Abstract
Human noroviruses are a major cause of epidemic and sporadic gastroenteritis worldwide, and can chronically infect immunocompromised patients. Efforts to develop effective vaccines and antivirals have been hindered by the uncultivable nature and extreme genetic diversity of human noroviruses. Although they remain a particularly challenging pathogen to study, recent advances in norovirus animal models and in vitro cultivation systems have led to an increased understanding of norovirus molecular biology and replication, pathogenesis, cell tropism, and innate and adaptive immunity. Furthermore, clinical trials of vaccines consisting of nonreplicating virus-like particles have shown promise. In this review, we summarize these recent advances and discuss controversies in the field, which is rapidly progressing towards generation of antiviral agents and increasingly effective vaccines.
Introduction
Human noroviruses (HuNoVs) are a leading cause of gastroenteritis outbreaks across the globe and of severe childhood diarrhea in the United States (Koo et al., 2013; Payne et al., 2013). HuNoV gastroenteritis is characteristically an acute illness. However, chronic HuNoV infection of immunocompromised persons presents a debilitating and often intractable problem (Bok and Green, 2012). Further, prolonged asymptomatic HuNoV infection and shedding may contribute to spread of the virus. Importantly, in animal models NoV infection can interact with allelic host genome variations to induce inflammatory bowel disease-like phenotypes (Basic et al., 2014; Cadwell et al., 2010), raising the possibility that NoV infection may trigger lasting effects in the gut long after the resolution of an acute illness.
Despite recent progress in HuNoV vaccine development, several key challenges remain in assessing the efficacy of vaccines and antiviral drugs. First, the lack of a robust HuNoV cell culture system limits direct study of these viruses (Duizer et al., 2004; Herbst-Kralovetz et al., 2013; Lay et al., 2010; Papafragkou et al., 2013; Straub et al., 2007). Second is the extreme genetic heterogeneity among strains (Green, 2013; Kroneman et al., 2013) and the emergence of new variants every 2-3 years as represented by the recent pandemic GII.4 Sydney HuNoV (Barclay et al., 2013). Finally, protective immunity to natural HuNoV infections is complicated by an apparent lack of heterotypic protection among strains (Bok et al., 2011; Wyatt et al., 1974). Moreover, there is evidence that homotypic responses are ineffective or short-lived at best (Johnson et al., 1990; Parrino et al., 1977), although circulating strains may elicit short-term herd immunity (Lindesmith et al., 2012).
Here, we summarize recent advances in the NoV field and discuss their potential in helping achieve successful prevention and control. We also point out controversies in the field regarding the relevance of NoV studies in animal models to human disease. As with any human infectious diseases, caveats and limitations are inherent to studying NoV in animal models and any conclusions drawn from animal studies will need to be validated in the natural host. Regardless of the ultimate answer as to how closely animal NoV infection mimics HuNoV infection, the murine norovirus (MuNoV) system provides a unique opportunity to answer fundamental questions about viral immunity especially in the intestine using a bona fide mouse virus and a genetically tractable experimental host. Here we emphasize recent findings with the greatest potential to advance vaccine and antiviral drug development as well as key remaining questions in NoV biology research.
Molecular Virology of Noroviruses
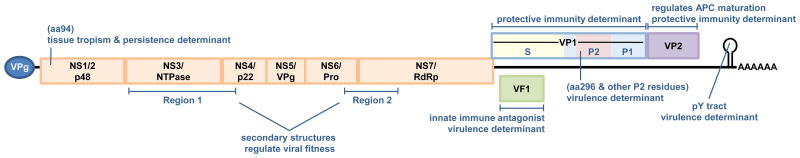
NoVs are small positive sense nonenveloped RNA viruses comprising one genus of the family Caliciviridae. The genus is segregated into at least five and possibly six genogroups (genogroups I-VI), of which genogroups I, II, and IV contain primarily human viruses associated with gastroenteritis (Green, 2013; Kroneman et al., 2013). Genogroups III, V, and VI are comprised of bovine NoVs, MuNoVs, and canine NoVs, respectively. NoV genogroups are further subdivided into genotypes, or clusters, based on genetic similarity. Specific strains are referred to by their genogroup and genotype (e.g., a GII.4 virus segregates in genogroup II and genotype 4). The viral genome is 7.4 to 7.7 kb in length and is organized into three or four open reading frames (ORFs) (Figure 1). The 5′ proximal ORF1 encodes a large polyprotein that is cleaved by a virus-encoded protease (Pro; NS6) into at least six mature nonstructural proteins including the viral RNA-dependent RNA polymerase (NS7; RdRp). NoVs have only a single major structural protein which is encoded by ORF2. This capsid protein, referred to as VP1, is organized into a well-conserved internal shell (S) domain and a protruding (P) domain forming dimeric VP1 arches; the P domain can be further subdivided into a P1 stalk subdomain and a hypervariable surface-exposed P2 subdomain that localizes to the tips of the arches (Prasad et al., 1994). ORF3 encodes a minor structural protein called VP2. The two structural proteins VP1 and VP2 are translated from a subgenomic RNA. For MuNoVs, a fourth ORF overlaps ORF2; translation from this alternative ORF4 produces a recently identified protein virulence factor 1 (VF1) (McFadden et al., 2011; Thackray et al., 2007). The 5′ ends of NoV genomic and subgenomic RNAs are covalently linked to a small virus-encoded protein known as VPg and the 3′ ends are polyadenlyated.
Figure 1. Norovirus virulence determinants.
The NoV genome is depicted. The 5′ proximal ORF1 (shown in orange) encodes a nonstructural polyprotein which is cleaved into six mature products by the virally encoded protease (NS6, or Pro). Other nonstructural proteins include NS1/2 (also referred to as p48), NS3 (NTPase), NS4 (p22), NS5 (VPg), and NS7 (the RNA-dependent RNA polymerase; RdRp). ORF2 encodes the capsid protein referred to as VP1; this protein can be divided into shell (S; shown in yellow) and protruding (P) domains, and the P domain further subdivided into the P1 stalk domain (shown in blue) and the hypervariable P2 domain comprising the tips of the arches (shown in red). ORF3 encodes the minor structural protein VP2, and ORF4 of MuNoV genomes encodes a newly defined protein called virulence factor 1, or VF1. NoV genomes are covalently linked to VPg at their 5′ ends and polyadenylated at their 3′ ends. Studies in the MuNoV model system have identified a number of NoV virulence determinants which are indicated along the viral genome.
Understanding the mechanisms of HuNoV genome translation and replication has been hampered by the lack of a cell culture system. Advances in the area, namely the development of a HuNoV replicon (Chang et al., 2006), the discovery of cultivable MuNoVs (Karst et al., 2003; Wobus et al., 2004), and transient in vitro expression assays using transfected viral genomes (Guix et al., 2007), have enabled significant progress. The MuNoV system has become the model of choice for the majority of molecular studies due to the availability of cell culture and reverse genetics systems (Chaudhry et al., 2007; Ward et al., 2007; Wobus et al., 2004). Thorne et al. recently reviewed NoV gene expression and replication (Thorne and Goodfellow, 2013), so here we briefly highlight work that could lead to new approaches in drug discovery and design.
The first step in intracellular NoV replication is translation of the nonstructural proteins from genomic RNA molecules. Several lines of evidence highlight a role for VPg in viral genome translation initiation: First, removal of VPg from the 5′ end of NoV genomes dramatically diminishes their infectivity (Chaudhry et al., 2006; Guix et al., 2007). Second, the NoV VPg interacts with cellular translation initiation factors (Chaudhry et al., 2006; Daughenbaugh et al., 2006, 2003; Goodfellow et al., 2005). Because VPg-mediated translation initiation is a process unique to the virus, it represents a strong candidate for antiviral drug design. After nonstructural protein synthesis, genome replication occurs via the viral RdRp in replication complexes. Characterization of the NoV RdRp has demonstrated both primer-dependent and de novo mechanisms of RNA synthesis (Belliot et al., 2005; Fukushi et al., 2004; Rohayem et al., 2006), the former utilizing the VPg protein as a peptide primer facilitating the covalent linkage of VPg to the 5′ end of the viral genome. The NoV RdRp is also a logical target for drug development based on its commonalities to other more widely studied viral RdRp enzymes for which many antiviral drugs have been developed. Indeed, one such drug – 2′-C-methylcytidine - has shown promise in treating MuNoV infections in the mouse model (Rocha-Pereira et al., 2013). The NoV replication complex is associated with virus-induced intracellular membranous vesicles (Hyde et al., 2009). The localization of MuNoV-1 nonstructural proteins to membranes of the early and late secretory pathway has suggested a role for these pathways in replication complex formation (Hyde and Mackenzie, 2010). Replication complex formation also involves the cytoskeletal network, allowing the establishment of the replication complex in close proximity to the microtubule organizing network (Hyde et al., 2012).
The HuNoV replicon system, developed by substituting the ORF2 gene of the GI.1 Norwalk virus with a neomycin resistance gene (Chang et al., 2006), has proven invaluable in the identification of small molecule inhibitors of viral replication (e.g., ribavirin, cyclic sulfamides, deubiquitinases) (Arias et al., 2013). Furthermore, analysis using this approach has increased understanding of the effect of NoV replication on host cell processes, highlighting the significant role of cholesterol levels on HuNoV replication (Chang, 2009). The observation that low cholesterol stimulates replication has since been corroborated using a pig model of HuNoV infection (Bui et al., 2013; Jung et al., 2012).
However, a number of important unanswered questions regarding NoV replication remain. These include the relevance of findings in animal viruses to replication of HuNoVs, questions that await the development of a robust HuNoV replication system. For all NoVs, key questions include the need for a detailed understanding of the function of cellular proteins required for NoV genome translation and replication, the processes by which NoV subgenomic RNA is synthesized and the mechanisms for formation and function of the membrane-bound replication complex. Moreover, the contribution and the impact of virus infection on cellular processes such as ubiquitination, nuclear-cytoplasmic export, cell death, and protein trafficking have yet to be fully explored. Gaining a better understanding of the cellular processes involved in, and required for, NoV replication may lead to therapeutic approaches that circumvent the problems associated with drug resistance commonly observed when targeting viral proteins directly.
Cell Tropism of Noroviruses
While most enteric pathogens infect intestinal epithelial cells (IECs) and NoV virions can be transcytosed through IECs, extensive attempts to cultivate HuNoVs and MuNoVs in epithelial cells have been unsuccessful (Duizer et al., 2004; Gonzalez-Hernandez et al., 2013; Marionneau et al., 2002; Wobus et al., 2004). Although one group has reported productive HuNoV infection using 3-dimensional organoid models of intestinal epithelium (Straub et al., 2007, 2011, 2013), multiple independent attempts to replicate these cell culture systems have failed (Herbst-Kralovetz et al., 2013; Papafragkou et al., 2013; Takanashi et al., 2013). Collectively, these data argue that ex vivo cultured IECs are likely not productively infected by NoVs. However, it is important to note that viral antigen can be detected in IECs of gnotobiotic piglets and calves infected perorally (p.o.) with a GII.4 HuNoV (Cheetham et al., 2006; Jung et al., 2012; Souza et al., 2008) and in STAT1-/- mice infected with a MuNoV (Mumphrey et al., 2007), suggesting that cellular tropism may be influenced by the immune status of the host and leaving open the possibility that IECs are targeted by NoVs in vivo.
Compelling evidence has accumulated in recent years demonstrating that a major target of NoVs is professional antigen presenting cells (APCs). MuNoVs efficiently replicate in primary dendritic cells and macrophages, as well as a number of macrophage-like cell lines, in vitro (Wobus et al., 2004). Furthermore, viral antigen has been detected in cells co-stained with a macrophage marker and cells morphologically resembling dendritic cells and macrophages in tissue sections from animals infected with a MuNoV (Mumphrey et al., 2007; Perdue et al., 2007; Ward et al., 2006; Wobus et al., 2004). With regard to HuNoVs, viral antigen was detected in lamina propria cells of an intestinal biopsy sample from a GI.1 HuNoV-infected person (Lay et al., 2010). Similarly, an inactivated GII.4 HuNoV was found to bind to lamina propria cells and submucosal Brunner's glands, but not IECs, when incubated with human duodenum tissue sections (Chan et al., 2011). Chimpanzees infected intravenously (i.v.) with a GI.1 HuNoV contain virus capsid antigen-positive intestinal dendritic cells, but not macrophages or epithelial cells (Bok et al., 2011). Finally, immunodeficient mice infected intraperitoneally (i.p.) with a pool of genogroup II HuNoVs display structural and nonstructural antigen-positive macrophage-like cells in their spleens and livers (Taube et al., 2013). While dendritic cells and macrophages are well-accepted targets of MuNoV infection, more recent data reveal that B cells are also permissive to NoVs. The B cell zones of Peyer's patches from MuNoV-1-infected STAT1-/- and IL-10-/- mice contain detectable viral nonstructural protein and viral genome, respectively (Basic et al., 2014; Mumphrey et al., 2007), and capsid-positive duodenal B cells are detectable in chimpanzees infected with a GI.1 HuNoV (Bok et al., 2011). Thus, all types of professional APCs and some lymphocytes appear to be permissive for NoVs. The ability of NoVs to infect intestinal immune cells undoubtedly has a significant impact on NoV pathogenesis and the host immune response to NoV infection. Unfortunately, efforts to propagate HuNoVs in APCs have so far been unsuccessful (Lay et al., 2010). It is important to note however that these data do not preclude a possible role for other cell types in NoV pathogenesis or immunity. Studies assessing the role for additional cell types are therefore an important priority.
Based on the developing model that NoVs productively infect intestinal immune cells but not, to date, IECs, NoVs may well employ strategies to overcome the intestinal epithelial barrier to access underlying target cells. Consistent with this, HuNoVs can be internalized by IECs in culture in the absence of productive infection [e.g., (Marionneau et al., 2002)]. Moreover, MuNoVs can be transcytosed across a monolayer of polarized confluent IECs in culture via M-like cells without productive infection or disruption of tight junctions (Gonzalez-Hernandez et al., 2013). M cells are specialized intestinal epithelial cells in the gut-associated lymphoid tissue that sample particulate antigens, including pathogens, in the lumen of the host to deliver them to underlying immune cells. Depletion of M cells from mice reduced MuNoV titers (Gonzalez-Hernandez et al., 2014), suggesting that MuNoVs exploit the special function of M cells to initiate infection of the host.
A major unresolved question in the area of NoV cell tropism is the mechanism of resistance to HuNoV replication in cultured cells. Based on the observation that a HuNoV virus-like particle (VLP) can bind to and be internalized by IECs but cannot replicate in these cells, it was initially speculated that the block occurs after viral entry (Marionneau et al., 2002). However, more recently it has been demonstrated that a HuNoV can overcome the restriction to in vitro growth if entry and genome uncoating are bypassed via genome transfection into non-susceptible cells, although this is inefficient (Guix et al., 2007). MuNoVs replicate efficiently in otherwise non-susceptible cells, including in human cells, upon viral genome transfection (Chaudhry et al., 2007), clearly demonstrating that early steps such as receptor binding, entry, and/or genome uncoating likely regulate susceptibility to NoV infection. The entry receptors used by NoVs have yet to be identified although there is extensive evidence that they use carbohydrates as attachment receptors. We will not discuss carbohydrate usage in this review since it has been the subject of numerous recent reviews [e.g., (Tan and Jiang, 2014)]. Many groups are aggressively testing other types and sources of human IECs and APCs, HuNoV strains, and innovative culture conditions to overcome this frustrating hurdle to NoV research.
Elucidating Norovirus Pathogenesis in Animal Models
The pathogenic mechanisms of HuNoV infection are not fully elucidated but advances in animal models are facilitating progress in this area (Table 1). HuNoVs can experimentally infect several animal species with variable clinical outcome. Gnotobiotic pigs and calves inoculated p.o. with a GII.4 or a GII.g/GII.12 HuNoV develop mild diarrhea (Cheetham et al., 2006; Souza et al., 2008; Takanashi et al., 2011) and thus can be used to study pathogenic mechanisms of NoV-induced disease. Animal models that support asymptomatic HuNoV infection are chimpanzees infected p.o. or i.v. with a GI.1 HuNoV (Bok et al., 2011; Wyatt et al., 1978), and immunocompromised RAG/gamma chain-deficient (RAG/γc-/-) mice on a Balb/c background infected i.p. with a pool of genogroup II viruses (Taube et al., 2013). In this mouse model of HuNoV infection, although mice do not develop disease, they display increased viral genome levels in the intestinal tract and systemic sites over input titers 1-2 days post-infection; and viral nonstructural protein can be detected in the liver and spleen (Figure 2), supporting in vivo viral propagation (Taube et al., 2013). However, it should be noted that further validation of this model is required before it can be applied for pre-clinical testing of therapeutics and vaccines.
Table 1.
Features of NoV animal models.
| Host | humans | chimpanzees | gnotobiotic pigs | gnotobiotic calves | Balb/c RAG/γc-/- mice | wild-type mice | interferon-/-mice | malnourished mice |
|---|---|---|---|---|---|---|---|---|
| Virus strain | HuNoVs | HuNoV GI.1 | HuNoV GII.4 | HuNoV GII.4 | HuNoV GII.4 pool | MuNoVs | MuNoVs | MuNoVs |
| Route | peroral | peroral; intravenous | peroral | peroral | intraperitoneal | Peroral | peroral | peroral |
| In vivo viral antigen | intestinal monocytes*, lamina propria cells | intestinal DC and B cells | IECs | IECs and intestinal Mϕ* | Mϕ* in spleen and liver | intestinal Mϕ and DC* | Mϕ and DC*; IECs | N/A |
| Intestinal disease | severe diarrhea and vomiting | asymptomatic | mild diarrhea | mild diarrhea | asymptomatic | asymptomatic | severe diarrhea | modest weight loss |
| Fecal shedding (dpi) | + (widely variable) | + (2-42) | + (1-4) | + (1-6) | - | + (1-≥56) | + | + (1-≥50) |
| Viremia | +/- | - | + | + | N/A | N/A | + | N/A |
| Stomach tropism# | N/A | N/A | N/A | N/A | + | +/- | + | + |
| Small intestinal tropism# | + | + | + | + | + | + | + | + |
| Large intestinal tropism# | N/A | N/A | N/A | N/A | + | + | + | + |
| MLN tropism# | N/A | N/A | N/A | N/A | + | + | + | + |
| Peripheral tissue tropism# | N/A | + | N/A | N/A | + | +/- | + | + |
| References | (Chan et al., 2011; Green, 2013; Lay et al., 2010) | (Bok et al., 2011; Wyatt et al., 1978) | (Cheetham et al., 2006) | (Souza et al., 2008) | (Taube et al., 2013) | (Arias et al., 2012; Karst et al., 2003; Mumphrey et al., 2007; Thackray et al., 2007) | (Karst et al., 2003; Mumphrey et al., 2007; Wobus et al., 2004) | (Hickman et al., 2014) |
Viral antigen detected in cells morphologically resembling monocytes, macrophages (Mφ), and dendritic cells (DC).
Tropism is defined as detection of viral antigen in a tissue section by immunohistochemistry or titration of infectious virus or viral genomes from the indicated tissue.
Figure 2. Human norovirus mouse model.
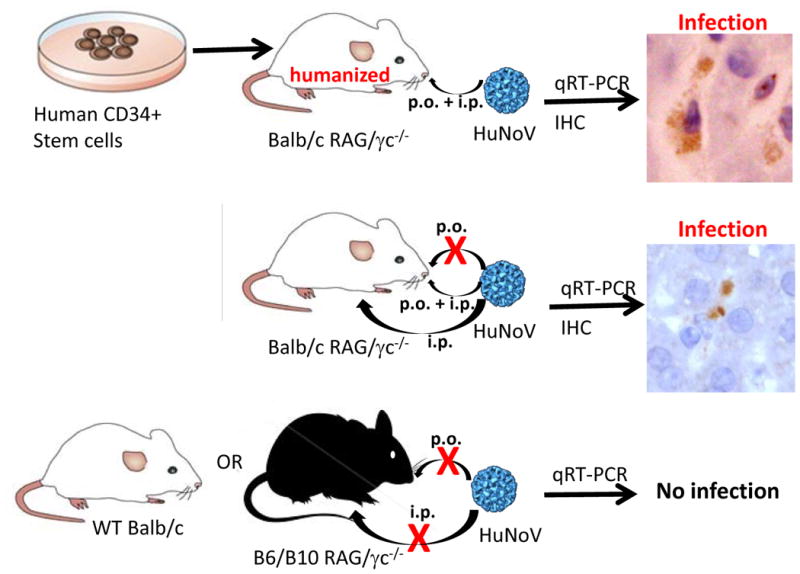
The schematic overview summarizes the findings of Taube et al. (Taube et al., 2013). (Top) Balb/c mice deficient in the recombination activating gene (RAG) and common gamma chain (γc) were “humanized” with human CD34+ positive stem cells following irradiation. Mice were infected with filtered HuNoV-containing stool by combined peroral (p.o.) and intraperitoneal (i.p.) routes. Infection was detected by measuring increased genome titers over input by qRT-PCR and viral protein expression by immunohistochemistry (IHC). The image shows two VP1-positive Kupffer cells. (Middle) Balb/c RAG/γc-deficient mice were infected with HuNoV-containing stool filtrate by the oral and/or intraperitoneal routes and infection measured as above. The image shows two NS6-positive cells in the spleen. No infection was seen following oral infection. (Bottom) B6/B10 RAG/γc-deficient mice or wild-type (wt) Balb/c were infected by the p.o. or i.p. routes but no increases in viral genome titers over input were detected, demonstrating both the immune status and genetic background are important susceptibility factors in this model.
In addition to animal models of HuNoV infection, the study of MuNoVs has provided substantial insights into NoV pathogenesis and immunity (Karst, 2010a; Karst et al., 2003; Wobus et al., 2006). MuNoVs are transmitted fecal-orally, replicate in the intestine, and cause quantifiable intestinal pathology but not overt diarrhea in wild-type murine hosts (Hickman et al., 2014; Hsu et al., 2005; Karst et al., 2003; Mumphrey et al., 2007). Malnourished mice are susceptible to more severe MuNoV infections as defined by modest weight loss, are impaired in controlling infection in certain tissues, and display delayed viral clearance compared to their healthy counterparts (Hickman et al., 2014). This model should be useful for defining the nature of NoV infections in the context of malnutrition.
Although HuNoVs can cause debilitating gastroenteritis, available evidence indicates that this disease is associated with only modest intestinal pathology (Blacklow et al., 1972; Dolin et al., 1975; Schreiber et al., 1973, 1974). No gross histological changes have been observed in the intestines of HuNoV-infected gnotobiotic pigs (Bui et al., 2013; Cheetham et al., 2006), chimpanzees (Bok et al., 2011), or immunocompromised mice (Taube et al., 2013); likewise MuNoV-infected mice show no gross histological abnormalities (Mumphrey et al., 2007). Similarly, HuNoV and MuNoV infections induce only modest inflammation of the intestinal lamina propria (Blacklow et al., 1972; Dolin et al., 1975; Mumphrey et al., 2007; Schreiber et al., 1973, 1974; Souza et al., 2008; Troeger et al., 2009) and apoptosis of IECs (Cheetham et al., 2006; Mumphrey et al., 2007; Troeger et al., 2009). Thus, one remaining question in NoV pathogenesis is how the virus causes severe gastroenteritis while inducing only modest intestinal pathology, inflammation, and apoptosis. Extrapolating to other types of infections, possible mechanisms include induction of pathogenic cytokines or expression of an enterotoxin.
Another question actively being investigated using animal models is the main site(s) of NoV replication. From these studies it may be possible that general features of NoV infection can be extrapolated, although not all features are conserved across all model systems (Table 1). NoVs are detected at very low levels in the stomachs of infected hosts but there is no evidence that the virus replicates in this fastidious environment. Further, NoVs can be detected along the length of the small and large intestine, although virus strain-specific preferences for specific intestinal segments exist. It should be noted that colonic infection by HuNoVs has not been analyzed so it remains unclear whether HuNoVs preferentially target specific regions of the intestine like MuNoVs. NoVs spread systemically at a low level to peripheral tissues potentially via the hematogenous route. Supporting viremia as the mode of spread is the reported detection of HuNoV genomes in the serum of infected people (Takanashi et al., 2009). However, it should be recognized that many groups have failed to detect HuNoV viremia so this may not be a common feature of infection. A requirement for dendritic cells for extraintestinal spread of a MuNoV has also been reported (Elftman et al., 2013). Finally, NoVs are shed fecally for variable lengths of time (discussed in more detail in section IV). As illuminated by MuNoV studies, there are virus strain-dependent variations in the magnitude and duration of shedding. For example, shedding is quite low for certain MuNoV strains but can reach ≥108 genomes per gram feces for other strains (Arias et al., 2012; Thackray et al., 2007), comparable to levels observed in HuNoV infections.
Elucidating NoV virulence determinants is another active research area (Figure 1). These studies have been facilitated by the identification of MuNoV strains with distinct pathogenic properties, MuNoV cell culture adaptation studies, and MuNoV RNA secondary structure analysis. One known MuNoV virulence determinant is the P domain of VP1 (Bailey et al., 2008; Strong et al., 2012); specifically lysine at position 296 is associated with virulence in STAT1-/- mice whereas a glutamate at this position is attenuating. However, the glutamate must afford a fitness advantage to the virus since it is routinely selected for in vitro and in vivo (Bailey et al., 2008; Hickman et al., 2014; Wobus et al., 2004). Another MuNoV virulence determinant is VF1 encoded by ORF4 which antagonizes the innate immune response by blocking the induction of type I IFN and regulating virus-induced apoptosis in cultured cells (McFadden et al., 2011; Thackray et al., 2007). MuNoV strains lacking VF1's immunoregulatory activities are attenuated in vivo (McFadden et al., 2011; Zhu et al., 2013). While human sapoviruses segregating in a distinct Caliciviridae genus encode VF1, HuNoVs do not. Evidence indicates that RNA structures in the NoV genome also regulate viral virulence. A polypyrimidine (pY) tract within a conserved stem-loop in the 3′ untranslated region of the MuNoV-1 genome, which binds cellular poly(rC) binding protein and polypyrimidine tract binding protein, is not required for virus replication in vitro, but its removal results in a partially attenuated virus in STAT1-/- mice (Bailey et al., 2010). Similarly, secondary structure elements in NS3/4 and NS6/7 of MuNoV-3 enhance viral fitness, although they are not required for replication in vitro or persistence establishment in immunocompetent mice (McFadden et al., 2013). It will be important in future investigations and as the necessary research tools become available to validate these factors as virulence determinants of HuNoVs.
Overall, the available animal models to study NoV pathogenesis each have strengths and weaknesses, as summarized in Table 2. Due to the significant limitations of studying HuNoVs in their natural host and in cultured cells, the field is reliant upon these animal models to uncover pathogenic mechanisms and host-virus interactions. Although no model system can perfectly mirror all aspects of infection in humans, there is a strong likelihood that both HuNoV infection of non-natural hosts and non-human MuNoV infection of its natural host will continue to inform many common features of HuNoV biology, including general pathogenic mechanisms, replication strategies, and immune responses.
Table 2.
Strengths and weaknesses of NoV animal models.
| Model | Strengths | Weaknesses |
|---|---|---|
|
| ||
| HuNoV infection of chimpanzees |
|
|
|
|
||
| HuNoV infection of gnotobiotic pigs & calves |
|
|
|
| ||
| HuNoV infection of Balb/c RAG/γc-/-mice |
|
|
|
| ||
| MuNoV infection of wild-type mice |
|
|
|
| ||
| MuNoV infection of interferon-/-mice |
|
|
Viral Persistence as a Possible Contributor to Spread
NoV infection in humans is generally considered an acute self-limiting infection in the majority of the population, with symptoms lasting between 24 and 72 hours. However, people continue to shed virus after symptom resolution and even asymptomatically infected people can shed virus (Patterson et al., 1993; Rockx et al., 2002). Moreover, a growing body of literature has highlighted the clinical importance of chronic long-term HuNoV infections in the immunocompromised (Bok and Green, 2012), and references within]. Patients on immunosuppressive therapy due to solid organ or hematopoietic stem cell transplantation may shed HuNoV for months to years (Alkhouri and Danziger-Isakov, 2011; Saif et al., 2011). In many cases this is accompanied by diarrhea, resulting in prolonged intestinal failure, often requiring nutritional support and further complicating patient treatment (Saif et al., 2011). The relevance and impact of chronic HuNoV shedders in outbreaks in both nosocomial environments and community settings has yet to be fully evaluated but preliminary evidence suggests that they can be a source of nosocomial outbreaks (Beersma et al., 2009). Nosocomial transmission between immunocompromised patients has also been observed, despite the use of stringent infection control measures (Kundu et al., 2013). Thus, prolonged shedding, in particular in the context of chronic HuNoV infections of immunocompromised patients, represents a putative factor in amplifying viral spread in the population.
A related and critical area of study in the NoV field is elucidating the role of chronic shedding in the emergence of new antigenic variants. It has been postulated by numerous groups that viral evolution in chronically infected people contributes to the emergence of phenotypically distinct viral variants [e.g., (Siebenga et al., 2008)]. In the absence of strong immune pressure, evidence suggests that the accumulation of mutations increases the potential to generate antigenic variants (Bull and White, 2011). Inter-host transmission studies suggest that minor variants with a frequency of less than 0.01% of the population may transmit between individuals (Bull and White, 2011), suggesting that inter-host transmission could be an important source selection and may contribute to the emergence of variants with the potential to escape herd immunity. Thus, chronically infected people may represent reservoirs of emergent HuNoV strains.
The MuNoV mouse model has provided insight into the viral reservoirs and genetic determinants of persistence. Similar to immunocompromised people chronically infected with a HuNoV, severely immunocompromised RAG-/- mice become chronically MNV-infected (Karst et al., 2003). Furthermore, malnourished mice that are delayed in clearing MuNoV infection display reduced antiviral antibody responses and enhanced viral diversity (Hickman et al., 2014), supporting the premise that immune impairment creates an amenable environment for NoV evolution. Even immunocompetent healthy mice become persistently infected with certain MuNoV strains. Persistent MuNoV infection in this context occurs primarily in the cecum and colon and is associated with fecal shedding (Arias et al., 2012; Nice et al., 2013). The nonstructural protein NS1/2 - and specifically aspartic acid to glutamic acid change at position 94 of NS1/2 -enhances colonic replication early after infection and allows establishment of life-long persistent fecal shedding (Figure 1) (Nice et al., 2013). This single amino acid change is accompanied by a significant structural rearrangement of the N-terminal domain of the protein (Borin et al., 2013). Unanswered questions relating to MuNoV persistence include elucidating the mechanism by which NS1/2 regulates colonic tropism and persistence; and determining whether the VF1 protein, previously shown to antagonize the innate immune response in MuNoV-1 (McFadden et al., 2011), contributes to viral persistence.
While there is some controversy in the field pertaining to the temporal definition of persistence and whether NoV infection meets these criteria, the shedding of NoVs long after the symptomatic stage of infection – and even from asymptomatically infected hosts – is clearly of potential importance in disease epidemiology. The mechanisms used by most viruses for acute replication differ from those utilized to evade the immune system and replicate continuously for persistence. Identifying and targeting viral mechanisms facilitating low-level prolonged infection could have a significant impact on prevention strategies by eliminating persistent shedding and thus disease incidence in the population as a whole.
Immune Mechanisms of Norovirus Control
The elucidation of immune mechanisms controlling primary NoV infections and mediating protective immunity to re-challenges has been greatly advanced by the use of NoV animal models, as reviewed in this section.
Immune control of primary norovirus infections
Consistent with the short duration of NoV symptoms, innate immunity - and in particular type I IFNs - is critical for controlling acute NoV infections. The MuNoV-1 system has also been used to explore the molecular basis of interferon (IFN) sensitivity (Changotra et al., 2009; Karst et al., 2003) and the role of MDA5 in NoV RNA sensing (McCartney et al., 2008). At least one mechanism of IFN action was linked to an inhibition of viral genome translation (Changotra et al., 2009). The effect of IFN-γ on MuNoV-1 replication has also indicated a role of the Atg5-Atg12/Atg16L1 autophagy protein complex in mediating the antiviral effect, independent of canonical degradative autophagy (Hwang et al., 2012). Further highlighting the protective role of type I IFN, inoculating gnotobiotic pigs with human IFN-α p.o. during GII.4 HuNoV infection significantly reduces fecal shedding (Jung et al., 2012). Furthermore, mice lacking type I and II IFN receptors, type I IFN receptor only, or STAT-1 have significantly higher titers than wild-type mice and succumb to lethal MuNoV infections (Karst et al., 2003; Thackray et al., 2012). This disease is associated with rapid weight loss, severe diarrhea, gastric bloating, and pathology in intestinal and peripheral tissues (Kahan et al., 2011; Karst et al., 2003; Mumphrey et al., 2007; Rocha-Pereira et al., 2013). The antiviral effect of type I IFN by macrophages and dendritic cells, as well as IFN regulatory factor (IRF-3) and IRF-7, contribute to MuNoV control but are dispensable in preventing lethal infections (Thackray et al., 2012). In the absence of type I IFN, a role for type II IFN in controlling MuNoV infections via a mechanism that requires IRF-1 and the autophagy protein Atg5 has also been revealed (Hwang et al., 2012; Karst et al., 2003; Maloney et al., 2012).
Components of the adaptive immune response also contribute to the control and clearance of primary NoV infections. People and animals infected with NoV develop mucosal and peripheral antiviral antibody responses that are important for viral clearance. Mice lacking functional B cells fail to clear MuNoV-1 from MLNs, and transfer of immune serum or immune B cells into persistently infected RAG1-/- mice reduces viral loads (Chachu et al., 2008a). Furthermore, MuNoV-3 immune serum mediates partial protection from a primary infection in wild-type and RAG1-/- mice (Zhu et al., 2013). HuNoV and MuNoV infections also stimulate CD4+ and CD8+ T cell responses (Lindesmith et al., 2005, 2010). Mice infected with a MuNoV develop an intestinal T cell response by 8 dpi (Tomov et al., 2013). Although MuNoV-1-infected mice deficient in CD4+ or CD8+ T cells ultimately clear the infection, transfer of immune CD4+ or CD8+ T cells into persistently infected RAG1-/- mice reduces the chronic viral burden (Chachu et al., 2008b; Tomov et al., 2013). Moreover, transfer of immune CD4+ T cells into naïve wild-type or RAG1-/- mice partially protects from primary infection (Zhu et al., 2013). Overall, these studies suggest that B cells, CD8+ T cells, and CD4+ T cells all contribute to the control of primary NoV infections.
Norovirus protective immunity determinants
The design of effective vaccines is driven by understanding the nature of the immune response that protects from a virulent infection. However, elucidating the determinants of protective immunity to a HuNoV challenge has been confounded by the lack of a cell culture system and the repeated exposure of individuals to distinct virus strains over time. Early human volunteer studies indicated that a subset of individuals fail to develop long-term protective immunity upon experimental GI.1 HuNoV infection (Johnson et al., 1990; Parrino et al., 1977), while in others resistance to GI.1 infection correlated with an early increase in mucosal IgA (Lindesmith et al., 2003). In contrast, GII.4 HuNoVs evolve in response to herd immunity (discussed below). Potential explanations for the apparently limited efficacy of the immune system at protecting against secondary HuNoV challenge include the possibility that NoVs elicit short-term herd immunity of sufficient duration to drive the emergence of antigenically distinct pandemic strains but these immune responses wane over time; and virus strain-specific and host genetic differences in protective immunity induction are dominant. There are supporting data for both explanations. In one human volunteer study, the duration of protective immunity to a GI.1 HuNoV lasted up to six months but waned after two years (Johnson et al., 1990). Protective immunity elicited by a single exposure of a MuNoV also substantially wanes within six months (Karst, 2010b; Zhu et al., 2013). A recent mathematical model estimated the duration of HuNoV immunity to be 4.1-8.7 years, again significantly shorter than the life-long immunity induced by some other virus infections (Simmons et al., 2013).
With regard to virus strain-specific differences, even intra-cluster MuNoV strains sharing 87% genetic identity differ remarkably in their ability to induce protective immunity (Liu et al., 2009; Zhu et al., 2013). This differential protective immunity induction is in part dictated by the ability of the MuNoV VP2 protein to regulate APC maturation (Figure 1). Protective immunity to MuNoV requires antiviral antibody and MHC class II-dependent CD4+ T cells, whereas type I IFN, type II IFN, and CD8+ T cells are dispensable (Zhu et al., 2013). Consistent with findings for other viruses, multiple exposures to a homologous NoV boosts the magnitude and duration of protective immunity. For example, a single exposure to live MuNoV-1 elicits weak, waning immunity (Karst, 2010b; Liu et al., 2009; Zhu et al., 2013), while a prime:boost elicits robust, long-lived immunity (Chachu et al., 2008b). While a single exposure to a GI.1 HuNoV elicits protective homotypic immunity in chimpanzees that lasts up to 10 months, a prime:boost regimen elicits protection lasting at least 24 months (Bok et al., 2011). Importantly, systemic vaccination with a non-replicating viral vector elicits protection against enteric infection in the murine system, raising important questions about the relative importance of mucosal versus systemic immunity for NoVs. Protective immunity in these models requires antiviral antibody and a CD4+ T cell response (Chachu et al., 2008a, 2008b). It will be important in the future to confirm whether MuNoV observations regarding immune and viral determinants of protective immunity translate to HuNoVs.
Noroviruses are Under Humoral Selection
Seroprevalence studies suggest that humans are exposed to HuNoVs frequently, with seropositivity rates reaching >90% worldwide in adulthood [e.g., (Son et al., 2013)]. Analysis of monoclonal antibodies raised against HuNoV VLPs has demonstrated the development of a wide spectrum of antibodies with strain-, genotype-, genogroup-, or intergenogroup-specific reactivities. While strain-specific epitopes are located in the hypervariable P2 domain of VP1 [e.g., (Allen et al., 2009; Debbink et al., 2012)], more broadly cross-reactive epitopes are located in conserved regions of the S and P1 domains [e.g., (Higo-Moriguchi et al., 2014)]. Although classical HuNoV neutralization assays cannot be performed due to the lack of a cell culture system, a widely used surrogate assay (‘blockade assay’) measures the ability of antibodies to block VLP binding to histo-blood group antigens (HBGAs), which are viral attachment factors on the intestinal epithelium (Harrington et al., 2002). The presence of such blockade antibodies correlates with resistance to infection [e.g., (Atmar et al., 2011)], providing support that this is a biologically relevant indicator of protective immunity. Such blockade studies have highlighted the correlation between the genetic evolution of HuNoV genotypes and the ability to escape blockade antibody responses. For example, the GII.4 cluster, which is most often associated with HuNoV outbreaks, undergoes genetic drift to cause epidemics every 2-3 years (Bull and White, 2011; Estes et al., 2006). By analyzing the ability of antibodies to block HBGA binding by epidemic GII.4 strains, it was determined that genetic differences between virus strains result in differential antibody recognition (Debbink et al., 2013; Lindesmith et al., 2011, 2012). Thus, herd immunity is one important factor that drives evolution of GII.4 strains. [a topic of frequent reviews, for e.g.: (Bull and White, 2011; Donaldson et al., 2010)].
While there is extensive evidence for GII.4 evolution in response to population immunity resulting in the emergence of antigenically distinct virus strains, accumulating data suggest genogroup- and even genotype-specific distinctions in HuNoV evolutionary potential. For example, genogroup I viruses are genetically stable compared to GII.4 viruses. Consistent with this, human sera from GI.1-challenged volunteers contained blockade responses effective against multiple genogroup I genotypes (Lindesmith et al., 2010). The GII.2 cluster also acquires limited antigenic changes in its VP1 protein (Swanstrom et al., 2014). Interestingly, GII.3 viruses which are predominantly associated with pediatric infections evolve as rapidly as GII.4 viruses at the genomic level but revert back to previously used residues at the protein level (Boon et al., 2011). Thus, serum antibody responses directed against GII.3 viruses are highly cross-reactive at the intra-genotype level (Mahar et al., 2014). The inability to evolve away from herd immunity may be one explanation for the limited prevalence of the GII.3 genotype in the adult population but future studies investigating blockade antibody responses are needed to support this hypothesis.
While the HuNoV antibody response is clearly complex, a pattern emerges whereby highly cross-reactive antibody responses are directed to conserved epitopes in the VP1 S and P1 domains while strain/variant-specific antibodies are directed to hypervariable regions of the P2 domain. When these hypervariable regions in the P2 domain overlap with HBGA binding sites, changes in antibody binding patterns correlate with changes in antibody blockade responses, which represent at least part of the neutralizing immune response. The relative ratio of each type of antibody response (cross-reactive vs. specific) and the antibody blockade response to heterologous viruses within and between genotypes appears to vary between genogroups I and II but future studies are needed to investigate additional genotypes. Such increased understanding of the antigenic relationships and evolution of the many HuNoV strains will greatly facilitate the development of effective HuNoV vaccines.
Development of Antiviral Drugs for Norovirus Infections
While no antiviral drugs for the prevention or treatment of NoV infections are approved for human use, technical advances in the field, especially the developments of GI.1 Norwalk virus-bearing replicon cells (Chang et al., 2006) and the MuNoV cell culture system (Wobus et al., 2004), have led to an explosion of research activities in this area. For detailed descriptions of antiviral approaches for controlling NoVs, the reader is referred to other recent reviews (Arias et al., 2013; Kaufman et al., 2014).
An increasing number of NoV protein structures are being solved, enabling the design of antiviral drugs through in silico modeling. Since many structural features are conserved across the NoV family, this approach has the potential to yield inhibitors effective against the wide diversity of NoV strains. To date, a multitude of candidate inhibitors, targeting primarily the viral Pro, RdRp, and VP1, have been tested in recombinant protein or cell-based assays, with activities typically in the low micromolar range (Arias et al., 2013; Kaufman et al., 2014). The only drug candidate tested in vivo so far is the nucleoside analog 2′-C-methylcytidine (2CMC) (Rocha-Pereira et al., 2013). Mice lacking type I and II IFN receptors infected with MuNoV-1 and treated twice daily subcutaneously with 50 mg/kg of 2CMC for 7 days show decreased viral replication in the intestine, shed fewer viruses, are protected from virus-induced diarrhea and mortality, develop protective immunity, and are protected from re-challenge. While 2CMC was withdrawn as a hepatitis C virus inhibitor because of side effects, these studies suggest that nucleoside analogues in use or in development against other viruses could be considered for development as treatment options for NoV infections and/or in limiting viral spread.
Attempts to modulate the host or target host-encoded viral-interacting proteins to limit NoV infections are in early stages. To date, inhibition of cellular deubiquitinases and addition of type I IFN have been tested in vivo for their ability to inhibit NoV infections. WP1130 is a promising small molecule inhibitor of a subset of deubiquitinases that reduces MuNoV-1 titers in the small intestine (Perry et al., 2012). The therapeutic activity of IFN-α against a HuNoV was demonstrated in gnotobiotic pigs (Jung et al., 2012); however the known side effects of IFN-α administration likely limit its general use as a NoV treatment option. Substances from natural products with known safety profiles and anti-microbial activities are another therapeutic avenue being pursued, although the mechanism(s) of action is generally poorly understood for these substances (Li et al., 2013).
In summary, an array of approaches and compounds are under investigation to develop effective and safe NoV therapeutics, including ones with pan-anti-NoV activities. Based on concerns about the emergence of viral variants displaying resistance to antiviral drugs when targeting a single viral protein, future studies will likely expand into combination therapy targeting multiple antiviral pathways simultaneously.
Norovirus Vaccine Development
Extensive studies have documented the immunogenicity of NoV VLPs comprised of the VP1 protein in animal models and humans [e.g., (Atmar and Estes, 2012; Richardson et al., 2013)]. Accordingly, ongoing HuNoV vaccine work focuses on immunizing with VLPs. Chimpanzees receiving GI.1 VLPs in a prime:boost intramuscular immunization regimen elicit homologous protection lasting at least 18 months (Bok et al., 2011). Gnotobiotic pigs administered GII.4 VLPs plus adjuvant in one oral followed by two intranasal inoculations are protected from live virus infection 28 days later (Souza et al., 2007). Mice receiving Venezuelan equine encephalitis viral replicons expressing the MuNoV-1 VP1 protein in a prime:boost footpad immunization regimen are protected for up to 6 months (Chachu et al., 2008b).
In addition to data from animal models, recent clinical trials in humans have shown promise. A randomized Phase I/II clinical trial testing the efficacy of GI.1 HuNoV VLPs plus adjuvant demonstrated modest protection. Volunteers administered either VLP + adjuvants (n=38) or placebo (n=39) in two intranasal doses were challenged with live virus three weeks later. Vaccinated subjects exhibited reduced gastroenteritis (37% of vaccinees versus 69% of placebo recipients) and incidence of infection (61% of vaccinees versus 82% of placebo recipients) (Atmar et al., 2011). In general, the presence of pre-challenge antibodies capable of blocking VLP binding to its respective HBGA correlated with protection against infection and illness. A more recent Phase I/II trial tested intramuscular prime:boost inoculation of a bivalent vaccine comprised of a GI.1 and a GII.4 VLP plus adjuvant followed by challenge with a GII.4 HuNoV. Incidence and severity of disease were significantly reduced in the vaccinated (n=56) vs. the placebo group (n=53) (Bernstein, 2013).
Recent work in the MuNoV model system demonstrated that malnourished mice develop a severely reduced mucosal IgA response to MNV-1 (although their antiviral serum IgG response is fairly normal); decreased mucosal antiviral antibody correlates with a lack of protective immunity to a secondary challenge as assessed by reductions in viral titers and intestinal fluid accumulation as a measure of disease (Hickman et al., 2014). These findings raise concerns that malnourished people will mount ineffective or significantly reduced immunity to a HuNoV vaccine, an outcome that would be consistent with other vaccines (von Bubnoff, 2011; Haque et al., 2014; Qadri et al., 2013). It will be important to consider the impact of inoculation route in future studies of vaccine efficacy in malnourished hosts.
Another major barrier to HuNoV vaccine development is the extreme genetic heterogeneity within the virus family. Several lines of investigation have revealed a lack of inter-genogroup cross-protection. For example, an early human challenge study demonstrated that prior GI.1 infection fails to elicit protection from a subsequent GII.1 infection (Wyatt et al., 1974). Similarly, chimpanzees immunized with genogroup II VLPs fail to generate protective immunity to a GI.1 challenge, although they elicit a robust homotypic serum antibody response (Bok et al., 2011). It is thus likely that an effective vaccine formulation will need to minimally include genogroup I and II VLPs. Based on the frequent emergence of dominant GII.4 HuNoV strains that appear to display antigenic variability it is also probable that the GII VLP will require periodic modifications, similar to the strategy used for updating seasonal influenza virus vaccines. Collectively, VLP-based NoV vaccines show great promise but concerns remain about the duration, breadth, and magnitude of the immune responses elicited by these nonreplicating antigens. Similar concerns have plagued the HIV and influenza virus fields for many years. New technologies for isolating human monoclonal antibodies that are broadly neutralizing against highly genetically diverse virus families have revolutionized these fields [reviewed by (Burton et al., 2012)]. The first human monoclonal antibodies against NoVs have been generated, with some of them being broadly reactive with a panel of GII.4 HuNoV strains (Lindesmith et al., 2012). Furthermore, work in the MuNoV model shows that administration of monoclonal antibodies effectively reduces viral titers (Kolawole et al., 2014). Thus, broadly reactive HuNoV antibodies may offer promise as treatment options or for new NoV vaccine strategies.
Outlook
The last decade has seen remarkable advances in the molecular virology and immunology of NoVs as summarized in this review. The field is now ripe to move forward, armed with knowledge that a major human disease may be overcome by sustained advances in the laboratory. The efficacy of the first human vaccine trial provides a major boost for this field, enabling one to envision a major reduction in the disease burden associated with these important human pathogens.
The tools for this progress are now largely in place due to rapid progress over the last 10-12 years. Primary among these is the finding that MuNoV is an enteric virus sharing many pathogenic properties with its human relatives that can be studied in the genetically tractable mouse system. Further, MuNoV replicates robustly in cultured cells, allowing the development of molecular clones of NoV strains with different properties and easy mutagenesis, which has shepherded the advancement of molecular NoV pathogenesis and immunity.
A major limitation of the MuNoV system remains the lack of acute gastroenteritis in wild-type mice infected with currently available strains. This has created controversy in the field regarding whether the mouse model has value for analysis of NoV biology and pathogenesis. Ultimately, all animal models have certain drawbacks but their utility to illuminate in vivo virus-host interactions provides otherwise unattainable insights into viral pathogenesis and the opportunity to derive in-depth understanding of fundamental processes in immunity and pathogenesis. Other animals models including nonhuman primates, pigs, and immunocompromised mice infected with HuNoVs can no doubt serve as parallel systems. Collectively, these animal models are illuminating determinants of NoV protective immunity and our understanding of the possible role of persistent NoV infection in viral spread. On the molecular virology side, newly developed tools include a replication system for an animal virus closely related to the human pathogens and replicon systems for HuNoVs.
Areas of NoV research that await investigation include (i) developing HuNoV propagation systems; (ii) elucidating mechanisms used by NoVs to elicit enteric pathology; (iii) designing anti-NoV drugs that are safe and effective, especially in critical target populations such as the immunocompromised; (iv) optimizing vaccine strategies to enhance their efficacy across the genetically diverse HuNoV strains; and finally, (v) interrogating the impact of NoV infection on the long-term health of the gut as the complex relationships between intestinal microbial communities, mucosal immunity, environmental factors, and host genetics are becoming better understood.
Acknowledgments
We thank Megan Baldridge and Tim Nice for critical reading of this manuscript. The nature of this review was to highlight recent advances in the norovirus field but not to exhaustively describe the history of norovirus research in its entirety. We thus sincerely apologize to those in the field whose work was not cited here due to formatting requirements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkhouri N, Danziger-Isakov L. Norovirus and severe chronic gastroenteritis in pediatric stem cell transplantation: The plot thickens. Pediatr Transplant. 2011;15:671–672. doi: 10.1111/j.1399-3046.2011.01522.x. [DOI] [PubMed] [Google Scholar]
- Allen DJ, Noad R, Samuel D, Gray JJ, Roy P, Iturriza-Gomara M. Characterisation of a GII-4 norovirus variant-specific surface-exposed site involved in antibody binding. Virol J. 2009;6 doi: 10.1186/1743-422X-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A, Bailey D, Chaudhry Y, Goodfellow IG. Development of a Reverse Genetics System for Murine Norovirus 3; Long-Term Persistence Occurs in the Caecum and Colon. J Gen Virol. 2012;93:1432–1441. doi: 10.1099/vir.0.042176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A, Emmott E, Vashist S, Goodfellow I. Progress towards the prevention and treatment of norovirus infections. Future Microbiol. 2013;8:1475–1487. doi: 10.2217/fmb.13.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar RL, Estes MK. Norovirus vaccine development: next steps. Expert Rev Vaccines. 2012;11:1023–1025. doi: 10.1586/erv.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, et al. Norovirus Vaccine against Experimental Human Norwalk Virus Illness. N Engl J Med. 2011;365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, Thackray LB, Goodfellow IG. A Single Amino Acid Substitution in the Murine Norovirus Capsid Protein Is Sufficient for Attenuation In Vivo. J Virol. 2008;82:7725–7728. doi: 10.1128/JVI.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, Karakasiliotis I, Vashist S, Chung LMW, Reese J, McFadden N, Benson A, Yarovinsky F, Simmonds P, Goodfellow I. Functional Analysis of RNA Structures Present at the 3′ Extremity of the Murine Norovirus Genome: the Variable Polypyrimidine Tract Plays a Role in Viral Virulence. J Virol. 2010;84:2859–2870. doi: 10.1128/JVI.02053-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay L, Wikswo ME, Gregoricus N, Vinje J, Lopman B, Parashar UD, Hall AJ, Leshem E. Notes from the Field: Emergence of New Norovirus Strain GII.4 Sydney — United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:55. [PMC free article] [PubMed] [Google Scholar]
- Basic M, Keubler LM, Buettner M, Achard MD, Breves GD, Schroder BD, Smoczek A, Jorns AD, Wedekind DD, Zschemisch NHD, et al. Norovirus Triggered Microbiota-driven Mucosal Inflammation in Interleukin 10-deficient Mice. Inflamm Bowel Dis March 2014. 2014;20:431–443. doi: 10.1097/01.MIB.0000441346.86827.ed. [DOI] [PubMed] [Google Scholar]
- Beersma MFC, Schutten M, Vennema H, Hartwig NG, Mes THM, Osterhaus ADME, van Doornum GJJ, Koopmans M. Norovirus in a Dutch tertiary care hospital (2002–2007): frequent nosocomial transmission and dominance of GIIb strains in young children. J Hosp Infect. 2009;71:199–205. doi: 10.1016/j.jhin.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Belliot G, Sosnovtsev SV, Chang KO, Babu V, Uche U, Arnold JJ, Cameron CE, Green KY. Norovirus Proteinase-Polymerase and Polymerase Are Both Active Forms of RNA-Dependent RNA Polymerase. J Virol. 2005;79:2393–2403. doi: 10.1128/JVI.79.4.2393-2403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein. IDWeek 2013: Norovirus. IDWeek 2014 2013 [Google Scholar]
- Blacklow NR, Dolin R, Fedson DS, Dupont H, Northrup RS, Hornick RB, Chanock RM. Acute infectious nonbacterial gastroenteritis: etiology and pathogenesis. Ann Intern Med. 1972;76:993–1008. doi: 10.7326/0003-4819-76-6-993. [DOI] [PubMed] [Google Scholar]
- Bok K, Green KY. Norovirus Gastroenteritis in Immunocompromised Patients. N Engl J Med. 2012;367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok K, Parra GI, Mitra T, Abente E, Shaver CK, Boon D, Engle R, Yu C, Kapikian AZ, Sosnovtsev SV, et al. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci. 2011;108:325–330. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon D, Mahar JE, Abente EJ, Kirkwood CD, Purcell RH, Kapikian AZ, Green KY, Bok K. Comparative Evolution of GII.3 and GII.4 Norovirus over a 31-Year Period. J Virol. 2011;85:8656–8666. doi: 10.1128/JVI.00472-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin BN, Tang W, Nice TJ, McCune BT, Virgin HW, Krezel AM. Murine norovirus protein NS1/2 aspartate to glutamate mutation sufficient for persistence reorients sidechain of surface exposed tryptophan within a novel structured domain. Proteins. 2013 doi: 10.1002/prot.24484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bubnoff A. A gut response to vaccines. IAVI Rep Newsl Int AIDS Vaccine Res. 2011;15:12–14. [PubMed] [Google Scholar]
- Bui T, Kocher J, Li Y, Wen K, Li G, Liu F, Yang X, LeRoith T, Tan M, Xia M, et al. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J Gen Virol. 2013;94:2005–2016. doi: 10.1099/vir.0.054080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RA, White PA. Mechanisms of GII.4 norovirus evolution. Trends Microbiol. 2011;19:233–240. doi: 10.1016/j.tim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly Neutralizing Antibodies Present New Prospects to Counter Highly Antigenically Diverse Viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Maloney NS, Liu TC, Ng ACY, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-Plus-Susceptibility Gene Interaction Determines Crohn's Disease Gene Atg16L1 Phenotypes in Intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, Virgin HW. Antibody Is Critical for the Clearance of Murine Norovirus Infection. J Virol. 2008a;82:6610–6617. doi: 10.1128/JVI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune Mechanisms Responsible for Vaccination against and Clearance of Mucosal and Lymphatic Norovirus Infection. PLoS Pathog. 2008b;4:e1000236. doi: 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MCW, Ho WS, Sung JJY. In Vitro Whole-Virus Binding of a Norovirus Genogroup II Genotype 4 Strain to Cells of the Lamina Propria and Brunner's Glands in the Human Duodenum. J Virol. 2011;85:8427–8430. doi: 10.1128/JVI.05016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO. Role of Cholesterol Pathways in Norovirus Replication. J Virol. 2009;83:8587–8595. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology. 2006;353:463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Changotra H, Jia Y, Moore TN, Liu G, Kahan SM, Sosnovtsev SV, Karst SM. Type I and Type II Interferons Inhibit the Translation of Murine Norovirus Proteins. J Virol. 2009;83:5683–5692. doi: 10.1128/JVI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry Y, Nayak A, Bordeleau ME, Tanaka J, Pelletier J, Belsham GJ, Roberts LO, Goodfellow IG. Caliciviruses Differ in Their Functional Requirements for eIF4F Components. J Biol Chem. 2006;281:25315–25325. doi: 10.1074/jbc.M602230200. [DOI] [PubMed] [Google Scholar]
- Chaudhry Y, Skinner MA, Goodfellow IG. Recovery of genetically defined murine norovirus in tissue culture by using a fowlpox virus expressing T7 RNA polymerase. J Gen Virol. 2007;88:2091–2100. doi: 10.1099/vir.0.82940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. Pathogenesis of a Genogroup II Human Norovirus in Gnotobiotic Pigs. J Virol. 2006;80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughenbaugh K, Wobus C, Hardy M. VPg of murine norovirus binds translation initiation factors in infected cells. Virol J. 2006;3:33. doi: 10.1186/1743-422X-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughenbaugh KF, Fraser CS, Hershey JWB, Hardy ME. The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J. 2003;22:2852–2859. doi: 10.1093/emboj/cdg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbink K, Donaldson EF, Lindesmith LC, Baric RS. Genetic Mapping of a Highly Variable Norovirus GII.4 Blockade Epitope: Potential Role in Escape from Human Herd Immunity. J Virol. 2012;86:1214–1226. doi: 10.1128/JVI.06189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbink K, Lindesmith LC, Donaldson EF, Costantini V, Beltramello M, Corti D, Swanstrom J, Lanzavecchia A, Vinjé J, Baric RS. Emergence of New Pandemic GII.4 Sydney Norovirus Strain Correlates with Escape from Herd Immunity. J Infect Dis. 2013;208:1877–1887. doi: 10.1093/infdis/jit370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin R, Levy AG, Wyatt RG, Thornhill TS, Gardner JD. Viral gastroenteritis induced by the Hawaii agent. Jejunal histopathology and serologic response. Am J Med. 1975;59:761–768. doi: 10.1016/0002-9343(75)90461-1. [DOI] [PubMed] [Google Scholar]
- Donaldson EF, Lindesmith LC, LoBue AD, Baric RS. Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Micro. 2010;8:231–241. doi: 10.1038/nrmicro2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MPG, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- Elftman MD, Gonzalez-Hernandez MB, Kamada N, Perkins C, Henderson KS, Nunez G, Wobus CE. Multiple effects of dendritic cell depletion on murine norovirus infection. J Gen Virol. 2013;94:1761–1768. doi: 10.1099/vir.0.052134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK, Prasad BV, Atmar RL. Noroviruses everywhere: has something changed? Curr Opin Infect Dis. 2006;19:467–474. doi: 10.1097/01.qco.0000244053.69253.3d. [DOI] [PubMed] [Google Scholar]
- Fukushi S, Kojima S, Takai R, Hoshino FB, Oka T, Takeda N, Katayama K, Kageyama T. Poly(A)- and Primer-Independent RNA Polymerase of Norovirus. J Virol. 2004;78:3889–3896. doi: 10.1128/JVI.78.8.3889-3896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hernandez MB, Liu T, Blanco LP, Auble H, Payne HC, Wobus CE. Murine Norovirus Transcytosis across an In Vitro Polarized Murine Intestinal Epithelial Monolayer Is Mediated by M-Like Cells. J Virol. 2013;87:12685–12693. doi: 10.1128/JVI.02378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hernandez MB, Liu T, Payne HC, Stencel-Baerenwald J, Ikizler M, Yagita H, Dermody TS, Williams IR, Wobus CE. Efficient norovirus and reovirus replication in the mouse intestine requires microfold (M) cells. J Virol JVI. 2014:00204–14. doi: 10.1128/JVI.00204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow I, Chaudhry Y, Gioldasi I, Gerondopoulos A, Natoni A, Labrie L, Laliberte JF, Roberts L. Calicivirus translation initiation requires an interaction between VPg and eIF4E. EMBO Rep. 2005;6:968–972. doi: 10.1038/sj.embor.7400510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KY. Fields Virology. Philadelphia: Lippincott, Williams, and Wilkins; 2013. Caliciviridae:The Noroviruses; pp. 582–608. [Google Scholar]
- Guix S, Asanaka M, Katayama K, Crawford SE, Neill FH, Atmar RL, Estes MK. Norwalk Virus RNA Is Infectious in Mammalian Cells. J Virol. 2007;81:12238–12248. doi: 10.1128/JVI.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Snider C, Liu Y, Ma JZ, Liu L, Nayak U, Mychaleckyj JC, Korpe P, Mondal D, Kabir M, et al. Oral polio vaccine response in breast fed infants with malnutrition and diarrhea. Vaccine. 2014;32:478–482. doi: 10.1016/j.vaccine.2013.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. Binding of Norwalk Virus-Like Particles to ABH Histo-Blood Group Antigens Is Blocked by Antisera from Infected Human Volunteers or Experimentally Vaccinated Mice. J Virol. 2002;76:12335–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst-Kralovetz MM, Radtke AL, Lay MK, Hjelm BE, Bolick AN, Sarker SS, Atmar RL, Kingsley DH, Arntzen CJ, Estes MK, et al. Lack of norovirus replication and histo-blood group antigen expression in 3-dimensional intestinal epithelial cells. Emerg Infect Dis. 2013;19:431–438. doi: 10.3201/eid1903.121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman D, Jones MK, Zhu S, Kirpatrick E, Ostrov DA, Wang X, Ukhanova M, Sun Y, Mai V, Salemi M, et al. The Effect of Malnutrition on Norovirus Infection. mBio. 2014;5:e01032–13. doi: 10.1128/mBio.01032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo-Moriguchi K, Shirato H, Someya Y, Kurosawa Y, Takeda N, Taniguchi K. Isolation of cross-reactive human monoclonal antibodies that prevent binding of human noroviruses to histo-blood group antigens. J Med Virol. 2014;86:558–567. doi: 10.1002/jmv.23734. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Wobus CE, Steffen EK, Riley LK, Livingston RS. Development of a Microsphere-Based Serologic Multiplexed Fluorescent Immunoassay and a Reverse Transcriptase PCR Assay To Detect Murine Norovirus 1 Infection in Mice. Clin Diagn Lab Immunol. 2005;12:1145–1151. doi: 10.1128/CDLI.12.10.1145-1151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, et al. Nondegradative Role of Atg5-Atg12/ Atg16L1 Autophagy Protein Complex in Antiviral Activity of Interferon Gamma. Cell Host Microbe. 2012;11:397–409. doi: 10.1016/j.chom.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JL, Mackenzie JM. Subcellular localization of the MNV-1 ORF1 proteins and their potential roles in the formation of the MNV-1 replication complex. Virology. 2010;406:138–148. doi: 10.1016/j.virol.2010.06.047. [DOI] [PubMed] [Google Scholar]
- Hyde JL, Sosnovtsev SV, Green KY, Wobus C, Virgin HW, Mackenzie JM. Mouse Norovirus Replication Is Associated with Virus-Induced Vesicle Clusters Originating from Membranes Derived from the Secretory Pathway. J Virol. 2009;83:9709–9719. doi: 10.1128/JVI.00600-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JL, Gillespie LK, Mackenzie JM. Mouse Norovirus 1 Utilizes the Cytoskeleton Network To Establish Localization of the Replication Complex Proximal to the Microtubule Organizing Center. J Virol. 2012;86:4110–4122. doi: 10.1128/JVI.05784-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis. 1990;161:18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- Jung K, Wang Q, Kim Y, Scheuer K, Zhang Z, Shen Q, Chang KO, Saif LJ. The Effects of Simvastatin or Interferon-α on Infectivity of Human Norovirus Using a Gnotobiotic Pig Model for the Study of Antivirals. PLoS ONE. 2012;7:e41619. doi: 10.1371/journal.pone.0041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan SM, Liu G, Reinhard MK, Hsu CC, Livingston RS, Karst SM. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology. 2011;421:202–210. doi: 10.1016/j.virol.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst SM. Caliciviruses: Molecular and Cellular Virology. Horizon Scientific Press; 2010a. Murine Norovirus Pathogenesis and Immunity; pp. 183–203. [Google Scholar]
- Karst SM. Pathogenesis of Noroviruses, Emerging RNA Viruses. Viruses. 2010b;2:748–781. doi: 10.3390/v2030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. STAT1-Dependent Innate Immunity to a Norwalk-Like Virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- Kaufman SS, Green KY, Korba BE. Treatment of norovirus infections: Moving antivirals from the bench to the bedside. Antiviral Res. 2014;105C:80–91. doi: 10.1016/j.antiviral.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolawole AO, Li M, Xia C, Fischer AE, Giacobbi NS, Rippinger CM, Proescher JBG, Wu SK, Bessling SL, Gamez M, et al. Flexibility in surface exposed loops in the virus capsid mediates escape from antibody neutralization. J Virol JVI. 2014:03685–13. doi: 10.1128/JVI.03685-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HL, Neill FH, Estes MK, Munoz FM, Cameron A, Dupont HL, Atmar RL. Noroviruses: The Most Common Pediatric Viral Enteric Pathogen at a Large University Hospital After Introduction of Rotavirus Vaccination. J Pediatr Infect Dis Soc. 2013;2:57–60. doi: 10.1093/jpids/pis070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroneman A, Vega E, Vennema H, Vinjé J, White PA, Hansman G, Green K, Martella V, Katayama K, Koopmans M. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol. 2013;158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Lockwood J, Depledge DP, Chaudhry Y, Aston A, Rao K, Hartley JC, Goodfellow I, Breuer J. Next-Generation Whole Genome Sequencing Identifies the Direction of Norovirus Transmission in Linked Patients. Clin Infect Dis. 2013;57:407–414. doi: 10.1093/cid/cit287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay MK, Atmar RL, Guix S, Bharadwaj U, He H, Neill FH, Sastry KJ, Yao Q, Estes MK. Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans. Virology. 2010;406:1–11. doi: 10.1016/j.virol.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Baert L, Uyttendaele M. Inactivation of food-borne viruses using natural biochemical substances. Food Microbiol. 2013;35:1–9. doi: 10.1016/j.fm.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- Lindesmith L, Moe C, LePendu J, Frelinger JA, Treanor J, Baric RS. Cellular and Humoral Immunity following Snow Mountain Virus Challenge. J Virol. 2005;79:2900–2909. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. Heterotypic Humoral and Cellular Immune Responses following Norwalk Virus Infection. J Virol. 2010;84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesmith LC, Donaldson EF, Baric RS. Norovirus GII.4 Strain Antigenic Variation. J Virol. 2011;85:231–242. doi: 10.1128/JVI.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, Debbink K, Lanzavecchia A, Baric RS. Immunogenetic Mechanisms Driving Norovirus GII.4 Antigenic Variation. PLoS Pathog. 2012;8:e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Kahan SM, Jia Y, Karst SM. Primary High-Dose Murine Norovirus 1 Infection Fails To Protect from Secondary Challenge with Homologous Virus. J Virol. 2009;83:6963–6968. doi: 10.1128/JVI.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar JE, Donker NC, Bok K, Talbo GH, Green KY, Kirkwood CD. Identification and characterization of antibody-binding epitopes on the norovirus GII.3 capsid. J Virol. 2014;88:1942–1952. doi: 10.1128/JVI.02992-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney NS, Thackray LB, Goel G, Hwang S, Duan E, Vachharajani P, Xavier R, Virgin HW. Essential Cell-Autonomous Role for Interferon (IFN) Regulatory Factor 1 in IFN-γ-Mediated Inhibition of Norovirus Replication in Macrophages. J Virol. 2012;86:12655–12664. doi: 10.1128/JVI.01564-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marionneau S, Ruvoën N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacois G, Huang P, Jiang X, Le Pendu J. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, IV, Colonna M. MDA-5 Recognition of a Murine Norovirus. PLoS Pathog. 2008;4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden N, Bailey D, Carrara G, Benson A, Chaudhry Y, Shortland A, Heeney J, Yarovinsky F, Simmonds P, Macdonald A, et al. Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4. PLoS Pathog. 2011;7:e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden N, Arias A, Dry I, Bailey D, Witteveldt J, Evans DJ, Goodfellow I, Simmonds P. Influence of genome-scale RNA structure disruption on the replication of murine norovirus— similar replication kinetics in cell culture but attenuation of viral fitness in vivo. Nucleic Acids Res. 2013;41:6316–6331. doi: 10.1093/nar/gkt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. Murine Norovirus 1 Infection Is Associated with Histopathological Changes in Immunocompetent Hosts, but Clinical Disease Is Prevented by STAT1-Dependent Interferon Responses. J Virol. 2007;81:3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice TJ, Strong DW, McCune BT, Pohl CS, Virgin HW. A Single-Amino-Acid Change in Murine Norovirus NS1/2 Is Sufficient for Colonic Tropism and Persistence. J Virol. 2013;87:327–334. doi: 10.1128/JVI.01864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papafragkou E, Hewitt J, Park GW, Greening G, Vinjé J. Challenges of Culturing Human Norovirus in Three-Dimensional Organoid Intestinal Cell Culture Models. PLoS ONE. 2013;8:e63485. doi: 10.1371/journal.pone.0063485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med. 1977;297:86–89. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- Patterson T, Hutchings P, Palmer S. Outbreak of SRSV gastroenteritis at an international conference traced to food handled by a post-symptomatic caterer. Epidemiol Infect. 1993;111:157–162. doi: 10.1017/s0950268800056776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, et al. Norovirus and Medically Attended Gastroenteritis in U.S. Children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue KA, Green KY, Copeland M, Barron E, Mandel M, Faucette LJ, Williams EM, Sosnovtsev SV, Elkins WR, Ward JM. Naturally Occurring Murine Norovirus Infection in a Large Research Institution. J Am Assoc Lab Anim Sci. 2007;46:39–45. [PubMed] [Google Scholar]
- Perry JW, Ahmed M, Chang KO, Donato NJ, Showalter HD, Wobus CE. Antiviral Activity of a Small Molecule Deubiquitinase Inhibitor Occurs via Induction of the Unfolded Protein Response. PLoS Pathog. 2012;8:e1002783. doi: 10.1371/journal.ppat.1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BV, Rothnagel R, Jiang X, Estes MK. Three-dimensional structure of baculovirus-expressed Norwalk virus capsids. J Virol. 1994;68:5117–5125. doi: 10.1128/jvi.68.8.5117-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine. 2013;31:452–460. doi: 10.1016/j.vaccine.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Richardson C, Bargatze RF, Goodwin R, Mendelman PM. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev Vaccines. 2013;12:155–167. doi: 10.1586/erv.12.145. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MSJ, Neyts J. The viral polymerase inhibitor 2′-C-methylcytidine inhibits Norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J Virol. 2013;87:11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B, de Wit M, Vennema H, Vinjé J, de Bruin E, van Duynhoven Y, Koopmans M. Natural History of Human Calicivirus Infection: A Prospective Cohort Study. Clin Infect Dis. 2002;35:246–253. doi: 10.1086/341408. [DOI] [PubMed] [Google Scholar]
- Rohayem J, Robel I, Jager K, Scheffler U, Rudolph W. Protein-Primed and De Novo Initiation of RNA Synthesis by Norovirus 3Dpol. J Virol. 2006;80:7060–7069. doi: 10.1128/JVI.02195-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif MA, Bonney DK, Bigger B, Forsythe L, Williams N, Page J, Babiker ZO, Guiver M, Turner AJ, Hughes S, et al. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: A cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant. 2011;15:505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- Schreiber DS, Blacklow NR, Trier JS. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N Engl J Med. 1973;288:1318–1323. doi: 10.1056/NEJM197306212882503. [DOI] [PubMed] [Google Scholar]
- Schreiber DS, Blacklow NR, Trier JS. The small intestinal lesion induced by Hawaii agent acute infectious nonbacterial gastroenteritis. J Infect Dis. 1974;129:705–708. doi: 10.1093/infdis/129.6.705. [DOI] [PubMed] [Google Scholar]
- Siebenga JJ, Beersma MFC, Vennema H, van Biezen P, Hartwig NJ, Koopmans M. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J Infect Dis. 2008;198:994–1001. doi: 10.1086/591627. [DOI] [PubMed] [Google Scholar]
- Simmons K, Gambhir M, Leon J, Lopman B. Duration of Immunity to Norovirus Gastroenteritis. Emerg Infect Dis. 2013;19:1260–1267. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Jeong HS, Cho M, Lee J, Lee H, Yoon K, Jeong AY, Jung S, Kim K, Cheon DS. Seroepidemiology of Predominant Norovirus Strains Circulating in Korea by Using Recombinant Virus-Like Particle Antigens. Foodborne Pathog Dis. 2013;10:461–466. doi: 10.1089/fpd.2012.1300. [DOI] [PubMed] [Google Scholar]
- Souza M, Costantini V, Azevedo MSP, Saif LJ. A human norovirus-like particle vaccine adjuvanted with ISCOM or mLT induces cytokine and antibody responses and protection to the homologous GII.4 human norovirus in a gnotobiotic pig disease model. Vaccine. 2007;25:8448–8459. doi: 10.1016/j.vaccine.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M, Azevedo MSP, Jung K, Cheetham S, Saif LJ. Pathogenesis and Immune Responses in Gnotobiotic Calves after Infection with the Genogroup II.4-HS66 Strain of Human Norovirus. J Virol. 2008;82:1777–1786. doi: 10.1128/JVI.01347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub T, Honer zu B, Orosz-Coghlan P, Dohnalkova A, Mayer B, Bartholomew R, Valdez C, Bruckner-Lea C, Gerba C, Abbaszadegan M, et al. In vitro cell culture infectivity assay for human noroviruses. Emerg Infect Dis. 2007;13:396–403. doi: 10.3201/eid1303.060549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub TM, Bartholomew RA, Valdez CO, Valentine NB, Dohnalkova A, Ozanich RM, Bruckner-Lea CJ, Call DR. Human Norovirus Infection of Caco-2 Cells Grown as a 3-Dimensional Tissue Structure. J Water Health. 2011;9:225–240. doi: 10.2166/wh.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub TM, Hutchison JR, Bartholomew RA, Valdez CO, Valentine NB, Dohnalkova A, Ozanich RM, Bruckner-Lea CJ. Defining cell culture conditions to improve human norovirus infectivity assays. Water Sci Technol J Int Assoc Water Pollut Res. 2013;67:863–868. doi: 10.2166/wst.2012.636. [DOI] [PubMed] [Google Scholar]
- Strong DW, Thackray LB, Smith TJ, Virgin HW. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J Virol. 2012;86:2950–2958. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom J, Lindesmith LC, Donaldson EF, Yount B, Baric RS. Characterization of Blockade Antibody Responses in GII.2.1976 Snow Mountain Virus-Infected Subjects. J Virol. 2014;88:829–837. doi: 10.1128/JVI.02793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi S, Hashira S, Matsunaga T, Yoshida A, Shiota T, Tung PG, Khamrin P, Okitsu S, Mizuguchi M, Igarashi T, et al. Detection, genetic characterization, and quantification of norovirus RNA from sera of children with gastroenteritis. J Clin Virol. 2009;44:161–163. doi: 10.1016/j.jcv.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Takanashi S, Wang Q, Chen N, Shen Q, Jung K, Zhang Z, Yokoyama M, Lindesmith LC, Baric RS, Saif LJ. Characterization of Emerging GII.g/GII.12 Noroviruses from a Gastroenteritis Outbreak in the United States in 2010. J Clin Microbiol. 2011;49:3234–3244. doi: 10.1128/JCM.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi S, Saif LJ, Hughes JH, Meulia T, Jung K, Scheuer KA, Wang Q. Failure of propagation of human norovirus in intestinal epithelial cells with microvilli grown in three-dimensional cultures. Arch Virol. 2013;159:257–266. doi: 10.1007/s00705-013-1806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Jiang X. Histo-blood group antigens a common niche for norovirus and rotavirus. Expert Rev Mol Med. 2014;16:null–null. doi: 10.1017/erm.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube S, Kolawole AO, Höhne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. A Mouse Model for Human Norovirus. mBio. 2013;4:e00450–13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray LB, Wobus CE, Chachu KA, Liu B, Alegre ER, Henderson KS, Kelley ST, Virgin HW. Murine Noroviruses Comprising a Single Genogroup Exhibit Biological Diversity despite Limited Sequence Divergence. J Virol. 2007;81:10460–10473. doi: 10.1128/JVI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray LB, Duan E, Lazear HM, Kambal A, Schreiber RD, Diamond MS, Virgin HW. Critical Role for Interferon Regulatory Factor 3 (IRF-3) and IRF-7 in Type I Interferon-Mediated Control of Murine Norovirus Replication. J Virol. 2012;86:13515–13523. doi: 10.1128/JVI.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne LG, Goodfellow IG. Norovirus gene expression and replication. J Gen Virol. 2013;95:278–291. doi: 10.1099/vir.0.059634-0. [DOI] [PubMed] [Google Scholar]
- Tomov VT, Osborne LC, Dolfi DV, Sonnenberg GF, Monticelli LA, Mansfield K, Virgin HW, Artis D, Wherry EJ. Persistent Enteric Murine Norovirus Infection Is Associated with Functionally Suboptimal Virus-Specific CD8 T Cell Responses. J Virol. 2013;87:7015–7031. doi: 10.1128/JVI.03389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger H, Loddenkemper C, Schneider T, Schreier E, Epple HJ, Zeitz M, Fromm M, Schulzke JD. Structural and functional changes of the duodenum in human norovirus infection. Gut. 2009;58:1070–1077. doi: 10.1136/gut.2008.160150. [DOI] [PubMed] [Google Scholar]
- Ward JM, Wobus CE, Thackray LB, Erexson CR, Faucette LJ, Belliot G, Barron EL, Sosnovtsev SV, Green KY. Pathology of Immunodeficient Mice With Naturally Occurring Murine Norovirus Infection. Toxicol Pathol. 2006;34:708–715. doi: 10.1080/01926230600918876. [DOI] [PubMed] [Google Scholar]
- Ward VK, McCormick CJ, Clarke IN, Salim O, Wobus CE, Thackray LB, Virgin HW, Lambden PR. Recovery of infectious murine norovirus using pol II-driven expression of full-length cDNA. Proc Natl Acad Sci. 2007;104:11050–11055. doi: 10.1073/pnas.0700336104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in Cell Culture Reveals a Tropism for Dendritic Cells and Macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus CE, Thackray LB, Virgin HW. Murine Norovirus: a Model System To Study Norovirus Biology and Pathogenesis. J Virol. 2006;80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RG, Dolin R, Blacklow NR, DuPont HL, Buscho RF, Thornhill TS, Kapikian AZ, Chanock RM. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis. 1974;129:709–714. doi: 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]
- Wyatt RG, Greenberg HB, Dalgard DW, Allen WP, Sly DL, Thornhill TS, Chanock RM, Kapikian AZ. Experimental infection of chimpanzees with the Norwalk agent of epidemic viral gastroenteritis. J Med Virol. 1978;2:89–96. doi: 10.1002/jmv.1890020203. [DOI] [PubMed] [Google Scholar]
- Zhu S, Regev D, Watanabe M, Hickman D, Moussatche N, Jesus DM, Kahan SM, Napthine S, Brierley I, Hunter RN, et al. Identification of Immune and Viral Correlates of Norovirus Protective Immunity through Comparative Study of Intra-Cluster Norovirus Strains. PLoS Pathog. 2013;9:e1003592. doi: 10.1371/journal.ppat.1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]