Abstract
Purpose
Based on a genome-wide association study (GWAS) of testicular dysgenesis syndrome (TDS) reporting possible association with TGFBR3, we analyzed GWAS data from a larger, phenotypically restricted cryptorchidism population for potential replication of this signal.
Materials and Methods
We excluded samples based on strict quality control criteria, leaving 844 cases and 2718 controls of European ancestry that were analyzed in 2 separate groups based on genotyping platform. Analyses included genotype imputation at the TGFBR3 locus, association analysis of imputed data with correction for population substructure, subsequent meta-analysis of Group 1 and 2 data and selective genotyping of independent cases (n=330) and controls (n=324) for replication. We also measured Tgfbr3 mRNA levels and performed TGFBR3/betaglycan immunostaining in rat fetal gubernaculum.
Results
We identified suggestive (p≤1×10−4) association of markers in/near TGFBR3 including rs9661103 (OR 1.40, 95% CI 1.20,1.64, p=2.71×10−5) and rs10782968 (OR 1.58, CI 1.26,1.98, p=9.36×10−5) in Groups 1 and 2, respectively. In subgroup analyses, we observed strongest association of rs17576372 (OR 1.42, CI 1.24,1.60; p=1.67×10−4) with proximal and rs11165059 (OR 1.32, CI 1.15,1.38; p=9.42×10−4) with distal testis position, signals in strong linkage disequilibrium with rs9661103 and rs10782968, respectively. Association of the prior GWAS signal (rs12082710) was marginal (OR 1.13, CI 0.99,1.28, p=0.09 for Group 1) and we were unable to replicate signals in our independent cohort. Tgfbr3/betaglycan was differentially expressed in wild type and cryptorchid rat fetal gubernaculum.
Conclusions
These data suggest complex or phenotype-specific association of cryptorchidism with TGFBR3 and the gubernaculum as a potential target of TGFβ signaling.
Keywords: Cryptorchidism, genetic association studies, TGFRB3, gubernaculum
INTRODUCTION
Cryptorchidism is a common genital anomaly identified in boys at birth or during childhood. The etiology is poorly understood and likely multifactorial, but associated genetic loci remain largely unknown. Familial aggregation suggests moderate genetic susceptibility and implicates the maternal environment as contributory to cryptorchidism risk.1,2 These levels of familial risk exceed those for many complex diseases and predict the potential for greater success in genome-wide analyses.3
Testicular descent is regulated by insulin-like 3 (INSL3) and androgens, Leydig cell-derived hormones that control development of the fetal gubernaculum.4 Exonic variants of INSL3, its receptor, relaxin/insulin-like family peptide receptor 2 (RXFP2), or other hormone pathway genes are rare in cryptorchidism cases, and their functional significance is poorly defined.5 Dalgaard and coworkers performed a genome-wide association study (GWAS) augmented by systems biology analysis methodology to identify genetic markers linked to the testicular dysgenesis syndrome (TDS), which includes cryptorchidism, hypospadias, testicular cancer and infertility.6 This approach did not identify genome-wide significant signals but had limited power to detect loci associated with isolated cryptorchidism. However, a TGFBR3 intronic single nucleotide polymorphism (SNP), rs12082710, showed suggestive evidence for association with TDS and cryptorchidism. Accordingly, we focused on TGFBR3 in an initial analysis of data from a larger GWAS cohort, and observed suggestive, phenotype-specific association of nonsyndromic cryptorchidism with this locus.
MATERIAL AND METHODS
Subjects and genotyping
Cases included subjects with cryptorchidism who underwent surgical repair at Nemours/Alfred I. DuPont Hospital for Children or The Children’s Hospital of Philadelphia (CHOP). Exclusion criteria included multiple congenital anomalies and/or diagnosis of a syndrome; other genital anomalies (hypospadias, chordee or other penile anomalies) and abdominal wall defects or major urogenital malformations. Control subjects recruited through the CHOP Health Care Network were males ≥ 7 years without a history of testicular disease, syndromes or other medical disorders potentially associated with cryptorchidism including inguinal hernia or hypospadias.
Basic demographic and phenotypic data were collected including age of diagnosis, race, ethnicity, laterality and the position of affected testes. Blood samples or excess tissue were collected and stored at −80°C or in RNAlater (Qiagen). As described in a previous report,7 we categorized the cases into different phenotypic subgroups. Nonscrotal position was defined as distal if the most severely affected cryptorchid testis was located at or beyond the external inguinal ring and proximal if at least one testis was located within the inguinal canal or abdomen. We assigned boys age ≤2 and >2 to early and late subgroups, respectively, based on the timing of surgery by a pediatric urologist. Informed consent was obtained for all participants based on approval by each participating center’s Institutional Review Board.
DNA was extracted from tissue or blood samples (5 PRIME, Gaithersburg, USA), and whole genome amplification (REPLI-g Mini Kit, Qiagen) performed for those with low DNA yield. Samples or adequate purity (OD 260/280=1.8-2.0 by Nanodrop1000 spectrophotometer) entered the standard genotyping workflow at the Center of Applied Genomics. Two genotyping platforms were used in the discovery stage, based on availability of control genotypes. In Group 1, 559 cases and 1772 controls was genotyped using the Illumina HumanHap550 v1, HumanHap550 v3 or Human610-Quad v1; these platforms share over 535K SNPs in common among all 3. In Group 2, 353 cases and 1149 controls were genotyped using the Illumina Human OmniExpress 12v1 platform.
Discovery Phase Data Analysis
Genome-wide genotyping data from Groups 1 and 2 were analyzed8 separately using PLINK (v1.07; http://pngu.mgh.harvard.edu/purcell/plink/).9 SNP content for each of the 3 genotyping platforms used for Group 1 cases was slightly different, therefore only overlapping SNPs (535,752) were used for further analysis. Individuals were excluded from further analysis using the following criteria: (1) discordance between reported sex and X and Y chromosome SNP data; (2) missing genotype rate >3%; (3) higher or lower than expected heterozygosity rate (greater than ± 3 SD from the mean) and (4) duplicates or relatives (based on estimate of proportion of alleles shared identical by descent >0.1875). SNPs were excluded based on the following criteria: (1) missing genotype rate >5%; (2) Hardy-Weinberg equilibrium (HWE) deviation in controls (p<0.00001); (3) significantly different missing genotype rates between cases and controls (p<0.00001); and (4) low minor allele frequency (MAF < 0.01). To select samples of European ancestry and control for population substructure, multidimensional scaling (MDS) analysis was performed in PLINK using European population SNP genotyping data from Stanford Human Genome Diversity Project (HGDP, http://www.hagsc.org/hgdp/files.html;10). We removed all samples that deviated from the means of the first or second components by more than 3 standard deviations. We performed separate association analyses for the remaining Group 1 and 2 samples using logistic regression with MDS components 1 and 2 as covariates.
We used HaploView to define the pattern of linkage disequilibrium (LD) at the TGFBR3 locus (v4.2, http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview11) and a targeted region was chosen for imputation (Chr1:91960000-92415000, GRCh37/hg19 assembly). Genotype data in PLINK format were converted to the IMPUTE2 (version 2.3.0) file format using GTOOL (version 0.7.5). We performed imputation using the 1000 Genome reference population (Sept. 2013 version) followed by association analysis of imputed data using IMPUTE2 and SNPTEST (version 2.5β), respectively.12,13 Imputed SNPs were removed from the SNPTEST results based on the following criteria: MAF <0.01; control HWE <0.00001 and frequentist_add_info (imputation quality score) <0.8. Meta-analyses of the remaining SNPs for Groups 1 and 2 was performed using META (version 1.5, http://www.stats.ox.ac.uk/~jsliu/meta.html) for all cases and controls, and for subgroups defined by testicular position, laterality and timing of presentation. Differences in phenotype frequencies between the two discovery groups were analyzed using Chi square tests. Results were mapped using the SNAP (SNP Annotation and Proxy Search) web tool14 and LD among the strongest signals was estimated using HaploView.
Replication Phase
DNA samples from boys with cryptorchidism and unaffected controls were obtained in a prior study of cryptorchidism in Sweden.15-17 We selected the top Group 1 and 2 TGFBR3 signals, or a SNP in complete LD, and the previously published marker, rs120827106, for genotyping using TaqMan assays (Life Technologies). Genotyping was performed in a 384-well format using the ABI 7900 Real Time Polymerase Chain Reaction (PCR) instrument. A reaction volume of 5 μl included 1.25μl water, 2.5μl 2X ABI genotyping master mix, 0.25μl 20X TaqMan genotyping assay and 1μl (15ng) genomic DNA. The PCR conditions were 95°C X 10m, followed by 50 cycles at 95°C X 15s and 60°C for 60s. The genotyping calls were made by ABI SDS-Automation Controller 2.3. Association tests were performed using online software (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
Real time quantitative RT-PCR (qRT-PCR) – text online
Immunostaining – text online
RESULTS
Based on quality control criteria, 56/559 cases, 158/1772 controls and 28,749/535,752 markers were removed in Group 1, and 12/353 cases, 45/1149 controls and 89,489/719,629 markers were removed in Group 2, leaving 844 cases and 2718 controls in the final analysis. Quantile-quantile (Q-Q) plots of the distribution of p values in Groups 1 and 2 did not show significant deviation from their expected distribution, with genomic inflation factor (λ) values of 1.014 and 1.017, respectively.
There were no genome-wide significant (p<5.0 × 10−8) signals in analyses of the 2 subsets in the discovery phase (Groups 1 and 2), but we identified TGFBR3 (chr1:92145900-92371559, reverse strand, GRCh37/hg19 assembly) as the only genomic region containing suggestive (p≤1×10−4) markers for both groups. In light of the previous data suggesting possible association of TGFBR3 with TDS and cryptorchidism,6 we focused on this region initially. Following imputation, the strongest signals were rs9661103 (p=2.71×10−5), located in intron 1, and rs10782968 (p=9.38×10−5), located ~101 kb downstream between TGFBR3 and CDC7, for Groups 1 and 2, respectively (Table 1, Figure 1). However, the reciprocal analysis showed no evidence for association of 3′ downstream or 5′ intragenic SNPs with Groups 1 and 2, respectively (Table 1), so these signals were weaker when we combined the Group 1 and 2 discovery data in a meta-analysis. In addition, we observed marginal (OR 1.13, 95% CI 0.99-1.28 p=0.09) association of rs12082710 with Group 1 but not Group 2 cases (Table 1). However, the risk allele frequency (RAF) in our control samples (0.62) was higher than previously reported (0.58-0.59) for this variant6 and therefore similar to the RAF in our case groups. In the replication phase, available genotypes of 305 cases and 324 unaffected controls collected from Caucasian subjects in Sweden failed to show association of any of these markers with cryptorchidism (Table 1).
Table 1.
Results of single marker association analysis in discovery and replication groups
| Marker | Position |
Risk
Allele |
Discovery Group 1 | Discovery Group 2 |
Discovery
Meta-analysis |
Replication | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| RAF |
OR (95%CI) p value |
RAF |
OR (95%CI) p value |
OR (95%CI) p value |
RAF |
OR (95%CI) p value |
||||||
| cases | controls | cases | controls | cases | controls | |||||||
| rs6687904 | 92025262 | G | 0.89 | 0.87 | 1.20 (0.96,1.50) 5.03E-02 |
0.90 | 0.85 | 1.50 (1.40,1.98) 1.91E-03 |
1.35 (1.14,1.60)
5.82E-04 |
|||
| rs925192 | 92038225 | T | 0.83 | 0.82 | 1.01 (0.84,1.22) 0.62 |
0.87 | 0.80 | 1.66 (1.29,2.13) 1.12E-04 |
1.23 (1.07,1.43) 5.29E-03 |
0.82 | 0.81 | 1.07 (0.80,1.43) 0.63 |
| rs10782968 | 92044755 | T | 0.79 | 0.78 | 1.02 (0.86,1.21) 0.62 |
0.84 | 0.76 |
1.58 (1.26,1.98)
9.38E-05 |
1.22 (1.06,1.39) 4.22E-03 |
|||
| rs12082710 | 92155337 | T | 0.65 | 0.62 | 1.13 (0.99,1.28) 0.09 |
0.62 | 0.62 | 1.01 (0.85,1.20) 0.74 |
1.07 (0.95,1.18) 0.27 |
0.62 | 0.61 | 1.04 (0.83,1.30) 0.83 |
| rs9661103 | 92335906 | C | 0.74 | 0.67 |
1.40 (1.20,1.64)
2.71E-05 |
0.70 | 0.69 | 1.01 (0.84,1.22) 0.88 |
1.23 (1.09,1.38) 7.94E-04 |
0.65 | 0.69 | 0.84 (0.66,1.06) 0.14 |
Markers shown include top signals (shown in bold) identified in Group 1 (rs9661103), Group 2 (rs10782968 and rs925192), in the meta-analysis (rs6687904) and in the study by Dalgaard et al (rs12082710). RAF, risk allele frequency; OR, odds ratio; CI, confidence interval, cont, controls.
Figure 1.
SNAP plots showing the association analysis results of imputed data from (A) Group 1 cases and controls, (B) Group 2 cases and controls and (C) Meta-analysis of all cases and controls.
We performed additional analyses to determine whether phenotypic heterogeneity between our 2 case groups could potentially account for these variable results. Testicular position, laterality and/or age at presentation data were available for ≥90% of affected individuals in each classification (Table 2). We noted increased frequencies of proximal, bilateral and early presentation cases in Group 1 as compared to Group 2, but these trends were not statistically significant. However, in meta-analyses of sub-phenotypes, we noted strongest evidence for association of testicular position (proximal vs. distal) with upstream/promoter and downstream regions of TGFBR3, respectively (Table 3, Figure 2). For the additional meta-analyses based on laterality and age of presentation, all associations were weaker (Table 3).
Table 2.
Frequency of specific phenotypes by group
| Phenotype | Group 1 | Group 2 | p value | |
|---|---|---|---|---|
| Position | Proximal | 167 (35%) | 107 (32%) | 0.47 |
| Distal | 313 (65%) | 224 (68%) | ||
| Laterality | Bilateral | 137 (28%) | 80 (24%) | 0.21 |
| Unilateral | 360 (72%) | 258 (76%) | ||
| Presentation | Early | 236 (48%) | 142 (42%) | 0.07 |
| Late | 255 (52%) | 198 (58%) |
Table 3.
Results of phenotype-specific meta-analysis of combined case-control groups
|
Phenotypes
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Position | Laterality | Presentation | ||||||
|
| ||||||||
| Marker | Position |
Risk
Allele |
Proximal | Distal | Bilateral | Unilateral | Early | Late |
|
| ||||||||
| OR (95%CI) p value |
OR (95%CI) p value |
OR (95%CI) p value |
OR (95%CI) p value |
OR (95%CI) p value |
OR (95%CI) p value |
|||
|
| ||||||||
| rs6687904 | 92025262 | G | 1.11 (0.84,1.37) 0.46 |
1.40 (1.20,1.61) 1.15E-03 |
1.26 (0.96,1.56) 0.13 |
1.35 (1.16,1.54) 2.16E-03 |
1.21 (0.98,1.45) 0.10 |
1.43 (1.21,1.65)
1.47E-03 |
| rs11165059 | 92067953 | A | 1.03 (0.82,1.25) 0.78 |
1.32 (1.15,1.48)
9.42E-04 |
1.23(0.99,1.7) 0.09 |
1.19 (1.04,1.35) 2.30E-02 |
1.19 (1.01,1.38) 0.06 |
1.21 (1.04,1.38) 3.25E-02 |
| rs17131560 | 92254485 | G | 1.59 (1.31,1.88) 9.00E-03 |
1.11 (0.86,1.35) 0.43 |
1.53 (1.16,1.90) 2.40E-02 |
1.14 (0.90,1.37) 0.29 |
1.51 (1.39,1.88)
1.37E-03 |
1.01 (0.74,1.28) 0.93 |
| rs9661103 | 92335906 | C | 1.34 (1.15,1.53) 2.80E-03 |
1.14 (1.00,1.28) 0.07 |
1.16 (0.95,1.37) 0.17 |
1.25 (1.11,1.39)
1.29E-03 |
1.16 (1.00,1.33) 0.07 |
1.26 (1.11,1.42) 2.93E-03 |
| rs17576372 | 92366174 | T |
1.42 (1.24,1.60)
1.67E-04 |
1.03 (0.90,1.17) 0.64 |
1.17 (0.97,1.37) 0.13 |
1.15 (1.02,1.28) 3.09E-02 |
1.13 (0.97,1.28) 0.13 |
1.17 (1.03,1.32) 3.03E-02 |
| rs6656817 | 92411560 | C | 1.24 (0.88,1.61) 0.24 |
1.20 (0.93,1.47) 0.18 |
2.14 (1.74,2.54)
2.00E-04 |
1.01 (0.76,1.27) 0.92 |
1.31 (1.00,1.62) 0.08 |
1.11 (0.82,1.40) 0.48 |
Markers shown include top signals (shown in bold) identified in phenotype subgroups including proximal (testes positioned proximal to external inguinal ring), distal (testes positioned distal to external inguinal ring), bilateral vs. unilateral, and early (age ≤2 years) vs. late (age >2 years) at presentation. OR, odds ratio; CI, confidence interval.
Figure 2.
SNAP plots of meta-analysis results of imputed data at the TGFBR3 locus. (A) Cryptorchidism cases with testes located proximal to the external inguinal ring and all controls and (B) Cryptorchidism cases with testes located distal to the external inguinal ring.
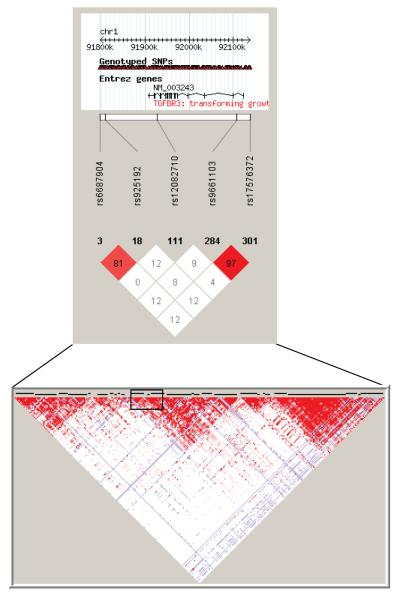
Using HaploView, we reviewed the LD patterns for the top signals in the 2 genotyping groups and the position subgroups. The strongest signals in the Group 1 (rs9661103) and Group 2 (rs10782968) analyses are not in LD with each other, but were correspondingly strongly linked (r2≥0.8) to the most significant SNPs that we identified in the proximal (rs17576372) and distal (rs6687904) meta-analyses (Figures 1-3), consistent with a higher frequency of proximal cases in Group 1. These results suggest that phenotypic bias may be one factor that accounts for the non-overlapping signals we identified in separate analyses of our discovery groups.
Figure 3.
Linkage disequilibrium (LD) plot of markers identified in the primary group and subgroup analyses that are available in HaploView (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview). Lower portion shows an overview of LD blocks within the imputed TGFBR3 locus, with the black rectangle centered on the 3′ end of the TGFBR3 gene.
In studies of rat gubernaculum, Tgfbr3 mRNA levels did not change significantly in wt samples between E17 and E21 (p=0.57), during the maximal developmental period (Figure 4). In contrast, Tgfbr3 expression increased after E17 (p=0.055) and was significantly higher in cryptorchid orl at E19 (p=0.011) and E21 (p=0.001) as compared to wt gubernaculum. In wt gubernacular explants, INSL3 exposure did not alter measured Tgfbr3 expression, but transcript levels increased significantly following DHT exposure (p<0.01 for 10nM DHT; Figure 4).
Figure 4.

Tgfbr3 mRNA expression in gubernaculum from wild type (wt; blue bars) and cryptorchid orl (green bars) rat fetuses. Baseline expression is shown for freshly isolated embryonic (E) day 17, 19 and 21 gubernacula and for E17 wt gubernacular explants established in culture followed by exposure to insulin-like 3 (INSL3; 0, 10 or 100 nM) or dihydrotestosterone (DHT; 0, 10 or 30 nM) for 24 hours. *p<0.05 and **p<0.01 for wt vs orl.
Immunostaining of the rat fetal gubernaculum showed diffuse TGFBR3 protein expression within the inner mesenchymal core but more intense staining of the developing peripheral muscle and outer mesothelial layer, as noted in E17 and E19 wt gubernacula (Figure 5B). The muscle layer was adjacent to the mesothelial layer at E17 and became progressively less peripheral and thicker during development. Prominent clusters of betaglycan (TGFBR3-A-positive) immunoreactivity consistent with the proteolytically cleaved extracellular domain (soluble betaglycan) showed variable expression at all time points along with co-expression of membrane-associated betaglycan and myosin in differentiated muscle. These extracellular collections were not identified using TGFBR3-B, an antibody targeting cytoplasmic betaglycan, and expression was less prominent by E21 in both wt and orl samples. These collections were particularly prominent in some orl samples, particularly within the mesenchymal core and in the peripheral mesothelial layer (Figure 5A).
Figure 5.
A TGFBR3-A (green; 1:50), myosin (red) and nuclei (DAPI; blue) in embryonic (E) day 21 gubernaculum shows an example of prominent soluble betaglycan collections that we observe in some fetuses (10X, scale bar, 200μm). B TGFBR3/betaglycan expression in E17, E19 and E21 gubernacula from wild type (wt) and cryptorchid orl fetuses (green). Staining for myosin (differentiated muscle; red) and TGFBR3-A (extracellular domain, top 2 rows) or TGFBR3-B (cystoplasmic domain, bottom 2 rows), both green (20X, scale bar, 100 μm).
DISCUSSION
Cryptorchidism is the most common reproductive anomaly identified in newborn boys but genetic susceptibility loci remain largely undefined. In the sole published GWAS that includes cryptorchidism, TGFBR3 was identified as a locus associated with TDS, and less strongly with isolated nonsyndromic cryptorchidism, although the data did not approach genome-wide significance.6 In the present analysis, we took advantage of larger sample size to provide increased power to detect an association of TGFBR3 with cryptorchidism. Although we showed nominal association of our Group 1 cohort with the previously reported signal, this association was lost with increasing sample size and did not replicate in a Swedish population. Surprisingly, we observed suggestive (p<10−4) association of each of our case-control groups with independent signals at or near the TGFBR3 locus. Our sub-phenotype analyses suggest stronger association of proximal and distal phenotypes with linked SNPs in the 5′ and downstream regions of the gene, respectively. HapMap data show minimal LD between these 3 genomic regions. It is possible that the strength of the observed association for any one signal may be limited by allelic heterogeneity and/or the unmeasured effects of variable environmental exposures.
Cryptorchidism is a complex disease, likely influenced by multilocus genetic, maternal and/or environmental factors. The genomic loci and pathways involved remain largely unknown, although many studies address this question in animal models. Gene targeting experiments in mice confirm requirement for Rxfp2 and Ar and confirm a role for Wnt and Notch signaling in gubernacular development, and confirm myogenesis as a key target of hormone action.21,22
TGFBR3/betaglycan is a biologically compelling risk locus for cryptorchidism because of its association with both fetal Leydig cell development and myogenesis.23,24 Betaglycan is a TGFβ coreceptor that facilitates or inhibits signaling depending on the context.25 The cytoplasmic domain facilitates signaling by TGFβ2 or TGFβ1, while the soluble, cleaved ectodomain can modulate TGFβ signaling by sequestering these growth factors. Betaglycan is expressed in interstitial and peritubular myoid cells in the fetal testes of mouse and human.26,27 Tgfbr3 −/− mice show disruption of tubular architecture and a transient delay in Leydig cell development with reduced expression of Insl3 and steroidogenesis genes.24 Notably, inactivation of Tgfβ2 in transgenic mice is associated with cryptorchidism without other external genital defects,28 suggesting a primary gubernacular defect rather than global hormone deficiency. This and previous studies showing expression of Tgfbr3 and Tgfb2, with increased expression in orl20 and in wt males following androgen exposure, also support a role for TGFβ signaling in gubernaculum development, as we.18 The present data suggest that membrane-associated betaglycan is expressed throughout the fetal rat gubernaculum but most strongly in the outer mesothelial layer and in association with areas of developing muscle, and that focal increased accumulation of soluble betaglycan occurs in mesothelium and mesenchyme of orl gubernacula. Although we have not fully elucidated the genomic loci that confer susceptibility to cryptorchidism, our prior studies suggest both androgen signaling and muscle patterning are altered in the orl strain.20,29
Inconsistency in the pattern of association of TGFBR3 with cryptorchidism may reflect various factors, notably insufficient sample size, allelic heterogeneity, population differences and/or phenotypic misclassification or variability. Regarding the latter, Dalgaard et al6 included subjects identified at birth or in medical records, but it is unclear whether all had persistent cryptorchidism requiring surgery. In their discussion of the concept of “synthetic” association of GWAS signals with rare, causal variants in the same genomic region, Dickson et al also hypothesized that even modest associations defined by GWAS may reveal loci that contain rare variants of larger effect.30 The present data warrant more intensive investigation to define potential causal variants and the role of betaglycan in reproductive development in experimental animal models. Hypotheses that should be addressed include the possibility that genomic loci participate in hormone signaling pathways that are also specific targets of environmental exposures and that pleiotropic effects of genes may explain the co-occurrence of testicular germ cell defects (cancer and subfertility) and cryptorchidism.
Supplementary Material
Acknowledgements
The authors would like to thank all our participants and their families for their gracious participation in this study.
References
- 1.Schnack TH, Zdravkovic S, Myrup C, et al. Familial Aggregation of Cryptorchidism--A Nationwide Cohort Study. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn081. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MS, Toft G, Thulstrup AM, et al. Cryptorchidism concordance in monozygotic and dizygotic twin brothers, full brothers, and half-brothers. Fertil Steril. 2010;93:124. doi: 10.1016/j.fertnstert.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Hemminki K, Li X, Sundquist K, et al. Familial risks for common diseases: etiologic clues and guidance to gene identification. Mutat Res. 2008;658:247. doi: 10.1016/j.mrrev.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen HE, Toppari J. Embryology and physiology of testicular development and descent. Pediatr Endocrinol Rev. 2014;11(Suppl 2):206. [PubMed] [Google Scholar]
- 5.Ferlin A, Zuccarello D, Zuccarello B, et al. Genetic alterations associated with cryptorchidism. JAMA. 2008;300:2271. doi: 10.1001/jama.2008.668. [DOI] [PubMed] [Google Scholar]
- 6.Dalgaard MD, Weinhold N, Edsgard D, et al. A genome-wide association study of men with symptoms of testicular dysgenesis syndrome and its network biology interpretation. J Med Genet. 2012;49:58. doi: 10.1136/jmedgenet-2011-100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold JS, Hossain J, Olivant-Fisher A, et al. Altered infant feeding patterns in boys with acquired nonsyndromic cryptorchidism. Birth Defects Res A Clin Mol Teratol. 2012;94:900. doi: 10.1002/bdra.23075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson CA, Pettersson FH, Clarke GM, et al. Data quality control in genetic case-control association studies. Nat Protoc. 2010;5:1564. doi: 10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg NA. Standardized subsets of the HGDP-CEPH Human Genome Diversity Cell Line Panel, accounting for atypical and duplicated samples and pairs of close relatives. Ann Hum Genet. 2006;70:841. doi: 10.1111/j.1469-1809.2006.00285.x. [DOI] [PubMed] [Google Scholar]
- 11.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 12.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3 (Bethesda) 2011;1:457. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AD, Handsaker RE, Pulit SL, et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollin C, Hesser U, Ritzen EM, et al. Testicular growth from birth to two years of age, and the effect of orchidopexy at age nine months: a randomized, controlled study. Acta Paediatr. 2006;95:318. doi: 10.1080/08035250500423812. [DOI] [PubMed] [Google Scholar]
- 16.Kollin C, Karpe B, Hesser U, et al. Surgical treatment of unilaterally undescended testes: testicular growth after randomization to orchiopexy at age 9 months or 3 years. J Urol. 2007;178:1589. doi: 10.1016/j.juro.2007.03.173. [DOI] [PubMed] [Google Scholar]
- 17.Kollin C, Stukenborg JB, Nurmio M, et al. Boys with undescended testes: endocrine, volumetric and morphometric studies on testicular function before and after orchidopexy at nine months or three years of age. J Clin Endocrinol Metab. 2012;97:4588. doi: 10.1210/jc.2012-2325. [DOI] [PubMed] [Google Scholar]
- 18.Barthold JS, Wang Y, Robbins A, et al. Transcriptome analysis of the dihydrotestosterone-exposed fetal rat gubernaculum identifies common androgen and insulin-like 3 targets. Biol Reprod. 2013;89:143. doi: 10.1095/biolreprod.113.112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson KJ, Robbins AK, Wang Y, et al. Insulin-like 3 exposure of the fetal rat gubernaculum modulates expression of genes involved in neural pathways. Biol Reprod. 2010;83:774. doi: 10.1095/biolreprod.110.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barthold JS, Robbins A, Wang Y, et al. Cryptorchidism in the Orl Rat Is Associated with Muscle Patterning Defects in the Fetal Gubernaculum and Altered Hormonal Signaling. Biol Reprod. 2014 doi: 10.1095/biolreprod.114.119560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaftanovskaya EM, Feng S, Huang Z, et al. Suppression of insulin-like3 receptor reveals the role of beta-catenin and Notch signaling in gubernaculum development. Mol Endocrinol. 2011;25:170. doi: 10.1210/me.2010-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaftanovskaya EM, Huang Z, Barbara AM, et al. Cryptorchidism in mice with an androgen receptor ablation in gubernaculum testis. Mol Endocrinol. 2012;26:598. doi: 10.1210/me.2011-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Casillas F, Riquelme C, Perez-Kato Y, et al. Betaglycan expression is transcriptionally up-regulated during skeletal muscle differentiation. Cloning of murine betaglycan gene promoter and its modulation by MyoD, retinoic acid, and transforming growth factor-beta. J Biol Chem. 2003;278:382. doi: 10.1074/jbc.M208520200. [DOI] [PubMed] [Google Scholar]
- 24.Sarraj MA, Escalona RM, Umbers A, et al. Fetal testis dysgenesis and compromised Leydig cell function in Tgfbr3 (beta glycan) knockout mice. Biol Reprod. 2010;82:153. doi: 10.1095/biolreprod.109.078766. [DOI] [PubMed] [Google Scholar]
- 25.Bilandzic M, Stenvers KL. Betaglycan: a multifunctional accessory. Mol Cell Endocrinol. 2011;339:180. doi: 10.1016/j.mce.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RA, Cambray N, Hartley PS, et al. Expression and localization of inhibin alpha, inhibin/activin betaA and betaB and the activin type II and inhibin beta-glycan receptors in the developing human testis. Reproduction. 2002;123:779. doi: 10.1530/rep.0.1230779. [DOI] [PubMed] [Google Scholar]
- 27.Sarraj MA, Chua HK, Umbers A, et al. Differential expression of TGFBR3 (betaglycan) in mouse ovary and testis during gonadogenesis. Growth Factors. 2007;25:334. doi: 10.1080/08977190701833619. [DOI] [PubMed] [Google Scholar]
- 28.Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson KJ, McCahan SM, Si X, et al. The orl rat with inherited cryptorchidism has increased susceptibility to the testicular effects of in utero dibutyl phthalate exposure. Toxicol Sci. 2008;105:360. doi: 10.1093/toxsci/kfn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickson SP, Wang K, Krantz I, et al. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;8:e1000294. doi: 10.1371/journal.pbio.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.