Abstract
We previously reported poorer survival among non-Hispanic blacks and Hispanics with idiopathic pulmonary fibrosis (IPF) compared to non-Hispanic whites at our center. In the current study, we hypothesized that these disparities would exist in a nationwide cohort of wait-listed patients with IPF. We performed a retrospective cohort study of 2635 patients with IPF listed for lung transplantation between 1995 and 2003 at 94 transplant centers in the United States. The age-adjusted mortality rate was higher among non-Hispanic blacks [hazard ratio (HR) = 1.24, 95% confidence interval (CI) 1.06–1.45, p = 0.009] and Hispanics (HR = 1.29, 95% CI 1.06–1.56, p = 0.01) compared to non-Hispanic whites. These findings persisted after adjustment for transplantation, medical comorbidities and socioeconomic status. Worse lung function at the time of listing appeared to explain some of these differences (HR for non-Hispanic blacks after adjustment for forced vital capacity percent predicted = 1.16, 95% CI 0.98–1.36, p = 0.09; HR for Hispanics = 1.21, 95% CI 0.99–1.48, p = 0.056). In summary, black and Hispanic patients with IPF have worse survival than whites after listing for lung transplant.
Keywords: Cohort, diffuse parenchymal lung disease, forced vital capacity, interstitial lung disease, lung transplantation
Introduction
Idiopathic pulmonary fibrosis (IPF) is characterized by progressive scarring and destruction of the lung parenchyma recognized histopathologically as usual interstitial pneumonia. Affected patients suffer from dyspnea, cough, and impaired exercise capacity, and have a median survival of less than 3 years (1–3). With an incidence of 7–10 per 100 000 per year and no effective medical therapy, IPF is the second most common indication for lung transplantation worldwide after emphysema (4,5). Still, an inadequate donor supply and strict candidacy criteria for transplantation limit the availability of this treatment modality (5,6).
Older age at diagnosis, male gender, reduced pulmonary function and impaired exercise capacity predict a worse outcome in IPF (1,2,7–12); yet, the impact of race and ethnicity on survival in IPF is largely unknown. Two studies have not shown significant associations between race and ethnicity and survival (13,14), whereas one study found that blacks had a lower age-adjusted mortality rate attributable to pulmonary fibrosis compared to whites (15). These population-based studies certainly included patients with forms of diffuse parenchymal lung disease other than IPF (4,14,15). Nonetheless, these are landmark studies of pulmonary fibrosis which have established the epidemiology of this disease, despite their recognized limitations. We recently reported that non-Hispanic black and Hispanic patients with IPF evaluated for lung transplantation at our center had a more than 3-fold higher risk of death than non-Hispanic white patients (16). However, it is unclear whether these disparities exist at other transplant centers.
In the current work, we studied a nationwide cohort of patients classified as having IPF at the time of listing for lung transplantation with the United Network for Organ Sharing (UNOS). We hypothesized that the risk of death would be higher among non-Hispanic black and Hispanic patients compared to non-Hispanic whites even with adjustment for transplantation and other measured factors that predict mortality in this condition. These data have been previously published in abstract form (17).
Methods
We performed a retrospective cohort study of non-Hispanic black, Hispanic and non-Hispanic white adult (≥18 years) Americans with IPF placed on the UNOS lung transplant waiting list between 1995 and 2003. This timeframe was chosen because (1) we aimed to study the most recent cohort possible with adequate follow-up time, and (2) the data fields collected by UNOS remained constant during this period. Baseline characteristics at the time of listing and the dates and types of transplant procedures performed were obtained from a Standard Transplant Analysis and Research file based on Organ Procurement and Transplantation Network data as of July 22, 2005. Demographic and clinical data were collected by clinical staff at 94 U.S. transplant centers. The designation of IPF was determined by transplant physicians at each center and did not require lung biopsy. This approach is similar to that taken in other large epidemiologic studies of pulmonary fibrosis and outcomes in lung transplant populations (4,14,15,18). Race and ethnicity designations were supplied by each center and coded in accordance with the Office of Management and Budget Directive (19,20). Poor performance status was defined as dependence on others for activities of daily living or hospitalization at the time of listing. Physiologic data included forced vital capacity percent predicted (FVC%), forced expiratory volume in 1 s percent predicted (FEV1%) and pulmonary hemodynamics. UNOS recorded the distance walked in 6 min as either greater than or less than 150 ft.
We obtained the median family income and the percentage of adults who completed high school and college in each patient’s residence zip code from the 2000 U.S. Census database (21), reflecting neighborhood-level socioeconomic status. Mortality was determined using a Social Security Death Index file generated on July 8, 2005 and the UNOS file. These are both validated indices (22). Subjects not reported as dead in either index were censored on the latest date reported to UNOS. The Columbia University Medical Center Institutional Review Board approved the study.
Statistical analysis
Continuous variables were summarized by mean ± standard deviation or median [interquartile range (IQR)]. Categorical variables were summarized by frequency and percentage. We used analysis of variance and t-tests with the Bonferroni correction to compare normally distributed continuous variables and Kruskal–Wallis and Wilcoxon rank sum tests to compare variables that did not meet this assumption. Categorical variables were compared using chi-squared tests.
The primary endpoint was time from the date of listing for transplantation to the date of death. We used Cox proportional hazards models to estimate the risks of death of non-Hispanic blacks and Hispanics compared to non-Hispanic whites. We built nested models with terms for race and ethnicity forced into the models. We used purposeful selection of covariates to identify factors that may have confounded or mediated observed associations between race/ethnicity and survival time. Covariates that changed the coefficients of race/ethnicity by >20% after inclusion were considered strong confounders. p-Value-based variable selection techniques were not performed. Time-varying covariates for transplantation status and for the first 30-day post-transplant period (a period characterized by a high mortality rate during which the assumption of proportional hazards may not be met) were employed (23–26). p-Values <0.05 were considered statistically significant. SAS 9.1 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
There were 3195 non-Hispanic black (‘black’), Hispanic or non-Hispanic white (‘white’) patients with IPF listed for lung transplantation between 1995 and 2003. We excluded those who underwent heart–lung transplantation (n = 32) or living donor lung transplantation (n = 6) as well as those with implausible dates (n = 4), leaving 3154 patients. We also excluded those with missing data for FVC% (n = 151), height or weight (n = 29), tobacco use (n = 105), performance status (n = 79), steroid use (n = 112), diabetes (n = 61) or hypertension (n = 116). Subjects with a missing zip code or a zip code that was not included in the year 2000 U.S. Census Summary File 3B were also excluded (n = 99). In total, 519 (16%) subjects were excluded for missing data, leaving a final cohort of 2635 subjects. Race and ethnicity did not differ between those excluded for missing data and those included in the analysis (11% black, 7% Hispanic and 82% white in both groups, p = 0.80). Subjects excluded due to missing data were also similar to those with complete data in terms of age (p = 0.41), gender (p = 0.42), FVC% (p = 0.37) and medical comorbidities (all p > 0.40). Subjects with missing data were somewhat less likely than those with complete data to undergo transplantation (38% vs. 46%, respectively, p < 0.01) and were slightly more likely to die during the study period (69% vs. 60%, respectively, p < 0.01).
Population
Characteristics of the final cohort at the time of listing are shown in Table 1. Eleven percent of the patients were black (n = 299), 7% were Hispanic (n = 173) and 82% were white (n = 2 163). Black and Hispanic patients were younger and more likely to be female than white patients. Black and Hispanic patients also had more medical comorbidities than whites, with higher frequencies of diabetes and poor performance status, but were less likely to have been smokers. Black patients were more likely to have treated hypertension than Hispanic or white patients. White patients were more likely to have private medical insurance or a college education and lived in higher income neighborhoods of higher educational attainment more often than blacks and Hispanics. Minority patients had a somewhat lower FVC% (44%, 45% and 51% for blacks, Hispanics and whites, respectively) and slightly higher pulmonary artery pressure at the time of listing (30 mmHg, 28 mmHg and 25 mmHg, respectively). Pulmonary capillary wedge pressure and cardiac index did not differ between groups. Hispanics (but not blacks) were more likely than whites to have a 6-min walk distance less than 150 ft.
Table 1.
Characteristics at the time of placement on the UNOS waiting list
| Variable | N | Black (n = 299) | Hispanic (n = 173) | White (n = 2163) | p Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 2635 | 46 ± 101 | 50 ± 101 | 54 ± 9 | <0.0001 |
| Female | 2635 | 169 (57%) | 85 (49%) | 755 (35%) | <0.0001 |
| Body mass index, kg/m2 | 2635 | 29 ± 6 | 28 ± 5 | 28 ± 5 | 0.14 |
| Comorbidities and functional status | |||||
| Diabetes mellitus | 2635 | 38 (13%) | 31 (18%) | 224 (10%) | 0.006 |
| Drug-treated hypertension | 2635 | 76 (25%) | 22 (13%) | 391 (18%) | 0.001 |
| Corticosteroid use >5mg/d | 2635 | 175 (59%) | 110 (64%) | 1348 (62%) | 0.41 |
| Smoking >10 pack-years | 2635 | 101 (34%) | 61 (35%) | 1051 (49%) | <0.0001 |
| Poor performance status | 2635 | 106 (69%) | 111 (64%) | 1311 (61%) | 0.02 |
| Physiologic data | |||||
| FVC% | 2635 | 44 ± 151 | 45 ± 161 | 51 ± 17 | <0.0001 |
| FEV1% | 2635 | 45 ± 171 | 47 ± 171 | 53 ± 17 | <0.0001 |
| Mean PAP, mmHg | 2009 | 30 ± 141 | 28 ± 121 | 25 ± 10 | <0.0001 |
| Pulmonary capillary wedge pressure, mm Hg | 2049 | 11 ± 6 | 11 ± 7 | 10 ± 5 | 0.09 |
| Cardiac index, L/min/m2 | 1879 | 2.7 ± 0.8 | 2.8 ± 0.7 | 2.8 ± 0.8 | 0.22 |
| 6-Min walk distance <150 ft | 2419 | 36 (13%) | 35 (22%) | 243 (12%) | 0.002 |
| Health insurance | |||||
| Private insurance | 2635 | 203 (68%) | 116 (67%) | 1893 (88%) | <0.0001 |
| Medicare | 2635 | 57 (19%) | 26 (15%) | 303 (14%) | 0.07 |
| Medicaid | 2635 | 54 (18%) | 36 (21%) | 93 (4%) | <0.0001 |
| Educational status2 | 2208 | <0.0001 | |||
| Less than 9 years | 22 (15%) | 8 (3%) | 38 (2%) | ||
| 9–12 Years | 56 (37%) | 114 (47%) | 714 (39%) | ||
| Some college | 48 (32%) | 55 (23%) | 460 (25%) | ||
| College graduate | 24 (16%) | 67 (27%) | 602 (33%) | ||
| Group-level socioeconomic status | |||||
| Median family income, U.S. $ | 2635 | 45 000 (34 4000–56 000)1 | 41 100 (33 200–53 200)1 | 52 700 (42 500–67 600) | <0.0001 |
| High school graduates, % | 2635 | 76 (63–85)1 | 76 (69–85)1 | 85 (78–91) | <0.0001 |
| College graduates, % | 2635 | 16 (11–25)1 | 16 (10–27)1 | 22 (15–36) | <0.0001 |
Data are mean ± SD, frequency (percentage) or median (interquartile range). p Values are from ANOVA, Kruskal–Wallis or chi-squared tests, as appropriate. FVC% = forced vital capacity % predicted; FEV1% = forced expiratory volume in 1 s % predicted; PAP = pulmonary artery pressure.
p < 0.05 compared to whites.
Percentages do not add to 100% because of rounding.
Transplantation
During the study period, 39% of blacks, 47% of Hispanics and 46% of whites underwent lung transplantation (p = 0.07). Among those who were transplanted, the median time from listing to lung transplantation did not differ between groups [250 days (IQR 99 to 494 days) for blacks; 225 days (IQR 110 to 533 days) for Hispanics and 215 days (IQR 86 to 455 days) for whites, p = 0.39]. Blacks and Hispanics were more likely to undergo double lung transplantation than were whites (37% and 39% vs. 26%, respectively for those transplanted, p = 0.001).
Survival
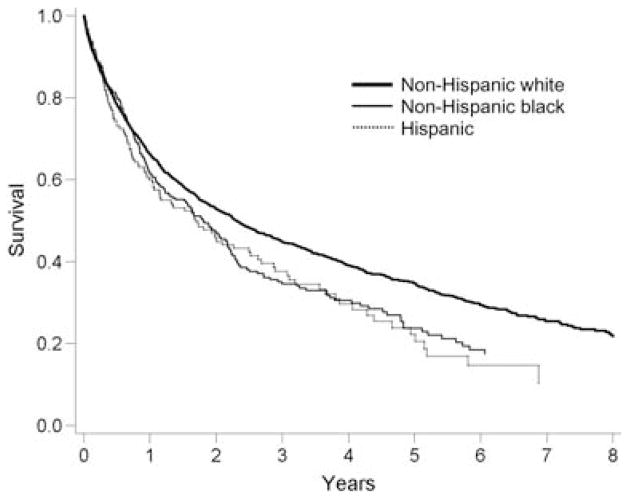
There were 1594 deaths during the study period. The median survival of the cohort was 1.7 years (IQR 7 months to 3.4 years). The age-adjusted mortality rate was higher for blacks and Hispanics compared to whites [Figure 1; Table 2; hazard ratio (HR) for blacks = 1.24, 95% confidence interval (CI) 1.06–1.45, p = 0.009; HR for Hispanics = 1.29, 95% CI 1.06–1.56, p = 0.01]. Differential timing or probability of lung transplantation did not explain the results, as adjustment for this factor did not change these effect estimates (Table 2, Model 2). Categorizing the transplant procedure as single or double did not influence the hazard ratios (data not shown).
Figure 1.
Age-adjusted survival curves.
Table 2.
Multivariate models of the risk of death
| Hazard ratio (95% CI) | p Value | |
|---|---|---|
| Model 11 | ||
| White | Referent group | |
| Black | 1.24 (1.06–1.45) | 0.009 |
| Hispanic | 1.29 (1.06–1.56) | 0.01 |
| Model 22 | ||
| White | Referent group | |
| Black | 1.24 (1.06–1.45) | 0.008 |
| Hispanic | 1.29 (1.06–1.56) | 0.01 |
| Model 33 | ||
| White | Referent group | |
| Black | 1.28 (1.09–1.50) | 0.003 |
| Hispanic | 1.31 (1.08–1.60) | 0.007 |
| Model 44 | ||
| White | Referent group | |
| Black | 1.24 (1.05–1.46) | 0.01 |
| Hispanic | 1.28 (1.05–1.56) | 0.01 |
| Model 55 | ||
| White | Referent group | |
| Black | 1.16 (0.98–1.36) | 0.09 |
| Hispanic | 1.21 (0.99–1.48) | 0.056 |
n = 2 635 in each model.
Model 1: Adjusted for age.
Model 2: Adjusted for age and transplantation.
Model 3: Adjusted for age, transplantation, gender, body mass index, diabetes, hypertension, smoking, performance status, year of listing and steroid use.
Model 4: Adjusted for variables in Model 3 + private insurance and group-level socioeconomic status (median family income and college education).
Model 5: Adjusted for variables in Model 4 + forced vital capacity % predicted.
Next, we adjusted for potentially important clinical covariates (gender, body mass index, diabetes, hypertension, steroid use, smoking status, year of listing and performance status) (Table 2, Model 3). Hispanic ethnicity and black race were still independently associated with an increased mortality rate after inclusion of these factors in the model. Further adjustment for type of health insurance and group-level socioeconomic indicators (median family income and community educational attainment) did not materially alter these findings (Table 2, Model 4). Additional adjustment for individual-level educational status resulted in similar findings for Hispanics but reduced the magnitude of the association for blacks (n = 2 208; HR for blacks = 1.17, 95% CI 0.98–1.41, p = 0.09; HR for Hispanics = 1.34, 95% CI 1.08–1.67, p = 0.007).
We then examined the role of differences in severity of lung disease and exercise tolerance at the time of listing. Inclusion of FVC% in the model reduced the associations between race and ethnicity and mortality, suggesting that differences in pulmonary function explained some of the disparities in outcome in the fully adjusted model (Table 2, Model 5). However, even in the FVC%-adjusted model, it appeared that minority patients may have had increased mortality rates compared to whites of borderline statistical significance (p = 0.09 for blacks and p = 0.056 for Hispanics).
Inclusion of pulmonary artery pressure instead of FVC% in Model 5 reduced the differences in risk for blacks compared to whites, albeit with a smaller sample size due to missing data (n = 2 009; HR for blacks = 1.17, 95% CI 0.96–1.41, p = 0.12; HR for Hispanics = 1.26, 95% CI 0.99–1.60, p = 0.06). Alternatively, adjustment for 6-min walk distance did not substantially change the effect estimates for race/ethnicity (n = 2 419; HR for blacks = 1.26, 95% CI 1.06–1.50, p = 0.01; HR for Hispanics = 1.23, 95% CI 0.99–1.52, p = 0.06). Minimal changes in the effect estimates after adjustment for these variables suggest a lack of confounding by these factors; however, missing data limited these analyses and resulted in widened 95% CIs.
The exclusion of patients with missing data from the final cohort could have affected the results. To address this, we examined the association of race and ethnicity status with the risk of death after including those with missing data. Age, gender and transplantation status were available for all 3154 subjects in this analysis. Black race and Hispanic ethnicity remained significantly associated with the age, gender and transplant-adjusted risk of death in the entire cohort of patients listed for lung transplantation (n = 3154; HR for blacks = 1.16, 95% CI 1.01–1.3, p = 0.04; HR for Hispanics = 1.24, 95% CI 1.04–1.5, p = 0.02).
When we censored at transplantation, the effect estimates were virtually identical with wider 95% CIs (due to the elimination of post-transplantation deaths from the analysis) (n = 2635; age- and gender-adjusted HR for blacks = 1.15, 95% CI 0.94–1.40, p = 0.16; HR for Hispanics = 1.33, 95% CI 1.04–1.69, p = 0.02). The race and ethnicity variables met the assumption of proportional hazards. Exclusion of potentially influential subjects from the models only increased the magnitude of the associations between black race and Hispanic ethnicity and the risk of death.
Discussion
We found that black and Hispanic patients classified as having IPF who were listed for lung transplantation in the United Status during the study period had higher mortality rates than whites independent of age, gender, medical comorbidities, insurance status, transplantation and socioeconomic status. These disparities in survival were attributable in part to worse lung function among both blacks and Hispanics compared to whites at the time of listing for lung transplantation. Although not surprising that lung function played some role in determining worse outcomes, this is the first demonstration that minority patients are more likely to have reduced lung function at the time of listing, disproportionately affecting their survival compared to whites. These findings are particularly disconcerting in light of all patients in this study having been listed for transplantation, therefore undergoing care and monitoring by transplant center physician and nursing staffs accustomed to treating patients with severe IPF. Lastly, receiving lung transplantation did not affect these findings, suggesting both pre- and post-transplant contributors to these disparities.
These results confirm and extend our previous report of increased mortality among minority IPF patients (16). IPF therefore joins the growing list of pulmonary diseases in which outcomes differ by race and ethnicity, such as asthma (27), lung cancer (20,28) and pulmonary arterial hypertension (29). While it is likely that not all patients in this cohort had IPF as it is currently defined, our classification is similar to the specificity of other large landmark epidemiologic studies (4,14,15).
Differences in pre-referral medical care, timing of referral and disease phenotype may have contributed to the disparities in disease severity and survival by race and ethnicity. There are currently no known medical therapies that improve survival in IPF. However, lower vaccination rates, poorly managed medical comorbidities, overuse of corticosteroids, underuse of supplemental oxygen and less access to pulmonary rehabilitation could explain the worse lung function and outcomes seen in minority patients in this study.
Barriers to obtaining high-quality medical care faced by minority patients and the physicians who serve them could lead to later referral and could account for our findings. It is well established that physicians who care for minority patients report less access to advanced imaging and specialist consultation despite similar insurance coverage (30). The probability of lung transplantation does not account for these findings, since our results did not change after adjustment for transplantation. The effects of pre-referral care and timing of referral may therefore adversely affect outcomes in minority patients even after undergoing this life-saving procedure. As the new lung allocation system in the United States prioritizes those patients having more severe disease, observed differences in survival may be affected, even though our findings were independent of the timing of transplantation.
Finally, race may serve as a surrogate for a genetic profile which produces a distinct disease phenotype. Indeed, the frequencies of key ‘overexpressing’ gene polymorphisms which drive inflammation and fibrosis vary by racial and ethnic groups (31–33). On the other hand, complex diseases such as IPF are characterized by complicated gene-environment interactions, so that variability in a single locus is unlikely to explain our results. In addition, it is implausible that one or several ‘high-risk’ genetic profiles cause similarly poor outcomes in the diversity of conditions which manifest racial disparities (e.g., coronary heart disease, lung cancer, asthma, pulmonary hypertension and now IPF). Independent of the quality of care or biologic differences, timely diagnosis or referral, specific cultural belief systems may also have contributed to differences in disease outcomes (28). In any case, the Department of Health and Human Services has targeted such differences in disease outcomes based on race and ethnicity whatever the mechanism (34). This study therefore identifies a new focus for future basic scientific and public health initiatives.
While it is widely accepted that IPF is typically diagnosed after the age of 50 and is more common among men than women (35), blacks and Hispanics in our study tended to be younger and were more likely to be female than whites in our study. Since the manifestations of pulmonary fibrosis in minority populations have not been previously described, these differences may represent a novel finding of a different demographic distribution of IPF among minorities compared to whites. Inclusion of patients with lung disease due to connective tissue disease preferentially in the minority groups might be an alternate explanation for the age and gender differences between groups. Even so, recent studies have found similar outcomes both before and after lung transplantation between patients with connective tissue disease-related lung disease and idiopathic interstitial pneumonia (36,37), lessening the impact of such misclassification on our findings.
We included post-transplantation time in our adjusted survival models with a simple yet powerful methodology which is the standard for studies of transplantation (23–26). Whereas censoring observations at the time of transplantation would be an alternative, such an approach neglects what is most important to patients, families, physicians and policy-makers, i.e. overall survival. The lifespan of a patient with IPF does not end at transplant, and the sequelae of IPF and pre-transplant management may affect post-transplant functional status and long-term outcome. For these reasons, post-transplant survival is important to include in studies of long-term outcomes of progressive and incurable lung diseases. Nevertheless, our findings were similar when we censored patient-time at transplantation.
There were several limitations. First, this study was retrospective and therefore suffers from the usual issues with this design. Second, some patients in our study may not have had IPF as it is currently defined, especially since the UNOS database preceded the release of the current diagnostic guidelines (38). There are no data to suggest that misclassification of IPF diagnosis would occur differentially by race. Therefore, the differences in outcome between groups may be even larger than those shown. In addition, the demonstration of such robust differences in outcome without knowledge of the histopathology and potential inciting factors increases the generalizability of the results. Most other large epidemiological studies of IPF have used similar methodology for defining IPF (4,14,15). Third, the UNOS database receives non-standardized race and ethnicity information when patients are listed, possibly resulting in misclassification. Fourth, although we adjusted for a variety of individual- and group-level covariates, residual or unmeasured confounding may be present. For example, we did not have access to the absolute values of forced vital capacity or to the various reference values (which may or may not have accounted for race) used by each center, possibly resulting in inadequate adjustment for lung function. Fifth, we excluded patients with missing data for certain variables. While the final cohort may have been somewhat healthier than the full cohort, race and ethnicity in the included and excluded groups were similar, and analysis of the full cohort showed identical results. Missing pulmonary artery pressure data for 24% of the final cohort may have limited our ability to detect an even stronger role of pulmonary hypertension in explaining the disparate outcomes, since pulmonary hypertension is an important predictor of survival in IPF (39–41).
Lastly, patients with IPF who are listed for transplantation are clearly highly selected. Referral to a tertiary-care lung transplant center and the stringent vetting process for listing for transplantation (including screening for adequate health care coverage, social and family support, self-care and compliance with a complicated medical regimen) would be expected to homogenize patient groups, tending to minimize differences between minority and white patients. Considering this selection process, the strength and consistency of our findings is therefore even more remarkable, as we would expect even greater differences in an unselected patient population with IPF. This is also the largest cohort study of IPF ever published to our knowledge, bolstering its generalizability.
In conclusion, we found an increased mortality rate among Hispanic and black patients with IPF listed for lung transplantation compared to whites after adjustment for a variety of factors and due, at least in part, to more severe disease at the time of listing. This study confirms our previous work showing that minority patients with IPF not only are more severely ill at the time of evaluation for lung transplantation, but also have a higher risk of death once listed. Our findings add IPF to the growing list of pulmonary diseases that have a disproportionate effect on black and Hispanic Americans. Future research should focus on elucidating the mechanisms of these disparities, with the ultimate goal of alleviating differences in survival. In the meantime, educational efforts should be targeted to physicians who care for minority patients with IPF encouraging early referral for subspecialist care and transplantation evaluation.
Acknowledgments
The authors wish to thank Steven Shea, MD, MS, for his thoughtful review of the manuscript and UNOS staff members Katarina Anderson, MS, Maureen McBride, PhD, and Sarah Taranto, BA, for their management of data collection.
This work was financially supported by National Institutes of Health grants HL072739 and HL67771, the Martin and Ellen Strahl Research Fund and the Jean Muir-Katz Research Fund. This work was supported in part by Health Resources and Services Administration contract 231-00-0115.
Drs. Wilt and Kawut are co-investigators in a clinical trial sponsored by InterMune.
Footnotes
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
References
- 1.King TE, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: Scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz DA, Helmers RA, Galvin JR, et al. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:450–454. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 4.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 5.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-second official adult lung and heart-lung transplant report–2005. J Heart Lung Transplant. 2005;24:956–967. doi: 10.1016/j.healun.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 6.The American Society for Transplant Physicians (ASTP)/American Thoracic Society (ATS)/European Respiratory Society (ERS)/International Society for Heart and Lung Transplantation (ISHLT) International guidelines for the selection of lung transplant candidates. Am J Respir Crit Care Med. 1998;158:335–339. doi: 10.1164/ajrccm.158.1.15812. [DOI] [PubMed] [Google Scholar]
- 7.Collard HR, King TE, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 9.Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: The prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 10.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 11.Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J. 2005;25:96–103. doi: 10.1183/09031936.04.00137203. [DOI] [PubMed] [Google Scholar]
- 12.Kawut SM, O’Shea MK, Bartels MN, Wilt JS, Sonett JR, Arcasoy SM. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir Med. 2005;99:1431–1439. doi: 10.1016/j.rmed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.King TE, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: Relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 14.Mapel DW, Hunt WC, Utton R, Baumgartner KB, Samet JM, Coultas DB. Idiopathic pulmonary fibrosis: Survival in population based and hospital based cohorts. Thorax. 1998;53:469–476. doi: 10.1136/thx.53.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979–1991. An analysis of multiple-cause mortality data. Am J Respir Crit Care Med. 1996;153:1548–1552. doi: 10.1164/ajrccm.153.5.8630600. [DOI] [PubMed] [Google Scholar]
- 16.Lederer DJ, Caplan-Shaw CE, O’Shea MK, et al. Racial and ethnic disparities in survival in lung transplant candidates with idiopathic pulmonary fibrosis. Am J Transplant. 2006;6:398–403. doi: 10.1111/j.1600-6143.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 17.Lederer DJ, Arcasoy SM, Barr RG, et al. Disparities in survival in minority patients with idiopathic pulmonary fibrosis [abstract] Proc Am Thor Soc. 2006;3:A523. [Google Scholar]
- 18.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Office of Management and Budget. [Accessed June 29, 2006]; Available from: http://www.whitehouse.gov/omb/fedreg/directive15.html.
- 20.Wisnivesky JP, McGinn T, Henschke C, Hebert P, Iannuzzi MC, Halm EA. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med. 2005;171:1158–1163. doi: 10.1164/rccm.200411-1475OC. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Commerce, Bureau of the Census. Census of Population and Housing, 2000 [United States]: Summary file 3, National [computer file] Vol. 2002 Washington, D.C: I.S. Department of Commerce, Bureau of the Census [producer]; Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2002. [Google Scholar]
- 22.Dickinson DM, Bryant PC, Williams MC, et al. Transplant data: Sources, collection, and caveats. Am J Transplant. 2004;4(Suppl 9):13–26. doi: 10.1111/j.1600-6135.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 23.Crowley J, Hu M. Covariance analysis of heart transplant survival data. J Am Stat Assoc. 1977;72:27–36. [Google Scholar]
- 24.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 25.Charman SC, Sharples LD, McNeil KD, Wallwork J. Assessment of survival benefit after lung transplantation by patient diagnosis. J Heart Lung Transplant. 2002;21:226–232. doi: 10.1016/s1053-2498(01)00352-7. [DOI] [PubMed] [Google Scholar]
- 26.Cox DR, Oakes D. Analysis of Survival Data. New York: Chapman and Hall; 1984. [Google Scholar]
- 27.Grant EN, Lyttle CS, Weiss KB. The relation of socioeconomic factors and racial/ethnic differences in US asthma mortality. Am J Public Health. 2000;90:1923–1925. doi: 10.2105/ajph.90.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis ML, Christie JD, Silvestri GA, Kaiser L, Santiago S, Hansen-Flaschen J. Racial differences pertaining to a belief about lung cancer surgery: Results of a multicenter survey. Ann Intern Med. 2003;139:558–563. doi: 10.7326/0003-4819-139-7-200310070-00007. [DOI] [PubMed] [Google Scholar]
- 29.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95:199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 31.Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS. TNF-alpha, TGF-beta, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. Report of the ASHI Minority Workshops: Part IV. Hum Immunol. 2004;65:1413–1419. doi: 10.1016/j.humimm.2004.07.240. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann SC, Stanley EM, Cox ED, et al. Ethnicity greatly influences cytokine gene polymorphism distribution. Am J Transplant. 2002;2:560–567. doi: 10.1034/j.1600-6143.2002.20611.x. [DOI] [PubMed] [Google Scholar]
- 33.Suthanthiran M, Li B, Song JO, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and(or target organ damage. Proc Natl Acad Sci USA. 2000;97:3479–3484. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2. Washington, D.C: Government Printing Office; 2000. [Google Scholar]
- 35.Johnston ID, Prescott RJ, Chalmers JC, Rudd RM. British Thoracic Society study of cryptogenic fibrosing alveolitis: Current presentation and initial management. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. Thorax. 1997;52:38–44. doi: 10.1136/thx.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocheril SV, Appleton BE, Somers EC, et al. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum. 2005;53:549–557. doi: 10.1002/art.21322. [DOI] [PubMed] [Google Scholar]
- 37.Rosas V, Conte JV, Yang SC, et al. Lung transplantation and systemic sclerosis. Ann Transplant. 2000;5:38–43. [PubMed] [Google Scholar]
- 38.American Thoracic Society, European Respiratory Society. . American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Resp Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 39.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 40.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 41.Whelan TP, Dunitz JM, Kelly RF, et al. Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. J Heart Lung Transplant. 2005;24:1269–1274. doi: 10.1016/j.healun.2004.10.014. [DOI] [PubMed] [Google Scholar]