Abstract
There is an increase in the cardiovascular disease (CVD) morbidity in individuals infected with HIV that may be due to inflammatory lipid modulation not captured by traditional lipid measures. The objective of this study was to perform advanced lipoprotein phenotyping inclusive of the high-density lipoprotein (HDL) cholesterol efflux capacity and lipoprotein particle concentration and size in a well-phenotyped group of 118 patients infected with HIV. We used simple and multivariable analyses to determine the associations between advanced lipoprotein parameters and known cardiometabolic risk factors. Participants were on stable antiretroviral therapy (ART) and had benign traditional lipid panels [median total cholesterol, low-density lipoprotein (LDL)-C, HDL-C, and triglycerides of 178 mg/dl, 108 mg/dl, 44 mg/dl, and 122.5 mg/dl, respectively]. However, advanced lipoprotein phenotyping demonstrated an elevation of LDL particle number (median of 1,233 nmol/liter) and a decrease in LDL size (median of 20.4 nm), along with a decrease in protective, large HDL particles (median of 3.15 μmol/liter) and reduced HDL cholesterol efflux capacity in comparison to controls of other studies. HDL cholesterol efflux capacity was associated with HDL levels (β=0.395, p<0.001), small LDL particle concentration (β=–0.198, p=0.031), insulin sensitivity by the Matsuda index (β=0.218, p=0.029), and the Framingham Risk Score (β=–0.184, p=0.046). We demonstrate an atherogenic lipoprotein profile by NMR spectroscopy and HDL efflux measurement in a group of HIV-infected patients on stable ART with normal lipid panels.
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality in HIV-infected individuals. This increased risk is due to many factors including the HIV infection itself, antiretroviral therapy (ART) used to treat the infection, and ongoing immune activation.1 Grunfield and colleagues found an atherogenic lipid profile, consisting of high total and low-density lipoprotein (LDL) cholesterol, high triglycerides, and low high-density lipoprotein (HDL) cholesterol, in untreated HIV patients,2 suggesting that the dyslipidemia was the direct result of the HIV infection.3 The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study group found a 26% increase in the incidence of myocardial infarction with the use of combination antiretroviral therapy4 as well as a positive association between the use of protease inhibitors and the risk of myocardial infarction,5 and these were thought to be due to modulations by ART. Although the use of statins and fibrates has helped to lower the elevated LDL and triglyceride levels,6 HDL characterization remains poorly understood in HIV-infected patients.
The role of HDL cholesterol (HDL-C) as a predictor of CVD has been shown in a variety of HIV-negative and HIV-positive epidemiologic studies. Gordon and colleagues examined four HIV-negative American studies and found an independent inverse relationship between HDL-C levels and rates of CVD events, demonstrating that a 1 mg/dl increase in HDL-C correlated with a CVD risk reduced by 2–3%.7 In a cross-sectional study of HIV-infected patients, Cotter and colleagues found that HDL-C was the second strongest predictor of overall CVD risk, behind age and ahead of smoking and total cholesterol.8 Although increasing the levels of HDL-C seemed to present promising therapeutic potential, trials using this technique failed to demonstrate clinical benefit,9 thereby calling into question the lack of usage of advanced lipoprotein phenotyping. These trials quantified HDL levels, but did not evaluate HDL composition (particle size and number) or function. Combined use of these advanced lipoprotein phenotypes has shown promise in other populations where chronic inflammation may modulate lipids10,11 and contribute to an elevated risk of CV events.
The use of proton nuclear magnetic resonance (NMR) spectroscopy expands upon the metrics of the traditional lipid panel, which just estimates concentration by measuring the size-specific lipoprotein particle information, quantifying the lipoprotein particle concentration and average particle size.12 In a prospective study analyzing the risk of CVD using NMR spectroscopy and advanced lipoprotein phenotyping in healthy women, Mora et al. observed that only large HDL particles were associated with CVD risk and that the magnitude of this inverse association was similar to that of HDL cholesterol, suggesting that the atheroprotective effects of HDL cholesterol may be due to the large HDL particles.13 One of HDL's strongest atheroprotective functions involves its role in the reverse cholesterol transport (RCT), the process in which HDL removes cholesterol from atherosclerotic plaques; the cholesterol is then eliminated from the body by the liver.14 The HDL cholesterol efflux capacity is the measure by which HDL removes cholesterol from the vessel wall thereby reducing acute cardiovascular events.15,16
To determine the significance of measuring the atheroprotective function of HDL in predicting CVD risk, a study by Khera and colleagues16 investigated the association between the cholesterol efflux capacity and two predictors of CVD in patients without HIV infection: carotid artery intima-media thickness (cIMT) and angiographically confirmed coronary artery disease (CAD). They found that the HDL cholesterol efflux capacity was a better predictor of the cIMT and angiographically confirmed CAD than HDL-C, and that HDL efflux capacity measured by a J774 macrophage assay was reliable in discriminating between CAD and non-CAD patients.16
Another clinical study examined two independent cohorts, one with stable patients following a coronary angiography and another group of patients referred to a cardioprotective clinic, and found that the HDL cholesterol efflux capacity was inversely associated with the prevalence of CAD in both cohorts.17,18 Therefore, given the increased CV risk in HIV and the paucity of data in HIV-infected individuals using advanced lipoprotein phenotyping, we assayed lipoprotein particle size and number by NMR and measured HDL cholesterol efflux capacity in a well-phenotyped group of HIV-infected individuals.
Materials and Methods
Study cohort
This population cohort derives from the original data of the Hawaii Aging with HIV Cardiovascular Study cohort, a 5-year longitudinal natural history cohort study designed to investigate the role of oxidative stress and inflammation in the pathogenesis of CVD in HIV-infected individuals. Details on enrollment and clinical characteristics are published elsewhere.19 Inclusion criteria for participation were documented HIV-positive status and having been on stable ART for at least 3 months. People were accepted into the study whether or not they had a history of CVD or diabetes. Current and past smoking status was taken from the patient report as yes or no responses. Inclusion into this subanalysis was restricted to subjects with measurements of the HDL cholesterol efflux capacity and NMR spectroscopy analysis (n=118). Signed consent for participation in the study was obtained from each person and IRB approval was obtained from the University of Hawaii.
Medical assessments obtained included vital signs, targeted physical examination, plasma HIV RNA, CD4+ T cell count, and lipid profile and glucose after a 12-h fast, and a 2-h oral glucose tolerance test (OGTT). Blood pressure was obtained in triplicate after 5 min of resting and averaged. Height, weight, body mass index, systolic and diastolic blood pressure, waist-hip ratio, and EKG were measured. Framingham Risk Score (FRS) was calculated using the National Cholesterol Education Program website (http://hp2010.nhlbihin.net/atpii/calculator.asp). Subjects were then classified into FRS risk categories. Subjects with FRS of less than 10% were categorized as low risk, 10–20% as intermediate risk, and ≥20% as high risk. Subjects with clinical CVD (including a history of myocardial infarction, angina, coronary disease-related cardiac surgery, or ischemic stroke) or those with diabetes as a CVD equivalent were automatically given a score of 20% and were classified as high risk. Clinical CVD was adjudicated by two physician-researchers (C.M.S. and D.C.C.). Insulin sensitivity was evaluated using the Matsuda index [insulin sensitivity index (ISI)], calculated as 10,000/square root of [fasting glucose (mmol/liter)×fasting insulin (mU/liter)]×[mean glucose×mean insulin during OGTT]. The Matsuda index is a dynamic measurement of insulin resistance and is strongly related to the ideal measurement of the euglycemic hyperinsulinemic clamp.20,21 The fasting lipid profile included total cholesterol, HDL-C, directly measured low-density lipoprotein (LDL-C) cholesterol, and total triglycerides by an enzymatic, colorimetric assay as previously reported.22 Lipoprotein particle concentration and diameters were measured using an automated NMR.23
Cholesterol efflux capacity measurements
Cholesterol efflux capacity was measured at a lipoprotein research laboratory at the NIH.10,11 J774 cells, derived from a murine macrophage cell line, were plated and radiolabeled with 2 μCi of 3H-cholesterol per milliliter. ABCA1 was up-regulated by means of a 6-h incubation with 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP. Subsequently, efflux mediums containing 2.8% apolipoprotein B (Apo-B)-depleted serum were added for 4 h. To prepare Apo-B-depleted serum, samples were thawed prior to Apo-B precipitation. Briefly, 40 parts polyethylene glycol solution (PEG; 20% PEG 8,000 MW in 200 mM glycine buffer, pH 7.4) was added to 100 parts serum and mixed by pipetting, then incubated at room temperature for 20 min before spinning in a microcentrifuge at 10,000 rpm for 30 min at 4°C. Apo-B-containing lipoproteins are pelleted by this procedure, and the supernatant, which contains the HDL fraction, is recovered and diluted in 14 mM MEM-HEPES (no bicarbonate)+0.15 mM cAMP to 2.8% (equivalent to 2% serum). All steps were performed in the presence of the acyl-coenzyme A cholesterol acyltransferase inhibitor CP113,818 (2 μg/ml). Liquid scintillation counting was used to quantify the efflux of radioactive cholesterol from the cells. The quantity of radioactive cholesterol incorporated into cellular lipids was calculated by means of the isopropanol extraction of control wells not exposed to patient serum. Percent efflux was calculated by the following formula: [(microcuries of 3H-cholesterol in mediums containing 2.8% apolipoprotein B-depleted serum – microcuries of 3H-cholesterol in serum-free mediums)÷microcuries of 3H-cholesterol in cells extracted before the efflux step]×100. All assays were performed in duplicate.10
Statistical analyses
The primary aim was to characterize HDL cholesterol efflux capacity and lipoprotein particle size, number, and composition in an HIV-infected population. Median and interquartile ranges were used to summarize the baseline measurements and clinical characteristics. Linear regression analyses were used to determine the association between each variable and the cholesterol efflux capacity. The multivariable linear regression analysis was adjusted using risk factors for CVD including FRS, HDL-C, BMI and Matsuda Index. All statistical analyses were conducted using SPSS and Microsoft Excel 2007 (IBM SPSS Statistics, Version 22, Armonk, NY). We performed sensitivity analyses excluding patients on statin therapy (n= 37), those with detectable viral loads (n= 17), those not using nucleoside reverse transcriptase inhibitors (NRTIs) (n= 7), those not using nonnucleoside reverse transcriptase inhibitors (NNRTIs) (n= 57), and those not using protease inhibitors (PIs) (n= 63) to test the robustness of our primary findings. An alpha of 0.05 was used in the single and multivariable analyses to determine significance. Our sample size was fixed due to the limitation of the number of participants who had plasma available for advanced lipoprotein phenotyping.
Results
Our patient cohort was composed of 118 HIV-infected individuals, all of whom were on stable ART (Table 1). The median age was 50 years and the number of males in the cohort was 102 (86.4%). The median number of years the patients had been living with HIV, determined by finding the difference between the year of the first HIV-positive blood test and the year of the examination date, was 15 years. The cohort had a median body mass index (BMI) of 25.7 kg/m2 (classified as mildly overweight) and a median weight of 77.8 kg. Out of the 118 patients, 36 (30.5%) were hypertensive, 25 (21.2%) were currently smoking at the time of the study, 60 (50.9%) had a history of high cholesterol, and 6 (5.1%) were diabetic. The median C-reactive protein (CRP) level was 0.89 μg/ml, the median CD4+ T cell count was 490 cells/mm3, and 101 (85.6%) of the patients had plasma HIV-1 RNA that was undetectable (<48 copies/ml). A total of 42 (35.6%) patients were currently taking lipid-lowering therapies (Table 1). The median Framingham Risk Score was 6%, suggesting a low-risk population for major adverse cardiovascular events.
Table 1.
Baseline Demographics and Clinical Characteristics
| HIV patients (n=118) | |
|---|---|
| Baseline demographics | |
| Age, years | 50 (45, 56) |
| Male, n (%) | 102 (86.4%) |
| White, n (%) | 70 (59.3%) |
| Not white, n (%) | 48 (40.7%) |
| Hypertension, n (%) | 36 (30.5%) |
| Currently smoking, n (%) | 25 (21.2%) |
| Waist size, cm | 92.4 (87.5, 99.0) |
| Weight, kg | 77.8 (67.4, 87.1) |
| Height, cm | 173.2 (166.9, 179.0) |
| Waist–hip ratio | 0.94 (0.91, 0.97) |
| BMI, kg/m2 | 25.75 (23.70, 27.85) |
| Diabetes mellitus, n (%) | 6 (5.1%) |
| CRP, μg/ml | 0.89 (0.39, 2.66) |
| History of high cholesterol, n (%) | 60 (50.85%) |
| Years living with HIV | 15 (8.25, 20.75) |
| Plasma HIV-1 RNA (n, % undetectable) | 101 (85.59%) |
| CD4+ T cell count, cells/mm3 | 490 (349.75, 639.50) |
| Antiretroviral therapy | |
| Total | 118 (100.0%) |
| Nucleoside reverse transcriptase inhibitors | 111 (94.1%) |
| Nonnucleoside reverse transcriptase inhibitors | 61 (51.7%) |
| Protease inhibitors | 55 (46.6%) |
| Integrase inhibitors | 15 (12.7%) |
| Lipid-lowering therapy | |
| Total | 42 (35.6%) |
| Statins | 37 (31.4%) |
| Fibrates | 7 (5.9%) |
| Niacin | 4 (3.4%) |
| Cholesterol absorption inhibitors | 1 (0.8%) |
| Omega 3 acid ethyl ester | 1 (0.8%) |
| Framingham Risk Score | 0.06 (0.02, 0.11) |
| Metabolic assessment | |
| Fasting glucose, mg/dl | 86 (81, 92) |
| Fasting insulin, mg/dl | 6.2 (4.08, 10.03) |
| Matsuda indexa | 6.095 (3.794, 9.896) |
| Lipid panel | |
| Total cholesterol, mg/dl | 178.0 (157.3, 207.0) |
| LDL-C, mg/dl | 108.0 (89.0, 132.0) |
| HDL-C, mg/dl | 44.0 (33.0, 54.8) |
| Non-HDL-C, mg/dl | 134.0 (108.0, 159.8) |
| Triglycerides, mg/dl | 122.5 (92.0, 178.3) |
| Cholesterol ratio (total/HDL-C) | 4.1962 (3.4298, 5.0935) |
| TG/HDL-C ratio | 2.9405 (1.8786, 4.9899) |
| LDL-C/HDL-C ratio | 2.554 (1.969, 3.228) |
| Apo-AI, mg/dl | 74.3 (56.3, 95.9) |
| Apo-AII, mg/dl | 21.7 (17.6, 29.6) |
| Apo-B, mg/dl | 3.7 (2.5, 6.0) |
| Apo-CII, mg/dl | 5.3 (3.5, 7.4) |
| Apo-CIII, mg/dl | 12.2 (8.3, 18.9) |
| Apo-E, mg/dl | 4.0 (3.1, 5.8) |
| NMR spectroscopy | |
| Concentration | |
| Very low-density lipoprotein particle (VLDL-P) concentration, nmol/liter | |
| Total VLDL-P | 76.85 (41.73, 105.45) |
| Large VLDL-P | 2.55 (1.30, 6.63) |
| Medium VLDL-P | 29.15 (13.43, 47.13) |
| Small VLDL-P | 35.30 (21.83, 51.25) |
| Low-density lipoprotein particle (LDL-P) concentration, nmol/liter | |
| Total LDL-P | 1,233.0 (996.8, 1458.3) |
| Large LDL-P | 356.5 (203.8, 539.3) |
| Small LDL-P | 771.0 (548.5, 958.3) |
| High-density lipoprotein particle (HDL-P) concentration, μmol/liter | |
| Total HDL-P | 32.05 (28.53, 38.25) |
| Large HDL-P | 3.2 (2.1, 5.0) |
| Medium HDL-P | 10.75 (7.55, 15.3.0) |
| Small HDL-P | 17.35 (14.00, 20.60) |
| Intermediate-density lipoprotein particle (IDL-P) concentration, nmol/liter | |
| Total IDL-P | 95 (49, 141) |
| Particle size | |
| VLDL, nm | 47.05 (42.98, 52.95) |
| LDL, nm | 20.4 (20.1, 20.8) |
| HDL, nm | 8.8 (8.7, 9.1) |
| HDL function | |
| Cholesterol efflux capacity | 0.975 (0.896, 1.090) |
N=101 patients.
Data are expressed as median (Q1, Q3).
BMI, body mass index; CRP, C-reactive protein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very-low-density lipoprotein; IDL, intermediate-density lipoprotein; TG, triglycerides; Apo, apolipoprotein.
The median fasting glucose and fasting insulin levels of the cohort were both within the normal range (86 mg/dl and 6.2 mg/dl, respectively). The median Matsuda index was 6.095, suggesting an insulin-sensitive group. The median total cholesterol, LDL-C, and HDL-C values were 178 mg/dl, 108 mg/dl, and 44 mg/dl, respectively, which are normal with the exception of a low-normal HDL level. The median triglyceride value of the patient cohort was 122.5 mg/dl. Table 1 provides the median values for the apolipoproteins of this patient cohort.
Through NMR spectroscopy methods, the median very-low-density lipoprotein (VLDL) was 76.85 nmol/liter, which is higher than the value seen in a study observing a younger HIV-positive patient population24 but similar to the value observed in a similarly aged HIV-positive study.25 The HDL particle (HDL-P) concentration was 32.05 μmol/liter, which was higher than the values seen in the younger HIV-positive study24 and similar to the similarly aged HIV-positive study.25 The median LDL particle (LDL-P) concentration was 1233 nmol/liter, which is higher than the values seen in control subjects of previous studies10 and similar to the values seen in the younger and similarly aged HIV-positive studies.24,25 Table 1 details the large, medium, and small particle concentrations of each of the particles. The large HDL-P and small HDL-P concentrations were 3.2 μmol/liter and 17.35 μmol/liter, respectively, which were similar to the values observed in other HIV-positive studies.24,25 Conversely, the medium HDL-P concentration was 10.75 μmol/liter, which is significantly higher than the value observed in the similarly aged HIV-positive study.25 The median LDL, HDL, and VLDL particle sizes were 20.4 nm, 8.8 nm, and 47.05 nm, respectively, and were similar to the values seen in previous HIV-positive studies.24 The median cholesterol efflux capacity of the cohort was a relatively low value of 0.975 in comparison to controls of other studies.11
The results of the simple linear regression analysis are summarized in Table 2. The cholesterol efflux capacity was strongly associated with total cholesterol and HDL-C. It was also negatively associated with the cholesterol ratio of total cholesterol to HDL-C. The cholesterol efflux capacity was not significantly associated with apolipoprotein A1, A2, or B but the directionality conflicts with prior studies.10,11,16 The cholesterol efflux capacity was negatively associated with patient weight and with fasting glucose levels. There was a negative association between the cholesterol efflux capacity and the FRS Score and FRS Class. The cholesterol efflux capacity was positively associated with the Matsuda index. For lipoprotein particle size and number values, the cholesterol efflux capacity was not significantly associated with any of the VLDL particle (VLDL-P) concentration values. Although there was no significant association with the total or large LDL-P concentration values, the cholesterol efflux capacity was negatively associated with the small LDL-P concentration (β=−0.198, p=0.031).
Table 2.
Unadjusted Simple Linear Regression Values of Associations with High-Density Lipoprotein Cholesterol Efflux Capacity
| Standardized beta coefficient | p-value | |
|---|---|---|
| Lipid panel | ||
| Total cholesterol, mg/dl | 0.259 | 0.005 |
| LDL-C, mg/dl | 0.086 | 0.353 |
| HDL-C, mg/dl | 0.395 | <0.001 |
| Triglycerides, mg/dl | 0.065 | 0.487 |
| Cholesterol ratio (total/HDL-C) | −0.184 | 0.046 |
| TG/HDL-C ratio | −0.042 | 0.655 |
| Apo-AI, ng/ml | −0.121 | 0.194 |
| Apo-AII, ng/ml | −0.148 | 0.110 |
| Apo-B, ng/ml | −0.170 | 0.066 |
| Apo-CII, ng/ml | −0.104 | 0.262 |
| Apo-CIII, ng/ml | −0.136 | 0.143 |
| Apo-E, ng/ml | −0.118 | 0.204 |
| Baseline demographics | ||
| Age, years | 0.003 | 0.975 |
| Waist size, cm | −0.136 | 0.142 |
| Weight, kg | −0.228 | 0.013 |
| Height, cm | −0.164 | 0.077 |
| BMI, kg/m2 | −0.164 | 0.075 |
| CRP, μg/ml | −0.061 | 0.510 |
| CD4+ T cell count, cells/mm3 | −0.032 | 0.730 |
| FRS score | −0.184 | 0.046 |
| FRS class | −0.186 | 0.044 |
| Lipid-lowering medications | 0.077 | 0.406 |
| Metabolic assessment | ||
| Fasting glucose, mg/dl | −0.208 | 0.024 |
| Fasting insulin, mg/dl | −0.139 | 0.134 |
| Matsuda indexa | 0.218 | 0.029 |
| NMR spectroscopy | ||
| VLDL particle (VLDL-P) conc., nmol/liter | ||
| Total VLDL-P | 0.127 | 0.170 |
| Large VLDL-P | 0.021 | 0.820 |
| Medium VLDL-P | 0.172 | 0.063 |
| Small VLDL-P | 0.029 | 0.756 |
| LDL particle (LDL-P) conc., nmol/liter | ||
| Total LDL-P | −0.052 | 0.579 |
| Large LDL-P | 0.165 | 0.074 |
| Small LDL-P | −0.198 | 0.031 |
| HDL particle (HDL-P) conc., μmol/liter | ||
| Total HDL-P | 0.399 | <0.001 |
| Large HDL-P | 0.337 | <0.001 |
| Medium HDL-P | 0.400 | <0.001 |
| Small HDL-P | −0.129 | 0.164 |
| IDL Particle conc., nmol/liter | ||
| Total IDL-P | 0.189 | 0.041 |
| Mean particle size, nm | ||
| VLDL | 0.003 | 0.971 |
| LDL | 0.174 | 0.059 |
| HDL | 0.253 | 0.006 |
N=101.
p-value<0.05.
The standardized beta coefficient is based on a one standard deviation of change in the exposure on the outcome and provides context to each variable's contribution to HDL efflux variance.
conc., concentration; CRP, C-reactive protein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very-low-density lipoprotein; IDL, intermediate-density lipoprotein; TG, triglycerides; Apo, apolipoprotein; FRS, Framingham Risk Score.
The cholesterol efflux capacity was also strongly associated with the HDL-P concentration (β=0.399, p<0.001), large HDL-P concentration (β=0.337, p<0.001), and medium HDL-P concentration (β=0.400, p<0.001). There was no significant association between the cholesterol efflux capacity and the small HDL-P concentration. There was also a positive association between the IDL-P concentration and the cholesterol efflux capacity. The cholesterol efflux capacity was strongly associated with HDL particle size and there were no significant associations between the cholesterol efflux capacity and LDL or VLDL particle size.
In the multivariable linear regression models, our dependent variable was the log of the HDL cholesterol efflux capacity (Fig. 1). Our independent variables were the FRS Score, HDL-C, BMI, and the Matsuda index. In this adjusted linear regression model (Table 3), as previously published HDL-C16 was highly associated with the cholesterol efflux capacity. The FRS Score, BMI, and Matsuda index were not significantly associated with the cholesterol efflux capacity.
FIG. 1.
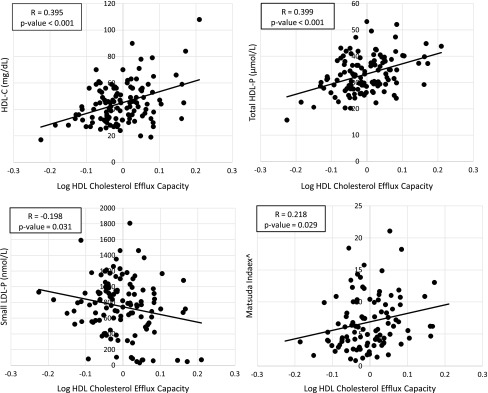
Scatter plots of unadjusted simple linear regressions of log HDL cholesterol efflux capacity and HDL-C, total HDL-P, small LDL-P, and Matsuda index. This figure plots the dependent variable (log HDL cholesterol efflux capacity) against the independent variables (HDL-C, total HDL-P, small LDL-P, and Matsuda index) for every patient in the cohort. The R value represents the correlation coefficient and is the same value as the standardized beta coefficient in Table 2. The p-value is taken from Table 2 and inserted below the R value. ^N=101. HDL, high-density lipoprotein; LDL, low-density lipoprotein; HDL-P, HDL particle concentration; LDL-P, LDL particle concentration.
Table 3.
Adjusted Multivariable Linear Regression of High-Density Lipoprotein Cholesterol Efflux Capacity by Framingham Risk Score, High-Density Lipoprotein Cholesterol, Body Mass Index, and Matsuda Index
| Variable | Standardized beta coefficient | p-value |
|---|---|---|
| Framingham risk score | −0.184 | 0.046 |
| Framingham risk score | −0.090 | 0.312 |
| HDL-C | 0.372 | <0.001 |
| Framingham risk score | −0.061 | 0.501 |
| HDL-C | 0.370 | <0.001 |
| BMI | −0.118 | 0.182 |
| Framingham risk score | 0.024 | 0.807 |
| HDL-C | 0.272 | 0.006 |
| BMI | −0.152 | 0.144 |
| Matsuda index | 0.115 | 0.286 |
p-value<0.05.
In this multivariable linear regression model, the dependent variable was the log of the HDL cholesterol efflux capacity and the independent variables that were sequentially added were the Framingham Risk Score, HDL-C, BMI, and the Matsuda index. The standardized beta coefficient is based on a one standard deviation of change in the exposure on the outcome and provides context to each variable's contribution to HDL efflux variance.
Our sensitivity analyses excluding patients on statin therapy (n=37), those with detectable viral load (n=17), those not using NRTI (n=7), and those not using NNRTI (n=57) confirmed the robustness of our primary findings. We also performed a sensitivity analysis excluding patients not using PI (n=63). In this analysis, the association between HDL-C and the HDL cholesterol efflux capacity was attenuated in the fully adjusted linear regression model but the primary estimates and patterns were similar (β=0.21, p=0.18).
Discussion
In this study we observed an atherogenic profile in lipoprotein composition and function in HIV-infected individuals despite normal traditional lipid panels. Specifically, we found a decrease in the HDL cholesterol efflux capacity accompanied by a shift in large HDL particle number and size. Furthermore, we observed a relationship between HDL function and insulin sensitivity, Framingham Risk Score, and advanced HDL particle parameters. These findings suggest that residual risk for cardiovascular comorbidity may be in part due to dysfunctional HDL.
Our results support the hypothesis that the size and type of HDL particles present in the bloodstream are significant predictors of the HDL cholesterol efflux capacity. Large HDL particles are associated with lower CVD risk26 and higher concentrations of large HDL particles are also associated with lower CVD risk. In our analysis, the large HDL-P was significantly associated with higher cholesterol efflux capacity, suggesting that larger HDL-P may be more mature and active in reverse cholesterol transport.
LDL-C is the strongest lipoprotein risk factor for CVD, so the composition of the LDL particles (LDL-P) is of great interest in HIV. Furthermore, understanding the relationship between the LDL-P and HDL efflux may provide insight into mechanisms of reverse cholesterol transport. The finding that the small LDL-P was a more significant predictor than LDL-C of HDL cholesterol efflux capacity suggests that the number and size of LDL cholesterol particles may confer CVD risk beyond simple cholesterol concentration. This correlation may be due to increased deposition in the subendothelial space, increased uptake by macrophages, increased susceptibility to oxidation, and/or decreased clearance because of reduced affinity for the LDL receptor.10 These analyses demonstrate the need to investigate the possible utilization of the size and type of HDL and LDL particles as independent predictors of the cholesterol efflux capacity in addition to CVD risk.
Along with the size and type of lipid subparticles, the findings also demonstrated a significant association with the concentration of HDL, supporting the hypothesis that HDL plays an important role in the reverse cholesterol transport process. Although both LDL and HDL play roles in this process, HDL-C was statistically significant in predicting the cholesterol efflux capacity whereas LDL-C was not statistically significant, suggesting that LDL-C operates detrimentally through separate mechanisms. HDL-C is antiatherogenic and is responsible for the efflux of cholesterol from the macrophages (foam cells) along the arterial wall for removal from the body. LDL-C, on the other hand, is responsible for the influx and for depositing cholesterol on the arterial walls, where the cholesterol is oxidized and ingested by macrophages to produce foam cells.3 These findings support the need for future research to investigate the interaction effect between HDL-C and LDL-C on HDL cholesterol efflux capacity.
Additionally, the cholesterol efflux capacity was significantly associated with fasting glucose and the Matsuda index, a validated measure of insulin sensitivity.20,21 This result supports the hypothesis that the conditions resulting from insulin resistance detrimentally influence cholesterol efflux capacity. A study conducted by Laakso and colleagues demonstrated that the serum HDL and HDL2 levels in non-insulin-dependent diabetics were lower in comparison to the nondiabetic control subjects.27 Lipoprotein lipase (LPL) hydrolyzes VLDL and chylomicron molecules, producing fragments that are transferred to maturing HDL molecules.28 Two studies examining HIV dyslipidemia and LPL activity found that decreased LPL-mediated clearance of VLDL-TG was associated with hypertriglyceridemia in mice given ritonavir29 and human HIV-positive patients on HAART-PI had low postheparin LDL activity that partially contributed to HIV dyslipidemia.30
In an HIV-negative study, Nikkila and colleagues found that obese diabetic subjects had significantly lower LPL activity in comparison to obese nondiabetics,31 which may allude to a decreased maturation of HDL molecules and decreased levels of HDL.27 Hepatic lipase (HL), which works to remove cholesterol from the plasma, is insulin dependent and an increase in its function in an insulin-resistant state would lead to decreased HDL-C levels.31 Since the cholesterol efflux capacity is positively associated with HDL-C, a decrease in the HDL-C levels in an insulin-resistant state may lead to a decrease in the cholesterol efflux capacity. Future research is needed to provide more thorough evidence of this relationship.
The significant associations between the cholesterol efflux capacity and lipid subparticles provide potential avenues for therapeutic interventions to increase the cholesterol efflux capacity and reduce CVD risk. Interventions that promote the cholesterol efflux capacity and RCT, such as infusions of reconstituted Apo-AI and up-regulating pathways of macrophage efflux with XLR agonists and miR-33 inhibitors, should be investigated for their relationship to the cholesterol efflux capacity and CVD outcomes.32 The influence of these interventions on HDL-C levels should not be the focus of these clinical studies. Instead, the importance should be placed on the changes in lipid subparticle measurements and the cholesterol efflux capacity. With the significant associations between the cholesterol efflux capacity and lipid subparticle measures and traditional risk factors of CVD, these findings help support the hypothesis that the cholesterol efflux capacity may play an important role in research to reduce CVD risk. As the investigation into the relationship between the cholesterol efflux capacity and CVD risk deepens, new therapeutic options, such as interventions to increase the levels of lipid-poor apolipoprotein AI (an acceptor in the initial steps of efflux) or of macrophage sterol transporters (efflux-promoting molecules), will arise and provide potential drugs to reduce the elevated risk of CVD in the HIV population.33
Our study is limited by its design as an observational study with a propensity for bias in population selection and measurement. However, the cohort is well phenotyped. There were no seronegative controls, but we are able to compare our values to other disease states.10,11 Finally, the lack of CVD outcomes in our study supports the necessity for future, extensive studies to measure the lipid subparticle measurements and the HDL cholesterol efflux capacity in an HIV-infected population with treatment effects of ART and CVD outcomes.10
This study is the first to demonstrate an association between HDL cholesterol efflux capacity and the size and type of HDL and LDL particles in an HIV-infected population. HDL concentration, but not LDL concentration, was significantly associated with cholesterol efflux capacity. Other predictors of cholesterol efflux capacity were the Matsuda index for insulin sensitivity, the Framingham Risk Score, and advanced HDL particle parameters. Our analysis provides evidence for the importance of researching HDL cholesterol efflux capacity to provide potential therapeutic options to reduce CVD risk.
Acknowledgments
This study was supported by an Intramural Grant from the NIH (N.N.M.), extramural NIH Grants R01HL095135 and U54RR026136 (C.M.S.). We thank our study participants and community physicians for their roles in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, Simon DI, Costa MA, Rodriguez B, Sieg SF, and Lederman MM: Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012;120:4599–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, and Feingold KR: Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992;74:1045–1052 [DOI] [PubMed] [Google Scholar]
- 3.Giannarelli C, Klein RS, and Badimon JJ: Cardiovascular implications of HIV-induced dyslipidemia. Atherosclerosis 2011;219:384–389 [DOI] [PubMed] [Google Scholar]
- 4.Friis-Møller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, Thiébaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD, and the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group: Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003 [DOI] [PubMed] [Google Scholar]
- 5.DAD Study Group, Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte AD, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, and Lundgren JD: Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723–1735 [DOI] [PubMed] [Google Scholar]
- 6.Shikuma CM, Yang Y, Glesby MJ, Meyer WA, 3rd, Tashima KT, Ribaudo HJ, Webb N, Bastow B, Kuritzkes DR, and Gulick RM: Metabolic effects of protease inhibitor-sparing antiretroviral regimens given as initial treatment of HIV-1 Infection. J Acquir Immune Defic Syndr 2007;44:540–550 [DOI] [PubMed] [Google Scholar]
- 7.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs JR, Jr, Bangdiwala S, and Tyroler HA: High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8–15 [DOI] [PubMed] [Google Scholar]
- 8.Cotter AG, Satchell CS, O'Halloran JA, Feeney ER, Sabin CA, and Mallon PWG: High-density lipoprotein levels and 10-year cardiovascular risk in HIV-1-infected patients. AIDS 2011;25:867–869 [DOI] [PubMed] [Google Scholar]
- 9.AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, and Weintraub W: Niacin in Patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267 [DOI] [PubMed] [Google Scholar]
- 10.Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, Raper A, Wilcox M, Baer A, DerOhannesian S, Wolfe M, Reilly MP, Rader DJ, VanVoorhees A, and Gelfand JM: Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012;224:218–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roe A, Hillman J, Butts S, Smith M, Rader D, Playford M, Mehta NN, and Dokras A: Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J Clin Endocrinol Metab 2014;99:E841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, and Ridker PM: Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes 2010;59:1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, and Ridker PM: Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy D. and Rader DJ: Update on strategies to increase HDL quantity and function. Nat Rev Cardiol 2009;6:455–463 [DOI] [PubMed] [Google Scholar]
- 15.Posadas-Sánchez R, Posadas-Romero C, Mendoza-Pérez E, Caracas-Portilla NA, Cardoso-Saldaña G, Medina-Urrutia A, Jorge-Galarza E, and Juárez-Rojas JG: Cholesterol efflux and metabolic abnormalities associated with low high-density-lipoprotein-cholesterol and high triglycerides in statin-treated coronary men with low-density lipoprotein-cholesterol <70 mg/dl. Am J Cardiol 2012;109:636–641 [DOI] [PubMed] [Google Scholar]
- 16.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, and Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, and Hazen SL: Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol 2013;33:1696–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera AV. and Rader DJ: Cholesterol efflux capacity: Full steam ahead or a bump in the road? Arterioscler Thromb Vasc Biol 2013;33:1449–1451 [DOI] [PubMed] [Google Scholar]
- 19.Shikuma CM, Seto T, Liang CY, Bennett K, DeGruttola V, Gerschenson M, Stein JH, Budoff M, Hodis HN, Delaney JA, Ogata-Arakaki D, Pramyothin P, and Chow D: Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res Hum Retroviruses 2012;28:793–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sciacqua A, Perticone M, Grillo N, Falbo T, Bencardino G, Angotti E, Arturi F, Parlato G, Sesti G, and Perticone F: Vitamin D and 1-hour post-load plasma glucose in hypertensive patients. Cardiovasc Diabetol 2014;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda M. and DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 22.Barbour JD, Jalbert EC, Chow DC, Gangcuangco LMA, Norris PJ, Keating SM, Heitman J, Nagamine L, Seto T, Ndhlovu LC, Nakamoto BK, Hodis HN, Parikh NI, and Shikuma CM: Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis 2014;232:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, and Goff DC, Jr: Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol 2011;5:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein JH, Komarow L, Cotter BR, Currier JS, Dubé MP, Fichtenbaum CJ, Gerschenson M, Mitchell CK, Murphy RL, Squires K, Parker RA, Torriani FJ, theACTG 5152s Study Team: Lipoprotein changes in HIV-infected antiretroviral-naïve individuals after starting antiretroviral therapy: ACTG Study A5152s Stein: Lipoprotein changes on antiretroviral therapy. J Clin Lipidol 2008;2:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, Neuhaus J, Paton NI, Friis-Moller N, Lampe F, Liappis AP, Neaton JD, the INSIGHT SMART Study Group: Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis 2009;207:524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan J, Qi Y, and Zhao D: [A meta-analysis on the association between high-density lipoprotein particle subfractions and cardiovascular disease events]. Zhonghua Xin Xue Guan Bing Za Zhi 2014;42:57–61 [PubMed] [Google Scholar]
- 27.Laakso M, Voutilainen E, Sarlund H, Aro A, Pyörälä K, and Penttilä I: Serum lipids and lipoproteins in middle-aged non-insulin-dependent diabetics. Atherosclerosis 1985;56:271–281 [DOI] [PubMed] [Google Scholar]
- 28.Borggreve SE, De Vries R, and Dullaart RPF: Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: Role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest 2003;33:1051–1069 [DOI] [PubMed] [Google Scholar]
- 29.den Boer MA, Berbée JF, Reiss P, van der Valk M, Voshol PJ, Kuipers F, Havekes LM, Rensen PC, and Romijn JA: Ritonavir impairs lipoprotein lipase-mediated lipolysis and decreases uptake of fatty acids in adipose tissue. Arterioscler Thromb Vasc Biol 2006;26:124–129 [DOI] [PubMed] [Google Scholar]
- 30.Yarasheski KE, Tebas P, Claxton S, Marin D, Coleman T, Powderly WG, and Semenkovich CF: Visceral adiposity, C-peptide levels, and low lipase activities predict HIV-dyslipidemia. Am J Physiol Endocrinol Metab 2003;285:E899–905 [DOI] [PubMed] [Google Scholar]
- 31.Nikkilä EA: High density lipoproteins in diabetes. Diabetes 1981;30:82–87 [PubMed] [Google Scholar]
- 32.Rader DJ. and Tall AR: The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med 2012;18:1344–1346 [DOI] [PubMed] [Google Scholar]
- 33.Heinecke JW: The not-so-simple HDL story: A new era for quantifying HDL and cardiovascular risk? Nat Med 2012;18:1346–1347 [DOI] [PubMed] [Google Scholar]


