Abstract
Monocytes/macrophages (MMs), mononuclear phagocytes, have been implicated in stroke-induced inflammation and injury. However, the presence of pro-inflammatory Ly-6Chigh and antiinflammatory Ly-6Clow monocyte subsets raises uncertainty regarding their role in stroke pathologic assessment. With recent identification of the spleen as an immediate reservoir of MMs, this current study addresses whether the spleen-derived MMs are required for stroke pathologic assessment. We observed that the spleen was contracted in poststroke animals and the contraction was accompanied by decreased number of Ly-6Chigh and Ly-6Clow subsets in the spleen. The deployment of these subsets from the spleen temporally coincided with respective increases in the ischemic brain. Compared to mice with the spleen, mice receiving a splenectomy just before the stroke displayed less accumulation of Ly-6Chigh and Ly-6Clow MMs in the brain. Despite the reduced accumulation of both subsets, infarct size and swelling were not reduced in the asplenic mice. The dissociative findings of infarct size and extent of MM infiltration in the postischemic brain indicate minimal involvement of spleen-derived total MMs in acute infarct development. Selective Ly-6Chigh or Ly-6Clow MM targeting is suggested to address the contribution of the individual subset to acute stroke pathologic assessment.
Keywords: ischemic stroke, Ly-6Chigh, Ly-6Clow, monocytes/macrophages, spleen, splenectomy
Introduction
Stroke-induced brain injury, a primary central nervous system event, is closely coupled with the infiltration of peripheral immune cells into the infarct area.1, 2, 3 Among peripheral immune cells, the presence of monocytes/macrophages (MMs) in the infarct area was thought to contribute to postischemic inflammation and injury.4, 5 For instance, studies showed that the extent of acute injury is positively correlated with the number of MMs in the injured brain after the stroke.6, 7 Additionally, decreased MM infiltration delayed disease progression in animal models of multiple sclerosis or spinal cord injury and increased axonal regeneration with functional benefit,8, 9, 10 suggesting MMs' detrimental role in neuronal pathologic assessment. In contrast, the depletion of MMs was associated with impaired wound healing and high mortality in myocardial infarction.11 Furthermore, decreased MM infiltration resulted in the depletion of circulating MMs or in the deficiency of chemokine receptor, which increased stroke-induced perilesional hemorrhage, with clinical deterioration in the mouse stroke model.12 These equivocal findings suggest an uncertain role of MMs in response to injury.
The spleen is the largest lymphatic organ in the body that functions in the removal of aging erythrocytes, recycling iron, producing antibodies, and elicitation of immunity.13, 14 The spleen has been considered to be an immediate reservoir of monocytes that mobilizes a large quantity of monocytes upon injury.15 Several studies reported the involvement of the spleen in inflammatory diseases. Not only is the number of monocytes in the spleen several fold higher than those found in circulation, but also the amount of monocytes in the injured heart after myocardial infarction exceeds those in circulation under homeostatic conditions. An additional support for the mobilization of spleen monocytes after the injury comes from the observation of a severely reduced accumulation of monocytes, but not neutrophils, following myocardial infarct in animals that have previously undergone a splenectomy (spx).15 Similarly, stroke caused the contraction of the spleen and reduced the number of splenic cells.16, 17 Moreover, there is a report that addressed the role of the spleen in leukocyte infiltration and injury in stroke. Spx 2 weeks before the stroke decreased the number of activated microglia, macrophages, and neutrophils in the injured area and was associated with reduced infarct size.18
Accumulating evidences suggest that human and mouse monocytes exhibit distinct subsets that are reminiscent of macrophage phenotypes.19, 20 The subset that expresses a high level of the hematopoietic cell differentiation antigen Ly-6C (Ly-6Chigh) also expresses the G-protein-linked membrane protein, CCR2. The Ly-6Chigh (CCR2+) monocytes are specifically recruited to an injury site and become classically activated M1 macrophages. The Ly-6Chigh (CCR2+) subset shows chemotaxis to monocyte chemoattractant protein-1 (MCP-1) that is produced in the inflamed tissue. Recruitment of this subset to inflammatory sites is believed to be CCR2 dependent, since monocytes from mice that do not express CCR2 do not traffic as efficiently into inflamed tissues as CCR2+ monocytes.19 In contrast, a low level of Ly-6C expressing monocytes (Ly-6Clow) expresses a high level of CX3CR1, a receptor for CX3CL1 (fractalkine), but this monocyte subset is devoid of CCR2 expression. This antiinflammatory Ly-6Clow (CCR2−/CX3CR1high) subset is recruited to normal tissues and develops into resident M2 macrophages that function in host defense and repair after injury.19 After myocardial infarction or Listeria monocytogenes infection, the early pro-inflammatory Ly-6Chigh (CCR2+) and late antiinflammatory Ly-6Clow (CCR2−) subsets are sequentially recruited to the injury site in a controlled manner for inflammation and repair/healing.19, 21
There is a relative paucity of in vivo studies related to monocyte mobilization from the periphery to brain infarct. The heterogeneity of monocyte subsets and temporally distinctive recruitment of each monocyte subset add the layers of complexity in immune cell trafficking. By analyzing MM subsets in the spleen and postischemic brain in normal and splenectomized mice, the current study investigates the role of the spleen in MM infiltration and injury in the acute ischemic brain. Here, we report that while disappearance of MMs in the spleen temporally coincided with appearance of the cells in the infarcted brain, spx did not reduced stroke-induced brain injury. The role of the spleen in ischemic injury requires careful reconsideration.
Materials and methods
Animals
The use of animals and procedures was approved by the Institutional Animal Care and Use Committee (IACUC) of Weill Medical College at Cornell University and in accordance with the IACUC, National Institutes of Health, and ARRIVE guidelines. Experiments were performed in 10- to 11-week-old male C57BL/6 mice (Jackson Lab, Bar Harbor, ME, USA). The mice were housed at the institute's animal facility, which monitors and maintains temperature, humidity, and 12-hour light/dark cycles. Maximum five mice were housed in a cage with an individual ventilating system and irradiated bedding (1/8″ Bed o'Cobs, The Anderson, Maumee, OH, USA). Sterilized food (PicoLab Rodent diet 5053, LabDiet, St Louise, MO, USA) and water were freely accessible in their cage. Animals were randomly selected for sham occlusion or middle cerebral artery occlusion (MCAO). Moreover, the identity of the mice that received sham-splenectomy (sham-spx) or spx was masked to surgeons who performed MCAO. The identity was revealed after data were collected.
Splenectomy
Mice were anesthetized with a mixture of isoflurane (1.5% to 2.0%) with oxygen and nitrogen (30%/70%) and an ∼1 cm incision was made on the left side of the abdominal cavity under the rib cage. Mice were randomly selected for an spx or sham procedure. The spleen was removed by cutting the mesentery and connective tissue and the splenic vessels were cauterized. For sham-control mice, incisions were made without removing the spleen.
Transient Middle Cerebral Artery Occlusion
Immediately after sham or spx, mice were subjected to transient ischemia by MCAO as previously described.22, 23 A fiber optic probe was glued to the parietal bone (2 mm posterior and 5 mm lateral to the bregma) and connected to a Laser-Doppler Flowmeter (Periflux System 5010; Perimed, Järfälla, Sweden) for continuous monitoring of cerebral blood flow (CBF) in the ischemic territory. For MCAO, a 6-0 Teflon-coated black monofilament surgical suture (Doccol, Redland, CA, USA) was inserted into the exposed external carotid artery, advanced into the internal carotid artery, and wedged into the cerebral arterial circle to obstruct the origin of the middle cerebral artery for 30 minutes. The filament was withdrawn to allow reperfusion. Buprenorphine, lidocaine, and buprivacaine were administered post ischemia as analgesics. Mice were then placed in a recovery cage until the animal regained consciousness and resumed activity. Using a rectal probe controlled by a masterflex pump and thermistor temperature controller (Cole-Parmer, Vernon Hills, IL, USA), animal's body temperatures were maintained at 37°C±0.5°C during MCAO and recovery after surgery. The mice were then returned to their home cages where they were previously housed together. For hydrating animals after surgery, hydrogel (ClearH2O, Portland, ME, USA) was provided with food. Only animals that exhibited >80% reduction in CBF during MCAO and >80% reperfusion 10 minutes after reperfusion were included in the study.
Leukocyte Harvest from the Spleen, Blood, Bone Marrow, and Brain
To collect cells from the spleen, the entire spleen was excised and minced using scissors and pipetting in phosphate-buffered saline (PBS). The mixture was passed through a 70 μm strainer and then centrifuged at 3,000 rpm for 10 minutes at 4°C to isolate splenocytes. For the collection of blood cells, mice were perfused with 20 mL PBS containing heparin and the entire solution containing blood was collected, followed by centrifugation at 3,000 rpm for 10 minutes at 4°C to obtain total blood cells. For bone marrow (BM)-derived cells, the tibia and femur bones were flushed with PBS using a syringe to obtain BM cells. To remove red blood cells, the cells from the spleen, blood, and BM were treated with a red blood cell lysis buffer (Sigma, MO, USA) followed by washing with PBS. To collect cells from the brain, mice were perfused with PBS and brains excluding olfactory bulb and cerebellum were excised. Each hemisphere was divided and homogenized in cold PBS. The homogenates were passed through a 70 μm cell strainer and centrifuged at 3,000 rpm for 10 minutes at 4°C. The homogenates were incubated with Earle's balanced salt solution (EBSS, Sigma) containing 0.37 g/mL papain (Worthington Biochemical Corp, Lakewood, NJ, USA) for 20 minutes at 37°C to obtain a single cell suspension followed by incubating with EBSS containing 30 mg/mL DNase I (Sigma) for 3 minutes at 37°C. The cells were suspended with EBSS with 1% of trypsin inhibitor (Sigma) and bovine serum albumin (Sigma), and centrifuged at 3,000 rpm for 10 minutes. The cells were suspended in 2 mL PBS, overlaid on 3 mL of histopaque (density, 1.5, Sigma), and further centrifuged at 400 g for 30 minutes at room temperature. The lower layer containing MMs was collected and washed with PBS. The isolated cells from each organ were fixed in 4% of paraformaldehyde for 15 minutes and kept at 4°C for further analyses.
Flow Cytometry Analysis
Flow cytometry analysis for MMs in peripheral organs was performed according to the methods as previously described.15, 24 After incubating with 100% fetal bovine serum overnight, the single cells from the spleen, blood, and BM were incubated with (i) a cocktail of phycoerythrin-conjugated antibodies (Lin-PE) against T cells (CD90.2), B cells (B220), natural killer cells (NK1.1, CD49B), and granulocytes (Ly-6G); (ii) allophycocyanin-conjugated CD11b (a marker high in myeloid cells MMs); and (iii) fluorescein isothiocyanate-conjugate Ly-6C. The selected gate for MMs (low phycoerythrin/high allophycocyanin) will be analyzed further for the distribution of Ly-6Clow and Ly-6Chigh MM subsets. For the measurement of apoptosis in the spleen, single cells prepared from the spleen were incubated with fluorescein isothiocyanate-conjugated Annexin V (Miltenyi Biotec, CA, USA, 130-092-052) for 15 minutes at room temperature in the dark. To detect myeloid lineage cells in the central nervous system, phycoerythrin-conjugated CD45 and allophycocyanin-conjugated CD11b were used to identify CD45high/CD11b+ population as infiltrating MMs in the brain,25, 26, 27 which was further analyzed for Ly-6Clow and Ly-6Chigh MM subsets by Ly-6C fluorescein isothiocyanate antibodies. The single cells from the brain that were incubated with the antibodies were washed in PBS and passed through a 40 μm cell strainer before flow cytometer analysis (Accuri C6, BD Biosciences, San Jose, CA, USA).
Adoptive Transfer of Green Fluorescent Protein-Positive Mononuclear Cells into C57BL/6 Mice
Splenocytes collected from GFP+ (green fluorescent protein positive) transgenic mice were overlaid on 3 mL of histopaque (density 1.8, Sigma) and centrifuged for 30 minutes at 400 g. The mononuclear cells laid between the layers were collected and washed with PBS. The cells (1 × 107) were transfused into the splenectomized male C57BL/6 mice via retro-orbital venous sinus 3 days after ischemia. Six hours after the infusion, the mice were perfused transcardially with 4% paraformaldehyde in 0.1 mol/L phosphate buffer. Brains were collected, postfixed overnight, transferred into a 30% sucrose solution, and sectioned in a cryostat at a 30-μm thickness for the immunohistochemistry.
Immunohistochemistry
Brain sections were washed in PBS solution at pH 7.4, and incubated with 1% bovine serum albumin and 5% normal goat serum for 1 hour at room temperature. The sections were then incubated with anti-rat CD11b (1:1,000, Millipore, Billerica, MA, USA, CBL1313) and anti-chicken GFP (1:1,000, Millipore, AB16901) overnight at 4°C. Sections were washed with PBS and then incubated with secondary antibodies conjugated with Alexa Fluor 488 Goat Anti-Chicken IgG (1:300, Life Technologies, Grand Island, NY, USA, A11039) or Alexa Fluor 594 Goat Anti-Rat IgG (1:300, Life Technologies, A11007) for 1 hour. After washing with PBS, the sections were mounted using ProLong Gold antifade reagent with DAPI (Life Technologies, P36935) and examined under a laser scanning confocal microscope (Carl Zeiss, Thornwood, NY, USA).
Tissue Collection for Infarct Volume and Gene and Protein Assessment
To obtain the tissue that contains the entire infarct territory in an unbiased manner, an unbiased stereological sampling strategy was used according to the method described in the previous study.22 Three and 7 days after MCAO, brains were excised, frozen, and serial sections spanning ∼6 mm rostrocaudal (roughly +2.8 mm and extending to −3.8 mm from bregma) were collected. The entire infarct region was cryosectioned for infarct volume measurement (20 μm thickness) and collected serially at 600 micron intervals. Infarct volume and hemispheric swelling were measured using Axiovision software (Zeiss, Germany). Infarct volume was corrected for swelling by a method described previously.28 Tissues between sections for infarct volume were serially cryosectioned for gene and protein assessment, cut in half and collected for each hemisphere.
Measurement of Gene and Protein Expression
Gene expression levels were quantified by real-time quantitative RT-PCR (qPCR) using fluorescent TaqMan technology as described previously.22, 23 Briefly, total RNA was extracted from brain tissues using Tri reagent (MRC, Cincinnati, OH, USA). RNAs were reverse transcribed using the QuantiTect Reverse Transcription Kit (QIAGEN, Valencia, CA, USA). PCR primers and probes specific for MCP-1, CCR2, and β-actin (an internal control) were obtained as TaqMan pre-developed optimized assay reagents for gene expression (Applied Biosystems, Foster City, CA, USA). The PCR reaction was performed using FastStart Universal Probe Master Mix (Roche), according to the manufacturer's instructions. Reactions were performed in 20 μL total volume and incubated at 95°C for 10 minutes followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The results were analyzed using the 7500 Fast Real-Time PCR System software (Applied Biosystems). Plasma MCP-1 levels were determined using commercially available kit according to the manufacturer's procedures (MCP-1 ELISA kit, R&D systems, MN, USA).
Data Analysis
Sample size for infarct measurement (minimum n=14/group) was calculated based on predicting detectable differences to reach power of 0.80 at a significance level of <0.05, assuming a 33% difference in mean and a 30% s.d. at the 95% confidence level. Infarct volume and percentage of hemispheric swelling were expressed as mean±95% confidence interval. Results from flow cytometry analysis and gene and protein expression were expressed as mean±s.e.m. Gene expression levels were presented as the β-actin normalized value according to the formula, value=2(Ct of β-actin−Ct of target gene). Comparison between the two groups was statistically evaluated using Student's t-test. Multiple comparisons were made using analysis of variance followed by a post hoc Newman–Keuls test. Differences were considered significant at P<0.05.
Results
Stroke Causes the Reduction in Spleen Size and the Number of Splenocytes
The spleen comprises ∼0.2% of body weight.29 We first determined changes in spleen size and cell numbers in response to stroke. Compared with spleen weight before the stroke, the weight of the spleen was reduced by 3 hours after stroke and remained contracted until 7 days after stroke (Figure 1A). From total leukocytes indicated by R1 (Figure 1B), MMs were identified by selectively gating the population (R2) with low expression of lineage marker (Lin) and high expression of CD11b (Figure 1B). The stroke reduced total number of leukocytes and MMs in the spleen at 1 day and the reduction persisted until 7 days after stroke (Figures 1C and 1D). The ratio of MMs over leukocytes showed a significant reduction in MMs at 1 and 3 days after ischemia (Figure 1E). Despite the early reduction of these cells at 1 day, the degree of apoptosis in the spleen leukocytes and MMs was similar between sham and stroked mice at this time (Figures 1F and 1G). The observation indicates that mobilization, rather than apoptosis, is a likely cause for the reduced cells in the spleen after transient focal ischemia.
Figure 1.
Stroke causes the reduction in spleen size and monocytes/macrophages (MMs). (A) Spleen weight before the stroke (pre), 3 hours, 1 day, 3 days, or 7 days after stroke. (B) Flow cytometry analyses of total leukocytes (R1) and total MMs (R2) in the spleen. R1 cells were further analyzed using a lineage marker (Lin) and CD11b antibodies to selectively gate the MM population (R2, Lin−/CD11b+). SSC, side scatter, index of cellular complexity; FSC, forward scatter, index of cell size. (C) Stroke-induced changes in total leukocytes (R1). (D) Stroke-induced changes in total MMs (R2). (E) Percentage (%) of MMs in total leukocytes, n=7 to 12/group, *P<0.05, **P<0.01, and ***P<0.001 versus pre; one-way analysis of variance. % Annexin V+ apoptotic cells in total leukocytes (F) and MMs (G) in the spleen at sham-stroke (Sham) or 1 day post stroke (1d-post). n=4 to 7/group, Student's t-test.
Stroke Differentially Reduces Pro- and Antiinflammatory MM Subsets in the Spleen
MMs in the spleen were further analyzed for pro-inflammatory (Ly-6Chigh) and antiinflammatory (Ly-6Clow) subsets. We detected two distinct peaks, which represent each subset (Figure 2A). The number of Ly-6Chigh MMs was similar to that of Ly-6Clow subset before the stroke. The stroke caused significant reductions in the Ly-6Chigh subset at 1 and 3 days after ischemia, but returned to pre-stroke baseline by 7 days (Figure 2B). However, the Ly-6Clow subset showed a reduction from 3 hours to 7 days after ischemia (Figure 2C). Ly-6Chigh/Ly-6Clow ratio was significantly higher at 3 hours and returned to the baseline (Supplementary Figure S1A). The results showed an acute involvement of Ly-6Chigh MMs from day 1 to day 3 and an immediate and sustained contribution of the Ly-6Clow subset in response to stroke.
Figure 2.
Stroke differentially reduces Ly-6Chigh and Ly-6Clow MM subsets in the spleen. The MM subsets were measured prior to ischemia (pre), 3 hours, 1 day, 3 days, or 7 days after stroke. (A) Flow cytometry analysis of MM subsets. Total MMs, indicated by R2, were further analyzed by Ly-6C expression. Cells without Ly-6C antibody serve as control (w/o antibody). Quantification of Ly-6Chigh (B) and Ly-6Clow subset (C) before and after stroke. n=7 to 12/group, **P<0.01, and ***P<0.001 versus pre. One-way analysis of variance. MMs, monocytes/macrophages.
Stroke Induces Changes in MM Subset Numbers in Circulation and Bone Marrow
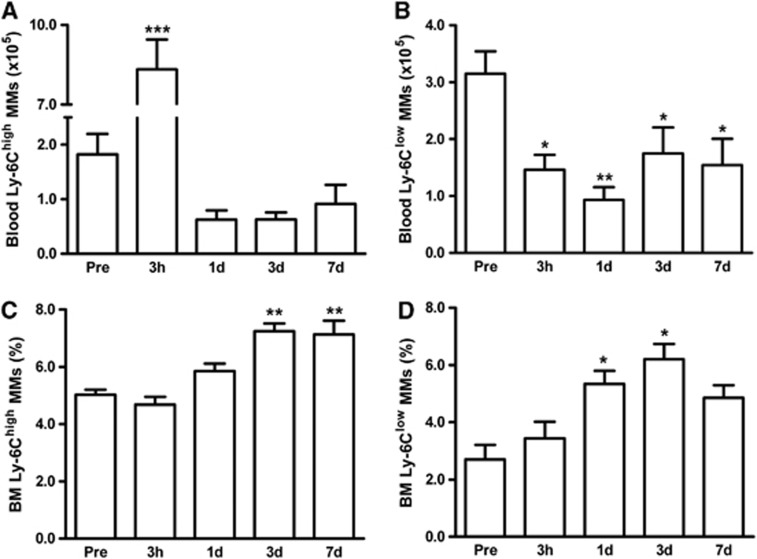
We investigated whether stroke induces changes in other peripheral organs besides the spleen. In circulation, stroke caused a sharp increase in Ly-6Chigh MMs at 3 hours, but a decline below preischemic levels from 1 to 7 days after ischemia (Figure 3A). Ly-6Clow MMs were reduced throughout the postischemic period (Figure 3B). Similar to the spleen, Ly-6Chigh/Ly-6Clow ratio in circulation was significantly higher at 3 hours and returned to the baseline (Supplementary Figure S1B). In the BM, stroke did not cause reduction of MMs but, overall, increased both MM subsets; Ly-6Chigh MMs at 3 and 7 days (Figure 3C) and Ly-6Clow subset at 1 and 3 days (Figure 3D). The continual depletion of MMs in the circulation and increased MM production in the BM suggest a dynamic response and participation of the peripheral organs after stroke.
Figure 3.
Stroke-induced changes of MM subsets in the circulation and BM. MM subsets in the blood or BM were measured in mice prior to ischemia (pre, n=5) and 3 hours (n=11), 1 day (n=13), 3 days (n=10), or 7 days (n=6) post ischemia. Flow cytometric analyses using a lineage marker (Lin) and CD11b antibodies to selectively gate MM population (R2, Lin−/CD11b+ shown in Figure 1B) followed by Ly-6C antibody incubation. Ly-6Chigh (A) and Ly-6Clow (B) MM subsets in whole blood. Percentage of Ly-6Chigh (C) and Ly-6Clow MMs (D) from BM. *P<0.05, **P<0.01, and ***P<0.001 versus pre; one-way analysis of variance. BM, bone marrow; MMs, monocytes/macrophages.
Ly-6Chigh and Ly-6Clow MMs Accumulate in the Postischemic Brain
To visualize MM trafficking into the infarct, splenic GFP+ mononuclear cells were adoptively transferred to C57BL/6 mice 3 days after stroke. Immunohistochemical examination showed numerous GFP+ cells that are colocalized with CD11b in the infarct (Figure 4A and a,b,d,e,f), but not in the contralateral hemisphere (Figures 4A and c), suggesting that the majority of GFP+ MMs are infiltrated cells from the periphery. We further quantify the extent of infiltration in the stroked brain. By differential expression of CD45, the infiltrating MMs (CD45high/CD11b+) have been distinguished from the resident microglia (CD45low/CD11b+).25, 26, 27 We found that the CD45high/CD11b+ population was absent in the naive brain (data not shown) and the contralateral hemisphere, but appears in the ipsilateral hemisphere (Figure 4B). The MM (CD45high/CD11b+) population was subjected to the analysis post ischemia. Total MMs in the stroked hemisphere were significantly increased at 1, 3, and 7 days with a peak at 3 days after ischemia (Figure 4C). Although, Ly-6Chigh and Ly-6Clow subsets were similarly increased during this time, absolute Ly-6Chigh MMs that were accumulated in the brain were several folds higher (Figures 5B and 5C). The ratio of Ly-6Chigh/Ly-6Clow was peaked at 1 day after ischemia (Supplementary Figure S1C). We also observed that mean Ly-6C expression in the recruited Ly-6Chigh subset at 1 day was decreased over time (Figure 5D), while its expression in the Ly-6Clow subset at this time was increased (Figure 5E). The data suggest a dynamic shift in the subset trafficking into the stroked brain and mean Ly-6C expression in recruited MMs.
Figure 4.
Stroke causes the accumulation of peripheral MMs in the ipsilateral hemisphere. (A) Photomicrographs of GFP and CD11b immunostaining. Brain sections of C57BL/6 mice that received GFP+ splenic mononuclear cells 3 days after stroke and killed 6 hours after the cell infusion. GFP+ (a), CD11b+ (b), and GFP/CD11b colocalization in contralateral hemisphere (c) or peri-infarct area (d), GFP+/CD11b+ cells (e) or GFP+/CD11b− cells (f) of the peri-infarct area in high magnification, blue, DAPI-stained nucleus (d,e,f); scale bar=100 μm. (B) Representative flow cytometry plots in the contralateral and ipsilateral hemisphere at 3 day post ischemia shows two distinct populations: infiltrated MMs (CD45high/CD11b+) and resident microglia (CD45low/CD11b+). Dotted lines indicate antibody controls. (C) Quantification of total MMs in the ipsilateral hemisphere prior to ischemia (pre, n=5), 3 hours (n=7), 1 day (n=11), 3 days (n=9), or 7 days (n=6) post ischemia. *P<0.05, **P<0.01, and ***P<0.001 versus contralateral hemisphere, Student's t-test. GFP, green fluorescent protein; MMs, monocytes/macrophages.
Figure 5.
Stroke causes the accumulation of both Ly-6Chigh and Ly-6Clow MMs in the brain. (A) Representative flow cytometry plots for two MM subsets by Ly-6C expression in the ipsilateral brain at 3 days post ischemia. R3, gate for infiltrated MMs. Dotted lines indicate antibody controls. Quantification of Ly-6Chigh (B) or Ly-6Clow MM subsets (C) prior to ischemia (pre, n=5), 3 hours (n=7), 1 day (n=11), 3 days (n=9), or 7 days (n=6) post ischemia. *P<0.05, **P<0.01, and ***P<0.001 versus contralateral hemisphere, Student's t-test. Mean Ly-6C fluorescence of Ly-6Chigh (D) and Ly-6Clow (E) subsets in the ipsilateral hemisphere (1, 3, and 7 days post). n=6 to 11/group (7 days post, n=6), ***P<0.001 versus 1 day post; one-way analysis of variance. MMs, monocytes/macrophages.
Splenectomy Decreases Ly-6Chigh and Ly-6Clow MM Accumulation in the Postischemic Brain
To address the involvement of spleen MMs in stroke pathologic assessment, we first determined the number of brain MMs in the mice that received sham-spx or spx just before MCAO. The relative CBF reduction during MCAO and reperfusion at 10 minutes was similar between sham and splenectomized animals (sham-spx versus spx, % CBF reduction, 85.0±0.7 versus 88.1±0.9; % CBF reperfusion at 10 minutes, 103.7±4.1 versus 115.9±7.1), showing that the severity of ischemia was similar between the groups. Poststroke mortality rates between the groups were also similar (sham-spx versus spx, % mortality, 9.2% versus 6.6%). Peripheral infection can severely modify ischemic outcome.30, 31 To exclude the possibility that spx alters the course of stroke-induced peripheral infection, we measured MCP-1 and CCR2 expression in the periphery of 3 days postischemic mice with or without the spleen. We found similar expression of MCP-1 and CCR2 gene expression in peritoneal macrophages and MCP-1 protein levels in the plasma between the groups (Supplementary Figure S2). The results indicate that spx unlikely triggers a different rate of infection in the stroked mice. Compared with the sham-spx mice, Ly-6Chigh and Ly-6Clow MMs in the ischemic brain were significantly reduced at 1 and 3 days in asplenic mice (Figures 6A and 6B). In addition, we observed that circulating Ly-6Chigh and Ly-6Clow MMs were increased in splenectomized mice at 7 days after ischemia (Supplementary Figure S3). These results suggest the spleen is a major source for the infiltrating MMs in the postischemic brain.
Figure 6.
Splenectomy reduces the number of both MM subsets in the ischemic brain. Quantification of Ly-6Chigh (A) and Ly-6Clow (B) MMs in the brain of sham-splenectomized (Sham) or splenectomized (Spx) mice at 3 hours (n=4 to 7), 1 day (n=8 to 11), 3 days (n=7 to 8), and 7 days (n=6) post ischemia. *P<0.05, **P<0.01 versus sham; Student's t-test. MMs, monocytes/macrophages.
Splenectomy did not Alter Stroke-Induced Acute Brain Injury
We further determined the effect of spx on stroke-induced acute injury. Infarct volume and percentage of hemispheric swelling showed no difference between sham and splenectomized mice 3 days after stroke (Figures 7A and 7B). Furthermore, stroke outcome assessed at 7 days showed no difference in infarct volume and swelling (Figures 7C and 7D) confirming that the spx does not affect stroke outcome in the transient MCAO model.
Figure 7.
Spleen-derived MMs are not a major cause of stroke-induced injury. (A–D) Infarct size and percentage of hemisphere swelling measured in sham-splenectomized (Sham) or splenectomized (Spx) mice at 3 days (A and B) and 7 days post ischemia (C and D). n=21 to 24/group (3 days post) or n=16 to 20/group (7 days post). Data expressed as mean±95% confidence interval. Student's t-test. MMs, monocytes/macrophages; NS, not significant.
Discussion
Accumulating evidence indicates an involvement of mononuclear phagocytes in central nervous system injury. However, the presence of discrete MM subsets and temporally distinct recruitment of individual subsets to the injured tissue required a close examination of their role in stroke pathologic assessment. Focusing on the spleen as a major source of MMs, this study addresses effects of spx on stroke-induced MM mobilization and ischemic brain injury. Several key findings from this study are: stroke deploys both Ly-6Chigh and Ly-6Clow subsets from the spleen; deployment of spleen-derived MMs temporally coincides with their accumulation in the postischemic brain with a greater accumulation of Ly-6Chigh MMs; and spx reduced MM accumulation in the ischemic brain without reducing infarct size. The dissociation between infarct size and the degree of MM infiltration in the absence of the spleen suggests that spleen-derived total MMs may not be a major contributor for acute infarct development.
Stroke-Induced Deployment of Spleen Monocytes/Macrophages
The spleen has been considered a major peripheral organ affected by inflammatory conditions, including stroke and myocardial infarction. The literatures show physical contraction and reduction of MMs in the spleen after injury.15, 17, 18 The reduction of splenic MMs is unlikely because of increased cell death or differentiation to macrophages/dendritic cells, but rather to redistribution to the blood and inflamed tissue.15 Our finding of decreased spleen size and MMs without increased apoptosis in the spleen after stroke (Figure 1) thus confirms the release and mobilization of the splenic MMs triggered by stroke rather than splenocyte apoptosis.
The timeframe for the mobilization of splenic MMs showed distinct patterns of deployment between the two subsets. We observed that the pro-inflammatory Ly-6Chigh MMs are involved acutely, as they were decreased at 1 and 3 days, but returned toward preischemic value. In contrast, the Ly-6Clow subset was decreased immediately upon stroke (i.e., 3 hours) and the reduction persisted throughout during 7 days after ischemia (Figure 2). The early and sustained reduction of the Ly-6Clow subset is likely a reflection of long-lasting involvement of antiinflammatory subset in response to injury. Although, monocyte egress from the spleen apparently involves interaction between angiotensin II in circulation and AT-1 receptor in monocytes,15, 32 the mechanisms that control the extent of deployment for individual subsets are currently unknown.
Blood vessels serve as a channel for the released spleen monocytes to enter the injury sites. Although, we observed an overall reduction of both subsets in circulation (Figures 3A and 3B), Ly-6Chigh MMs showed a selective increase at 3 hours (Figure 3A). The early rise of Ly-6Chigh MMs in blood may indicate a potential source of the Ly-6Chigh subset from other organs/tissues before the involvement of the spleen. Alternatively, the subset accumulates in the blood for subsequent infiltration to the injury site, while a sufficient chemokine gradient is established in the inflamed tissues. In BM, where monocytes are produced, we observed late rises of MM subsets (Figures 3C and 3D). The relatively late rises in BM suggest that the BM increased production of monocytes to replenish MM pools in response to the injury and that the organ, unlike the spleen, is not an immediate source for monocyte deployment.
Accumulation of MM Subsets in the Ischemic Brain
The study supports the trafficking of splenic MMs to the infarct, as the accumulation of MMs in the ischemic brain temporally coincides with their disappearance from the spleen. During the 7 days after stroke, the accumulation of both Ly-6Chigh and Ly-6Clow MMs peaked at 3 days (Figures 5B and 5C). Although, both subsets shared similar temporal patterns of infiltration, one major difference between the subsets was the quantity of cells, evidenced by several fold greater accumulation of Ly-6Chigh compared with Ly-6Clow MMs (Supplementary Figure S1C). This observation is consistent with the pro-inflammatory property of Ly-6Chigh monocytes that respond to the MCP-1 gradient formed in the injury sites. However, Ly-6Clow monocytes function in patrolling and crawling in vasculature and are recruited later to the injured site.21, 33 A recent study indicates that the Ly-6Clow monocytes recruit neutrophils to mediate focal necrosis of endothelial cells. The study also showed that the Ly-6Clow monocytes are retained within the vessel lumen without extravasation,34 which may result in reduced accumulation in the injured tissue. Regardless of the degree of infiltration in each subset, an intriguing observation from the current study is that there is a shift of mean Ly-6C expression in recruited MMs in the ischemic brain. We found that over time, Ly-6C expression was steadily reduced in the Ly-6Chigh subset, but increased in the Ly-6Clow subset (Figures 5D and 5E). This shift within an individual subset may partly reflect differentiation of recruited monocytes to tissue macrophages or potential conversion from the Ly-6Chigh to the Ly-6Clow subset.35, 36, 37
Effect of Splenectomy in Stroke-Induced MM Infiltration and Injury
Studies have implicated the involvement of the spleen in the ischemic injury.16, 17, 18 In addressing the role of spleen in the mobilization of monocyte to the infarct, we observed that the removal of the spleen profoundly affected the number of MMs accumulated in the postischemic brain. Although, other studies did not address heterogeneity of MM subsets, our analysis of subsets revealed that the reduction occurs in both Ly-6Chigh and Ly-6Clow subsets at 1 and 3 days (Figure 6). This observation supports the view regarding the spleen's involvement in an acute setting as an important source for the early deployment of MMs upon injury. Our finding of a similar number of Ly-6Chigh and Ly-6Clow MMs at 7 days between sham and splenectomized animals (Figure 6) is an additional evidence that the spleen is less involved at 7 days, possibly accounted for by increased production of monocytes in the BM at this time (Figure 3).
In addressing the role of the spleen in acute stroke outcome, we performed spx just before the stroke. Our finding contradicts the view regarding the benefits of spx in stroke by a reported study where they performed the spx 2 weeks before the permanent stroke.18 It is likely that the 2-week interval may trigger compensation in the circulating immune cells, which may exert the differential effect at the time of permanent occlusion. Indeed, the literature shows that spx increases lymphocytes, neutrophils, and monocytes in the circulation.38, 39, 40 Consistent with the reports, we also observed increased Ly-6Chigh and Ly-6Clow MMs in the circulation in splenectomized mice at 7 days after ischemia (Supplementary Figure S3). Thus, the reported beneficial effect may arise from compensated or altered immune cell homeostasis during the 2-week asplenic period before stroke. Future investigations to explain the outcome differences by different stroke models and spx-induced immune compensation are warranted.
In summary, the dissociation between MM accumulation and infarct size in splenectomized mice suggests that spleen-derived total MMs do not significantly contribute to acute infarct development. Moreover, accumulation of both pro- and antiinflammatory MM subsets, albeit to a different degree, in the postischemic brain suggests a potential synergistic or offsetting effect between the subsets during infarct development. Thus, spx as a strategy to reduce stroke injury requires careful reconsideration. To define the role of an individual MM subset for stroke-induced brain injury, further studies on selective targeting to suppress an MM subset while sparing the other are warranted.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by NIH grants HL82511, NS07396 (SC), and the Burke Foundation.
Supplementary Material
References
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Schabitz WR, Ringelstein EB, Kiefer R. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience. 2009;161:806–812. doi: 10.1016/j.neuroscience.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, et al. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71:743–752. doi: 10.1002/ana.23529. [DOI] [PubMed] [Google Scholar]
- Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Kim E, Bhosle S, Mehta H, Cho S. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation. 2010;7:92. doi: 10.1186/1742-2094-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Ajmo CT, Jr., Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Macrophage heterogeneity and tissue lipids. J Clin Invest. 2007;117:89–93. doi: 10.1172/JCI30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Febbraio M, Bao Y, Tolhurst AT, Epstein JM, Cho S. CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol. 2012;71:753–764. doi: 10.1002/ana.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28:4661–4670. doi: 10.1523/JNEUROSCI.0982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Ishii H, Bai Z, Itokazu T, Yamashita T. Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One. 2012;7:e41892. doi: 10.1371/journal.pone.0041892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation. 2008;5:46. doi: 10.1186/1742-2094-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Molina DK, DiMaio VJ. Normal organ weights in men: part II-the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2011;33:368–372. doi: 10.1097/PAF.0b013e31823d29ad. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- Nikolic T, de Bruijn MF, Lutz MB, Leenen PJ. Developmental stages of myeloid dendritic cells in mouse bone marrow. Int Immunol. 2003;15:515–524. doi: 10.1093/intimm/dxg050. [DOI] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JA, Dacie JV, Shapley R. The effect of splenectomy on the leucocyte count. Br J Haematol. 1968;14:225–231. doi: 10.1111/j.1365-2141.1968.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Rozing J, Brons NH, Bennner R. Effects of splenectomy on the humoral immune system. A study in neonatally and adult splenectomized mice. Immunology. 1978;34:909–917. [PMC free article] [PubMed] [Google Scholar]
- Weng J, Brown CV, Rhee P, Salim A, Chan L, Demetriades D, et al. White blood cell and platelet counts can be used to differentiate between infection and the normal response after splenectomy for trauma: prospective validation. J Trauma. 2005;59:1076–1080. doi: 10.1097/01.ta.0000189001.00670.d2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.