Abstract
Directed cell migration is a physical process that requires dramatic changes in cell shape and adhesion to the extracellular matrix. For efficient movement, these processes must be spatiotemporally coordinated. To a large degree, the morphological changes and physical forces that occur during migration are generated by a dynamic filamentous actin (F-actin) cytoskeleton. Adhesion is regulated by dynamic assemblies of structural and signaling proteins that couple the F-actin cytoskeleton to the extracellular matrix. Here, we review current knowledge of the dynamic organization of the F-actin cytoskeleton in cell migration and the regulation of focal adhesion assembly and disassembly with an emphasis on how mechanical and biochemical signaling between these two systems regulate the coordination of physical processes in cell migration.
Keywords: F-actin cytoskeleton, adhesion maturation, traction force, adhesion strength, focal adhesion
INTRODUCTION
Cell migration plays a crucial role in many aspects of healthy physiology, and its misregulation can lead to a variety of pathologies. During embryonic development, the migration of cells drives changes in tissue shape during gastrulation, and neuronal development requires the migration of neural crest cells (Keller 2005, Klambt 2009, Locascio & Nieto 2001). Both immune response and wound healing require directed cell migration in response to chemical and mechanical gradients (David et al. 2007). Such directed cell migration is misregulated in many diseases, including inflammatory and vascular diseases, and contributes to cancer metastasis (Yamaguchi et al. 2005).
Cell migration requires highly coordinated changes in cell morphology and interactions with the extracellular matrix (ECM). Although complex, this process can be broken down into four discrete steps: protrusion of the leading cell edge, adhesion to the ECM, generation of traction stresses against adhesions, and finally, release of rear adhesions and cell body contraction (Lauffenburger & Horwitz 1996). For efficient cell movement, these processes must be tightly coordinated. In highly motile cells, the rate of movement of the cell body is nearly the same as the rate of protrusion, whereas in slowly moving cells, the release of rear adhesions dominates the rate of cell body advance. The migration rate of many cell types is optimized at intermediate levels of adhesion, and modulating the density or mechanical compliance of the ECM alters migration speed in a biphasic fashion (Gaudet et al. 2003, Lo et al. 2000, Palecek et al. 1997, Pelham & Wang 1997, Peyton & Putnam 2005). Perturbations to the level of intracellular contractility alter the amount of adhesion required for optimal migration (Gupton & Waterman-Storer 2006) and indicate a feedback between cellular adhesion and traction.
The morphological and physical behaviors of migrating cells are largely driven by a dynamic filamentous actin (F-actin) cytoskeleton, which is coupled to the ECM via dynamic assemblies of structural and signaling proteins known as focal adhesions (FAs). The spatiotemporal regulation of these physical structures is driven by a variety of signaling molecules and their downstream effectors; the most prominent of these are the Rho family GTPases (see Raftopoulou & Hall 2004 for a review). The GTPase activity of Rac and Cdc42 largely regulates the assembly of protrusive actin-based organelles and promotes the formation of small adhesions near the cell periphery (Nobes & Hall 1995, Nobes et al. 1995). Likewise, the activity of Rho promotes the assembly of contractile actomyosin structures (Nobes & Hall 1995, Ridley & Hall 1992) that play a central role in cellular traction and tension-mediated changes in FA composition and morphology.
In addition to the Rho GTPases, it is now clear that numerous mechanical and biochemical cues mediate the interplay between the F-actin cytoskeleton and integrin-mediated adhesion to regulate the coordination of cell protrusion, adhesion, and contraction. Here, we review current understanding of how the dynamic actomyosin cytoskeleton is coordinated with FA dynamics to support directed cell migration.
ACTIN DYNAMICS IN MIGRATING CELLS
The F-actin cytoskeleton in migrating mesenchymal cells forms two distinct functional modules that mediate the protrusive and contractile steps in the migration cycle (Figure 1) (Gupton et al. 2005, Ponti et al. 2004, Salmon et al. 2002). The protrusive module may consist of either a lamellipodial F-actin network or filopodial F-actin bundles at the leading cell edge (Faix & Rottner 2006, Gupton & Gertler 2007, Pollard & Borisy 2003). The contractile module, also known as the lamella, consists of a variety of F-actin–myosin II structures behind the leading edge in the cell body (Abercrombie et al. 1971, Cramer et al. 1997, Heath & Holifield 1993, Ponti et al. 2004). Within these two modules, actin filaments exhibit specific patterns of assembly-disassembly and translocation dynamics that are dictated by the differential distribution and activities of actin regulatory proteins (DesMarais et al. 2002, Ponti et al. 2004). These patterns are crucial to the ability of these modules to generate the directional forces that drive whole-cell movement.
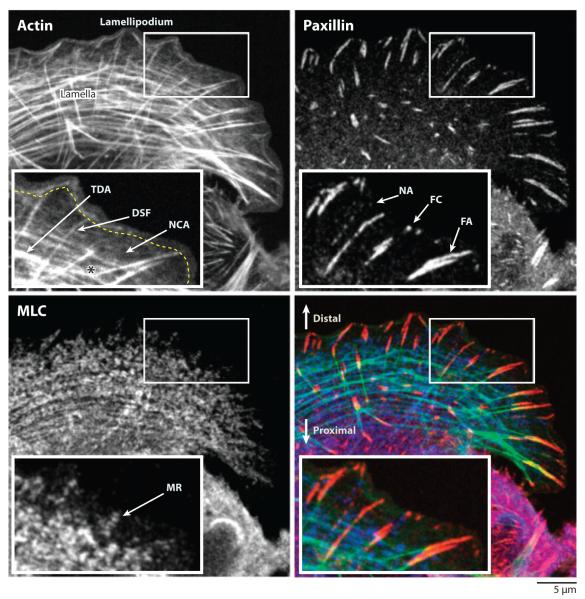
Figure 1.
Organization of the actomyosin cytoskeleton and adhesions in the leading edge of a migrating human osteosarcoma (U2OS) cell shown via double indirect immunolabeling of paxillin (red) and myosin light chain (MLC, blue) as well as fluorescent phalloidin staining of actin filaments (green). In each image the boxed area is shown magnified in the inset. (Upper left) The yellow dashed line highlights the lamellipodium-lamella border, and the asterisk highlights a junction between a dorsal stress fiber (DSF) and a transverse dorsal arc (TDA). NCA, network-contraction array; NA, nascent adhesion; FC, focal complex; FA, focal adhesion; MR, myosin II ribbon. In the merged image (lower right), the distal and proximal directions are highlighted.
Cell protrusion is driven by a thin, broad and flat lamellipodium and/or by thin cylindrical filopodia. The lamellipodium, which extends ~2–4 μm away from the cell leading edge, forms by the rapid polymerization of a cross-linked, dendritic F-actin array that is mediated primarily by the Arp2/3 complex and cofilin (Anaout 2002, Bailly et al. 1999, Ichetovkin et al. 2002, Pollard & Borisy 2003, Small 1988). This produces a gel-like F-actin network along the leading edge that is mostly free of microtubules, intermediate filaments, and membranous organelles (Abercrombie et al. 1971). The addition of actin monomers onto the barbed ends of lamellipodial actin filaments, which face the leading edge (Okabe & Hirokawa 1991, Symons & Mitchison 1991, Wang 1985), generates a pushing force, likely by a tethered Brownian ratchet mechanism (Alberts & Odell 2004, Mogilner & Oster 1996), that drives the leading edge membrane forward but also pushes the network backward, away from the cell edge, in a process termed retrograde F-actin flow (Forscher & Smith 1988, Heath 1983, Holifield et al. 1990, Lin & Forscher 1995, Pollard & Borisy 2003, Ponti et al. 2004). During retrograde flow, capping protein caps the barbed ends of F-actin, which are then severed and depolymerized primarily by the action of cofilin family proteins (Iwasa & Mullins 2007, Miyoshi et al. 2006) such that at the base of the lamellipodium, >90% of the filaments assembled at the leading edge are depolymerized (Ponti et al. 2004, Watanabe & Mitchison 2002). This polarized assembly at the leading cell edge coupled with proximal disassembly results in a treadmilling lamellipodial F-actin array.
Likewise, filopodia are treadmilling F-actin bundles formed by the directed assembly of fascin-mediated F-actin bundles at the leading edge (Abercrombie et al. 1971, Mallavarapu & Mitchison 1999). The molecular mechanisms mediating filopodial actin filament assembly likely involve mDia/formin and/or vasodilator-stimulated phosphoprotein (VASP) family proteins but are currently quite controversial (Faix & Rottner 2006, Gupton & Gertler 2007, Svitkina et al. 2003). The disassembly of filopodial bundles is not heavily studied, but may involve myosin II–mediated bundle breakage (Medeiros et al. 2006) or may be incomplete, such that filopodial filaments contribute to the formation of contractile structures in the lamella (Anderson et al. 2008, Hotulainen & Lappalainen 2006).
Independent of the mechanisms of F-actin assembly and disassembly, protrusive lamellipodial and filopodial structures exhibit similar F-actin dynamics featuring barbed-end filament polymerization at the plasma membrane, polymerization-driven retrograde filament flow, and proximal filament depolymerization to produce treadmilling F-actin structures at the cell edge. Importantly, in both cases leading edge protrusion results when filament assembly outpaces retrograde flow (Giannone et al. 2007, Lin & Forscher 1995, Machacek & Danuser 2006, Mallavarapu & Mitchison 1999, Ponti et al. 2004).
A few micrometers behind the leading edge, F-actin in the contractile lamella contains myosin II and tropomyosin as well as other cytoskeletal elements and membranous organelles (Abercrombie et al. 1971, DesMarais et al. 2002, Gupton et al. 2007, Ponti et al. 2004, Verkhovsky et al. 1995). Depending on cell type, two different types of contractile structures can dominate the contractile lamella: contractile bundles or network-contraction arrays. The three types of contractile bundles are ventral and dorsal stress fibers, which are prominent in most migrating mesenchymal cells; transverse arcs (also called dorsal arcs), which are prominent in fibroblasts and osteosarcoma cells (Heath 1983, Hotulainen & Lappalainen 2006); and graded polarity bundles, which are prominent in primary chick heart fibroblasts (Cramer et al. 1997). Network-contraction arrays are prominent in fish keratocytes, fibroblasts, and epithelial cells (Gupton & Waterman-Storer 2006, Svitkina et al. 1997, Verkhovsky et al. 1995). Stress fibers and transverse arcs display a periodic α-actinin/myosin II pattern interspersed by short (~1–5 μm) F-actin filaments of alternating polarity, reminiscent of sarcomeric structures in muscle cells (Hotulainen & Lappalainen 2006, Sanger et al. 1983). These contractile bundles are differentiated by their orientation to the leading cell edge, with stress fibers aligned in the direction of migration and transverse arcs oriented perpendicularly (Heath 1983). Graded polarity bundles are also aligned with the cell migration axis, but they display nonperiodic α-actinin and myosin II and are composed of longer (10–15 μm), overlapping filaments whose polarity gradually changes along the length of the bundle (Cramer et al. 1997). Finally, network-contraction arrays consist of clusters or “ribbons” of bipolar myosin II minifilaments embedded in a cross-linked F-actin network that exhibit a density gradient from distal to proximal lamella (Gupton & Waterman-Storer 2006; Svitkina et al. 1997; Verkhovsky et al. 1995, 1999). Network-contraction arrays are located within the leading third of the cell and may terminate in a transverse arc (Svitkina et al. 1997, Verkhovsky et al. 1995).
The actin dynamics within the lamella contractile structures are reflective of their roles in cell motility. Transverse arcs, dorsal stress fibers, graded polarity bundles, and network-contraction arrays are all highly dynamic structures associated with motile cells. F-actin within these structures undergoes myosin II–dependent retrograde flow that is approximately tenfold slower than the polymerization-dependent flow in the lamellipodium (Hotulainen & Lappalainen 2006, Ponti et al. 2004, Schaub et al. 2007). In dorsal stress fibers, continuous formin-mediated assembly of bundled filaments at FAs near the cell edge is coupled to the contractile rearward flow of the bundle (Hotulainen & Lappalainen 2006). In network-contraction arrays, filament assembly occurs throughout the array, but disassembly exhibits a gradient such that maximal disassembly is concentrated in the proximal lamella and dorsal arcs (Gupton & Waterman-Storer 2006, Gupton et al. 2007, Vallotton et al. 2004). Thus, the network-contraction array, dorsal stress fibers, and transverse arcs work together to form an integrated treadmilling contractile lamella. This module is characterized by F-actin assembly at the distal lamella edge in FAs, myosin II–driven filament retrograde flow, and filament disassembly within the contractile transverse arcs in the proximal lamella.
By contrast, graded polarity bundles span from the cell front to the rear and have distinct F-actin dynamics. Graded polarity bundles contain two dynamic populations of filaments: one that stays stationary with respect to the substrate, and one that moves forward via myosin II activity (Cramer et al. 1997). This suggests that myosin II–mediated contraction pulls antiparallel actin filament sets past each other, one set anchored to the ECM and the other possibly pulling the cell rear forward. Finally, ventral stress fibers do not exhibit major remodeling on a timescale relevant to cell migration but do exhibit smaller-scale dynamics that are suggestive of their sarcomeric organization: Distances between α-actinin bands decrease under conditions that allow or promote contraction and increase under stretching or relaxation conditions (Colombelli et al. 2009, Kumar et al. 2006, Peterson et al. 2004). As such, ventral stress fibers tend to be associated with less motile cell types (Couchman & Rees 1979), where they transmit cellular prestress to the ECM to maintain tensional homeostasis with the cellular environment (Kumar et al. 2006).
ADHESION DYNAMICS IN MIGRATING CELLS
Cell migration requires a regulated, dynamic adhesion assembly-disassembly cycle. For productive advance of the cell leading edge, lamellipodial protrusion must be tightly spatiotemporally coupled to adhesion. Conversely, for productive advance of the cell body, contraction must be coupled to deadhesion. Adhesion of a protruding cell edge is initiated by nascent adhesions, smaller than the ~0.25 μm resolution of the light microscope, that assemble concurrent with lamellipodial protrusion (Choi et al. 2008). As the leading edge advances, a subpopulation of nascent adhesions disassembles within minutes (Choi et al. 2008), and the remainder grow and mature into focal complexes (~0.5 μm) and then FAs (1–5 μm) (Figure 2). FAs then either disassemble within the cell body within 10–20 minutes (Gupton & Waterman-Storer 2006, Laukaitis et al. 2001, Webb et al. 2004, Zaidel-Bar et al. 2003) or mature further into stable fibrillar adhesions that do not promote migration but are involved in ECM remodeling (Laukaitis et al. 2001, Webb et al. 2004, Zaidel-Bar et al. 2003). Thus, the maturation and turnover of adhesions have been described as a morphological cycle that involves protein recruitment and elongation followed by protein dissociation and shrinkage.
Figure 2.
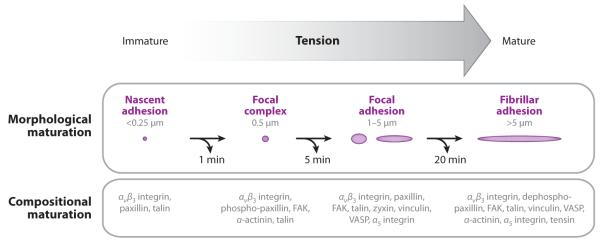
Schematic diagram comparing the morphological phases of adhesion maturation (above, adhesion represented by purple ovals; length scales indicated are focal adhesion lengths) with the compositional phases of adhesion maturation (below, protein components listed) in response to increasing mechanical tension (gray arrow). At each of these steps, adhesion turnover can occur (curved black arrows) after a certain amount of time (timescale below each arrow). FAK, focal adhesion kinase; VASP, vasodilator-stimulated phosphoprotein.
In conjunction with the morphological change during adhesion maturation, a less well-defined process occurs that is thought to involve a sequential cascade of compositional changes (Figure 2; Zaidel-Bar et al. 2004, Zamir & Geiger 2001). The formation of integrin microclusters of <10 molecules that diffuse in the plasma membrane are believed to initiate FAs (Wiseman et al. 2004). Enhanced affinity of integrin extracellular heads for the ECM, which is known as activation, occurs through association of their cytoplasmic tails with the vinculin- and actin-binding protein talin (Tadokoro et al. 2003). Activated integrins may then either bind to the ECM, which immobilizes them, or they may associate with the dynamic actin at the leading edge and, in doing so, move in a directed fashion within the plasma membrane. This is thought to contribute to a searching behavior for conducive binding sites in the ECM (Galbraith et al. 2007) and to result in direct coupling of actin-based protrusion and adhesion (Schneider et al. 2009). Soon after integrin-ECM engagement, an unknown mechanism promotes recruitment of the adapter protein paxillin to integrin clusters to form nascent adhesions, which recruit more integrins and promote further integrin clustering (Laukaitis et al. 2001, Webb et al. 2004, Wiseman et al. 2004). Nascent adhesion growth is accompanied by recruitment of the actin-bundling protein, α-actinin (Choi et al. 2008), which with talin may establish a link between ECM-bound integrins and the actin cytoskeleton (Lee et al. 2004). These nascent adhesions undergo rapid disassembly within the lamellipodium unless they are stimulated to proceed through maturation (Choi et al. 2008).
Mechanical tension stimulates the transition of nascent adhesions to focal complexes and FAs (Balaban et al. 2001, Chrzanowska-Wodnicka & Burridge 1996). Either Rho-mediated myosin II activity or external forces applied to the cell can supply the tension driving FA maturation (Choi et al. 2008, Chrzanowska-Wodnicka & Burridge 1996, Galbraith et al. 2002, Riveline et al. 2001, Vicente-Manzanares et al. 2007). α-actinin-mediated F-actin bundling in nascent adhesions, together with myosin II-mediated retrograde actin flow, transmit pulling forces from the lamella actin cytoskeleton to the integrin-ECM linkage. This tension is thought to promote the formation of small adhesion-associated actomyosin bundles where the cytoskeletal adapter proteins vinculin and zyxin bind (Choi et al. 2008). Adhesion-associated actin bundles elongate in a force- and mDia/formin-dependent manner, and this is critical to the formation of the lamella actomyosin network (Gupton et al. 2007, Hotulainen & Lappalainen 2006, Riveline et al. 2001). Thus, tension-mediated adhesion maturation plays a central role in supplying new actin filament growth for formation of the contractile lamella, thus distinguishing it from the lamellipodial protrusion machine (Alexandrova et al. 2008, Shemesh et al. 2005).
In addition, tension on ECM-engaged integrins promotes β1 integrin head binding to synergy sites on fibronectin (Friedland et al. 2009). This secondary integrin-ECM binding induces the presumed recruitment and documented phosphorylation and activation of focal adhesion kinase (FAK), a nonreceptor protein tyrosine kinase (Friedland et al. 2009, Shi & Boettiger 2003). In addition to initiating integrin-mediated signaling, tyrosine phosphorylation of focal complex proteins, including paxillin and p130cas (Ballestrem et al. 2006), sets up scaffolds for phosphotyrosine-binding SH-2 domain–containing proteins whose binding further contributes to adhesion growth. In addition, some studies show compositional differences between small and large FAs (Laukaitis et al. 2001; Zaidel-Bar et al. 2003, 2007a; Zimerman et al. 2004), although the order of protein addition and the requirement for myosin II activity and/or tension in FA recruitment is not known. A subset of mature adhesions in the central part of the cell are then further matured into long, thin fibrillar adhesions owing to the dephosphorylation of paxillin on tyrosines 31 and 118, which promotes adhesion stability and recruitment of tensin (Volberg et al. 2001, Zaidel-Bar et al. 2007b, Zamir et al. 1999).
How does tension on nascent adhesions promote their growth? At the level of protein structure, directional forces on FA proteins are thought to drive localized unfolding or conformational changes that unmask protein binding sites (Vogel & Sheetz 2006). The binding of new proteins to unmasked sites results in FA growth and reinforces the linkage between the ECM and cytoskeleton. Studies examining global differences in the cysteine reactivity of proteins in contracted and relaxed cells indicate that many proteins alter their conformation in response to mechanical stimulation ( Johnson et al. 2007). How this affects the structure and function of a single protein has just begun to be explored. For example, molecular dynamics simulations support the notion that directional force on integrin cytoplasmic tails induces separation of the α and β subunits (Zhu et al. 2008), which could promote activation and allow binding of cytoplasmic proteins. In addition, in vitro experimental evidence suggests that forced unfolding of talin promotes vinculin binding (del Rio et al. 2009), whereas stretching p130cas unmasks a tyrosine substrate for Src family kinases (Sawada et al. 2006). However, whether these specific mechanisms operate in cells is not known.
FA disassembly or “turnover” is of central importance to cell migration and thus has been a subject of intense study. Turnover is likely the default pathway for nascent adhesions if they are not stimulated to mature by sufficient tension. Once tension-stimulated, however, signal transduction largely governs the decision of focal complexes/adhesions to mature further or turn over. These pathways are thought somehow to reduce adhesion strength below the level of contractile force applied to the FA, causing the adhesion to disengage from the ECM, slide, and disassemble (Goldyn et al. 2009, Wehrle-Haller & Imhof 2003).
FAK (and its homolog Pyk2) is a master regulator of adhesion turnover. Cells lacking FAK (and Pyk2) possess large, stable peripheral adhesions and exhibit tail retraction defects during migration. How FAK promotes adhesion disassembly is debatable. One possibility is that it reverses pathways of FA growth, i.e., by local downregulation of myosin II tension. For example, FAK-mediated phosphorylation and activation of p190RhoGAP may decrease Rho and Rho kinase (ROCK)-mediated myosin II activity (Arthur & Burridge 2001, Holinstat et al. 2006, Schober et al. 2007). Alternatively, FAK may promote adhesion turnover by upregulation of Rac1 activity at adhesions, which by its Rho antagonism (Arthur & Burridge 2001, Sander et al. 1999) may block tension-mediated FA maturation. The FAK-mediated phosphorylation of paxillin kinase linker (PKL)/GIT1 promotes formation of a Rac-activating complex of PKL/Git1, the Rac1 guanine-nucleotide exchange factor (GEF) βPix, and the Rac effector Pak to form a PKL/Pix/Pak complex that is targeted to forming adhesions through their interaction with paxillin (Brown et al. 2005, Nayal et al. 2006, Schober et al. 2007). In addition, tyrosine-phosphorylated p130cas and paxillin bind the adapter Crk, which in turn recruits the Rac GEF DOCK180 to promote Rac activation (Brugnera et al. 2002, Cote & Vuori 2002). Finally, some evidence suggests that FAK regulates the dynamin-mediated endocytosis of integrins (Ezratty et al. 2005).
Whereas tyrosine kinase activity promotes adhesion disassembly and turnover, tyrosine phosphatases such as Shp-2 and RPTPα are thought to be critical for inhibiting turnover and driving adhesion maturation, although they are less well studied than the kinases (Bur-ridge et al. 2006; von Wichert et al. 2003a,b; Zaidel-Bar et al. 2007b). Alternative mechanisms for adhesion disassembly include calpain-mediated proteolysis of talin (Franco et al. 2004) and microtubule-mediated adhesion relaxation (Kaverina et al. 1999, Krylyshkina et al. 2002).
INTEGRATION OF ACTIN DYNAMICS AND ADHESION: FOCAL ADHESIONS AS A MECHANICAL CLUTCH
What is the purpose of the retrograde actin flow dynamics that diminish the protrusive capability of newly polymerized actin at the leading edge? A leading hypothesis is that mechanical coupling of retrograde actin motion inside the cell through FAs to the ECM could immobilize the filament meshwork relative to the ECM for two possible outcomes (Figure 3): (a) transmission of filament polymerization at the leading edge (blue monomers in Figure 3a) into a protrusion of the plasma membrane (Figure 3c) or (b) transmission of myosin-driven pulling forces (yellow myosin in Figure 3c) into traction against the ECM to pull the cell body forward (Harris 1973, Jurado et al. 2005, Suter & Forscher 2000). It is suggested that this coupling could be locally variable and regulatable by a “molecular clutch” to allow tunable transmission of myosin- or polymerization-driven retrograde flow into leading edge protrusion and/or traction (Mitchison & Kirschner 1988). Given a constant rate of contraction and actin polymerization, a high degree of engagement between actin and the ECM would result in abrogation of retrograde flow, high force generation, and persistent leading edge protrusion (Figure 3c), whereas disengagement would correspond to fast retrograde flow, low traction force, and no net movement of the cell edge (Figure 3b).
Figure 3.
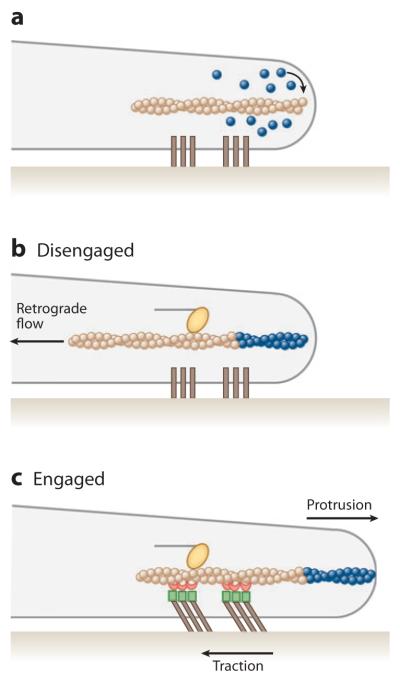
Schematic diagram of the molecular clutch at the leading edge of a migrating cell. (a) The blue spheres are actin monomers assembling onto the barbed end of the actin filament (arrow) at the leading edge; dark brown bars are transmembrane integrins. (b) If the clutch is disengaged, the newly assembled actin (blue spheres), together with the action of myosin motors (yellow oval), drives the filament rearward, away from the leading edge in retrograde flow. (c) If the clutch is engaged, indirect interactions occur between actin-binding focal adhesion molecules (red) and integrin-binding focal adhesion molecules (green) to immobilize the filament. The forces of myosin on actin thus are transmitted through the focal adhesion into traction on the extracellular matrix, and new actin polymerization drives the leading edge forward in a protrusion.
One simple prediction of the molecular clutch model is that, in the presence of uniform myosin II–driven contraction, the rate of cell protrusion should be inversely correlated with the retrograde flow rate. This has been verified in several cell types including neuronal growth cones (Lin & Forscher 1995), fibroblast filopodia (Mallavarapu & Mitchison 1999), fish skin keratocytes ( Jurado et al. 2005), and epithelial cells (Ponti et al. 2004). More generally, retrograde flow is rapid in stationary or slowly moving cells (Henson et al. 1999, Lin & Forscher 1995, Ponti et al. 2004, Salmon et al. 2002, Wang 1985) and in the absence of integrin-mediated adhesion (Alexandrova et al. 2008) but is minimal in rapidly moving keratocytes (Theriot & Mitchison 1991). Indeed, during FA assembly, decreases in F-actin retrograde flow speed and increases in traction stress are observed (Alexandrova et al. 2008, Gardel et al. 2008).
In many cell types, F-actin retrograde flow is not completely abrogated at FAs (Hu et al. 2007, Jurado et al. 2005, Theriot & Mitchison 1992) but is still associated with large traction stresses (Gardel et al. 2008). Imaging of protein dynamics within FAs has further shown that several actin-binding and FA proteins, including α-actinin, zyxin, vinculin, and talin, also move retrogradely with the actin to varying degrees (Brown et al. 2006, Guo & Wang 2007, Hu et al. 2007). By contrast, other proteins in FAs, such as integrin, paxillin, and FAK, are stationary with respect to the underlying substrate. These data indicate that FAs act more like a “slip clutch” such that the F-actin/FA/ECM interface enables dynamic rearrangements while still enabling force transmission and cell protrusion.
How might molecules within a dynamic, tunable molecular clutch be expected to behave? In a simplistic scenario, to disengage actin from integrins bound to the ECM, we would expect that certain critical FA molecules might exhibit a regulated dissociation from the actin filaments, core proteins, or the integrins. Based on the current literature, talin and vinculin are the best molecular candidates for mediating a tunable force-transmitting structural linkage from actin to the ECM (Calderwood & Ginsberg 2003, Critchley 2004, Jiang et al. 2003). Vinculin exhibits regulated binding to talin and actin (Gilmore & Burridge 1996), whereas talin can bind directly both to actin (Kaufmann et al. 1991) and to the cytoplasmic tails of β integrins, which induces the high affinity state of the integrin (Calderwood et al. 1999, Kaufmann et al. 1991). Thus, talin could single-handedly mediate engagement of actin to the ECM through integrins. Tension applied to talin molecules in vitro promotes the binding of vinculin, suggesting that the interaction strength between the FA and F-actin may strengthen under increased mechanical load by promoting vinculin/talin/actin links (del Rio et al. 2009). At FAs, vinculin and talin were shown to be partially coupled to actin retrograde flow (Brown et al. 2006, Hu et al. 2007), suggesting that by their dynamic association to ECM-bound components or F-actin-bound components, they modulate a dynamic link between F-actin and the ECM. Consistent with this, cell lines that are depleted of both isoforms of talin (talin1 and talin2) exhibit impaired FA formation, rapid F-actin retrograde flow, and impaired traction stresses (Zhang et al. 2008).
How F-actin dynamics build tension and how ECM mechanics regulate the FA slip clutch are largely unknown, but recent literature is beginning to elucidate some aspects. The magnitude of the retrograde flow of FA proteins, such as zyxin and VASP, depends on external mechanical cues, which suggests that retrograde flow and the slip clutch might be used to maintain an internal tensional homeostasis (Guo & Wang 2007). Clearly, such a tunable force-transmitting link between the cytoskeletons of cells and their extracellular environments could be a general model that applies not only to retrograde flow in cell migration but to other mechanoactive cell functions. Recent computational and experimental work has shown that a dynamic slip clutch is a type of mechanosensor in which different ECM mechanics lead to very different force and adhesive behaviors (Chan & Odde 2008).
MECHANICS OF FOCAL ADHESIONS
Regulation of Cellular Adhesion Strength
The adhesion strength characterizes the magnitude of force that can be sustained across a FA before one or multiple binding interfaces within the FA fail. Adhesion strength is modulated by numerous chemical, mechanical, and structural processes that occur at the onset of integrin binding to the ECM and continue within the FA plaque during assembly and maturation. Many of these strengthening processes are accelerated by the application of mechanical force and are crucial for the ability of cells to sense and respond to internal or external mechanical forces (Bershadsky et al. 2003, Geiger et al. 2009).
The strength of integrin-ECM bonds is strongly modulated by the type, density, and organization of the bonds. For instance, adhesive bonds formed with α5β1 integrins are stronger than those formed with αvβ3 integrins (Petrie et al. 2006). For any type of integrin-ECM bond, the adhesion strength increases as the density of ECM ligand increases (Gallant et al. 2005, Palecek et al. 1997). This enhanced adhesion strength is dependent on the ability of integrin to “cluster” with close lateral spacing on the order of 100 nm (Maheshwari et al. 2000, Selhuber-Unkel et al. 2008).
One means of force-mediated adhesion strengthening at the level of individual receptor-ligand bonds is for the bond lifetime to increase under applied force, a phenomenon known as catch bond behavior (Thomas 2008). In the past several years catch bond behavior has been observed for several different types of receptor-ligand pairs involved in cell adhesion. For bonds between α5β1 integrin and its ECM ligand, fibronectin, the application of forces up to tens of picoNewtons prolongs the bond lifetime owing to force-induced changes in the conformation of the integrin head that are akin to activation (Kong et al. 2009). Applied force also strengthens α5β1-fibronectin bonds by engaging a synergy site in fibronectin (Friedland et al. 2009). Thus, tension-dependent changes in protein conformation are important for enhanced strength of adhesive bonds under applied force.
Force-accelerated strengthening of adhesive interactions can also be mediated by changes in bond composition or density. Upon binding of αvβ3 integrin to fibronectin, talin 1 mediates transient interactions with the actin cytoskeleton (Giannone et al. 2003, Jiang et al. 2003). Under sustained tension, accumulation of vinculin correlates with a strengthened connection with the contractile actin cytoskeleton to build resistance to externally applied forces (Galbraith et al. 2002). Sustained force also initiates several signal transduction cascades, including RPTPα, Shp2, and FAK, that regulate FA assembly, maturation, and turnover, which play critical roles in the strengthening of adhesions in response to external mechanical force (Michael et al. 2009, Shi & Boettiger 2003, von Wichert et al. 2003a, 2003b). Surprisingly, only a small fraction (30%) of the total adhesive strength relies on FA growth; the bond strength of the integrin-ECM ligand accounts for nearly 70% of the adhesion strength (Gallant et al. 2005).
Regulation of Cellular Traction Stress
FAs transmit forces generated within the actin cytoskeleton to exert traction forces on the ECM. Traction stresses are generally oriented toward the cell body and in the direction of the underlying actin dynamics (Dembo & Wang 1999, Gardel et al. 2008). A large percentage of traction stresses require myosin II enzymatic activity; however, the persistence of traction stresses in the absence of myosin II activity indicates a role for alternative mechanisms of intracellular force generation including forces generated by F-actin network polymerization (Cai et al. 2006, Gardel et al. 2008). Although some degree of traction is required for cell migration, measured traction stresses vastly exceed estimates for the amount of resistive drag imposed on a motile cell in a primarily viscous environment (Mierke et al. 2008). These forces may become increasingly important for migration in a physiological, three-dimensional viscoelastic environment.
The organization and magnitude of traction forces exerted at cell-substrate adhesions are highly dependent on migratory state, cell morphology, and underlying actin dynamics. Highly motile cells (e.g., keratocytes, neutrophils, and Dictyostelium) typically exert lower traction stresses than their slower-moving counterparts (e.g., fibroblasts). The organization of traction stresses in fast-moving cells is coordinated with the direction of migration such that low traction stresses are exerted within the lamellipodium at the cell front and stronger contractile forces, often perpendicular to the direction of migration, are exerted in the rear (Lee et al. 1994, Oliver et al. 1999). The rear myosin-based contractile stresses are important for maintaining cell polarity (Lombardi et al. 2007) and rapidly disrupting rear adhesion (Burton et al. 1999). Pharmacological inhibition or genetic perturbation of myosin II markedly reduces traction stress (Beningo et al. 2006, Cai et al. 2006) and impairs the persistence of directed migration (Lo et al. 2004, Vicente-Manzanares et al. 2007).
In fibroblasts, large traction stresses are distributed around the cell periphery at discrete foci associated with FA plaques (Beningo et al. 2001). In these cells, high traction exerted near the periphery appears dominant and advances the cell body by pulling (Munevar et al. 2001). The myosin II isoform responsible for driving retrograde actin flow, nonmuscle myosin IIA, is also required for nearly 60% of traction stress generation and production, whereas traction only weakly requires the presence of nonmuscle myosin IIB (Cai et al. 2006). The regulation of myosin II activity that is responsible for traction stress in both fibroblasts and endothelial cells occurs predominantly through the Rho-kinase pathway (Beningo et al. 2006, Shiu et al. 2004). In migrating fibroblasts, the highest force per unit area of adhesion is localized to small, nascent adhesions near the advancing cell edge, whereas elongated FAs near the cell center or rear exert lower stresses (Beningo et al. 2001). By contrast, in quiescent fibroblasts, traction force is proportional to the FA area (Balaban et al. 2001, Goffin et al. 2006, Tan et al. 2003). Increases in both the density and stiffness of ECM ligand enhance the total amount of traction force generated by the cell on its ECM (Gaudet et al. 2003, Rajagopalan et al. 2004, Wang et al. 2002, Yeung et al. 2005), which is consistent with FA mechanosensitivity mediating a mechanical feedback between ECM and intracellular contractility.
FEEDBACK BETWEEN ADHESION AND F-ACTIN DYNAMICS
Feedback from mechanical and biochemical signals within FAs and the F-actin cytoskeleton coordinates the behaviors of the protrusive and contractile modules by promoting and sustaining the proper spatial and temporal control. Because adhesion assembly and maturation are regulated by numerous tension-dependent processes, this feedback often involves organization of actin structures that can sustain tension at focal complexes and adhesions. For instance, the lamellipodial actin forms a transient link between the cell edge and contractile actomyosin networks in more central cell regions that, when connected, applies tension to nascent adhesions and, when broken, allows for leading edge protrusion (Giannone et al. 2007). Lamellipodial activity is stimulated at dynamic adhesions near the leading cell edge by PAK-mediated changes in paxillin phosphorylation, which recruits both a Rac GEF (PIX) and active Rac effector (PAK) (Nayal et al. 2006) to locally stimulate Rac-mediated actin polymerization and adhesion formation (Machesky & Hall 1997) while reducing myosin activity through PAK-mediated inhibition of myosin light chain kinase (Sanders et al. 1999). Direct binding of Arp2/3 to vinculin and FAK may also promote localization of lamellipodial actin at leading edge FAs (DeMali et al. 2002, Serrels et al. 2007). In the absence of RhoA/ROCK activity, FA maturation is impaired, as evidenced by reduced phosphorylation of FAK and paxillin (Sinnett-Smith et al. 2001). Instead, integrin-mediated adhesion is enhanced and is accompanied by enhanced phosphotyrosine signaling through Pyk-2 and paxillin, as well as by unconstrained membrane protrusions (Worthylake & Burridge 2003; for reviews see Le Clainche & Carlier 2008, Vicente-Manzanares et al. 2009).
Tension-mediated signaling at adhesions plays an important role in distinguishing the protrusive lamellipodium from the contractile lamellar actin (Alexandrova et al. 2008, Shemesh et al. 2005). Mechanical stresses applied to FAs inhibit Rac activation (Katsumi et al. 2002). Instead, mechanical forces generated through ROCK-dependent myosin contractility or application of external force promote FA elongation and adhesion-associated actin bundles in an mDia-dependent manner (Gupton et al. 2007, Hotulainen & Lappalainen 2006, Riveline et al. 2001). ROCK-dependent inactivation of cofilin via LIM-kinase likely mediates stabilization of actin filaments within contractile F-actin structures (Amano et al. 2001, Pritchard et al. 2004). The Rho/mDia pathway, in turn, likely regulates FA turnover by localizing c-Src to mature FAs (Yamana et al. 2006; for a review see Huveneers & Danen 2009).
An understanding of the feedback between the actin cytoskeleton and FAs provides insight into how cells sense properties of their ECM. As the mechanical stiffness and density of ECM ligands increase, Rho activity is enhanced (Huang & Ingber 2005), and more pronounced contractile actomyosin bundles associate with a higher density of stable FAs (Gupton & Waterman-Storer 2006, Yeung et al. 2005). At low levels of cell adhesion, the separation between the protrusive lamellipodia and the contractile lamella is poorly defined (Alexandrova et al. 2008, Gupton & Waterman-Storer 2006). By contrast, at the highest levels of adhesion, retrograde flow in the lamellar region is nearly fully inhibited and FAs are stabilized, indicating that myosin II–mediated retrograde flow can become stalled in the presence of stabilized adhesion. The cell migration rate is optimized at intermediate adhesion levels, which promote adhesion turnover and the characteristic dynamics of the contractile actomyosin module (Gupton & Waterman-Storer 2006).
ACKNOWLEDGMENTS
M.L.G. acknowledges support from a Burroughs Wellcome Fund at the Scientific Interface and an NIH Director’s Pioneer Award (DP10D00354). C.M.W. is supported by the National Heart, Lung, and Blood Institute of NIH.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abercrombie M, Heaysman JEM, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp. Cell Res. 1971;67:359–67. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Alberts JB, Odell GM. In silico reconstitution of Listeria propulsion exhibits nano-saltation. PLoS Biol. 2004;2:2054–66. doi: 10.1371/journal.pbio.0020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister JJ, et al. Comparative dynamics of retrograde actin flow and focal adhesions: Formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE. 2008;3:e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem. J. 2001;354:149–59. doi: 10.1042/0264-6021:3540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaout M. Integrin structure: new twists and turns in dynamic cell adhesion. Immunol. Rev. 2002;186:125–40. doi: 10.1034/j.1600-065x.2002.18612.x. [DOI] [PubMed] [Google Scholar]
- Anderson TW, Vaughan AN, Cramer LP. Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol. Biol. Cell. 2008;19:5006–18. doi: 10.1091/mbc.E08-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001;12:2711–20. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M, Macaluso F, Cammer M, Chan A, Segall JE, Condeelis JS. Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J. Cell Biol. 1999;145:331–45. doi: 10.1083/jcb.145.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J. Cell Sci. 2006;119:866–75. doi: 10.1242/jcs.02794. [DOI] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001;153:881–88. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch. Biochem. Biophys. 2006;456:224–31. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, et al. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell Sci. 2006;119:5204–14. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol. Biol. Cell. 2005;16:4316–28. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 2002;4:574–82. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- Burridge K, Sastry SK, Sallee JL. Regulation of cell adhesion by protein-tyrosine phosphatases. J. Biol. Chem. 2006;281:15593–96. doi: 10.1074/jbc.R500030200. [DOI] [PubMed] [Google Scholar]
- Burton K, Park JH, Taylor DL. Keratocytes generate traction forces in two phases. Mol. Biol. Cell. 1999;10:3745–69. doi: 10.1091/mbc.10.11.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Biais N, Giannone G, Tanase M, Jiang G, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 2006;91:3907–20. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, Ginsberg MH. Talin forges the links between integrins and actin. Nat. Cell Biol. 2003;5:694–97. doi: 10.1038/ncb0803-694. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 1999;274:28071–74. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–91. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008;10:1039–50. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, et al. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell Sci. 2009;122:1665–79. doi: 10.1242/jcs.042986. [DOI] [PubMed] [Google Scholar]
- Cote J-F, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 2002;115:4901–13. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- Couchman JR, Rees DA. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J. Cell Sci. 1979;39:149–65. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- Cramer LP, Siebert M, Mitchison TJ. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J. Cell Biol. 1997;136:1287–305. doi: 10.1083/jcb.136.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley DR. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem. Soc. Trans. 2004;32:831–36. doi: 10.1042/BST0320831. [DOI] [PubMed] [Google Scholar]
- David MR, Ronen A, Mark HG. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 2007;218:126–34. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 2002;159:881–91. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 1999;76:2307–16. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesMarais V, Ichetovkin I, Condeelis J, Hitchcock-DeGregori SE. Spatial regulation of actin dynamics: a tropomyosin-free, actin-rich compartment at the leading edge. J. Cell Sci. 2002;115:4649–60. doi: 10.1242/jcs.00147. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 2005;7:581–90. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- Faix J, Rottner K. The making of filopodia. Curr. Opin. Cell Biol. 2006;18:18–25. doi: 10.1016/j.ceb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Forscher P, Smith SJ. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 1988;107:1505–16. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 2004;6:977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–44. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Galbraith JA. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science. 2007;315:992–95. doi: 10.1126/science.1137904. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J. Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant ND, Michael KE, Garcia AJ. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell. 2005;16:4329–40. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, Waterman CM. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet C, Marganski WA, Kim S, Brown CT, Gunderia V, et al. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys. J. 2003;85:3329–35. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–75. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, Jiang G, Sutton DH, Critchley DR, Sheetz MP. Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J. Cell Biol. 2003;163:409–19. doi: 10.1083/jcb.200302001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature. 1996;381:531–35. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 2006;172:259–68. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J. Cell Sci. 2009;122:3644–51. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W-H, Wang Y-L. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol. Biol. Cell. 2007;18:4519–27. doi: 10.1091/mbc.E07-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Anderson KL, Kole TP, Fischer RS, Ponti A, et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J. Cell Biol. 2005;168:619–31. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Eisenmann K, Alberts AS, Waterman-Storer CM. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J. Cell Sci. 2007;120:3475–87. doi: 10.1242/jcs.006049. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci. STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–74. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Harris AK. Cell surface movements related to cell locomotion. Ciba Found. Symp. 1973;14:3–26. doi: 10.1002/9780470719978.ch2. [DOI] [PubMed] [Google Scholar]
- Heath JP. Behaviour and structure of the leading lamella in moving fibroblasts. I. Occurrence and centripetal movement of arc-shaped microfilament bundles beneath the dorsal cell surface. J. Cell Sci. 1983;60:331–54. doi: 10.1242/jcs.60.1.331. [DOI] [PubMed] [Google Scholar]
- Heath JP, Holifield BF. On the mechanisms of cortical actin flow and its role in cytoskeletal organisation of fibroblasts. Symp. Soc. Exp. Biol. 1993;47:35–56. [PubMed] [Google Scholar]
- Henson JH, Svitkina TM, Burns AR, Hughes HE, MacPartland KJ, et al. Two components of actin-based retrograde flow in sea urchin coelomocytes. Mol. Biol. Cell. 1999;10:4075–90. doi: 10.1091/mbc.10.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holifield BF, Ishihara A, Jacobson K. Comparative behavior of membrane protein-antibody complexes on motile fibroblasts: implications for a mechanism of capping. J. Cell Biol. 1990;111:2499–512. doi: 10.1083/jcb.111.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP. J. Biol. Chem. 2006;281:2296–305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006;173:383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–15. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–76. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Danen EHJ. Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Ichetovkin I, Grant W, Condeelis J. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr. Biol. 2002;12:79–84. doi: 10.1016/s0960-9822(01)00629-7. [DOI] [PubMed] [Google Scholar]
- Iwasa JH, Mullins RD. Spatial and temporal relationships between actin-filament nucleation, capping, and disassembly. Curr. Biol. 2007;17:395–406. doi: 10.1016/j.cub.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–37. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Tang H-Y, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663–66. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado C, Haserick JR, Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol. Biol. Cell. 2005;16:507–18. doi: 10.1091/mbc.E04-10-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, et al. Effects of cell tension on the small GTPase Rac. J. Cell Biol. 2002;158:153–64. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S, Piekenbrock T, Goldmann WH, Barmann M, Isenberg G. Talin binds to actin and promotes filament nucleation. FEBS Lett. 1991;284:187–91. doi: 10.1016/0014-5793(91)80681-r. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 1999;146:1033–44. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Cell migration during gastrulation. Curr. Opin. Cell Biol. 2005;17:533–41. doi: 10.1016/j.ceb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Klambt C. Modes and regulation of glial migration in vertebrates and invertebrates. Nat. Rev. Neurosci. 2009;10:769–79. doi: 10.1038/nrn2720. [DOI] [PubMed] [Google Scholar]
- Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 2009;185:1275–84. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylyshkina O, Kaverina I, Kranewitter W, Steffen W, Alonso MC, et al. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. J. Cell Biol. 2002;156:349–60. doi: 10.1083/jcb.200105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 2006;90:3762–73. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol. 2001;153:1427–40. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clainche C, Carlier M-F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- Lee H-S, Bellin RM, Walker DL, Patel B, Powers P, et al. Characterization of an actin-binding site within the talin FERM domain. J. Mol. Biol. 2004;343:771–84. doi: 10.1016/j.jmb.2004.08.069. [DOI] [PubMed] [Google Scholar]
- Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting keratocytes. J. Cell Biol. 1994;127:1957–64. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14:763–71. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell. 2004;15:982–89. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A, Nieto MA. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr. Opin. Genet. Dev. 2001;11:464–69. doi: 10.1016/s0959-437x(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Lombardi ML, Knecht DA, Dembo M, Lee J. Traction force microscopy in Dictyostelium reveals distinct roles for myosin II motor and actin-crosslinking activity in polarized cell movement. J. Cell Sci. 2007;120:1624–34. doi: 10.1242/jcs.002527. [DOI] [PubMed] [Google Scholar]
- Machacek M, Danuser G. Morphodynamic profiling of protrusion phenotypes. Biophys. J. 2006;90:1439–52. doi: 10.1529/biophysj.105.070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell Biol. 1997;138:913–26. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 2000;113:1677–86. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J. Cell Biol. 1999;146:1097–106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006;8:216–26. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Michael KE, Dumbauld DW, Burns KL, Hanks SK, Garcia AJ. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol. Biol. Cell. 2009;20:2508–19. doi: 10.1091/mbc.E08-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierke CT, Rösel D, Fabry B, Brábek J. Contractile forces in tumor cell migration. Eur. J. Cell Biol. 2008;87:669–76. doi: 10.1016/j.ejcb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–72. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Tsuji T, Higashida C, Hertzog M, Fujita A, et al. Actin turnover-dependent fast dissociation of capping protein in the dendritic nucleation actin network: evidence of frequent filament severing. J. Cell Biol. 2006;175:947–55. doi: 10.1083/jcb.200604176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner A, Oster G. The physics of lamellipodial protrusion. Eur. Biophys. J. 1996;25:47–53. [Google Scholar]
- Munevar S, Wang Y, Dembo M. Traction force microscopy of migrating normal and H-Ras transformed 3T3 fibroblasts. Biophys. J. 2001;80:1744–57. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006;173:587–89. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins Rho and Rac by growth factor receptors. J. Cell Sci. 1995;108(Pt. 1):225–33. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hirokawa N. Actin dynamics in growth cones. J. Neurosci. 1991;11:1918–29. doi: 10.1523/JNEUROSCI.11-07-01918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver T, Dembo M, Jacobson K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J. Cell Biol. 1999;145:589–604. doi: 10.1083/jcb.145.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–40. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–65. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LJ, Rajfur Z, Maddox AS, Freel CD, Chen Y, et al. Simultaneous stretching and contraction of stress fibers in vivo. Mol. Biol. Cell. 2004;15:3497–508. doi: 10.1091/mbc.E03-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie TA, Capadona JR, Reyes CD, García AJ. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459–70. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell Physiol. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–86. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- Pritchard CA, Hayes L, Wojnowski L, Zimmer A, Marais RM, Norman JC. B-Raf acts via the ROCKII/LIMK/cofilin pathway to maintain actin stress fibers in fibroblasts. Mol. Cell Biol. 2004;24:5937–52. doi: 10.1128/MCB.24.13.5937-5952.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Rajagopalan P, Marganski WA, Brown XQ, Wong JY. Direct comparison of the spread area, contractility, and migration of Balb/c 3T3 fibroblasts adhered to fibronectin- and RGD-modified substrata. Biophys. J. 2004;87:2818–27. doi: 10.1529/biophysj.103.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001;153:1175–86. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon WC, Adams MC, Waterman-Storer CM. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 2002;158:31–37. doi: 10.1083/jcb.200203022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, Van Der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999;147:1009–22. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–85. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM, Jockusch BM. Differences in the stress fibers between fibroblasts and epithelial cells. J. Cell Biol. 1983;96:961–69. doi: 10.1083/jcb.96.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub S, Bohnet S, Laurent VM, Meister J-J, Verkhovsky AB. Comparative maps of motion and assembly of filamentous actin and myosin II in migrating cells. Mol. Biol. Cell. 2007;18:3723–32. doi: 10.1091/mbc.E06-09-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider IC, Hays CK, Waterman CM. Epidermal growth factor-induced contraction regulates paxillin phosphorylation to temporally separate traction generation from de-adhesion. Mol. Biol. Cell. 2009;20:3155–67. doi: 10.1091/mbc.E09-03-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 2007;176:667–80. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhuber-Unkel C, López-García M, Kessler H, Spatz JP. Cooperativity in adhesion cluster formation during initial cell adhesion. Biophys. J. 2008;95:5424–31. doi: 10.1529/biophysj.108.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 2007;9:1046–56. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- Shemesh T, Geiger B, Bershadsky AD, Kozlov MM. Focal adhesions as mechanosensors: a physical mechanism. Proc. Natl. Acad. Sci. USA. 2005;102:12383–88. doi: 10.1073/pnas.0500254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol. Biol. Cell. 2003;14:4306–15. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu Y-T, Li S, Marganski WA, Usami S, Schwartz MA, et al. Rho mediates the shear-enhancement of endothelial cell migration and traction force generation. Biophys. J. 2004;86:2558–65. doi: 10.1016/S0006-3495(04)74311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith J, Lunn JA, Leopoldt D, Rozengurt E. Y-27632, an inhibitor of Rho-associated kinases, prevents tyrosine phosphorylation of focal adhesion kinase and paxillin induced by bombesin: dissociation from tyrosine phosphorylation of p130cas. Exp. Cell Res. 2001;266:292–302. doi: 10.1006/excr.2001.5219. [DOI] [PubMed] [Google Scholar]
- Small JV. The actin cytoskeleton. Electron. Microsc. Rev. 1988;1:155–74. doi: 10.1016/s0892-0354(98)90010-7. [DOI] [PubMed] [Google Scholar]
- Suter DM, Forscher P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J. Neurobiol. 2000;44:97–113. [PubMed] [Google Scholar]
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S-I, et al. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 2003;160:409–21. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons MH, Mitchison TJ. Control of actin polymerization in live and permeabilized fibroblasts. J. Cell Biol. 1991;114:503–13. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, et al. Talin binding to integrin β tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 2003;100:1484–89. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot JA, Mitchison TJ. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–31. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- Theriot JA, Mitchison TJ. Comparison of actin and cell surface dynamics in motile fibroblasts. J. Cell Biol. 1992;119:367–77. doi: 10.1083/jcb.119.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. Catch bonds in adhesion. Annu. Rev. Biomed. Eng. 2008;10:39–57. doi: 10.1146/annurev.bioeng.10.061807.160427. [DOI] [PubMed] [Google Scholar]
- Vallotton P, Gupton SL, Waterman-Storer CM, Danuser G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc. Natl. Acad. Sci. USA. 2004;101:9660–65. doi: 10.1073/pnas.0300552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J. Cell Biol. 1995;131:989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Network contraction model for cell translocation and retrograde flow. Biochem. Soc. Symp. 1999;65:207–22. [PubMed] [Google Scholar]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration—the actin connection. J. Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007;176:573–80. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–75. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Volberg T, Romer L, Zamir E, Geiger B. pp60c-src and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J. Cell Sci. 2001;114:2279–89. doi: 10.1242/jcs.114.12.2279. [DOI] [PubMed] [Google Scholar]
- von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 2003a;22:5023–35. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wichert G, Jiang G, Kostic A, De Vos K, Sap J, Sheetz MP. RPTP-α acts as a transducer of mechanical force on αv/β3-integrin-cytoskeleton linkages. J. Cell Biol. 2003b;161:143–53. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 2002;282:C606–16. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Wang YL. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of tread-milling. J. Cell Biol. 1985;101:597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Mitchison TJ. Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science. 2002;295:1083–86. doi: 10.1126/science.1067470. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–61. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Imhof BA. Actin, microtubules and focal adhesion dynamics during cell migration. Int. J. Biochem. Cell Biol. 2003;35:39–50. doi: 10.1016/s1357-2725(02)00071-7. [DOI] [PubMed] [Google Scholar]
- Wiseman PW, Brown CM, Webb DJ, Hebert B, Johnson NL, et al. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J. Cell Sci. 2004;117:5521–34. doi: 10.1242/jcs.01416. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 2003;278:13578–84. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005;17:559–64. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol. Cell. Biol. 2006;26:6844–58. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003;116:4605–13. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004;32:416–20. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007a;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J. Cell Sci. 2007b;120:137–48. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 2001;114:3583–90. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 1999;112(Pt. 11):1655–69. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 2008;10:1062–68. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo B-H, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell. 2008;32:849–61. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman B, Volberg T, Geiger B. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell Motil. Cytoskelet. 2004;58:143–59. doi: 10.1002/cm.20005. [DOI] [PubMed] [Google Scholar]