Summary
Background
Listeriosis, caused by Listeria monocytogenes, is an important foodborne disease that can be difficult to control and commonly results in severe clinical outcomes. We aimed to provide the first estimates of global numbers of illnesses, deaths, and disability-adjusted life-years (DALYs) due to listeriosis, by synthesising information and knowledge through a systematic review.
Methods
We retrieved data on listeriosis through a systematic review of peer-reviewed and grey literature (published in 1990–2012). We excluded incidence data from before 1990 from the analysis. We reviewed national surveillance data where available. We did a multilevel meta-analysis to impute missing country-specific listeriosis incidence rates. We used a meta-regression to calculate the proportions of health states, and a Monte Carlo simulation to generate DALYs by WHO subregion.
Findings
We screened 11 722 references and identified 87 eligible studies containing listeriosis data for inclusion in the meta-analyses. We estimated that, in 2010, listeriosis resulted in 23 150 illnesses (95% credible interval 6061–91 247), 5463 deaths (1401–21 497), and 172 823 DALYs (44 079–676 465). The proportion of perinatal cases was 20·7% (SD 1·7).
Interpretation
Our quantification of the global burden of listeriosis will enable international prioritisation exercises. The number of DALYs due to listeriosis was lower than those due to congenital toxoplasmosis but accords with those due to echinococcosis. Urgent efforts are needed to fill the missing data in developing countries. We were unable to identify incidence data for the AFRO, EMRO, and SEARO WHO regions.
Funding
WHO Foodborne Diseases Epidemiology Reference Group and the Université catholique de Louvain.
Introduction
Listeriosis is caused by the Gram-positive ubiquitous bacterium Listeria monocytogenes, which was first recognised as a foodborne pathogen in the early 1980s.1 Since its discovery, it has been identified as a cause of major foodborne outbreaks. Unlike most other foodborne pathogens, L monocytogenes can grow in food with fairly low moisture content and high salt concentration. Most importantly, L monocytogenes grows at refrigeration temperatures, by contrast with many other foodborne pathogens. This ability to persist and multiply in the food environment makes L monocytogenes especially difficult to control.2
Clinical listeriosis mainly occurs in particular at-risk groups: pregnant women, elderly people, immunocompromised people, unborn babies, and neonates (through vertical transmission from the mother or, more rarely, at birth by ascending colonisation from the vagina).3 In healthy people, L monocytogenes infections might cause febrile gastroenteritis, which is usually mild and self-limiting. Mainly in patients with impaired cell-mediated immunity, listeriosis can lead to severe illnesses, including severe sepsis, meningitis, or encephalitis, and thereby cause lifelong consequences and even death.4–6 Infection during pregnancy can result in spontaneous abortions or stillbirths.7 Preterm birth is also a common consequence of listeriosis in pregnant women.8,9
Most cases of listeriosis are sporadic and have been reported in high-income countries, where incidence is quite low but fatality rate is high.10 Important outbreaks have also occurred—for example, an outbreak of listeriosis from cantaloupes in Colorado, USA, in 2011 resulted in infection of 147 people and 33 deaths, making it the deadliest recorded US foodborne outbreak since the US Centers for Disease Control and Prevention (CDC) began tracking outbreaks in the 1970s.11–13
Listeriosis often results in admission to intensive-care units, which makes L monocytogenes the third most costly foodborne pathogen in the USA per case in 2010, after Clostridium botulinum and Vibrio vulnificus.14 Ivanek and colleagues15 estimated that the annual cost of L monocytogenes in the USA was US$2·3 billion to 22 billion, and the annual benefit of listeria food safety measures was $0·01 billion to 2·4 billion.
Only a few countries have assessed the listeriosis burden in terms of disability-adjusted life-years (DALYs),16–18 and the global burden of listeriosis has never been estimated. However, DALYs can be used to compare diseases and health conditions, and thereby help policy makers to allocate resources. To understand the global burden of foodborne diseases, including listeriosis, WHO therefore established an advisory body, the Foodborne Disease Epidemiology Reference Group (FERG).19 The aim of our study was to estimate the annual global number of illnesses, deaths, and DALYs due to listeriosis, to contribute to the FERG initiative. We synthesised existing information and knowledge through a systematic literature review and meta-analysis, which was incorporated into calculations of the disease burden.
Methods
Search strategy and selection criteria
We did a systematic review to identify all relevant information about the global burden of listeriosis. We searched PubMed, WHOLIS, Sciverse Scopus, CAB abstracts (BIDS), OpenGrey, and Conference proceedings citation index (Web of knowledge) for references published between Jan 1, 1990, and May 21, 2012. We did not set any language restrictions. Papers in languages we could not read were translated by native speakers. For one report in Malay, no native speakers could be identified, so we used Google Translate.
We developed the search terms in accordance with the Medical Subject Headings thesaurus, using a combination of test searches and via collaboration between independent researchers and knowledge users. Search terms were designed to capture a range of terms and outcomes associated with listeriosis (appendix). The appendix summarises further details on the databases and Boolean operators that were checked.
Additionally, for each of the member states of WHO for which we did not identify incidence data, we reviewed national surveillance data where available, via national websites. We identified the national websites and surveillance data through a Google search in French, Dutch, English, or the official language of the country using Google Translate (if no website was identified in French, Dutch, or English). The appendix summarises the search terms. We contacted countries for which we did not identify websites or national surveillance data by contacting the ministry of health or health professionals in the country.
Finally, for each of the WHO subregions for which we did not identify incidence data, we consulted the WHO Collaborating Centre for Foodborne Listeriosis to assist in filling of data gaps. After deleting duplicates, we screened titles, abstracts, or entire articles for exclusion criteria. Screening was done independently by two authors (CMN, BD). Any disagreement about eligibility between reviewers was resolved by a third author (NS). The first two authors extracted data from included papers using a data extraction form reviewed by the other co-authors (appendix), and we excluded incidence data from before 1990 from the analysis. We hand-searched bibliographies of included documents for additional references. Our procedures accorded with the PRISMA guidelines for reporting systematic reviews (appendix).
Listeriosis disease model
For a quantitative assessment of the listeriosis disease burden, we constructed a disease model for perinatal and non-perinatal listeriosis, based on identified qualitative information about pathological and clinical symptoms, and the availability of quantitative data.20 This model enabled the quantification of the global burden of listeriosis, expressed in DALYs (figure 1).21
Figure 1. Outcome tree for perinatal and non-perinatal listeriosis.
Each block represents a node in the computational disease model, and arrows represent transition probabilities between nodes. Red boxes contribute years of life lost caused by premature death (YLLs), green boxes contribute years lived with disability (YLDs), and blue boxes have no contribution to the disability-adjusted life-years. In addition to this baseline model, we did a scenario analysis in which stillbirths were excluded from the burden estimates.
We defined a case of listeriosis as isolation of L monocytogenes from a normally sterile site (eg, blood or cerebrospinal fluid) or from products of conception (eg, placental or fetal tissue), and septicaemia referred to severe sepsis. We defined perinatal cases as maternofetal, including pregnancy-associated cases and cases in newborn babies during the first month of life. We counted a maternofetal infection as one case. We defined stillbirths as a death in a fetus between 24 weeks and 41 weeks of gestation. Because of the controversy regarding the inclusion of stillbirths in disease burden estimates,22 we also did a scenario analysis in which we excluded stillbirths from the burden estimates.
Statistical analyses
We developed a multilevel random-effects model to impute missing country-level incidence values.23 In this model, the number of cases yijk in each study k from country j belonging to WHO subregion i was assumed to follow a Poisson distribution with parameter λijk = θijk × nijk/100 000, with θijk the listeriosis incidence per 100 000 people and nijk the study-specific population size. Furthermore, we modelled the natural logarithm of the incidence per 100 000 people as the sum of a global intercept α0, a region-specific random effect ti, a country-specific random effect uij, and a study-specific random effect vijk: log θijk = α0 + ti + uij + vijk.
For the random effects, independent Normal distributions with means of zero and variances (σt2, σu2, σv2) were assumed. We implemented the multilevel random-effects model in a Bayesian framework, using Normal (μ=0,σ2=10 000), a prior distribution for the global intercept α0, and a Uniform (min=0,max=10) prior distribution for each of the random-effect standard errors (σt, σu, σv). We did the analysis in WinBUGS 1.4.3.24 The appendix contains the code developed to analyse the data.
We imputed incidence values for countries with no data, on the basis of the posterior predictive distributions from the multilevel random effects model. For countries in a WHO subregion where no countries had data, we imputed the log-incidence as multiple random draws from a normal distribution with mean equal to the global intercept α0, and variance equal to the sum of the between-region variance σt2 and the between-country variance σu2. For countries in a WHO subregion where at least one of the other countries had data, we imputed the log-incidence as multiple random draws from a Normal distribution, with mean equal to the sum of the global intercept α0 and the region-specific random effect ti, and variance equal to the between-country variance σu2. In summary, these imputations corresponded to the predicted distribution for an average country within an average WHO subregion for countries in a WHO subregion where no countries had data, and an average country within the particular WHO subregion for countries where at least one of the other countries had data. We did these analyses in R 3.0.1. We did no imputations for countries with available incidence data; the incidence data used in the further analyses are therefore a combination of actual data and imputed estimates.
We combined values for the transition probabilities listed in figure 1 extracted from the studies identified in the systematic review into a single estimate with corresponding uncertainty using a random effects meta-regression model. For every study, we assigned an indicator variable matching the study quality, with 0 representing prospective cohort studies, multiplier studies, or case-based notifiable studies, and 1 representing notifiable outbreaks or other (eg, case-control studies). We included this indicator as a fixed effect in the meta-regression model. We did the analyses in R 3.0.1 using the Metafor package.25
Health outcomes for perinatal and non-perinatal listeriosis were death, septicaemia, CNS infection, and neurological sequelae after a CNS infection. We derived disability weights (DWs) for septicaemia, CNS infection, and neurological sequelae from DWs for different health states in the Global Burden of Disease (GBD) 2010 study (table 1).26 For septicaemia, we used the GBD 2010 DW for severe acute episode of infectious disease. For CNS infection, we used a combination of four GBD 2010 DWs: severe acute episode of infectious disease, severe intellectual disability, average of the DWs severe epilepsy and treated epilepsy with recent seizures, and moderate motor impairment. For neurological sequelae, we used a combination of three GBD 2010 DWs: average of the ten DWs for hearing loss, average of the five DWs for vision loss, and average of the four DWs for stroke with long-term consequences. To create DWs for CNS infection and neurological sequelae, we did an expert elicitation of eight members of the Belgian Association of Neurology via a web-based questionnaire. We asked members to estimate the probability of occurrence of each health state, or combinations of health states, after perinatal or non-perinatal listeriosis. We established the DWs for the combinations of health states using a described multiplicative method.27 We applied the Las Vegas method, in which we asked experts in CNS infections to distribute 100 points over the different possible outcomes and combinations.28 We obtained a weighted overall DW per expert by combining the individual DWs and the assigned probabilities. Last, we did a bootstrap analysis on these weighted DWs to derive the 95% CIs, to account for the between-expert variability.
Table 1. Disability weights used to calculate the global burden of listeriosis.
| Disability weight (95% CI) | |
|---|---|
| Perinatal or non-perinatal septicaemia | |
| (A) Infectious disease: acute episode (severe)—disability weight used for septicaemia | 0·210* (0·139–0·298) |
| Perinatal or non-perinatal CNS infection | |
| (A) Infectious disease: acute episode (severe) | 0·210* |
| (B) Intellectual disability (severe) | 0·126* |
| (C) Epilepsy (severe and treated, with recent seizures) | 0·488*† |
| (D) Motor impairment (moderate) | 0·076* |
| (A) and (B) | 0·340‡ |
| (A) and (C) | 0·540‡ |
| (A) and (D) | 0·270‡ |
| (B) and (C) | 0·553‡ |
| (B) and (D) | 0·192‡ |
| (C) and (D) | 0·527‡ |
| (A) and (B) and (C) | 0·646‡ |
| (A) and (B) and (D) | 0·362‡ |
| (B) and (C) and (D) | 0·626‡ |
| (A) and (B) and (C) and (D) | 0·673‡ |
| Disability weight used for CNS infection | 0·426§ (0·368-0·474¶) |
| Perinatal or non-perinatal neurological sequelae | |
| (A) Hearing loss | 0·047*† |
| (B) Vision loss | 0·087*† |
| (C) Stroke: long-term consequences | 0·303*† |
| (A) and (B) | 0·130‡ |
| (B) and (C) | 0·364‡ |
| (A) and (C) | 0·336‡ |
| (A) and (B) and (C) | 0·394‡ |
| Disability weight used for neurological sequelae | 0·292§ (0·272–0·316¶) |
GBD 2010.26
Averaged disability weight.
Multiplicative methodology.
Using expert elicitation.
Bootstrap analysis.
We calculated DALYs according to the standard formulae,29,30 without age-weighting or time-discounting. We based life expectancies on the Coale-Demeny model life table West.31 We applied no sex distinction. We accounted for parameter uncertainty using Monte Carlo simulations of the input parameters, based on 10 000 samples. To enable comparisons of our results with others studies, we did scenario analyses in which DALYs were calculated on the basis of a 3% discount rate, with and without age-weighting.32 We did not correct for comorbidity. We used a duration of 7 days for septicaemia, 182 days for CNS infection, and 7 years for neurological sequelae.16 We did DALY calculations in R 3.0.1, using the DALY package version 1.2.0.33 We generated the global maps with the rworldmap package.34
Role of funding source
We consulted the WHO advisory body FERG during the study design, who assisted with obtaining of identified references, and provided feedback about preliminary results. The Université catholique de Louvain assisted with reference searching and funded the study. All authors had full access to all study data, and the analysis, interpretation, and the decision to publish were solely the responsibility of the authors.
Results
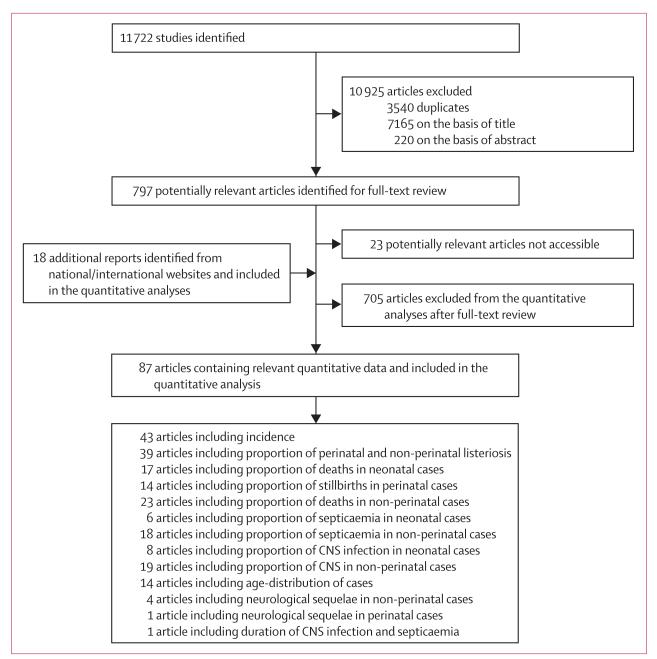
Our systematic review identified 11 722 studies, of which we included 87 in the quantitative analysis (figure 2, appendix). Incidence data were available for seven of the 14 WHO subregions. The WHO Collaborating Centre for Foodborne Listeriosis did not provide additional incidence data. In AMRO A, incidence data were available for two of three countries, in EURO A for 23 of 27 countries, in WPRO A for four of five countries, in AMRO B, for one of 26 countries, in EURO B for eight of 16 countries, in WPRO B for one of 22 countries and in EURO C for six of nine countries (panel).
Figure 2. Study selection.
The studies are referenced in the appendix.
For 75% of the studies included in the different meta-analyses we assigned a good study quality weight of zero. We could not identify any useful incidence data for 85 countries (Afghanistan, Algeria, Angola, Bahrain, Bangladesh, Benin, Bhutan, Bolivia, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo, Côte d’Ivoire, Democratic Republic of the Congo, Djibouti, Ecuador, Egypt, Equatorial Guinea, Eritrea, Ethiopia, Gabon, The Gambia, Ghana, Guatemala, Guinea, Guinea-Bissau, Haiti, India, Indonesia, Iran, Iraq, Jordan, Kenya, Kuwait, Lebanon, Lesotho, Liberia, Libya, Madagascar, Malawi, Maldives, Mali, Mauritania, Mauritius, Morocco, Mozambique, Myanmar, Namibia, Nepal, Nicaragua, Niger, Nigeria, North Korea, Oman, Pakistan, Peru, Qatar, Rwanda, São Tomé and Príncipe, Saudi Arabia, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, South Sudan, Sri Lanka, Sudan, Swaziland, Syria, Tanzania, Thailand, Timor Leste, Togo, Tunisia, Uganda, United Arab Emirates, Yemen, Zambia, Zimbabwe). These countries correspond to a population of 3 320 865 627 (48% of the global population in 2010).
We estimated that L monocytogenes caused 23 150 illnesses worldwide in 2010 (table 2), on the basis of the 2010 population of 6 860 035 412. The highest estimated listeriosis incidence rate was in the AMRO B subregion (table 2), and the lowest estimated incidence was in the EURO B WHO subregion (table 2). We also estimated that listeriosis led to 5463 deaths globally in 2010 (table 2).
Table 2. Listeriosis cases and deaths caused by listeriosis in 2010 by WHO subregion.
|
Incident cases
|
Deaths
|
|||
|---|---|---|---|---|
| Net values | Rates (per 100 000) | Net values | Rates (per 100 000) | |
| AFRO D | 1711 (0–9760) | 0·433 (0·000–2·470) | 404 (0–2322) | 0·102 (0·000–0·588) |
| AFRO E | 1913 (0–10 912) | 0·433 (0·000–2·470) | 451 (0–2596) | 0·102 (0·000–0·588) |
| AMRO A | 1330 (910–2104) | 0·374 (0·256–0·592) | 314 (207–508) | 0·088 (0·058–0·143) |
| AMRO B | 2298 (92–10 023) | 0·469 (0·019–2·046) | 544 (22–2386) | 0·111 (0·004–0·487) |
| AMRO D | 362 (0–2066) | 0·433 (0·000–2·470) | 85 (0–491) | 0·102 (0·000–0·588) |
| EMRO B | 715 (0–4079) | 0·433 (0·000–2·470) | 169 (0–970) | 0·102 (0·000–0·588) |
| EMRO D | 1851 (0–10 560) | 0·433 (0·000–2·470) | 437 (0–2512) | 0·102 (0·000–0·588) |
| EURO A | 1491 (1238–1765) | 0·342 (0·284–0·405) | 352 (277–436) | 0·081 (0·063–0·100) |
| EURO B | 96 (49–206) | 0·042 (0·022–0·091) | 23 (11–49) | 0·010 (0·005–0·022) |
| EURO C | 209 (86–593) | 0·089 (0·037–0·253) | 49 (20–140) | 0·021 (0·008–0·060) |
| SEARO B | 1428 (0–8146) | 0·433 (0·000–2·470) | 337 (0–1938) | 0·102 (0·000–0·588) |
| SEARO D | 6399 (0–36 505) | 0·433 (0·000–2·470) | 1510 (0–8684) | 0·102 (0·000–0·588) |
| WPRO A | 205 (137–290) | 0·129 (0·087–0·183) | 48 (31–70) | 0·030 (0·020–0·044) |
| WPRO B | 3141 (1986–6887) | 0·192 (0·121–0·420) | 741 (447–1632) | 0·045 (0·027–0·100) |
| Global total | 23 150 (6061–91247) | 0·337 (0·088–1·330) | 5463 (1401–21 497) | 0·080 (0·020–0·313) |
Data are mean (95% credible interval). Country groupings and child and adult mortality groupings are from WHO. WHO subregions are listed in the panel.
We estimated 2% of non-perinatal cases to be in individuals aged 1–4 years, 4% in those aged 5–14 years, 10% in those aged 15–34 years, 6% in those aged 35–44 years, 7% in those aged 45–54 years, 13% in those aged 55–64 years, 20% in those aged 65–74 years, 20% in those aged 75–84 years, and 18% in those aged 85 years or older.
Of all listeriosis cases, 20·7% (95% CI 17·4–23·9) were perinatal infections, and 79·3% (75·4–83·3) were non-perinatal. Septicaemia was the most common outcome in perinatal cases, occurring in 30·7% (12·6–48·9) of infected neonates. In total, 15·2% (11·2–19·2) of neonates with listeriosis developed CNS infections, of whom 43·8% (20·2–67·3) showed neurological sequelae. 9·2% (5·8–12·6) of all perinatal cases resulted in neonatal deaths (among livebirths) and 5·7% (2·0–9·4) resulted in stillbirths, for an overall case fatality of 14·9%. Of all non-perinatal listeriosis cases, 61·6% (57·4–65·9) resulted in septicaemia and 30·7% (26·9–34·6) resulted in CNS infections. Of the nonperinatal cases affected by CNS infection, 13·7% (2·4–25·1) developed neurological sequelae. In total, 25·9% (21·9–29·9) of the non-perinatal cases resulted in death.
On the basis of expert elicitations and bootstrap analysis, we obtained DW of 0·426 for CNS infection and 0·292 for neurological sequelae (table 1).
We estimated that, in 2010, listeriosis resulted in 172 823 DALYs (95% CrI 44 079–676 465; table 3) and 2·519 DALYs per 100 000 people (0·643–9·861; table 4). Years of life lost (YLLs) accounted for 98% of the total DALYs. The highest burden occurred in AMRO B, where listeriosis resulted in 3·512 DALYs per 100 000 people (0·140–15·532). The lowest rate was in EURO B, with an estimated 0·311 DALYs per 100 000 people (0·155–0·673). Results of the scenario analysis showed that inclusion of stillbirths increased the average DALYs total by 14·7% (tables 3, 4; figures 3, 4).
Table 3. Summary of listeriosis burden in 2010 by WHO subregion.
| Years lived with disability (net values) |
Years of life lost (net values) |
Disability-adjusted life-years (net values) |
|
|---|---|---|---|
| AFRO D | 263 (0–1533) | 12 504 (1–71 215) | 12 767 (1–72 726) |
| AFRO E | 294 (0–1714) | 13 984 (1–79 641) | 14 278 (1–81 331) |
| AMRO A | 204 (112–367) | 9730 (6358–15 882) | 9934 (6496–16 215) |
| AMRO B | 354 (13–1602) | 16 846 (672–74 350) | 17 200 (686–76 070) |
| AMRO D | 56 (0–325) | 2647 (0–15 075) | 2703 (0–15 395) |
| EMRO B | 110 (0–641) | 5226 (0–29 763) | 5336 (0–30 395) |
| EMRO D | 284 (0–1659) | 13 530 (1–77 053) | 13 814 (1–78 688) |
| EURO A | 229 (146–344) | 10 903 (8464–13 723) | 11 132 (8656–13 991) |
| EURO B | 15 (7–33) | 703 (351–1523) | 718 (359–1553) |
| EURO C | 32 (11–95) | 1526 (609–4362) | 1558 (621–4447) |
| SEARO B | 219 90–1279) | 10 437 (1–59 539) | 10 656 (1–60 701) |
| SEARO D | 983 90–5734) | 46 769 (3–266 357) | 47 752 (3–272 009) |
| WPRO A | 31 (18–52) | 1497 (967–2188) | 1528 (988–2233) |
| WPRO B | 482 (244–1117) | 22 965 (13 739–50 696) | 23 447 (14 059–51 688) |
| Global total | 3556 (815–14 542) | 169 267 (43 106-661 907) | 172 823 (44 079–676 465) |
Data are mean (95% credible interval). Country groupings and child and adult mortality groupings are shown in the panel.
Table 4. Global burden of listeriosis, with and without the inclusion of stillbirths.
| Years lived with disability (rate per 100 000) |
Years of life lost (rate per 100 000) |
Disability-adjusted life years (rate per 100 000) |
|
|---|---|---|---|
| Without stillbirths | 0·052 (0·012–0·212) | 2·145 (0·547–8·447) | 2·197 (0·560–8·621) |
| With stillbirths | 0·052 (0·012–0·212) | 2·467 (0·628–9·649) | 2·519 (0·643–9·861) |
Data are mean (95% credible interval).
Figure 3. Disability-adjusted life-years (DALYs) per 100 000 people for listeriosis (with stillbirths) by WHO subregion.
DALYs (A) and SD of estimated DALYs (B). NA=not applicable.
Figure 4. Disability-adjusted life-years (DALYs) per 100 000 people for listeriosis (without stillbirths) by WHO subregion.
DALYs (A) and SD of estimated DALYs (B). NA=not applicable.
Discussion
Our meta-analysis provides the first estimates of the global burden of listeriosis, and enables the relative burden of listeriosis to be put in perspective. Compared with other foodborne pathogens, L monocytogenes causes fewer infections than do non-typhoidal salmonella (93·8 million cases, 95% CI 61·8–131·6 million),35 Salmonella Typhi (21·7 million cases),36 or Toxoplasma gondii related to congenital cases (190 100 cases [179 300–206 300]);37 L monocytogenes causes a similar number of infections to Echinococcus multilocularis (18 235 cases, 11 900–28 200).38 L monocytogenes also caused fewer deaths than did S Typhi (216 500 deaths)36 or non-typhoidal salmonella (155 000 deaths, 39 000–303 000).35 The number of DALYs due to listeriosis was lower than that due to congenital toxoplasmosis (1·20 million DALYs),37 but accords with DALYs due to echinococcosis (144 000 DALYs, 95% CrI 69 000–286 000).39 However, unlike these other diseases, listeriosis is mainly foodborne and is a major problem for the food industry, because it is difficult to control in the production environment.
Havelaar and colleagues18 estimated that listeriosis caused 0·58 DALYs per 100 000 people in 2009 in the Netherlands. We estimated a higher rate—2·555 DALYs per 100 000 people (95% CrI 1·987–3·211) in EURO A—but we used updated DWs to generate DALYs and included stillbirths in the burden calculation. Cressey and colleagues17 estimated that listeriosis caused 217 DALYs in New Zealand in 2007 (about 5·24 DALYs per 100 000 inhabitants). We estimated a lower rate than this, of 0·963 DALYs per 100 000 people (0·623–1·408), because we included the lower incidence reported in Japan in WPRO A and a 3% discount rate in DALYs calculation. In Greece, listeriosis was estimated to cause 4·1 YLLs (95% CrI 0·45–9·7) per 1 million people in an average year, and DALYs due to listeriosis were mainly established by the YLLs.40 We similarly noted that the YLLs accounted for the highest part of the total DALYs, but we estimated a higher YLL per million people (25·03, 19·43–31·50) because we included stillbirths.
We noted that inclusion of stillbirths in the DALY calculations for listeriosis increased the global burden by 14·7%. By contrast, despite substantial interest in the number of intrapartum fetal deaths, stillbirths are not included in the disease burden estimates in GBD 2010.41 Bhutta and colleagues42 estimated that, worldwide, 2·65 million stillbirths occur annually, of which 98% are in low-income and middle-income countries. In 2011, Frøen and colleagues43 launched an initiative to spotlight stillbirths; our study contributes to this goal.
Our study proposes a multilevel meta-analysis model for imputation of missing country-specific incidences, taking into account variability at the study, country, and WHO subregion levels. To our knowledge, such a multiple imputation model has not yet been applied in other global burden of disease studies. Other studies applied simple imputation techniques, for example by imputing the median value of the same or of a neighbouring region (eg, the work on visual impairment by Resnikoff and colleagues44), which ignores the variability of the data, and thus the uncertainty in the imputations. Our multiple imputation method could be extended further by inclusion of a correction for under-reporting, if a realistic model was available for the amount of under-reporting and for the variability in under-reporting within and between regions.
Our study uncovered important data gaps. Most importantly, we were unable to identify incidence data for the AFRO, EMRO, and SEARO WHO regions. We therefore relied on several data sources and assumptions to produce global estimations. The major assumption of our imputation model is that missing data were missing at random, meaning that the unobserved values were not associated with the probability of being missing.45 In our case, this assumption implied that, within each subregion, countries with data provided unbiased information on those without data, and that, across subregions, subregions with data provided unbiased information on those without data. We recognise that this assumption might be problematic, because we could only retrieve data from high-income and middle-income subregions. However, without information on the systematic difference in listeriosis incidence across subregions, it is almost impossible to account for this unless arbitrary assumptions are made, which cannot be checked with observed data, and which might greatly affect the final results. Data from these subregions are therefore urgently needed. This absence of data, and our resultant great uncertainty, was shown in the large CrIs for these subregions.
Our model did not take into account the food habits (eg, storage) or practices of importation that could differ between countries. We also did not correct for under-reporting because this information was only available for some countries in EURO A and AMRO A WHO subregions, is typically low (under-reporting multiplier of about 1·1),46 and is impossible to estimate for other countries with the available data. We think that use of multipliers for under-reporting that are derived from studies in others countries raises questions about the comparability of multipliers and health-care systems of countries. However, most of the studies included in the different meta-analyses had a good study quality weight score of 0—this means that most datapoints somehow accounted for under-reporting.
Moreover, incidence data on listeriosis could vary depending on the surveillance system of the country (passive or active, case mandatory reported or not) and on the definition of case based on isolation of bacteria or detection by PCR or immunological test. Additionally, gastrointestinal listeriosis exists and has been proven to be linked with septicaemia and CNS infections.47 By excluding gastrointestinal listeriosis from our calculations, we have underestimated the burden of listeriosis. However, we believe that this underestimation is probably minor, because acute gastroenteritis is typically less severe than are the outcomes of invasive listeriosis included in our analyses, and because the listeriosis burden seems to be dominated by the fatal outcomes. Nevertheless, we acknowledge the importance of recognition and correct management of such cases to avoid invasive forms of listeriosis.
In view of the small amount of data in some regions, we did not account for the trends of increasing or decreasing listeriosis incidence that could have occurred in some countries. In the USA, Voetsch and colleagues48 estimated that by 2001, compared with 1996–98, the incidence of listeriosis had decreased by about 37%. In Europe, the number of listeriosis cases slowly increased in the period 2008–12,49 and in Australia the notification rate of listeriosis has remained stable in 1991–2000.50
We also did not include all possible sequelae in our outcome tree designed to calculate DALYs, because we deemed some to be rare or mild, or because there was not enough data. Listeriosis can also lead to other atypical forms, such as skin infections, pneumonia, or peritonitis.51–53
We also did not correct our estimates for comorbidities; non-perinatal listeriosis cases are often linked with comorbidities such as cancer and diabetes. Some authors, such as Havelaar and colleagues,18 corrected the number of DALYs for comorbidities by reducing the life expectancy of non-perinatal listeriosis cases.
For some health outcomes such as neurological sequelae caused by CNS infection we identified few references, necessitating that we interpret these proportions carefully.
No DWs were available for the CNS infections and neurological sequelae, and we had to obtain these from eight experts in CNS infection. More optimum DWs should be designed in future.
We took the probabilities of development of symptoms and the case fatality ratios to be constant across the world. More data, particularly from developing countries, are needed to take into account regional differences in clinical outcome of listeriosis.
This study is the first attempt to quantify the global burden of listeriosis, and will enable listeriosis to be included in international prioritisation exercises. Nevertheless, because of the scarce data on listeriosis incidence, great uncertainty remains about the real effect of listeriosis worldwide. We encourage further studies, especially in the AFRO, EMRO, and SEARO WHO regions, to increase efforts to generate and share local data about listeriosis incidence.54,55 As additional data become available, an update of our analysis should be done.
Supplementary Material
Webappendix 1: Overall list of search terms
Webappendix 2: Databases and Boolean operators (last run May 21, 2012)
Webappendix 3: Exclusion criteria and data extracted
Webappendix 4: PRISMA Checklist for the Systematic Review and Meta-analysis to Estimate the Global Burden of listeriosis circa 2012
Webappendix 5: the code developed to analyse the data in WinBUGS 1.4.3.
Webappendix 6: Bibliographic details of references containing information for estimation of the global burden of listeriosis
Webappendix 7 : Study selection and references added into the quantitative analysis. References numbers are given in the webappendix 6.
Panel: WHO subregions included in this study.
AFRO D
Algeria, Angola, Benin, Burkina Faso, Cameroon, Cape Verde, Chad, Comoros, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Madagascar, Mali, Mauritania, Mauritius, Niger, Nigeria, São Tomé and Príncipe, Senegal, Seychelles, Sierra Leone, and Togo
AFRO E
Botswana, Burundi, Central African Republic, Congo, Côte d’Ivoire, Democratic Republic of the Congo, Eritrea, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Uganda, Tanzania, Zambia, and Zimbabwe
AMRO A
Canada, Cuba, and USA
AMRO B
Antigua and Barbuda, Argentina, Bahamas, Barbados, Belize, Brazil, Chile, Colombia, Costa Rica, Dominica, Dominican Republic, El Salvador, Grenada, Guyana, Honduras, Jamaica, Mexico, Panama, Paraguay, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Uruguay, and Venezuela
AMRO D
Bolivia, Ecuador, Guatemala, Haiti, Nicaragua, and Peru
EMRO B
Bahrain, Iran, Jordan, Kuwait, Lebanon, Libya, Oman, Qatar, Saudi Arabia, Syria, Tunisia, and United Arab Emirates
EMRO D
Afghanistan, Djibouti, Egypt, Iraq, Morocco, Pakistan, Somalia, South Sudan, Sudan, and Yemen
EURO A
Andorra, Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, and UK
EURO B
Albania, Armenia, Azerbaijan, Bosnia and Herzegovina, Bulgaria, Georgia, Kyrgyzstan, Montenegro, Poland, Romania, Serbia, Slovakia, Tajikistan, Macedonia, Turkey, Turkmenistan, and Uzbekistan
EURO C
Belarus, Estonia, Hungary, Kazakhstan, Latvia, Lithuania, Moldova, Russia, and Ukraine.
SEARO B
Indonesia, Sri Lanka, and Thailand
SEARO D
Bangladesh, Bhutan, North Korea, India, Maldives, Myanmar, Nepal, and Timor Leste
WPRO A
Australia, Brunei Darussalam, Japan, New Zealand, and Singapore
WPRO B
Cambodia, China, Cook Islands, Fiji, Kiribati, Laos, Malaysia, Marshall Islands, Federated States of Micronesia, Mongolia, Nauru, Niue, Palau, Papua New Guinea, Philippines, South Korea, Samoa, Solomon Islands, Tonga, Tuvalu, Vanuatu, and Vietnam
A=very low child mortality, very low adult mortality. B=low child mortality, low adult mortality. C=low child mortality, high adult mortality. D=high child mortality, high adult mortality. E=high child mortality, very high adult mortality.
Acknowledgments
The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization and of the US Centers for Disease Control and Prevention (CDC). We thank WHO’s Foodborne Diseases Epidemiology Reference Group (FERG), Carine Van Malderen (Université catholique de Louvain, Belgium), Patricia M Griffin (CDC, Atlanta), Shannon Majowicz (University of Waterloo, Canada) and Joachim Blocher (Department of Neurology, University Medical Center Göttingen, Germany) for intellectual support. We thank the CDC for assistance looking for missing PDFs and creating search strings, Daniel S Graciaa, Sabina Stoilova, Tanja Kuchenmüller, Jihee Lee, Tomasz Seliwiorstow, Anna I Vedel Sørensen, and Erika Ota for their invaluable help in obtaining and translating foreign-language literature. All authors had full access to all study data, and the analysis, interpretation, and decision to publish were solely the responsibility of the authors.
Footnotes
Declaration of interests CMN, BD, JH, FJA, AH, MK, and NS serve without compensation as members of FERG. All authors declare no competing interests.
See Online for appendix
References
- 1.Schlech WF, 3rd, Lavigne PM, Bortolussi RA, et al. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983;308:203–06. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- 2.Havelaar AH, van Rosse F, Bucura C, et al. Prioritizing emerging zoonoses in the Netherlands. PLoS One. 2010;5:e13965. doi: 10.1371/journal.pone.0013965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamont RF, Sobel J, Mazaki-Tovi S, et al. Listeriosis in human pregnancy: a systematic review. J Perinat Med. 2011;39:227–36. doi: 10.1515/JPM.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLauchlin J, Mitchell RT, Smerdon WJ, Jewell K. Listeria monocytogenes and listeriosis: a review of hazard characterisation for use in microbiological risk assessment of foods. Int J Food Microbiol. 2004;92:15–33. doi: 10.1016/S0168-1605(03)00326-X. [DOI] [PubMed] [Google Scholar]
- 5.Doganay M. Listeriosis: clinical presentation. FEMS Immunol Med Microbiol. 2003;35:173–75. doi: 10.1016/S0928-8244(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 6.Disson O, Lecuit M. Targeting of the central nervous system by Listeria monocytogenes. Virulence. 2012;3:213–21. doi: 10.4161/viru.19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston WH, Morton SA, Wong MH, Roy TE. Septicaemia of the newborn due to Listeria monocytogenes. Can Med Assoc J. 1955;73:402–05. [PMC free article] [PubMed] [Google Scholar]
- 8.Becroft DM, Farmer K, Seddon RJ, et al. Epidemic listeriosis in the newborn. BMJ. 1971;3:747–51. doi: 10.1136/bmj.3.5777.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awofisayo A, Amar C, Ruggles R, Elson R, Adak GK, Grant KA. Pregnancy-associated listeriosis in England and Wales. Epidemiol Infect. 2014 doi: 10.1017/S0950268814000594. published online March 20. http://dx.doi.org/10.1017/S0950268814000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillespie IA, Mook P, Little CL, Grant KA, McLauchlin J. Human listeriosis in England, 2001-2007: association with neighbourhood deprivation. Euro Surveill. 2010;15:7–16. doi: 10.2807/ese.15.27.19609-en. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August-September 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1357–58. [PubMed] [Google Scholar]
- 12.CDC [accessed Dec 31, 2013];Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado. 2011 http://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/120811/index.html.
- 13.Gerner-Smidt P, Whichard JM. Foodborne disease trends and reports. Foodborne Pathog Dis. 2009;6:749–51. doi: 10.1089/fpd.2009.9997. [DOI] [PubMed] [Google Scholar]
- 14.Scharff RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot. 2012;75:123–31. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- 15.Ivanek R, Gröhn YT, Tauer LW, Wiedmann M. The cost and benefit of Listeria monocytogenes food safety measures. Crit Rev Food Sci Nutr. 2004;44:513–23. doi: 10.1080/10408690490489378. [DOI] [PubMed] [Google Scholar]
- 16.Kemmeren JM, Mangen MJJ, Van Duynhoven YTHP, Havelaar AH. [accessed July 15, 2014];Priority setting of foodborne pathogens. Disease burden and costs of selected enteric pathogens. 2006 http://www.rivm.nl/bibliotheek/rapporten/330080001.pdf.
- 17.Cressey P, Lake R. [accessed July 15, 2014];Risk ranking: estimates of the burden of foodborne disease for New Zealand. 2007 http://foodsafety.govt.nz/elibrary/industry/Risk_Ranking-Science_Research.pdf.
- 18.Havelaar AH, Haagsma JA, Mangen MJ, et al. Disease burden of foodborne pathogens in the Netherlands, 2009. Int J Food Microbiol. 2012;156:231–38. doi: 10.1016/j.ijfoodmicro.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Kuchenmüller T, Hird S, Stein C, Kramarz P, Nanda A, Havelaar AH. Estimating the global burden of foodborne diseases—a collaborative effort. Euro Surveill. 2009;14:1–4. doi: 10.2807/ese.14.18.19195-en. [DOI] [PubMed] [Google Scholar]
- 20.Mangen MJ, Plass D, Havelaar AH, et al. the BCoDE consortium The pathogen- and incidence-based DALY approach: an appropriate [corrected] methodology for estimating the burden of infectious diseases. PLoS One. 2013;8:e79740. doi: 10.1371/journal.pone.0079740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devleesschauwer B, Havelaar AH, Maertens de Noordhout C, et al. DALY calculation in practice: a stepwise approach. Int J Public Health. 2014;59:571–74. doi: 10.1007/s00038-014-0553-y. [DOI] [PubMed] [Google Scholar]
- 22.Jamison DT, Shahid-Salles SA, Jamison J, Lawn JE, Zupan J. Incorporating deaths near the time of birth into estimates of the global burden of disease. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, editors. Global Burden of Disease and Risk Factors. World Bank; Washington (DC): 2006. [PubMed] [Google Scholar]
- 23.Lesaffre E, Lawson BL. Bayesian biostatistics. John Wiley & Sons; New York: 2012. (Statistics in practice). [Google Scholar]
- 24.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS–a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–37. [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36:1–48. [Google Scholar]
- 26.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wackerly D, Mendenhall W, 3rd, Scheaffer R. Mathematical statistics with applications. 7th edn Thomson Brooks/Cole; Boston, MA: 2008. [Google Scholar]
- 28.Cardoen S, Van Huffel X, Berkvens D, et al. Evidence-based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathog Dis. 2009;6:1083–96. doi: 10.1089/fpd.2009.0291. [DOI] [PubMed] [Google Scholar]
- 29.Devleesschauwer B, Havelaar AH, Maertens de Noordhout C, et al. Calculating disability-adjusted life years to quantify burden of disease. Int J Public Health. 2014;59:565–69. doi: 10.1007/s00038-014-0552-z. [DOI] [PubMed] [Google Scholar]
- 30.Murray CJ, Lopez AD, Mathers CD, Lopez AD, editors. Summary measures of population health: concepts, ethics, measurement and applications. WHO; Geneva: 2002. [Google Scholar]
- 31.Coale AJ, Demeny P. Regional model life tables and stable populations. Princeton University Press; Princeton: 1966. [Google Scholar]
- 32.Haagsma JA, Polinder S, Stein CE, Havelaar AH. Systematic review of foodborne burden of disease studies: quality assessment of data and methodology. Int J Food Microbiol. 2013;166:34–47. doi: 10.1016/j.ijfoodmicro.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Devleesschauwer B, McDonald S, Haagsma J, Praet N, Havelaar A, Speybroeck N. [accessed July 15, 2014];DALY: the DALY calculator—a GUI for stochastic DALY calculation in R. R package version 1.2.0. http://cran.r-project.org/package=DALY.
- 34.A South 2011 rworldmap: a new R package for mapping global data. R J. 2011;3/1:35–43. [Google Scholar]
- 35.Majowicz SE, Musto J, Scallan E, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:88289. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 36.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 37.Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ. 2013;91:501–08. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. doi: 10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 40.Gkogka E, Reij MW, Havelaar AH, Zwietering MH, Gorris LG. Risk-based estimate of effect of foodborne diseases on public health, Greece. Emerg Infect Dis. 2011;17:1581–90. doi: 10.3201/eid1709.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–66. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 42.Bhutta ZA, Yakoob MY, Lawn JE, et al. the Lancet’s Stillbirths Series steering committee Stillbirths: what difference can we make and at what cost? Lancet. 2011;377:1523–38. doi: 10.1016/S0140-6736(10)62269-6. [DOI] [PubMed] [Google Scholar]
- 43.Frøen JF, Cacciatore J, McClure EM, et al. Stillbirths: why they matter. Lancet. 2011;377:1353–66. doi: 10.1016/S0140-6736(10)62232-5. [DOI] [PubMed] [Google Scholar]
- 44.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin BR. Inference and Missing data. Biometrika. 1976;63:581–92. [Google Scholar]
- 46.Thomas MK, Murray R, Flockhart L, et al. Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog Dis. 2013;10:639–48. doi: 10.1089/fpd.2012.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allerberger F, Wagner M. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect. 2010;16:16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- 48.Voetsch AC, Angulo FJ, Jones TF, et al. Reduction in the incidence of invasive listeriosis in foodborne diseases active surveillance network sites, 1996-2003. Clin Infect Dis. 2007;44:513–20. doi: 10.1086/511006. [DOI] [PubMed] [Google Scholar]
- 49.European Food Safety Authority and European Centre for Disease Prevention and Control [accessed July 9, 2014];The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. http://ecdc.europa.eu/en/publications/Publications/EU-summary-report-zoonoses-food-borne-outbreaks-2012.pdf.
- 50.Lin M, Roche P, Spencer J, et al. [accessed July 9, 2014];Australia’s notifiable disease status, 2000. Annual report of the National Notifiable Diseases Surveillance System. doi: 10.33321/cdi.2002.26.14. https://www.health.gov.au/internet/main/publishing.nsf/Content/cda-2002-cdi2602-pdf-cnt.htm/$FILE/cdi2602b.pdf. [DOI] [PubMed]
- 51.Lambotte O, Fihman V, Poyart C, Buzyn A, Berche P, Soumelis V. Listeria monocytogenes skin infection with cerebritis and haemophagocytosis syndrome in a bone marrow transplant recipient. J Infect. 2005;50:356–58. doi: 10.1016/j.jinf.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 52.De Sá FR, Sztajnbok J, De Almeida JF, Troster EJ, Vaz FA. Listeria monocytogenes pneumonia in a cirrhotic child. Int J Clin Pract. 2004;58:536–38. doi: 10.1111/j.1368-5031.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad M, Krishnan A, Kelman E, Allen V, Bargman JM. Listeria monocytogenes peritonitis in a patient on peritoneal dialysis: a case report and review of the literature. Int Urol Nephrol. 2008;40:815–19. doi: 10.1007/s11255-008-9411-2. [DOI] [PubMed] [Google Scholar]
- 54.Devleesschauwer B, Ale A, Duchateau L, et al. Understanding the burden of disease in Nepal: a call for local evidence. J Nepal Health Res Counc. 2013;11:221–24. [PubMed] [Google Scholar]
- 55.Devleesschauwer B, Ale A, Torgerson P, et al. The burden of parasitic zoonoses in Nepal: a systematic review. PLoS Negl Trop Dis. 2014;8:e2634. doi: 10.1371/journal.pntd.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Webappendix 1: Overall list of search terms
Webappendix 2: Databases and Boolean operators (last run May 21, 2012)
Webappendix 3: Exclusion criteria and data extracted
Webappendix 4: PRISMA Checklist for the Systematic Review and Meta-analysis to Estimate the Global Burden of listeriosis circa 2012
Webappendix 5: the code developed to analyse the data in WinBUGS 1.4.3.
Webappendix 6: Bibliographic details of references containing information for estimation of the global burden of listeriosis
Webappendix 7 : Study selection and references added into the quantitative analysis. References numbers are given in the webappendix 6.