safe and effective age-specific adjuvants and adjuvanted vaccines to be given in early life.
Characterize the role of epigenetic mechanisms in susceptibility to infection and risk of developing atopic disorders.
Assess the scope and mechanisms underlying trained innate immunity (innate memory) and its potential relevance to vaccine-induced heterologous immunity in early life.
Assess the effect of vaccines on the ontogeny of immune polarization.
Assess the impact of modulation of maternal immune system, microbiome, environment, nutrition and region of birth/infancy on immune ontogeny.
Immunity is not static but rather changes with age. Increased awareness and characterization of age-dependent changes of innate immune ontogeny, including definition of the underlying cellular and molecular mechanisms, will be of substantial clinical importance for development of novel age-specific anti-infectives, vaccine design and treatment of atopic disease. The continuing high global burden of infections in the very young and consequent need for additional safe and effective early life anti-infectives and vaccines provide a compelling rationale and motivation for on-going basic and translational studies in the area of neonatal and infant immune ontogeny.
Highlights.
There is a continuing high global burden of infections in the very young.
Immunity is not static; it changes with age, with many unique features in early life.
Newborns and young infants have distinct immune ontogeny and responses to microbes.
Emerging in vitro assays may more accurately model age-specific human immune ontogeny.
Development of novel age-specific vaccine formulations and delivery systems is likely warranted.
Acknowledgments
The authors thank the members of the Levy Laboratory for helpful discussions and feedback. O.L. is supported by U.S. National Institutes of Health (NIH) grant 1R01AI100135-01, as well as Global Health (OPPGH5284) and Grand Challenges Explorations (OPP1035192) awards from the Bill & Melinda Gates Foundation. The Levy Laboratory has received sponsored research support from VentiRx Pharmaceuticals, 3M Drug Delivery Systems, MedImmune and Crucell (Johnson & Johnson). O.L. is an inventor on a licensed patent for use of the anti-infective protein rBPI21 to mitigate radiation injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hostetter MK. What we don’t see. N Engl J Med. 2012;366(14):1328–34. doi: 10.1056/NEJMra1111421. [DOI] [PubMed] [Google Scholar]
- 2.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 3.Prabhu Das M, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12(3):189–94. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 4.Bortolussi R, Henneke P, Kollmann T. Host defense against common early life-threatening infections. Clin Dev Immunol. 2013;2013:350808. doi: 10.1155/2013/350808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Argenio DA, Wilson CB. A decade of vaccines: Integrating immunology and vaccinology for rational vaccine design. Immunity. 2010;33(4):437–40. doi: 10.1016/j.immuni.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473(7348):463–9. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3(90):90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30(12):585–91. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy O, Goriely S, Kollmann TR. Immune response to vaccine adjuvants during the first year of life. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollmann TR, et al. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37(5):771–83. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adkins B. Heterogeneity in the CD4 T Cell Compartment and the Variability of Neonatal Immune Responsiveness. Curr Immunol Rev. 2007;3(3):151–159. doi: 10.2174/157339507781483496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 13.Maynard CL, et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–41. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tourneur E, Chassin C. Neonatal immune adaptation of the gut and its role during infections. Clin Dev Immunol. 2013;2013:270301. doi: 10.1155/2013/270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belkaid Y, Hand TW. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescott S. Developmental immunology and vaccines: cellular immune development and future vaccine strategies. Expert Rev Vaccines. 2004;3(4):339–42. doi: 10.1586/14760584.3.4.339. [DOI] [PubMed] [Google Scholar]
- 17.Levast B, et al. Animal models for neonatal diseases in humans. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.11.089. [DOI] [PubMed] [Google Scholar]
- 18.Scharfe-Nugent A, et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188(11):5706–12. doi: 10.4049/jimmunol.1103454. [DOI] [PubMed] [Google Scholar]
- 19.Werling D, et al. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 2009;30(3):124–30. doi: 10.1016/j.it.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun. 1992;60(8):3446–7. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philbin VJ, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130(1):195–204. e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelone DF, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60 (2):205–9. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 24.Jansen K, et al. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J Immunol Methods. 2008;336(2):183–92. doi: 10.1016/j.jim.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisciandro JG, et al. Ontogeny of Toll-like and NOD-like receptor-mediated innate immune responses in Papua New Guinean infants. PLoS One. 2012;7(5):e36793. doi: 10.1371/journal.pone.0036793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smolen KK, et al. Pattern recognition receptor-mediated cytokine response in infants across 4 continents. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burl S, et al. Age-dependent maturation of toll-like receptor-mediated cytokine responses in gambian infants. PLoS One. 2011;6(4):e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reikie BA, et al. Ontogeny of Toll-Like Receptor Mediated Cytokine Responses of South African Infants throughout the First Year of Life. PLoS One. 2012;7(9):e44763. doi: 10.1371/journal.pone.0044763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Upham JW, et al. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002;70(12):6583–8. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen M, et al. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One. 2010;5(4):e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Biggelaar AH, et al. Neonatal pneumococcal conjugate vaccine immunization primes T cells for preferential Th2 cytokine expression: a randomized controlled trial in Papua New Guinea. Vaccine. 2009;27(9):1340–7. doi: 10.1016/j.vaccine.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phuanukoonnon S, et al. A neonatal pneumococcal conjugate vaccine trial in Papua New guinea: study population, methods and operational challenges. P N G Med J. 2010;53(3–4):191–206. [PubMed] [Google Scholar]
- 33.Zhang JP, et al. Human neonatal peripheral blood leukocytes demonstrate pathogen-specific coordinate expression of TLR2, TLR4/MD2, and MyD88 during bacterial infection in vivo. Pediatr Res. 2010;68(6):479–83. doi: 10.1203/PDR.0b013e3181f90810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomat WS, et al. Safety and immunogenicity of neonatal pneumococcal conjugate vaccination in papua new guinean children: a randomised controlled trial. PLoS One. 2013;8(2):e56698. doi: 10.1371/journal.pone.0056698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kollmann TR, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183(11):7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews NC, Power UF, Reen DJ. Neonatal human autologous dendritic cells pulsed with recombinant protein antigen prime the generation of non-polarized CD4 T-cell effectors. Int Immunol. 2007;19(6):703–12. doi: 10.1093/intimm/dxm025. [DOI] [PubMed] [Google Scholar]
- 37.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68(10):813–22. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Belderbos ME, et al. Plasma-mediated immune suppression: a neonatal perspective. Pediatr Allergy Immunol. 2012 doi: 10.1111/pai.12023. [DOI] [PubMed] [Google Scholar]
- 39.Dowling DJ, et al. The Ultra-Potent and Selective TLR8 Agonist VTX-294 Activates Human Newborn and Adult Leukocytes. PLoS One. 2013;8(3):e58164. doi: 10.1371/journal.pone.0058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giese C, Marx U. Human immunity in vitro - Solving immunogenicity and more. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 42.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110(1):18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 43.Fielding CA, et al. Viral IL-6 blocks neutrophil infiltration during acute inflammation. J Immunol. 2005;175(6):4024–9. doi: 10.4049/jimmunol.175.6.4024. [DOI] [PubMed] [Google Scholar]
- 44.Prosser A, et al. Phagocytosis of neonatal pathogens by peripheral blood neutrophils and monocytes from newborn preterm and term infants. Pediatr Res. 2013 doi: 10.1038/pr.2013.145. [DOI] [PubMed] [Google Scholar]
- 45.Filias A, et al. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr. 2011;11:29. doi: 10.1186/1471-2431-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy O. Impaired innate immunity at birth: deficiency of bactericidal/permeability-increasing protein (BPI) in the neutrophils of newborns. Pediatr Res. 2002;51(6):667–9. doi: 10.1203/00006450-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Strunk T, et al. Reduced levels of antimicrobial proteins and peptides in human cord blood plasma. Arch Dis Child Fetal Neonatal Ed. 2009;94(3):F230–1. doi: 10.1136/adc.2008.143438. [DOI] [PubMed] [Google Scholar]
- 48.Yost CC, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–27. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76(5):909–25. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 50.Nussbaum C, et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. J Leukoc Biol. 2013;93(2):175–84. doi: 10.1189/jlb.0912468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettengill M, et al. Soluble ecto-5′-nucleotidase (5′NT), alkaline phosphatase, and adenosine deaminase (ADA1) activities in neonatal blood favor elevated extracellular adenosine. J Biol Chem. 2013 doi: 10.1074/jbc.M113.484212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–94. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 53.Almeida-Oliveira A, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72(4):319–29. doi: 10.1016/j.humimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Guilmot A, et al. Natural killer cell responses to infections in early life. J Innate Immun. 2011;3(3):280–8. doi: 10.1159/000323934. [DOI] [PubMed] [Google Scholar]
- 55.Marcoe JP, et al. TGF-beta is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat Immunol. 2012;13(9):843–50. doi: 10.1038/ni.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivarsson MA, et al. Differentiation and functional regulation of human fetal NK cells. J Clin Invest. 2013;123(9):3889–901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13(2):75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 59.Elahi S, et al. Immunosuppressive CD71 erythroid cells compromise neonatal host defence against infection. Nature. 2013 doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perrone S, et al. Nucleated red blood cell count in term and preterm newborns: reference values at birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F174–5. doi: 10.1136/adc.2004.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 62.Dowling D, Hamilton CM, O’Neill SM. A comparative analysis of cytokine responses, cell surface marker expression and MAPKs in DCs matured with LPS compared with a panel of TLR ligands. Cytokine. 2008;41(3):254–62. doi: 10.1016/j.cyto.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Levy O, et al. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173(7):4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 64.Pedraza-Sanchez S, et al. Reduced frequency of a CD14+ CD16+ monocyte subset with high Toll-like receptor 4 expression in cord blood compared to adult blood contributes to lipopolysaccharide hyporesponsiveness in newborns. Clin Vaccine Immunol. 2013;20(7):962–71. doi: 10.1128/CVI.00609-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraft JD, et al. Neonatal macrophages express elevated levels of interleukin-27 that oppose immune responses. Immunology. 2013;139(4):484–93. doi: 10.1111/imm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 67.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39(1):26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 68.Dakic A, et al. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172(2):1018–27. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 69.Sun CM, et al. Ontogeny and innate properties of neonatal dendritic cells. Blood. 2003;102(2):585–91. doi: 10.1182/blood-2002-09-2966. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, et al. Neonatal Plasmacytoid Dendritic Cells (pDCs) Display Subset Variation but Can Elicit Potent Anti-Viral Innate Responses. PLoS One. 2013;8(1):e52003. doi: 10.1371/journal.pone.0052003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schuller SS, et al. Preterm neonates display altered plasmacytoid dendritic cell function and morphology. J Leukoc Biol. 2013;93(5):781–8. doi: 10.1189/jlb.1011525. [DOI] [PubMed] [Google Scholar]
- 72.Upham JW, et al. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74(2):1106–12. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corbett NP, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One. 2010;5(11):e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levy O. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res. 2005;11(2):113–6. doi: 10.1179/096805105X37376. [DOI] [PubMed] [Google Scholar]
- 75.Goriely S, et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199(7):1011–6. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vanden Eijnden S, et al. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36 (1):21–6. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 77.Lo-Man R. Regulatory B cells control dendritic cell functions. Immunotherapy. 2011;3(4 Suppl):19–20. doi: 10.2217/imt.11.34. [DOI] [PubMed] [Google Scholar]
- 78.Prescott SL, et al. Neonatal interleukin-12 capacity is associated with variations in allergen-specific immune responses in the neonatal and postnatal periods. Clin Exp Allergy. 2003;33(5):566–72. doi: 10.1046/j.1365-2222.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 79.Lisciandro JG, et al. Neonatal antigen-presenting cells are functionally more quiescent in children born under traditional compared with modern environmental conditions. J Allergy Clin Immunol. 2012;130(5):1167–1174. e10. doi: 10.1016/j.jaci.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 80.Lisciandro JG, et al. Comparison of neonatal T regulatory cell function in Papua New Guinean and Australian newborns. Pediatr Allergy Immunol. 2012;23 (2):173–80. doi: 10.1111/j.1399-3038.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 81.Lewis DB, Wilson CB. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Remington JS, Wilson CB, Baker CJ, editors. Infectious diseases of the fetus and newborn infant. 7. Elsevier Saunders; Philiadelphia: 2011. pp. 80–191. [Google Scholar]
- 82.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–17. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naderi N, et al. Cord blood dendritic cells prevent the differentiation of naive T-helper cells towards Th1 irrespective of their subtype. Clin Exp Med. 2009;9(1):29–36. doi: 10.1007/s10238-008-0020-2. [DOI] [PubMed] [Google Scholar]
- 84.Tulic MK, et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol. 2011;127(2):470–478. e1. doi: 10.1016/j.jaci.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 85.Doganci A, et al. In the presence of IL-21 human cord blood T cells differentiate to IL-10 producing Th1 but not Th17 or Th2 cells. Int Immunol. 2012 doi: 10.1093/intimm/dxs097. [DOI] [PubMed] [Google Scholar]
- 86.Palin AC, et al. Human Neonatal Naive CD4+ T Cells Have Enhanced Activation-Dependent Signaling Regulated by the MicroRNA miR-181a. J Immunol. 2013 doi: 10.4049/jimmunol.1202534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore SE, et al. Early-life and contemporaneous nutritional and environmental predictors of antibody response to vaccination in young Gambian adults. Vaccine. 2012;30(32):4842–8. doi: 10.1016/j.vaccine.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costa G, Kouskoff V, Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33(5):215–23. doi: 10.1016/j.it.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants are phenotypically and functionally distinct from adult recent thymic emigrants. Blood. 2009;113(22):5635–43. doi: 10.1182/blood-2008-08-173658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330(6011):1695–9. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 92.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213–20. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 93.Rhorer J, et al. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine. 2009;27(7):1101–10. doi: 10.1016/j.vaccine.2008.11.093. [DOI] [PubMed] [Google Scholar]
- 94.Capozzo AV, et al. Neonatal immunization with a Sindbis virus-DNA measles vaccine induces adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies. J Immunol. 2006;176(9):5671–81. doi: 10.4049/jimmunol.176.9.5671. [DOI] [PubMed] [Google Scholar]
- 95.Premenko-Lanier M, et al. DNA vaccination of infants in the presence of maternal antibody: a measles model in the primate. Virology. 2003;307(1):67–75. doi: 10.1016/s0042-6822(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 96.Premenko-Lanier M, et al. Protection against challenge with measles virus (MV) in infant macaques by an MV DNA vaccine administered in the presence of neutralizing antibody. J Infect Dis. 2004;189(11):2064–71. doi: 10.1086/420792. [DOI] [PubMed] [Google Scholar]
- 97.Pan CH, et al. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J Virol. 2010;84(8):3798–807. doi: 10.1128/JVI.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamath AT, et al. Adult-like anti-mycobacterial T cell and in vivo dendritic cell responses following neonatal immunization with Ag85B-ESAT-6 in the IC31 adjuvant. PLoS One. 2008;3(11):e3683. doi: 10.1371/journal.pone.0003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamath AT, et al. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS One. 2009;4(6):e5771. doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramirez K, et al. Mucosally delivered Salmonella typhi expressing the Yersinia pestis F1 antigen elicits mucosal and systemic immunity early in life and primes the neonatal immune system for a vigorous anamnestic response to parenteral F1 boost. J Immunol. 2009;182(2):1211–22. doi: 10.4049/jimmunol.182.2.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramirez K, et al. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal Immunol. 2010;3(2):159–71. doi: 10.1038/mi.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reikie BA, et al. A single immunization near birth elicits immediate and lifelong protective immunity. Vaccine. 2010;29(1):83–90. doi: 10.1016/j.vaccine.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Mastelic B, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189(12):5764–72. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 104.Mold JE, McCune JM. At the crossroads between tolerance and aggression: Revisiting the “layered immune system” hypothesis. Chimerism. 2011;2(2):35–41. doi: 10.4161/chim.2.2.16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–61. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 106.Ku CL, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204(10):2407–22. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quintin J, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12 (2):223–32. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van den Biggelaar AH, Pomat WS. Immunization of newborns with bacterial conjugate vaccines. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 109.Blencowe H, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 110.Liu L, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 111.Sharma AA, et al. The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol. 2012;145(1):61–8. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(Suppl 1):S4–9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 113.Flanagan KL, et al. The challenge of assessing infant vaccine responses in resource-poor settings. Expert Rev Vaccines. 2010;9(6):665–74. doi: 10.1586/erv.10.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olszak T, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chassin C, et al. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8(4):358–68. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 116.van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508(7494):123–7. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sumino K, et al. Antiviral IFN-gamma responses of monocytes at birth predict respiratory tract illness in the first year of life. J Allergy Clin Immunol. 2012;129(5):1267–1273. e1. doi: 10.1016/j.jaci.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.King CL, et al. B cell sensitization to helminthic infection develops in utero in humans. J Immunol. 1998;160(7):3578–84. [PubMed] [Google Scholar]
- 119.Manzoni P, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. Jama. 2009;302(13):1421–8. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 120.Levy O, et al. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177(3):1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Belderbos ME, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133(2):228–37. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Belderbos ME, et al. Neonatal plasma polarizes TLR4-mediated cytokine responses towards low IL-12p70 and high IL-10 production via distinct factors. PLoS One. 2012;7(3):e33419. doi: 10.1371/journal.pone.0033419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wynn JL, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750–8. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13(4):333–2. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13(4):325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Levy O, et al. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108(4):1284–90. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Forster-Waldl E, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58 (1):121–4. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 128.La Pine TR, et al. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B Streptococci. Pediatr Res. 2003;54(2):276–81. doi: 10.1203/01.PDR.0000072515.10652.87. [DOI] [PubMed] [Google Scholar]
- 129.Geiger R, et al. Circulating interleukin-1 receptor antagonist levels in neonates. Eur J Pediatr. 1996;155(9):811–4. doi: 10.1007/BF02002913. [DOI] [PubMed] [Google Scholar]
- 130.Granland C, et al. NOD1 and NOD2 expression and function in very preterm infant mononuclear cells. Acta Paediatr. 2014 doi: 10.1111/apa.12559. [DOI] [PubMed] [Google Scholar]
- 131.Martino D, Holt P, Prescott S. A novel role for interleukin-1 receptor signaling in the developmental regulation of immune responses to endotoxin. Pediatr Allergy Immunol. 2012;23(6):567–72. doi: 10.1111/j.1399-3038.2012.01287.x. [DOI] [PubMed] [Google Scholar]
- 132.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14 (1):24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haines CJ, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206(2):275–85. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rowe JH, et al. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–6. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 137.de Roock S, et al. Defective TH17 development in human neonatal T cells involves reduced RORC2 mRNA content. J Allergy Clin Immunol. 2013;132(3):754–756. e3. doi: 10.1016/j.jaci.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 138.Cosmi L, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205(8):1903–16. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maggi L, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40(8):2174–81. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 140.Dijkstra KK, et al. T17 differentiation capacity develops within the first 3 months of life. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.022. [DOI] [PubMed]
- 141.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 142.McLoughlin RM, et al. Longitudinal relationship of early life immunomodulatory T cell phenotype and function to development of allergic sensitization in an urban cohort. Clin Exp Allergy. 2012;42(3):392–404. doi: 10.1111/j.1365-2222.2011.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chambers MC, Schneider DS. Pioneering immunology: insect style. Curr Opin Immunol. 2012;24(1):10–4. doi: 10.1016/j.coi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 144.Kleinnijenhuis J, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aaby P, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–52. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 146.Brynjolfsson SF, et al. Concomitant administration of Mycobacterium bovis BCG with the meningococcal C conjugate vaccine to neonatal mice enhances antibody response and protective efficacy. Clin Vaccine Immunol. 2011;18(11):1936–42. doi: 10.1128/CVI.05247-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Echeverry A, et al. Yersinia enterocolitica promotes robust mucosal inflammatory T-cell immunity in murine neonates. Infect Immun. 2010;78(8):3595–608. doi: 10.1128/IAI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adkins B, Contractor N. Immune responses of female BALB/c and C57BL/6 neonatal mice to vaccination or intestinal infection are unaltered by exposure to breast milk lycopene. J Nutr. 2011;141(7):1326–30. doi: 10.3945/jn.110.136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kronforst KD, et al. A neonatal model of intravenous Staphylococcus epidermidis infection in mice <24 h old enables characterization of early innate immune responses. PLoS One. 2012;7(9):e43897. doi: 10.1371/journal.pone.0043897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mao Q, et al. A neonatal mouse model of coxsackievirus A16 for vaccine evaluation. J Virol. 2012;86(22):11967–76. doi: 10.1128/JVI.00902-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bjarnarson SP, et al. The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice. J Immunol. 2012;189(3):1265–73. doi: 10.4049/jimmunol.1200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bjarnarson SP, et al. Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness. PLoS One. 2013;8(9):e72588. doi: 10.1371/journal.pone.0072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jupelli M, et al. The contribution of interleukin-12/interferon-gamma axis in protection against neonatal pulmonary Chlamydia muridarum challenge. J Interferon Cytokine Res. 2010;30(6):407–15. doi: 10.1089/jir.2009.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jupelli M, et al. Endogenous IFN-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J Immunol. 2008;180(6):4148–55. doi: 10.4049/jimmunol.180.6.4148. [DOI] [PubMed] [Google Scholar]
- 155.Lines JL, et al. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol. 2010;185(5):2980–8. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kurkjian C, et al. Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun. 2012;80(8):2835–46. doi: 10.1128/IAI.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bogaert D, et al. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect Immun. 2009;77(4):1613–22. doi: 10.1128/IAI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yu X, et al. Effect of intrauterine lipopolysaccharide infusion on Toll-like receptor 4 signaling transduction pathway in lungs of perinatal rats. Zhonghua Er Ke Za Zhi. 2009;47(9):667–71. [PubMed] [Google Scholar]
- 159.Birchenough GM, et al. Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic Escherichia coli. Infect Immun. 2013;81(9):3264–75. doi: 10.1128/IAI.00268-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Polewicz M, et al. Influence of maternal antibodies on active pertussis toxoid immunization of neonatal mice and piglets. Vaccine. 2011;29(44):7718–26. doi: 10.1016/j.vaccine.2011.07.135. [DOI] [PubMed] [Google Scholar]
- 161.Kiros TG, et al. Induction, regulation and physiological role of IL-17 secreting helper T-cells isolated from PBMC, thymus, and lung lymphocytes of young pigs. Vet Immunol Immunopathol. 2011;144(3–4):448–54. doi: 10.1016/j.vetimm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 162.Wen K, et al. CD4+ CD25- FoxP3+ regulatory cells are the predominant responding regulatory T cells after human rotavirus infection or vaccination in gnotobiotic pigs. Immunology. 2012;137(2):160–71. doi: 10.1111/j.1365-2567.2012.03617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Butler JE, et al. Antibody repertoire development in fetal and neonatal piglets. XVI. Influenza stimulates adaptive immunity, class switch and diversification of the IgG repertoire encoded by downstream Cgamma genes. Immunology. 2013;138(2):134–44. doi: 10.1111/imm.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X, et al. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood. 2010;116(20):4168–74. doi: 10.1182/blood-2010-03-273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rosario M, et al. Safety and immunogenicity of novel recombinant BCG and modified vaccinia virus Ankara vaccines in neonate rhesus macaques. J Virol. 2010;84(15):7815–21. doi: 10.1128/JVI.00726-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Skinner JM, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine. 2011;29(48):8870–6. doi: 10.1016/j.vaccine.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 167.Polack FP, et al. Poor immune responses of newborn rhesus macaques to measles virus DNA vaccines expressing the hemagglutinin and fusion glycoproteins. Clin Vaccine Immunol. 2013;20(2):205–10. doi: 10.1128/CVI.00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hood JD, et al. Immunoprofiling toll-like receptor ligands: Comparison of immunostimulatory and proinflammatory profiles in ex vivo human blood models. Hum Vaccin. 2010;6(4):322–35. doi: 10.4161/hv.6.4.10866. [DOI] [PubMed] [Google Scholar]
- 169.Strunk T, et al. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One. 2010;5(4):e10111. doi: 10.1371/journal.pone.0010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strunk T, et al. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr Res. 2012;72(1):10–8. doi: 10.1038/pr.2012.48. [DOI] [PubMed] [Google Scholar]
- 171.Yan SR, et al. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72(3):1223–9. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Du J, et al. TLR8 agonists stimulate newly recruited monocyte-derived cells into potent APCs that enhance HBsAg immunogenicity. Vaccine. 2010;28(38):6273–81. doi: 10.1016/j.vaccine.2010.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scott JA, et al. Pneumococcal conjugate vaccine given shortly after birth stimulates effective antibody concentrations and primes immunological memory for sustained infant protection. Clin Infect Dis. 2011;53(7):663–70. doi: 10.1093/cid/cir444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Greenway F, Wang S, Heiman M. A novel cobiotic containing a prebiotic and an antioxidant augments the glucose control and gastrointestinal tolerability of metformin: a case report. Benef Microbes. 2014;5(1):29–32. doi: 10.3920/BM2012.0063. [DOI] [PubMed] [Google Scholar]