Abstract
Aims
Kidney cells in patients with diabetic nephropathy are reported to be senescent. However, the mechanisms that regulate cellular senescence in the diabetic kidney are still unknown. In the present study, we evaluated the contribution of high glucose to renal cell senescence in streptozotocin (STZ)-induced diabetic mice.
Methods
Non-diabetic and streptozotocin (STZ, 10 mg kg–1 day–1 for 7 days, i.p.)-induced type 1 diabetic C57BL/6 J mice and cultured human proximal tubular cells were used in this study.
Results
Hyperglycemia dramatically increased the renal expression of p21 but not other CDK inhibitors such as p16 and p27 at 4 weeks after STZ injection. These changes were accompanied by an increase in senescence-associated β-galactosidase staining in tubular epithelial cells. Administration of insulin at doses that maintained normoglycemia or mild hypoglycemia suppressed the changes induced by STZ. Insulin did not affect the senescent markers in non-diabetic mice. Exposure of cultured human proximal tubular cells to 25 mmol/L, but not 8 mmol/L, glucose medium increased the expression of senescence markers, which was suppressed by knock-down of p21 or sodium glucose cotransporter (SGLT) 2.
Conclusions
These results suggest that hyperglycemia causes tubular senescence via a SGLT2- and p21-dependent pathway in the type 1 diabetic kidney.
Keywords: Cellular senescence, Diabetic nephropathy, p21, Insulin, Proximal tubular cells, Sodium glucose cotransporter 2
1. Introduction
Cellular senescence, which is defined as an irreversible cessation of mitosis, is observed in the aging kidney (Melk et al., 2003, 2004; Ohtani et al., 2007). The development of cellular senescence is associated with a reduction of telomere length, reduced expression of cyclin and cyclin-dependent kinase (CDK), and increased expression of CDK inhibitors, such as p16 and p21 (Hayflick, 2003). CDK inhibitors, which inhibit the CDK function and induce the cessation of cell cycle progression, are critical factors for the induction of cellular senescence. Meanwhile, there is increasing evidence to suggest that some stress and/or pathological conditions cause cellular senescence, even in early generations of cells, and that stress-induced cellular senescence contributes to the development of lesions, such as atherosclerosis and cancer (Kunieda et al., 2006; Minamino et al., 2002; Yoshimoto et al., 2013).
Previous studies have revealed increased expression of CDK inhibitors, such as p21, in the diabetic kidney or in high glucose-treated cultured cells (Chuang et al., 2007; Kuan, al-Douahji, & Shankland, 1998; Okada et al., 2006; Satriano et al., 2010; Terada et al., 1999; Vallon, 2011). Satriano et al. reported that type 1 diabetic rats exhibited renal senescence with increases in p16, p21 and p27 in cortical tubules and that oxidative stress could be one of the mechanisms for proximal tubular senescence (Satriano et al., 2010). We also reported that type 2 diabetic KKAy mice exhibited renal cell senescence with increased p21 expression (Lei et al., 2012). Notably, tubular cells in the kidney of patients with early stage diabetic nephropathy were reported to be senescent (Verzola et al., 2008). These findings suggest that diabetes increases the expression of CDK inhibitors and cellular senescence in the kidney.
We recently reported that chronic aldosterone/mineralocorticoid receptor activation caused cellular senescence in the proximal tubules of the kidney via the p53/p21 pathway (Fan et al., 2011). Importantly, we also reported that p21 in proximal tubular cells contributed to proximal tubular cell injury by decreasing cellular repair activities after the injury, accelerating apoptosis, and increasing the expression of inflammation- and fibrosis-related factors (Fan et al., 2011; Kitada et al., 2012). In addition, previous studies reported that mice with knockout (KO) of CDK inhibitors, such as p21 and p27, showed alleviated proteinuria, glomerular hypertrophy, and tubulointerstitial damage in diabetic nephropathy (Al-Douahji et al., 1999; Wolf, Schanze, Stahl, Shankland, & Amann, 2005) and 5/6 nephrectomy (Megyesi, Price, Tamayo, & Safirstein, 1999) models. These findings suggest that the CDK inhibitors are involved in the development of kidney injury and it could be involved in the pathophysiology of diabetic nephropathy. However, the mechanisms by which diabetes increases the expression of CDK inhibitors and cellular senescence in the kidney are still unclear.
The purpose of this study was to determine the role of hyperglycemia in the increase in CDK inhibitor expression and cellular senescence. We investigated the effects of high glucose and insulin treatment on renal cell senescence in streptozotocin (STZ)-induced diabetic mice. In addition, we used cultured human proximal tubular cells to examine the mechanisms by which hyperglycemia (i.e., high glucose in the glomerular filtrate) induces renal cell senescence.
2. Materials and methods
2.1. Animals
All animal experiments were performed under the guidelines for the care and use of animals established by Kagawa University. Male C57/BL6J mice (CLEA, Tokyo, Japan) were divided into five groups and treated as follows: (1) vehicle alone (untreated non-diabetic group), (2) STZ alone (STZ-induced diabetic group), (3) vehicle plus insulin (0.1 U/day for >30 days; insulin-treated non-diabetic group), (4) STZ plus low-dose insulin (0.1–0.2 U/day for >30 days; low-dose insulin-treated diabetic group), or (5) STZ plus high-dose insulin (0.3 U/day for > 30 days; high-dose insulin-treated diabetic group). At 6 weeks of age, the mice received an intraperitoneal injection of STZ at a dose of 10 mg kg–1 day–1 (Sigma Chemical St. Louis, MO) dissolved in 10 mmol/L citrate buffer (pH 4.5) for 7 days. The vehicle-treated mice received citrate buffer alone. We used a relatively lower dose of STZ than used in previous reports (Ichihara et al., 2006; Tesch & Allen, 2007) and applied multiple injections to avoid STZ toxicity that can induce acute kidney injury (AKI) (Kraynak et al., 1995). A preliminary study showed that there were no signs of AKI, with no detectable changes in plasma creatinine, blood urea nitrogen, or renal morphology, 7 days after injection with this STZ dose regimen. Blood glucose levels were measured daily in this period from tail vein blood using a Glutest-Ace monitor (Sanwa Kagaku, Nagoya, Aichi, Japan), and confirmed the adequacy of each STZ injection. Insulin was administered using Linbit insulin implants (LinShin Canada, Scarborough, Ontario, Canada) immediately after confirming the hyperglycemia in STZ group. In addition, we confirmed the efficacy of insulin treatment by measuring the blood glucose level at one day after insulin implantation. Type 1 diabetes, defined as blood glucose >250 mg/dL (1–3 days after the last STZ injection), was induced in 24/25 mice using this procedure.
At 4 weeks after the last day of STZ injection, we measured systolic blood pressure using a tail-cuff Plethysmograph (model BP-98A; Softron, Tokyo, Japan). We also collected 24-h urine in a metabolic cage after a 24-h acclimatization period. Mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.), and blood was withdrawn from the abdominal aorta. The right kidney was snap-frozen in liquid nitrogen and stored at &minus80 °C until processing for RNA analysis. The left kidney was fixed in neutral 15% formalin or immersed in OCT compound (Sakura Finetek, Tokyo, Japan), and was frozen in chilled acetone for senescence-associated β-galactosidase (SAβ-Gal) staining. In preliminary experiments, we examined the time-dependent changes in renal senescence after STZ injection and found that renal senescence, evaluated by SAβ-Gal staining, was detectable from 4 weeks after injection.
2.2. Senescence-associated β-galactosidase (SAβ-Gal) staining
SAβ-Gal activity was measured using a senescence detection kit (BioVision, Milpitas, CA) according to the manufacturer's protocol. Briefly, the kidney frozen in OCT compound was cut into 15-μm-thick sections. Frozen sections or cells were fixed for 15 min at room temperature in fixative solution, washed twice with PBS, and incubated for 12 h at 37 °C in freshly prepared X-Gal staining solution. We then removed the staining solution, immersed the samples in 70% glycerol, and assessed the development of blue color. In animal experiments, we evaluated the ratio of the blue-stained area in each sample relative to its total area in five randomly chosen renal cortical area per each kidney section, and it is expressed as a percentage (magnification, 200×). To determine senescent cell numbers in cell culture experiments, the number of SAβ-Gal-positive cells (defined as those stained blue) were counted in five randomly chosen fields per section at a magnification of 100×.
2.3. Real-time reverse transcription–polymerase chain reaction (RT-PCR)
mRNA was extracted from the whole kidney by the phenolchloroform extraction method. The mRNA expression levels of β-actin, p21, p27, p53, p16, SIRT1 and nicotinamide phosphoribosyltransferase (Nampt) in kidney were analyzed by RT-PCR using an ABI Prism 7000 with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The following sense and antisense primers were used: mouse p27, 5′-TAACCCAGCCGGATTGTCTG-3′ and 5′-GCGGTGCCTTTAAT TGGGTC-3′; mouse Nampt, 5′-CAGCAGAGCACAGTACCATAACG-3′ and 5′-ACCACAGACACAGGCACTGATG-3′. The oligonucleotide primer sequences of β-actin, p21, p53, p16 and SIRT1 were as previously described (Fan et al., 2011; Nakano et al., 2012). All data are shown as the relative differences between untreated non-diabetic mice after normalization for β-actin expression.
2.4. Immunohistochemistry
p21 was analyzed by immunohistochemistry. After deparaffinization in xylene and rehydration in graded ethanol, the kidney sections were immersed in proteinase K (Dako, Glostrup, Denmark) for 10 min. The slides were treated with 0.3% hydrogen peroxide for 15 min to block endogenous peroxide activity, and rinsed briefly in PBS. The sections were incubated for 15 min at room temperature with 10% normal goat serum. Then, the sections were incubated one hour at room temperature with a primary anti-p21 antibody (1:100; ab2961; Abcam, Cambridge, UK) and reacted with Histofine Simple-Stain MAX-PO (Nichirei, Tokyo, Japan) for 30 min at room temperature. The sections were then developed with diaminobenzidine using Simple stain DAB buffer (Nichirei). After the immunohistochemistry, the slides were stained with hematoxylin to stain the nucleus or periodic acid-Schiff (PAS) reagent to stain the brush border in the proximal tubules for analyzing the location of p21-positive tubular cells. The number of p21-positive cells (defined as those stained brown) in the renal cortical area was counted in five randomly chosen fields per section at a magnification of 200× by using hematoxylin-stained sections.
2.5. Other analytical procedures
Plasma insulin (Mice Insulin ELISA Kit; Shibayagi, Gunma, Japan) and urinary albumin (Mice Albumin ELISA Kit; Shibayagi) levels were measured using commercially available kits.
2.6. Cell culture and treatment
We used Caucasian non-hypertensive patient-derived human proximal tubular cells (HPTCs) immortalized by temperature-sensitive SV40 large T antigen containing adenovirus. The HPTCs express sodium glucose cotransporter (SGLT) 2 rather than SGLT1 (3.29 ± 0.56 fold, n = 4, RT-PCR). In our previous report, we demonstrated that the HPTCs were able to approach the Hayflick limit, a limitation of cell mitosis, which is not observed in immortalized cells at 37 °C (Fan et al., 2011). The HPTCs were maintained in a growth medium consisting of a 1:1 ratio of Click's Medium and RPM1-1640 (Quality Biological, Gaithersburg, MD), supplemented with 1%insulin/transferrin/selenium, 40 ng/mL dexamethasone, 10 ng/mL epidermal growth factor, 2% FBS, and 2% penicillin, in humidified atmosphere of 5% CO2 at 33 °C. After reaching 50% confluence in growth medium, the cells were transferred to 0.2% FBS in Click's Medium/RPMI-1640 containing 1% insulin/transferrin/selenium and 40 ng/mL dexamethasone for 24 h at 37 °C. Finally, the cells were incubated in medium containing 8 or 25 mmol/L glucose or mannitol (as an osmotic control) with or without transfection with p21, SGLT2 or scrambled small interfering RNA (siRNA). In another series of experiments, cells were treated with insulin (100 nmol/L) and 25 mmol/L glucose. Three days after incubation with 8 or 25 mmol/L glucose, the cells were prepared for western blotting or SAβ-Gal staining.
For the glucose uptake treatment, HPTCs were transfected with scrambled or SGLT2 siRNA for 24 hours. Cells were then incubated in Krebs-Ringer-Hepes buffer (15 mmol/L of Hepes [pH 7.4], 105 mmol/L of NaCl, 5 mmol/L of KCl, 1.4 mmol/L of CaCl2, 1 mmol/L of KH2PO4, 1.4 mmol/L of MgSO4, and 10 mmol/L of NaHCO3) for 2 hours. Next, cells were incubated with 0.8 mmol/L 2-deoxy-D-glucose containing 1 μCi/mL2-deoxy-d-[3H] glucose for 1 hour. Transport was stopped by removal of the buffer, followed by 3 washes with ice-cold PBS. Cells were disrupted with 0.4 mol/L of NaOH, neutralized with HCl, and the amount of labeled glucose taken up was determined by scintillation counting.
2.7. Western blotting
The protein expression of p21 was measured by western blotting. Protein samples (50 μg) were separated by 15% SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with an antibody specific for p21 (1:1000; Millipore, Temecula, CA). Equal loading was confirmed by reprobing the membranes with an antibody against β-actin (1:10,000; Sigma Chemicals, St. Louis, MO). IRDye-labeled anti-mouse IgG antibody (1:15,000; Li-Cor, Lincoln, NE) was used to detect p21 and β-actin with an Odyssey System (Li-Cor). p21 expression was normalized for β-actin protein expression.
2.8. RNA interference
siRNAs targeting p21 and SGLT2 (Invitrogen, San Diego, CA) were transfected using Lipofectamine 2000 (Invitrogen). Subconfluent (40–50%) HPTCs in antibiotic-free growth medium were transfected in Click's Medium/RPMI-1640 containing 5 μL Lipofectamine 2000 with 100 pmol siRNA per well (6 wells/plate) for 24 h, and medium was replaced with growth medium.
2.9. Statistical analysis
All values are expressed as the mean ± standard error of the mean (S.E.M.). Data were processed using InStat (Graph-PAD Software for Science, San Diego, CA). For statistical analysis, we used one-way analysis of variance followed by Tukey's multiple comparison tests. Differences were considered significant at P < 0.05.
3. Results
3.1. Blood glucose, plasma insulin levels and albuminuria
As expected, blood glucose levels were increased in the STZ-induced diabetic group compared with the vehicle group (Table 1). Blood glucose levels were decreased by low and high dose insulin treatment (Table 1). The diabetic group also showed a decrease in plasma insulin levels, which was overcome by the insulin implant (Table 1). Plasma insulin levels were significantly higher in the insulin-treated non-diabetic group compared with the untreated non-diabetic group (Table 1).
Table 1.
Body weight, systolic blood pressure, plasma insulin level and albuminuria at 4 weeks after the STZ and/or insulin injection.
| Vehicle | STZ | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Untreated (n = 8) | Insulin (n = 7) | Untreated (n = 8) | Low insulin (n = 8) | High insulin (n = 8) | |
| BW | 25.3 ± 0.4** | 25.4 ± 0.2 | 20.8 ± 0.5 | 24.3 ± 0.3** | 23.6 ± 0.5** |
| SBP (mmHg) | 111 ± 4 | 114 ± 3 | 115 ± 4 | 108 ± 4 | 105 ± 2 |
| Blood glucose baseline (mmol/L) | 7.6 ± 0.3 | 7.7 ± 0.4 | 7.3 ± 0.3 | 7.3 ± 0.3 | 7.8 ± 0.4 |
| Blood glucose week-2 (mmol/L) | 7.9 ± 0.2** | 4.3 ± 0.3## | 20.5 ± 1.0 | 9.2 ± 0.5** | 3.8 ± 0.5** |
| Blood glucose week-4 (mmol/L) | 7.6 ± 0.3** | 4.0 ± 0.3## | 19.1 ± 0.7 | 10.3 ± 0.9** | 3.1 ± 0.2** |
| Insulin (pmol/L) | 166 ± 13 | 2371 ± 367## | 49 ± 2 | 518 ± 94 | 2310 ± 273** |
| Albuminuria (μg/day) | 12.4 ± 0.9** | 12.9 ± 0.8 | 199 ± 22 | 27.3 ± 3.1** | 28.0 ± 4.7** |
Values represent the mean ± S.E.M.
STZ, streptozotocin; BW, body weight; SBP, systolic blood pressure.
P < 0.01, compared with untreated STZ group.
P < 0.01, compared with untreated vehicle group.
The STZ-induced diabetic group significantly increased albuminuria compared with non-diabetic groups (Table 1). The insulin treatment significantly decreased the albuminuria in the STZ-treated groups.
3.2. Renal senescence
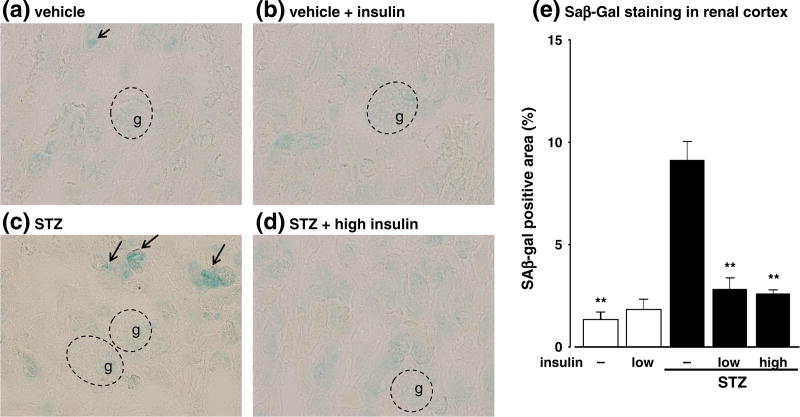
SAβ-Gal staining, which labels senescent cells, was detected in the kidneys, particularly in the cortical tubules, of the STZ-induced diabetic group, but not in the kidneys of the non-diabetic group (Fig. 1a and c). The extent of SAβ-Gal staining in the STZ-induced diabetic mice was diminished by low (data not shown) and high doses of insulin (Fig. 1d). Conversely, insulin did not affect SA-βGal staining in non-diabetic mice (Fig. 1b).
Fig. 1.
Effects of streptozotocin (STZ) and insulin on senescence-associated β-galactosidase (SAβ-Gal) staining (a–d) (magnification, 200×) and quantitative evaluations (e) in the renal cortex at 4 weeks after STZ injection. Pictures are taken in the cortical area and glomeruli are indicated by g. Data are expressed as means ± S.E.M. (n = 6 per group). **P < 0.01 vs. STZ-induced diabetic mice.
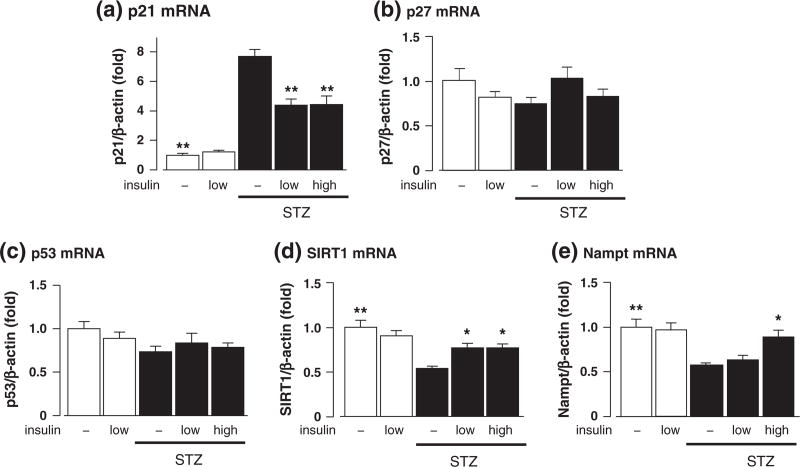
There were significant increases in the mRNA expression of p21 and decreases in the mRNA expression of SIRT1, a deacetylase that inactivates p53, and Nampt, an enzyme that synthesize the substrate of SIRT1, in the kidney of STZ-induced diabetic mice (Fig. 2a, d, and e). These changes were significantly attenuated by low and high doses of insulin (Fig. 2a, d and e). STZ and insulin did not affect the mRNA expressions of p53 and p27 (Fig. 2b and c). Conversely, there were no changes in the mRNA expression levels of p21, p53, p27, SIRT1, or Nampt in insulin-treated non-diabetic mice (Fig. 2a-e). We could not accurately evaluate p16 mRNA level in all groups because its expression level was below the limit of detection.
Fig. 2.
Effects of streptozotocin (STZ) and insulin on the mRNA levels of p21 (a), p27 (b), p53 (c), SIRT1 (d), and nicotinamide phosphoribosyltransferase (Nampt) (e) in the kidney at 4 weeks after STZ injection. Data are expressed as means ± S.E.M. (n = 7–8 per group). *P < 0.05 and **P < 0.01 vs. STZ-induced diabetic mice.
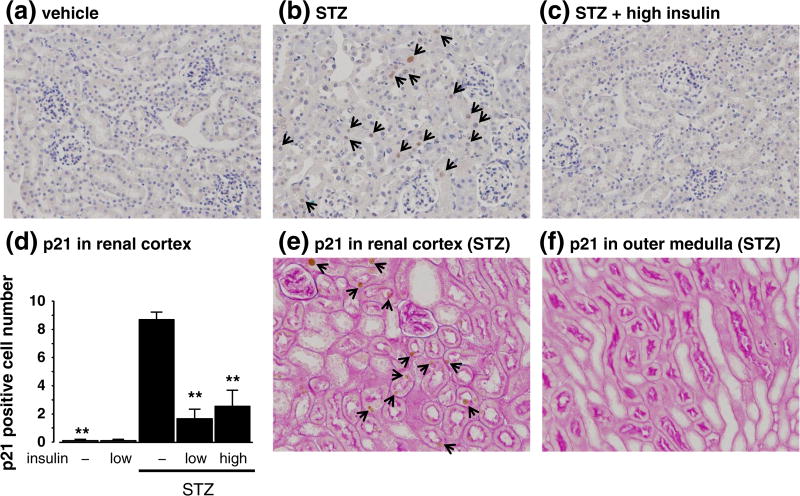
In non-diabetic groups and/or insulin-treated diabetic groups, immunohistochemical staining of p21 were almost at undetectable levels (Fig. 3a, c and d). On the other hand, untreated diabetic mice exhibited p21 staining in the nuclei of proximal tubules in the renal cortical area (Fig. 3b and d). In renal cortical, convoluted proximal tubules, but not in outer medullary straight tubules, exhibited p21-positive nuclei which were confirmed by PAS-stained brush border possessing on the apical membrane of proximal tubules (Fig. 3e and f).
Fig. 3.
Effects of streptozotocin (STZ) and insulin on immunohistochemical staining of p21. Hematoxylin (a-c) staining was performed for counting p21-positive cell number in the renal cortex. (magnification, 200×). Periodic acid-Schiff staining (e and f) was performed for the localization of p21-positive cells in the kidney of STZ group (magnification, 200×). Quantitative analysis (d) are expressed as means ± S.E.M. (n = 4 per group). **P < 0.01 vs. STZ-induced diabetic mice.
3.3. Effects of high glucose on cellular senescence and p21 expression in cultured HPTCs
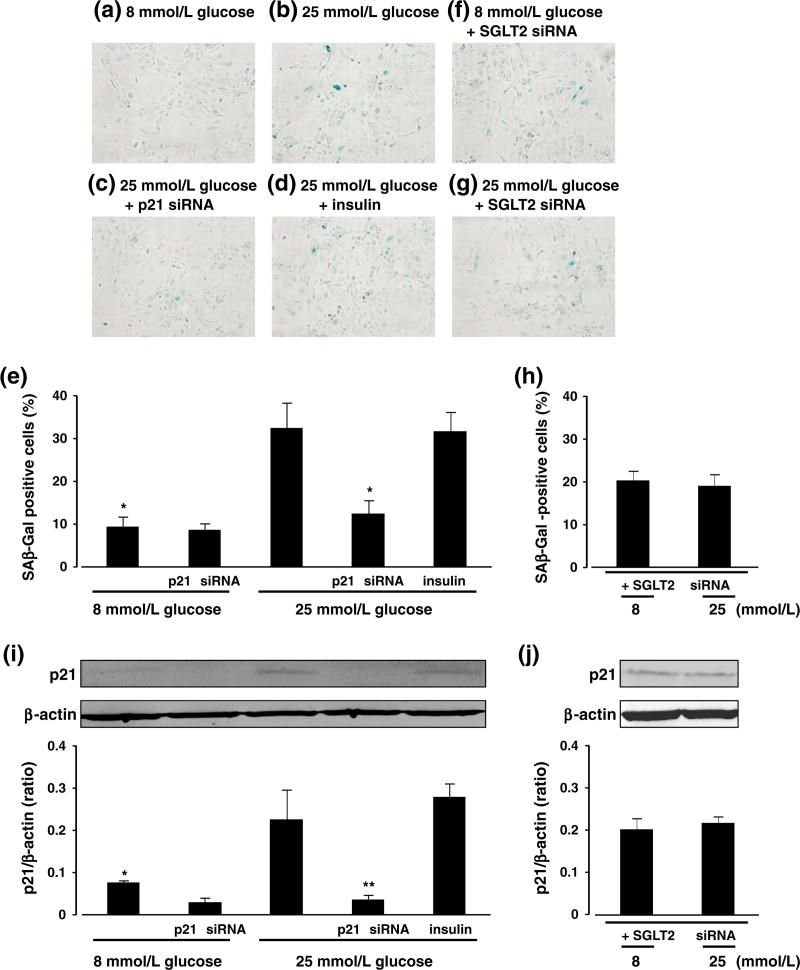
High glucose (25 mmol/L) increased the number of SAβ-Gal-positive HPTCs and p21 protein expression compared with 8 mmol/L glucose at 3 days after treatment (Fig. 4a, b, e and i). These effects of high glucose on HPTCs were attenuated by siRNAs targeting p21 (Fig. 4c, e and i). Conversely, mannitol, used as an osmotic control, did not affect the number of SAβ-Gal-positive HPTCs (8.14 ± 1.52%, n = 3). Furthermore, the effects of high glucose on senescent parameters were not affected by insulin (Fig. 4d, e and i), eliminating the possibility that direct effects of insulin on proximal tubular cells could influence the senescent changes.
Fig. 4.
Effects of 25 mmol/L glucose medium and insulin on senescence-associated β-galactosidase (SAβ-Gal) staining (a-e) and p21 protein expression (i) in cultured human proximal tubular cells. Effects of sodium glucose transporter (SGLT) 2 siRNA on SAβ-Gal staining (f-h) and p21 protein expression (j) in cultured human proximal tubular cells exposed to 25 mmol/L glucose. Data are expressed as means ± S.E.M. (n = 4-6 per group). *P < 0.05 and **P < 0.01 vs. 25 mmol/L glucose.
Finally, high glucose did not increase the number of SAβ-Gal positive cells or p21 protein expression following SGLT2 knock-down (Fig. 4f-h and j). We measured the SGLT2 mRNA expression and glucose uptake in cultured HPTCs to confirm the efficacy of SGLT2 siRNA transfection. SGLT2 siRNA significantly reduced the SGLT2 mRNA level (0.21 ± 0.02 fold, n = 4, P < 0.01) and the amount of glucose uptake (0.66 ± 0.09 fold, n = 6, P < 0.05) in cultured HPTCs compared with scrambled siRNA.
4. Discussion
In the present study, we showed that hyperglycemia, but not hyperinsulinemia, induces cellular senescence in the kidney of mice in the early stage of type 1 diabetes. Furthermore, insulin did not affect high glucose-induced cellular senescence in cultured HPTCs, suggesting that the control of blood glucose levels by insulin, rather than local direct effects of insulin on renal cells (Minamino & Komuro, 2007), may help to prevent cellular senescence in the type 1 diabetic kidney. In addition, hyperglycemia increased the renal p21 mRNA levels, but not the other CDK inhibitors, such as p16 and p27. Moreover, the knockdown of p21 by siRNA clearly suppressed the high glucose-induced cellular senescence in cultured HPTCs. These results suggest that hyperglycemia causes proximal tubular cell senescence via a p21-dependent pathway.
It has been reported that hyperinsulinemia and hyperglycemia may contribute to tissue injury, particularly in cardiac diseases (Karason, Sjostrom, Wallentin, & Peltonen, 2003; Shimizu et al., 2010). Miyauchi et al. (Miyauchi et al., 2004) reported that insulin/Akt signaling caused cellular senescence in human endothelial cells, suggesting that insulin signaling is involved in the development of cellular senescence in the vessel. In the present study, we showed that hyperinsulinemia itself did not induce senescent-like changes in the kidney of insulin-treated mice or in cultured HPTCs. Thus, our results indicate that elevated plasma insulin levels do not induce cellular senescence in the kidney of type 1 diabetic mice, at least, within the present study duration. A limitation of our study is that we used a model of type 1 diabetes and the tissues had normal insulin sensitivity. Type 2 diabetes, which is associated with the combination of hyperinsulinemia with insulin resistance, likely involves different insulin-dependent signaling activities compared with type 1 diabetes that may augment high glucose-induced cellular senescence (Hitomi et al., 2011; Sherajee et al., 2012).
Importantly, the present study revealed the importance of SGLT2 on high glucose-induced cellular senescence. Most of the glucose present in the glomerular filtrate is reabsorbed in proximal tubules via SGLT1 and SGLT2. SGLT2, which is mainly expressed in segments S1 and S2, reabsorbs > 90% of glucose in the luminal fluid into proximal tubular cells (Santer & Calado, 2010; Wright & Turk, 2004), while most of the remaining glucose is reabsorbed by SGLT1 in segment S3 (Santer & Calado, 2010; Wright & Turk, 2004) in the normoglycemic state. Because glucose in the plasma is freely filtered from glomeruli, the proximal tubules are exposed to very high glucose levels in the diabetic state. In addition, the cortical proximal tubules, majority of which are S1 and S2 segments, exhibited the increases in p21 expression in the diabetic kidney whereas the outer medullary proximal tubules are not exhibited the p21 expression. Therefore, in the present study, we examined whether exposure to high glucose levels causes cellular senescence via an SGLT2-dependent mechanism in cultured HPTCs. In fact, high glucose did not increase p21 expression or the number of SAβ-Gal-positive cells in SGLT2 siRNA-transfected HPTCs. These results suggest that hyper-reabsorption of glucose through SGLT2 contributes to hyperglycemia-induced proximal tubular cell senescence. On the other hand, SGLT2 siRNA transfection itself slightly increased p21 expression and the number of SAβ-Gal-positive cells compared with scrambled siRNA transfection. This indicates that physiological glucose supply through SGLT2 may be an essential factor for maintaining proper proximal tubular cell mitosis. Considering these findings, it will be interesting to determine the effects of SGLT2 inhibitors, which are expected to be introduced as new antihyperglycemic drugs (Pfister, Whaley, Zhang, & List, 2011; Santer & Calado, 2010), on renal cell senescence in vivo. It would also be important to determine whether therapeutic doses of SGLT2 inhibitors attenuate cellular senescence through the reduction of “glucose stress” in proximal tubules and also through the subsequent blood glucose-lowering effect, and whether excessive doses of SGLT2 inhibitors induce cellular senescence, which may partially explain a recent study reporting the lack of reno-protection in SGLT2-KO mice against STZ-induced type 1 diabetes (Vallon et al., 2013).
p53 is a well-characterized anti-tumor molecule that induces cell cycle arrest by regulating the transcription of p21 (Campisi, 2005; Shay & Wright, 2005). SIRT1 deacetylates p53 and reduces its activity as a transcriptional factor. It has been reported that high glucose reduces the expression of SIRT1 (Mortuza, Chen, Feng, Sen, & Chakrabarti, 2013; Zhuo et al., 2011). Our present study also showed that the expression levels of SIRT1 and Nampt, an important regulator of SIRT1 activation (Imai & Kiess, 2009; Kim et al., 2011), were decreased in STZ-induced diabetic mice. Therefore, although the mRNA expression of p53 was not affected by hyperglycemia, the decrease in SIRT1 activity in hyperglycemic kidney might cause p53 re-acetylation and activate the p21 transcription in the proximal tubules of diabetic kidney.
Previous studies and the present findings showed that cellular senescence was observed in the renal tubules of diabetic kidney although the pathophysiological roles of senescent cells in the diabetic kidney in vivo remained incompletely understood (Satriano et al., 2010; Verzola et al., 2008). Meanwhile, we previously revealed that p21-dependent senescent cells induced decreasing cellular repair activities after the injury, accelerating apoptosis, and increasing the expression of inflammation- and fibrosis-related factors in aldosterone-induced renal injury (Fan et al., 2011; Kitada et al., 2012). Thus, p21-dependent senescent cells may also contribute to the development of diabetic nephropathy via inducing the above pathways such as inflammation and fibrosis, which are common pathology of the diabetic nephropathy. Further examinations regarding the roles of p21-dependent senescent cells in the diabetic kidney are required in the future study.
Cellular senescence of kidney proximal tubules was previously reported in STZ-induced type 1 diabetic rats (Satriano et al., 2010). The dose of STZ used in that study (65 mg/kg, one dose) was reported to induce DNA damage and have AKI-like effects (Kraynak et al., 1995). Considering that the number of senescent cells is increased in the AKI kidney (Humphreys et al., 2011; Kraynak et al., 1995), the results of the earlier study (Satriano et al., 2010) might be due to either AKI or diabetes. However, that study reported that the CDK inhibitors such as p21 were not increased at 4 days after STZ injection, whereas these were increased at 10 days after STZ injection (Satriano et al., 2010), suggesting little contribution of STZ toxicity to renal senescence. To minimize the impact of STZ toxicity in the murine model, we used a low dose of STZ (10 mg kg–1 day–1 for 7 days) in the present study. In addition, we confirmed that the reduction of blood glucose level by insulin could prevent senescence in proximal tubular cells in STZ-induced diabetic mice. Previous paper and our present findings coordinately indicate that hyperglycemia rather than STZ toxicity induces cellular senescence in the type 1 diabetic kidney (Satriano et al., 2010).
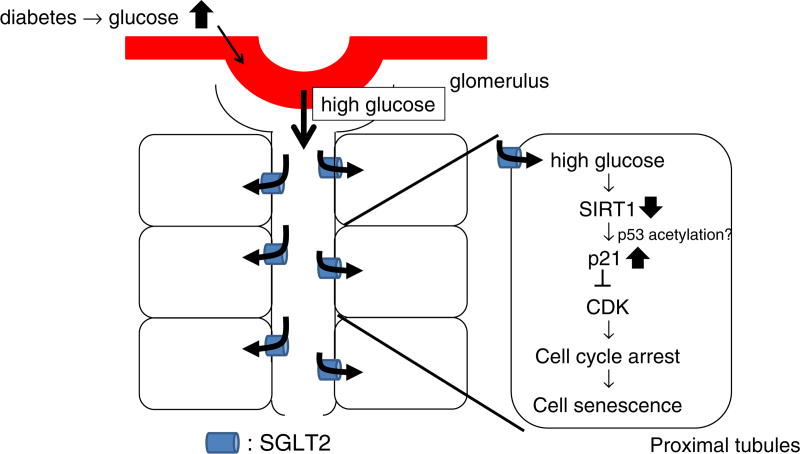
In conclusion, we examined cellular senescence in diabetic nephropathy and demonstrated that hyperglycemia, not hyperinsulinemia, induced cellular senescence via a SGLT2- and p21-dependent pathway (Fig. 5). The present findings suggest that SGLT2 and/or p21 could be a new therapeutical target for the prevention of renal senescence in diabetic nephropathy although the in vivo roles of SGLT2 and p21 in diabetes-induced senescence remain to be determined.
Fig. 5.
Working hypothesis. Hyperreabsorption of glucose via sodium glucose transporter (SGLT)2 induces cellular senescence via a p21-dependent pathway. CDK; cydin-dependent kinase.
Acknowledgments
None.
Grants: This work was supported by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23790299: to Daisuke Nakano).
Footnotes
Disclosure: There is no conflict of interest in this study.
References
- Al-Douahji M, Brugarolas J, Brown PA, Stehman-Breen CO, Alpers CE, Shankland SJ. The cyclin kinase inhibitor p21WAFl/CIPl is required for glomerular hypertrophy in experimental diabetic nephropathy. Kidney International. 1999;56:1691–1699. doi: 10.1046/j.1523-1755.1999.00728.x. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Chuang TD, Guh JY, Chiou SJ, Chen HC, Hung WC, Chuang LY. Sp1 and Smad3 are required for high glucose-induced p21 (WAF1) gene transcription in LLC-PK1 cells. Journal of Cellular Biochemistry. 2007;102:1190–1201. doi: 10.1002/jcb.21346. [DOI] [PubMed] [Google Scholar]
- Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, et al. Aldosterone/Mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology. 2011;152:680–688. doi: 10.1210/en.2010-0829. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Living forever and dying in the attempt. Experimental Gerontology. 2003;38:1231–1241. doi: 10.1016/j.exger.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hitomi H, Kaifu K, Fujita Y, Sofue T, Nakano D, Moriwaki K, et al. Angiotensin II shifts insulin signaling into vascular remodeling from glucose metabolism in vascular smooth muscle cells. American Journal of Hypertension. 2011;24:1149–1155. doi: 10.1038/ajh.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, et al. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1 a receptor-deficient mice. Journal of the American Society of Nephrology. 2006;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Frontiers in Bioscience. 2009;14:2983–2995. doi: 10.2741/3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karason K, Sjostrom L, Wallentin I, Peltonen M. Impact of blood pressure and insulin on the relationship between body fat and left ventricular structure. European Heart Journal. 2003;24:1500–1505. doi: 10.1016/s0195-668x(03)00312-9. [DOI] [PubMed] [Google Scholar]
- Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP, Lee S, et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. American Journal of Physiology Renal Physiology. 2011;301:F427–F435. doi: 10.1152/ajprenal.00258.2010. [DOI] [PubMed] [Google Scholar]
- Kitada K, Nakano D, Hitomi H, Kobori H, Deguchi K, Mori H, et al. Aldosterone induces p21-regulated apoptosis via increased synthesis and secretion of tumour necrosis factor-alpha in human proximal tubular cells. Clinical and Experimental Pharmacology and Physiology. 2012;39:858–863. doi: 10.1111/1440-1681.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak AR, Storer RD, Jensen RD, Kloss MW, Soper KA, Clair JH, et al. Extent and persistence of streptozotocin-induced DNA damage and cell proliferation in rat kidney as determined by in vivo alkaline elution and BrdUrd labeling assays. Toxicology and Applied Pharmacology. 1995;135:279–286. doi: 10.1006/taap.1995.1234. [DOI] [PubMed] [Google Scholar]
- Kuan CJ, al-Douahji M, Shankland SJ. The cyclin kinase inhibitor p21WAF1, CIP1 is increased in experimental diabetic nephropathy: potential role in glomerular hypertrophy. Journal of the American Society of Nephrology. 1998;9:986–993. doi: 10.1681/ASN.V96986. [DOI] [PubMed] [Google Scholar]
- Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- Lei B, Nakano D, Fan YY, Kitada K, Hitomi H, Kobori H, et al. Add-on Aliskiren elicits stronger renoprotection than high-dose Valsartan in type 2 diabetic KKAy mice that do not respond to low-dose Valsartan. Journal of Pharmacological Sciences. 2012;119:131–138. doi: 10.1254/jphs.12031fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megyesi J, Price PM, Tamayo E, Safirstein RL. The lack of a functional p21 (WAF1/CIP1) gene ameliorates progression to chronic renal failure. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10830–10835. doi: 10.1073/pnas.96.19.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, et al. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney International. 2003;63:2134–2143. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney International. 2004;65:510–520. doi: 10.1111/j.1523-1755.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circulation Research. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO Journal. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano D, Lei B, Kitada K, Hitomi H, Kobori H, Mori H, et al. Aldosterone does not contribute to renal p21 expression during the development of angiotensin II-induced hypertension in mice. American Journal of Hypertension. 2012;25:354–358. doi: 10.1038/ajh.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Imamura Y, Yamakoshi K, Hirota F, Nakayama R, Kubo Y, et al. Visualizing the dynamics of p21(Waf1/Cip1) cyclin-dependent kinase inhibitor expression in living animals. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15034–15039. doi: 10.1073/pnas.0706949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Wada J, Hida K, Eguchi J, Hashimoto I, Baba M, et al. Thiazolidinediones ameliorate diabetic nephropathy via cell cycle-dependent mechanisms. Diabetes. 2006;55:1666–1677. doi: 10.2337/db05-1285. [DOI] [PubMed] [Google Scholar]
- Pfister M, Whaley JM, Zhang L, List JF. Inhibition of SGLT2: a novel strategy for treatment of type 2 diabetes mellitus. Clinical Pharmacology and Therapeutics. 2011;89:621–625. doi: 10.1038/clpt.2011.16. [DOI] [PubMed] [Google Scholar]
- Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clinical Journal of the American Society of Nephrology. 2010;5:133–141. doi: 10.2215/CJN.04010609. [DOI] [PubMed] [Google Scholar]
- Satriano J, Mansoury H, Deng A, Sharma K, Vallon V, Blantz RC, et al. Transition of kidney tubule cells to a senescent phenotype in early experimental diabetes. American Journal of Physiology Cell Physiology. 2010;299:C374–C380. doi: 10.1152/ajpcell.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- Sherajee SJ, Fujita Y, Rafiq K, Nakano D, Mori H, Masaki T, et al. Aldosterone induces vascular insulin resistance by increasing insulin-like growth factor-1 receptor and hybrid receptor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:257–263. doi: 10.1161/ATVBAHA.111.240697. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. Journal of Clinical Investigation. 2010;120:1506–1514. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y, Inoshita S, Nakashima O, Tamamori M, Ito H, Kuwahara M, et al. Cell cycle inhibitors (p27Kip1 and p21CIP1) cause hypertrophy in LLC-PK1 cells. Kidney International. 1999;56:494–501. doi: 10.1046/j.1523-1755.1999.00568.x. [DOI] [PubMed] [Google Scholar]
- Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton, Vic.) 2007;12:261–266. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Rose M, Gerasimova M, Satriano J, Piatt KA, Koepsell H, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. American Journal of Physiology Renal Physiology. 2013;304:F156–F167. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. American Journal of Physiology Renal Physiology. 2008;295:F1563–F1573. doi: 10.1152/ajprenal.90302.2008. [DOI] [PubMed] [Google Scholar]
- Wolf G, Schanze A, Stahl RA, Shankland SJ, Amann K. p27(Kip1) Knockout mice are protected from diabetic nephropathy: evidence for p27(Kipl) haplotype insufficiency. Kidney International. 2005;68:1583–1589. doi: 10.1111/j.1523-1755.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflügers Archiv. 2004;447:510–518. doi: 10.1007/s00424-003-1063-6. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J, et al. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cellular Physiology and Biochemistry. 2011;27:681–690. doi: 10.1159/000330077. [DOI] [PubMed] [Google Scholar]