Abstract
Embryo implantation involves the intimate interaction between an implantation-competent blastocyst and a receptive uterus, which occurs in a limited time period known as the window of implantation. Emerging evidence shows that defects originating during embryo implantation induce ripple effects with adverse consequences on later gestation events, highlighting the significance of this event for pregnancy success. Although a multitude of cellular events and molecular pathways involved in embryo-uterine crosstalk during implantation have been identified through gene expression studies and genetically engineered mouse models, a comprehensive understanding of the nature of embryo implantation is still missing. This review focuses on recent progress with particular attention to physiological and molecular determinants of blastocyst activation, uterine receptivity, blastocyst attachment and uterine decidualization. A better understanding of underlying mechanisms governing embryo implantation should generate new strategies to rectify implantation failure and improve pregnancy rates in women.
Keywords: blastocyst activation, uterine receptivity, blastocyst attachment, embryo implantation, decidualization
1. Introduction
In mammals, a new life begins with the union of an egg with a sperm, a process known as fertilization (Wassarman, 1999). Following fertilization, the zygote undergoes several rounds of divisions and morphogenesis to form the blastocyst, an embryonic stage with two distinct cell lineages: the outer specialized trophectodermal epithelium and the inner cell mass (Cockburn and Rossant, 2010; Wang and Dey, 2006). The blastocyst participates in the first physical and physiological interaction with the maternal endometrium to initiate implantation (Red-Horse et al., 2004; Wang and Dey, 2006). A bidirectional crosstalk is essential for normal implantation thus the success of pregnancy, since perturbations will generate adverse outcomes for subsequent development, including decidualization and placentation, with potential loss of the pregnancy (Chen et al., 2011; Song et al., 2002; Wilcox et al., 1999; Ye et al., 2005).
Early pregnancy loss, occurring during the periimplantation period before pregnancy is recognized clinically, is a relatively common phenomenon in humans (Cockburn and Rossant, 2010; Norwitz et al., 2001). For example, even in natural conception, the maximum chance of successful pregnancy occurring in a given menstrual cycle is limited to about 30% (Zinaman et al., 1996). Only 50 to 60 percent of all conceptions advance beyond 20 weeks of gestation (Norwitz et al., 2001). Among the pregnancies that are lost, implantation failure is the major cause, reaching approximate 75% (Wilcox et al., 1988). Furthermore, 1 out of 7 couples worldwide are suffering from infertility (Forti and Krausz, 1998). Despite significant developments in in vitro fertilization and embryo transfer (IVF-ET) technology that have overcome many underlying causes of infertility, pregnancy success rates remain relatively low, mainly due to implantation failure (Miller et al., 2012; Norwitz et al., 2001; Wilcox et al., 1993). Therefore, it is imperative to address this global issue by investigating the mysteries of embryo implantation.
Successful implantation requires synchronization between the acquisition of implantation competency by the blastocyst and a receptive state in the uterine endometrium (Dey et al., 2004;Tranguch et al., 2005b; Wang and Dey, 2006). These two events are precisely regulated by maternal hormones, in particular, ovarian estrogen and progesterone (Conneely et al., 2002; Curtis Hewitt et al., 2002). Molecular and genetic evidence indicates that ovarian hormones together with locally produced signaling molecules, including cytokines, growth factors, homeobox transcription factors, lipid mediators and morphogen genes, function through autocrine, paracrine and juxtacrine interactions to specify the complex process of implantation (Dey et al., 2004). However, the hierarchical landscape of the molecular signaling pathways that govern embryo-uterine interactions during early pregnancy remains to be explored in depth.
The crosstalk between the blastocyst and the uterus can only occur during a brief period, namely the “window of implantation” (Ma et al., 2003; Paria et al., 1993; Rogers and Murphy, 1989; Yoshinaga, 1980). In response to the implanting embryo, the surrounding uterine stroma undergoes cellular transformation, a process known as decidualization, to accommodate embryonic growth and invasion (Lim and Wang, 2010). Locally induced decidua provides a positive feedback to support embryo survival. It is also thought that the decidua functions as a barrier against maternal immunological responses to the semi-allogenic embryo. However, it remains largely unclear how the blastocyst escapes maternal immune surveillance at the time of implantation. With the emergence of advanced technologies, a global analysis of gene and protein expression in the implanting embryo and uterus has been undertaken in several studies to unravel the molecular networks that control implantation in mice, as well as in humans (Hamatani et al., 2004b; Haouzi et al., 2011; Hu et al., 2008; Kao et al., 2002; Reese et al., 2001; Riesewijk et al., 2003; Yoon et al., 2004; Yoshioka et al., 2000). However, due to experimental difficulties and ethical restrictions, our understanding of human implantation still relies predominantly on animal models, particularly the mouse. Gene-knockout mouse models provide valuable information that has been used to construct a tentative molecular basis of implantation. Since embryo implantation is a dynamic developmental process that integrates many signaling molecules into a precisely orchestrated program, it is important to understand the hierarchical landscape of the pathways governing these processes to generate new strategies to correct implantation failure and improve pregnancy rates in women. This review will examine our understanding of signaling cascades that regulate embryo implantation and decidualization derived from gene expression studies and genetically engineered mouse models.
2. Maternal hormonal environment required for embryo implantation
In the majority of eutherian mammals, implantation occurs in a fixed interval of time after ovulation when the corpus luteum is fully formed (Finn and Martin, 1974). In humans, this is during the luteal phase of the menstrual cycle, while in rodents, it is in the diestrous phase of the estrous cycle. It has been well established that estrogen and progesterone are principal hormones in this process. According to their dynamic fluctuating levels, the reproductive cycle is divided into three stages (Finn and Martin, 1974; Wang and Dey, 2006). The first stage is the proestrous or follicular phase in women during which estrogen levels are very high (Michael, 1976; Yoshinaga et al., 1969). The second stage is a period when the levels of both hormones are low immediately after ovulation. Finally, the luteal stage is when both progesterone and estrogen are secreted from the corpus luteum. Embryo implantation occurs towards the end of the luteal phase. For example, at this stage in mice, the level of progesterone is gradually increased, owing to an enhanced secretion from newly formed corpora luteum, accompanied by a preimplantation surge of estrogen on day 4 of pregnancy (day 1=day of vaginal plug), while embryo implantation takes place at the midnight of day 4 (McCormack and Greenwald, 1974; Wang and Dey, 2006) (Figure 1A). Based on the preimplantation ovarian steroid profiles, priming with exogenous estrogen and progesterone can confer on the uterus of ovariectomized mice a receptive state (Lim et al., 1997;Paria et al., 1999b). These hormones direct the preparation of the uterus for implantation in mice and rats (Harper and Walpole, 1967; Roblero et al., 1987; Vinijsanun and Martin, 1990). It is generally accpted that progesterone is required for implantation nearly in all the animals studied, while the role of two estrogen surges at the proestrous and luteal phase prior to embryo implantation remains controversial (Dey et al., 2004; Finn and Martin, 1972; Tranguch et al., 2006; Wang and Dey, 2006).
Figure 1.
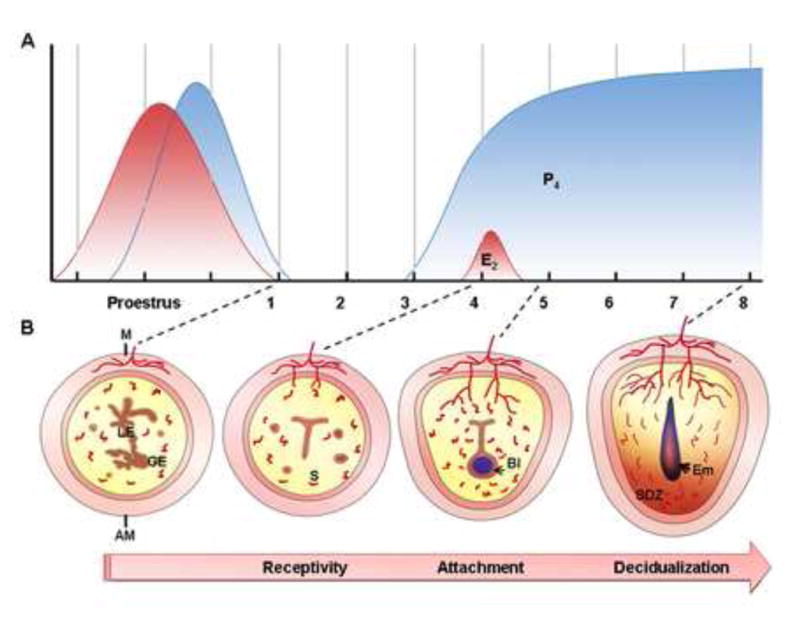
Hormonal control of embryo implantation in mice. (A) Steroid hormone patterns are illustrated during indicated days of the estrous cycle, uterine receptivity and early pregnancy. Estrogen secretion (red curve) is high at ovulation after the luteinizing hormone surge. Soon afterwards, progesterone (blue curve) increases beginning in the late afternoon of proestrus. If mating is successful, the newly formed corpora luteum, stimulated by mating behavior, will secrete progesterone from day 3 onward. On day 4, a small surge of estrogen cooperates with progesterone to induce uterine receptivity. Blastocyst implantation occurs at midnight of day 4. After implantation, progesterone is required for decidualization, placentation and completion of pregnancy. (B) Diagrams depicting cross-sections of the preimplantation uterus (Day 1, Day 4) and implantation sites (day 5, day 8). On day 1, the luminal epithelium of the nonreceptive uterus is highly branched. On day 4, the uterus is receptive with the opposing luminal epithelium that closes around an implanting blastocyst. On day 5, the mural trophectoderm of the blastocyst attaches to the antimesometrial luminal epithelium. The stromal cells underlying the invading embryo then proliferate and differentiate to form an avascular primary decidual zone (PDZ) on the afternoon of day 5. Stroma cells next to the PDZ continue proliferation and differentiation to form a well-vascularized secondary decidual zone (SDZ) by day 8. AM, antimesometrial side; Bl, blastocyst; Em, embryo; E2, estradiol-17β; GE, glandular epithelium; LE, luminal epithelium; M, mesometrial side; P4, progesterone; S, stroma.
It was once thought that progesterone alone mediates implantation. However, the observation that suckling mice and rats with normal progesterone secretion have a facultative delayed implantation suggests that there may be another hormone involved in implantation (Malafaya et al., 2008; Mantalenakis and Ketchel, 1966; McLaren, 1968; Whitten, 1955; Yoshinaga and Adams, 1966). This suspicion is supported by the evidence that implantation can be induced in lactating rats by injection of a small dose of estrogen (Krehbiel, 1941). Later studies provide direct evidence for the role of luteal estrogen in normal implantation (Cochrane and Meyer, 1957; Whitten, 1958), showing that the timing of ovariectomy before or after luteal phase estrogen is critical for the induction of delayed implantation. For example, the blastocyst implants normally when ovariectomy is performed after preimplantation ovarian estrogen secretion, whereas if ovariectomy takes place before estrogen secretion, the embryo does not implant and the uterus enters into a condition of delayed implantation. With progesterone supplementation, the blastocyst remains quiescent, but it can be induced to implant by exogenous estrogen (Paria et al., 1993). These findings clearly indicate that preimplantation estrogen secretion is crucial for blastocyst implantation into a progesterone-primed receptive uterus. Since preovulatory estrogen is secreted in most species, it is thought that proestrous estrogen optimizes the subsequent response just before implantation (Barkley et al., 1979).
Notably, the requirement for ovarian estrogen in implantation is species-specific. In species such as guinea pig, rhesus monkey, rabbit and golden hamster, progesterone alone is adequate for implantation (Harper et al., 1969; Heap and Deanesly, 1967; Heap et al., 1981; Kwun and Emmens, 1974; Psychoyos, 1973, 1986). However, participation of estrogen in implantation of these species may not be completely excluded. A hypothesis has been proposed that blastocysts in these species could synthesize and secrete estrogen locally to initiate implantation (Dey et al., 2004; Wang and Dey, 2006). In agreement with this, aromatase, an enzyme for estrogen synthesis, is detected in the blastocyst of hamster and rabbit, while such an aromatase is absent in mice (Dickmann et al., 1975;Hoversland et al., 1982a; Reese et al., 2008; Sengupta et al., 1983; Sholl et al., 1983). It remains unclear whether blastocyst-uterus attachment during implantation requires ovarian estrogen in humans.
In recent years, genetically engineered mouse models have provided valuable clues to understand the roles of progesterone and estrogen during embryo implantation. The function of progesterone is mainly mediated by its receptor, PR (encoded by Pgr gene), which has two isoforms, PRA and PRB (Edwards, 2005). Both isoforms are expressed in the uterus (Mote et al., 2006). Female mice lacking both PRA and PRB are infertile with many defects in ovarian and uterine functions (Lydon et al., 1995), while these functions are normal in PRB deficient females (Mulac-Jericevic et al., 2000), indicating that essential progesterone-regulated functions in uteri are primarily mediated by PRA. Estrogen functions in the uterus primarily through nuclear estrogen receptors (Tan et al., 1999). ER also has two isoforms, known as ERα (encoded by Esr1 gene) and ERβ (encoded by Esr2 gene) (Krege et al., 1998). Previous studies using knockout mice for ERs have demonstrated their differential functions in uterine biology (Hewitt et al., 2005;Lee et al., 2012a; Lubahn et al., 1993). ERα is the most important mediator of estrogen signaling during early pregnancy since ERα knockout mice are unable to support implantation (Hewitt et al., 2005; Krege et al., 1998;Lee et al., 2012a; Lubahn et al., 1993). Although ERβ knockout mice are fertile with normal implantation, increasing evidences show that it is also important in uterine biology (Krege et al., 1998;Lee et al., 2012a; Su et al., 2012; Wada-Hiraike et al., 2006). For example, ERβ is expressed in the endometrial endothelium and may participate in implantation through regulating angiogenic and vasomotor changes (Huang et al., 2010; Su et al., 2012). In agreement with this, the expression level of ERβ is significantly lower in women with infertility (Altmae et al., 2010). In addition, ERβ is also believed to be a potent player in human labor onset due to its high expression in the myometrium and the cervix (Huang et al., 2010; Su et al., 2012).
3. Embryonic preparation for implantation necessitates “blastocyst activation”
Acquisition of implantation competency by the blastocyst is a prerequisite for successful implantation (Paria et al., 1993). In mice, the blastocyst escapes from its zona pellucida and attaches to the uterine epithelium at day 4.5 of pregnancy (Das et al., 1994; McCormack and Greenwald, 1974; Wang and Dey, 2006). However, this sequence is interrupted in delayed implantation. Except for the facultative delay in lactating mice and rats (Enzmann et al., 1932; Hamlett, 1935; Kirkham, 1918), implantation delay occurs naturally as an obligate delay in nearly 100 mammalian species, notably the mustelids and marsupials (Lopes et al., 2004; Mead, 1993; Renfree and Shaw, 2000; Thom et al., 2004). But in some species such as the hamster, guinea pig, rabbit and pig, delayed implantation does not occur (Dey et al., 2004). It is unclear whether this phenomenon exists in humans. Delayed implantation can be induced experimentally in mice and rats by ovariectomy before the preimplantation ovarian estrogen surge and maintained by injection of progesterone (Huet and Dey, 1987; Yoshinaga and Adams, 1966), providing a powerful model for the study of implantation.
During delayed implantation, the blastocyst is metabolically dormant and incompetent to initiate attachment in the uterus (Nieder and Weitlauf, 1985; Van Blerkom et al., 1978). Although embryos develop into blastocysts and undergo zona dissolution, they show signs of being inactive with subnormal metabolic activity, reduced cell divisions with low DNA synthesis (Lopes et al., 2004). Ultrastructural observations of the trophoblast cells in dormant blastocysts reveal several morphological fine structure changes. For example, the ribosomes become monosomes, the endoplasmic reticulum is less profiled and the Golgi apparatus is not well developed (Renfree and Shaw, 2000; Wu and Meyer, 1974). The blastocyst can maintain this dormant state within the uterine cavity for days or even weeks (Lee et al., 2011a). But under appropriate conditions, the blastocyst is rapidly activated to resume its development with attachment and invasion. The active blastocyst also shows distinct morphological differences from the dormant blastocyst. For example, a more irregular surface with more microvilli is observed in activated trophoblast cells, with accumulating glycogen granules in the cytoplasm (Naeslund et al., 1980). Using the murine delayed implantation model and blastocyst transfer techniques, it has been demonstrated that dormant blastocysts fail to implant in the receptive uterus, highlighting the notion that the blastocyst’s state of activity determines the “window” of implantation in mice (Paria et al., 1993). However, it is still largely unknown how blastocysts undergo dormancy and survive for an extended period or how they become reactivated to acquire implantation competency.
Since implantation is a two-way interaction, it has been speculated that there is a growth-impeding substance in the uterine secretions that target the embryos (Surani, 1975). In support of this hypothesis, a blastocyst in delay that is subsequently activated reverts to its delayed state if replaced in the uterine cavity of a mouse in delayed implantation (Naeslund et al., 1980). Another explanation of embryo dormancy or diapause is that suboptimal conditions exist for blastocyst growth. Several in vitro experiments support this view. For instance, depletion of glucose and/or arginine and leucine from a conventional medium impedes blastocyst growth (Nieder and Weitlauf, 1985). Although these observations are valuable for the clues about blastocyst activation, the underlying molecular and cellular mechanisms are still unknown.
The advent of cDNA microarray technology and genomic sequencing approaches has made global analysis of differential gene expression between dormant and active blastocysts possible (Hamatani et al., 2004b). Hamatani and coworkers find that 229 genes are differentially expressed between dormant and activated blastocysts, suggesting that the physiological states are molecularly distinguishable. The major functional categories of altered genes include the cell cycle, cell signaling, and energy metabolic pathways. Current knowledge on mechanisms governing the process of blastocyst activation for implantation is summarized in Figure 2.
Figure 2.
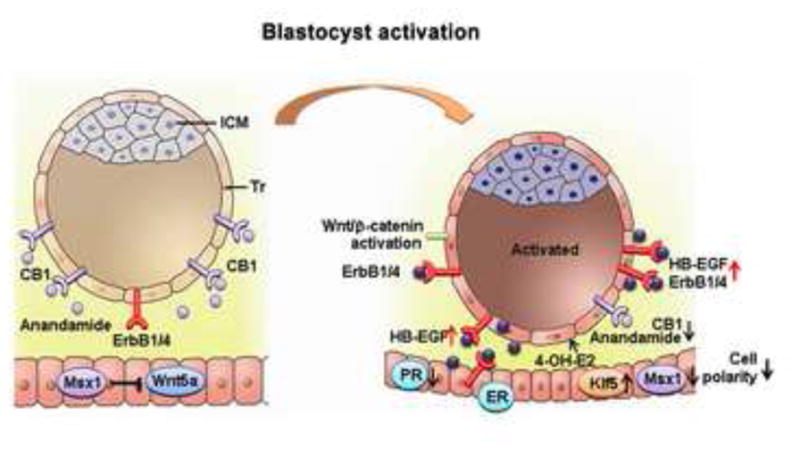
Signals participating in blastocyst activation and uterine epithelial preparation for receptivity. Achievement of blastocyst implantation competency (blastocyst activation) involves steroid signaling, cannabinoid signaling and Wnt signaling pathways. Acquisition of uterine receptivity under the influence of ovarian progesterone and estrogen is associated with a flattening of the luminal epithelium and a loss of polarity at the site of embryo attachment. Several genes regulating uterine transformation are differentially regulated, as illustrated here and detailed in the text. CB1, brain-type cannabinoid receptor-1; ErbB1/4, epidermal growth factor receptor 1/4; ER, estrogen receptor; HB-EGF, heparin-binding EGF-like growth factor; ICM, inner cell mass; Klf5, kruppel-like factor 5; Msx1, muscle segment homeobox 1; 4-OH-E2, 4-hydroxyestradiol; PR, progesterone receptor; Tr, trophectoderm; Wnt5a, wingless-related MMTV integration site 5a.
3.1 Estrogenic derivatives
The dormant blastocysts in delayed implantation in mice will resume implantation upon an injection of estrogen, suggesting the importance of estrogen in mediating both the process of uterine receptivity and blastocyst activation (Paria et al., 1993). Since estrogen receptor is present in preimplantation mouse embryos (Hou and Gorski, 1993; Hou et al., 1996), it was previously thought that estrogen could trigger blastocyst activation for implantation. However, a specific ER antagonist can not inhibit the active status of the blastocyst, and estrogen fails to activate dormant blastocysts in culture, suggesting the participation of a pathway distinct from the classical nuclear ERs signaling (Paria et al., 1998). A further study shows that 4-hydroxyestradiol (4-OH-E2), a catechol metabolite of endogenous estrogen, is effective in initiating blastocyst activation through stimulating the synthesis of prostaglandin (PG) (Paria et al., 1998).
A haloestrogen, 2-fluoroestrdiol-17β (2-FL-E2), is a potent estrogen but acts as an inhibitor of catecholestrogen synthesis. It has been previously shown to stimulate uterine growth and estrogen-targeting gene expression in mice (Dey et al., 1986; Mitchell et al., 1993; Paria et al., 1990; Paria et al., 1998). To distinguish the site-specific function of estrogen and catecholestrogen in uterine preparation versus blastocyst activation for implantation, experiments employing 2-FL-E2 have demonstrated that, unlike native estrogen, 2-FL-E2 fails to induce implantation in progesterone-primed delayed implantation mice, even at a relatively high dose (Paria et al., 1998). Moreover, dormant blastocysts fail to implant after their transfer into uteri of progesterone-treated delayed recipients receiving an injection of 2-FL-E2, whereas normal day 4 blastocysts show implantation after transfer into recipient receiving the same treatment (Paria et al., 1998). These results indicate that 2-FL-E2 can induce the uterus into a receptive status, but fails to activate the dormant blastocyst mainly due to its inability to transform into catecholestrogen. In this respect, catecholestrogen alone can re-induce blastocyst activation and attachment reaction in delayed implanting mice (Hoversland et al., 1982b; Kantor et al., 1985).
Along with this finding, the receptive uterus is capable of transforming the native estrogen into catecholestrogen prior to embryo implantation (Paria et al., 1990; Paria et al., 1998). CYP1B1, an enzyme involved in NADPH-dependent 4-hydroxylation of estrogens, is expressed in uterine stroma cells on day 4 of pregnancy in mice (Paria et al., 1998; Reese et al., 2001). Observations of the presence of CYP1B1 activity in the progesterone-treated uterus and Cyp1b1 transcripts in the receptive uterus strongly suggest that catecholestrogen locally produced in the uterus directs blastocyst activation for implantation. Collectively, ovarian estrogen interacting via the nuclear ERs is required for the preparation of the receptive uterus, whereas its catechol metabolite 4-hydroxyestradiol produced locally in the uterus activates the blastocyst for implantation. Future studies are warranted to further reveal the molecular mechanisms by which catecholestrogen exerts bioactivity and whether there is a specific receptor solely for catecholestrogen in the blastocyst and the uterus during implantation. It is also equally important to address whether and how ovarian steroid hormones and their metabolites interact with other local factors to initiate embryo implantation during early pregnancy.
3.2 Cannabinoid signaling
Psychoactive cannabinoids are major components in marijuana and exert most of their effects through activation of G protein-coupled cell surface receptors, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), encoded in mice by the genes Cnr1 and Cnr2, respectively (Wang et al., 2006a). With the discovery of cannabinoid receptors, two endogenous cannabinoid ligands, arachidonoylethanolamide (also known as anandamide) and 2-arachidonolglycerol, have been identified that can be synthesized in the uterus during early pregnancy (De Petrocellis et al., 2004; Maccarrone and Finazzi-Agro, 2002; Paria et al., 1996). The receptor-mediated activity of anandamide depends on its extracellular concentration, which largely depends on its intracellular degradation by the endocannabinoid-degrading enzyme, fatty acid amide hydrolase (FAAH) (Cravatt et al., 2001; Ueda et al., 2000; Wang et al., 2006c).
Endocannabinoid signaling has recently been highlighted as an important lipid-mediated pathway that directs preimplantation embryo development and the timely homing of embryos into the uterus (Paria et al., 2001b; Wang and Dey, 2005;Wang et al., 2004a; Wang et al., 2006b). Although both CB1 and CB2 are expressed in preimplantation embryos (Paria et al., 1995), cellular and pharmological experiments have demonstrated that CB1 is the functional receptor subtype in the preimplantation embryos. For example, two-cell embryos cultured in the presence of natural, synthetic or endocannabinoids fail to develop into the blastocyst stages, while a CB1-selective antagonist can reverse this inhibitory effect (Schmid et al., 1997; Wang et al., 1999). These findings indicate that the mouse embryo is a potential target for cannabinoid signaling. During the periimplantation period, the levels of uterine anandamide and blastocyst CB1 receptor are precisely regulated. Both the ligand and CB1 receptor are maintained at high levels in the nonreceptive uterus and dormant blastocysts, whereas with the attainment of uterine receptivity and blastocyst activation, they are coordinately downregulated (Das et al., 1995; Guo et al., 2005; Schmid et al., 1997). This is further supported by the finding showing a higher level of anandamide in leukemia inhibitory factor deficient (Lif-/-) mice with implantation failure than those in wild-type mice (Paria et al., 2001b). Similar fluctuations in anandamide levels during early pregnancy have been observed in women undergoing IVF-ET treatment, showing that a significantly lower level of anandamide during implantation is required for successful pregnancy (El-Talatini et al., 2009).
Indeed, a very narrow range of anandamide regulates blastocyst function by differentially modulating mitogen-activated protein kinase (MAPK) signaling and Ca2+-channel activity via CB1 (Wang et al., 2003). For example, anandamide at a low concentration induces the activation of MAPK signaling and confers implantation competency on the blastocyst, whereas a higher concentration dampens this response by inhibiting Ca2+ mobilization (Wang et al., 2003). Thus, it is conceivable that critical levels of endocannabinoids produced in the uterus synchronize blastocyst activation with uterine receptivity for implantation. Aberrant levels of uterine endocannabinoids or blastocyst CB1 receptors interfere with the normal embryo-uterine dialogue during implantation, resulting in early pregnancy loss. It is noteworthy that spontaneous pregnancy losses are associated with elevated anandamide levels in women (Habayeb et al., 2008; Maccarrone et al., 2002; Maccarrone et al., 2000), reinforcing the concept that endocannabinoid signaling is an important determinant of embryonic fate during implantation.
3.3 Wnt signaling
The highly conserved Wnt signaling pathway is a critical mediator of cell-cell interactions during embryogenesis (van Amerongen and Nusse, 2009), it is thus assumed to be involved in the process of implantation, referring to intricate interplay between the blastocyst and the uterus. Wnt signaling is branched into canonical and noncanonical pathways according to its distinct functions (van Amerongen and Nusse, 2009). With respect to canonical Wnt pathway, the Wnt ligands transduce their signals through combining with the frizzled (Fzd) receptor and low density lipoprotein receptor-related protein (LRP) Lrp5/6 co-receptors complex (Logan and Nusse, 2004). This binding will liberate the cytoplasmic β-catenin, which is under continuous proteasome-mediated degradation before the pathway is activated (Metcalfe and Bienz, 2011). Then intracelluar accumulated β-catenin will translocate into the nucleus and form a complex with the lymphoid enhancer-binding factor/T cell-specific transcription factor (LEF/TCF) to guide the transcription of target genes (Behrens et al., 1996). The non-canonical pathway is independent of β-catenin via solely binding to Fzd receptors, and the downstream effectors are diverse, including Ca2+/Planar Cell Polarity (PCP) pathway, Rho signaling, Wnt-PKA signaling and so on (Barrow, 2006; Semenov et al., 2007). The secreted frizzled related proteins (sFRPs), which have similar sequence signatures to Fzd receptors in the cysteine-rich domain to bind the ligand but lack the transmembrane domain to conduct the signal, will inhibit the bioactivities of Wnt proteins (Rattner et al., 1997; Suzuki et al., 2004). Alternatively, the co-receptor Lrp5/6 can also be regulated by the Dickkopf proteins (Dkks) to downregulate cell-surface LRP receptors through interacting with Kremen (Bafico et al., 2001; Glinka et al., 1998; Mao and Niehrs, 2003; Mao et al., 2002; Mao et al., 2001; Semenov et al., 2001). The complexity and redundancy of the Wnt family of proteins, receptors, extracellular antagonists and intracellular signaling components suggest that the nature of the inter- and intra-cellular Wnt machinery determines the orchestration of this signal pathway during development.
Previous studies have revealed specific expression pattern of Wnt pathway members in early embryos and uteri during the periimplantation period in mice (Hamatani et al., 2004a; Kemp et al., 2005; Mohamed et al., 2004; Mohamed et al., 2005; Paria et al., 2001a; Wang et al., 2004c; Zeng et al., 2004), and the dispensable role of canonical Wnt signaling in blastocyst formation is well studied and reviewed previously (Chen et al., 2009; Sonderegger et al., 2010). Notably, during implantation, the canonical Wnt signaling is required for blastocyst competency. Female mice with oocyte specific deletion of β-catenin give reduced number of pups compared with the wild-type littermates, as embryos are lost during the blastocyst stage, implying the role of β-catenin-dependent pathway during the periimplantation stage (De Vries et al., 2004). Interfering the Wnt pathway with the sFRP molecule also disturbs the implantation process (Mohamed et al., 2005). Using the strategy of adenoviral mediated Dkk1, our previous study has clearly demonstrated that silencing canonical Wnt/β-catenin signaling does not adversely affect the uterine preparation for receptivity, but remarkably blocks blastocyst competency for implantation in mice (Xie et al., 2008). The significance of this pathway in blastocysts is first evidenced from the findings utilizing delayed implantation model, showing that the activity of nuclear β-catenin signaling is different between the dormant and reactivated blastocysts (Xie et al., 2008). Moreover, Wnt3a is able to induce intracellular accumulation and nuclear translocation of β-catenin in trophectoderm cells and concurrently induces the expression of peroxisome proliferator-activated receptor (PPAR) δ, a nuclear receptor for prostacyclin (Xie et al., 2008). Through pharmological gain of function study we further reveals that canonical Wnt signaling synergizes with PG signaling to confer blastocyst competency to implantation (Xie et al., 2008). This is consistent with the early finding that expression of cyclooxygenase 2 (COX-2), a rate-limiting enzyme of PG biosynthesis, is substantially upregulated in activated blastocysts after treatment with catecholestrogen (Paria et al., 1998). Collectively, these findings constitute direct evidence that Wnt signaling is at least one pathway determining blastocyst competency for implantation.
3.4 Embryo-derived signals for implantation
Although it is clear that a maternal-embryonic dialogue is essential for the initiation of embryo implantation and that the state of the blastocyst determines the implantation window (Paria et al., 1993), there is a long-standing quest for specific embryo-derived signaling molecules that can functionally influence the uterus, as well as other reproductive organs (Lim and Dey, 2009). Global gene analysis of the dormant versus active blastocysts demonstrates that heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) encoded by Hbegf gene is significantly up-regulated during blastocyst activation (Hamatani et al., 2004b). Prior studies demonstrate that Hbegf is expressed in the luminal epithelium at the site of blastocyst apposition approximate 6 hours prior to the blastocyst attachment reaction in mice (Das et al., 1994; Paria et al., 1999a). Moreover, increasing expression of its receptors (ErbB1 and ErbB4) and ligand binding activity has been observed in the blastocysts that are competent for implantation (Das et al., 1997; Paria et al., 1993; Raab et al., 1996). Most interestingly, blastocyst-size Affi-gel beads presoaked with HB-EGF protein that are transferred intraluminally into a pseudopregnant uterus can induce its own gene expression in uterine cells surrounding the beads and increase vascular permeability, similar to the physiological changes induced by normal blastocysts (Hamatani et al., 2004b; Paria et al., 2001a). Signaling by HB-EGF back to the embryo, in turn, activates the program of trophoblast differentiation required for adhesive functions during subsequent attachment and invasion (Wang et al., 2000). These observations suggest that HB-EGF conducts an auto-induction loop between the implanting blastocyst and the uterus via paracrine and juxtacrine manners. Maternal deficiency of HB-EGF targeted to the uterus defers the window of implantation and compromises pregnancy outcome, while amphiregulin, another heparin-binding member of the EGF family, partially compensates for the loss of HB-EGF during implantation (Xie et al., 2007). In human, HB-EGF is highly expressed in the receptive endometrium (Birdsall et al., 1996; Leach et al., 1999; Stavreus-Evers et al., 2002; Yoo et al., 1997) and its receptor ErbB4 is localized on the surface of the trophectoderm in periimplantation blastocysts (Chobotova et al., 2002b), indicating that HB-EGF-ErbB4 signaling also mediates the trophectoderm-uterine epithelium interaction during implantation in humans. HB-EGF continues to regulate trophoblast development and survival during placentation in the first trimester of human gestation (Jessmon et al., 2009).
As described above, aberrant endocannabinoid signaling is detrimental for preimplantation development and blastocyst implantation (Paria et al., 2001b; Wang et al., 1999). As an endogenous cannabinoid ligand, anandamide is spatiotemporally regulated in the uterus during early pregnancy, i.e., its expression levels are lower in the receptive uterus than for non-receptive uterus and at the implantation site than for interimplantation site (Paria et al., 2001b; Wang et al., 1999). Regarding the machinery controlling appropriate levels of anandamide in the implanting uterus, it is worth noting that the blastocyst can rapidly release a non-identified, cell permeable lipid that is able to activate uterine FAAH, protecting against the detrimental effects of excessive uterine endocannabinoids (Maccarrone et al., 2004).
Another intriguing example of embryonic regulation of uterine function during implantation is revealed by asynchronous embryo transfer studies. When preimplantation embryos at different developmental stage are transferred into the oviducts of the same recipient, embryos of advanced stage implant in advance of younger embryos prior to the anticipated time when implantation normally occurs (Ueda et al., 2003). This finding indicates that the presence of embryos in the reproductive tract can prime the endometrium through unknown embryonic factors, leading to an expansion of the implantation window. A previous study employing non-human primates reports that super-imposition of endometrial receptivity on embryonic stimuli causes remarkable changes in endometrial expression of pro-inflammatory cytokines and immunosuppressive factors, which regulate endometrial transformation conducive for embryo survival, growth and development (Nimbkar-Joshi et al., 2009). A similar phenomenon has been observed in Lif null pregnant mice, in which implantation fails to occur, but the uterine luminal epithelium exhibits COX-2 expression at the apposition site of blastocysts (Fouladi-Nashta et al., 2005; Song et al., 2000), reinforcing the notion that embryo-derived signaling molecules are able to regulate uterine preparation for implantation. It is a challenge to unravel the nature of embryonic signals influencing uterine functions. One of the limiting factors is the availability of adequate amount of tissues for analysis. With the advent of microscale proteomics and genomics, it becomes possible to identify embryonic signals during implantation (Aghajanova et al., 2012; Dominguez et al., 2010; Katz-Jaffe et al., 2006; Tang et al., 2009). Identification of putative secreted embryonic signaling molecules could be validated by employing the uterine blastocyst-size bead transfer approach, as previously described (Hamatani et al., 2004b;Paria et al., 2001a).
After the attachment, the embryo directly contacts with the endometrium and the uterine stroma cells initiate the decidualization that embed and protect the embryo. Decidualization can also occur in response to artificial stimulus by oil injection or endometrium scratch in the receptive uterus to form deciduoma, which exclude the absolute requirement of the conceptus (Loeb, 1906, 1908). However, some studies uncover the morphological and time schedule difference between the deciduoma and the decidua, which hint the involvement of embryo-derived signals (Deanesly, 1971; Lundkvist and Nilsson, 1982; Welsh and Enders, 1985). The disappearance of primary decidual zone which forms a barrier surrounding the embryo and reduced immune cell population in deciduoma further emphasize the importance of embryo-uterus dialogue (Herington and Bany, 2007b; Wang et al., 2004d). Using microarray approaches, the differentially expressed genes responsive to (Bany and Cross, 2006; Herington and Bany, 2007a; Herington and Bany, 2007b, 2009; Herington et al., 2009; Kashiwagi et al., 2007; McConaha et al., 2011)the living embryo versus artificial stimuli further prove the paracrine signals from the embryo regulating the decidua development (Bany and Cross, 2006; Herington and Bany, 2007a; Herington and Bany, 2007b, 2009; Herington et al., 2009; Kashiwagi et al., 2007; McConaha et al., 2011). Collectively, these findings highlight the necessity of embryo-derived signals for normal implantation and decidualization.
4. Uterine receptivity: unique status of uterine differentiation conducive for embryo implantation
In placental mammals, the uterus is receptive to blastocyst implantation during a spatiotemporally restricted “window” when the uterine environment is favorable for blastocyst implantation (Yoshinaga, 1988). In mice, this period is limited to day 4 of pregnancy (Wang and Dey, 2006). The uterus cannot initiate implantation before this period (days 1–3). Immediately after the receptive state, the uterus spontaneously enters a refractory phase (day 5 onward) where the uterine environment is hostile for blastocyst survival (Carson et al., 2000; Yoshinaga, 1988). Similarly, in humans, the receptivity period occurs between days 20 and 24 of a regular menstrual cycle (days 6 to 10 after ovulation LH surge) (Bergh and Navot, 1992; Lessey, 2011; Noyes et al., 1975; Psychoyos, 1973; Rashid et al., 2011). The key to uterine receptivity is the dynamic and precisely controlled molecular and cellular events that drive blastocyst growth, attachment and the subsequent events of implantation. This dynamic process involves a variety of genes that include cytokines, homeobox transcription factors, and developmental genes working together with ovarian steroids to specify uterine receptivity.
4.1 Steroid hormones
Ovarian steroids progesterone and estrogen are principal hormones that direct uterine receptivity. Synchronized production of progesterone and estrogen mediates structural and functional changes in the uterus that enable the blastocyst to attach and initiate the process of implantation. On day 1 of pregnancy in mice, under the influence of preovulatory ovarian estrogen, the uterine epithelial cells undergo extensive proliferation that to some extent continues through day 2. Rising progesterone levels secreted from the newly formed corpora luteum initiate stromal cell proliferation from day 3 onward (Huet-Hudson et al., 1989; Huet et al., 1989). On the morning of day 4, when the uterus enters the pre-receptive stage, the production of a small amount of estrogen is crucial for the uterus to attain receptivity (Tranguch et al., 2005b). At that time, the uterine epithelial cells gradually lose their polarity, and meanwhile, the plasma membranes of the epithelial cells become smooth and flattened at the site of blastocyst apposition (Martin et al., 1970).
To some extent, the window of uterine receptivity is flexible and can be modified under different hormonal environments. In mice, the blastocyst can initiate implantation outside of the normal “window” of uterine receptivity (Song et al., 2007). For example, blastocysts can initiate the attachment reaction in the non-receptive uterus when transferred on day 5 of pseudopregnancy, but implantation will not occur when normal blastocysts are transferred into the day 6 pseudopregnant uterus (Song et al., 2007). Exogenous progesterone supplementation can prolong the implantation window to day 6 with sustained LIF expression (Song et al., 2007). However, this deferred embryo implantation leads to embryonic demise before birth in mice, and is often associated with higher risk of early pregnancy losses in humans (Wilcox et al., 1999).
Estrogen is a critical determinant specifying the duration of the window of uterine receptivity for implantation. Using different doses of estrogens in the delayed implantation model, estrogen extends the window of uterine receptivity at a low threshold, whereas physiologically higher levels rapidly close the implantation window, transforming the uterus into a refractory state (Ma et al., 2003). Although whether blastocyst attachment reaction requires ovarian estrogen or not is still ambiguous in humans, exposure to high levels of estrogen, which could be resulting from ovarian stimulation with clomiphene citrate in IVF, leads to implantation failure and embryo resorption (Ertzeid and Storeng, 2001; Shapiro et al., 2011). This reduced implantation rate in IVF cycles could be due to asynchrony between the endometrium and the blastocyst when exposed to high levels of estrogen (Devroey et al., 2004).
4.2 Cytokines
Of the cytokines that have been studied, LIF is most pertinent to implantation (Kimber, 2005; Stewart et al., 1992; White et al., 2007). The LIF receptor shares gp130 as a common signal-transduction partner with other cytokine receptors. Expression of LIF is biphasic on day 4, initially in the uterine glands and later in the stromal cells surrounding the blastocyst during the attachment reaction (Ni et al., 2002; Song and Lim, 2006; Song et al., 2000). This expression pattern indicates that LIF has dual roles, first in uterine preparation and later in the attachment reaction (Song et al., 2000; Stewart et al., 1992). Lif-deficient female mice exhibit implantation failure and supplementation with LIF rescues this defect (Chen et al., 2000; Stewart et al., 1992). Moreover, pharmological blocking LIF action in the uterus significantly reduces the phosphorylation of the downstream signaling molecule signal transducer and activator of transcription (STAT) 3 in the uterus, resulting in implantation failure (Menkhorst et al., 2011; Mohamet et al., 2009; White et al., 2007). The importance of LIF signaling in implantation is further evidenced by the observations that inactivation of gp130 and STAT3 also causes implantation failure (Catalano et al., 2005; Cheng et al., 2001; Daikoku et al., 2011; Ernst et al., 2001). In humans, LIF is expressed at high levels in the glandular epithelium of the secretory endometrium (Rashid et al., 2011), with a significant increase in the luminal and glandular epithelium at mid-secretory phase (Leach et al., 2012). It is reported that an optimal level of LIF is required for blastocyst implantation in women (Menkhorst et al., 2011; Terakawa et al., 2011). Moreover, clinical evidence shows that LIF deficiency is associated with unexplained recurrent abortion and infertility in women (Dey et al., 2004; Ernst et al., 2001; Hambartsoumian, 1998). Overall, significant gains have been made in our understanding of the function of LIF, a critical determinant of embryo implantation.
4.3 Homeobox transcription factors
Homeobox genes are evolutionarily conserved transcriptional regulators that control embryonic morphogenesis and differentiation (Krumlauf, 1994). In mice, homeobox A (Hoxa) genes, Hoxa10 and Hoxa11, are expressed in uterine stromal cells during receptivity and upregulated upon decidualization in a steroid hormone-responsive manner (Benson et al., 1996; Gendron et al., 1997; Hsieh-Li et al., 1995). This specific expression pattern indicates that these two transcription factors may have dual roles, first in uterine receptivity and then in decidualization. Both Hoxa10 and Hoxa11 mutant mice are infertile due to implantation defects (Bagot et al., 2001; Gendron et al., 1997; Lim et al., 1999b). Hoxa11-/- uteri have a more severe phenotype than Hoxa10-/- mice, and the absence of Lif expression in Hoxa11-/- uteri reinforces its role as a crucial participant in uterine receptivity and later events of implantation (Gendron et al., 1997). Regarding the pathophysiological significance of Hox genes in human implantation, expression of HOXA10 and HOXA11 in the endometrium increases significantly in the midluteal phase when the uterus is receptive for embryo attachment (Gui et al., 1999; Taylor et al., 1998;Taylor et al., 1999b), and is significantly lower in infertile women (Eun Kwon and Taylor, 2004; Fischer et al., 2011; Matsuzaki et al., 2009; Taylor et al., 1999a).
Nonclassical Hox genes may also be important in implantation. Ablation of H6 homeobox 3 (Hmx3) in mice leads to implantation failure (Wang et al., 1998). Msx1, another homeobox gene, is transiently expressed in the mouse luminal epithelium and glandular epithelium on the morning of day 4 of pregnancy (Daikoku et al., 2004; Pavlova et al., 1994), but its expression is dramatically downregulated to undetectable levels upon the termination of uterine receptivity (Daikoku et al., 2004; Pavlova et al., 1994). However, in Lif-/- mice, Msx1 is consistently expressed in the uterine epithelium even on day 6 of pregnancy, suggesting that LIF signaling is essential for the down-regulation of Msx1 (Daikoku et al., 2004). This is confirmed by the observation of sustained Msx1 expression in uteri with conditional depletion of gp130 (Daikoku et al., 2011). Conditional deletion of uterine Msx1 impairs implantation. Histological analysis of Msx1-/- implantation sites reveals that the luminal epithelium lacks well-defined crypts for blastocyst homing and attachment (Daikoku et al., 2011). Moreover, a double knockout of uterine Msx1 and Msx2 results in complete implantation failure with altered luminal epithelial cell polarity and impaired stromal-epithelial communication (Daikoku et al., 2011; Nallasamy et al., 2012), pointing toward a compensatory role of Msx2 in the establishment of uterine receptivity in the absence of Msx1. Nonetheless, these results suggest that Msx1/2 genes are critical for conferring uterine epithelial integrity and uterine receptivity in mice.
4.4 Developmental genes
The dialogue between the blastocyst and uterus shares features with reciprocal epithelial-mesenchymal interactions that occur during embryogenesis, and involves evolutionarily conserved signaling pathways mediated by Indian hedgehog (IHH) proteins, bone morphogenetic proteins (BMPs), Wnts, and their cognate receptors (Paria et al., 2001a).
IHH is a member of the hedgehog family which fine-tune cell proliferation, differentiation and cell-cell communication in many processes such as organogenesis, stem cell maintenance and oncogenesis (Ingham and McMahon, 2001; Ryan and Chiang, 2012). The progesterone-dependent expression of Ihh is restricted to the epithelium, whereas the effectors of IHH, patched-1 (ptch1) and transcription factors GLI-Krüppel family member (Gli) 1–3 are coordinately expressed in the underlying stroma (Matsumoto et al., 2002; Takamoto et al., 2002). Conditional deletion of Ihh in the uterus results in implantation failure due to a lack of progesterone-facilitated stromal proliferation, angiogenesis and decidualization, phenotypically mimicking that of PR knockout mice (Franco et al., 2010a; Lee et al., 2006). These findings indicate that IHH is an essential mediator for PR action in the uterus, participating in the communication between the uterine epithelium and the stroma required for embryo implantation. Microarray analysis demonstrates that IHH mRNA is synchronously regulated with PR during the human menstrual cycle (Talbi et al., 2006). Indeed, the temporal elevation of endometrial IHH and GLI1 during the secretory phase, and their modulation by CDB-2914, a selective PR modulator (Wei et al., 2010), suggest that progesterone regulated hedgehog signaling is a key regulator of human endometrial differentiation and implantation. Moreover, endometrial IHH expression is reduced in women with endometriosis, indicative of progesterone resistance (Smith et al., 2011).
BMPs are the largest family of morphogens belonging to the transforming growth factor-β (TGF-β) superfamily of growth modulators (Zamani and Brown, 2011), and transcripts corresponding to several BMP family members are expressed in mouse uteri (Paria et al., 2001a; Ying and Zhao, 2000). Among all the BMPs expressed in the uterus, only BMP2 is induced in response to progesterone, with intense expression in the stromal cells surrounding the implanted embryo (Paria et al., 2001a). In vitro studies have demonstrated that addition of recombinant BMP2 to undifferentiated stromal cells markedly enhances the decidualization by stimulating Smad signaling pathway, whereas silencing the expression of BMP2 in these cells efficiently blocks the decidualization in both mice and humans (Lee et al., 2007; Li et al., 2007). Moreover, the biological activity of BMP2 is mediated by proprotein convertase 6 (PC6) during decidualization; therefore, addition of recombinant active BMP2 partially rescues the decidualization arrest caused by PC6 inhibition (Heng et al., 2010). This is consistent with the previous findings that PC6 is highly expressed and regulated during implantation and decidualization, and that deletion or knockdown of PC6 inhibits decidualization, causing implantation failure and female infertility (Nie et al., 2005; Okada et al., 2005; Tang et al., 2005).
Interestingly, uterine expression of the PR-interacting protein, Krüppel-like factor (KLF) 9, is significantly enhanced upon conditional ablation of Bmp2 (Lee et al., 2007; Li et al., 2007). Previous studies have demonstrated that KLF9 is highly expressed in the predecidual stroma and becomes undetectable in the decidua, while its deficiency induces subfertility in mice and reduces uterine progesterone sensitivity (Simmen et al., 2004; Velarde et al., 2005). Increasing evidence points toward the existence of a negative feedback loop between KLF9 and BMP2 to maintain PR function in stromal cells for successful implantation, where deregulation of this interaction can compromise the attainment of uterine receptivity and cause infertility (Pabona et al., 2010).
Gene expression profiling experiments have identified Wnt4 as a downstream target of BMP2-induced decidualization (Li et al., 2007). Wnt4 is expressed primarily in the luminal epithelium during the preimplantation period and then re-localizes to the stromal cells surrounding the implanting embryo and expands its expression to the decidua (Daikoku et al., 2004; Hayashi et al., 2009). Conditional deletion of Wnt4 in the uterus renders female mice subfertile due to defective embryo implantation and subsequent decidualization (Franco et al., 2011a). Molecular analysis demonstrates that Bmp2 along with other decidual marker genes such as follistatin are downregulated in Wnt4-null uteri (Franco et al., 2011a). These data indicate a complex correlation between Wnt4 and BMP2 during decidualization.
In addition to Wnt4, many other components of Wnt signaling pathway are spatiotemporally regulated in the periimplantation uterus and are believed to be crucial to implantation (Chen et al., 2009; Hayashi et al., 2009). For example, Wnt5a is expressed in the uterine subepithelial stroma albeit at lower levels in the epithelium (Hayashi et al., 2009; Hou et al., 2004; Mericskay et al., 2004). However, its expression is upregulated in both the epithelium and stroma in Msx1-/- and Msx1/Msx2-/- uteri (Daikoku et al., 2011; Nallasamy et al., 2012). In addition, application of Wnt5a in vitro compromises blastocyst invasion and trophoblast outgrowth when co-cultured with uterine epithelial cells (Daikoku et al., 2011). These findings suggest that Wnt5a may affect epithelial function and, thus, direct blastocyst trophectoderm differentiation. A recent study show that Wnt7b is strongly upregulated in uterine epithelia after periimplantation estrogen secretion and that its expression can be induce by injection of estrogen in ovariectomized mice (Hayashi et al., 2009). Therefore, it is likely that Wnt7b is a direct mediator of uterine function in response to ovarian estrogen. However, a definitive role of Wnt7b in uterine receptivity warrants further investigation through the use of a conditional deletion mouse model, since Wnt7b null mice die during midgestation due to a failure of chorioallantoic fusion (Parr et al., 2001). Wnt7a is also expressed in the luminal epithelium with maximal expression on day 5 and declines thereafter at both implantation and inter-implantation sites (Hayashi et al., 2009). In contrast to Wnt7b, uterine expression of Wnt7a is not under the control of ovarian estrogen. Adult Wnt7a-deficient mice are viable, but infertile, owing to a lack of endometrial glands, suggesting that Wnt7a is crucial for normal formation and maintenance of uterine cellular architecture (Carta and Sassoon, 2004; Miller and Sassoon, 1998; Parr and McMahon, 1998). Sfrp4, a Wnt inhibitory protein, is expressed in the uterine stroma during the receptive phase (Daikoku et al., 2004). A marked downregulation of sfrp4 in Lif/ uteri indicates that sFRP4 could participate in regulating uterine preparation for implantation (Daikoku et al., 2004). This is further supported by the observation that sFPR4 expression remains low in the endometrium of women with recurrent implantation failure (Revel et al., 2011). Unlike sFRP4, expression of sFRP2 is downregulated at the implantation site, and intraluminal infusion of sFRP2 protein disrupts normal implantation (Mohamed et al., 2005). This differential expression of Wnts and their inhibitory molecules underscores that a precisely regulated Wnt system is crucial to uterine preparation for implantation.
5. Cell-cell interactions: the nature of embryo implantation
Embryo implantation is a dynamic developmental event that involves a series of physical and physiological interactions among the blastocyst trophectoderm and various endometrial cell-types, including the luminal and glandular epithelial and stromal cells (Aplin and Kimber, 2004; Carson, 2008; Enders, 2000; Lim and Dey, 2009). Implantation proceeds through three stages: apposition, adhesion/attachment, and penetration (Carson et al., 2000; Schlafke and Enders, 1975). During the apposition stage, the trophectoderm comes into close proximity with the luminal epithelium. With uterine lumen closure, firm attachment between the trophectoderm and luminal epithelium is initiated. In mice, the attachment reaction occurs on day 4 of pregnancy at midnight, coinciding with a localized increase of endometrial vascular permeability. Intravenous injection of macromolecular blue dye will clearly mark the sites of blastocyst implantation along the uterine horn due to this vascular permeability (Lundkvist, 1978a, b, 1979; Lundkvist and Ljungkvist, 1977). After the attachment reaction, the implanting embryo initiates penetration through the luminal epithelium into the stromal bed (Carson et al., 2000; Schlafke and Enders, 1975; Welsh and Enders, 1987). Therefore, implantation is a bulk of cell-cell interactions between the blastocyst and the uterus under the primary influence of ovarian steroids (Figure 3).
Figure 3.
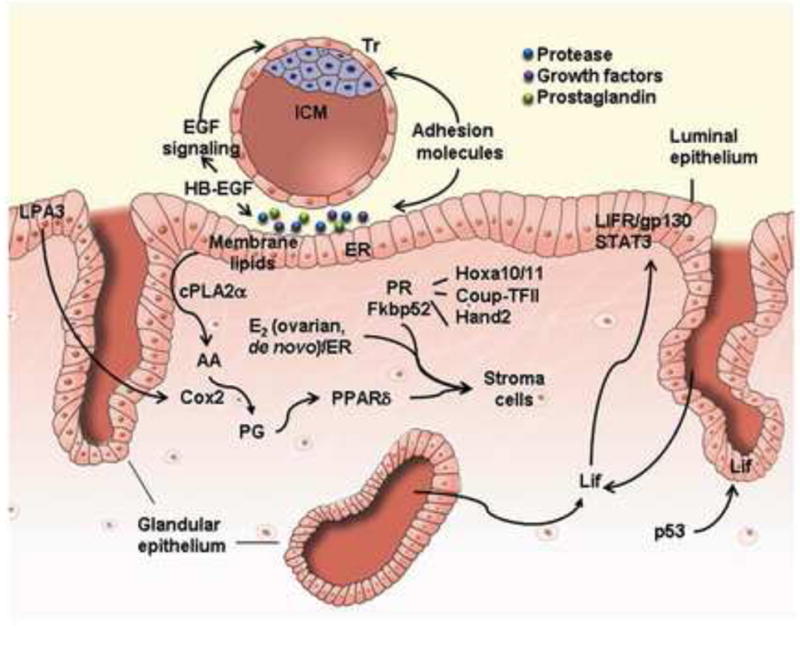
Signaling networks that regulate embryo implantation. Embryo implantation is a dynamic developmental event which involves physical and physiological interactions among the blastocyst trophectoderm and the uterine luminal and glandular epithelial cells, as well as participation by stromal cells. In this cartoon, critical signals that regulate these interactions are portrayed, as described in the text. AA, arachidonic acid; cPLA2α, cytosolic phospholipase A 2α; COUP-TFII, chicken ovalbumin upstream promoter transcription factor-2; COX2, cyclooxygenase-2; E2, 17β-estradiol; ER, estrogen receptor; Hand2, Heart- and neural crest derivatives-expressed protein 2; Hoxa10/11, homeobox A10/11; ICM, inner cell mass; LIF, leukemia inhibitory factor; LIFR, LIF receptor; LPA3, lysophosphatidic acid receptor 3; PG, prostaglandin; PPARδ; peroxisome proliferator-activating receptor δ; PR, progesterone receptor; STAT3, signal transducers and activators of Transcription 3; Tr, trophectoderm;.
5.1 Uterine luminal closure for blastocyst apposition
During the preimplantation period, uterine luminal fluid provides a medium for transporting preimplantation embryos into the uterine horn, and absorption of uterine luminal fluid on day 4 of pregnancy facilitates the process of luminal closure and blastocyst apposition within the uterine cavity, establishing intimate contact of the blastocyst and the uterine epithelium (Figure 1B). In mice and rats, ovarian estrogen stimulates fluid secretion, while progesterone induces fluid absorption prior to attachment reaction. Interaction between the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-activated Cl- channel, and the epithelial Na+ channel (ENaC) has been proposed as the major mechanism regulating uterine fluid secretion and reabsorption (Salleh et al., 2005). Previous studies demonstrate that ENaC is primarily localized to the apical membrane of both luminal and glandular epithelia, while CFTR is predominantly expressed in the stromal cells (Chan et al., 2002; Ruan et al., 2012; Yang et al., 2004). Estrogen induces CFTR expression, but represses ENaC, resulting in fluid accumulation in the uterine lumen, whereas progesterone acts oppositely in regulating these two genes for fluid reabsorption in the uterus (Nobuzane et al., 2008; Zheng et al., 2004). Abnormal uterine fluid accumulation and implantation failure have also been observed when CFTR expression is aberrantly upregulated by inflammation in mice (He et al., 2010).
The invading embryos can release trypsin, a serine protease known to activate ENaC (Kleyman et al., 2009; Vallet et al., 1997). A most recent study demonstrates that the activation of ENaC in the mouse uterus is critical for implantation due to its requirement for PG production and release (Ruan et al., 2012). A related study is conducted on serum and glucocorticoid inducible kinase-1 (SGK1), a key regulator of sodium transport in mammalian epithelia (Fejes-Toth et al., 2008). SGK1 functions through directly activating ENaC and enhancing ENaC expression by inhibiting the ubiquitin ligase, neural precursor cell expressed developmentally down-regulated protein (NEDD) 4–2 (Lang et al., 2006). In mice, Sgk1 mRNA levels transiently decline in the luminal epithelium during the window of endometrial receptivity (Fisher and Giudice, 2011; Salker et al., 2011). Intraluminal delivery of an overexpressing Sgk1 vector abolishes normal implantation, with markedly upregulated expression of ENaC α subunit (Salker et al., 2011). In this respect, SGK1 functions as an important enzyme to modulate the appropriate expression and activity of ENaC, which is conducive to uterine fluid absorption prior to implantation. Overall, these findings add a new line of evidence showing that a compensatory expression profile and balanced activity of uterine CFTR and ENaC provide a molecular mechanism, by which maximal fluid absorption and uterine luminal closure can be achieved to facilitate blastocyst apposition.
5.2 The trophectoderm-uterine epithelium interaction
It is thought that adhesion molecules play key roles in blastocyst apposition and attachment during implantation. Previous studies have demonstrated a group of adhesion molecules, such as integrins, the trophinin-bystin-tastin complex, selectins and cadherins, are involved in these processes (Armant, 2011; Kimber and Spanswick, 2000; Singh and Aplin, 2009).
The integrins are versatile and have the ability to bind arginine-glycine-aspartic acid (RGD) sequences in extracellular ligands including fibronectin, osteopontin, vitronectin and others (Giancotti and Ruoslahti, 1999). An attachment mechanism involving the integrins might require a bifunctional bridging ligand to span between the receptors on the embryonic and uterine cell surfaces (Singh and Aplin, 2009). Many integrins have been proposed to have important roles during implantation, including αvβ3, α9β1, αvβ1, α1β1, α3β1, α6β1, αvβ5, and αvβ6 (Aplin, 1997). Among these integrins, the integrin β3 subunit is most broadly studied in both humans and mice. In the human endometrium, the integrin β3 subunit is highly expressed in the luminal and glandular epithelium during the mid-secretory phase, suggesting its potential function in endometrial receptivity (Lessey et al., 1995; Liu et al., 2012). Indeed, it has been demonstrated that the aberrant expression of integrin αvβ3 is associated with infertility and recurrent pregnancy loss (Lessey et al., 1995; Liu et al., 2012). In mice, αvβ3 is expressed in the uterine luminal epithelium and the blastocyst during implantation, and injection of RGD peptides or neutralizing antibody against the αv or β3 subunit into the mouse uterine cavity reduces the rate of implantation compared to injection of BSA or non-RGD peptides, suggesting its participation in mediating the trophectoderm-luminal epithelium interaction (Aplin et al., 1996; Illera et al., 2000; Sutherland et al., 1993). There is evidence that integrin signaling modulates trophoblast adhesion to extracellular matrices via activation of phospholipase C-γ to initiate phosphoinositide signaling and intracellular calcium mobilization during blastocyst implantation (Wang et al., 2002, 2007b). “Outside-in” integrin signaling could select appropriate “inside-out” integrin mobilization depending on the extracellular matrix composition (Armant, 2005). Recent evidence shows that integrin β3 expression in the trophectoderm can be repressed by microRNA lethal-7a (let-7a) during blastocyst dormancy with reversal during reactivation for implantation (Liu et al., 2012).
Trophinin and tastin form a cell adhesion complex through an intermediary protein bystin that directly binds trophinin and tastin (Suzuki et al., 1998). Previous studies have demonstrated that the trophinin-bystin-tastin complex mediates a unique homophilic adhesion mechanism between trophoblasts and endometrial epithelial cells at their respective apical cell membranes (Fukuda et al., 1995; Suzuki et al., 1998). Trophinin, an intrinsic membrane protein, is expressed in trophectoderm cells of embryos and in uterine epithelial cells in mice during the periimplantation period (Fukuda et al., 1995; Suzuki et al., 2000). In humans, trophinin expression is restricted to the apical plasma membranes of the endometrial epithelium at the early secretory phase (Sugihara et al., 2007). In a non-human primate model, trophinin is strongly expressed in the trophectoderm of the rhesus macaque blastocyst (Fukuda et al., 1995). Blastocyst-secreted human chorionic gonadotropin (hCG) induces endometrial expression of trophinin, indicative of the spatiotemporal mechanisms that govern embryo-uterine crosstalk for their synchronous development during implantation (Nakayama et al., 2003; Sugihara et al., 2008). Bystin is expressed in the luminal and glandular epithelium in the mouse uterus during the periimplantation period and is detected in hatching blastocysts (Fukuda et al., 2008; Fukuda and Nozawa, 1999). However, its expression apparently disappears from the blastocyst during implantation, suggesting that its absence is permissive for blastocyst activation (Aoki et al., 2006). Indeed, dissociation of trophinin from bystin by the trophinin-binding peptide, GWRQ, which mimics trophinin homophilic binding, permits activation of ErbB4 to induce trophectoderm activation in human embryo implantation (Sugihara et al., 2007). At the same time, homophilic ligation of trophinin induces tyrosine phosphorylation of PKC-δ and its nuclear translocation in uterine epithelial cells, leading to their apoptosis that is conducive for trophoblast invasion (Armant, 2011). These findings collectively suggest that trophinin, through its dynamic ligation and dissociation from interacting proteins bystin and tastin, potentially mediates the attachment of the blastocyst to uterine epithelial cells at the time of implantation and initiates key physiological changes in both maternal and embryonic cells.
L-selectin, a carbohydrate-binding protein, has been proposed as part of a system that mediates the initial adhesion of human blastocysts to the uterine epithelium (Genbacev et al., 2003; Wang et al., 2008). L-selectin was previously thought to be expressed only in hematopoietic cells due to its essential role in adhesion between lymphocytes and high endothelial venules (Berg et al., 1993). Intriguingly, human trophoblasts also express functional L-selectin, while its ligand oligosaccharides are detected mainly in pinopodes, the apical cellular protrusions of the endometrial epithelium where blastocyst adhesion initiates (Genbacev et al., 2003; Nejatbakhsh et al., 2012). These findings indicate a potential interaction between L-selectin in human blastocysts and oligosaccharide ligands on the endometrial epithelium as an initial step in human implantation. Blocking L-selectin with specific antibodies leads to impaired adhesion of trophoblasts to the endometrial epithelium (Genbacev et al., 2003). Moreover, endometrial expression of L-selectin ligands is significantly different between fertile and infertile women in natural cycles (Foulk et al., 2007; Margarit et al., 2009), and impaired expression of L-selectin ligands reduces the chance of successful implantation (Genbacev et al., 2003; Shamonki et al., 2006). High expression of L-selectin ligands in the secretory endometrium is likely associated with robust endometrial receptivity for embryo implantation in humans (Shamonki et al., 2006; Wang et al., 2008). However, gene knockout mice deficient in fucosyltransferases responsible for the synthesis of fucosylated oligosaccharides ligand of L-selectin are fertile, suggesting that fucosylated carbohydrates, including L-selectin, are dispensable for implantation in mice, although they are expressed in the uterine epithelial cells (Domino et al., 2001).
E-cadherin, a Ca2+-dependent transmembrane adhesion molecule, mediates intercellular adhesion and dynamic changes to the cytoskeleton through its interaction with cytoplasmic catenins (Goodrich and Strutt, 2011). E-cadherin is a critical factor for blastocyst formation, since embryos lacking the E-cadherin gene fail to establish adhesion junctions in the trophectoderm and die in the periimplantation period (De Vries et al., 2004; Larue et al., 1994). On the maternal side, E-cadherin is highly expressed in the luminal epithelium prior to implantation, but is transiently downregulated before blastocyst invasion into the stroma, suggesting that remodeling the adhesion junctions between epithelial cells is a critical event during embryo implantation (Paria et al., 1999c; Thie et al., 1996; Thie et al., 1995; Thie et al., 1998). There is evidence that loosening of cell-cell junctions in the mouse uterine epithelium through downregulation of E-cadherin is a prerequisite for blastocyst attachment (Li et al., 2002; Thie et al., 1996). Indeed, E-cadherin is persistently expressed in the luminal epithelium of uterine-specific Msx1/Msx2 ablated mice which show implantation failure (Daikoku et al., 2011; Nallasamy et al., 2012). Similarly, a recent observation shows that the expression of E-cadherin is remarkably downregulated in endometrial epithelial cells of the mid-secretory endometrium in women with endometriosis (Matsuzaki et al., 2010). Uterine-specific deletion of E-cadherin results in female infertility due to defective implantation and decidualization. The deficient mice lose adhesion junctions and tight junctions in the uterine epithelium, creating a disorganized cellular structure that is incapable of supporting embryo attachment and invasion (Reardon et al., 2012). Collectively, these findings indicate that E-cadherin plays critical roles during embryonic and uterine preparation for implantation.
5.3 The epithelial-stromal interaction
Uterine tissue consists of three major layers: an outer muscle layer, the inner luminal epithelium and the stromal bed in between (Wang and Dey, 2006). Synchronization of estrogen and progesterone directs the uterus into the receptive state, accompanied by morphological and functional changes in the epithelium and the stroma. Increasing attention has been paid to understand how these two hormones execute their differential effects on the two major endometrial cell types, and the molecular basis of stromal-epithelial interactions essential for uterine receptivity (Cooke et al., 1986; Cunha et al., 2004;Kurita et al., 2000b). The synergistic and antagonistic interactions of ovarian progesterone and estrogen on uterine cell proliferation and differentiation are portrayed in Figure 4.
Figure 4.
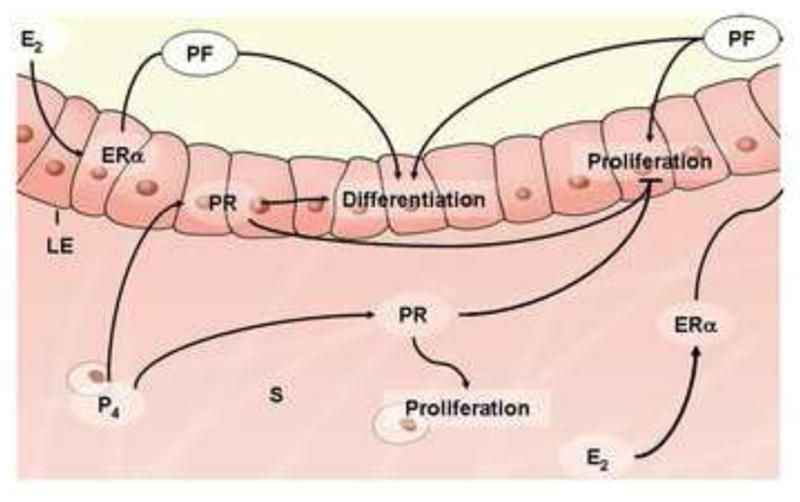
Synergistic and antagonistic interactions of ovarian progesterone and estrogen on uterine cell proliferation and differentiation. Estrogen-stimulated uterine epithelium proliferation requires the presence of functional ER in the stroma through a paracrine/autocrine manner. Moreover, estrogen-induced differentiation of the uterine epithelium requires ER in both the epithelium and the stroma. Progesterone acts through stromal and epithelial PRs to inhibit the proliferative response of epithelium to estrogen, while inducing the proliferation of the underlying stroma. E2, 17β-estradiol; ERα, nuclear estrogen receptor-α; LE, luminal epithelium; P4, progesterone; PF, paracrine factor; PR, progesterone receptor; S, stroma.
5.3.1 Uterine epithelial responsiveness to estrogen signaling
ER is expressed in both epithelial and stromal cells of adult uteri, and it was initially assumed that estrogen exerts its function directly through ER in the corresponding compartments (Cooke et al., 1998). The crucial finding that estrogen stimulates the proliferation of neonatal mouse uterine epithelium, which does not express ER, indicates that estrogen might stimulate uterine epithelial mitogenesis indirectly (Cooke et al., 1998). Employing stroma-epithelium separation/recombination systems (Cunha, 2008) using uteri from adult ERα-deficient mice and neonatal ER intact wild-type mice, a previous study finds that estrogen-induced epithelial proliferation is a paracrine event mediated by stromal ER, not epithelial ER (Cooke et al., 1997). The tissue-specific knockout technique provides an excellent approach to systematically study the uterine responsiveness to estrogen. Selective deletion of ERα in the uterine epithelium (UtEpiaERKO) using Wnt7a-Cre and ER-loxp mouse models demonstrates that stromal ERα is responsible for estrogen-induced epithelial proliferation (Winuthayanon et al., 2010). However, an immediately arising question is how estrogen acting through stromal ERα induces epithelial proliferation.
Paracrine actions of polypeptide growth factors, such as insulin-like growth factor (IGF) 1, EGF or TGFα, are believed to be integral components of uterine responses to estrogens. IGF1, a key growth factor induced and activated by estrogen in the uterine stroma, is necessary for estrogen-induced uterine epithelial DNA synthesis through IGF1 receptor signaling in the luminal epithelium (Chen et al., 2005; Kapur et al., 1992; Kurita et al., 2005; Zhu and Pollard, 2007). A previous study has shown that estrogen-stimulated proliferation of uterine epithelial cells is compromised in Igf1 knockout mice, suggesting the role of IGF1 in mediating estrogen action in the endometrium (Adesanya et al., 1999; Sato et al., 2002). These studies collectively support a paracrine mechanism of estrogen-mediated epithelial proliferation that solely requires functional ERα in the underlying stroma. After estrogen treatment, PR is dramatically downregulated in the epithelium and increased in the stroma in wild-type and UtEpiaERKO mice, whereas the ER antagonist ICI 182,780 (ICI) inhibits this effect in both genotypes (Kurita et al., 2001; Winuthayanon et al., 2010), suggesting that stromal ERα is also required for estrogen-induced down-regulation of uterine epithelial PR. These findings indicate that estrogen acts on stromal ERα to stimulate the proliferation of uterine epithelium through production of paracrine factors, such as IGF1.
Although uterine epithelial ERα is dispensable for estrogen-induced epithelial proliferation, it is essential for complete biological and biochemical responses, since selective deletion of uterine epithelial ERα results in compromised uterine weight increase in response to estrogen and epithelial apoptosis after initial proliferation (Winuthayanon et al., 2010). Differentiation of the uterine epithelium, as indicated by secretory products, such as lactoferrin (LF) and Mucin-1, requires functional ERα in both the stroma and the epithelium, and may be a direct effect via ERα plus a paracrine/autocrine effect via synthesis of secreted factors (Buchanan et al., 1999; Kurita et al., 2000a; Kurita et al., 2000b). These observations suggest that differentiation of the uterine epithelium requires functional ER in both the epithelium and the stroma.
5.3.2 Stromal responsiveness to progesterone signaling
PR-null uteri exhibit a phenotype similar to ovariectomized mice exposed to prolonged estrogen treatment, which is ascribed to an essential modulatory action of PR in the uterus (Lydon et al., 1995). Recombination experiments using uterine tissues from PR-null and wild-type mice demonstrate that stromal PR is required to attenuate estrogen’s proliferative effect on the endometrial epithelium (Kurita et al., 1998). In recent years, numerous genes have been identified that mediate progesterone activity via PR. Immunophilin FK506 binding protein-4 (FKbp52), a co-chaperone required for appropriate uterine PR function (Daikoku et al., 2005), has overlapping expression with PR in uterine stroma (Tranguch et al., 2005a). Fkbp52-/- mice exhibit implantation failure with an exaggerated estrogenic influence in the epithelium (Tranguch et al., 2005a; Yang et al., 2006). At the histological and cellular level, Fkbp52-/- uteri display aberrant epithelial proliferation and lower stromal proliferation on day 4 of pregnancy, pointing towards a phenomenon of progesterone resistance (Tranguch et al., 2005a; Yang et al., 2006). Moreover, ER activity is mostly unaffected and the implantation defect can be rescued by treatment with high dose of progesterone in Fkbp52 null females (Tranguch et al., 2007).
Chicken ovalbumin upstream promoter transcription factor II (Coup-TFII, also known as NR2F2), a member of the nuclear receptor superfamily, is highly expressed in the uterine stroma (Takamoto et al., 2005), and its expression is controlled by progesterone-IHH-PTCH1 signaling from the epithelium to the stroma (Franco et al., 2011b; Kurihara et al., 2007). In addition, uterine conditional knockout of the Coup-TFII gene results in implantation and decidualization failure and enhanced epithelial estrogen receptor activity (Kurihara et al., 2007; Lee et al., 2010; Simon et al., 2009). These findings suggest that stromal Coup-TFII is an essential PR mediator during implantation and decidualization.
The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2 (Hand2), is disclosed by microarray gene profiling analysis of progesterone-responsive transcription in mouse uteri (Bagchi et al., 2005; Li et al., 2011). Progesterone induces the expression of Hand2 in the uterine stroma (Li et al., 2011). Selective ablation of Hand2 in uterine cells induces sustained fibroblast growth factor (FGF) expression and its activities in stimulating preimplantation estrogen-induced epithelial proliferation, leading to implantation failure in mice (Li et al., 2011). This finding indicates that Hand2 is a critical regulator of uterine stromal-epithelial communication initiated by steroid signaling that fosters uterine receptivity for implantation.
Despite the well-established concept that stromal PR mediates progesterone activity to counter the proliferative response of the epithelium to estrogen, specific roles of epithelial PR in uterine biology have been largely ignored. A most recent study using Wnt7a-Cre/PRloxp mouse models for uterine epithelial PR ablation demonstrates that epithelial PR is essential for uterine epithelial-stromal crosstalk via directly regulating the transcription of progesterone target genes in the epithelium, such as Ihh (Franco et al., 2011b). Furthermore, loss of epithelial PR results in a complete pregnancy failure due to impaired decidualization and uncontrolled estrogen-induced epithelial cell proliferation (Franco et al., 2011b), highlighting that the epithelium is essential for normal stromal-decidual transformation. This finding clearly demonstrates that epithelial PR is an essential regulator of the stromal-epithelial interaction. In conclusion, progesterone acts through both epithelial and stromal PRs to antagonize the proliferative response of the epithelium to estrogen, while it induces the stromal proliferation.
5.4 Uterine glands for embryo implantation
The initial development of the female reproductive tract occurs during embryogenesis soon after gastrulation (Kobayashi and Behringer, 2003; Orvis and Behringer, 2007; Spencer et al., 2012). In laboratory rodents, domestic animals and humans, the uterus is not fully developed or differentiated at birth; thus, postnatal uterine morphogenesis is a critical event for normal uterine functions in adult life (Gray et al., 2001a). Uterine glands are thought to be the principal source of uterine secretions that are required for blastocyst survival and implantation, as well as the establishment and maintenance of pregnancy (Bazer, 1975). Using uterine gland knockout ewe model, it has been demonstrated that uterine gland and its secretion during early pregnancy are essential for conceptus survival (Gray et al., 2001a; Gray et al., 2001b; Gray et al., 2001c). Molecular and genetic studies have demonstrated that growth factors, cytokine factors and potential histotrophic factors produced by uterine glands are able to regulate the endometrial function.
5.4.1 Uterine adenogenesis
Coordinated development of the endometrial glands from the luminal epithelium, known as uterine adenogenesis, is a predominant event during postnatal uterine morphogenesis. The process of adenogenesis involves three typical steps, budding, penetration and branching. The glandular epithelium derived from the luminal epithelium differentiates and buds, followed by the penetration of the glandular tubes into the uterine stroma, and then the glandular elements keep branching and coiling to expand throughout the endometrial stroma (Bartol et al., 1999; Bartol et al., 1993). In mice, epithelial invaginations or gland buds appear at postnatal day (PND) 5, nascent endometrial glands are present at PND 7, and complex glands extending from the luminal epithelium into the endometrial stroma are established by PND 12 (Branham et al., 1985; Brody and Cunha, 1989). In contrast, endometrial gland development in humans begins during fetal development within the womb, continues from birth to age 6, and is fully developed by puberty (Koff, 1933).
The process of uterine adenogenesis involves intrinsic epithelial-stromal interactions and is precisely orchestrated by multifactorial gene networks (Baker et al., 1996; Miller and Sassoon, 1998; Reardon et al., 2012). Exposure of mice to native estrogen, estrogen analogs or progesterone during critical developmental periods inhibits endometrial adenogenesis by disrupting the normal expression of morphoregulatory genes (Cooke et al., 2012; Filant et al., 2012; Hayashi et al., 2011; Miller et al., 1998; Stewart et al., 2011; Talbi et al., 2006). Ablation of these key genes in mice reduces or even eliminates uterine glands (Table 1). Interestingly, abnormalities of uterine glands are often accompanied by defects in the uterine stroma and luminal epithelium, leading to implantation failure. For example, uterine conditional depletion of Dicer, an RNase III endonuclease that is essential for the biogenesis of microRNAs and small interfering RNAs, leads to female sterility and underdeveloped small uteri (Hawkins et al., 2012). The most prominent features of Dicer-deficient uteri are a complete lack of uterine glands and enhanced stromal apoptosis with failure to proliferate in response to progesterone (Hawkins et al., 2012). Females with conditional knockout of prohibitin 2 (also known as repressor of estrogen receptor activity, Rea) in the uterus show implantation and decidualization failure due to altered endometrial gland morphogenesis (Park et al., 2012b). In addition, Wnt5a plays a crucial role in the generation of uterine glands and is required for cellular and molecular responses to exogenous estrogen (Mericskay et al., 2004). During postnatal uterine development, Wnt5a participates in a regulatory loop with other uterine patterning genes, including Wnt7a, Hoxa10 and Hoxa11 (Mericskay et al., 2004). Mice with uterine-specific ablation of Wnt4, Wnt7a or E-cadherin show defective implantation associated with a reduction or absence of uterine glands (Dunlap et al., 2011;Franco et al., 2011a; Hayashi et al., 2011).
Table 1.
Genes required for uterine adenogenesis
| Gene | Gene encoded | Knockout phenotype in females | Ref. |
|---|---|---|---|
| Ctnnb1 | β-catenin | Few uterine glands | (Jeong et al., 2009) |
| Cdh1 | E-Cadherin | A disorganized cellular structure of the epithelium and ablation of endometrial glands | (Reardon et al., 2012) |
| Dicer | Dicer | No glands | (Hawkins et al., 2012) |
| Foxa2 | Forkhead box A2 | Severe reduction in the number of endometrial glands | (Jeong et al., 2010) |
| Hoxa11 | Homeobox A11 (TF) | Decreased number of endometrial glands and abnormal glandular differentiation | (Gendron et al., 1997) |
| Igf1 | Insulin-like growth factor 1 (IGF1) | Reduction in abundance and complexity of the secretory glandular elements | (Baker et al., 1996) |
| REA | Repressor of estrogen receptor activity | Severely compromised gland morphogenesis | (Park et al., 2012b) |
| Wnt4 | Wingless-related MMTV integration site 4 | Decreased number of uterine glands | (Franco et al., 2011a) |
| Wnt5a | Wingless-related MMTV integration site 5a | Uterine gland formation failure due to lack of stromal signals | (Mericskay et al., 2004) |
| Wnt7a | Wingless-related MMTV integration site 7a | No uterine glands due to lack of epithelial signals | (Dunlap et al., 2011; Miller and Sassoon, 1998) |
5.4.2 Glandular- luminal epithelial interaction
Of the products secreted by uterine glands, LIF is one of the most important molecules. It has been well documented that membrane-associated LIFR and gp130 are required for LIF signaling (Layton et al., 1994; Narazaki et al., 1994). In mice, the expression of LIFR is restricted to the luminal epithelium during the preimplantation period and remains highly intense on day 5 when implantation occurs (Cheng et al., 2001; Ni et al., 2002; Song and Lim, 2006). In contrast, gp130 is expressed in the gland and the stroma on day 4, however, its expression is induced in the luminal epithelium, similar to LIFR, on day 5 (Bhatt et al., 1991; Song and Lim, 2006). This overlapping expression pattern of LIFR and gp130 in the luminal epithelium at the attachment site provides robust evidence for the paracrine nature of glandular LIF acting on uterine epithelial cells (Cheng et al., 2001; Stewart et al., 1992). Failure of gp130 activation in the luminal epithelium in Lif-/- mice reinforces the notion that LIF-driven glandular-epithelial interaction is essential for normal implantation. Moreover, glandular LIF is essential for the downregulation of Msx1 expression in the uterine luminal epithelium, since the expression of Msx1 persists in the epithelium in Lif null mice during the periimplantation period (Daikoku et al., 2004). Reciprocally, the expression of Lif in the glandular epithelium is also downregulated with loss of uterine Msx1 gene (Daikoku et al., 2011), suggesting that there is a regulatory loop between Lif and Msx1 genes. However, implantation failure resulting from Msx1 deletion cannot be rescued by administration of LIF, suggesting that this mutual regulation is not mediated by the same mechanism (Daikoku et al., 2011). The remarkable positive-negative feedback loop between Msx1 and LIF reflects, to some extent, the complexity of glandular-epithelial interactions during implantation.
5.4.3 Glandular-stromal interaction
Since uterine glands are embedded within the stromal bed, it is conceivable that glandular-derived signaling can influence stromal function in a paracrine manner, and vice versa. Forkhead box A2 (Foxa2) is expressed specifically in the uterine glandular epithelium (Besnard et al., 2004). Uterine-specific ablation of this gene in mice results in defective uterine gland formation and dysfunctional uterine stroma unable to support embryo invasion and decidualization (Bazer, 2010; Jeong et al., 2010). Notably, LIF expression is significantly decreased in Foxa2 deficient uteri, whereas administration of recombinant LIF can partially restore decidualization in null females (Bazer, 2010; Jeong et al., 2010), suggesting that Foxa2 may be required for normal glandular LIF expression. Mice systemically lacking Wnt7a have a stratified luminal epithelium surrounded by a shallow stroma layer devoid of glands, in contrast to the simple columnar structure in the wild-type (Miller and Sassoon, 1998). The conditional uterine ablation of Wnt7a after birth disrupts endometrial gland development, resulting in a failure of blastocyst implantation (Dunlap et al., 2011). Both Foxa2 and LIF are severely reduced in the Wnt7a-/- uterus during the periimplantation period (Dunlap et al., 2011). These findings collectively highlight the necessity of endometrial gland-derived signals for normal stromal function during implantation.
The tumor suppressor p53 is crucial for embryonic implantation and decidual development (Hirota et al., 2010; Hu et al., 2007). Loss of p53 decreases uterine LIF levels, whereas supplementation of exogenous LIF restores normal implantation in p53-/- females (Hu et al., 2007). Experiments using human p53 knock-in mice carrying a single nucleotide polymorphism (SNP) at codon 72 (arginine/proline) reveal that mice with the arginine allele exhibit higher uterine LIF levels during implantation than those with the proline allele (Feng et al., 2011), indicating that p53 can regulate the transcription of Lif. However, it is worth noting that p53 is primarily expressed in uterine stromal cells during the periimplantation period (Yue et al., 2005), whereas Lif is initially expressed in the uterine glandular epithelium (Bhatt et al., 1991; Song et al., 2000; Stewart et al., 1992). These studies hint that signals from the stroma can also influence the physiological function of uterine glands during implantation.
6. Molecular basis of decidualization
In response to implantation, stromal cells surrounding the implanting embryo undergo extensive proliferation and subsequent differentiation into polyploidy decidual cells (Lim and Wang, 2010; Ramathal et al., 2010). The differentiating stromal cells initially form an avascular primary decidual zone (PDZ) encasing the fetus in the afternoon of day 5 (Paria et al., 1999c;Wang et al., 2004d). Stromal cells next to the PDZ continue to proliferate and then differentiate to from a well-vascularized secondary decidual zone (SDZ) (Figure 1B). It is believed that a fully developed decidua is important for the provision of nutrition to the developing embryo and to provide a barrier that prevents uncontrolled trophoblast invasion and protects the embryo from the maternal rejection prior to the formation of functional placenta (Peng et al., 2008; Rogers et al., 1983;Wang et al., 2004d; Welsh and Enders, 1987).
6.1 Steroid hormones: central players in decidualization
Ovarian progesterone signaling through PRA is essential for postimplantation uterine development and decidualization (Conneely et al., 2002). However, the potential role of PRB during decidualization cannot be ruled out since mice lacking both PRB and p21, a cyclin dependent kinase (CDK) inhibitor, are infertile with decidualization defects (Das, 2009). In addition to FKBP52, transcriptional coregulators are also crucial in mediating PR activity, such as the steroid receptor coactivator (SRC)-1, SRC-2 and SRC-3 (McKenna and O’Malley, 2002; Spencer et al., 1997). These cofactors have certain overlapping and distinct physiological functions in the female reproductive system. For example, mice with a null mutation of either SRC-1 or SRC-2 show progesterone resistance and comprised decidualization, whereas mice lacking both SRC-1 and SRC-2 are infertile with a complete blockage of decidualization (Jeong et al., 2007; Mukherjee et al., 2006a; Mukherjee et al., 2006b; Xu and Li, 2003; Xu et al., 1998). In contrast, SRC-3 knockout mice display delay puberty, but have normal decidualization (Xu et al., 2000b). Interestingly, SRC-2 also acts as a coactivator of PPARδ, a nuclear hormone receptor activated during stromal differentiation by COX-2-derived prostacyclin during implantation and decidualization (Lim et al., 1999a; Lim et al., 2004).
Since progesterone alone is adequate to maintain the decidual response in experimentally induced decidualization after ovariectomy (Paria et al., 1999b), it was thought that estrogen is dispensable in this process. Conversely, decidualization is compromised by administration of ICI after embryo attachment, suggesting a contribution of an intrauterine source of estrogen in the decidua (Curtis et al., 1999). Moreover, later studies show that blocking P450 aromatase, a key enzyme that converts androgen to estrogen and is expressed in decidual stromal cells (Simpson et al., 1994), inhibits the process of decidualization, suggesting that de novo synthesized estrogen in the uterus is essential for normal decidualization (Das et al., 2009). As a downstream target of estrogen, Fos-related antigen 1 (FRA-1), a member of the Fos family of transcription factors, has recently been shown to be a critical estrogen mediator during decidualization (Das et al., 2012). FRA-1 is expressed in the differentiating uterine stroma surrounding the implanted embryo and this expression is significantly repressed in response to letrozole. Loss of FRA-1 inhibits stromal cell differentiation and migration (Das et al., 2012).
6.2 Epithelial signals controlling stromal decidualization
By employing rodent models with experimentally ablated uterine epithelium, evidence has been produced that the uterine luminal epithelium transmits signals required for the induction of decidualization (Lejeune et al., 1981). After blastocyst attachment, the luminal epithelial cells surrounding the invading blastocyst undergo apoptosis (Parr et al., 1987; Schlafke et al., 1985; Welsh and Enders, 1991) to facilitate embryo invasion and access to maternal blood supply (Pampfer and Donnay, 1999; Parr et al., 1987). The apoptotic degeneration of the uterine epithelium involves embryo-derived signaling molecules, such as transforming growth factor β (Kamijo et al., 1998; Mahesh et al., 1996) and trophinin (Tamura et al., 2011), and is mediated by caspase 3 (Joswig et al., 2003; Zhang and Paria, 2006). Recent studies have demonstrated that KLF5, a zinc finger-containing transcription factor is primarily expressed in the luminal epithelium throughout the periimplantation stage (Ema et al., 2008; Sun et al., 2012). Conditional ablation of Klf5 leads to implantation failure, suggesting that KLF5 contributes to the establishment of uterine receptivity. In addition, Klf5-null mice show decidualization defects, primarily due to a failure of the trophoblast cells to penetrate the luminal epithelium (Sun et al., 2012). Conversely, premature death of endometrial epithelium from aberrantly upregulated expression of CFTR in response to high levels of estrogen contributes to impaired embryo implantation (Yang et al., 2011). These findings highlight the necessity of appropriately differentiated luminal epithelium for inducing the decidual response.
With the advance of uterine specific gene depletion models, a number of key regulators have been identified involving in epithelial-stromal interactions that initiate decidualization (Lim and Wang, 2010; Ramathal et al., 2010; Wetendorf and DeMayo, 2012; Yang et al., 2011) (Figure 5). Ihh is expressed in the uterine epithelium under the control of progesterone (Lee et al., 2006; Matsumoto et al., 2002). In addition to its essential role in uterine receptivity, Ihh-null mice also exhibit decidualization failure in response to an artificial stimulus (Lee et al., 2006). Since both the IHH receptor Ptch1 and the downstream effectors, Gli1–3 and COUP-TFII, are expressed in the uterine stroma, it is presumed that IHH signals from the luminal epithelium to the stroma through Ptch1 and activates downstream transcription factors. Indeed, ablation of COUP-TFII also results in severe decidual defects, suggesting that this cascade is required for decidualization (Kurihara et al., 2007; Lee et al., 2006). Interestingly, the decidual defects in these mice can be partially rescued by intraluminal supplementation of recombinant BMP2, suggesting that BMP2 also participates in IHH signaling and operates as a downstream effector of COUP-TFII in decidualization (Kurihara et al., 2007). Collectively, these studies indicate that the progesterone-induced IHH/Ptch1/COUP-TFII/BMP2 pathway mediates the epithelial-stromal crosstalk that is critical for implantation and decidualization.
Figure 5.
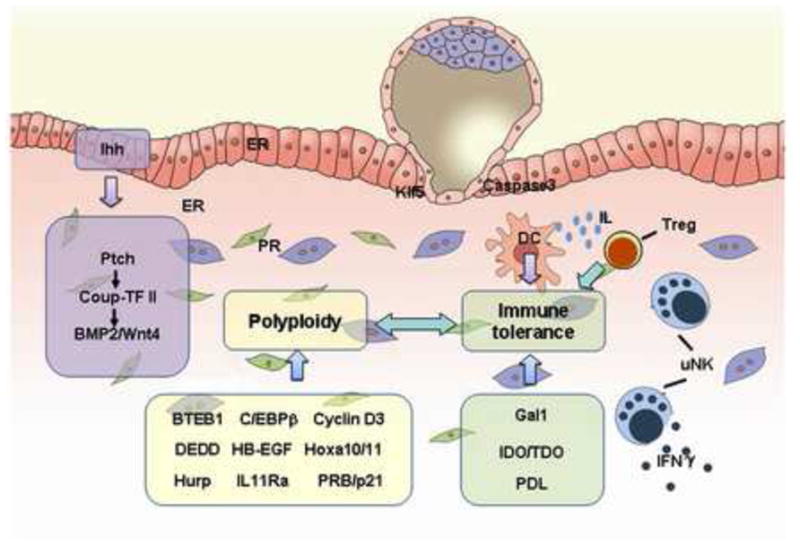
Factors governing decidualization and immune tolerance after embryo invasion. Decidualization involves coordination of several processes, including luminal epithelium apoptosis at the site of embryo invasion, epithelial-stromal dialogue initiating decidualization and decidual cell polyploidization. Many molecules, such as cytokine receptors, morphogens, hormones and transcription factors, regulate this process. The primary role of decidua is to protect the genetically ‘foreign’ semi-allogeneic embryo from attack by the maternal immune system. Several immune-related molecules and cells that contribute to decidual development and immune tolerance are depicted in this cartoon and detailed in the text. BMP2, bone morphogenetic protein 2; BTEB1, basic transcription element-binding protein1; COUP-TFII, chicken ovalbumin upstream promoter transcription factor-2; DC: dendritic cell; DEDD, death effector domain-containing protein; ER, estrogen receptor; HB-EGF, heparin-binding EGF-like growth factor; Hoxa10, homeobox A10; Gal1, galectin-1; Hurp, hepatoma upregulated protein; IDO, indoleamine 2,3-dioxygenase 1; IFNγ, interferon γ; Ihh, Indian hedgehog; IL11Ra, interleukin-11 receptor alpha; Klf5, Kruppel-like factor 5; uNK, uterine nature killer cell; PR, progesterone receptor; pRB, retinoblastoma protein; p21, cyclin-dependent kinase inhibitor 1A; Ptch, patched homolog 1; TDO, tryptophan 2,3-dioxygenase ; Wnt4, wingless-related MMTV integration site 4.
6.3 Cell-cycle regulators during decidualization
Polyploidization is a hallmark of decidualization that utilizes a specialized cell cycle progression (Das, 2009; Dey et al., 2004). It is speculated that polyploidy limits the life span of decidual cells, but ensures that these cells meet the demand for postimplantation embryonic growth (Ansell et al., 1974; Davoli and de Lange, 2011; Hemberger, 2008; Sachs and Shelesnyak, 1955). Cyclin D3 is a key cell cycle regulator in stromal cell proliferation, differentiation and polyploidization (Das, 2009; Das et al., 1999). Its deficiency in mice leads to defective decidualization (Das, 2009; Das et al., 1999). Moreover, previous study has identified several other regulators crucial for decidualization executing their function through cyclin D3. For instance, female deficiency of Hoxa10, which is highly expressed in developing decidua, exhibits impaired decidualization with aberrant regulation of cyclin D3 and loss of region-specific expression of CDK4 and CDK6 in the decidua bed (Rahman et al., 2006). Female deficiency of basic transcription element-binding protein 1 (BTEB1), a potential upstream regulator of Hoxa10, also exhibits decidualization defects with dysregulation of both Hoxa10 and cyclin D3 expression in the uterus (Pabona et al., 2012; Simmen et al., 2004). Interleukine-11 receptor α (IL-11Rα) is the α-chain of the receptor for IL-11. A null mutant of this gene in mice results in decidual degeneration with derailed endoreplication due to a reduced cyclin D3 expression (Bilinski et al., 1998; Li et al., 2008; Menkhorst et al., 2009; Robb et al., 1998). In addition, p21 and survivin are downstream targets of IL-11-stimulated decidualization (Li et al., 2008). Observation of decidual defects in mice lacking both p21 and PRB indicates a critical role of p21 in decidualization (Li et al., 2008). A recent study has demonstrated that the death effector domain-containing protein (DEDD), which can stabilize cyclin D3, is essential for normal uterine decidualization. Its deficiency leads to impaired decidual development accompanied by attenuated polyploidy (Arai et al., 2007; Mori et al., 2011). HB-EGF also facilitates stromal cell polyploidy by upregulating cyclin D3 expression (Das, 2009; Tan et al., 2004). All these findings point towards the central role of cyclin D3 during decidual cells proliferation and polyploidization.
In addition, many cell-cycle associated genes, such as hepatoma upregulated protein (Hurp) and CCAAT/enhancer binding protein β (C/EBPβ), have also been proved to be essential for decidual development (Tsou et al., 2003). Hurp is highly expressed in G2/M phase of the cell cycle and participates in regulating spindle formation and chromosome congression during mitosis (Bassal et al., 2001). Female mice lacking Hurp are completely infertile due to a failed decidual reaction caused by a defect in stromal proliferation that leads to implantation failure (Tsai et al., 2008). C/EBPβ is rapidly induced in the stromal cells surrounding the implanted blastocyst and the expression is highly increased during decidualization. Ablation of C/EBPβ gene in female mice results in infertility with a complete lack of decidual formation (Bagchi et al., 2006; Ramathal et al., 2011; Wang et al., 2010a). Further studies show that C/EBPβ functions during decidualization through modulating the expression of multiple key cell cycle regulatory factors that control G2 to M transition of the proliferating stromal cells (Bagchi et al., 2006; Ramathal et al., 2011; Wang et al., 2010a) (Figure 5).
6.4 Molecular and cellular aspects of immune tolerance during decidualization
Decidua is a privileged site for coordinating the process of immune tolerance to protect the genetically ‘foreign’ semi-allogeneic embryo from attack by the maternal immune system. Indeed, the avascular PDZ, composed of several layers of semi-epithelialized stromal cells, acts as a physical barrier to restrict the access of immunologically competent maternal cells and/or their secretions (Tung et al., 1986; Wang et al., 2004d). A large number of molecules and immune cell types participate in immune surveillance and tolerance during pregnancy (Dosiou and Giudice, 2005; Moffett and Loke, 2006; Taglauer et al., 2010; Trowsdale and Betz, 2006; Yoshinaga, 2012) (Figure 5).
6.4.1 Genes regulating immune tolerance
The maternal circulating tryptophan concentration falls with the progression of pregnancy and the establishment of maternal tolerance, and this tryptophan catabolism can suppress the T cell activity and defend the conceptus against maternal rejection (Fuchs et al., 1996; Kamimura et al., 1991; Munn et al., 1996; Munn et al., 1998). The first clear example of allospecific abortion during early pregnancy comes from the study on the effect of 1-methyl-tryptophan, a pharmacologic agent that inhibits the activity of indoleamine 2,3-dioxygenase (IDO) enzyme, which catabolizes tryptophan, on murine pregnancy (Cady and Sono, 1991; Munn et al., 1998). A systemic administration of 1-methyl-tryptophan that causes continuously high tryptophan concentration induces fetal deterioration by embryonic day 9.5 in female mice mated with allogeneic males but not with syngeneic males (Munn et al., 1998). IDO is expressed in dendritic cells, macrophages and trophoblasts in mice and humans (Munn et al., 1998; Saito et al., 2007), and its activity is also detectable in the decidua (Jeddi-Tehrani et al., 2009; Kudo et al., 2004). A high tryptophan-degrading activity is present in the early conceptus on days 7 and 8 of pregnancy in mice, whereas IDO is not expressed during this early gestational stage, but appeared 2–3 days later concomitant with the formation of the placenta (Suzuki et al., 2001). Tryptophan 2,3-dioxygenase (TDO), another key catabolizing enzyme of tryptophan, contributes to this early tryptophan-degrading activity (Suzuki et al., 2001). Expression of TDO is induced in the decidualizing stroma surrounding the implanted embryo. With the progression of pregnancy, its expression is further upregulated and shifts to the mesometrial decidua, a site where future placenta will form (Suzuki et al., 2001; Tatsumi et al., 2000).These findings demonstrate that TDO and IDO present in the endometrium and trophoblasts concomitant with decidualization is necessary to prevent immunological rejection of fetal allografts during pregnancy recognition and maintenance. Moreover, 1-methyl-tryptophan- induced abortion occurs when the T and B cell-deficient (Rag-I-/-) mother carries CD8+ T cells specific for a fetal major histocompatibility complex (MHC) antigen inherited from the father, whereas does not occur when the mother carries syngeneic fetuses, suggesting that this fetal allograft rejection triggered by 1-methyl-tryptophan is T cell-dependent and antibody-independent activation of complement deposition (Mellor et al., 2001). Interestingly, IDO-deficient females produce litters of normal sizes at normal rates, and resistance to 1-methyl-tryptophan injection (Baban et al., 2004), implying that some other molecules, such as uterine TDO, elicit the compensatory function to protect the allogeneic fetuses. Alternatively, this phenomenon can be argued that abortion induced by IDO-catabolized metabolic products from 1-methyl-tryptophan is allospecific and T cell-dependent.
Other molecules associated with immune tolerance have been found in succession, such as programmed death ligand 1 (PDL1). PDL1 is an inhibitory T cell costimulatory molecule, which has been proved to be pivotal in regulating immune responses in many systems (Khoury and Sayegh, 2004; Rothstein and Sayegh, 2003). Interestingly, its expression in the uterus is restricted to the decidua basalis (Guleria et al., 2005). This specific expression pattern suggests a role of PDL1 in regulating the maternal alloimmune response. Blockage of PDL1 signaling with specific monoclonal antibody leads to a significant increase in the rejection rate of allogeneic concepti in B cell-deficient but not in Rag-I-/- mothers, and therefore this fetal rejection was T cell- but not B cell-dependent (Guleria et al., 2005; Guleria and Sayegh, 2007). Furthermore, ablation of PDL1 in female mice results in severe reduction in allogeneic fetal survival rates (Guleria et al., 2005; Guleria and Sayegh, 2007). These observations together indicate that the PDL1 is crucial in fetomaternal immune tolerance.
Galectins (Gal) are a family of β-galactoside-binding lectins which execute their immune functions through orchestrating the proliferation and survival of effector T cells, and among them, Gal-1 can directly induce the death of T cells, which is favorable to fetal survival in the maternal uterus (Blaser et al., 1998; Chung et al., 2000; Rabinovich et al., 2007; Stillman et al., 2006). In mouse uteri, the expression of Gal-1 is regulated by ovarian steroids and increased in the decidua during early pregnancy (Hirota et al., 2012). Gal-1-deficient mice show higher rates of fetal loss just when the female mice are mated with the allogeneic males (Blois et al., 2007). A functional crosstalk between the immune and endocrine systems exists in the regulation of fetomaternal tolerance (Arck et al., 2007). For example, Gal-1 treatment prevents the stress-induced decrease of circulating progesterone (Blois et al., 2007). Reciprocally, treatment with progesterone increases Gal-1 expression in the myometrium and decidua of stress-challenged pregnancies (Blois et al., 2007). Previous studies have shown that exogenous progesterone can rescue implantation failure in Fkbp52-/- mice, but the embryo resorption rate remains high at midgestation (Tranguch et al., 2005a; Tranguch et al., 2007; Yang et al., 2006). This is consistent with recent findings that Fkbp52 deficiency results in significantly down-regulated Gal-1 expression, whereas concomitant supplementation of recombinant Gal-1 can suppress the high incidence of fetal resorptions in Fkbp52-/- females (Hirota et al., 2012). These results provide robust evidence for the crosstalk between progesterone-FKBP52-PR signaling and Gal-1 in immune surveillance during midgestation pregnancy.
Complement activation is a component of innate T-cell driven immunity and its accurate regulation is necessary for protecting tissues from inflammation (Mellor et al., 2001; Molina, 2005). Complement component receptor 1-like protein (Crry), a murine negative regulator of complement activation, is highly expressed in the trophoblast cells as early as embryonic day 7.5, as well as in the decidual tissues during early pregnancy (Hagmann, 2000; Xu et al., 2000a). Systemic deletion of Crry leads to embryonic lethality due to abnormal complement deposition in the placenta (Mellor et al., 2001; Molina, 2005; Xu et al., 2000a). However, when this mutation is backcrossed into complement component 3 (C3)-deficient background, the knockout fetus can survive until adulthood, indicating that the Crry-/- embryonic lethality is resulted from the activation of complement system (Xu et al., 2000a). It is further proved that the Crry-/- embryonic lethality is mediated exclusively by the activation of maternal C3, independent of another component C5 (Mao et al., 2003). Moreover, C3 activation has been identified as the main cause of pathogenesis of antiphospholipid syndrome with recurrent fetal loss in humans (Holers et al., 2002).
6.4.2 Immune cells during decidualization
Stromal differentiation and angiogenesis during decidualization are crucial for pregnancy establishment and maintenance. Decidual leukocytes are thought to play a role not only in creating maternal immune tolerance, but also in decidual development and remodeling during early pregnancy. Leukocytes comprise a large part of cell population in decidual bed during early pregnancy both in humans and mice (Croy et al., 2012; Lash et al., 2010a). Among these leukocytes, the major player involved in maternal immune tolerance is the uterine natural killer (uNK) cells, which become activated and dramatically increased during decidualization, accounting for about 65–70% of the decidual immune cells (Croy et al., 2002; Dosiou and Giudice, 2005; Lash et al., 2010b). Unlike T and B lymphocytes, uNK cells are devoid of somatically rearranged antigen-sensing receptors (Natarajan et al., 2002), but express CD56 brightly and lack CD16 and L-selectin, which distinguish them from peripheral NK cells (Searle et al., 1999). uNK cells proliferate rapidly between days 5–7 of pregnancy in mice within the uterus, where they execute the differentiation program under the control of local microenvironment (Chantakru et al., 2002; Croy et al., 2006). Although murine trophoblast cells express target molecules recognized by the uNK cells (Chatterjee-Hasrouni et al., 1984), they remain unharmed in the presence of the decidual uNK cells, which is also blocked by prostaglandin secreted from the decidual cells (Parhar et al., 1989). The physiological role of uNK cells is to provide growth support for stromal cells to differentiate into decidual cells by modifying spiral arteries (Charalambous et al., 2012; Greenwood et al., 2000; Hanna et al., 2006; Herington and Bany, 2007b), which are critical for sufficient fetus nutrition supply (Adamson et al., 2002). Ablation of uNK cells in mice often induces severe abnormalities in the uterus, such as absence of metrial gland, hypocellularity and edema of decidual bed and blockade of normal modification of spiral arteries (Ashkar et al., 2000; Guimond et al., 1998). This defect is uNK-specific as bone marrow transplants from severe combined immunodeficient (SCID) mice, which are T and B cell-deficient, can rescue this phenotype (Guimond et al., 1998). In humans, extravillous cytotrophoblast cells express a unique combination of MHC class I molecules, namely human leukocyte antigen (HLA)-C, -E and -G, and among these only HLA-C is polymorphic (Moffett-King, 2002). Another crucial phenotype of uNK cells is that they express highly diverse killer immunoglobulin-like receptors (KIRs) that recognize polymorphic HLA-C. Different KIR and HLA-C haplotype combinations can limit or foster uNK activation and uterine vasculature remodeling, and notably, are associated with the etiology of pre-eclampsia (Hiby et al., 2010; Redman and Sargent, 2005).
Antigen-presenting cells (APCs) are the second abundant leukocyte population in the decidua, displaying foreign antigen complexes with MHC on their surfaces, and these complexes bind to T-cell receptors (TCRs) to activate T cells (Cella et al., 1997). Among APCs, CD11chi dendritic cells (DCs) expressing both MHC class I and II molecules are the most potent inducers of immune responses. DCs have been confirmed to exist in the nonpregnant and pregnanant endometrium in both humans and rodents (Blois et al., 2004; Gardner and Moffett, 2003; Zarnani et al., 2007). In mice, DCs are located subadjacent to the luminal epithelium at early pregnancy, and randomly distributed in the stroma and around the epithelium afterwards (Zarnani et al., 2007). Inducible ablation of DCs can be achieved by the administration of diphtheria toxin (DT) in transgenic mice carrying diphtheria toxin receptor (DTR) under the control of CD11c promoter, and therefore the CD11c-expressing DC cells are susceptible to acute ablation (Jung et al., 2002; Saito et al., 2001). By using this approach, several groups demonstrate that depletion of DCs impairs the proliferation and differentiation of endometrial stromal cells during decidulization (Krey et al., 2008; Plaks et al., 2008; Pollard, 2008). Moreover, abalation of DCs also affects the accumulation of mature uNK cells (Krey et al., 2008). An impaired trafficking of CD11c+CD11b+ DC cells to the pregnant uterus are accompanied with aberrant uNK differentiation in lower numbers and with smaller cell size (Behrends et al., 2008; Karsten et al., 2009), suggesting that DC-derived signals may directly mediate uNK homing and maturation in the pregnant endometrium, as reviewed (Blois et al., 2011). A milestone for understanding the fetomaternal immune tolerance is a unique Act-mOVA transgenic mouse model, which express the transmembrane form of ovalbumin (OVA) under the control of the ubiquitous β-actin promoter. Unexpectly, the OVA transgene is expressed in the labyrinthine trophoblasts and particularly by invasive trophoblasts at the fetomaternal interface (Ehst et al., 2003; Erlebacher et al., 2007). As a result, the nontransgenic females bearing the transgenic conceptus will encounter the antigen OVA, which serves as a surrogate fetal or placental alloantigen and allows for visualizing antigen prsentation at mouse fetomaternal interface (Erlebacher et al., 2007). Employing this model, Erlebacher et al. demonstrate that T cells have a specific and robust proliferative responses in female mice bearing OVA-expressing conceptus (Erlebacher et al., 2007). However, the OVA antigen presentation is mediated exclusively by APCs of maternal rather than fetal origin, and this is important because allorecognition relying on maternal APCs is minor and avoids a major threat to fetal survival by T cells recognizing fetal MHC complexes (Erlebacher et al., 2007; Moldenhauer et al., 2009). Moreover, CD8+ cytoxic T cells that indirectly recognize fetal antigen can undergo clonal deletion and cannot prime for their effector function (Erlebacher et al., 2007). More strikingly, the decidual DCs become trapped and immobile within the pregnant uterus, and as a result, these resident DCs cannot reach the lymphatic vessels, which solely exist in the myometrium (Chakraborty and Pulendran, 2009; Collins et al., 2009). Therefore, maternal T cells appear to be ignorant of fetal antigens and immnue surveilance at the fetomaternal interface, as comprehensively reviewed by Erlebacher recently (Erlebacher, 2010).
Regulatory T cells (Treg cells), also known as suppressor T cells, are a population of CD4+CD25+ T cells, which contribute to maintaining in vivo tolerance by suppressing immune response in an antigen-nonspecific manner (Sakaguchi et al., 1995; Shevach, 2011). Such immune activities of Treg cells suggest their involvement in impeding the maternal allogeneic response against the fetal antigen. Recent studies suggest that circulating and uterine CD4+CD25+ Treg cells increase during pregnancy in both humans and mice (Aluvihare et al., 2004; Heikkinen et al., 2004; Robertson et al., 2009; Sasaki et al., 2004; Zenclussen, 2005; Zhu et al., 2005). Therefore, it is not surprising that absence of Treg cells will lead to pregnancy loss due to uncontrolled immune rejection of the fetus. For example, in Treg-deficient BALB/c nude (nu/nu) females, transferring Treg-depleted polyclonal T cells leads to fetal resorption in allogeneic pregnancy (Aluvihare et al., 2004). Consistently, Treg depletion with anti-CD25 monoclonal antibodies during implantation phase or early pregnancy also results in implantation failure or rapid embryo resorption in allogeneic pregnancy (Darrasse-Jeze et al., 2006; Shima et al., 2010), whereas Treg cells are dispensible for the maintenance of the late stage of maternal tolerance to the allogeneic fetus (Shima et al., 2010). Notably, seminal fluid at mating also contributes to maternal immune tolerance to paternal alloantigens via mediating the expansion of Treg cell pool in the uterine draining lymph nodes at the onset of pregnancy (Robertson et al., 2009). In addition to CD4 and CD25, endogenous Treg cells also specifically express the forkhead box P3 (Foxp3), the key control gene in their development (Fontenot et al., 2003; Hori et al., 2003), and Foxp3 dysfunction causes autoimmune disease in humans and mice (Sakaguchi, 2005). Ablation of Foxp3-expressing Treg cells with diphtheria toxin also fractures maternal tolerance to fetal antigen and triggers fetal resorption with robust expansion of fetal antigen-specific CD4+ and CD8+ T cells (Rowe et al., 2011). In humans, a decrease of Treg cell population and their expression of transcript factor Foxp3 has been reported in paitents with miscarriage or unexplained infertility (Jasper et al., 2006; Sasaki et al., 2004; Yang et al., 2008). Remarkably, conserved noncoding DNA sequence 1 (CNS1) present only in placental mammals, has recently been characterized as a Foxp3 enhancer (Zheng et al., 2010), and CNS1-deficient females exhibit increased fetal resorption in allogeneic pregnancy, resulting from failed accumulation of paternal alloantigen-specific Treg cells in the decidua (Samstein et al., 2012). During the pregnancy, sustained expansion of Treg cells contributes to the maternal immune tolerance, thus it seems that the ability of host defense against infection will reduce (Rowe et al., 2011; Rowe et al., 2012a). A recent study highlights the specificity of Treg cells during pregnancy, which uncovers the myth to dissociate their beneficial and detrimental impacts. Treg cells have a memory after the first pregnancy and these fetal-specific Treg cells can maintain tolerance to pre-existing fetal antigen more rapidly and effectly, which could be resistant to partial maternal Foxp3 cell ablation (Rowe et al., 2012b).
The T helper type 1 (Th1)-Th2 theory proposed in 1986 (Mosmann et al., 1986) defines that Th1 cells-derived cytokines such as IFN-γ predominantly promote cell-mediated immunity, whereas cytokines produced by Th2 cells such as IL-4 induce humoral immunity and the production of immunoglobulin antibodies. It has been once considered that successful pregnancy is dependent upon the Th1/Th2 balance, in that Th2 cytokines favor the fetal growth whereas Th1 cytokines hamper it (Piccinni and Romagnani, 1996; Saito, 2000; Zenclussen, 2005; Zenclussen et al., 2005). T cells from the decidua of women with miscarriage can produce predominant Th1-type cytokines with decreased Th2-type cytokines (Piccinni et al., 1998; Piccinni and Romagnani, 1996; Raghupathy et al., 1999), and this is also the case in mice with impaired gestation (Zenclussen, 2005). Moreover, previous studies show that Th1/Th2 cytokines can regulate uterine expression of FAAH and LIF and trophoblast release of hCG (Karasu et al., 2011; Saito, 2000), all of which are known to play roles during implantation. However, the Th1-Th2 paradigm is questioned since IFN-Y that is detrimental to human pregnancy facilitates uterine vascular modification, decidualization and uNK cells differentiation in mice (Ashkar et al., 2000). Mice lacking Th2-type cytokines IL-10 and IL-4 show apparently normal pregnancy (Svensson et al., 2001), further shaking the Th1-Th2 theory.
In fact, this traditional Th1-Th2 paradigm has evolved into a more complicated theory. Recent evidence shows that naive CD4+ T helper cells differentiate into four effector subsets, Th1, Th2, Th17 and Treg cells, which secret distinct combinations of cytokines (Tato and O’Shea, 2006). It is becoming extensively recognized that cytokines are of multiple cellular originations, rather than solely secreted from one subset of leukocyte (Coffman, 2006). This view helps to resolve the longstanding paradox of Th1-Th2 theory. Adaptive immune defense tailored to different pathogens is orchestrated by Th1, Th2 and Th17, whereas Treg cells perform an immunosuppression role, as discussed above. The development of these subsets is under the control of distinct lineage specifying genes. For example, STATs, T-box transcription factor (T-bet) and GATA binding protein 3 (GATA-3) are crucial for the differentiation along the Th1 and Th2 pathways, while retinoic acid receptor (RAR)-related orphan nuclear receptor RORYt is the master regulator of Th17 cells, as reviewed excellently (Lehar and Bevan, 2004; Reiner, 2007; Tato and O’Shea, 2006). Notably, a very recent study shows the differentiation of Th2 lineage is controlled by an epigenetic transcriptional silencing pathway (Allan et al., 2012). The Th1/Th2/Th17 and Treg paradigm in implantation has also drawn extensive attention of reproductive immunologists (Lee et al., 2012b; Saito et al., 2010). Increasing evidence shows an elevated prevalence of IL-17-producing cells and/or Th17 immune response in patients with pregnancy complications, miscarriage (Nakashima et al., 2012; Wang et al., 2010b) and preeclampsia (Darmochwal-Kolarz et al., 2012; Darmochwal-Kolarz et al., 2002; Santner-Nanan et al., 2009; Toldi et al., 2011). Similar as Th1 cells, Th17 cells tend to reject the conceptus alloantigens and are thus harmful to pregnancy maintenance in humans (Liu et al., 2011). Interestingly, progesterone suppresses the development of Th17 cells via inhibiting STAT3 activation in response to IL-6 (Lee et al., 2011b). However, a recent study fails to observe a predominant Th1 and Th17 response in women with repeated IVF failure (Persson et al., 2012), suggesting a diversified etiology of gestation diseases. Overall, the hitherto useful Th1/Th2/Th17 and Treg paradigm can be expanded into a well-orchestrated cytokine network conducive for maternal immune tolerance during pregnancy recognition and adaption.
Effector T cells are the main cell type causing immune rejection owing to their cytolytic and cytokine-producing effector function. At the fetomaternal interface, as mentioned, decidua-entraped DC cells contribute to the prevention of fetal rejection during early pregnancy via minimizing the activation of naive T cells (Collins et al., 2009; Erlebacher et al., 2007). Moreover, the fetus can aviod the T cell attackment even when antigen-specific cytotoxic T lymphocyte (CTL) activity is experimentally induced in late gestation (Collins et al., 2009), pointing towards the existence of a fail-safe mechanism protecting the conceptus from activated CTLs. In support of this, a recent study demonstrates that effector T cells fail to infiltrate into the decidua in response to fetal challenge, and instead are trapped within the myometeial layer of the pregnant uteri (Nancy et al., 2012; Reinhardt et al., 2003). This impaired accumulation of cytolytic T cells in the decidua is in part attributable to the epigenetic silencing of key T cell-attracting chemokine genes, and as a result the process of decidualization reduces the capacity of the decidual cells to produce T cell chemoattractants (Nancy et al., 2012; Reinhardt et al., 2003). For example, the expression of chemoattractants for T cell infiltration, such as CCL5 and CXCL9, are synergistically induced in the myometrium, whereas their expression remains at low levels in the dedidua, via a repressive promoter regulatory status presented by the histone H3 trimethyl lysine 27 (H3K27me3) mark (Nancy et al., 2012; Reinhardt et al., 2003). In humans, T cells have also been reported to be relatively rare in the decidua, and chemoattractant CXCL10 is expressed only locally in decidual tissues, in association with periglandular leukocyte aggregations (Bulmer et al., 2010; Marlin et al., 2011; Red-Horse et al., 2001; Volchek et al., 2010).
7. Emerging concept: the quality of implantation determines the quality of ongoing pregnancy
Although our understanding of reciprocal interactions between the blastocyst and the uterus during implantation has advanced during past decades through the identification of numerous molecules involved in the process (Dey et al., 2004; Lim and Wang, 2010; Paria et al., 2002; Wang and Dey, 2006), these discoveries may be just a tip of the iceberg. Recent progress highlights an emerging concept that the quality of implantation determines the quality of ongoing pregnancy.
Embryo implantation initiates the first physical interaction between the blastocyst and the uterus, thus, programming all subsequent development of both the embryo and uterus. Any errors at this early stage will profoundly contribute to early pregnancy loss or various pregnancy complications (Dey, 2005). Previous studies using embryo transfer technique demonstrate that normal mouse blastocysts transferred after the window of implantation exhibit a dramatic increase of implantation failure and fetal absorption (Song et al., 2002), establishing the concept that a short delay in the embryo attachment to the uterus creates adverse effects that are compounded in later development. This view is further supported by findings from genetically engineered mouse models.
For example, a dysfunctional cytoplasmic phospholipase A 2α (cPLA2α)-COX-2-PG signaling axis defers on-time implantation, coupled with impaired decidualization and placentation, that eventually compromises term pregnancy in mice (Lim et al., 1999a; Lim et al., 1997; Song et al., 2002; Wang et al., 2004b; Wang et al., 2007a). Female mice lacking lysophosphatidic acid receptor 3 (LPA3) also exhibit a deferral of embryo implantation with a crowded embryo distribution and retarded feto-placental development (Ye et al., 2005), phenotypically mimicking cPLA2α deficient mice (Song et al., 2002). The spatiotemporal expression of LPA3 receptor partially overlaps with that of cPLA2α and COX-2 at the site of implantation (Chakraborty et al., 1996; Lim et al., 1997; Song et al., 2002; Wang et al., 2004b; Ye et al., 2005). More strikingly, Lpa3-/- females show an aberrant expression of COX-2 and treatment with PGs can restore the on-time implantation in mutant females (Chen et al., 2011; Daikoku et al., 2011; Hama et al., 2007; Liu and Armant, 2004; Nallasamy et al., 2012; Sun et al., 2012; Xie et al., 2007; Ye et al., 2011), highlighting the necessity of this lipid signaling for on-time implantation.
This new concept has high clinical relevance since the risk of early pregnancy loss increases with later implantation in humans (Liu and Rosenwaks, 1991; Wilcox et al., 1999). In women during normal menstrual cycle or undertaken IVF treatment, if implantation occurs beyond the normal window, pregnancy loss is frequently observed, indicating a high association of occult pregnancy failure with deferral of implantation (Macklon et al., 2002). However, these data also demonstrate a considerable range in the time, up to several days, e.g. days 6–8 after ovulation, when human implantation can take place without negative consequences, emphasizing the latitude that must be programmed into mammalian development to account for the uncertainties of when interactions between the maternal and embryonic components will occur. The necessity to synchronize the independently developing embryo and female reproductive tract is, therefore, paramount and facilitated through rapid post-transcriptional regulation of physiological responses to crucial elements in the local microenvironment (Armant, 2005).
Interestingly, a recent study further demonstrates that aberrant implantation caused by transient adrenoceptor activation retains normal on-time implantation but disrupts embryo spacing, with comprised embryo development and pregnancy loss occurred at midgestation (Chen et al., 2011), reinforcing the concept that embryo implantation at the right time and the right place are the prerequisites for successful pregnancy. Moreover, IVF pregnancies are often complicated by a high risk of the vanishing twin syndrome (Pinborg et al., 2006), one cause of which might be embryo crowding at implantation and subsequent resorption of one fetus by the twin (Chen et al., 2011; Daikoku et al., 2011; Liu and Armant, 2004; Nallasamy et al., 2012; Sun et al., 2012; Xie et al., 2007; Ye et al., 2011). Placenta previa is a human pregnancy complication in which the placenta is situated close to or completely covering the cervix (Allahdin et al., 2011; Bulletti and de Ziegler, 2006; Rao et al., 2012), affecting approximate 0.5% of human pregnancies. And one risk factor for this complication could also be aberrant embryo spacing (Wang and Dey, 2006). Therefore, answers to the question of how embryo spacing in mouse models will provide clues into the etiology of pregnancy disease in humans, such as vanishing twin syndrome and placenta previa.
7. Implications for human fertility
Most of what is understood about the molecular signaling pathways that govern embryo-uterine interactions has been obtained using animal models because human experimentation is limited by ethical considerations and restrictions on research with human embryos (Berlanga et al., 2011; Lim and Wang, 2010). Although humans and rodents are evolutionarily different, they share many physiological similarities with respect to embryo implantation. For example, blastocyst hatching or zona pellucida dissolution occurs in both species to ensure proper contact and orientation of the blastocyst within the uterine lumen (Chen et al., 2012). After blastocyst apposition and spacing, blastocyst attachment initiates a precisely regulated embryo-uterine dialogue that occurs within a defined window of implantation in both species (Bazer et al., 2009; Singh and Aplin, 2009; Wang and Dey, 2006). Stroma cells underlying the luminal epithelium undergo extensive decidualization upon trophoblast penetration to protect and support the development of embryos in both humans and mice, although stromal-decidual transformation begins independently of an embryo in women during the mid-secretory phase of the menstrual cycle (Bazer et al., 2009). These similarities strengthen the value of the tremendous advances in implantation research achieved using mouse models.
Many signaling molecules found to be crucial for embryo implantation in mice are of high clinical significance for human implantation and pregnancy outcome (Table 2). For example, LIF, a crucial cytokine in mouse implantation, appears essential in humans, since LIF gene mutations are often associated with unexplained infertility and repeated implantation failures (Aghajanova, 2004; Aghajanova et al., 2009; Steck et al., 2004). Moreover, women with high endometrial LIF immunoreactivity during the window of implantation are more likely to become pregnant than those with weaker expression in IVF-ET program (Serafini et al., 2009). Anandamide at high levels is detrimental to blastocyst differentiation and uterine receptivity in mice (Saito, 2000; Sun and Dey, 2009; Wang et al., 2006a). Clinical evidence suggests that high peripheral levels of anandamide are correlated with recurrent pregnancy losses in women (Bari et al., 2011; Habayeb et al., 2008; Maccarrone, 2009; Maccarrone et al., 2002; Maccarrone et al., 2000). While the lipid signaling cascade of LPA3-cPLA2α-COX-2-PG is essential for normal implantation and decidualization in mice (Lim et al., 1999a; Lim et al., 1997; Song et al., 2002; Wang et al., 2004b; Wang et al., 2007a; Ye et al., 2005), the physiological significance of PG and LPA signaling in human embryo implantation is supported by observations that women with repeated implantation failure have reduced levels of PG and LPA (Achache et al., 2010; Marions and Danielsson, 1999). HB-EGF activation of ErbB1/4 receptors involves bidirectional interactions between the blastocyst and the uterus prior to implantation in mice (Das et al., 1994; Hamatani et al., 2004b; Paria et al., 1999a; Paria et al., 2001a; Raab et al., 1996; Wang et al., 2000; Xie et al., 2007), and a similar machinery functions during human implantation and placentation (Birdsall et al., 1996; Chobotova et al., 2005; Chobotova et al., 2002a; Chobotova et al., 2002b; Gonzalez et al., 2011; Jessmon et al., 2009; Leach et al., 1999; Lessey et al., 2002; Yoo et al., 1997). Moreover, aberrant expression of HB-EGF in human trophoblasts is associated with preeclampsia (Aghajanova et al., 2008a; Leach et al., 2002). In addition, homeobox transcription factors and developmental morphogenesis genes essential for uterine function and implantation in mice exhibit dynamic expression patterns in human endometrium and potential association with certain pregnancy complications (Bombail et al., 2010; Cheng et al., 2008; Kodama et al., 2010; Koler et al., 2009; Li et al., 2007; Mirkin et al., 2005; Taylor, 2000; Taylor et al., 1998; Taylor et al., 1997; Tulac et al., 2006).
Table 2.
Molecules implicated in human infertility: clues from mice
| Factors | Putative function in human fertility | Ref. |
|---|---|---|
| Anandamide | High levels of anandamide correlate with recurrent pregnancy losses | (Bari et al., 2011; Maccarrone, 2009) |
| Cox | Expressed in the endometrium during the implantation period, participate In PG synthesis in the endometrium, associated with endometrial receptivity | (Achache et al., 2010; Marions and Danielsson, 1999) |
| Hbegf | Highly expressed in the receptive endometrium, suggesting a role in implantation; Preeclampsia | (Leach et al., 2002) |
| Hoxa10/Hoxa11 | Upregulated during the secretory phase, suggesting a potential role in uterine receptivity | (Taylor, 2000; Taylor et al., 1998; Taylor et al., 1997) |
| Integrin αvβ3 Lif | Aberrant expression correlated with infertility and recurrent pregnancy loss Lif mutation associated with unexplained infertility and repeated implantation failure occurred | (Illera et al., 2000) (Serafini et al., 2009) |
| Msx1 | Dynamically expressed in the human endometrium around the time of implantation, suggesting a potential role in determining uterine receptivity | (Kao et al., 2002; Mirkin et al., 2005) |
| p53 | Reduced expression level in preterm labor | (Balasch and Gratacos, 2012; Roberts et al., 1994) |
| Sgk1 | Markedly low expression in recurrent pregnancy loss endometrium | (Salker et al., 2011) |
8. Perspectives and closing remarks
Human population growth is a global issue that has become increasingly important since the last half of the twentieth century. By 2020, there will be approximate 1.2 billion people (15% of the world’s population) entering their child-bearing years (Rao, 2008). Meanwhile, about 15% of couples at reproductive age face the risk of infertility. Moreover, human reproduction is an inefficient process with only about 30% success rate in each normal menstrual cycle, while embryo implantation is a major limiting event. Therefore, unraveling the complexities of implantation will help to address these two contrasting global issues (Wang and Dey, 2006).
Mapping the molecular landscape of implantation is necessary for fully understanding this process. Although studies in the past few years have established the role of various signaling pathways, whether these pathways function independently or converge into larger networks needs to be further explored. In recent years, the identification of specific genes that regulate implantation has been largely advanced by the PR-Cre mouse model (Soyal et al., 2005), where candidate genes can be ablated in PR-expressing tissues, including the uterus. Employing this mouse model, a number of genes with embryonic lethal phenotypes have been recognized to be essential for adult uterine biology and implantation (Table 3) (Afshar et al., 2012; Daikoku et al., 2008; Das, 2012; Hawkins et al., 2012; Kim et al., 2010a; Kim et al., 2010b; Laws et al., 2008; Park et al., 2012a; Park et al., 2012b). In addition, transgenic or knock-in lines with Cre recombinase driven by the promoter of Wnt7a, small proline-rich protein 2F (Sprr2f) or anti-Müllerian hormone receptor type 2 (AMHR2) known respectively as Wnt7a-Cre, Sprr2f-Cre and AMHR2-Cre mice, have been used for conditional depletion of genes in the uterine epithelium (Contreras et al., 2010; Franco et al., 2011b; Winuthayanon et al., 2010) or in mesenchyme-derived stroma (Deutscher and Hung-Chang Yao, 2007; Jamin et al., 2002; Orvis et al., 2008). However, currently available Cre mouse lines are not ideal for investigating the potential roles of genes involved in the development of the female reproductive tract during embryo implantation in adulthood, since it will be difficult to distinguish the outcome of any early pregnancy defects from an impaired postnatal uterine development (Dunlap et al., 2011; Jeong et al., 2009). Therefore, development of new Cre mouse lines is warranted for conditional gene deletion in epithelial and stromal cells, respectively.
Table 3.
Genes critical to implantation and decidualization: results from conditional knockout models
| Gene | Gene Product | Knockout phenotype in females | Refs |
|---|---|---|---|
| Bmp2 | Bone morphogenetic protein 2 | Incapable of undergoing the decidual reaction | (Lee et al., 2007) |
| Cdh1 | E-cadherin | Implantation failure; failed to response to artificially induced decidualization | (Reardon et al., 2012) |
| Ctnnb1 | β-catenin | Implantation failure | (Jeong et al., 2009) |
| Dicer | Dicer | Enhanced stromal apoptosis; impaired uterine stromal cell proliferation in response to progesterone | (Hawkins et al., 2012) |
| Errfi1 | ERBB receptor feedback inhibitor 1 | Implantation failure due to enhanced ER activity in epithelium | (Kim et al., 2010a) |
| Foxa2 | Forkhead box A2 | Implantation failure, severe impairment to respond to the artificially induced decidualization | (Jeong et al., 2010) |
| Gja1 | Connexin 43 | Comprised decidualization; neovascularization defects | (Laws et al., 2008) |
| Hbegf | Heparin-binding EGF-like growth factor | Sub-fertile with deferred implantation | (Xie et al., 2007) |
| Hand2 | Heart and neural crest derivatives expressed transcript 2 | Impaired PR function; uterus receptivity defects | (Li et al., 2011) |
| Ihh | Indian hedgehog | Implantation failure | (Lee et al., 2006) |
| Src2 | Steroid receptor coactivator 2 | Infertile due to impaired PR function mediated by SRC2 | (Mukherjee et al., 2006a; Mukherjee et al., 2006b) |
| Klf5 | Kruppel-like factor 5 | Defective implantation; comprised decidualization | (Sun et al., 2012) |
| K-ras | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | Endometrial Cancer | (Kim et al., 2010b) |
| Pten | Phosphatase and tensin homolog | Endometrial Cancer | (Daikoku et al., 2008) |
| Msx1/2 | Muscle segment homeobox gene (Msh) family members 1/2; | Implantation failure as altered uterine luminal epithelial cell polarity | (Daikoku et al., 2011; Nallasamy et al., 2012) |
| Nodal | NODAL | Abnormal decidua basalis at midgestation and aberrant placental development | (Park et al., 2012a) |
| Notch1 | Notch1 | Comprised decidualization | (Afshar et al., 2012) |
| Nr2f2 | Chicken ovalbumin Upstream promoter transcription factor II | Implantation failure | (Kurihara et al., 2007) |
| p53 | Transformation related protein 53 | Uterine decidual senescence; preterm birth | (Hirota et al., 2010) |
| Rea | Repressor of estrogen receptor activity | Implantation and decidualization failure due to uterine development defects | (Park et al., 2012b) |
| Smo | Smoothened | Uterine hypertrophy; luminal epithelial stratify; impaired decidualization | (Franco et al., 2010b) |
| Wnt4 | Wingless-related MMTV integration site 4 | Implantation defect; failed to undergo the artificially induced decidual response | (Franco et al., 2011a) |
| Wnt7a | Wingless-related MMTV integration site 7a | Implantation failure | (Dunlap et al., 2011) |
To improve the diagnosis and treatment of infertility is one of the ultimate goals of understanding the molecular basis of implantation. Embryo quality and endometrial receptivity are two determinants for pregnancy success in patients undergoing IVF-ET program. With respect to embryos, clinical data show that 70% of embryos selected for transfer fail to implant. To increase pregnancy rates, multiple embryos are transferred, which is responsible for complications due to multiple gestation pregnancy (Hernandez, 2001; Tiitinen, 2012). A pre-selection of high quality embryos by monitoring embryonic metabolic activity and morphology can significantly reduce IVF complications, while maintaining acceptable pregnancy rates (Gardner and Sakkas, 2003; Giorgetti et al., 1995; Rijnders and Jansen, 1998). Newly-developed time-lapse imaging and embryo secretomics technologies can be useful in assessing embryo quality. In addition, improvement of culture medium and embryo freezing techniques are of equal importance (Ciray et al., 2012; Pool et al., 2012; Rato et al., 2012), particularly since the risk of low birth weight in IVF-ET conceived newborns is greatly reduced in frozen embryo cycles (Kalra et al., 2011).
Transferring the embryo into the non-receptive endometrium largely accounts for low pregnancy rates in IVF-ET programs (Miller et al., 2012). Despite numerous advances in our understanding on the underlying molecular and cellular mechanisms that are essential for endometrial receptivity, it has not yet been possible for clinicians to accurately predict when receptivity is attained in each individual. Thus, it is essential to identify reliable biomarkers to define the status of endometrial receptivity. Large-scale technologies, such as proteomics and secretomics, can also be used to screen novel biomarkers for dating endometrium (Aghajanova et al., 2008b; Borthwick et al., 2003; Carson et al., 2002; Diaz-Gimeno et al., 2011; Hannan et al., 2010; Haouzi et al., 2009; Horcajadas et al., 2007; Horcajadas et al., 2004; Mirkin et al., 2005; Riesewijk et al., 2003). Characterization of biomarkers of endometrial receptivity will help to formulate new strategies of non-hormonal contraception. Moreover, a better understanding of the window of implantation in humans will suggest new interventions to correct receptivity defects and extend the receptivity window in IVF-ET programs.
There is an urgent need to verify in humans about the molecules known to be essential for implantation in mice through expression and functional analysis under various pathophysiological conditions. These efforts will be advanced through large-scale approaches, using mass spectrometry or genome-wide screening to discover essential regulators in humans. Mouse models will continue to be important as functional tools to demonstrate the involvement of embryo-uterine crosstalk during early pregnancy. A better understanding of the physiological and molecular mechanisms responsible for implantation will, thus, help improve modern reproductive therapies and develop novel contraceptives.
Acknowledgments
Work incorporated in this article was partially supported by the National Basic Research Program of China (2011CB944400, 2010CB945002), the National Natural Science Foundation (30825015, 81130009), the National Institutes of Health (HD071408) and the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. We apologize for unintended omission of any relevant references.
References
- Achache H, Tsafrir A, Prus D, Reich R, Revel A. Defective endometrial prostaglandin synthesis identified in patients with repeated implantation failure undergoing in vitro fertilization. Fertil Steril. 2010;94(4):1271–1278. doi: 10.1016/j.fertnstert.2009.07.1668. [DOI] [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250(2):358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- Adesanya OO, Zhou J, Samathanam C, Powell-Braxton L, Bondy CA. Insulin-like growth factor 1 is required for G2 progression in the estradiol-induced mitotic cycle. Proc Natl Acad Sci U S A. 1999;96(6):3287–3291. doi: 10.1073/pnas.96.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, Radnor R, Miele L, Fazleabas A. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012;26(1):282–294. doi: 10.1096/fj.11-184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L. Leukemia inhibitory factor and human embryo implantation. Ann N Y Acad Sci. 2004;1034:176–183. doi: 10.1196/annals.1335.020. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Altmae S, Bjuresten K, Hovatta O, Landgren BM, Stavreus-Evers A. Disturbances in the LIF pathway in the endometrium among women with unexplained infertility. Fertil Steril. 2009;91(6):2602–2610. doi: 10.1016/j.fertnstert.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Bjuresten K, Altmae S, Landgren BM, Stavreus-Evers A. HB-EGF but not amphiregulin or their receptors HER1 and HER4 is altered in endometrium of women with unexplained infertility. Reprod Sci. 2008a;15(5):484–492. doi: 10.1177/1933719108314624. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008b;19(2):204–211. doi: 10.1016/j.semcdb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod. 2012;86(1):1–21. doi: 10.1095/biolreprod.111.092775. [DOI] [PubMed] [Google Scholar]
- Allahdin S, Voigt S, Htwe TT. Management of placenta praevia and accreta. J Obstet Gynaecol. 2011;31(1):1–6. doi: 10.3109/01443615.2010.532248. [DOI] [PubMed] [Google Scholar]
- Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, Roche D, Maison C, Quivy JP, Almouzni G, Amigorena S. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487(7406):249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- Altmae S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16(3):178–187. doi: 10.1093/molehr/gap102. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–274. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Ansell JD, Barlow PW, McLaren A. Binucleate and polyploid cells in the decidua of the mouse. J Embryol Exp Morphol. 1974;31(1):223–227. [PubMed] [Google Scholar]
- Aoki R, Suzuki N, Paria BC, Sugihara K, Akama TO, Raab G, Miyoshi M, Nadano D, Fukuda MN. The Bysl gene product, bystin, is essential for survival of mouse embryos. FEBS Lett. 2006;580(26):6062–6068. doi: 10.1016/j.febslet.2006.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD. Adhesion molecules in implantation. Rev Reprod. 1997;2(2):84–93. doi: 10.1530/ror.0.0020084. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Kimber SJ. Trophoblast-uterine interactions at implantation. Reprod Biol Endocrinol. 2004;2(48) doi: 10.1186/1477-7827-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD, Spanswick C, Behzad F, Kimber SJ, Vicovac L. Integrins beta 5, beta 3 and alpha v are apically distributed in endometrial epithelium. Mol Hum Reprod. 1996;2(7):527–534. doi: 10.1093/molehr/2.7.527. [DOI] [PubMed] [Google Scholar]
- Arai S, Miyake K, Voit R, Nemoto S, Wakeland EK, Grummt I, Miyazaki T. Death-effector domain-containing protein DEDD is an inhibitor of mitotic Cdk1/cyclin B1. Proc Natl Acad Sci U S A. 2007;104(7):2289–2294. doi: 10.1073/pnas.0611167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck P, Hansen PJ, Mulac Jericevic B, Piccinni MP, Szekeres-Bartho J. Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am J Reprod Immunol. 2007;58(3):268–279. doi: 10.1111/j.1600-0897.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Armant DR. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev Biol. 2005;280(2):260–280. doi: 10.1016/j.ydbio.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant DR. Life and death responses to trophinin-mediated adhesion during blastocyst implantation. Cell Cycle. 2011;10(4):574–575. [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192(2):259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61(2):67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3(7):683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Bagchi IC, Li Q, Cheon YP, Mantena SR, Kannan A, Bagchi MK. Use of the progesterone receptor antagonist RU 486 to identify novel progesterone receptor-regulated pathways in implantation. Semin Reprod Med. 2005;23(1):38–45. doi: 10.1055/s-2005-864032. [DOI] [PubMed] [Google Scholar]
- Bagchi MK, Mantena SR, Kannan A, Bagchi IC. Control of Uterine Cell Proliferation and Differentiation by C/EBPb: Functional Implications for Establishment of Early Pregnancy. Cell Cycle. 2006;5(9):922–925. doi: 10.4161/cc.5.9.2712. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Kliman HJ, Taylor HS. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn. 2001;222(3):538–544. doi: 10.1002/dvdy.1209. [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10(7):903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- Balasch J, Gratacos E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol. 2012;24(3):187–193. doi: 10.1097/GCO.0b013e3283517908. [DOI] [PubMed] [Google Scholar]
- Bany BM, Cross JC. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev Biol. 2006;294(2):445–456. doi: 10.1016/j.ydbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci. 2011;16:498–516. doi: 10.2741/3701. [DOI] [PubMed] [Google Scholar]
- Barkley MS, Geschwind, Bradford GE. The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol Reprod. 1979;20(4):733–738. doi: 10.1095/biolreprod20.4.733. [DOI] [PubMed] [Google Scholar]
- Barrow JR. Wnt/PCP signaling: a veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol. 2006;17(2):185–193. doi: 10.1016/j.semcdb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, Spencer TE. Uterine differentiation as a foundation for subsequent fertility. J Reprod Fertil Suppl. 1999;54:287–302. [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Spencer TE, Vallet JL, Christenson RK. Early uterine development in pigs. J Reprod Fertil Suppl. 1993;48:99–116. [PubMed] [Google Scholar]
- Bassal S, Nomura N, Venter D, Brand K, McKay MJ, van der Spek PJ. Characterization of a novel human cell-cycle-regulated homologue of Drosophila dlg1. Genomics. 2001;77(1–2):5–7. doi: 10.1006/geno.2001.6570. [DOI] [PubMed] [Google Scholar]
- Bazer FW. Uterine protein secretions: Relationship to development of the conceptus. J Anim Sci. 1975;41(5):1376–1382. doi: 10.2527/jas1975.4151376x. [DOI] [PubMed] [Google Scholar]
- Bazer FW. Uterine adenogenesis and pregnancy: multiple roles for Foxa2 in mice. Biol Reprod. 2010;83(3):319–321. doi: 10.1095/biolreprod.110.086694. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138(2):195–209. doi: 10.1530/REP-09-0158. [DOI] [PubMed] [Google Scholar]
- Behrends J, Karsten CM, Wilke S, Robke A, Kruse A. Identification of ITGA4/ITGB7 and ITGAE/ITGB7 expressing subsets of decidual dendritic-like cells within distinct microdomains of the pregnant mouse uterus. Biol Reprod. 2008;79(4):624–632. doi: 10.1095/biolreprod.107.067041. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122(9):2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Berg E, McEvoy L, Berlin C, Bargatze R, Butcher E. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366(6456):695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- Bergh P, Navot D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 1992;58(3):537. doi: 10.1016/s0015-0282(16)55259-5. [DOI] [PubMed] [Google Scholar]
- Berlanga O, Bradshaw HB, Vilella-Mitjana F, Garrido-Gomez T, Simon C. How endometrial secretomics can help in predicting implantation. Placenta. 2011;32(Suppl 3):S271–275. doi: 10.1016/j.placenta.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5(2):193–208. doi: 10.1016/j.modgep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A. 1991;88(24):11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski P, Roopenian D, Gossler A. Maternal IL-11Ralpha function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12(14):2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall MA, Hopkisson JF, Grant KE, Barlow DH, Mardon HJ. Expression of heparin-binding epidermal growth factor messenger RNA in the human endometrium. Mol Hum Reprod. 1996;2(1):31–34. doi: 10.1093/molehr/2.1.31. [DOI] [PubMed] [Google Scholar]
- Blaser C, Kaufmann M, Muller C, Zimmermann C, Wells V, Mallucci L, Pircher H. Beta-galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur J Immunol. 1998;28(8):2311–2319. doi: 10.1002/(SICI)1521-4141(199808)28:08<2311::AID-IMMU2311>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod. 2004;70(4):1018–1023. doi: 10.1095/biolreprod.103.022640. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Blois SM, Klapp BF, Barrientos G. Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J Reprod Immunol. 2011;88(2):86–92. doi: 10.1016/j.jri.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Bombail V, Gibson DA, Collins F, MacPherson S, Critchley HO, Saunders PT. A Role for the orphan nuclear receptor estrogen-related receptor alpha in endometrial stromal cell decidualization and expression of genes implicated in energy metabolism. J Clin Endocrinol Metab. 2010;95(10):E224–228. doi: 10.1210/jc.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9(1):19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- Branham WS, Sheehan DM, Zehr DR, Ridlon E, Nelson CJ. The postnatal ontogeny of rat uterine glands and age-related effects of 17 beta-estradiol. Endocrinology. 1985;117(5):2229–2237. doi: 10.1210/endo-117-5-2229. [DOI] [PubMed] [Google Scholar]
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am J Anat. 1989;186(1):1–20. doi: 10.1002/aja.1001860102. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Setiawan T, Lubahn DB, Taylor JA, Kurita T, Cunha GR, Cooke PS. Tissue compartment-specific estrogen receptor-alpha participation in the mouse uterine epithelial secretory response. Endocrinology. 1999;140(1):484–491. doi: 10.1210/endo.140.1.6448. [DOI] [PubMed] [Google Scholar]
- Bulletti C, de Ziegler D. Uterine contractility and embryo implantation. Curr Opin Obstet Gynecol. 2006;18(4):473–484. doi: 10.1097/01.gco.0000233947.97543.c4. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54(2–3):281–294. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291(2):326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- Carson DD. Molecular and cell biology of embryo-uterine interactions: mammalian embryo implantation. Semin Cell Dev Biol. 2008;19(2):160. doi: 10.1016/j.semcdb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223(2):217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8(9):871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Carta L, Sassoon D. Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol Reprod. 2004;71(2):444–454. doi: 10.1095/biolreprod.103.026534. [DOI] [PubMed] [Google Scholar]
- Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci U S A. 2005;102(24):8585–8590. doi: 10.1073/pnas.0502343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9(1):10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16(2):107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Pulendran B. Restraining order for dendritic cells: all quiet on the fetal front. J Clin Invest. 2009;119(7):1854–1857. doi: 10.1172/JCI39946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LN, Tsang LL, Rowlands DK, Rochelle LG, Boucher RC, Liu CQ, Chan HC. Distribution and regulation of ENaC subunit and CFTR mRNA expression in murine female reproductive tract. J Membr Biol. 2002;185(2):165–176. doi: 10.1007/s00232-001-0117-y. [DOI] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168(1):22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- Charalambous F, Elia A, Georgiades P. Decidual spiral artery remodeling during early post-implantation period in mice: investigation of associations with decidual uNK cells and invasive trophoblast. Biochem Biophys Res Commun. 2012;417(2):847–852. doi: 10.1016/j.bbrc.2011.12.057. [DOI] [PubMed] [Google Scholar]
- Chatterjee-Hasrouni S, Parhar R, Lala PK. An evaluation of the maternal natural killer cell population during the course of murine pregnancy. Cell Immunol. 1984;84(2):264–275. doi: 10.1016/0008-8749(84)90098-4. [DOI] [PubMed] [Google Scholar]
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19(8):1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141(12):4365–4372. doi: 10.1210/endo.141.12.7855. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Elad D, Jaffa AJ, Cao Y, Ye X, Duan E. Navigating the site for embryo implantation: Biomechanical and molecular regulation of intrauterine embryo distribution. Mol Aspects Med. 2012 doi: 10.1016/j.mam.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Lu J, Wang Q, Wang S, Cao Y, Wang H, Duan E. Embryo-uterine cross-talk during implantation: the role of Wnt signaling. Mol Hum Reprod. 2009;15(4):215–221. doi: 10.1093/molehr/gap009. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Peng H, Lei L, Kuang H, Zhang L, Ning L, Cao Y, Duan E. Transient {beta}2-adrenoceptor activation confers pregnancy loss by disrupting embryo spacing at implantation. J Biol Chem. 2011;286(6):4349–4356. doi: 10.1074/jbc.M110.197202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Smith SK, Charnock-Jones DS. Transcript profile and localization of Wnt signaling-related molecules in human endometrium. Fertil Steril. 2008;90(1):201–204. doi: 10.1016/j.fertnstert.2007.05.077. [DOI] [PubMed] [Google Scholar]
- Cheng JG, Chen JR, Hernandez L, Alvord WG, Stewart CL. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci U S A. 2001;98(15):8680–8685. doi: 10.1073/pnas.151180898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobotova K, Karpovich N, Carver J, Manek S, Gullick WJ, Barlow DH, Mardon HJ. Heparin-binding epidermal growth factor and its receptors mediate decidualization and potentiate survival of human endometrial stromal cells. J Clin Endocrinol Metab. 2005;90(2):913–919. doi: 10.1210/jc.2004-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, Barlow DH, Mardon HJ. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002a;87(12):5769–5777. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobotova K, Spyropoulou I, Carver J, Manek S, Heath JK, Gullick WJ, Barlow DH, Sargent IL, Mardon HJ. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech Dev. 2002b;119(2):137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Chung CD, Patel VP, Moran M, Lewis LA, Miceli MC. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J Immunol. 2000;165(7):3722–3729. doi: 10.4049/jimmunol.165.7.3722. [DOI] [PubMed] [Google Scholar]
- Ciray HN, Aksoy T, Goktas C, Ozturk B, Bahceci M. Time-lapse evaluation of human embryo development in single versus sequential culture media-a sibling oocyte study. J Assist Reprod Genet. 2012 doi: 10.1007/s10815-012-9818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane RL, Meyer RK. Delayed nidation in the rat induced by progesterone. Proc Soc Exp Biol Med. 1957;96(1):155–159. doi: 10.3181/00379727-96-23418. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest. 2010;120(4):995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman RL. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat Immunol. 2006;7(6):539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119(7):2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, Tagao O, Bardeesy N, Takahashi M, Settleman J, Wong KK, Castrillon DH. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Model Mech. 2010;3(3–4):181–193. doi: 10.1242/dmm.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Lubahn DB, Cunha GR. Mechanism of estrogen action: lessons from the estrogen receptor-alpha knockout mouse. Biol Reprod. 1998;59(3):470–475. doi: 10.1095/biolreprod59.3.470. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94(12):6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Ekman GC, Kaur J, Davila J, Bagchi IC, Clark SG, Dziuk PJ, Hayashi K, Bartol FF. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod. 2012;86(3):63. doi: 10.1095/biolreprod.111.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Uchima FD, Fujii DK, Bern HA, Cunha GR. Restoration of normal morphology and estrogen responsiveness in cultured vaginal and uterine epithelia transplanted with stroma. Proc Natl Acad Sci U S A. 1986;83(7):2109–2113. doi: 10.1073/pnas.83.7.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98(16):9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q. Decidual natural killer cells: key regulators of placental development (a review) J Reprod Immunol. 2002;57(1–2):151–168. doi: 10.1016/s0165-0378(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Croy BA, Chen Z, Hofmann AP, Lord EM, Sedlacek AL, Gerber SA. Imaging of Vascular Development in Early Mouse Decidua and Its Association with Leukocytes and Trophoblasts. Biol Reprod. 2012 doi: 10.1095/biolreprod.112.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76(6):578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67(5):417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biol Reprod. 2002;67(4):1268–1277. doi: 10.1095/biolreprod67.4.1268. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Clark J, Myers P, Korach KS. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor alpha knockout mouse uterus. Proc Natl Acad Sci U S A. 1999;96(7):3646–3651. doi: 10.1073/pnas.96.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R, Dey SK. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell. 2011;21(6):1014–1025. doi: 10.1016/j.devcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68(14):5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18(5):1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19(3):683–697. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol. 2012;93(2):75–81. doi: 10.1016/j.jri.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Darmochwal-Kolarz D, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am J Reprod Immunol. 2002;48(6):381–386. doi: 10.1034/j.1600-0897.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102(1):106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Das A, Li Q, Laws MJ, Kaya H, Bagchi MK, Bagchi IC. Estrogen-induced expression of Fos-related antigen 1 (FRA-1) regulates uterine stromal differentiation and remodeling. J Biol Chem. 2012;287(23):19622–19630. doi: 10.1074/jbc.M111.297663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci U S A. 2009;106(30):12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction. 2009;137(6):889–899. doi: 10.1530/REP-08-0539. [DOI] [PubMed] [Google Scholar]
- Das SK. Contribution of maternal NODAL to term pregnancy. Biol Reprod. 2012;86(6):193. doi: 10.1095/biolreprod.112.100271. [DOI] [PubMed] [Google Scholar]
- Das SK, Das N, Wang J, Lim H, Schryver B, Plowman GD, Dey SK. Expression of betacellulin and epiregulin genes in the mouse uterus temporally by the blastocyst solely at the site of its apposition is coincident with the “window” of implantation. Dev Biol. 1997;190(2):178–190. doi: 10.1006/dbio.1997.8694. [DOI] [PubMed] [Google Scholar]
- Das SK, Lim H, Paria BC, Dey SK. Cyclin D3 in the mouse uterus is associated with the decidualization process during early pregnancy. J Mol Endocrinol. 1999;22(1):91–101. doi: 10.1677/jme.0.0220091. [DOI] [PubMed] [Google Scholar]
- Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci U S A. 1995;92(10):4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120(5):1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141(5):765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, Solter D, Knowles BB. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131(18):4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- Deanesly R. The differentiation of the decidua at ovo-implantation in the guinea-pig contrasted with that of the traumatic deciduoma. J Reprod Fertil. 1971;26(1):91–97. doi: 10.1530/jrf.0.0260091. [DOI] [PubMed] [Google Scholar]
- Deutscher E, Hung-Chang Yao H. Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev Biol. 2007;307(2):227–236. doi: 10.1016/j.ydbio.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devroey P, Bourgain C, Macklon NS, Fauser BC. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15(2):84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Dey S, Johnson D, Pakrasi P, Liehr J. Estrogens with reduced catechol-forming capacity fail to induce implantation in the rat. Royal Society of Medicine. 1986:215–218. doi: 10.3181/00379727-181-42243. [DOI] [PubMed] [Google Scholar]
- Dey SK. Reproductive biology: fatty link to fertility. Nature. 2005;435(7038):34–35. doi: 10.1038/435034a. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Diaz-Gimeno P, Horcajadas JA, Martinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, Simon C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95(1):50–60. 60 e51–15. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- Dickmann Z, Dey SK, Gupta JS. Steroidogenesis in rabbit preimplantation embryos. Proc Natl Acad Sci U S A. 1975;72(1):298–300. doi: 10.1073/pnas.72.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez F, Gadea B, Mercader A, Esteban FJ, Pellicer A, Simon C. Embryologic outcome and secretome profile of implanted blastocysts obtained after coculture in human endometrial epithelial cells versus the sequential system. Fertil Steril. 2010;93(3):774–782. e771. doi: 10.1016/j.fertnstert.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Domino SE, Zhang L, Gillespie PJ, Saunders TL, Lowe JB. Deficiency of reproductive tract alpha(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 alpha(1,2)fucosyltransferase locus. Mol Cell Biol. 2001;21(24):8336–8345. doi: 10.1128/MCB.21.24.8336-8345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26(1):44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- Dunlap KA, Filant J, Hayashi K, Rucker EB, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong JW, Spencer TE. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 2011;85(2):386–396. doi: 10.1095/biolreprod.111.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- El-Talatini MR, Taylor AH, Konje JC. Fluctuation in anandamide levels from ovulation to early pregnancy in in-vitro fertilization-embryo transfer women, and its hormonal regulation. Hum Reprod. 2009;24(8):1989–1998. doi: 10.1093/humrep/dep065. [DOI] [PubMed] [Google Scholar]
- Ema M, Mori D, Niwa H, Hasegawa Y, Yamanaka Y, Hitoshi S, Mimura J, Kawabe Y, Hosoya T, Morita M, Shimosato D, Uchida K, Suzuki N, Yanagisawa J, Sogawa K, Rossant J, Yamamoto M, Takahashi S, Fujii-Kuriyama Y. Kruppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell. 2008;3(5):555–567. doi: 10.1016/j.stem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Enders AC. Trophoblast-uterine interactions in the first days of implantation: models for the study of implantation events in the human. Semin Reprod Med. 2000;18(3):255–263. doi: 10.1055/s-2000-12563. [DOI] [PubMed] [Google Scholar]
- Enzmann E, Saphir N, Pincus G. Delayed pregnancy in mice. Anat Rec. 1932;54(3):325–341. [Google Scholar]
- Erlebacher A. Immune surveillance of the maternal/fetal interface: controversies and implications. Trends Endocrinol Metab. 2010;21(7):428–434. doi: 10.1016/j.tem.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117(5):1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Inglese M, Waring P, Campbell IK, Bao S, Clay FJ, Alexander WS, Wicks IP, Tarlinton DM, Novak U, Heath JK, Dunn AR. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194(2):189–203. doi: 10.1084/jem.194.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod. 2001;16(2):221–225. doi: 10.1093/humrep/16.2.221. [DOI] [PubMed] [Google Scholar]
- Eun Kwon H, Taylor HS. The role of HOX genes in human implantation. Ann N Y Acad Sci. 2004;1034:1–18. doi: 10.1196/annals.1335.001. [DOI] [PubMed] [Google Scholar]
- Fejes-Toth G, Frindt G, Naray-Fejes-Toth A, Palmer LG. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol. 2008;294(6):F1298–1305. doi: 10.1152/ajprenal.00579.2007. [DOI] [PubMed] [Google Scholar]
- Feng Z, Zhang C, Kang HJ, Sun Y, Wang H, Naqvi A, Frank AK, Rosenwaks Z, Murphy ME, Levine AJ, Hu W. Regulation of female reproduction by p53 and its family members. FASEB J. 2011;25(7):2245–2255. doi: 10.1096/fj.10-180166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filant J, Zhou H, Spencer TE. Progesterone inhibits uterine gland development in the neonatal mouse uterus. Biol Reprod. 2012;86(5):146, 141–149. doi: 10.1095/biolreprod.111.097089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn C, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7(1):82–86. doi: 10.1093/biolreprod/7.1.82. [DOI] [PubMed] [Google Scholar]
- Finn CA, Martin L. The control of implantation. J Reprod Fertil. 1974;39(1):195–206. doi: 10.1530/jrf.0.0390195. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Kayisili U, Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil Steril. 2011;95(3):1133–1136. doi: 10.1016/j.fertnstert.2010.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SJ, Giudice LC. SGK1: a fine balancing act for human pregnancy. Nat Med. 2011;17(11):1348–1349. doi: 10.1038/nm.2549. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Forti G, Krausz C. Clinical review 100: Evaluation and treatment of the infertile couple. J Clin Endocrinol Metab. 1998;83(12):4177–4188. doi: 10.1210/jcem.83.12.5296. [DOI] [PubMed] [Google Scholar]
- Fouladi-Nashta AA, Jones CJ, Nijjar N, Mohamet L, Smith A, Chambers I, Kimber SJ. Characterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain mice. Dev Biol. 2005;281(1):1–21. doi: 10.1016/j.ydbio.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Foulk RA, Zdravkovic T, Genbacev O, Prakobphol A. Expression of L-selectin ligand MECA-79 as a predictive marker of human uterine receptivity. J Assist Reprod Genet. 2007;24(7):316–321. doi: 10.1007/s10815-007-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011a;25(4):1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Broaddus RR, White LD, Lanske B, Lydon JP, Jeong JW, DeMayo FJ. Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod. 2010a;82(4):783–790. doi: 10.1095/biolreprod.109.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Rubel CA, Creighton CJ, White LD, Broaddus RR, Lewis MT, Lydon JP, Jeong JW, DeMayo FJ. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol Reprod. 2010b;82(5):991–999. doi: 10.1095/biolreprod.109.081513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP, Demayo FJ. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2011b doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Schrocksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H. Activated cellular immunity and decreased serum tryptophan in healthy pregnancy. Adv Exp Med Biol. 1996;398:149–153. doi: 10.1007/978-1-4613-0381-7_24. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Miyoshi M, Nadano D. The role of bystin in embryo implantation and in ribosomal biogenesis. Cell Mol Life Sci. 2008;65(1):92–99. doi: 10.1007/s00018-007-7302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda MN, Nozawa S. Trophinin, tastin, and bystin: a complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. 1999:229. doi: 10.1055/s-2007-1016230. [DOI] [PubMed] [Google Scholar]
- Fukuda MN, Sato T, Nakayama J, Klier G, Mikami M, Aoki D, Nozawa S. Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev. 1995;9(10):1199–1210. doi: 10.1101/gad.9.10.1199. [DOI] [PubMed] [Google Scholar]
- Gardner D, Sakkas D. Assessment of embryo viability: the ability to select a single embryo for transfer--a review. Placenta. 2003;24:S5–S12. doi: 10.1016/s0143-4004(03)00136-x. [DOI] [PubMed] [Google Scholar]
- Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003;69(4):1438–1446. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299(5605):405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol Reprod. 1997;56(5):1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, Roulier R. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10(9):2427–2431. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Neufeld J, Reimann K, Wittmann S, Samalecos A, Wolf A, Bamberger AM, Gellersen B. Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1 beta. Mol Hum Reprod. 2011;17(7):421–433. doi: 10.1093/molehr/gar015. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138(10):1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod. 2001a;65(5):1311–1323. doi: 10.1095/biolreprod65.5.1311. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bazer FW, Spencer TE. Effects of neonatal progestin exposure on female reproductive tract structure and function in the adult ewe. Biol Reprod. 2001b;64(3):797–804. doi: 10.1095/biolreprod64.3.797. [DOI] [PubMed] [Google Scholar]
- Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod. 2001c;64(6):1608–1613. doi: 10.1095/biolreprod64.6.1608. [DOI] [PubMed] [Google Scholar]
- Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21(7):693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5(9):866–873. doi: 10.1093/molehr/5.9.866. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187(2):217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202(2):231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178(6):3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang H, Okamoto Y, Ueda N, Kingsley PJ, Marnett LJ, Schmid HH, Das SK, Dey SK. N-acylphosphatidylethanolamine-hydrolyzing phospholipase D is an important determinant of uterine anandamide levels during implantation. J Biol Chem. 2005;280(25):23429–23432. doi: 10.1074/jbc.C500168200. [DOI] [PubMed] [Google Scholar]
- Habayeb OM, Taylor AH, Finney M, Evans MD, Konje JC. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA. 2008;299(10):1135–1136. doi: 10.1001/jama.299.10.1135. [DOI] [PubMed] [Google Scholar]
- Hagmann M. Embryos attacked by mom’s natural defenses. Science. 2000;287(5452):408. doi: 10.1126/science.287.5452.408. [DOI] [PubMed] [Google Scholar]
- Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, Kanai M, Ye X, Chun J, Matsuki N, Suzuki H, Shibasaki M, Arai H. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol Reprod. 2007;77(6):954–959. doi: 10.1095/biolreprod.107.060293. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004a;6(1):117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci U S A. 2004b;101(28):10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39(2):137–143. doi: 10.1111/j.1600-0897.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Hamlett G. Delayed implantation and discontinuous development in the mammals. The Quarterly Review of Biology. 1935;10(4):432–447. [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Stephens AN, Rainczuk A, Hincks C, Rombauts LJ, Salamonsen LA. 2D-DiGE analysis of the human endometrial secretome reveals differences between receptive and nonreceptive states in fertile and infertile women. J Proteome Res. 2010;9(12):6256–6264. doi: 10.1021/pr1004828. [DOI] [PubMed] [Google Scholar]
- Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, De Vos J, Hamamah S. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24(6):1436–1445. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi D, Dechaud H, Assou S, Monzo C, de Vos J, Hamamah S. Transcriptome analysis reveals dialogues between human trophectoderm and endometrial cells during the implantation period. Hum Reprod. 2011;26(6):1440–1449. doi: 10.1093/humrep/der075. [DOI] [PubMed] [Google Scholar]
- Harper MJ, Dowd D, Elliott AS. Implantation and embryonic development in the ovariectomized-adrenalectomized hamster. Biol Reprod. 1969;1(3):253–257. doi: 10.1095/biolreprod1.3.253. [DOI] [PubMed] [Google Scholar]
- Harper MJ, Walpole AL. Mode of action of I.C.I. 46,474 in preventing implantation in rats. J Endocrinol. 1967;37(1):83–92. doi: 10.1677/joe.0.0370083. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, Andreu-Vieyra CV, Kim TH, Jeong JW, Hodgson MC, Chen R, Creighton CJ, Lydon JP, Gunaratne PH, Demayo FJ, Matzuk MM. Dysregulation of Uterine Signaling Pathways in Progesterone Receptor-Cre Knockout of Dicer. Mol Endocrinol. 2012 doi: 10.1210/me.2012-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA, Rucker EB, Johnson GA, Spencer TE. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. (23) 2009;80(5):989–1000. doi: 10.1095/biolreprod.108.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yoshioka S, Reardon SN, Rucker EB, Spencer TE, DeMayo FJ, Lydon JP, MacLean JA. WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod. (32) 2011;84(2):308–319. doi: 10.1095/biolreprod.110.088161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Tsang LL, Ajonuma LC, Chan HC. Abnormally up-regulated cystic fibrosis transmembrane conductance regulator expression and uterine fluid accumulation contribute to Chlamydia trachomatis-induced female infertility. Fertil Steril. 2010;93(8):2608–2614. doi: 10.1016/j.fertnstert.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Heap RB, Deanesly R. The increase in plasma progesterone levels in the pregnant guinea-pig and its possible significance. J Reprod Fertil. 1967;14(2):339–341. doi: 10.1530/jrf.0.0140339. [DOI] [PubMed] [Google Scholar]
- Heap RB, Flint AP, Hartmann PE, Gadsby JE, Staples LD, Ackland N, Hamon M. Oestrogen production in early pregnancy. J Endocrinol. 1981;(89 suppl):77–94. [PubMed] [Google Scholar]
- Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136(2):373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemberger M. IFPA award in placentology lecture - characteristics and significance of trophoblast giant cells. Placenta. 2008;29(suppl A):S4–9. doi: 10.1016/j.placenta.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Heng S, Paule S, Hardman B, Li Y, Singh H, Rainczuk A, Stephens AN, Nie G. Posttranslational activation of bone morphogenetic protein 2 is mediated by proprotein convertase 6 during decidualization for pregnancy establishment. Endocrinology. 2010;151(8):3909–3917. doi: 10.1210/en.2010-0326. [DOI] [PubMed] [Google Scholar]
- Herington JL, Bany BM. The conceptus increases secreted phosphoprotein 1 gene expression in the mouse uterus during the progression of decidualization mainly due to its effects on uterine natural killer cells. Reproduction. 2007a;133(6):1213–1221. doi: 10.1530/REP-07-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bany BM. Effect of the conceptus on uterine natural killer cell numbers and function in the mouse uterus during decidualization. Biol Reprod. 2007b;76(4):579–588. doi: 10.1095/biolreprod.106.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bany BM. Do molecular signals from the conceptus influence endometrium decidualization in rodents? J Exp Zool B Mol Dev Evol. 2009;312(8):797–816. doi: 10.1002/jez.b.21308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Underwood T, McConaha M, Bany BM. Paracrine signals from the mouse conceptus are not required for the normal progression of decidualization. Endocrinology. 2009;150(9):4404–4413. doi: 10.1210/en.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez ER. Avoiding multiple pregnancies: sailing uncharted seas. Hum Reprod. 2001;16(4):615–616. doi: 10.1093/humrep/16.4.615. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120(11):4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Burnum KE, Acar N, Rabinovich GA, Daikoku T, Dey SK. Galectin-1 markedly reduces the incidence of resorptions in mice missing immunophilin FKBP52. Endocrinology. 2012;153(5):2486–2493. doi: 10.1210/en.2012-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120(3):803–815. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, Salmon JE. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195(2):211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horcajadas JA, Pellicer A, Simon C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update. 2007;13(1):77–86. doi: 10.1093/humupd/dml046. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Dominguez F, Cervero A, Pellicer A, Simon C. Determinants of endometrial receptivity. Ann N Y Acad Sci. 2004;1034:166–175. doi: 10.1196/annals.1335.019. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Hou Q, Gorski J. Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development. Proceedings of the National Academy of Sciences. 1993;90(20):9460–9464. doi: 10.1073/pnas.90.20.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Paria BC, Mui C, Dey SK, Gorski J. Immunolocalization of estrogen receptor protein in the mouse blastocyst during normal and delayed implantation. Proc Natl Acad Sci U S A. 1996;93(6):2376–2381. doi: 10.1073/pnas.93.6.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18(12):3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoversland RC, Dey SK, Johnson DC. Aromatase activity in the rabbit blastocyst. J Reprod Fertil. 1982a;66(1):259–263. doi: 10.1530/jrf.0.0660259. [DOI] [PubMed] [Google Scholar]
- Hoversland RC, Dey SK, Johnson DC. Catechol estradiol induced implantation in the mouse. Life Sci. 1982b;30(21):1801–1804. doi: 10.1016/0024-3205(82)90316-2. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, Potter SS. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121(5):1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, Ma XH, Ni H, Lei W, Yang ZM. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008;283(34):23473–23484. doi: 10.1074/jbc.M800406200. [DOI] [PubMed] [Google Scholar]
- Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- Huang GS, Gunter MJ, Arend RC, Li M, Arias-Pulido H, Prossnitz ER, Goldberg GL, Smith HO. Co-expression of GPR30 and ERbeta and their association with disease progression in uterine carcinosarcoma. Am J Obstet Gynecol. 2010;203(3):242, e241–245. doi: 10.1016/j.ajog.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125(3):1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- Huet YM, Andrews GK, Dey SK. Modulation of c-myc protein in the mouse uterus during pregnancy and by steroid hormones. Prog Clin Biol Res. 1989;294:401–412. [PubMed] [Google Scholar]
- Huet YM, Dey SK. Role of early and late oestrogenic effects on implantation in the mouse. J Reprod Fertil. 1987;81(2):453–458. doi: 10.1530/jrf.0.0810453. [DOI] [PubMed] [Google Scholar]
- Illera MJ, Cullinan E, Gui Y, Yuan L, Beyler SA, Lessey BA. Blockade of the alpha(v)beta(3) integrin adversely affects implantation in the mouse. Biol Reprod. 2000;62(5):1285–1290. doi: 10.1095/biolreprod62.5.1285. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32(3):408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12(5):301–308. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- Jeddi-Tehrani M, Abbasi N, Dokouhaki P, Ghasemi J, Rezania S, Ostadkarampour M, Rabbani H, Akhondi M, Tahmasebi Fard Z, Zarnani A. Indoleamine 2, 3-dioxygenase is expressed in the endometrium of cycling mice throughout the oestrous cycle. J Reprod Immunol. 2009;80(1):41–48. doi: 10.1016/j.jri.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, DeMayo FJ. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 2010;83(3):396–403. doi: 10.1095/biolreprod.109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28(1):31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O’Malley BW, DeMayo FJ. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148(9):4238–4250. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- Jessmon P, Leach RE, Armant DR. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev. 2009;76(12):1116–1127. doi: 10.1002/mrd.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joswig A, Gabriel HD, Kibschull M, Winterhager E. Apoptosis in uterine epithelium and decidua in response to implantation: evidence for two different pathways. Reprod Biol Endocrinol. 2003;1:44. doi: 10.1186/1477-7827-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118(4):863–871. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo T, Rajabi MR, Mizunuma H, Ibuki Y. Biochemical evidence for autocrine/paracrine regulation of apoptosis in cultured uterine epithelial cells during mouse embryo implantation in vitro. Mol Hum Reprod. 1998;4(10):990–998. doi: 10.1093/molehr/4.10.990. [DOI] [PubMed] [Google Scholar]
- Kamimura S, Eguchi K, Yonezawa M, Sekiba K. Localization and developmental change of indoleamine 2,3-dioxygenase activity in the human placenta. Acta Med Okayama. 1991;45(3):135–139. doi: 10.18926/AMO/32206. [DOI] [PubMed] [Google Scholar]
- Kantor BS, Dey SK, Johnson DC. Catechol oestrogen induced initiation of implantation in the delayed implanting rat. Acta Endocrinol (Copenh) 1985;109(3):418–422. doi: 10.1530/acta.0.1090418. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- Kapur S, Tamada H, Dey SK, Andrews GK. Expression of insulin-like growth factor-I (IGF-I) and its receptor in the peri-implantation mouse uterus, and cell-specific regulation of IGF-I gene expression by estradiol and progesterone. Biol Reprod. 1992;46(2):208–219. doi: 10.1095/biolreprod46.2.208. [DOI] [PubMed] [Google Scholar]
- Karasu T, Marczylo T, Maccarrone M, Konje J. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum Reprod Update. 2011;17(3):347–361. doi: 10.1093/humupd/dmq058. [DOI] [PubMed] [Google Scholar]
- Karsten CM, Behrends J, Wagner AK, Fuchs F, Figge J, Schmudde I, Hellberg L, Kruse A. DC within the pregnant mouse uterus influence growth and functional properties of uterine NK cells. Eur J Immunol. 2009;39(8):2203–2214. doi: 10.1002/eji.200838844. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, DiGirolamo CM, Kanda Y, Niikura Y, Esmon CT, Hansen TR, Shioda T, Pru JK. The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology. 2007;148(9):4173–4184. doi: 10.1210/en.2007-0268. [DOI] [PubMed] [Google Scholar]
- Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril. 2006;86(3):678–685. doi: 10.1016/j.fertnstert.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233(3):1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20(5):529–538. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- Kim TH, Lee DK, Franco HL, Lydon JP, Jeong JW. ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biol Reprod. 2010a;82(4):706–713. doi: 10.1095/biolreprod.109.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Wang J, Lee KY, Franco HL, Broaddus RR, Lydon JP, Jeong JW, Demayo FJ. The Synergistic Effect of Conditional Pten Loss and Oncogenic K-ras Mutation on Endometrial Cancer Development Occurs via Decreased Progesterone Receptor Action. J Oncol. 2010b:139087. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber SJ. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction. 2005;130(2):131–145. doi: 10.1530/rep.1.00304. [DOI] [PubMed] [Google Scholar]
- Kimber SJ, Spanswick C. Blastocyst implantation: the adhesion cascade. Semin Cell Dev Biol. 2000;11(2):77–92. doi: 10.1006/scdb.2000.0154. [DOI] [PubMed] [Google Scholar]
- Kirkham WB. Observations on the relation between suckling and the rate of embryonic development in mice. J Exp Zool. 1918;27(1):49–55. [Google Scholar]
- Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284(31):20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4(12):969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Kodama A, Yoshino O, Osuga Y, Harada M, Hasegawa A, Hamasaki K, Takamura M, Koga K, Hirota Y, Hirata T, Takemura Y, Yano T, Taketani Y. Progesterone decreases bone morphogenetic protein (BMP) 7 expression and BMP7 inhibits decidualization and proliferation in endometrial stromal cells. Hum Reprod. 2010;25(3):751–756. doi: 10.1093/humrep/dep455. [DOI] [PubMed] [Google Scholar]
- Koff AK. Development of the vagina in the human fetus. Contrib Embryol. 1933;24(140):59–91. [PubMed] [Google Scholar]
- Koler M, Achache H, Tsafrir A, Smith Y, Revel A, Reich R. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod. 2009;24(10):2541–2548. doi: 10.1093/humrep/dep193. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel RH. The effects of theelin on delayed implantation in the pregnant lactating rat. Anat Rec. 1941;81(3):381–392. [Google Scholar]
- Krey G, Frank P, Shaikly V, Barrientos G, Cordo-Russo R, Ringel F, Moschansky P, Chernukhin IV, Metodiev M, Fernandez N, Klapp BF, Arck PC, Blois SM. In vivo dendritic cell depletion reduces breeding efficiency, affecting implantation and early placental development in mice. J Mol Med (Berl) 2008;86(9):999–1011. doi: 10.1007/s00109-008-0379-2. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Spyropoulou I, Redman CW, Takikawa O, Katsuki T, Hara T, Ohama K, Sargent IL. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. 2004;61(2):87–98. doi: 10.1016/j.jri.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Lee K, Saunders PT, Cooke PS, Taylor JA, Lubahn DB, Zhao C, Makela S, Gustafsson JA, Dahiya R, Cunha GR. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol Reprod. 2001;64(1):272–283. doi: 10.1095/biolreprod64.1.272. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 2000a;62(4):831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000b;62(4):821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, Gold LI. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation. 2005;73(6):313–322. doi: 10.1111/j.1432-0436.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139(11):4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- Kwun JK, Emmens CW. Hormonal requirements for implantation and pregnancy in the ovariectomized rabbit. Aust J Biol Sci. 1974;27(3):275–283. doi: 10.1071/bi9740275. [DOI] [PubMed] [Google Scholar]
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86(4):1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proceedings of the National Academy of Sciences. 1994;91(17):8263. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash G, Robson S, Bulmer J. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010a;31:S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010b;(31 Suppl):S87–92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Laws MJ, Taylor RN, Sidell N, DeMayo FJ, Lydon JP, Gutstein DE, Bagchi MK, Bagchi IC. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135(15):2659–2668. doi: 10.1242/dev.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton MJ, Lock P, Metcalf D, Nicola NA. Cross-species receptor binding characteristics of human and mouse leukemia inhibitory factor suggest a complex binding interaction. J Biol Chem. 1994;269(25):17048–17055. [PubMed] [Google Scholar]
- Leach RE, Jessmon P, Coutifaris C, Kruger M, Myers ER, Ali-Fehmi R, Carson SA, Legro RS, Schlaff WD, Carr BR, Steinkampf MP, Silva S, Leppert PC, Giudice L, Diamond MP, Armant DR. High throughput, cell type-specific analysis of key proteins in human endometrial biopsies of women from fertile and infertile couples. Hum Reprod. 2012;27(3):814–828. doi: 10.1093/humrep/der436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84(9):3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, Armant DR. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet. 2002;360(9341):1215–1219. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]
- Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24(5):930–940. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HR, Kim TH, Choi KC. Functions and physiological roles of two types of estrogen receptors, ERalpha and ERbeta, identified by estrogen receptor knockout mouse. Lab Anim Res. 2012a;28(2):71–76. doi: 10.5625/lar.2012.28.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Oh HA, Song H, Jun JH, Roh CR, Xie H, Dey SK, Lim HJ. Autophagy regulates embryonic survival during delayed implantation. Endocrinology. 2011a;152(5):2067–2075. doi: 10.1210/en.2010-1456. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011b;187(4):1778–1787. doi: 10.4049/jimmunol.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38(10):1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27(15):5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol. 2012b;67(4):311–318. doi: 10.1111/j.1600-0897.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- Lehar SM, Bevan MJ. Polarizing a T cell response. Nature. 2004;430(6996):150–151. doi: 10.1038/430150a. [DOI] [PubMed] [Google Scholar]
- Lejeune B, Van Hoeck J, Leroy F. Transmitter role of the luminal uterine epithelium in the induction of decidualization in rats. J Reprod Fertil. 1981;61(1):235–240. doi: 10.1530/jrf.0.0610235. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Assessment of endometrial receptivity. Fertil Steril. 2011;96(3):522–529. doi: 10.1016/j.fertnstert.2011.07.1095. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63(3):535–542. [PubMed] [Google Scholar]
- Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62(4):446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- Li F, Devi YS, Bao L, Mao J, Gibori G. Involvement of cyclin D3, CDKN1A (p21), and BIRC5 (Survivin) in interleukin 11 stimulation of decidualization in mice. Biol Reprod. 2008;78(1):127–133. doi: 10.1095/biolreprod.107.063313. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282(43):31725–31732. doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang J, Armant DR, Bagchi MK, Bagchi IC. Calcitonin down-regulates E-cadherin expression in rodent uterine epithelium during implantation. J Biol Chem. 2002;277(48):46447–46455. doi: 10.1074/jbc.M203555200. [DOI] [PubMed] [Google Scholar]
- Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999a;13(12):1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999b;13(6):1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Dey SK. HB-EGF: a unique mediator of embryo-uterine interactions during implantation. Exp Cell Res. 2009;315(4):619–626. doi: 10.1016/j.yexcr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HJ, Moon I, Han K. Transcriptional cofactors exhibit differential preference toward peroxisome proliferator-activated receptors alpha and delta in uterine cells. Endocrinology. 2004;145(6):2886–2895. doi: 10.1210/en.2004-0011. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Wang H. Uterine disorders and pregnancy complications: insights from mouse models. J Clin Invest. 2010;120(4):1004–1015. doi: 10.1172/JCI41210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Rosenwaks Z. Early pregnancy wastage in IVF (in vitro fertilization) patients. Journal of Assisted Reproduction and Genetics. 1991;8(2):65–72. doi: 10.1007/BF01138657. [DOI] [PubMed] [Google Scholar]
- Liu WM, Pang RT, Cheong AW, Ng EH, Lao K, Lee KF, Yeung WS. Involvement of microRNA lethal-7a in the regulation of embryo implantation in mice. PLoS One. 2012;7(5):e37039. doi: 10.1371/journal.pone.0037039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, Luo LH, Luan HB. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65(5):503–511. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp Cell Res. 2004;296(2):317–326. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Loeb L. Wounds of the pregnant uterus. Royal Society of Medicine. 1906:93–94. [Google Scholar]
- Loeb L. The production of Deciduomata. Journal of the American Medical Association. 1908;50(23):1897. [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lopes FL, Desmarais JA, Murphy BD. Embryonic diapause and its regulation. Reproduction. 2004;128(6):669–678. doi: 10.1530/rep.1.00444. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist O. Morphological studies of the stromal changes after artificial induction of the decidual reaction in rats. Cell Tissue Res. 1978a;194(2):287–296. doi: 10.1007/BF00220395. [DOI] [PubMed] [Google Scholar]
- Lundkvist O. Ultrastructural studies of the endometrial stromal cells in rats during estradiol-induced implantation after an experimental delay. Biol Reprod. 1978b;18(2):306–316. doi: 10.1095/biolreprod18.2.306. [DOI] [PubMed] [Google Scholar]
- Lundkvist O. Morphometric estimation of stromal edema during delayed implantation in the rat. Cell Tissue Res. 1979;199(2):339–348. doi: 10.1007/BF00236143. [DOI] [PubMed] [Google Scholar]
- Lundkvist O, Ljungkvist I. Morphology of the rat endometrial stroma at the appearance of the pontamine blue reaction during implantation after an experimental delay. Cell Tissue Res. 1977;184(4):453–466. doi: 10.1007/BF00220969. [DOI] [PubMed] [Google Scholar]
- Lundkvist Ö, Nilsson BO. Endometrial ultrastructure in the early uterine response to blastocysts and artificial deciduogenic stimuli in rats. Cell Tissue Res. 1982;225(2):355–364. doi: 10.1007/BF00214688. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100(5):2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M. Endocannabinoids: friends and foes of reproduction. Prog Lipid Res. 2009;48(6):344–354. doi: 10.1016/j.plipres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bisogno T, Valensise H, Lazzarin N, Fezza F, Manna C, Di Marzo V, Finazzi-Agro A. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol Hum Reprod. 2002;8(2):188–195. doi: 10.1093/molehr/8.2.188. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, DeFelici M, Klinger FG, Battista N, Fezza F, Dainese E, Siracusa G, Finazzi-Agro A. Mouse blastocysts release a lipid which activates anandamide hydrolase in intact uterus. Mol Hum Reprod. 2004;10(4):215–221. doi: 10.1093/molehr/gah034. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agro A. Endocannabinoids and their actions. Vitam Horm. 2002;65:225–255. doi: 10.1016/s0083-6729(02)65066-6. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet. 2000;355(9212):1326–1329. doi: 10.1016/S0140-6736(00)02115-2. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8(4):333–343. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW, Hendry LB. Diverse modes of action of progesterone and its metabolites. J Steroid Biochem Mol Biol. 1996;56(1–6):209–219. doi: 10.1016/0960-0760(95)00238-3. [DOI] [PubMed] [Google Scholar]
- Malafaya PB, Santos TC, van Griensven M, Reis RL. Morphology, mechanical characterization and in vivo neo-vascularization of chitosan particle aggregated scaffolds architectures. Biomaterials. 2008;29(29):3914–3926. doi: 10.1016/j.biomaterials.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Mantalenakis SJ, Ketchel MM. Frequency and extent of delayed implantation in lactating rats and mice. J Reprod Fertil. 1966;12(2):391–394. doi: 10.1530/jrf.0.0120391. [DOI] [PubMed] [Google Scholar]
- Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302(1–2):179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417(6889):664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao D, Wu X, Deppong C, Friend LD, Dolecki G, Nelson DM, Molina H. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 2003;19(6):813–822. doi: 10.1016/s1074-7613(03)00321-2. [DOI] [PubMed] [Google Scholar]
- Margarit L, Gonzalez D, Lewis PD, Hopkins L, Davies C, Conlan RS, Joels L, White JO. L-selectin ligands in human endometrium: comparison of fertile and infertile subjects. Hum Reprod. 2009;24(11):2767–2777. doi: 10.1093/humrep/dep247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marions L, Danielsson KG. Expression of cyclo-oxygenase in human endometrium during the implantation period. Mol Hum Reprod. 1999;5(10):961–965. doi: 10.1093/molehr/5.10.961. [DOI] [PubMed] [Google Scholar]
- Marlin R, Nugeyre MT, Duriez M, Cannou C, Le Breton A, Berkane N, Barre-Sinoussi F, Menu E. Decidual soluble factors participate in the control of HIV-1 infection at the maternofetal interface. Retrovirology. 2011;8:58. doi: 10.1186/1742-4690-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Finn CA, Carter J. Effects of progesterone and oestradiol-17 beta on the luminal epithelium of the mouse uterus. J Reprod Fertil. 1970;21(3):461–469. doi: 10.1530/jrf.0.0210461. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245(2):280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Darcha C, Pouly JL, Mage G. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24(12):3180–3187. doi: 10.1093/humrep/dep306. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Darcha C, Maleysson E, Canis M, Mage G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J Clin Endocrinol Metab. 2010;95(7):3437–3445. doi: 10.1210/jc.2009-2713. [DOI] [PubMed] [Google Scholar]
- McConaha ME, Eckstrum K, An J, Steinle JJ, Bany BM. Microarray assessment of the influence of the conceptus on gene expression in the mouse uterus during decidualization. Reproduction. 2011;141(4):511–527. doi: 10.1530/REP-10-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JT, Greenwald GS. Evidence for a preimplantation rise in oestradiol-17beta levels on day 4 of pregnancy in the mouse. J Reprod Fertil. 1974;41(2):297–301. doi: 10.1530/jrf.0.0410297. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108(4):465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- McLaren A. A study of balstocysts during delay and subsequent implantation in lactating mice. J Endocrinol. 1968;42(3):453–463. doi: 10.1677/joe.0.0420453. [DOI] [PubMed] [Google Scholar]
- Mead RA. Embryonic diapause in vertebrates. J Exp Zool. 1993;266(6):629–641. doi: 10.1002/jez.1402660611. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2(1):64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- Menkhorst E, Salamonsen L, Robb L, Dimitriadis E. IL11 antagonist inhibits uterine stromal differentiation, causing pregnancy failure in mice. Biol Reprod. 2009;80(5):920–927. doi: 10.1095/biolreprod.108.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkhorst E, Zhang JG, Sims NA, Morgan PO, Soo P, Poulton IJ, Metcalf D, Alexandrou E, Gresle M, Salamonsen LA, Butzkueven H, Nicola NA, Dimitriadis E. Vaginally administered PEGylated LIF antagonist blocked embryo implantation and eliminated non-target effects on bone in mice. PLoS One. 2011;6(5):e19665. doi: 10.1371/journal.pone.0019665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131(9):2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling-two contrasting models. J Cell Sci. 2011;124(21):3537–3544. doi: 10.1242/jcs.091991. [DOI] [PubMed] [Google Scholar]
- Michael SD. Plasma prolactin and progesterone during the estrous cycle in the mouse. Royal Society of Medicine. 1976:254–257. doi: 10.3181/00379727-153-39522. [DOI] [PubMed] [Google Scholar]
- Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat Genet. 1998;20(3):228. doi: 10.1038/3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125(16):3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL, Kitawaki J, Lessey BA. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod. (3) 2012;27(3):881–888. doi: 10.1093/humrep/der452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20(8):2104–2117. doi: 10.1093/humrep/dei051. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6(8):584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Dufort D, Clarke HJ. Expression and estradiol regulation of Wnt genes in the mouse blastocyst identify a candidate pathway for embryo-maternal signaling at implantation. Biol Reprod. 2004;71(2):417–424. doi: 10.1095/biolreprod.103.025692. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102(24):8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamet L, Heath JK, Kimber SJ. Determining the LIF-sensitive period for implantation using a LIF-receptor antagonist. Reproduction. 2009;138(5):827–836. doi: 10.1530/REP-09-0113. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182(12):8080–8093. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- Molina H. Complement regulation during pregnancy. Immunol Res. 2005;32(1–3):187–192. doi: 10.1385/IR:32:1-3:187. [DOI] [PubMed] [Google Scholar]
- Mori M, Kitazume M, Ose R, Kurokawa J, Koga K, Osuga Y, Arai S, Miyazaki T. Death effector domain-containing protein (DEDD) is required for uterine decidualization during early pregnancy in mice. J Clin Invest. 2011;121(1):318–327. doi: 10.1172/JCI44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond M, Giedlin M, Coffman R. Two types of murine helper T cell clone I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- Mote PA, Arnett-Mansfield RL, Gava N, deFazio A, Mulac-Jericevic B, Conneely OM, Clarke CL. Overlapping and distinct expression of progesterone receptors A and B in mouse uterus and mammary gland during the estrous cycle. Endocrinology. 2006;147(12):5503–5512. doi: 10.1210/en.2006-0040. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Amato P, Allred DC, Fernandez-Valdivia R, Nguyen J, O’Malley BW, DeMayo FJ, Lydon JP. Steroid receptor coactivator 2 is essential for progesterone-dependent uterine function and mammary morphogenesis: insights from the mouse–implications for the human. J Steroid Biochem Mol Biol. 2006a;102(1–5):22–31. doi: 10.1016/j.jsbmb.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O’Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006b;26(17):6571–6583. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Munn DH, Pressey J, Beall AC, Hudes R, Alderson MR. Selective activation-induced apoptosis of peripheral T cells imposed by macrophages. A potential mechanism of antigen-specific peripheral lymphocyte deletion. J Immunol. 1996;156(2):523–532. [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Naeslund G, Lundkvist O, Nilsson BO. Transmission electron microscopy of mouse blastocysts activated and growth-arrested in vivo and in vitro. Anat Embryol (Berl) 1980;159(1):33–48. doi: 10.1007/BF00299253. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Shima T, Inada K, Ito M, Saito S. The balance of the immune system between T cells and NK cells in miscarriage. Am J Reprod Immunol. 2012;67(4):304–310. doi: 10.1111/j.1600-0897.2012.01115.x. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Aoki D, Suga T, Akama TO, Ishizone S, Yamaguchi H, Imakawa K, Nadano D, Fazleabas AT, Katsuyama T, Nozawa S, Fukuda MN. Implantation-dependent expression of trophinin by maternal fallopian tube epithelia during tubal pregnancies: possible role of human chorionic gonadotrophin on ectopic pregnancy. Am J Pathol. 2003;163(6):2211–2219. doi: 10.1016/S0002-9440(10)63579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallasamy S, Li Q, Bagchi MK, Bagchi IC. Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet. 2012;8(2):e1002500. doi: 10.1371/journal.pgen.1002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336(6086):1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narazaki M, Witthuhn BA, Yoshida K, Silvennoinen O, Yasukawa K, Ihle JN, Kishimoto T, Taga T. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Natl Acad Sci U S A. 1994;91(6):2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- Nejatbakhsh R, Kabir-Salmani M, Dimitriadis E, Hosseini A, Taheripanah R, Sadeghi Y, Akimoto Y, Iwashita M. Subcellular localization of L-selectin ligand in the endometrium implies a novel function for pinopodes in endometrial receptivity. Reprod Biol Endocrinol. 2012;10(1):46. doi: 10.1186/1477-7827-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Ding NZ, Harper MJ, Yang ZM. Expression of leukemia inhibitory factor receptor and gp130 in mouse uterus during early pregnancy. Mol Reprod Dev. 2002;63(2):143–150. doi: 10.1002/mrd.10168. [DOI] [PubMed] [Google Scholar]
- Nie G, Li Y, Wang M, Liu YX, Findlay JK, Salamonsen LA. Inhibiting uterine PC6 blocks embryo implantation: an obligatory role for a proprotein convertase in fertility. Biol Reprod. 2005;72(4):1029–1036. doi: 10.1095/biolreprod.104.036889. [DOI] [PubMed] [Google Scholar]
- Nieder GL, Weitlauf HM. Effects of metabolic substrates and ionic environment on in-vitro activation of delayed implanting mouse blastocysts. J Reprod Fertil. 1985;73(1):151–157. doi: 10.1530/jrf.0.0730151. [DOI] [PubMed] [Google Scholar]
- Nimbkar-Joshi S, Rosario G, Katkam RR, Manjramkar DD, Metkari SM, Puri CP, Sachdeva G. Embryo-induced alterations in the molecular phenotype of primate endometrium. J Reprod Immunol. 2009;83(1–2):65–71. doi: 10.1016/j.jri.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Nobuzane T, Tashiro S, Kudo Y. Morphologic effects of epithelial ion channels on the mouse uterus: differences between raloxifene analog (LY117018) and estradiol treatments. Am J Obstet Gynecol. 2008;199(4):363, e361–366. doi: 10.1016/j.ajog.2008.03.047. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Okada H, Nie G, Salamonsen LA. Requirement for proprotein convertase 5/6 during decidualization of human endometrial stromal cells in vitro. J Clin Endocrinol Metab. 2005;90(2):1028–1034. doi: 10.1210/jc.2004-0904. [DOI] [PubMed] [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306(2):493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, Huang S, Roberts AB, Umans L, Huylebroeck D, Zwijsen A, Wang D, Martin JF, Behringer RR. Functional redundancy of TGF-beta family type I receptors and receptor-Smads in mediating anti-Mullerian hormone-induced Mullerian duct regression in the mouse. Biol Reprod. 2008;78(6):994–1001. doi: 10.1095/biolreprod.107.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabona JM, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, Zelenko Z, Giudice LC, Simmen RC. Kruppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2012;97(3):E376–392. doi: 10.1210/jc.2011-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabona JM, Zeng Z, Simmen FA, Simmen RC. Functional differentiation of uterine stromal cells involves cross-regulation between bone morphogenetic protein 2 and Kruppel-like factor (KLF) family members KLF9 and KLF13. Endocrinology. 2010;151(7):3396–3406. doi: 10.1210/en.2009-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampfer S, Donnay I. Apoptosis at the time of embryo implantation in mouse and rat. Cell Death Differ. 1999;6(6):533–545. doi: 10.1038/sj.cdd.4400516. [DOI] [PubMed] [Google Scholar]
- Parhar RS, Yagel S, Lala PK. PGE2-mediated immunosuppression by first trimester human decidual cells blocks activation of maternal leukocytes in the decidua with potential anti-trophoblast activity. Cell Immunol. 1989;120(1):61–74. doi: 10.1016/0008-8749(89)90174-3. [DOI] [PubMed] [Google Scholar]
- Paria BC, Chakraborty C, Dey SK. Catechol estrogen formation in the mouse uterus and its role in implantation. Mol Cell Endocrinol. 1990;69(1):25–32. doi: 10.1016/0303-7207(90)90085-m. [DOI] [PubMed] [Google Scholar]
- Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci U S A. 1995;92(21):9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Deutsch DD, Dey SK. The uterus is a potential site for anandamide synthesis and hydrolysis: differential profiles of anandamide synthase and hydrolase activities in the mouse uterus during the periimplantation period. Mol Reprod Dev. 1996;45(2):183–192. doi: 10.1002/(SICI)1098-2795(199610)45:2<183::AID-MRD11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999a;126(9):1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993;90(21):10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Lim H, Wang XN, Liehr J, Das SK, Dey SK. Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse. Endocrinology. 1998;139(12):5235–5246. doi: 10.1210/endo.139.12.6386. [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001a;98(3):1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296(5576):2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, Bonner TI, Zimmer A, Dey SK. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001b;276(23):20523–20528. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- Paria BC, Tan J, Lubahn DB, Dey SK, Das SK. Uterine decidual response occurs in estrogen receptor-alpha-deficient mice. Endocrinology. 1999b;140(6):2704–2710. doi: 10.1210/endo.140.6.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Zhao X, Das SK, Dey SK, Yoshinaga K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev Biol. 1999c;208(2):488–501. doi: 10.1006/dbio.1999.9206. [DOI] [PubMed] [Google Scholar]
- Park CB, DeMayo FJ, Lydon JP, Dufort D. NODAL in the uterus is necessary for proper placental development and maintenance of pregnancy. Biol Reprod. 2012a;86(6):194. doi: 10.1095/biolreprod.111.098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Yoon S, Zhao Y, Park SE, Liao L, Xu J, Lydon JP, Demayo FJ, O’Malley BW, Bagchi MK, Katzenellenbogen BS. Uterine Development and Fertility Are Dependent on Gene Dosage of the Nuclear Receptor Coregulator REA. Endocrinology. 2012b;153(8):3982–3994. doi: 10.1210/en.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237(2):324–332. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395(6703):707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Parr EL, Tung HN, Parr MB. Apoptosis as the mode of uterine epithelial cell death during embryo implantation in mice and rats. Biol Reprod. 1987;36(1):211–225. doi: 10.1095/biolreprod36.1.211. [DOI] [PubMed] [Google Scholar]
- Pavlova A, Boutin E, Cunha G, Sassoon D. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development. 1994;120(2):335–345. doi: 10.1242/dev.120.2.335. [DOI] [PubMed] [Google Scholar]
- Peng S, Li J, Miao C, Jia L, Hu Z, Zhao P, Zhang Y, Chen Q, Duan E. Dickkopf-1 secreted by decidual cells promotes trophoblast cell invasion during murine placentation. Reproduction. 2008;135(3):367–375. doi: 10.1530/REP-07-0191. [DOI] [PubMed] [Google Scholar]
- Persson M, Ekerfelt C, Jablonowska B, Jonsson Y, Ernerudh J, Jenmalm MC, Berg G. Immunological status in patients undergoing in vitro fertilisation: responses to hormone treatment and relation SHIP to outcome. J Reprod Immunol. 2012 doi: 10.1016/j.jri.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4(9):1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Romagnani S. Regulation of fetal allograft survival by hormone-controlled Th1-and Th2-type cytokines. Immunol Res. 1996;15(2):141–150. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Lidegaard Ø, Andersen AN. The vanishing twin: a major determinant of infant outcome in IVF singleton births. Br J Hosp Med. 2006;67(8):417–420. doi: 10.12968/hmed.2006.67.8.21976. [DOI] [PubMed] [Google Scholar]
- Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118(12):3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Uterine DCs are essential for pregnancy. J Clin Invest. 2008;118(12):3832–3835. doi: 10.1172/JCI37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool TB, Schoolfield J, Han D. Human embryo culture media comparisons. Methods Mol Biol. 2012;912:367–386. doi: 10.1007/978-1-61779-971-6_21. [DOI] [PubMed] [Google Scholar]
- Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973;31:201–256. doi: 10.1016/s0083-6729(08)60999-1. [DOI] [PubMed] [Google Scholar]
- Psychoyos A. Uterine Receptivity for Nidationa. Ann N Y Acad Sci. 1986;476(1):36–42. doi: 10.1111/j.1749-6632.1986.tb20920.x. [DOI] [PubMed] [Google Scholar]
- Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122(2):637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Liu FT, Hirashima M, Anderson A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol. 2007;66(2–3):143–158. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1-and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196(2):122. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Li M, Li P, Wang H, Dey SK, Das SK. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev Biol. 2006;290(1):105–117. doi: 10.1016/j.ydbio.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal C, Wang W, Hunt E, Bagchi IC, Bagchi MK. Transcription factor CCAAT enhancer-binding protein beta (C/EBPbeta) regulates the formation of a unique extracellular matrix that controls uterine stromal differentiation and embryo implantation. J Biol Chem. 2011;286(22):19860–19871. doi: 10.1074/jbc.M110.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KA. Putting sexual and reproductive health on the agenda. Int J Gynaecol Obstet. 2008;102(3):221–222. doi: 10.1016/j.ijgo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Rao KP, Belogolovkin V, Yankowitz J, Spinnato JA. Abnormal placentation: evidence-based diagnosis and management of placenta previa, placenta accreta, and vasa previa. Obstet Gynecol Surv. (2) 2012;67(8):503–519. doi: 10.1097/OGX.0b013e3182685870. [DOI] [PubMed] [Google Scholar]
- Rashid NA, Lalitkumar S, Lalitkumar PG, Gemzell-Danielsson K. Endometrial receptivity and human embryo implantation. Am J Reprod Immunol. 2011;66(Suppl 1):23–30. doi: 10.1111/j.1600-0897.2011.01048.x. [DOI] [PubMed] [Google Scholar]
- Rato ML, Gouveia-Oliveira A, Plancha CE. Influence of post-thaw culture on the developmental potential of human frozen embryos. J Assist Reprod Genet. 2012;29(8):789–795. doi: 10.1007/s10815-012-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94(7):2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon SN, King ML, MacLean JA, Mann JL, DeMayo FJ, Lydon JP, Hayashi K. CDH1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol Reprod. (2) 2012;86(5):141, 141–110. doi: 10.1095/biolreprod.112.098871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Drake PM, Gunn MD, Fisher SJ. Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol. 2001;159(6):2199–2213. doi: 10.1016/S0002-9440(10)63071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114(6):744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276(47):44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- Reese J, Wang H, Ding T, Paria BC. The hamster as a model for embryo implantation: insights into a multifaceted process. Semin Cell Dev Biol. 2008;19(2):194–203. doi: 10.1016/j.semcdb.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129(1):33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197(6):751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfree MB, Shaw G. Diapause. Annu Rev Physiol. 2000;62:353–375. doi: 10.1146/annurev.physiol.62.1.353. [DOI] [PubMed] [Google Scholar]
- Revel A, Achache H, Stevens J, Smith Y, Reich R. MicroRNAs are associated with human embryo implantation defects. Hum Reprod. 2011;26(10):2830–2840. doi: 10.1093/humrep/der255. [DOI] [PubMed] [Google Scholar]
- Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simon C. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9(5):253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13(10):2869–2873. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4(3):303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- Roberts CL, Algert CS, March LM. Delayed childbearing–are there any risks? The Medical journal of Australia. 1994;160(9):539. [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80(5):1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblero LS, Fernandez O, Croxatto HB. The effect of RU486 on transport, development and implantation of mouse embryos. Contraception. 1987;36(5):549–555. doi: 10.1016/0010-7824(87)90007-2. [DOI] [PubMed] [Google Scholar]
- Rogers P, Murphy C. Uterine receptivity for implantation: human studies. 1989:231–238. [Google Scholar]
- Rogers PA, Murphy CR, Rogers AW, Gannon BJ. Capillary patency and permeability in the endometrium surrounding the implanting rat blastocyst. Int J Microcirc Clin Exp. 1983;2(3):241–249. [PubMed] [Google Scholar]
- Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol Rev. 2003;196:85–108. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10(1):54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Listeria monocytogenes Cytoplasmic Entry Induces Fetal Wastage by Disrupting Maternal Foxp3(+) Regulatory T Cell-Sustained Fetal Tolerance. PLoS Pathog. 2012a;8(8):e1002873. doi: 10.1371/journal.ppat.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012b doi: 10.1038/nature11462. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YC, Guo JH, Liu X, Zhang R, Tsang LL, Dong JD, Chen H, Yu MK, Jiang X, Zhang XH, Fok KL, Chung YW, Huang H, Zhou WL, Chan HC. Activation of the epithelial Na(+) channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat Med. 2012 doi: 10.1038/nm.2771. [DOI] [PubMed] [Google Scholar]
- Ryan KE, Chiang C. Hedgehog secretion and signal transduction in vertebrates. J Biol Chem. 2012;287(22):17905–17913. doi: 10.1074/jbc.R112.356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L, Shelesnyak MC. The development and suppression of polyploidy in the developing and suppressed deciduoma in the rat. J Endocrinol. 1955;12(2):146–151. doi: 10.1677/joe.0.0120146. [DOI] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19(8):746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47(2):87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Shima T, Ito M. REVIEW ARTICLE: Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- Saito S, Shima T, Nakashima A, Shiozaki A, Ito M, Sasaki Y. What is the role of regulatory T cells in the success of implantation and early pregnancy? J Assist Reprod Genet. 2007;24(9):379–386. doi: 10.1007/s10815-007-9140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25)Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- Salker MS, Christian M, Steel JH, Nautiyal J, Lavery S, Trew G, Webster Z, Al-Sabbagh M, Puchchakayala G, Foller M, Landles C, Sharkey AM, Quenby S, Aplin JD, Regan L, Lang F, Brosens JJ. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med. 2011;17(11):1509–1513. doi: 10.1038/nm.2498. [DOI] [PubMed] [Google Scholar]
- Salleh N, Baines DL, Naftalin RJ, Milligan SR. The hormonal control of uterine luminal fluid secretion and absorption. J Membr Biol. 2005;206(1):17–28. doi: 10.1007/s00232-005-0770-7. [DOI] [PubMed] [Google Scholar]
- Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, de St Groth BF, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183(11):7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Sato T, Wang G, Hardy MP, Kurita T, Cunha GR, Cooke PS. Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology. 2002;143(7):2673–2679. doi: 10.1210/endo.143.7.8878. [DOI] [PubMed] [Google Scholar]
- Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod. 1975;12(1):41–65. doi: 10.1095/biolreprod12.1.41. [DOI] [PubMed] [Google Scholar]
- Schlafke S, Welsh AO, Enders AC. Penetration of the basal lamina of the uterine luminal epithelium during implantation in the rat. Anat Rec. 1985;212(1):47–56. doi: 10.1002/ar.1092120107. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 1997;94(8):4188–4192. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle RF, Jones RK, Bulmer JN. Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biol Reprod. 1999;60(4):871–878. doi: 10.1095/biolreprod60.4.871. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131(7):1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11(12):951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Sengupta J, Paria BC, Manchanda SK. Effect of an estrogen antagonist on development of blastocysts and implantation in the hamster. J Exp Zool. 1983;225(1):119–122. doi: 10.1002/jez.1402250114. [DOI] [PubMed] [Google Scholar]
- Serafini PC, Silva ID, Smith GD, Motta EL, Rocha AM, Baracat EC. Endometrial claudin-4 and leukemia inhibitory factor are associated with assisted reproduction outcome. Reprod Biol Endocrinol. 2009;7:30. doi: 10.1186/1477-7827-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamonki MI, Kligman I, Shamonki JM, Schattman GL, Hyjek E, Spandorfer SD, Zaninovic N, Rosenwaks Z. Immunohistochemical expression of endometrial L-selectin ligand is higher in donor egg recipients with embryonic implantation. Fertil Steril. 2006;86(5):1365–1375. doi: 10.1016/j.fertnstert.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–348. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- Shevach EM. The resurrection of T cell-mediated suppression. J Immunol. 2011;186(7):3805–3807. doi: 10.4049/jimmunol.1100364. [DOI] [PubMed] [Google Scholar]
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85(2):121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Sholl SA, Orsini MW, Hitchins DJ. Estrogen synthesis and metabolism in the hamster blastocyst, uterus and liver near the time of implantation. J Steroid Biochem. 1983;19(2):1153–1161. doi: 10.1016/0022-4731(83)90410-7. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;279(28):29286–29294. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- Simon L, Spiewak KA, Ekman GC, Kim J, Lydon JP, Bagchi MK, Bagchi IC, DeMayo FJ, Cooke PS. Stromal progesterone receptors mediate induction of Indian Hedgehog (IHH) in uterine epithelium and its downstream targets in uterine stroma. Endocrinology. 2009;150(8):3871–3876. doi: 10.1210/en.2008-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Singh H, Aplin JD. Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. J Anat. 2009;215(1):3–13. doi: 10.1111/j.1469-7580.2008.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Alnifaidy R, Wei Q, Nieman LK. Endometrial Indian hedgehog expression is decreased in women with endometriosis. Fertil Steril. 2011;95(8):2738–2741. e2731–2733. doi: 10.1016/j.fertnstert.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation–review. Placenta. 2010;31(10):839–847. doi: 10.1016/j.placenta.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Han K, Lim H. Progesterone supplementation extends uterine receptivity for blastocyst implantation in mice. Reproduction. 2007;133(2):487–493. doi: 10.1530/REP-06-0330. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction. 2006;131(2):341–349. doi: 10.1530/rep.1.00956. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14(8):1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129(12):2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: insights into mechanisms and developmental disruption. Mol Cell Endocrinol. 2012;354(1–2):34–53. doi: 10.1016/j.mce.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389(6647):194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8(8):765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- Steck T, Giess R, Suetterlin MW, Bolland M, Wiest S, Poehls UG, Dietl J. Leukaemia inhibitory factor (LIF) gene mutations in women with unexplained infertility and recurrent failure of implantation after IVF and embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2004;112(1):69–73. doi: 10.1016/s0301-2115(03)00315-4. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Fisher SJ, Wang Y, Stewart MD, Hewitt SC, Rodriguez KF, Korach KS, Behringer RR. Uterine gland formation in mice is a continuous process, requiring the ovary after puberty, but not after parturition. Biol Reprod. 2011;85(5):954–964. doi: 10.1095/biolreprod.111.091470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176(2):778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- Su EJ, Xin H, Monsivais D. The emerging role of estrogen receptor-beta in human reproduction. Semin Reprod Med. 2012;30(1):62–70. doi: 10.1055/s-0031-1299598. [DOI] [PubMed] [Google Scholar]
- Sugihara K, Kabir-Salmani M, Byrne J, Wolf DP, Lessey B, Iwashita M, Aoki D, Nakayama J, Fukuda MN. Induction of trophinin in human endometrial surface epithelia by CGbeta and IL-1beta. FEBS Lett. 2008;582(2):197–202. doi: 10.1016/j.febslet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Sugihara K, Sugiyama D, Byrne J, Wolf DP, Lowitz KP, Kobayashi Y, Kabir-Salmani M, Nadano D, Aoki D, Nozawa S, Nakayama J, Mustelin T, Ruoslahti E, Yamaguchi N, Fukuda MN. Trophoblast cell activation by trophinin ligation is implicated in human embryo implantation. Proc Natl Acad Sci U S A. 2007;104(10):3799–3804. doi: 10.1073/pnas.0611516104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Dey SK. Cannabinoid/Endocannabinoid signaling impact on early pregnancy events. Curr Top Behav Neurosci. 2009;1:255–273. doi: 10.1007/978-3-540-88955-7_10. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang L, Xie H, Wan H, Magella B, Whitsett JA, Dey SK. Kruppel-like factor 5 (KLF5) is critical for conferring uterine receptivity to implantation. Proc Natl Acad Sci U S A. 2012;109(4):1145–1150. doi: 10.1073/pnas.1118411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surani MA. Hormonal regulation of proteins in the uterine secretion of ovariectomized rats and the implications for implantation and embryonic diapause. J Reprod Fertil. 1975;43(3):411–417. doi: 10.1530/jrf.0.0430411. [DOI] [PubMed] [Google Scholar]
- Sutherland AE, Calarco PG, Damsky CH. Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development. 1993;119(4):1175–1186. doi: 10.1242/dev.119.4.1175. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36(4):417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Nadano D, Paria BC, Kupriyanov S, Sugihara K, Fukuda MN. Trophinin expression in the mouse uterus coincides with implantation and is hormonally regulated but not induced by implanting blastocysts. Endocrinology. 2000;141(11):4247–4254. doi: 10.1210/endo.141.11.7738. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Zara J, Sato T, Ong E, Bakhiet N, Oshima RG, Watson KL, Fukuda MN. A cytoplasmic protein, bystin, interacts with trophinin, tastin, and cytokeratin and may be involved in trophinin-mediated cell adhesion between trophoblast and endometrial epithelial cells. Proc Natl Acad Sci U S A. 1998;95(9):5027–5032. doi: 10.1073/pnas.95.9.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, Minatogawa Y. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. 2001;355(2):425–429. doi: 10.1042/0264-6021:3550425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Arvola M, Sällström MA, Holmdahl R, Mattsson R. The Th2 cytokines IL-4 and IL-10 are not crucial for the completion of allogeneic pregnancy in mice. J Reprod Immunol. 2001;51(1):3–7. doi: 10.1016/s0165-0378(01)00065-1. [DOI] [PubMed] [Google Scholar]
- Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2010;54(2–3):421–430. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19(9):2299–2308. doi: 10.1210/me.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16(10):2338–2348. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Tamura N, Sugihara K, Akama TO, Fukuda MN. Trophinin-mediated cell adhesion induces apoptosis of human endometrial epithelial cells through PKC-delta. Cell Cycle. 2011;10(1):135–143. doi: 10.4161/cc.10.1.14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140(11):5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265(1):181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6(5):377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Tang M, Mikhailik A, Pauli I, Giudice LC, Fazelabas AT, Tulac S, Carson DD, Kaufman DG, Barbier C, Creemers JW, Tabibzadeh S. Decidual differentiation of stromal cells promotes Proprotein Convertase 5/6 expression and lefty processing. Endocrinology. 2005;146(12):5313–5320. doi: 10.1210/en.2005-0684. [DOI] [PubMed] [Google Scholar]
- Tato CM, O’Shea JJ. Immunology: what does it mean to be just 17? Nature. 2006;441(7090):166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Higuchi T, Fujiwara H, Nakayama T, Egawa H, Itoh K, Fujii S, Fujita J. Induction of tryptophan 2,3-dioxygenase in the mouse endometrium during implantation. Biochem Biophys Res Commun. 2000;274(1):166–170. doi: 10.1006/bbrc.2000.3115. [DOI] [PubMed] [Google Scholar]
- Taylor HS. The role of HOX genes in human implantation. Hum Reprod Update. 2000;6(1):75–79. doi: 10.1093/humupd/6.1.75. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999a;14(5):1328–1331. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999b;84(3):1129–1135. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57(6):1338–1345. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- Terakawa J, Wakitani S, Sugiyama M, Inoue N, Ohmori Y, Kiso Y, Hosaka YZ, Hondo E. Embryo implantation is blocked by intraperitoneal injection with anti-LIF antibody in mice. J Reprod Dev. 2011;57(6):700–707. doi: 10.1262/jrd.11-048h. [DOI] [PubMed] [Google Scholar]
- Thie M, Fuchs P, Denker HW. Epithelial cell polarity and embryo implantation in mammals. Int J Dev Biol. 1996;40(1):389–393. [PubMed] [Google Scholar]
- Thie M, Harrach-Ruprecht B, Sauer H, Fuchs P, Albers A, Denker HW. Cell adhesion to the apical pole of epithelium: a function of cell polarity. Eur J Cell Biol. 1995;66(2):180–191. [PubMed] [Google Scholar]
- Thie M, Rospel R, Dettmann W, Benoit M, Ludwig M, Gaub HE, Denker HW. Interactions between trophoblast and uterine epithelium: monitoring of adhesive forces. Hum Reprod. 1998;13(11):3211–3219. doi: 10.1093/humrep/13.11.3211. [DOI] [PubMed] [Google Scholar]
- Thom MD, Johnson DDP, Macdonald DW. The evolution and maintenance of delayed implantation in the Mustelidae (Mammalia: Carnivora) Evolution. 2004;58(1):175–183. doi: 10.1111/j.0014-3820.2004.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Tiitinen A. Prevention of multiple pregnancies in infertility treatment. Best Pract Res Clin Obstet Gynaecol. 2012 doi: 10.1016/j.bpobgyn.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Toldi G, Rigó J, Jr, Stenczer B, Vásárhelyi B, Molvarec A. Increased Prevalence of IL-17-Producing Peripheral Blood Lymphocytes in Pre-eclampsia. Am J Reprod Immunol. 2011;66(3):223–229. doi: 10.1111/j.1600-0897.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005a;102(40):14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci. 2005b;62(17):1964–1973. doi: 10.1007/s00018-005-5230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Smith DF, Dey SK. Progesterone receptor requires a co-chaperone for signalling in uterine biology and implantation. Reprod Biomed Online. 2006;13(5):651–660. doi: 10.1016/s1472-6483(10)60655-4. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- Tsai CY, Chou CK, Yang CW, Lai YC, Liang CC, Chen CM, Tsai TF. Hurp deficiency in mice leads to female infertility caused by an implantation defect. J Biol Chem. 2008;283(39):26302–26306. doi: 10.1074/jbc.C800117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou AP, Yang CW, Huang CY, Yu RC, Lee YC, Chang CW, Chen BR, Chung YF, Fann MJ, Chi CW, Chiu JH, Chou CK. Identification of a novel cell cycle regulated gene, HURP, overexpressed in human hepatocellular carcinoma. Oncogene. 2003;22(2):298–307. doi: 10.1038/sj.onc.1206129. [DOI] [PubMed] [Google Scholar]
- Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab. 2006;91(4):1453–1461. doi: 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- Tung HN, Parr MB, Parr EL. The permeability of the primary decidual zone in the rat uterus: an ultrastructural tracer and freeze-fracture study. Biol Reprod. 1986;35(4):1045–1058. doi: 10.1095/biolreprod35.4.1045. [DOI] [PubMed] [Google Scholar]
- Ueda N, Puffenbarger RA, Yamamoto S, Deutsch DG. The fatty acid amide hydrolase (FAAH) Chem Phys Lipids. 2000;108(1–2):107–121. doi: 10.1016/s0009-3084(00)00190-0. [DOI] [PubMed] [Google Scholar]
- Ueda O, Yorozu K, Kamada N, Jishage K, Kawase Y, Toyoda Y, Suzuki H. Possible expansion of “Window of Implantation” in pseudopregnant mice: time of implantation of embryos at different stages of development transferred into the same recipient. Biol Reprod. 2003;69(3):1085–1090. doi: 10.1095/biolreprod.103.017608. [DOI] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389(6651):607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Chavez DJ, Bell H. Molecular and cellular aspects of facultative delayed implantation in the mouse. Ciba Found Symp. 1978;(64):141–172. doi: 10.1002/9780470720479.ch7. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC. Null mutation of Kruppel-like factor9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biol Reprod. 2005;73(3):472–481. doi: 10.1095/biolreprod.105.041855. [DOI] [PubMed] [Google Scholar]
- Vinijsanun A, Martin L. Effects of progesterone antagonists RU486 and ZK9873 on embryo transport, development and implantation in laboratory mice. Reprod Fertil Dev. 1990;2(6):713–727. doi: 10.1071/rd9900713. [DOI] [PubMed] [Google Scholar]
- Volchek M, Girling JE, Lash GE, Cann L, Kumar B, Robson SC, Bulmer JN, Rogers PA. Lymphatics in the human endometrium disappear during decidualization. Hum Reprod. 2010;25(10):2455–2464. doi: 10.1093/humrep/deq224. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta-/- mice. Proc Natl Acad Sci U S A. 2006;103(48):18350–18355. doi: 10.1073/pnas.0608861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Sheng JZ, He RH, Qian YL, Jin F, Huang HF. High expression of L-selectin ligand in secretory endometrium is associated with better endometrial receptivity and facilitates embryo implantation in human being. Am J Reprod Immunol. 2008;60(2):127–134. doi: 10.1111/j.1600-0897.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat. 2005;77(1–4):84–102. doi: 10.1016/j.prostaglandins.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK, Maccarrone M. Jekyll and hyde: two faces of cannabinoid signaling in male and female fertility. Endocr Rev. 2006a;27(5):427–448. doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004a;10(10):1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- Wang H, Ma WG, Tejada L, Zhang H, Morrow JD, Das SK, Dey SK. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J Biol Chem. 2004b;279(11):10649–10658. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci U S A. 2003;100(25):14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xie H, Dey SK. Endocannabinoid signaling directs periimplantation events. AAPS J. 2006b;8(2):E425–432. doi: 10.1007/BF02854916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xie H, Guo Y, Zhang H, Takahashi T, Kingsley PJ, Marnett LJ, Das SK, Cravatt BF, Dey SK. Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest. 2006c;116(8):2122–2131. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xie H, Sun X, Tranguch S, Zhang H, Jia X, Wang D, Das SK, Desvergne B, Wahli W, DuBois RN, Dey SK. Stage-specific integration of maternal and embryonic peroxisome proliferator-activated receptor delta signaling is critical to pregnancy success. J Biol Chem. 2007a;282(52):37770–37782. doi: 10.1074/jbc.M706577200. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Armant DR. Integrin signaling regulates blastocyst adhesion to fibronectin at implantation: intracellular calcium transients and vesicle trafficking in primary trophoblast cells. Dev Biol. 2002;245(2):270–279. doi: 10.1006/dbio.2002.0644. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Armant DR. Trophoblast adhesion of the peri-implantation mouse blastocyst is regulated by integrin signaling that targets phospholipase C. Dev Biol. 2007b;302(1):143–153. doi: 10.1016/j.ydbio.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Schultz JF, Armant DR. Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development. 2000;127(1):33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- Wang J, Paria BC, Dey SK, Armant DR. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol Reprod. 1999;60(4):839–844. doi: 10.1095/biolreprod60.4.839. [DOI] [PubMed] [Google Scholar]
- Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, Davis RW, Zernicka-Goetz M. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004c;6(1):133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- Wang W, Li Q, Bagchi IC, Bagchi MK. The CCAAT/enhancer binding protein beta is a critical regulator of steroid-induced mitotic expansion of uterine stromal cells during decidualization. Endocrinology. 2010a;151(8):3929–3940. doi: 10.1210/en.2009-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Van De Water T, Lufkin T. Inner ear and maternal reproductive defects in mice lacking the Hmx3 homeobox gene. Development. 1998;125(4):621–634. doi: 10.1242/dev.125.4.621. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Hao CF, Yin GJ, Bao SH, Qiu LH, Lin QD. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010b;84(2):164–170. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Wang X, Matsumoto H, Zhao X, Das SK, Paria BC. Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J Cell Sci. 2004d;117(1):53–62. doi: 10.1242/jcs.00826. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Mammalian fertilization: molecular aspects of gamete adhesion, exocytosis, and fusion. Cell. 1999;96(2):175–183. doi: 10.1016/s0092-8674(00)80558-9. [DOI] [PubMed] [Google Scholar]
- Wei Q, Levens ED, Stefansson L, Nieman LK. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB-2914. J Clin Endocrinol Metab. 2010;95(12):5330–5337. doi: 10.1210/jc.2010-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. Light and electron microscopic examination of the mature decidual cells of the rat with emphasis on the antimesometrial decidua and its degeneration. Am J Anat. 1985;172(1):1–29. doi: 10.1002/aja.1001720102. [DOI] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. Trophoblast-decidual cell interactions and establishment of maternal blood circulation in the parietal yolk sac placenta of the rat. Anat Rec. 1987;217(2):203–219. doi: 10.1002/ar.1092170213. [DOI] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. Chorioallantoic placenta formation in the rat: I Luminal epithelial cell death and extracellular matrix modifications in the mesometrial region of implantation chambers. Am J Anat. 1991;192(3):215–231. doi: 10.1002/aja.1001920302. [DOI] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357(1–2):108–118. doi: 10.1016/j.mce.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CA, Zhang JG, Salamonsen LA, Baca M, Fairlie WD, Metcalf D, Nicola NA, Robb L, Dimitriadis E. Blocking LIF action in the uterus by using a PEGylated antagonist prevents implantation: a nonhormonal contraceptive strategy. Proc Natl Acad Sci U S A. 2007;104(49):19357–19362. doi: 10.1073/pnas.0710110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten WK. Endocrine studies on delayed implantation in lactating mice. J Endocrinol. 1955;13(1):1–6. doi: 10.1677/joe.0.0130001. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Endocrine studies on delayed implantation in lactating mice; role of the pituitary in implantation. J Endocrinol. 1958;16(4):435–440. doi: 10.1677/joe.0.0160435. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Wilcox LS, Peterson HB, Haseltine FP, Martin MC. Defining and interpreting pregnancy success rates for in vitro fertilization. Fertil Steril. 1993;60(1):18–25. doi: 10.1016/s0015-0282(16)56030-0. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107(45):19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JT, Meyer RK. Ultrastructural changes of rat blastocysts induced by estrogen during delayed implantation. Anat Rec. 1974;179(2):253–271. doi: 10.1002/ar.1091790208. [DOI] [PubMed] [Google Scholar]
- Xie H, Tranguch S, Jia X, Zhang H, Das SK, Dey SK, Kuo CJ, Wang H. Inactivation of nuclear Wnt-beta-catenin signaling limits blastocyst competency for implantation. Development. 2008;135(4):717–727. doi: 10.1242/dev.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A. 2007;104(46):18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000a;287(5452):498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17(9):1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proceedings of the National Academy of Sciences. 2000b;97(12):6379. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279(5358):1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89(3):656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Ajonuma LC, Tsang LL, Lam SY, Rowlands DK, Ho LS, Zhou CX, Chung YW, Chan HC. Differential expression and localization of CFTR and ENaC in mouse endometrium during pre-implantation. Cell Biol Int. 2004;28(6):433–439. doi: 10.1016/j.cellbi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Jiang X, Dong J, Guo J, Chen H, Tsang LL, Chung YW, Zhang X, Chan HC. Abnormally enhanced cystic fibrosis transmembrane conductance regulator-mediated apoptosis in endometrial cells contributes to impaired embryo implantation in controlled ovarian hyperstimulation. Fertil Steril. 2011;95(6):2100–2106. e2101–2102. doi: 10.1016/j.fertnstert.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER, Shou W. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20(11):2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435(7038):104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Herr DR, Diao H, Rivera R, Chun J. Unique uterine localization and regulation may differentiate LPA3 from other lysophospholipid receptors for its role in embryo implantation. Fertil Steril. 2011;95(6):2107–2113. 2113 e2101–2104. doi: 10.1016/j.fertnstert.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad1, during mouse decidualization. Biol Reprod. 2000;63(6):1781–1786. doi: 10.1095/biolreprod63.6.1781. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21(1):102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Choi DH, Lee WS, Cha KY, Kim SN, Lee KA. A molecular basis for embryo apposition at the luminal epithelium. Mol Cell Endocrinol. 2004;219(1–2):95–104. doi: 10.1016/j.mce.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Inhibition of implantation by advancement of uterine sensitivity and refractoriness. In: Leroy F, Finn CA, Psychoyos A, Hubinot PO, editors. Blastocysts-endometrium relationships: progress in reproductive biology. Karger; Basel, Switzerland: 1980. pp. 189–199. [Google Scholar]
- Yoshinaga K. Uterine receptivity for blastocyst implantation. Ann N Y Acad Sci. 1988;541:424–431. doi: 10.1111/j.1749-6632.1988.tb22279.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Two concepts on the immunological aspect of blastocyst implantation. J Reprod Dev. 2012;58(2):196–203. doi: 10.1262/jrd.2011-027. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil. 1966;12(3):593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Hawkins R, Stocker J. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969;85(1):103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Matsuda F, Takakura K, Noda Y, Imakawa K, Sakai S. Determination of genes involved in the process of implantation: application of GeneChip to scan 6500 genes. Biochem Biophys Res Commun. 2000;272(2):531–538. doi: 10.1006/bbrc.2000.2818. [DOI] [PubMed] [Google Scholar]
- Yue L, Daikoku T, Hou X, Li M, Wang H, Nojima H, Dey SK, Das SK. Cyclin G1 and cyclin G2 are expressed in the periimplantation mouse uterus in a cell-specific and progesterone-dependent manner: evidence for aberrant regulation with Hoxa-10 deficiency. Endocrinology. 2005;146(5):2424–2433. doi: 10.1210/en.2004-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32(3):387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi-Tehrani M. Kinetics of murine decidual dendritic cells. Reproduction. 2007;133(1):275–283. doi: 10.1530/rep.1.01232. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC. CD4(+)CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol. 2005;65(2):101–110. doi: 10.1016/j.jri.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S, Zambon Bertoja A, Fest S, Hontsu S, Ueha S, Matsushima K, Leber J. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. 2005;36(1):82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272(2):483. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Paria BC. Importance of uterine cell death, renewal, and their hormonal regulation in hamsters that show progesterone-dependent implantation. Endocrinology. 2006;147(5):2215–2227. doi: 10.1210/en.2005-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XY, Chen GA, Wang HY. Expression of cystic fibrosis transmembrane conductance regulator in human endometrium. Hum Reprod. 2004;19(12):2933–2941. doi: 10.1093/humrep/deh507. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci U S A. 2007;104(40):15847–15851. doi: 10.1073/pnas.0705749104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Zhou YH, Wang MY, Jin LP, Yuan MM, Li DJ. Blockade of CD86 signaling facilitates a Th2 bias at the maternal-fetal interface and expands peripheral CD4+CD25+ regulatory T cells to rescue abortion-prone fetuses. Biol Reprod. 2005;72(2):338–345. doi: 10.1095/biolreprod.104.034108. [DOI] [PubMed] [Google Scholar]
- Zinaman MJ, Clegg E, Brown CC, O’connor J, Selevan S. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65(3):503. [PubMed] [Google Scholar]


