Abstract
Premature birth is a significant cause of infant and child morbidity and mortality. In the United States, the premature birth rate, which had steadily increased during the 1990s and early 2000s, has decreased annually for four years and is now approximately 11.5%. Human viability, defined as gestational age at which the chance of survival is 50%, is currently approximately 23–24 weeks in developed countries. Infant girls, on average, have better outcomes than infant boys. A relatively uncomplicated course in the intensive care nursery for an extremely premature infant results in a discharge date close to the prenatal EDC. Despite technological advances and efforts of child health experts during the last generation, the extremely premature infant (less than 28 weeks gestation) and extremely low birth weight infant (ELBW) (< 1000 grams) remain at high risk for death and disability with 30–50% mortality and, in survivors, at least 20–50% risk of morbidity. The introduction of CPAP, mechanical ventilation, and exogenous surfactant increased survival and spurred the development of neonatal intensive care in the 1970s through the early 1990s. Routine administration of antenatal steroids during premature labor improved neonatal mortality and morbidity in the late 1990s. The recognition that chronic postnatal administration of steroids to infants should be avoided may have improved outcomes in the early 2000s. Evidence from recent trials attempting to define the appropriate target for oxygen saturation in preterm infants suggests arterial oxygen saturation between 91–95% (compared to 85–89%) avoids excess mortality. However, final analyses of data from these trials have not been published, so definitive recommendations are still pending The development of neonatal neurocognitive care visits may improve neurocognitive outcomes in this high-risk group. Long-term follow up to detect and address developmental, learning, behavioral, and social problems is critical for children born at these early gestational ages.
The striking similarities in response to extreme prematurity in the lung and brain imply that agents and techniques that benefit one organ are likely to also benefit the other. Finally, since therapy and supportive care continue to change, the outcomes of ELBW infants are ever evolving. Efforts to minimize injury, preserve growth, and identify interventions focused on antioxidant and anti-inflammatory pathways are now being evaluated. Thus, treating and preventing long-term deficits must be developed in the context of a “moving target.”
Introduction
In 2010, more than one in 10 of the world’s infants, of more than 15 million children, were born prematurely.1 More than a million of those children died secondary to complications associated with premature birth. Prematurity is the single most important cause of death in the first month of life and is a factor in over 75% of pediatric deaths in the neonatal period. As the second leading cause of death in children under five years old,2 prematurity remains a global health problem. In addition, prematurity is associated with learning and motor disabilities and with visual and hearing impairment, contributing to approximately half of disabilities in children. Although preterm birth has actually decreased in the United States over the past five years (see below), worldwide rates have increased over the last decade.1–4
The challenges that “graduates” of the neonatal intensive care unit (NICU) present in the setting of anesthesiology and surgery will be discussed from several perspectives: 1) general outcomes of the extremely premature infant; 2) respiratory consequences of prematurity in the pediatric patient; 3) neurological outcomes and therapies associated with neonatal neurological intensive care therapies; and 4) selected aspects of term and preterm newborn brain imaging. This review is intended to provide background and historical perspective to the management of infants whose medical needs present a multitude of challenges for various health care professionals.
Outcomes of the Extremely Premature Infant
Several definitions are important for clarity in the discussion of premature birth outcomes. First, gestational age is defined as the age of the fetus in terms of pregnancy duration in weeks, measured from the first day of the last menstrual period and, by convention, gestation is recorded as completed weeks and never rounded up. For example, an infant who is born at 32 weeks and four days is defined as being 32 weeks. The definition of the “estimated date of confinement” (EDC), also known as the due date, is 40 weeks added to the first day of the last menstrual period and estimates the day when the infant will be born.5 “Postmenstrual age” (PMA) is the time elapsed between the first day of the last menstrual period and the current day,5 and PMA can also be calculated as the gestational age plus the time elapsed after birth (chronologic age). PMA is used clinically during the perinatal period beginning after the day of birth. “Post-conceptual age” is not synonymous with PMA.5 “Corrected age,” also called the adjusted age, describes children up to three years old who were born preterm. “Corrected age” represents the age of the child since the EDC. “Term” birth is classically defined as 37 to 42 weeks. Recently, a National Institute of Child Health and Human Development (NICHD)-led group proposed subgrouping births between 37 and 39 weeks’ gestation as “early term.”6 “Preterm birth” is defined as any birth prior to 37 weeks’ completed gestation or fewer than 259 days since the first day of the mother’s last menstrual period.2, 3, 5, 6 Preterm birth is often subdivided further based on birth gestational age. “Late preterm” infants are those born 34 to less than 37 weeks gestation, “moderate preterm” is designated as 32 to less than 34 weeks gestation, “very preterm” is designated 28 to less than 32 weeks gestation, and “extremely preterm,” which is the primary focus of this discussion, is less than 28 weeks gestational age2, 3, 5, 6 (Table 1).
Table 1.
Terminology of Prematurity
| Label | Definition (week’s completed gestation) |
|---|---|
| Extremely Preterm | < 28 |
| Very Preterm | 28 to <32 |
| Moderate Preterm | 32 to <34 |
| Late Preterm | 34 to <37 |
| Early-Term | 37 to <39 |
| Term | 38 to <41 |
| Late-Term | 41 to <42 |
| Post-Term | >42 |
| SGA | Weight less than 10th percentile for gestational age |
| LGA | Weight greater than 90th percentile for gestational age |
| VLBW | Less than 1500 grams |
| ELBW | Less than 1000 grams |
SGA = Small for gestational age
LGA = Large for gestational age
VLBW = Very low birth weight
ELBW = Extremely low birth weight
Other useful definitions include “small for gestational age” (SGA), defined as weight less than 10th percentile at a given fetal gestational age. By this definition, less than 2,500 grams at birth is considered SGA. “Large for gestational age” (LGA) is defined as weight greater than the 90th percentile for duration of gestation. Infants greater than 4,500 grams at term birth are LGA.7 The terms SGA and LGA age do not distinguish among the various ideologies of these conditions. Low birth weight infants can be further classified into very low birth weight (VLBW) which includes infants less than 1500 grams and extremely low birth weight infants (ELBW) which are infants less than 1000 grams.
A “live birth” is defined as the complete expulsion or extraction of the product of human conception regardless of the duration of pregnancy, and following such expulsion the infant breathes or shows other evidence of life such as a beating heart, pulsation of the umbilical cord, or definitive movement of voluntary muscles. “Live birth” does not designate whether the umbilical cord has been cut or the placenta remains attached. Heartbeats need to be distinguished from transient cardiac contractions. Respirations need to be distinguished from fleeting respiratory efforts or gasps. “Fetal death” is death of the product of human conception prior to complete expulsion or extraction from the mother regardless of the duration of pregnancy. Death is indicated by the fact that after expulsion/extraction, the fetus does not breathe or show any other evidence of life, such as a beating heart or pulsation of the umbilical cord.
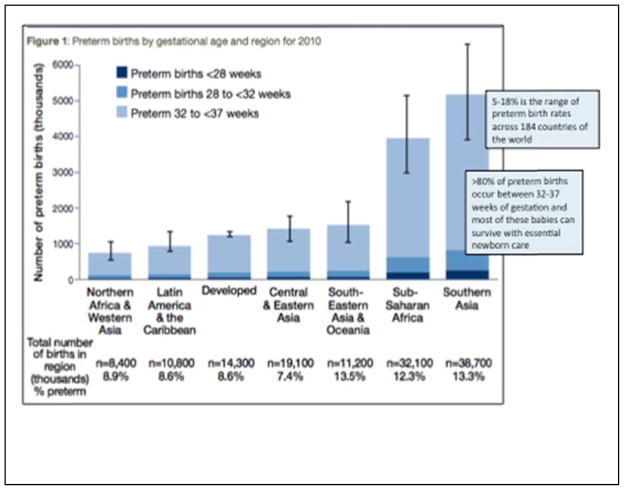
Viability is often defined as the gestational age at which there is a 50% chance of survival with or without medical care;2 therefore, under current conditions, viability in developed, high income countries of the world is somewhere between 22–24 weeks, whereas viability is closer to 34 weeks gestational age in low- and middle-income countries.2 Premature birth rate and the relative proportions within the subcategories of extremely preterm, very preterm, and late preterm births vary throughout the world (Figure 1). The highest rate of premature birth (close to 18%) is noted in Southeastern Asia.2 Worldwide, most preterm births are late preterm.
Figure 1.
Modified with permission from World Health Organization: Born too soon: the global action report on preterm birth. WHO Press, Geneva, Switzerland 2012 (2).
Survival and Morbidity
In the United States in 2011, approximately half a million (11.7%) of the 4 million total births were preterm (Figure 2). The subgroup of extremely preterm births comprise approximately 6% of all preterm births and are less than 1% of all births. Infants born less than 34 weeks comprise almost 60% of infant deaths.4 Recently, the premature birthrate has decreased. Preliminary data for 2012 show a preterm birthrate of 11.5%, compared to a maximum of 12.5% in 2009.4 The premature birth rate in the United States ranks in the middle of other nations.2 In addition, ethnic and racial disparities in the premature birthrate remain throughout the world. In the United States, non-Hispanic blacks have a premature birthrate closer to 16%, and non-Hispanic whites have rates closer to 10%.8
Figure 2.

Figure drawn using data from Centers for Disease Control and Prevention (4).
Over the last generation, a dramatic decline in infant mortality has been associated with medical innovations in the management of neonates, particularly those born preterm. The specialty of anesthesiology has contributed significantly to those innovations. These contributions include initiation of rational intravenous fluid therapies; development of artificial airways and breathing circuits; application of mechanical ventilation and airway distending pressure; and development of a resuscitation scoring system, the Apgar score, to aid in the evaluation of the resuscitative efforts of the newborn.
Following application of the airway distending pressure by Gregory,9 many centers in the United States and the developed world began aggressive programs to develop intensive care programs to support the smallest premature infants and to develop follow-up clinics to evaluate the success of these intensive care efforts. In a cohort of 61 infants born between 500–750 grams in the latter half of the 1970’s, girls (71.4% survival) had better outcomes compared to boys (18.2% survival) and small increments of increasing birth weight and gestational age were strongly associated with improved outcomes.10
Changes in clinical outcomes of extremely premature infants have been significantly influenced by changes in medical care (Table 2). Continuous positive airway pressure (CPAP), mechanical ventilation, exogenous surfactant, and antenatal steroids have markedly improved survival, whereas postnatal steroid administration to premature infants worsened outcome.11, 12 The adverse effects of postnatal steroids became apparent in trials designed to lessen the severity of chronic lung disease in premature infants. Although these studies have shown that a course of high-dose dexamethasone tapered over weeks is associated with smaller head circumference, weak motor skills, lower IQ scores, and clinically significant disabilities, the role of single-dose or short-term use of either hydrocortisone or dexamethasone has not been established.11–13
Table 2.
Extremely Low Birth Weight Infant Outcomes
| Innovation | Time |
|---|---|
| CPAP, Mechanical Ventilation | 1980s |
| Exogenous Surfactant | Early 1990s |
| Antenatal Steroids | Mid/Late 1990s |
| Avoiding Postnatal Steroids | Early2000s |
| Targeted Oxygen Therapy | Mid 2000s |
| Systematic Care/Experience | Continuous |
CPAP = Continuous positive airway pressure
Most recently, randomized control trials have been published to better delineate the lowest inspired oxygen concentration (comparing target ranges for oxygen saturation of 85–89% with 91–95%) that maximizes survival and minimizes eye, pulmonary, and neurocognitive morbidities in premature infants.14–16 That is, oxygen therapy has been associated with improved survival but higher incidence of chronic lung disease and retinopathy of prematurity (ROP), especially in extremely premature infants.
The results of these randomized controlled, multicenter studies have yielded conflicting evidence. The SUPPORT trial (surfactant, positive pressure, and pulse oximetry) conducted in the United States noted that the lower SpO2 target group (85 – 89%) had a lower incidence of ROP (8.6% v 17.9%) but a higher mortality (22.1% v 18.2%) compared to the target group of 91 to 95% (14). Similar findings were noted in BOOST (Benefits of Oxygen Saturation Targeting) II, a collaboration of United Kingdom, Australia, and New Zealand.16 However, in the Canadian Oxygen Trial (COT) involving 578 infants, no significant difference in the rate of death or disability at 18 months15 were identified. Similar findings of no difference in death, major disability, ROP, or chronic lung disease between the two target saturation groups was also reported in the six regional NICUs from New Zealand (Boost-NZ).17, 18
The optimal oxygen saturation during surgery and anesthesia has not been defined. Some of these disparities in these large multicenter studies may have been related to a technical problem with the Massimo pulse oximeter. During these studies, a change in the algorithm was made when it was discovered that the pulse oximeter was reading 2 – 3% higher than the actual valve. However, it appears that small differences in SpO2 target range may influence mortality. Thus, at present in the premature infant, targeting SpO2 to less than 90% should be avoided. A critical factor in the management of these infants is the inability to maintain saturation in the targeted range. For example in the oxygen targeting study involving CPAP, infants targeted for oxygen saturations of 88–92% achieved their goal saturation only 31% of the time.19
The innovation epoch framework (Table 2) provides a useful backdrop against which to examine the changing results of outcome studies over the last 20 years. For example, the initial National Institutes of Health and Human Development Neonatal Network study20 reported the morbidity and mortality of 1,765 premature infants (birth weight 500–1500 grams) in the period following the widespread implementation of NICUs and mechanical respiratory support, but before the introduction of exogenous surfactant. Survival dramatically improved with each week of gestational age and for each 100-gram increment in birth weight. Specifically, survival in the 500–600 gram group was only 20% compared to 56% in the 700–800 gram birth weight group (Figure 3). Non-survivors tended to die within in the first two weeks of life, and mortality after 28 days of age was 8%. Of note, length of stay correlated with the gestational age; if the course in the NICU was stable, the extremely premature infant was discharged at approximately the time of the EDC. As observed in earlier reports, infant girls had better outcomes compared to male counterparts. Finally, data from these earlier studies often included patients admitted to intensive care nurseries and not infants who died in the delivery room or during transport to the intensive care unit, which tended to skew survival statistics (Figure 4).
Figure 3.
Extremely Low Birth Weight Infant Outcomes of the National Institute of Child Health and Human Development (NICHD) Neonatal Network. Eight centers participated in the NICHD Neonatal Network, 1,765 infants born in the middle to late 1980s. Survival significantly improved with each week of increase in gestational age and with each 100-gram increment increase in birth weight (data not shown). Figure drawn from data presented in Hack et al. Pediatrics. 1991; 87:587 (20).
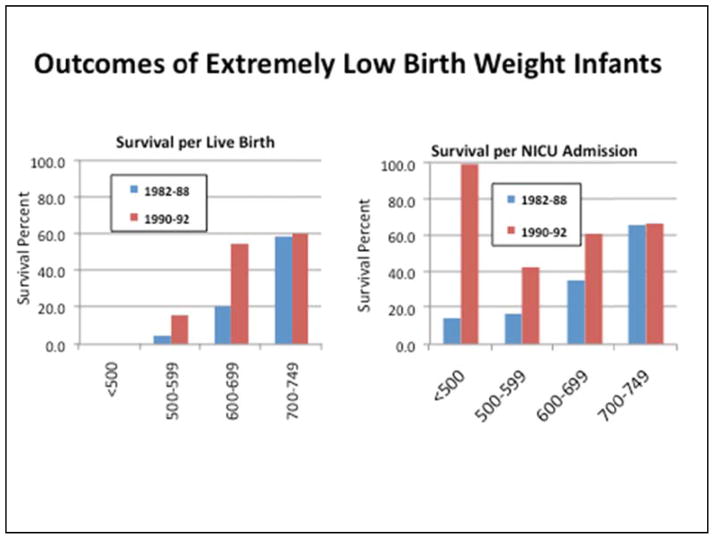
Figure 4.
The figure shows how survival statistics may be distorted depending on the reference denominator used in evaluating mortality risk. Older cohort observations often included only patients admitted to intensive care. These studies did not account for infants who succumbed in the delivery room or on the way to intensive care. In the figure in the right hand panel, survival is referenced to live births (population-based cohort), demonstrating very poor outcome in the less than 500–600 gram subgroup. When survival is referenced to pediatric intensive care unit (ICU) admissions as it is on the left sided panel, outcomes seem much better. Clinicians involved in the surgical and intensive care management of these patients often have this distorted view of survival because he or she sees only the children admitted for care. Figure 6 also displays the significant improvement in survival that occurred with the widespread availability and use of exogenous surfactant. Figure drawn from data presented in Hack et al. Pediatrics. 1996;98: 931–937 (21).
From 1986 to 2004, several large population-based cohort outcome studies (Table 3)20–43 included approximately 14,700 extremely low birth weight (ELBW) infants from North America, Western Europe, the United Kingdom, Australia, and Japan. Outcomes of observation studies were pooled and then referenced to the Table 2 epochs to show changes in survival over time (Figure 5). The regression lines for each gestational between 23 to 26 weeks document improved survival rates over time. Using the 50% survival rate standard, the viability appears to have improved from approximately 25–26 weeks in the 1990s to approximately 23–24 weeks by the mid-2000s.
Table 3.
Large Observational Outcome Cohort Studies from 1986–2004
| Country | Birth Cohort Treatment Epoch | Reference |
|---|---|---|
| USA | 1980s | Hack M. 1991 20 |
| USA | 1980s | Hack M. 1996 21 |
| Australia | Early 1990s | Doyle LW. 1997 22 |
| USA | Early 1990s | Hack M. 1996 21 |
| UK | Early 1990s | Tin W. 1997 23 |
| Australia | Early 1990s | Sutton L. 1999 24 |
| USA | Mid 1990s | Hintz SR. 2005 25 |
| UK | Mid 1990s | Draper ES. 1999 26 |
| Denmark | Mid 1990s | Kamper J. 2004 27 |
| Northern Sweden | Mid 1990s | Hakensson S. 2004 28 |
| Southern Sweden | Mid 1990s | Hakensson S. 2004 28 |
| UK/Ireland | Mid 1990s | Wood NS. 2000 29 |
| Netherlands | Mid 1990s | den Ouden AL. 2000 30 |
| Finland | Late 1990s | Tommiska V. 2007 31 |
| Australia | Late 1990s | Doyle LW. 2004 32 |
| France | Late 1990s | Larroque B. 2004 33 |
| USA | Late 1990s | Hintz SR. 2005 25 |
| Belgium | Late 1990s | Vanhaesebrouck P. 2004 34 |
| Finland | Late 1990s | Tommiska V. 2007 31 |
| Norway | Late 1990s | Markestad T. 2005 35 |
| USA | Early 2000s | Hintz SR. 2011 36 |
| Germany | Early 2000s | Kutz P. 2009 37 |
| USA | Early 2000s | Mercier CE. 2010 38 |
| Sweden | Mid 2000s | Serenius F. 2013 39 |
| Japan | Mid 2000s | Ishii N. 2013 40 |
| Australia | Mid 2000s | Doyle LW. 2010 41 |
USA = United States of America
UK = United Kingdom
Figure 5.
Extremely Low Birth Weight Infant Survival. Outcomes of observation studies were pooled, then referenced to the Table 2 epochs to allow visual evaluation the association of changes in survival over time with the innovations. The regression lines for each gestational age 23 through 26 weeks suggest that survival has improved over time. Using the 50% survival rate standard (black dashed line), the figure suggests that viability has improved from approximately 25–26 weeks in the 1990s to between 23–24 weeks by the mid-2000s. Graphic drawn from data in references (20–43).
Increased survival of extremely premature infants has raised the concern for an increase in the number of children who incur severe acute and chronic morbidities (e.g. intraventricular hemorrhage [IVH], necrotizing enterocolitis [NEC], bronchopulmonary dysplasia [BPD], chronic lung disease, severe visual impairment, hearing impairment, cerebral palsy, and cognitive developmental delay) (Table 4). Eichenwald and Stark44 have systematically documented the critical importance of gestational age on survival in a cohort born between 1997 and 2002 by noting short-term survival without complications (NEC, BPD, severe IVH) as a function of narrow ranges of birth weights (~20%, 501–750 g; ~50%, 751–1000 g; ~70%, 1001–1250 g; ~90%, 1251–1500). Comparing several eras (1991–1996 vs. 1997–2002), the authors concluded that improved survival was accompanied by persistently high rates of morbidity. In a more recent publication, Stoll45 reported similar results, but expanded the dataset to include infants between 22–24 weeks of gestation and added several parameters to gauge outcomes (periventricular leukomalacia, retinopathy of prematurity > stage 3, early/late sepsis/meningitis, NEC, BPD, and severe IVH). Comparing short-term outcomes in 2003, 2005, and 2007, the study confirmed that rates of survival without morbidity remained stable over this time period for each gestational age (0%, 22 weeks; 5–11%, 24 weeks; 20–22%, 25 weeks; 32–34%, 26 weeks; 44–46%, 27 weeks; 54–62%, 28 weeks).
Table 4.
Morbidity Risks
| ELBW Morbidity | Risk | 95% CI |
|---|---|---|
| Acute/NICU | ||
| Any ROP | 63.7 | 60.6 – 66.6 |
| Severe ROP | 12.3 | 11.3 – 13.3 |
| IVH (Grade III-IV) | 14.1 | 13.1 – 15.2 |
| Surgical NEC | 10.1 | 9.3 – 11.0 |
| Chronic Problems | ||
| BPD (O2 at 28 weeks) | 42.2 | 40.4 – 43.9 |
| BPD (O2 at 36 weeks) | 38.7 | 36.9 – 40.5 |
| Blindness | 0.8 | 0.36 – 1.62 |
| Hearing loss | 3.1 | 2.1 – 4.4 |
| Cerebral Palsy | 6.1 | 4.7 – 7.7 |
| Cognitive Delay | 7.4 | 5.2 – 9.4 |
Data from the three recent randomized trials of titrated oxygen therapy (14–16) are pooled to provide point estimates of morbidity risks with 95% confidence intervals. The risk of bronchopulmonary dysplasia (BPD), defined as “supplemental oxygen needed at 28 weeks postmenstrual age,” is 42%. A more strict definition of oxygen BPD, “supplemental oxygen greater than 30% at 36 weeks postmenstrual age” places the risk at 39% of patients.
CI = Confidence Interval
ELBW = Extremeley low birth weight
NICU = Neonatal intensive care unit
ROP =retinopathy of prematurity
IVH =intraventricular hemorrhage
NEC =necrotizing enterocolitis
Of note, pooled data from recent randomized, controlled trials of titrated oxygen therapy14–16 suggest that from 2000–2010, morbidity may have gradually improved in extremely premature infants (Table 4). Because patients may have more than one morbidity, some experts suggest that examining outcomes in this population requires examining the percentage of patients who are spared various morbidities (Figure 6).34
Figure 6.
The percentage of extremely premature infants who are spared from various morbidities. The figure represents the cumulative short-term outcome scale at the time of discharge for admitted infants with a gestational age (GA) of 24 to 26 weeks. At the time of hospital discharge, approximately 50% of infants born at 25 weeks GA leave the hospital without any major neurologic disability versus only 20% of infants born at 24 weeks. Figure modified with permission from Vanhaesebrouck P et al. Pediatrics 2004;114:663–675 (34).
Thus, even with technological advances the extremely premature infant remains at considerable risk for death (30–50% mortality) and disability (20–50%).14–16, 36 Abitbol and Rodriquez46 noted that preterm birth significantly influences the “developmental programming” of health and disease so that abnormalities in organogenesis secondary to preterm birth clearly may influence function throughout a lifetime.
Respiratory Outcomes
Alveolar lung development continues into the postnatal period. Prematurity coupled with inflammation, hyperoxia, and volutrauma and barotrama from mechanical ventilation can interrupt normal pulmonary development and thus create a clinical scenario of chronic lung disease with pathophysiological effects that can extend beyond infancy into adulthood. Bronchopulmonary dysplasia (BPD) has been referred to as the “chronic lung disease of the premature”. Although multiple definitions have been proposed, one commonly accepted version includes oxygen dependence at 28 postnatal days and severity and stratification as mild, moderate, or severe at 36 weeks post-conceptual age (gestational age < 32 weeks) or at 56 days (gestational age> 32 weeks) based on supplemental oxygen delivery (i.e., room air, FIO2 0.22–0.29, and FiO2 > 0.30, respectively).47
Understanding the etiology of BPD requires a review of normal lung development. A simplified sequence of development includes several stages: embryonic, pseudoglandular (8–17 weeks), canalicular (16–23 weeks), saccular (23–32 weeks), and alveolar (overlaps saccular-postnatal)47 (Figure 7). Because the entire bronchiolar tree is established by 17–18 weeks gestation, destruction of or severe injury to airways postnatally (e.g., supplemental oxygen and/or ventilation) in the extremely premature infant are likely to impart permanent sequelae. Another critical event associated with BPD centers on pulmonary vascularization, which is the hallmark of the canalicular stage of development. Active development of the distal pulmonary circulation includes appearance of lung capillaries by 20 weeks, a time closely proximate to the gestational age of the ELBW infant. Injury to the lung at this phase predisposes the infant to irreversible pulmonary vascular injury and delayed or arrested growth of alveoli.47, 48
Figure 7.
Normal Development of the Lung. The five stages of normal development of the lung; weeks 0 to 6 of gestation comprise the embryonic period, weeks 6 to 16 the pseudoglandular period, weeks 16 to 24 the canalicular period, and weeks 24 to term (40 weeks) the saccular period. Pulmonary circulation develops in parallel with lung development (115).
Over the last decade, “new” BPD has been contrasted to “old” BPD. The distinction between new and old has evolved as a result of changes in postnatal care, as well as increased survival of more immature infants (i.e. infants born at earlier stages of pulmonary development). The so-called “new BPD” primarily develops in the ELBW infant (less than 1000 g) born during the late canalicular or early saccular phase.47 In contrast, “Old BPD” includes infants from the pre-surfactant era, of an older gestational age, born during the late saccular and alveolar stages of lung development, and exposed to vigorous mechanical ventilation and supplemental oxygen. The primary pathology of “old BPD” includes intense airway inflammation, fibrosis with severe epithelial injury, and smooth muscle hyperplasia. In contrast, “new BPD” of the ELBW infants evolves in the setting of gentler ventilator strategies, prenatal steroids, and postnatal surfactant. With birth at mid-gestation, ELBW infants undergo the complex process of lung development postnatally. At this immature stage of development, the lung is extremely susceptible to injury secondary to inflammation/infection, ventilatory support (including CPAP), and even appropriate supplemental oxygen. When postnatal damage occurs during the early saccular or late canalicular stages, the pathology includes fewer but larger alveoli, abnormal vascular growth, but less prominent inflammation, smooth muscle hypertrophy, and fibroproliferation. Thus, “new BPD” is characterized by the prominent arrest of both alveolar septation and vascular development and subsequent reduction in surface area for gas exchange.47–49 Despite the well-described differences in the pathology of “new” compared to “old” BPD, children born prematurely in different eras seem to have remarkably similar long-term functional pulmonary abnormalities.50 That is, over the last 30 years, survivors of BPD seem to display similar patterns of clinical dysfunction that persist into late childhood and early adulthood.
The circulation of the developing lung is critical in establishing the distal airspaces; pulmonary blood vessels promote alveolar growth and development. Vascular endothelial growth factor (VEGF) has been linked to both normal vascular and parenchymal lung development (Figure 8), and, in experimental BPD models, disrupting VEGF signaling is associated with structural abnormalities.51 Abman has suggested that abnormal VEGF signaling may serve as a common pathway for injury associated with a variety of prenatal (e.g., chorioamnionitis, intrauterine growth retardation [IUGR], genetic susceptibility) and postnatal (e.g., mechanical ventilation, supplemental oxygen) factors that converge to produce decreased angiogenesis and alveolarization characteristic of BPD.51 For example, the abnormal VEGF signaling in the placenta of IUGR fetuses may also predispose to developing pulmonary hypertension postnatally, especially in the setting of BPD. Check reported that at least half of patients with BPD-associated pulmonary hypertension had a birth weight below the 25th percentile.52 In a recent review, Mourani and Abman noted that “pulmonary vascular disease secondary to disruption of normal pulmonary vascular development after preterm birth is an important determinant of the pathobiology of BPD and contributes significantly to morbidity and mortality.”53 The high incidence of pulmonary hypertension among infants with BPD supports the concept that impaired angiogenesis decreases alveolarization.
Figure 8.

Vascular endothelial growth factor (VEGF) signaling in the pathogenesis of neonatal pulmonary vascular disease. Blood vessels in the lung actively promote alveolar growth during development. VEGF contributes to normal lung development and early disruption leads to structural abnormalities. Lung angiogenesis promotes lung development (51).
Several recent reviews54–57 note that the incidence of pulmonary hypertension among ELBW infants ranges between 17–43% and mortality between 14–38%. Of importance, only 5% of those with severe BPD and pulmonary hypertension survive to two to three years of age. In a prospective study, Bhat58 reported pulmonary hypertension in 18% of a cohort of ELBW infants (24–27 weeks gestation). Similar to others, he noted that IUGR and severe BPD were highly associated with the diagnosis of pulmonary hypertension. Although 31% of infants were diagnosed by four weeks, 66% were not identified until three to four months. In 58%, pulmonary hypertension persisted to discharge from the intensive care nursery. Although the ELBW infant encounters the highest risk for BPD and associated pulmonary hypertension, accurately predicting outcomes in specific patients remains elusive; i.e. not all infants with severe BPD develop pulmonary hypertension, while others with milder BPD may develop pulmonary hypertension.
Bronchopulmonary Dysplasia: Incidence and Long term Outcomes
Outcomes in ELBW infants born between 2004–2007 demonstrate that even with optimal therapy (e.g., prenatal steroids, appropriate prenatal antibiotics, early treatment with surfactant, “gentle” ventilatory strategies, meticulous monitoring of oxygen administration), BPD persists as a major source of both short- and long-term morbidity.45 Stoll and colleagues noted that after a 24-week gestation, all infants incurred some degree of BPD (mild, 26%; moderate, 35%; severe, 39%), whereas in the 28-week gestation group, 39% developed BPD (mild, 16%; moderate, 15%; severe, 8%) (Table 5).
Table 5.
Incidence of Bronchopulmonary Dysplasia (%)
| 23 Weeks | 26 Weeks | 28 Weeks | Total | |
|---|---|---|---|---|
| Mild | 26 | 35 | 16 | 27 |
| Moderate | 35 | 26 | 15 | 23 |
| Severe | 39 | 17 | 8 | 18 |
After 23-weeks’ gestation, all infants incurred some degree of bronchopulmonary dysplasia (BPD) (mild, 26%; moderate, 35%; severe, 39%). Although still prevalent, BPD was less common in the 28-week gestation group (mild, 16%; moderate, 15%; severe, 8%). Incidence of BPD varies markedly among subgroups of infants based on gestational age. Data from (45).
Similar to findings from studies from earlier eras, airway obstruction currently persists as a common long-term outcome of the ELBW infant. Based on studies of 11-year olds from the Epicure cohort (<25 weeks gestation, born in 1995), Fawke50 noted that abnormally low pulmonary function (FEV1, FEF25–75, and FEV1/FVC more than 2 SD lower than predicted) occurred in 66% of ELBW infants with BPD compared to 32% of ELBW infants without BPD and 9% of term infants. Of significance, airway obstruction was only partially reversible with bronchodilators, especially in the ex-ELBW infants with BPD. Similarly, Joshi and colleagues also documented a higher incidence of exercise-induced, bronchodilator-responsive wheezing in eight-to-12 year old ex-prematures (<32 weeks gestation) with histories of BPD.59 Although airway obstruction and/or hyper-reactivity dominate the reported abnormal pulmonary outcome of ex-ELBW infants, decreased alveolar surface area and decreased effective pulmonary blood flow have also been noted.60, 61
In two distinct cohorts, Vollsæter et al. 62 reported FEV1 over time among three groups: term control, ex-ELBW infants with no BPD, and ex-ELBW with BPD in two separate cohorts. One cohort was studied at age 10 and 18 years (born between 1991–1995) and an older cohort at 18 and 25 years (born between 1982–1985) (Figure 9). In these two distinct cohorts of ELBW infants (e.g., later time period more likely to have received prenatal steroids and postnatal surfactant), Vollsaeter identified significant airway obstruction that tracked (i.e., no improvement in function) from age 10–18 in one group and between 18–25 years in the other. Thus, pulmonary injury associated with ELBW implies persistent dysfunction into adulthood that did not improve over time.
Figure 9.

Tracking of Forced expiratory volume in 1 s (FEV1) in normal and extremely low birth weight infants (with and without bronchopulmonary dysplasia [BPD]) into early adulthood. FEV1 in preterm-born subjects, including subjects with BPD and without BPD, compared to subjects born at term (62).
An analogous finding of “tracking of pulmonary dysfunction over time” was noted in the Tucson Children’s Respiratory Study, which followed a normal, unselected population (N=1246) from birth to 22 years, and reported that infants who were in the lowest quartile of function (FEV1, FEF25–75, FEV1/FVC) at birth remained in that quartile at 11, 16, and 22 years of age.63 Thus, factors that occur in utero or in the neonatal period may have pulmonary effects that persist into adult life. For example, Postma suggested that chronic lung disease (i.e., chronic obstructive pulmonary disease, “COPD”) may actually originate during fetal development.64
In summary, the lifelong pulmonary injury associated with prematurity is exaggerated in the setting of a history of BPD. The severity of pulmonary dysfunction seems to persist throughout life, with clear separation into two groups of ex-ELBW infants (those with vs. those without BPD), both of which are distinct from normal, full-term controls. In the adult, the pulmonary function of the ex-ELBW infant is abnormal compared to the age-matched ex-term infant, but not as severely abnormal as that associated with the ex-ELBW infant with BPD.59, 62 The airway obstruction of the ex-premature, especially with BPD, that persists into late childhood and early adulthood is commonly characterized by coughing and wheezing. Such airway hyper-responsiveness may be only partially responsive to treatment (i.e., bronchodilators, anti-inflammatory agents). The long-term outcome of pulmonary hypertension associated with BPD has not been completely defined. BPD may be “the earliest & perhaps the longest lasting lung disease in humans.”49
Although asthma and chronic lung disease of the ex-premature share common clinical features, each has a distinct underlying pathology and etiology. Instead of the eosinophil-mediated inflammation and atopy typical of asthma, the ex-premature’s recurrent broncho-obstructive symptoms result from abnormal growth and development of the architecture of the lung.47, 65 Instead of bronchoconstriction, small conductive airways may collapse during expiration because of inadequate supporting parenchyma. High-resolution computed tomography suggests that adolescent and young adult survivors of BPD have lung changes more closely resembling those of pulmonary emphysema than those seen in asthma.
Neurologic Outcomes
The neurologic sequelae of prematurity and its treatment impart life-long structural and functional impairments. Similar to the response of the lung, injury to the brain correlates highly with gestational age. Even with the dramatic changes in management over the last two decades (e.g., prenatal steroids, avoiding postnatal steroids, easy access to surfactant), the rates of neurodevelopmental abnormalities among ex-preterm infants remain high.66 For example, Kobaly and colleagues67 have documented that even with clinical practice changes between the years 2000–2003, neurodevelopmental outcomes (mental developmental index [MDI], psychomotor developmental [PDI] index) did not improve compared to earlier eras, even though the incidence of neurosensory impairment (e.g., deafness requiring aid, blindness, persistent hypo- or hypertonia) decreased. In addition, children with BPD continued to have poorer cognitive performance compared to children without BPD.
Vulnerability of the Preterm Brain to Injury
Pervasive and persistent neuropsychologlogical deficits are better understood in the context of normal in utero-third trimester brain development. During this period, dramatic cortical, dendritic, and axonal ramifications, as well as glial proliferation and differentiation occur, along with synaptogenesis and myelination. The combined effect of this neurodevelopment is a four- to five-fold increase in the volume of cortical grey and white matter.68 During the same time period of cortical development, there is a similar proliferation, growth, and migration of granule cells occur in the cerebellum.69 At 30 weeks gestation, the brain has achieved only half of its full-term weight70 (Figure 10), while the cerebellum has only reached 35–40% of its expected volume at 40 weeks71 (Figure 11).
Figure 10.
Normal Brain Growth. Brain weight from 20 to 40 (term) gestational weeks is expressed as a percent of term brain weight. For example, at 34 gestational weeks, the overall brain weight is 65% of term weight (70).
Figure 11.
Normal Cerebellar Growth. Note that cerebellar volume as a function of gestational age reveals the dramatic increase between 24 to 40 weeks’ gestation (71).
Similar to development of the lung, blood vessels in the brain develop in parallel with the parenchyma. During the last 16 weeks of gestation, the periventricular network expands with growth of long and short penetrator vessels and formation of extensive anastomoses. At mid-gestation, blood flow to the white matter is only 25% of that to the cortex. Of note, in the ELBW infant, cerebral blood flow is often passive, with more than 95% of ELBW infants demonstrating pressure passive flow at least 20–50% of the time.72 In recognizing the disturbed autoregulation in the ELBW infant, Greisen emphasizes that the lower limit of blood pressure associated with intact cerebral autoregulation is especially difficult to pinpoint,73 at least in part due to the enormous variability in “normal” blood pressure in ELBW infants. Consistent with these data, diffuse white matter injury is identified in >50% of ELBW infants. Deep grey matter growth failure has been noted to accompany white matter injury.74, 75 Perinatal injuries disrupt the coordinated growth of the whole brain,76, 77 thus lending a pathologic correlation and possible explanation of the diffuse deficits in higher (executive) cognitive function in survivors of ELBW.
Thus, as with advances in neonatal medicine focused on the avoiding lung injury, developments in supportive care of the central nervous system have contributed to improved survival for critically ill neonates. Nonetheless, similar to the persistent high rate of chronic lung disease, this vulnerable population still remains at high risk for brain injury and consequent adverse outcomes, including cerebral palsy, cognitive disabilities, and epilepsy.78
Volpe emphasizes that “brain injury and impaired brain development” are “inextricably intertwined.”77 Similar to other investigators,36, 38 he notes that injury at a critical period of rapid development and growth interferes with establishing the intricate microstructural connections throughout the brain (e.g., between basal ganglia and cortex, thalamus and cortex, cerebellum and cortex, pons and cortex, and intracortical). Analogous to long-term pulmonary dysfunction, structural abnormalities of the ELBW infant’s brain persist into late childhood, adolescence, and adulthood and correlate with neuropsychological abnormalities. Decreased volumes of the frontal and temporal lobes and the caudate and/or cerebellum have been associated with functional abnormalities. Of importance, disturbed connectivity associated with cognitive impairment increases the risk for inattention and/or hyperactivity, anxiety, and other social and emotional problems.79, 80
Neurodevelopmental disability related to cognition is often categorized with the Bayley Scales of Infant Development, a tool used to quantify developmental outcome during the first three years of life.21, 29, 81 Cognitive delay, as defined by mean Bayley scores and the proportion of subjects scoring below 70, is increased in extremely premature infants. Pooled estimates from large population-based cohort studies conducted through the late 1990s to the early 2000s suggest an increasing incidence of cognitive delay, with the risk in some studies approaching 50%. However, pooled estimates from large observational studies suggest a risk of 25.2% (95% CI: 20.4–30.5)14, 20–43, 82 (Figure 12).
Figure 12.
Bayley Developmental Index. The Bayley score is a developmental test based on a play behavioral examination. The raw score is converted to a scale, which in turn can be referenced to normalized values. The pink shaded area represents the normal range (between 84–115). Scores below the red dashed line, at 70, indicate severe developmental disability, while scores above the green line indicate superior performance. When Bayley scores are used to quantify developmental outcomes, the point estimate, or effect size, is represented by the mean score ± standard deviation, or alternatively, the proportion of infants who score below a score of 70 can be used as the metric. Graphic drawn from data of Hack M. et al. Pediatrics 1996; 98: 931–937 (21).
The early reports from the NICHD cohort (births <32 weeks between 1993–1998) reported that periventricular leukomalacia, BPD, multiple births, male gender, non-white race, maternal education less than 12 years, and IVH were risk factors associated with cognitive delay. Postnatal steroids increased the risk and antenatal steroids decreased the risk of neurodevelopmental disability.83 Other collaborative studies of long-term neurocognitive outcome involving multiple cohorts from various eras confirm that ex-ELBW infants who reached school age scored lower scores on all cognitive and academic scales,28, 66, 84, 85 including abnormalities of both attention and emotional problems. Even in the 2004 cohort,28 although most patients had received antenatal steroids, postnatal surfactant, magnesium, and caffeine, 38% developed BPD and 3.7% incurred severe IVH (grade III, IV). In a more recent cohort, Kumar et al.84 noted that although 33% of ex-ELBW infants were categorized as having an “unimpaired outcome” at 30 months, Mental Developmental Index (MDI) scores were markedly skewed toward the low end of normal. That is, only 17% of these infants had an MDI>101, suggesting that this group with “normal cognitive function” were in fact disadvantaged compared to the control group of ex-term infants.
The neuropsychological sequelae of preterm birth have been noted to persist into adolescence and adulthood. Based on data from the Multicenter Randomized Indomethacin IVH Prevention Trial, Luu and colleagues noted that adolescents had an increased incidence of deficits in “executive function,” which are high-level mental activities that correlate with ability to regulate behavior and cognition for goal-directed activity (e.g., verbal fluency inhibition, cognitive flexibility).85
Cerebral Palsy and the Preterm Infant
Cerebral palsy (CP) is a non-progressive motor disability that results in movement disorders such as plegias, spasticity, and dystonia. The exact cause is unknown but prematurity is significantly associated with CP and it affects 2.5 out of every 1,000 children in the United States.86 The injury may occur in utero, at birth, or after birth up to about three years of age.86, 87 Children born prematurely are at an increased risk, and comprise about half of those affected. CP is often used as a marker for overall neurologic outcomes in association with prematurity.88
Observational studies from the 1980s through middle of the 1990s suggested that the occurrence of CP was increasing in association with increased survival of extremely premature babies. However, registry data from studies in Europe, Australia, and Canada89, 90 indicate that since the late 1990s, the incidence of CP has decreased.89, 90 Available pooled data from cohort studies (14, 20–43, 82) used to examine survival suggest the risk of CP in this patient population is 10.4% (95% CI: 7.3–13.4%) However, more recent data from three large international randomized controlled titrated oxygen therapy trials14–16 suggests that the risk is lower than 6% (95% CI: 6.7–7.7%). A study conducted by the NICHD noted that periventricular leukomalacia, severe IVH (grade 3–4), administration of postnatal steroids, and male gender were risk factors associated with the development of CP, whereas the use of antenatal steroids was associated with a decreased risk.83
Neonatal Neurocritical Intensive Care and Neuroimaging
Neonatal neurocritical care has developed in response to goals of care for the preterm infant shifting from a single-minded focus on cardiopulmonary support to optimization of neurodevelopmental outcomes. Along with this focused brain care, advanced brain imaging has become the standard of care to facilitate diagnosis for both congenital and acquired conditions and to improve predicting prognosis.91, 92 Ultrasound, magnetic resonance imaging, and computed tomography are the three most commonly used imaging modalities.
Brain injury in preterm neonates admitted to a neurocritical care unit may present clinically as encephalopathy (8%), seizures (31%), or a precipitous decrease in hematocrit in the case of IVH (27%).93 Although encephalopathy and seizures are common presenting signs, some neonates are asymptomatic. With the advent of improved imaging technology, unsuspected brain injury or developmental anomalies are often identified on routine imaging (Table 6), most commonly on screening ultrasound (US). In fact, US is recommended for all neonates at high risk (ie. <30 weeks gestational age)94 to screen for intracranial pathologies (e.g., IVH, periventricular hemorrhagic infarct, ventriculomegaly, large cerebellar hemorrhages, and cystic white matter injury).94–96 Since it is readily performed at the bedside, US is often the initial diagnostic modality and is therefore easily performed urgently when critical neurosurgical conditions (e.g., posterior fossa bleed or hydrocephalus), are suspected, or when more definitive imaging by MRI must be delayed.78 Of interest, US-detectable injury correlates with later development of cerebral palsy.
Table 6.
Neonatal clinical conditions that present high risk for brain injury or developmental anomalies and may require detailed magnetic resonance brain imaging to determine diagnosis and prognosis
| Suspected acute acquired brain injury |
| • Encephalopathy |
| • Seizures |
| • Intracranial infection |
| • Inborn error of metabolism |
| Abnormal ultrasound findings |
| • High grade intraventricular hemorrhage |
| • Suspected parenchymal injury |
| • Ventriculomegaly or suspected malformation |
| Abnormal examination |
| • Hypotonia or hypertonia |
| • Multiple congenital defects or dysmorphic features |
| • Microcephaly or macrocephlay |
| High risk for brain injury |
| • Congenital heart defect, especially during and after corrective surgery |
| • Postnatal cardiopulmonary arrest |
| • Central nervous system vascular malformation |
| • Symptomatic hypoglycemia |
| • Extreme prematurity (<28 weeks gestation at birth) |
| • Need for extracorporeal membrane oxygenation (ECMO) |
MRI is more sensitive and specific than US for defining both qualitative and quantitative data about the developing and injured brain relevant for detecting subtle pathologies associated with cognitive impairment, including focal, non-cystic white matter injury, diffuse white matter injury, and small cerebellar hemorrhages.97–104 Based on increasing evidence that MRI-detected injury may be more accurate than ultrasound for defining diagnosis and in estimating prognosis,105, 106 some experts recommend imaging for all children born <28 weeks gestation;107 however, routine use of MRI for all preterm neonates remains controversial.
MRI can be accomplished without sedation by feeding and swaddling and using a beanbag positioning device.108 However, in spite of careful preparation, at least 25–30% of newborns require sedation or general anesthesia to achieve high quality, motion-free images. While it is recognized that anesthetizing these infants for imaging studies poses risks and challenges, the information often assists primary care takers and family members with management decisions.
Computed tomography (CT) requires a relatively high dose of radiation due to the high water content of the neonatal brain, especially the premature. Although some guidelines recommend CT in case of suspected intracranial hemorrhage,95 many experts recommend avoiding the risks of radiation exposure, especially when MRI is available.109
Protecting the Newborn Brain
Prompt access to resuscitation and initiation of supportive care during the high-risk perinatal period (i.e., labor, birth, and postnatal) are essential for protecting the developing brain. Guidelines by the International Liaison Committee on Resuscitation (ILCOR) and the Neonatal Resuscitation Program (NRP) guide initial resuscitation.110 Principles for maintaining normal cardiopulmonary function during the initial resuscitation are relevant to neonates at high risk for brain injury, since hemodynamic stability is essential to prevent secondary brain injury (Table 7) and adverse postoperative outcomes.111
Table 7.
Supportive care for the at-risk developing brain
| Supportive Care | Principles | Consequence of Mismanagement |
|---|---|---|
| Oxygenation | Avoid hyperoxia |
|
| Ventilation | Avoid hypocapnea |
|
| Circulatory support | Maintain stable physiologic blood pressure |
|
| Temperature control | Maintain normothermia |
|
| Glucose management | Maintain physiologic glucose levels |
|
Inducing mild hypothermia (whole-body cooling to 33.5°C ± 0.5°C, or selective head cooling) for 72 hours initiated within six hours of birth among term neonates with signs of perinatal asphyxia and encephalopathy has been reported to reduce brain injury,112, 113 risk of death, and/or major developmental disability at age 18 (risk ratio [RR] 0.76; 95% CI, 0.69–0.84). Furthermore, hypothermia increases the rate of survival with normal neurological function (RR 1.63; 1.36–1.95).114 Current investigations are underway to examine the therapeutic effect of late-onset hypothermia (after six hours), of cooling preterm neonates (33–35 weeks gestational age), and longer (120 hours) or deeper (32 °C) cooling. In addition, a number of promising neuroprotective agents (erythropoietin and darbepoitin, melatonin, xenon, topiramate, allopurinol, magnesium sulfate, stem cells) are being investigated in animal models and clinical trials.
Summary
In conclusion, premature birth is a significant cause of infant and child morbidity and mortality. In the United States, the premature birth rate, which had steadily increased during the 1990s and early 2000s, has decreased annually for four years and is now approximately 11.5%. Human viability, defined as gestational age at which the chance of survival is 50%, is now approximately 23–24 weeks in developed countries. Infant girls, on average, have better outcomes than infant boys. A relatively uncomplicated course in the intensive care nursery for an extremely premature infant results in a discharge date close to the prenatal EDC. Despite technological advances and efforts of child health experts during the last generation, the extremely premature infant (less than 28 weeks gestation) and extremely low birth weight infant (ELBW) (< 1000 grams) remain at high risk for death and disability with 30–50% mortality and, in survivors, at least 20–50% risk of morbidity. The introduction of CPAP, mechanical ventilation, and exogenous surfactant increased survival and spurred the development of neonatal intensive care in the 1970s through the early 1990s. Routine administration of antenatal steroids during premature labor improved neonatal mortality and morbidity in the late 1990s. The recognition that chronic postnatal administration of steroids to infants should be avoided may have improved outcomes in the early 2000s. Evidence from recent trials attempting to define the appropriate target for oxygen saturation in preterm infants suggests that arterial oxygen saturation between 91–95% (compared to 85–89%) avoids excess mortality. However, final analyses of data from these trials have not been published, so definitive recommendations are still pending The development of neonatal neurocognitive care visits may improve neurocognitive outcomes in this high-risk group. Long-term follow up to detect and address developmental, learning, behavioral, and social problems is critical for children born at these early gestational ages.
The striking similarities in response to extreme prematurity in the lung and brain imply that agents and techniques that benefit one organ are likely to also benefit the other. Finally, since therapy and supportive care continue to change, the outcomes of ELBW infants are ever evolving. Efforts to minimize injury, preserve growth, and identify interventions focused on antioxidant and anti-inflammatory pathways are now being evaluated. Thus, treating and preventing long-term deficits must be developed in the context of a “moving target.”
Acknowledgments
Funding: Hannah Glass, MDCM, MAS receives support from K23 NS066137 and the Neonatal Brain Research Center.
Footnotes
See Disclosures at end of article for Author Conflicts of Interest.
Reprints will not be available from the authors.
Much of this material was discussed and presented as a part of a SPA meeting last October in San Francisco.
DISCLOSURES:
Name: Hannah C. Glass, MDCM, MAS
Contribution: The author helped write the manuscript.
Attestation: This author approved the final manuscript.
Conflicts of Interest: Hannah Glass, MDCM, MAS receives support from K23 NS066137 and the Neonatal Brain Research Center.
Name: Andrew T. Costarino, MD
Contribution: The author helped write the manuscript.
Attestation: This author approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Stephen A. Stayer, MD
Contribution: The author helped write the manuscript.
Attestation: This author approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Claire Brett, MD
Contribution: The author helped write the manuscript.
Attestation: This author approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Franklyn Cladis, MD, FAAP
Contribution: The author helped write the manuscript.
Attestation: This author approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Peter J. Davis, MD, FAAP
Contribution: The author helped write the manuscript.
Attestation: This author approved the final manuscript.
Conflicts of Interest: The author has no conflicts of interest to declare.
Contributor Information
Hannah C. Glass, Department of Pediatrics, UCSF Benioff Children’s Hospital, San Francisco, California.
Andrew T. Costarino, Jefferson Medical College, Thomas Jefferson University, Philadelphia, Pennsylvania; Department of Pediatric Anesthesiology, The Alfred I. duPont Hospital for Children, Wilmington, Delaware.
Stephen A. Stayer, Baylor College of Medicine, Texas Children’s Hospital, Houston, Texas.
Claire Brett, Department of Anesthesiology and Perioperative Care, University of California, San Francisco, San Francisco, California.
Franklyn Cladis, Children’s Hospital of Pittsburgh of UPMC, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
Peter J. Davis, Children’s Hospital of Pittsburgh of UPMC, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
References
- 1.Organization WH. Preterm birth fact sheet No363. [Google Scholar]
- 2.World Health Organization. March of Dimes; The Partnership for Maternal NCHStCBtstgaropb 12/13/13. [Google Scholar]
- 3.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics. 2013;131(3):548–58. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engle WA. Age terminology during the perinatal period. Pediatrics. 2004;114(5):1362–4. doi: 10.1542/peds.2004-1915. [DOI] [PubMed] [Google Scholar]
- 6.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445–6. doi: 10.1001/jama.2013.6235. [DOI] [PubMed] [Google Scholar]
- 7.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New interuterine growth curves based on United States data. Pediatrics. 2010;125:e214. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data 2012. National Vital Statistics. 2013:1–19. [PubMed] [Google Scholar]
- 9.Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med. 1971;284(24):1333–40. doi: 10.1056/NEJM197106172842401. [DOI] [PubMed] [Google Scholar]
- 10.Hirata T, Epcar JT, Walsh A, Mednick J, Harris M, McGinnis MS, Sehring S, Papedo G. Survival and outcome of infants 501 to 750 gm: a six-year experience. J Pediatr. 1983;102(5):741–8. doi: 10.1016/s0022-3476(83)80250-9. [DOI] [PubMed] [Google Scholar]
- 11.Hirata T, Epcar JT, Walsh A, Mednick J, Harris M, McGinnis MS, Sehring S, Papedo G. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350(13):1304–13. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 12.O’Shea TM, Washburn LK, Nixon PA, Goldstein DJ. Follow-up of a randomized, placebo-controlled trial of dexamethasone to decrease the duration of ventilator dependency in very low birth weight infants: neurodevelopmental outcomes at 4 to 11 years of age. Pediatrics. 2007;120(3):594–602. doi: 10.1542/peds.2007-0486. [DOI] [PubMed] [Google Scholar]
- 13.Kersbergen KJ, de Vries LS, van Kooij BJ, Isgum I, Rademaker KJ, van Bel F, Huppi PS, Dubois J, Groenendaal F, Benders MJ. Hydrocortisone treatment for bronchopulmonary dysplasia and brain volumes in preterm infants. J Pediatr. 2013;163(3):666–71 e1. doi: 10.1016/j.jpeds.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Vaucher YE, Peralta-Carcelen M, Finer NN, Carlo WA, Gantz MG, Walsh MC, Laptook AR, Yoder BA, Faix RG, Das A, Schibler K, Rich W, Newman NS, Vohr BR, Yolton K, Heyne RJ, Wilson-Costello DE, Evans PW, Goldstein RF, Acarregui MJ, Adams-Chapman I, Pappas A, Hintz SR, Poindexter B, Dusick AM, McGowan EC, Ehrenkranz RA, Bodnar A, Bauer CR, Fuller J, O’Shea TM, Myers GJ, Higgins RD. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367(26):2495–504. doi: 10.1056/NEJMoa1208506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, Solimano A, Roberts RS Canadian Oxygen Trial (COT) Group. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309(20):2111–20. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 16.Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, Battin M, Bowler U, Broadbent R, Cairns P, Davis PG, Deshpande S, Donoghoe M, Doyle L, Fleck BW, Ghadge A, Hague W, Halliday HL, Hewson M, King A, Kirby A, Marlow N, Meyer M, Morley C, Simmer K, Tin W, Wardle SP, Brocklehurst P. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368(22):2094–104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- 17.Darlow BA, Marschner SL, Donoghoe M, Battin MR, Broadbent RS, Elder MJ, Hewson MP, Meyer MP, Ghadge A, Graham P, McNeill NJ, Kuschel CA, Tarnow-Mordi WO. Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. J Pediatr. 2014;165(1):30–5 e2. doi: 10.1016/j.jpeds.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt B, Whyte RK, Roberts RS. Trade-off between lower or higher oxygen saturations for extremely preterm infants: the first benefits of oxygen saturation targeting (BOOST) II trial reports its primary outcome. J Pediatr. 2014;165(1):6–8. doi: 10.1016/j.jpeds.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Vento M. Oxygen supplementation in the neonatal period: changing the paradigm. Neonatology. 2014;105(4):323–31. doi: 10.1159/000360646. [DOI] [PubMed] [Google Scholar]
- 20.Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics. 1991;87(5):587–97. [PubMed] [Google Scholar]
- 21.Hack M, Friedman H, Fanaroff AA. Outcomes of extremely low birth weight infants. Pediatrics. 1996;98(5):931–7. [PubMed] [Google Scholar]
- 22.Outcome at 2 years of children 23–27 weeks’ gestation born in Victoria in 1991–92. The Victorian Infant Collaborative Study Group. J Paediatr Child Health. 1997;33(2):161–5. [PubMed] [Google Scholar]
- 23.Tin W, Wariyar U, Hey E. Changing prognosis for babies of less than 28 weeks’ gestation in the north of England between 1983 and 1994. Northern Neonatal Network. BMJ. 1997;314(7074):107–11. doi: 10.1136/bmj.314.7074.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton L, Bajuk B. Population-based study of infants born at less than 28 weeks’ gestation in New South Wales, Australia, in 1992–3. New South Wales Neonatal Intensive Care Unit Study Group. Paediatr Perinat Epidemiol. 1999;13(3):288–301. doi: 10.1046/j.1365-3016.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 25.Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD. Changes in neurodevelopmental outcomes at 18 to 22 months’ corrected age among infants of less than 25 weeks’ gestational age born in 1993–1999. Pediatrics. 2005;115(6):1645–51. doi: 10.1542/peds.2004-2215. [DOI] [PubMed] [Google Scholar]
- 26.Draper ES, Manktelow B, Field DJ, James D. Prediction of survival for preterm births by weight and gestational age: retrospective population based study. BMJ. 1999;319(7217):1093–7. doi: 10.1136/bmj.319.7217.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamper J, Feilberg Jorgensen N, Jonsbo F, Pedersen-Bjergaard L, Pryds O. The Danish national study in infants with extremely low gestational age and birthweight (the ETFOL study): respiratory morbidity and outcome. Acta Paediatr. 2004;93(2):225–32. doi: 10.1080/08035250310022298. [DOI] [PubMed] [Google Scholar]
- 28.Hakansson S, Farooqi A, Holmgren PA, Serenius F, Hogberg U. Proactive management promotes outcome in extremely preterm infants: a population-based comparison of two perinatal management strategies. Pediatrics. 2004;114(1):58–64. doi: 10.1542/peds.114.1.58. [DOI] [PubMed] [Google Scholar]
- 29.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343(6):378–84. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 30.den Ouden ALAS. Death rates in very preterm babies. Tijdschr Kindergeneeskd. 68:241–6. [Google Scholar]
- 31.Tommiska V, Heinonen K, Lehtonen L, Renlund M, Saarela T, Tammela O, Virtanen M, Fellman V. No improvement in outcome of nationwide extremely low birth weight infant populations between 1996–1997 and 1999–2000. Pediatrics. 2007;119(1):29–36. doi: 10.1542/peds.2006-1472. [DOI] [PubMed] [Google Scholar]
- 32.Doyle LW. Neonatal intensive care at borderline viability--is it worth it? Early Hum Dev. 2004;80(2):103–13. doi: 10.1016/j.earlhumdev.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Larroque B, Breart G, Kaminski M, Dehan M, Andre M, Burguet A, Grandjean H, Ledesert B, Leveque C, Maillard F, Matis J, Roze JC, Truffert P. Survival of very preterm infants: Epipage, a population based cohort study. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F139–44. doi: 10.1136/adc.2002.020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanhaesebrouck P, Allegaert K, Bottu J, Debauche C, Devlieger H, Docx M, Francois A, Haumont D, Lombet J, Rigo J, Smets K, Vanherreweghe I, Van Overmeire B, Van Reempts P. The EPIBEL study: outcomes to discharge from hospital for extremely preterm infants in Belgium. Pediatrics. 2004;114(3):663–75. doi: 10.1542/peds.2003-0903-L. [DOI] [PubMed] [Google Scholar]
- 35.Markestad T, Kaaresen PI, Ronnestad A, Reigstad H, Lossius K, Medbo S, Zanussi G, Engelund IE, Skjaerven R, Irgens LM. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics. 2005;115(5):1289–98. doi: 10.1542/peds.2004-1482. [DOI] [PubMed] [Google Scholar]
- 36.Hintz SR, Kendrick DE, Wilson-Costello DE, Das A, Bell EF, Vohr BR, Higgins RD. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics. 2011;127(1):62–70. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutz P, Horsch S, Kuhn L, Roll C. Single-centre vs. population-based outcome data of extremely preterm infants at the limits of viability. Acta Paediatr. 2009;98(9):1451–5. doi: 10.1111/j.1651-2227.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 38.Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. 2010;97(4):329–38. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serenius F, Kallen K, Blennow M, Ewald U, Fellman V, Holmstrom G, Lindberg E, Lundqvist P, Marsal K, Norman M, Olhager E, Stigson L, Stjernqvist K, Vollmer B, Stromberg B. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309(17):1810–20. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 40.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M. Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics. 2013;132(1):62–71. doi: 10.1542/peds.2012-2857. [DOI] [PubMed] [Google Scholar]
- 41.Doyle LW, Roberts G, Anderson PJ. Outcomes at age 2 years of infants < 28 weeks’ gestational age born in Victoria in 2005. J Pediatr. 2010;156(1):49–53 e1. doi: 10.1016/j.jpeds.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Delobel-Ayoub M, Kaminski M, Marret S, Burguet A, Marchand L, N’Guyen S, Matis J, Thiriez G, Fresson J, Arnaud C, Poher M, Larroque B. Behavioral outcome at 3 years of age in very preterm infants: the EPIPAGE study. Pediatrics. 2006;117(6):1996–2005. doi: 10.1542/peds.2005-2310. [DOI] [PubMed] [Google Scholar]
- 43.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 44.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358(16):1700–11. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 45.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, 3rd, Watterberg KL, Saha S, Das A, Higgins RD. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol. 2012;8(5):265–74. doi: 10.1038/nrneph.2012.38. [DOI] [PubMed] [Google Scholar]
- 47.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–55. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 48.Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology. 2009;95(4):353–61. doi: 10.1159/000209301. [DOI] [PubMed] [Google Scholar]
- 49.Carraro S, Filippone M, Da Dalt L, Ferraro V, Maretti M, Bressan S, El Mazloum D, Baraldi E. Bronchopulmonary dysplasia: the earliest and perhaps the longest lasting obstructive lung disease in humans. Early Hum Dev. 2013;89 (Suppl 3):S3–5. doi: 10.1016/j.earlhumdev.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 50.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182(2):237–45. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. 2010;661:323–35. doi: 10.1007/978-1-60761-500-2_21. [DOI] [PubMed] [Google Scholar]
- 52.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33(7):553–7. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013;25(3):329–37. doi: 10.1097/MOP.0b013e328360a3f6. [DOI] [PubMed] [Google Scholar]
- 54.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):124–31. doi: 10.1053/j.semperi.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collaco JM, Romer LH, Stuart BD, Coulson JD, Everett AD, Lawson EE, Brenner JI, Brown AT, Nies MK, Sekar P, Nogee LM, McGrath-Morrow SA. Frontiers in pulmonary hypertension in infants and children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2012;47(11):1042–53. doi: 10.1002/ppul.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinhorn RH, Kinsella JP, Abman SH. Beyond pulmonary hypertension: sildenafil for chronic lung disease of prematurity. Am J Respir Cell Mol Biol. 2013;48(2):iii–v. doi: 10.1165/rcmb.2012-0441ED. [DOI] [PubMed] [Google Scholar]
- 57.Baker CD, Abman SH, Mourani PM. Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Pediatr Allergy Immunol Pulmonol. 2014;27(1):8–16. doi: 10.1089/ped.2013.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129(3):e682–9. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joshi S, Powell T, Watkins WJ, Drayton M, Williams EM, Kotecha S. Exercise-induced bronchoconstriction in school-aged children who had chronic lung disease in infancy. J Pediatr. 2013;162(4):813–8 e1. doi: 10.1016/j.jpeds.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 60.Lum S, Kirkby J, Welsh L, Marlow N, Hennessy E, Stocks J. Nature and severity of lung function abnormalities in extremely pre-term children at 11 years of age. Eur Respir J. 2011;37(5):1199–207. doi: 10.1183/09031936.00071110. [DOI] [PubMed] [Google Scholar]
- 61.Narang I, Bush A, Rosenthal M. Gas transfer and pulmonary blood flow at rest and during exercise in adults 21 years after preterm birth. Am J Respir Crit Care Med. 2009;180(4):339–45. doi: 10.1164/rccm.200809-1523OC. [DOI] [PubMed] [Google Scholar]
- 62.Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013;68(8):767–76. doi: 10.1136/thoraxjnl-2012-202980. [DOI] [PubMed] [Google Scholar]
- 63.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370(9589):758–64. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Postma DSBA, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2014 doi: 10.1016/S0140-6736(14)60446-3. [DOI] [PubMed] [Google Scholar]
- 65.Filippone M, Carraro S, Baraldi E. The term “asthma” should be avoided in describing the chronic pulmonary disease of prematurity. Eur Respir J. 2013;42(5):1430–1. doi: 10.1183/09031936.00055913. [DOI] [PubMed] [Google Scholar]
- 66.Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131(4):e1053–61. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- 67.Kobaly K, Schluchter M, Minich N, Friedman H, Taylor HG, Wilson-Costello D, Hack M. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121(1):73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- 68.Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43(2):224–35. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 69.Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–95. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 70.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81–8. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24(9):1085–104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61(4):467–73. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 73.Greisen G. To autoregulate or not to autoregulate--that is no longer the question. Semin Pediatr Neurol. 2009;16(4):207–15. doi: 10.1016/j.spen.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, Maalouf E, Duggan P, Ajayi-Obe M, Hajnal J, Allsop JM, Boardman J, Rutherford MA, Cowan F, Edwards AD. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, Hajnal J, Allsop JM, Rutherford MA, Edwards AD. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 2006;32(1):70–8. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 76.McQuillen PS, Ferriero DM. Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol. 2005;15(3):250–60. doi: 10.1111/j.1750-3639.2005.tb00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Volpe JJ. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16(4):167–78. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volpe J. Neurology of the Newborn. 4. Philadelphia: WB Saunders; 2008. [Google Scholar]
- 79.Counsell SJ, Boardman JP. Differential brain growth in the infant born preterm: current knowledge and future developments from brain imaging. Semin Fetal Neonatal Med. 2005;10(5):403–10. doi: 10.1016/j.siny.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(5 Pt 2):11R–8R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- 81.Bayley N. Bayley Scales of Infant Development. 2. Psychological Corp; San Antonio, TX: 1993. [Google Scholar]
- 82.De Groote I, Vanhaesebrouck P, Bruneel E, Dom L, Durein I, Hasaerts D, Laroche S, Oostra A, Ortibus E, Roeyers H, van Mol C. Outcome at 3 years of age in a population-based cohort of extremely preterm infants. Obstet Gynecol. 2007;110(4):855–64. doi: 10.1097/01.AOG.0000284447.43442.55. [DOI] [PubMed] [Google Scholar]
- 83.Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–43. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 84.Kumar P, Shankaran S, Ambalavanan N, Kendrick DE, Pappas A, Vohr BR, Poindexter BB, Das A, Higgins RD. Characteristics of extremely low-birth-weight infant survivors with unimpaired outcomes at 30 months of age. J Perinatol. 2013;33(10):800–5. doi: 10.1038/jp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luu TM, Ment L, Allan W, Schneider K, Vohr BR. Executive and memory function in adolescents born very preterm. Pediatrics. 2011;127(3):e639–46. doi: 10.1542/peds.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.CDC. Cerebral Palsy. Centers for Disease Control and Prevention; [Accessed 12/14/13]. http://www.cdc.gov/ncbddd/cp/facts.html. [Google Scholar]
- 87.Nelson KB. Can we prevent cerebral palsy? N Engl J Med. 2003;349(18):1765–9. doi: 10.1056/NEJMsb035364. [DOI] [PubMed] [Google Scholar]
- 88.Hack M, Costello DW. Decrease in frequency of cerebral palsy in preterm infants. Lancet. 2007;369(9555):7–8. doi: 10.1016/S0140-6736(07)60006-3. [DOI] [PubMed] [Google Scholar]
- 89.Reid SM, Carlin JB, Reddihough DS. Rates of cerebral palsy in Victoria, Australia, 1970 to 2004: has there been a change? Dev Med Child Neurol. 2011;53(10):907–12. doi: 10.1111/j.1469-8749.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- 90.Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, Krageloh-Mann I. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369(9555):43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- 91.Bonifacio SL, Glass HC, Peloquin S, Ferriero DM. A new neurological focus in neonatal intensive care. Nat Rev Neurol. 2011;7(9):485–94. doi: 10.1038/nrneurol.2011.119. [DOI] [PubMed] [Google Scholar]
- 92.de Vries LS, van Haastert IC, Benders MJ, Groenendaal F. Myth: cerebral palsy cannot be predicted by neonatal brain imaging. Semin Fetal Neonatal Med. 2011;16(5):279–87. doi: 10.1016/j.siny.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Glass HC, Bonifacio SL, Peloquin S, Shimotake T, Sehring S, Sun Y, Sullivan J, Rogers E, Barkovich AJ, Rowitch D, Ferriero DM. Neurocritical care for neonates. Neurocrit Care. 2010;12(3):421–9. doi: 10.1007/s12028-009-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144(6):815–20. doi: 10.1016/j.jpeds.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 95.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, Pinto-Martin J, Rivkin M, Slovis TL. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726–38. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 96.Perlman JM, Rollins N. Surveillance protocol for the detection of intracranial abnormalities in premature neonates. Arch Pediatr Adolesc Med. 2000;154(8):822–6. doi: 10.1001/archpedi.154.8.822. [DOI] [PubMed] [Google Scholar]
- 97.Leijser LM, de Bruine FT, van der Grond J, Steggerda SJ, Walther FJ, van Wezel-Meijler G. Is sequential cranial ultrasound reliable for detection of white matter injury in very preterm infants? Neuroradiology. 2010;52(5):397–406. doi: 10.1007/s00234-010-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Partridge JC, Vigneron DB, Barkovich AJ. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24(8):1661–9. [PMC free article] [PubMed] [Google Scholar]
- 99.Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24(5):805–9. [PMC free article] [PubMed] [Google Scholar]
- 100.Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. 2009;252(1):190–9. doi: 10.1148/radiol.2521081525. [DOI] [PubMed] [Google Scholar]
- 101.Tam EW, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, Glass HC, Piecuch RE, Barkovich AJ. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr. 2011;158(2):245–50. doi: 10.1016/j.jpeds.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El-Dib M, Massaro AN, Bulas D, Aly H. Neuroimaging and neurodevelopmental outcome of premature infants. Am J Perinatol. 2010;27(10):803–18. doi: 10.1055/s-0030-1254550. [DOI] [PubMed] [Google Scholar]
- 103.Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol. 2011;36(1):22–41. doi: 10.1080/87565641.2011.540530. [DOI] [PubMed] [Google Scholar]
- 104.Maalouf EF, Duggan PJ, Counsell SJ, Rutherford MA, Cowan F, Azzopardi D, Edwards AD. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107(4):719–27. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 105.Counsell SJ, Tranter SL, Rutherford MA. Magnetic resonance imaging of brain injury in the high-risk term infant. Semin Perinatol. 2010;34(1):67–78. doi: 10.1053/j.semperi.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 106.Barkovich AJ. MR imaging of the neonatal brain. Neuroimaging Clin N Am. 2006;16(1):117–35. viii–ix. doi: 10.1016/j.nic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Mirmiran M, Barnes PD, Keller K, Constantinou JC, Fleisher BE, Hintz SR, Ariagno RL. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics. 2004;114(4):992–8. doi: 10.1542/peds.2003-0772-L. [DOI] [PubMed] [Google Scholar]
- 108.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38(3):260–4. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 109.Brenner DJ. Should we be concerned about the rapid increase in CT usage? Rev Environ Health. 2010;25(1):63–8. doi: 10.1515/reveh.2010.25.1.63. [DOI] [PubMed] [Google Scholar]
- 110.Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, Guinsburg R, Hazinski MF, Morley C, Richmond S, Simon WM, Singhal N, Szyld E, Tamura M, Velaphi S. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126(5):e1319–44. doi: 10.1542/peds.2010-2972B. [DOI] [PubMed] [Google Scholar]
- 111.McCann ME, Schouten AN, Dobija N, Munoz C, Stephenson L, Poussaint TY, Kalkman CJ, Hickey PR, de Vries LS, Tasker RC. Infantile postoperative encephalopathy: perioperative factors as a cause for concern. Pediatrics. 2014;133(3):e751–7. doi: 10.1542/peds.2012-0973. [DOI] [PubMed] [Google Scholar]
- 112.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonifacio SL, Glass HC, Vanderpluym J, Agrawal AT, Xu D, Barkovich AJ, Ferriero DM. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr. 2011;158(3):360–5. doi: 10.1016/j.jpeds.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 115.Smith LJ, McKay KO, van Asperen PP, Selvadurai H, Fitzgerald DA. Normal development of the lung and premature birth. Paediatr Respir Rev. 2010;11(3):135–42. doi: 10.1016/j.prrv.2009.12.006. [DOI] [PubMed] [Google Scholar]