Summary
Multifactorial and polygenic inheritance is commonly recognized for many genetic conditions including physical anomalies, complex congenital malformation syndromes, and even common disorders such as adult-onset diabetes mellitus. It has only recently been suggested as a mechanism for inheritance in inborn errors of metabolism. This article reviews the phenomenon of multiple partial enzyme deficiencies leading to clinical relevant biochemical derangements (synergistic heterozygosity) and its implications for other more common disorders such as diabetes and obesity.
Introduction
The human genome consists of some three billion nucleotide base pairs encoding approximately 25 000 genes. Nearly 10 000 genetic disorders have now been recognized due to alteration in the function of a single gene, affecting as many as 1% of all births worldwide (Korf et al 2008). Neatly organized into online catalogues, the increasingly complete morbid map of the human genome can lull clinical geneticists into a false sense of security that we will soon understand all we need to know about genetic disease (http://www.ncbi.nlm.nih.gov/). Single gene defects, however, offer only a partial view of the complexity of genetic disease. An even greater burden on human health is related to the dysfunction of genes at multiple genetic loci, resulting in such polygenic and multifactorial conditions as cleft lip and palate and isolated congenital heart defects. More recently, the accumulation of multiple derangements in sequential functions of a biological pathway has been recognized as a molecular mechanism for disease, best typified by the multihit process resulting in the development of cancer (Vogelstein and Kinzler 2004). Inborn errors of metabolism have traditionally been viewed as single gene disorders, though modifying genetic and environmental factors are recognized (Scriver and Waters 1999). Recently, we documented patients with clinical evidence of energy metabolism disorders who exhibited concurrent partial enzymatic deficiencies in more than one energy-generating pathway and showed that some of these patients were heterozygous carriers for mutations in multiple genes in these functionally related pathways (Vockley et al 2000). This led to the hypothesis that such mutations cumulatively can cause physiologically relevant reduction of flux through metabolic pathways, a genetic phenomenon we termed synergistic heterozygosity. Synergistic heterozygosity is, in reality, a special example of epistasis or multifactorial inheritance, where multiple loci (heterozygosity for deleterious mutations in metabolic pathway genes) interact to produce a phenotype when the organism is environmentally stressed. This article will examine the implications of this hypothesis in the diagnosis, treatment, and understanding of metabolic disease with energy metabolism as an example and its potential impact on more common disorders such as diabetes and obesity.
Inborn errors of mitochondrial energy metabolism
Energy metabolism is a complex process that requires the activities of hundreds of proteins located in multiple cellular compartments, most prominently mitochondria and the cytoplasm. Mitochondrial β-oxidation requires the transport of activated acyl-CoA moieties into the mitochondria, followed by sequential removal of 2-carbon acetyl-CoA units (Ghisla 2004; Vockley et al 2002; Vockley and Whiteman 2002) (Figs. 1 and 2). These products, in turn, are used as fuel for the tricarboxylic acid cycle or the production of ketone bodies. Energy derived from the various steps of these cycles is efficiently transformed into ATP through oxidative phosphorylation (Shoffner 2001; Smeitink et al 2001). β-Oxidation represents an important source of energy for the body during times of fasting and metabolic stress. Additional energy during fasting is generated through glycogenolysis and gluconeogenesis, while catabolism of simple sugars provides steady-state basal energy for cellular functions. Two recent studies integrating analysis of gene expression and proteomic data from mouse mitochondria have identified a network of at least 90 co-regulated genes involved in oxidative phosphorylation, β-oxidation, and the TCA cycle (Mootha et al 2003; Su et al 2002).
Fig. 1.
The carnitine transport cycle. Carnitine palmitoyltransferases I and II (CPT I and II, respectively) work in concert with carnitineacylcarnitine translocase (CAT) in the inner mitochondrial membrane to transport long-chain acyl-CoAs into the mitochondrial matrix. The yellow circle represents the plasma membrane high-affinity carnitine transporter
Fig. 2.
The mitochondrial matrix components of fatty acid oxidation. Reactions are as described in the text. ETF is electron transfer flavoprotein and FAD is flavin–adenine dinucleotide
At least 25 enzymes and specific transport proteins are involved in mitochondrial fatty acid metabolism and genetic deficiencies of many of these enzymes have been described (Bennett et al 2000; Vockley et al 2002; Vockley and Whiteman 2002). Entry of long chain acyl-CoA substrates is generally considered to be the rate-limiting step of β-oxidation, specifically their conjugation to carnitine by carnitine palmitoyltransferase I (CPT I) (McGarry 2001; McGarry and Foster 1980). The first and rate-limiting step in β-oxidation within the mitochondrial matrix is the extraction of two electrons from an acyl-CoA substrate by α-carbon chain lengthspecific acyl-CoA dehydrogenases (ACADs) (Thorpe and Kim 1995). The subsequent three enzymatic steps are performed by a combination of multi- and monofunctional enzymes depending again on the length and structure of the primary carbon backbone of the substrate (Ghisla 2004).
Oxidative phosphorylation involves a highly organized set of over 150 proteins embedded within the inner mitochondrial membrane and organized into five functional complexes that comprise the respiratory chain (Shoffner 2001). The oxidative phosphorylation complexes are in turn organized into higher-order supercomplexes that lead to greater functional efficiency (Heinemeyer et al 2007; Schagger 2002; Stroh et al 2004). These supercomplexes have been shown to support more rapid electron transfer rates both in vitro and in intact mitochondrial preparations, an effect postulated to be mediated through substrate channelling, catalytic enhancement, sequestration of reactive intermediates, stabilization of protein complexes, increase of the capacity of the inner mitochondrial membrane for protein insertion, and generation of mitochondrial cristae (Link et al 1986; Stroh et al 2004; Wallace 1992, 1993).
The physiological relationship between mitochondrial oxidative phosphorylation and β-oxidation has long been established, but evidence for a physical connection between the two pathways is scant. β-Oxidation has been postulated to occur in the context of a multi-protein complex, but this has been poorly characterized and no evidence exists to link it physically with the respiratory chain. Probably the strongest suggestion for such an interaction comes from studying patients with clinical deficiencies of the ETC. Many of these patients accumulate metabolites in blood and urine suggestive of dysfunction of multiple β-oxidation enzymes (Bennett et al 1994; Enns et al 2000; Vockley et al 2000). Moreover, approximately 25% of fibroblast cultures from patients with complex I deficiency show a concomitant decrease compared with controls in the ability to oxidize palmitate (Venizelos et al 1998). In mammals, reducing equivalents from ACADs are transferred via the electron transfer flavoprotein (ETF) to ETF:coenzyme Q oxidoreductase (Ruzicka and Beinert 1975). Submitochondrial particles have been shown to catalyse the transfer of electrons from ETF to complex III through ETF:coenzyme Q oxidoreductase and the coenzyme Q pool (Frerman 1987). A protein complex containing the ACADs, ETF, ETF: coenzyme Q oxidoreductase (ETF dehydrogenase, ETFD), and complex III has been identified but poorly characterized (Sumegi et al 1991), and ETF and ETFD have been shown to form a stable complex (Parker and Engel 2000). Finally, long chain 3-hydroxyacyl-CoA dehydrogenase has been shown to interact directly with ETC complex I at the inner mitochondria membrane (Kispal et al 1986; Sumegi and Srere 1984).
Metabolic cycles and systems biology
The pathophysiology of a gene mutation in an IEM has typically been viewed in terms of its consequences on the function of a single enzyme. In reality, the effects are more correctly viewed as pleiotropic at a number of levels. For example, in null mice with deletion of the PPARα gene, the expression of multiple genes in several energy pathways and organelles was affected (Goetzman et al 2005). Metabolic pathways are complex networks of interacting molecules consisting of individual components that must coordinately control their activities in the context of routine physiological function and reaction to stress. A complete quantitative description of the regulation of such networks is not possible, but by grouping related processes into a small number of functional modules, it becomes possible to describe the essential regulatory features within a system, as well as its response to internal and external stimuli such as mutations and environment, respectively. Metabolic control analysis has been used to characterize a number of complex systems, including classical metabolic pathways (Fell 1992; Heinrich and Rapoport 1974; Kacser and Burns 1973, 1995). Most applications have sought to measure control coefficients, which describe how strongly each reaction influences the different variables (usually flux or concentration) in a system (Ainscow and Brand 1999a, b; Brand et al 1990; Brown et al 1990; Hafner et al 1990).
Regulation of flux through a metabolic pathway in vitro is usually determined by a rate-limiting enzyme. However, the in vivo situation is more complicated, and metabolic control analysis instead seeks to describe flux through a biochemical pathway in quantitative terms related to the relative (cumulative) effects of all component enzymes and transporters involved in the pathway, along with changes in metabolic states (Fell 1992; Quant 1993). For example, metabolic control analysis studies indicate that, while the potential for CPT I to control flux through mitochondrial β-oxidation is high, the ability of malonyl-CoA (an inhibitor of CPT I) to regulate flux through the pathway is dependent on several factors such as age and the relative malonyl-CoA concentration versus other metabolites (Krauss and Quant 1996). Moreover, malonyl-CoA concentration in heart is high enough to completely inhibit β-oxidation according to predictions derived purely from the in vitro enzymatic properties of the CPT I (McGarry 2001; McGarry and Foster 1980). In fact, metabolic flux experiments performed with isolated mitochondria in stage 3 respiration conditions (simulating a high-energy output state) have shown that CPT I activity must be inhibited >50% before flux through β-oxidation begins to decline (Eaton et al 2001). Thus, to understand the full clinical effects of partial reductions of components in a metabolic pathway, one must consider them in combination rather than individually.
Simple phenotypes in yeast models offer some insight into the need to consider gene interactions in predicting the effect of any one mutation on an organism. Recent experiments studying the effect on cell growth in yeast of all possible combinations of double knockouts of 890 pairs of metabolic genes have shown that some of the combinations led to poorer growth than either alone. Some combinations, however, improved growth. Moreover, groupings of ‘aggravating’ and ‘buffering’ combinations led to the identification of both predictable and unpredicted functional relationships (Segre et al 2005).
These experiments point to a need to consider another level of gene interaction when attempting to understand the effects of mutations on an organism. This global approach evaluates the interaction of complex pathways and has been designated ‘systems biology’ (Csete and Doyle 2002; Davidson et al 2002; Kitano 2002; Tong et al 2004). While an understanding of genes and proteins continues to be important, the focus is on understanding a system’s structure and dynamics. Because a system is not just an assembly of genes and proteins, its properties cannot be fully understood merely by drawing diagrams of their interconnections or even calculating their control coefficients. Rather, it is necessary to understand both the interaction of the individual parts and as those unique qualities imparted to the system through interaction of the components.
Complex phenotypes and IEMs
Traditionally, the pool of patients with episodic symptoms suggestive of a defect in energy metabolism (such as recurrent fasting or stress-induced episodic muscle pain) who remain without a diagnosis after extensive clinical biochemical and enzymatic evaluation has proved a fruitful group from which to identify new enzymes and their deficiencies (Tonin et al 1990). From this same pool, however, we have described a selection of patients who appear to exhibit a different phenomenon (Vockley et al 2000). These cases are remarkable in that concomitant reductions have been identified in more than one enzyme involved in four different energy metabolism pathways including fatty acid β-oxidation, oxidative phosphorylation, glycogenolysis, and highenergy phosphate recycling. Thus, the co-existence of multiple single-gene mutations in an individual appears to have resulted in complex phenotypes characterized by the emergence of symptoms atypical of the heterozygote state of any of the individual underlying disorders (Vladutiu 2000, 2001). Indeed, symptoms in these patients have not been directly predictable on the basis of the individual defects. In addition to our own patients, a few other cases of apparent complex deficiencies in energy metabolism consistent with the concept of synergistic heterozygosity have been reported (Rubio et al 1998, 2000). It is, however, difficult to move beyond phenomenology strictly through case reports. Thus, attention has shifted to more testable systems that can be manipulated experimentally.
Multiple mutations in animal models
A number of mouse models of deficiencies in β-oxidation proteins have been created or identified. These include defects in the carnitine transporter, CPT1a, VLCAD, LCAD, SCAD, MCAD, long and short chain 3-hydroxyacyl-CoA dehydrogenase, and mitochondrial β-oxidation trifunctional protein (Cox et al 2001; Guerra et al 1998; Hinsdale et al 1993; Kelly et al 1995, 1997; Kurtz et al 1998; Wood et al 1989). In general, the animals homozygous for single mutations are good models for deficiencies of these same proteins in humans, as they exhibit similar biochemical and clinical phenotypes. Specifically, the mice are well when maintained at room temperature with an ad libitum diet but become hypoglycaemic when either fasted or cold stressed. In addition, cold-stressed animals become hypothermic. Most importantly, heterozygote carriers of these same deficiencies show no or reduced symptoms compared with homozygotes when placed under physiological stress (Schuler et al 2005). In contrast, mice with two or more heterozygous mutant alleles in one of several loci (VLCAD, LCAD, SCAD, PPARα, CPTIa) become hypothermic under identical conditions (Fig. 3). Interestingly, double and triple heterozygous mice have milder biochemical derangements than any of the homozygous deficient animals. These experiments suggest that the accumulation of one mutant allele at two or more loci can lead to the development of clinical and biochemical abnormalities not associated with heterozygosity for any one of the alleles, i.e., synergistic heterozygosity.
Fig. 3.
Synergistic heterozygosity in mouse models of fatty acid oxidation defects. Shown in the figure are the percentage fatalities due to cold intolerance in mice with double and triple heterozygous mutations in VLCAD/LCAD/SCAD genes. This is compared with wild-type mice with no fatalities and homozygous LCAD-deficient mice, which demonstrate 100% fatality during cold challenge. Wild-type (n = 8), VLCAD+/− (n = 8), LCAD+/− (n = 10), SCAD+/− (n = 10), VLCAD+/−/LCAD+/− (n = 9), LCAD+/− /SCAD+/− (n = 14), VLCAD+/−/LCAD+/−/SCAD+/− (n = 3). The entire study was published in Schuler et al (2005)
Global changes induced by single enzyme defects
Incorporating system biology concepts into the study of inborn errors of metabolisms not only introduces complexities at the level of multiple gene dysfunction but also requires consideration of systemic changes induced by individual gene defects. For example, many of the symptoms in type I tryrosinaemia are not due to direct toxicity of the immediate precursor that accumulates as a result of the fumarylacetoacetate hydrolase deficiency, but rather are due to the secondary effects of accumulation of the alternative product succinylacetone. Recognition of this phenomenon led to the successful development of a treatment for type I tyrosinaemia (2-[2-nitro-4-trifluoromethylbenzoyl]-1,3-cyclohexanedione) that inhibits 4-hydroxyphenylpyruvic acid dioxygenase and prevents the accumulation of succinylacetone (Holme and Lindstedt 1995). Changes in expression of a number of genes have been reported in an animal model of VLCAD deficiency. Acyl-CoA synthase, adipophilin, activator protein 2, cytochrome c, and the peroxisome proliferator activated receptor γ coactivator-1 were increased immediately after birth, prior to the development of overt histological lipidosis, while acyl-CoA synthetase was markedly downregulated in the adult heart (Exil et al 2003). Using expression array analysis, we find that the genes in the pathways for mitochondrial fatty acid oxidation and inflammatory response are upregulated in VLCAD-deficient animals, while the respiratory chain genes are generally downregulated. In contrast, while fatty acid oxidation and oxidative phosphorylation are also upregulated and downregulated, respectively, in LCAD-deficient animals, the inflammatory response pathways are not altered. Rather, the expression of bile acid and cholesterol synthesis enzymes genes is downregulated and upregulated, respectively, in these animals (but not with VLCAD deficiency). Thus understanding the physiological ramifications of enzyme deficiencies requires a transition from attention only on the primary defect to a systems-oriented approach.
The scope of the problem
The risk for being homozygous for a mutation on both alleles of a single locus is of course the disease frequency, defined genetically as q2 where q = the frequency of mutant alleles in the population. In contrast, the risk for being heterozygous for a mutation at any one of multiple loci (of equal frequency) is the sum of the carrier frequencies of mutant alleles at each locus: (n)(2q), where n = the number loci under consideration, and 2q is the risk for being a heterozygote for a mutant allele with a gene frequency of q. The likelihood of being heterozygous for mutations in multiple loci, then, is the independent risk of being heterozygote at one of those loci multiplied by the risk of being heterozygote at the remaining loci = [(n)(2q)][(n−1)(2q)] = (n)(n−1)(qn), assuming an equal frequency of q for mutant alleles at both loci. The frequency of heterozygosity at any three alleles is [(n)(n−1)(n−2)(qn)], etc. In a metabolic system composed of 20 loci (an approximation of β-oxidation) each with a mutant allele frequency 0f 0.01 or 0.005, the incidence of being homozygous for a mutation at any one locus or compound heterozygous for a mutant allele at any two, three, four, or five loci is as shown in Table 1. The difference in frequency between the homozygous and multiple heterozygous states becomes more pronounced for more loci and with a lower incidence of mutations. If the combination of mutant alleles leads to symptoms, this is defined as synergistic heterozygosity. Although this represents a simplified version of a metabolic system, it illustrates the main point: that individuals are more likely to be heterozygous for mutations at two or more loci than homozygous for mutations at any one locus. It thus becomes critical to be able to evaluate the physiological relevance of this situation through functional assays of flux through the metabolic cycle under consideration.
Table 1.
Predicted frequency of homozygous mutant and compound heterozygous mutant individuals for 20 loci at the given mutant allele frequency
| Allele frequency | Homozygosity at one allele | Frequency of heterozygosity at number of loci = n | ||||
|---|---|---|---|---|---|---|
| n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | ||
| 0.01 | 1.0 × 10−4 | 2.0 × 10−2 | 3.8 × 10−2 | 6.8 × 10−3 | 1.2 × 10−3 | 1.9 × 10−4 |
| 0.005 | 2.5 × 10−5 | 1.0 × 10−2 | 9.5 × 10−3 | 8.6 × 10−4 | 7.3 × 10−5 | 5.8 × 10−6 |
Physiological paradigms for synergistic heterozygosity
What are the cellular mechanisms for synergistic heterozygosity? There are two broad possibilities. The first is best described as a disruption in the mass action of the pathways involved. At its simplest, this mechanism implies that partial reduction in an enzyme activity leads to a reduction of product and thus of substrate for downstream reactions (Fig. 4). Accumulation of such reductions at multiple steps would exaggerate this phenomenon and ultimately reduce overall flux through the pathway. Of course, this is an oversimplification of the dynamics of metabolic pathways and does not take into account rate-limiting reactions, substrate or product inhibition, metabolite channelling, or changes in gene expression or protein translation that might be induced. Nevertheless, the analogy to a pipe of ever-decreasing diameter restricting the flow of water to a greater extent at its distal end is a useful paradigm. The alternative mechanism is a biophysical one and is comparable to a dominant negative model. Here the individual enzymes that compose a metabolic pathway must function as a multiprotein complex that may in turn physically interact with other pathway protein complexes. In this situation, reduction of one protein in the complex due to a heterozygous, unstable mutation could disrupt the structure of the complex and lead to a reduction of the overall function of the pathway. The effect of the reduction of additional subunits would be expected to be additive. Of course, true dominant negative effects could also be seen with stable mutant proteins.
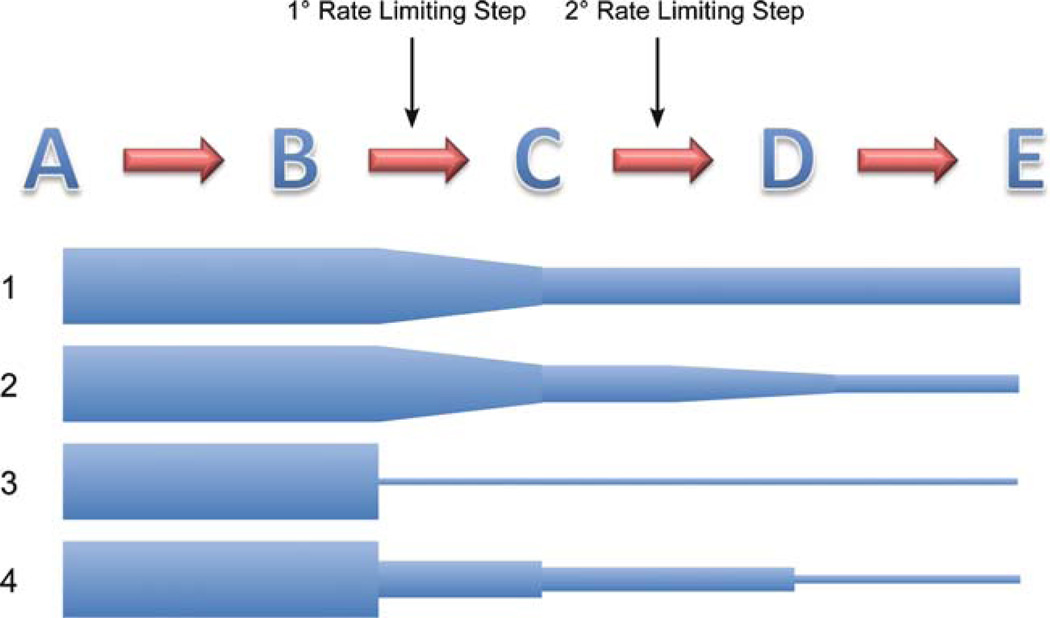
Fig. 4.
A theoretical metabolic pathway. Line 1 shows the flux through a pathway with five theoretical steps and one primary control point (rate-limiting step) and a secondary one. The height of the blue shapes represents the rate of flux through the pathway. The secondary control point might not normally be physiologically relevant unless flux through the cycle is altered through the primary control point for some reason (line 2). Line 3 shows a representation of a typical complete enzymatic block seen in a homozygous inborn error of metabolism. In line 4, multiple partial blocks in the pathway can lead to an overall decreased flux through the pathway and output of product (synergistic heterozygosity)
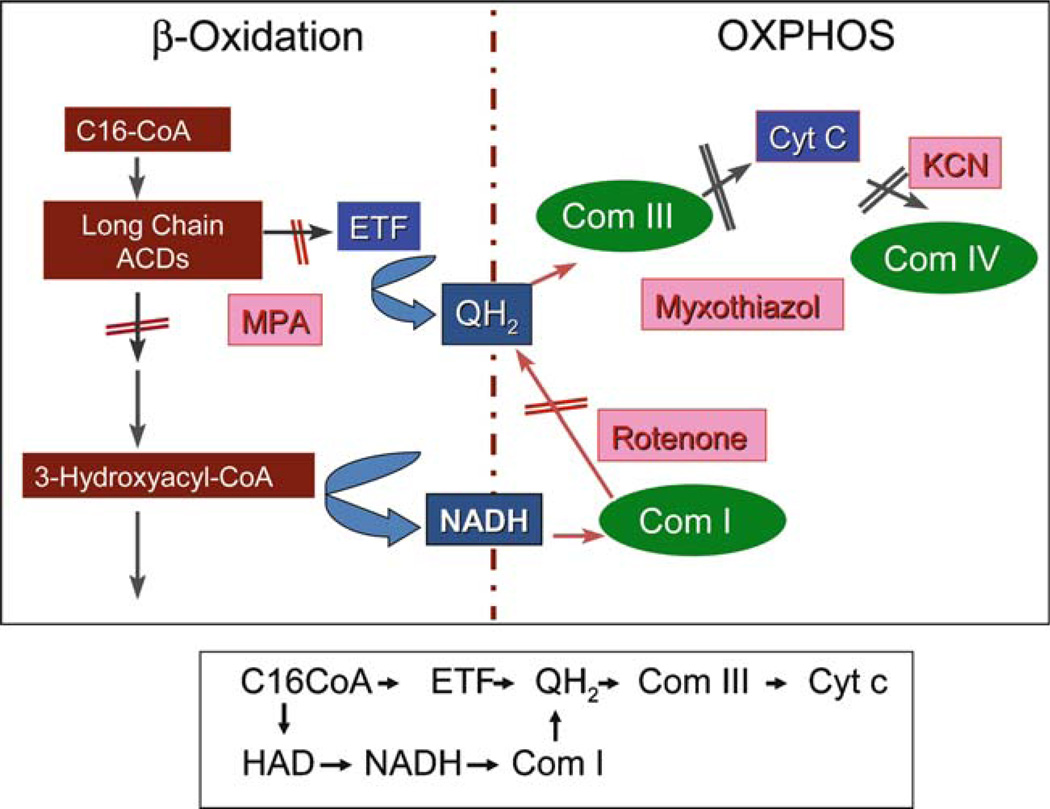
To examine these possibilities in the case of energy metabolism, we have begun to examine the physical relationship between mitochondrial fatty acid oxidation and oxidative phosphorylation (OXPHOS, Fig. 5). First, rat liver or skeletal muscle mitochondria were isolated, the mitochondrial membranes were gently dissolved with a mild detergent, and the resultant extracts were subjected to blue native acrylamide gel electrophoresis to separate OXPHOS complexes and supercomplexes. The gels were then transferred to nylon membranes and probed via western blotting with various antisera to β-oxidation enzymes. Extracts were also subjected to sucrose density centrifugation and fractions were analysed by blue native gel electrophoresis or enzymatic assays. VLCAD, LCAD, LCHAD, MCAD, SCAD, ETF, and ETFD were all found to be associated with respiratory chain supercomplexes in different patterns. When palmitoyl-CoA was added to the sucrose gradient fractions containing respiratory chain supercomplexes in the presence of KCN, cytochrome c was reduced. Cytochrome c reduction could be completely inhibited by myxothiazol (a complex III inhibitor) and MPA (methylpropionic acid, an inhibitor of the long chain acyl-CoA dehydrogenases), and only partially inhibited by rotenone (a complex I inhibitor). Thus, reducing equivalents generated by acyl-CoA dehydrogenase and the 3-hydroxyacyl-CoA dehydrogenase steps can each reduce cytochrome c. HPLC analysis of sucrose gradient fractions containing the linked FAO-OXPHOS complexes showed that palmitoyl- and octanoyl-CoA were completely oxidized without the appearance of intermediate βoxidation products, indicative of metabolic channelling. These results provide direct evidence of physical interaction between β-oxidation and OXPHOS. Moreover, it appears that our preparation contains a complete, functional, multiprotein β-oxidation complex that supports metabolic channelling of physiological substrates. Examination of mitochondrial extracts from animals with combinations of heterozygous mutations in β-oxidation and OXPHOS genes should provide an opportunity to characterize the effects of these mutations on pathway function and complex stability.
Fig. 5.
Interactions between mitochondrial fatty acid oxidation and oxidative phosphorylation (OXPHOS). Palmitoyl-CoA is used as substrate for an enzymatic reaction with a submitochondrial high-molecular-weight fraction from sucrose gradient, and electron transfer to ETF (indicative of ACAD activity) and cytochrome c (indicative of transfer of electrons from either oxidized acyl-CoAs or 3-hydroxyacyl-CoAs to the respiratory chain) is measured. Inhibition with methylpropionyl-CoA (MPA), myxothiazol, or rotenone in the reaction allows differentiation of the transfer of electrons through OXPHOS complexes I and III
Implications for the future
How relevant are multigene interactions likely to be in metabolic medicine? While the answer to this question will probably remain elusive for the immediate future, there is good reason to consider it. As many as half of all patients with recurrent rhabdomyolysis remain without a diagnosis following an extensive metabolic evaluation, as do a large number of patients with hypoketotic hypoglycaemia (Tonin et al 1990). New disorders in this group of patients undoubtedly remain to be discovered, but some fraction may also have their symptoms as a result of multigene effects including synergistic heterozygosity. Perhaps even more significantly, dysfunction in energy metabolism is increasingly being recognized as playing a role in such common conditions as type 2 diabetes mellitus and obesity (Kelley et al 1999). In light of this, we have recently begun an investigation to identify metabolic abnormalities in patients with these two conditions by analyzing blood acylcarnitine profiles form individuals with morbid obesity with or without type 2 diabetes. Dried plasma specimens spotted onto filter paper were assayed for butyl derivatives of acylcarnitines by the same tandem mass spectrometry method used for newborn blood spot screening. We found that numerous acylcarnitine species were significantly elevated in the plasma of obese and type 2 noninsulin-dependent diabetics (type 2 DM) as compared to lean participants, but none of the changes were of the magnitude found in inborn errors of metabolism (Fig. 6). Surprisingly, the acylcarnitine profile did not suggest limited carnitine palmitoyltransferase I activity, a key regulatory step in fatty acid oxidation. Rather, the pattern of acylcarnitine accumulation showed two prominent patterns. First, both the obese and diabetic participants showed a similar accumulation of long-chain acylcarnitines consistent with decreased entry of substrate into the β-oxidation cycle following transport into mitochondria. Diabetic individuals displayed an additional accumulation of many shorter chain and hydroxyacylcarnitines, suggestive of inefficient interactions between β-oxidation and oxidative phosphorylation. All participants showed a very high correlation between glucose dysregulation and the accumulation of succinylcarnitine, indicating an as of yet unexplained association of insulin resistance with this species. Finally, the plasma pool of acylcarnitines was rapidly responsive to alterations in physiological state (i.e. insulin infusion), leading to a dramatic decrease in nearly all species. However, even with a euglycaemic insulin clamp, long-chain acylcarnitine species did not decrease as much in participants with type 2 DM as in lean or obese participants. Thus, plasma acylcarnitine profiles in obese individuals are consistent with the presence of a generalized defect in fatty acid oxidation that is even more pronounced in participants with type 2 diabetes.
Fig. 6.
Acylcarnitine species are abnormal in obese and type 2 diabetic (Type 2) individuals versus lean participants. Saturated = sum of saturated acylcarnitine species from C4 through C18; Unsaturated = sum of unsaturated (one double bond) acylcarnitine species from C6:1 through C18:1; PUFA = sum of polyunsaturated acylcarnitine C18-C22 acylcarnitines. ⌘, significantly different from lean. ❖, significantly different from obese.
Conclusion
The first century of metabolic medicine began with the simple observations of Garrod that led to his profound recognition of the nature of inborn errors of metabolism. The reverberations of his insight have echoed beyond the completion of the human genome project with its unmet promise that we would know all about Homo sapiens by knowing its entire genetic sequence. It is now clear that life in a protein world is more complex than predicted by nucleic acids. The protein diversity of humans far exceeds that of our nucleic acids, and the organization of proteins into functional pathways further magnifies this complexity. Understanding modifiers of inborn errors of metabolism caused by single gene defects as well as the role of smaller alterations in cellular metabolism on human health remain major challenges for the future. In this context, synergistic heterozygosity becomes a useful model, amenable to experimental manipulations to examine these issues. All in all, the second century of metabolic medicine should prove to be as exciting as the first!
Acknowledgements
The author was funded in part by NIH grant R01 DK78775 and the Matthew Fisch Fund of the Children’s Hospital of Pittsburgh Foundation. Thanks to Drs Phil Wood and Stephanie Mihalik for providing previously unpublished figures (3 and 6) for this review.
Abbreviations
- ACAD
acyl-CoA dehydrogenase
- CPT I
carnitine palmitoyltransferase I
- DM
diabetes mellitus
- ETC
electron transfer chain
- ETF
electron transfer flavoprotein
- FAO
fatty acid oxidation
- IEM
inborn error of metabolism
- OXPHOS
oxidative phosphorylation
- TCA
tricarboxylic acid
Footnotes
Competing interests: None declared
Presented at the 20008 SSIEM Annual Symposium in Lisbon, Portugal, 2 to 5 September 2008.
References
- Ainscow EK, Brand MD. The responses of rat hepatocytes to glucagon and adrenaline. Application of quantified elasticity analysis. Eur J Biochem. 1999a;265:1043–1055. doi: 10.1046/j.1432-1327.1999.00820.x. [DOI] [PubMed] [Google Scholar]
- Ainscow EK, Brand MD. Top-down control analysis of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur J Biochem. 1999b;263:671–685. doi: 10.1046/j.1432-1327.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Weinberger MJ, Sherwood WG, Burlina AB. Secondary 3-hydroxydicarboxylic aciduria mimicking long chain 3-hydroxyacyl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 1994;17:283–286. doi: 10.1007/BF00711808. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Rinaldo P, Strauss AW. Inborn errors of mitochondrial fatty acid oxidation. Crit Rev Clin Lab Sci. 2000;37:1–44. doi: 10.1080/10408360091174169. [DOI] [PubMed] [Google Scholar]
- Brand MD, D’Alessandri L, Reis HM, Hafner RP. Stimulation of the electron transport chain in mitochondria isolated from rats treated with mannoheptulose or glucagon. Arch Biochem Biophys. 1990;283:278–284. doi: 10.1016/0003-9861(90)90643-d. [DOI] [PubMed] [Google Scholar]
- Brown GC, Hafner RP, Brand MD. A ‘top-down’ approach to the determination of control coefficients in metabolic control theory. Eur J Biochem. 1990;188:321–325. doi: 10.1111/j.1432-1033.1990.tb15406.x. [DOI] [PubMed] [Google Scholar]
- Cox KB, Hamm DA, Millington DS, et al. Gestational, pathologic and biochemical differences between very long-chain acyl-CoA dehydrogenase deficiency and long-chain acyl-CoA dehydrogenase deficiency in the mouse. Hum Mol Genet. 2001;10:2069–2077. doi: 10.1093/hmg/10.19.2069. [DOI] [PubMed] [Google Scholar]
- Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295:1664–1669. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Rast JP, Oliveri P, et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- Eaton S, Fukumoto K, Paladio Duran N, et al. Carnitine palmitoyl transferase I and the control of myocardial beta-oxidation flux. Biochem Soc Trans. 2001;29:245–250. doi: 10.1042/0300-5127:0290245. [DOI] [PubMed] [Google Scholar]
- Enns GM, Bennett MJ, Hoppel CL, et al. Mitochondrial respiratory chain complex I deficiency with clinical and biochemical features of long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. J Pediatr. 2000;136:251–254. doi: 10.1016/s0022-3476(00)70111-9. [DOI] [PubMed] [Google Scholar]
- Exil VJ, Roberts RL, Sims H, et al. Very-long-chain acylcoenzyme a dehydrogenase deficiency in mice. Circ Res. 2003;93:448–455. doi: 10.1161/01.RES.0000088786.19197.E4. [DOI] [PubMed] [Google Scholar]
- Fell DA. Metabolic control analysis: a survey of its theoretical and experimental development. Biochem J. 1992;286:313–330. doi: 10.1042/bj2860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerman FE. Reaction of electron-transfer flavoprotein ubiquinone oxidoreductase with the mitochondrial respiratory chain. Biochim Biophys Acta. 1987;893:161–169. doi: 10.1016/0005-2728(87)90035-1. [DOI] [PubMed] [Google Scholar]
- Ghisla S. Beta-oxidation of fatty acids. A century of discovery. Eur J Biochem. 2004;271:459–461. doi: 10.1046/j.1432-1033.2003.03952.x. [DOI] [PubMed] [Google Scholar]
- Goetzman ES, Tian L, Wood PA. Differential induction of genes in liver and brown adipose tissue regulated by peroxisome proliferator-activated receptor-alpha during fasting and cold exposure in acyl-CoA dehydrogenasedeficient mice. Mol Genet Metab. 2005;84:39–47. doi: 10.1016/j.ymgme.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest. 1998;102:1724–1731. doi: 10.1172/JCI4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner RP, Brown GC, Brand MD. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the ‘top-down’ approach of metabolic control theory. Eur J Biochem. 1990;188:313–319. doi: 10.1111/j.1432-1033.1990.tb15405.x. [DOI] [PubMed] [Google Scholar]
- Heinemeyer J, Braun HP, Boekema EJ, Kouril R. A structural model of the cytochrome C reductase/oxidase supercomplex from yeast mitochondria. J Biol Chem. 2007;282:12240–12248. doi: 10.1074/jbc.M610545200. [DOI] [PubMed] [Google Scholar]
- Heinrich R, Rapoport TA. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974;42:89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hinsdale ME, Kelly CL, Wood PA. Null allele at bcd-1 locus in BALB/cByJ mice is due to a deletion in the shortchain acyl-CoA dehydrogenase gene and results in missplicing of messenger RNA. Genomics. 1993;16:605–611. doi: 10.1006/geno.1993.1237. [DOI] [PubMed] [Google Scholar]
- Holme E, Lindstedt S. Diagnosis and management of tyrosinemia type I. Curr Opin Pediatr. 1995;7:726–732. doi: 10.1097/00008480-199512000-00017. [DOI] [PubMed] [Google Scholar]
- Kacser H, Burns JA. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kacser H, Burns JA. The control of flux. Biochem Soc Trans. 1995;23:341–366. doi: 10.1042/bst0230341. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Kelly CL, Rhead WJ, Kutschke DA, et al. Functional correction of short-chain acyl-CoA dehydrogenase deficiency in transgenic mice. Am J Hum Genet. 1995;57(Supplement):A52. doi: 10.1093/hmg/6.9.1451. [DOI] [PubMed] [Google Scholar]
- Kelly CL, Rhead WJ, Kutschke WK, et al. Functional correction of short-chain acyl-CoA dehydrogenase deficiency in transgenic mice—implications for gene therapy of human mitochondrial enzyme deficiencies. Hum Mol Genet. 1997;6:1451–1455. doi: 10.1093/hmg/6.9.1451. [DOI] [PubMed] [Google Scholar]
- Kispal G, Sumegi B, Alkonyi I. Isolation and characterization of 3-hydroxyacyl coenzyme A dehydrogenase-binding protein from pig heart inner mitochondrial membrane. J Biol Chem. 1986;261:14209–14213. [PubMed] [Google Scholar]
- Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Korf B, Rimoin D, O’Connor J, Pyeritz R. Nature and frequency of genetic disease. In: Rimoin D, O’Connor J, Pyeritz R, Korf B, editors. Principles and Practice of Medical Genetics. Amsterdam: Elsevier; 2008. pp. 49–51. [Google Scholar]
- Krauss S, Quant PA. Regulation and control in complex, dynamic metabolic systems: experimental application of the top-down approaches of metabolic control analysis to fatty acid oxidation and detogenesis. J Theor Biol. 1996;182:381–388. doi: 10.1006/jtbi.1996.0177. [DOI] [PubMed] [Google Scholar]
- Kurtz DM, Rinaldo P, Rhead WJ, et al. Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci U S A. 1998;95:15592–15597. doi: 10.1073/pnas.95.26.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link TA, Schagger H, von Jagow G. Analysis of the structures of the subunits of the cytochrome bc1 complex from beef heart mitochondria. FEBS Lett. 1986;204:9–15. doi: 10.1016/0014-5793(86)81378-3. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Travels with carnitine palmitoyltransferase I: from liver to germ cell with stops in between. Biochem Soc Trans. 2001;29:241–245. doi: 10.1042/0300-5127:0290241. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Bunkenborg J, Olsen JV, et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/s0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- Parker A, Engel P. Preliminary evidence for the existence of specific functional assemblies between enzymes of the beta-oxidation pathway and the respiratory chain. Biochem J. 2000;345:429–435. [PMC free article] [PubMed] [Google Scholar]
- Quant PA. Experimental application of top-down control analysis to metabolic systems. Trends Biochem Sci. 1993;18:26–30. doi: 10.1016/0968-0004(93)90084-z. [DOI] [PubMed] [Google Scholar]
- Rubio JC, Martin MA, Bautista J, et al. Myophosphorylase deficiency associated with a defect in complex I of the mitochondrial respiratory chain. J Neurol Sci. 1998;161:110–113. doi: 10.1016/s0022-510x(98)00263-9. [DOI] [PubMed] [Google Scholar]
- Rubio JC, Martin MA, del Hoyo P, et al. Molecular analysis of Spanish patients with AMP deaminase deficiency. Muscle Nerve. 2000;23:1175–1178. doi: 10.1002/1097-4598(200008)23:8<1175::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Ruzicka FJ, Beinert H. A new membrane iron-sulfur flavoprotein of the mitochondrial electron transfer system. The entrance point of the fatty acyl dehydrogenation pathway? Biochem Biophys Res Commun. 1975;66:622–631. doi: 10.1016/0006-291x(75)90555-0. [DOI] [PubMed] [Google Scholar]
- Schagger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta. 2002;1555:154–159. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- Schuler AM, Gower BA, Matern D, Rinaldo P, Vockley J, Wood PA. Synergistic heterozygosity in mice with inherited enzyme deficiencies of mitochondrial fatty acid beta-oxidation. Mol Genet Metab. 2005;85:7–11. doi: 10.1016/j.ymgme.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Scriver CR, Waters PJ. Monogenic traits are not simple: lessons from phenylketonuria. Trends Genet. 1999;15:267–272. doi: 10.1016/s0168-9525(99)01761-8. [DOI] [PubMed] [Google Scholar]
- Segre D, Deluna A, Church GM, Kishony R. Modular epistasis in yeast metabolism. Nat Genet. 2005;37:77–83. doi: 10.1038/ng1489. [DOI] [PubMed] [Google Scholar]
- Shoffner JM. Oxidative phosphorylation diseases. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors; Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edn. New York: McGraw-Hill; 2001. pp. 2367–2424. assoc. eds. [Google Scholar]
- Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nature Rev Genet. 2001;2:342–352. doi: 10.1038/35072063. [DOI] [PubMed] [Google Scholar]
- Stroh A, Anderka O, Pfeiffer K, et al. Assembly of respiratory complexes I, III, and IV into NADH oxidase supercomplex stabilizes complex I in Paracoccus denitrificans. J Biol Chem. 2004;279:5000–5007. doi: 10.1074/jbc.M309505200. [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, et al. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumegi B, Srere PA. Complex I binds several mitochondrial NAD-coupled dehydrogenases. J Biol Chem. 1984;259:15040–15045. [PubMed] [Google Scholar]
- Sumegi B, Porpaczy Z, Alkonyi I. Kinetic advantage of the interaction between the fatty acid beta-oxidation enzymes and the complexes of the respiratory chain. Biochim Biophys Acta. 1991;1081:121–812. doi: 10.1016/0005-2760(91)90016-b. [DOI] [PubMed] [Google Scholar]
- Thorpe C, Kim JJ. Structure and mechanism of action of the acyl-CoA dehydrogenases. FASEB J. 1995;9:718–725. doi: 10.1096/fasebj.9.9.7601336. [DOI] [PubMed] [Google Scholar]
- Tong AH, Lesage G, Bader GD, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- Tonin P, Lewis P, Servidei S, DiMauro S. Metabolic causes of myoglobinuria. Ann Neurol. 1990;27:181–185. doi: 10.1002/ana.410270214. [DOI] [PubMed] [Google Scholar]
- Venizelos N, von Dobeln U, Hagenfeldt L. Fatty acid oxidation in fibroblasts from patients with defects in beta-oxidation and in the respiratory chain. J Inherit Metab Dis. 1998;21:409–415. doi: 10.1023/a:1005310809714. [DOI] [PubMed] [Google Scholar]
- Vladutiu G. Complex phenotypes in metabolic muscle diseases. Muscle Nerve. 2000;23:1157–1159. doi: 10.1002/1097-4598(200008)23:8<1157::aid-mus1>3.0.co;2-o. doi:10.1002/1097-4598(200008)23:8<1157::AID-MUS1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Vladutiu GD. Heterozygosity: an expanding role in proteomics [Review] Mol Genet Metab. 2001;74:51–63. doi: 10.1006/mgme.2001.3240. [DOI] [PubMed] [Google Scholar]
- Vockley J, Whiteman DA. Defects of mitochondrial betaoxidation: a growing group of disorders. Neuromuscul Disord. 2002;12:235–246. doi: 10.1016/s0960-8966(01)00308-x. [DOI] [PubMed] [Google Scholar]
- Vockley J, Rinaldo P, Bennett MJ, Matern D, Vladutiu GD. Synergistic heterozygosity: disease resulting from multiple partial defects in one or more metabolic pathways. Mol GenetMetab. 2000;71:10–18. doi: 10.1006/mgme.2000.3066. [DOI] [PubMed] [Google Scholar]
- Vockley J, Singh RH, Whiteman DA. Diagnosis and management of defects of mitochondrial beta-oxidation. Curr Opin Clin Nutr Metab Care. 2002;5:601–609. doi: 10.1097/00075197-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial diseases—genotype versus phenotype. Trends Gene. 1993;9:128–133. doi: 10.1016/0168-9525(93)90207-x. [DOI] [PubMed] [Google Scholar]
- Wood PA, Amendt BA, Rhead WJ, Millington DS, Inoue F, Armstrong D. Short chain acyl-CoA dehydrogenase deficiency in mice. Pediatr Res. 1989;25:38–43. doi: 10.1203/00006450-198901000-00010. [DOI] [PubMed] [Google Scholar]