Abstract
Reactive oxygen species (ROS) have been implicated in a variety of age-related diseases including multiple cardiovascular disorders. However, translation of ROS scavengers (anti-oxidants) into the clinic has not been successful. These anti-oxidants grossly reduce total levels of cellular ROS including ROS that participate in physiological signaling. In this review, we challenge the traditional anti-oxidant therapeutic approach that targets ROS directly with novel approaches that improve mitochondrial functions to more effectively treat cardiovascular diseases.
Keywords: Mitochondria, reactive oxygen species (ROS), fission, mitophagy, protein kinase C (PKC)
INTRODUCTION
The heart is a tissue rich in mitochondria with approximately 30% of cardiomyocyte volume occupied by these ATP-generating organelles.1 The large mitochondrial mass also orchestrates a myriad of signaling pathways related to metabolite generation, stress response and cell death. Thus, it is not surprising that mitochondrial dysfunction is implicated in numerous cardiovascular-related diseases,2 making mitochondria attractive therapeutic targets.
As hubs of cellular signaling cascades, mitochondria participate in a diverse array of pathways that are clinically relevant. For example, the contribution of mitochondria to intracellular Ca2+ is necessary for proper myocardial contraction and relaxation.3 In addition, mitochondria are master regulators of cell life versus death decisions. Opening of the mitochondrial permeability transition pore (mPTP) as well as the subsequent collapse of the proton gradient and matrix swelling lead to the release of pro-apoptotic proteins. Such opening of the mPTP is implicated during ischemia/reperfusion (IR) injury.4 Finally, not only are mitochondria major producers of ROS, they are also the main target of ROS-induced signaling. In this review, we focus on mitochondria and ROS-related mechanisms as novel targets for therapeutics in cardiovascular diseases.
SECTION I – ROS as a Clinical Target
ROS are a class of reactive molecules that were originally ascribed a pathological role in the cell. In addition to increasing the oxidative environment and thus irreversibly modifying organelles and macromolecules, ROS also trigger important signaling events. Members of this class include the hydroxyl radical (HO•), peroxynitrite (OONO—), superoxide (O2•-), hydrogen peroxide (H2O2), and others.5 ROS accumulate both inside and outside of the cell. For example, ROS are generated in the cytoplasm and the plasma membrane by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and by xanthine oxidase as well as uncoupled nitric oxide synthase in the cytoplasm. In addition, monocytes and neutrophils use myeloperoxidase to produce ROS, which upon release can affect neighboring cells from the outside. All of these ROS production sites have implications in cardiovascular disease. Interested readers are directed to Sugamura and Keaney's review.6 However, our discussion focuses only on ROS generated by the mitochondria.
Around 0.1-0.2% of oxygen consumed is associated with leakage of electrons and production of ROS.7 At least ten sites of mitochondrial ROS production that are not only limited to the electron transport chain have been identified using isolated mitochondria from rat skeletal muscle. These include complex I, complex II, complex III, 2-oxoglutarate, pyruvate dehydrogenase, branched-chain 2-oxoacid dehydrogenase, mitochondrial glycerol phosphate dehydrogenase, ETF (electron-transferring-flavoprotein) dehydrogenase, and dihydroorotate dehydrogenase.8 The relative contribution of ROS by each of these sites was overestimated in in vitro studies, which masked the dynamic changes in contribution of each site under different cellular conditions. For example, while the quinol site in complex I and the flavin site in complex II equally contribute to the majority of ROS production under basal conditions, the flavin site in complex I dominates ROS production under conditions that mimic aerobic exercise.8 The dynamic nature of ROS production at the ten mitochondrial sites is yet to be investigated under various conditions mimicking cardiovascular disorders. An understanding of the sites that contribute the majority of ROS accumulation under pathological conditions would allow for a more directed therapeutic approach for targeting pathological ROS production.
Experiments demonstrating the damaging effects of ROS date back to the 1940's.9 The role of ROS has been since implicated in a variety of pathological processes and conditions, including aging, DNA mutagenesis, inflammation, and multiple cell-death pathways. In 1956, Harman proposed the “free radical theory,” which suggested that ROS accumulate spontaneously as well as in response to the environment. Diseases associated with aging, such as many cardiovascular disorders, can be traced to the effects that these ROS have on normal cellular functions.10 Much support for these early studies has accumulated. One example is the use of knockout mice of manganese superoxide dismutase (SOD2); mice lacking SOD2, an enzyme that converts superoxide within the mitochondria to hydrogen peroxide, die within ten days with dilated cardiomyopathy.11 It is therefore not surprising that multiple anti-oxidants have been tested in clinical trials to reduce the oxidative burden of cardiovascular diseases.
In 2011, Sugamura and Keaney summarized clinical trials for anti-oxidants in the context of cardiovascular disorders and concluded that targeting oxidative stress using ROS scavengers is an ineffective therapeutic strategy. While ROS scavengers are effective at reducing cellular ROS levels, they are in general ineffective and sometimes harmful in the context of cardiovascular pathology.6 Table 1 provides an update of their summary. ROS scavengers, such as N-acetylcysteine (NAC), have mixed efficacy outcomes. For example, intravenous NAC prior to angioplasty and orally delivered NAC following the procedure reduced nephrotoxicity in patients with acute myocardial infarction (MI), whereas an intravenous NAC treatment was ineffective at reducing contrast-induced nephropathy in patients with acute coronary syndrome.12,13 NAC oral supplement given twice daily for four weeks increased forearm blood flow but did not affect patient outcome following both heart and renal failure.14 A promising effect for NAC in improving cardiovascular function has only been shown in the study involving 354 patients undergoing angioplasty following acute MI.13 A follow-up clinical study for the effect of NAC in patients undergoing angioplasty on adverse outcomes is currently recruiting patients.15
Table 1.
Summary of selected clinical trials using anti-oxidants as therapeutics for cardiovascular diseases (based on the review by Sugamura and Keaney).6
| Anti-oxidant | Indication | Outcome | Comments | Ref. |
|---|---|---|---|---|
|
Primary Prevention
| ||||
| α-lipoic acid | N/A | Recruiting | 21 | |
| β-carotene | Ineffective or harmful | Completed (higher incidence of lung cancer) | 6 * | |
| Vitamin C | Effective | Lowered cardiovascular disease risk | 6 * | |
| Ineffective | Completed | 6 * | ||
| Vitamin E | Effective | Associated with lower cardiovascular disease risk | 6 * | |
| Ineffective or harmful | Completed (increased HF) | 6 * | ||
|
Secondary Prevention
| ||||
| α-lipoic acid | Type II diabetes / CAD | N/A | Recruiting | 20 |
| β-carotene | MI | Harmful | Increased CAD risk | 6 * |
| Allopurinol | HF | Effective | Reversed endothelial dysfunction | 6 * |
| HF | Ineffective | Completed | 6 * | |
| Co-enzyme Q10 | Idiopathic dilated cardiomyopathy - Children | Effective | Improved diastolic function | 171 |
| Edaravone | MI | Effective | Decreased infarct size and reperfusion arrhythmia | 6 * |
| L-carnitine | CAD | Effective | Increase anti-oxidant activity | 16 |
| L-carnitine | MI | Effective | Reduced level of cardiac MI markers | 17 |
| Melatonin | MI | N/A | Recruiting | 22,172 |
| MI | Effective | Reduced nephrotoxicity | 173 | |
| N-acetylcysteine | HF & renal failure | Ineffective | Increased blood flow to forearm | 14 |
| ACS | Ineffective | Completed | 12 | |
| Post angiography | N/A | Recruiting | 15 | |
| Oxypurinol | HF | Ineffective | Completed | 6 * |
| Probucol | Post PCI | Effective | Prevented restenosis | 6 * |
| CAD - Women | Harmful | Increased mortality | 6 * | |
| Vitamin C | CAD | Effective | Improved endothelial function | 6 * |
| Variant angina | Effective | Attenuated vasomotor dysfunction | 6 * | |
| CAD | Effective | Reduced CAD events | 6 * | |
| Hemodialysis | Effective | Reduced cardiovascular events | 6 * | |
| Vitamin E | MI | Ineffective | Completed | 6 * |
| Variant angina | Effective | Reversed endothelial dysfunction | 6 * | |
| HF | Ineffective | Completed | 6 * | |
| Succinobucol | ACS | Ineffective or harmful | Completed (increased onset atrial fibrillation and bleeding events) | 6 * |
HF=heart failure; MI=myocardial infarction; CAD=coronary artery disease; ACS=acute coronary syndrome; PCI= Percutaneous coronary intervention.
Referenced trials can be found in Table 1 of Sugamura and Keaney's review.
In addition to NAC, the anti-oxidant L-carnitine (4-N-trimethylammonium-3-hydroxybutyric acid) has been tested in the clinic using biomarkers for oxidative stress and heart damage with promising results. In patients with coronary artery disease, L-carnitine reduced the levels of the toxic aldehyde, malondialdehyde (MDA), and increased the expression of anti-oxidant enzymes including catalase, glutathione peroxidase, and superoxide dismutase.16 In patients with non-ST elevation MI, L-carnitine reduced the release of both creatine-MB and troponin-I.17 However, the effect of L-carnitine on overall clinical outcome has not been determined. Other anti-oxidants such as α-lipoic acid (the level of which significantly declines with age)18 and melatonin (an anti-oxidant and a neurohormone produced by the pineal gland)19 are currently being tested in clinical trials for cardiovascular-related indications.20–22 Finally, other ROS scavengers that have been used in other indications such as NXY-059 (Cerovive, a hydrophilic free radical spin trap agent) in stroke are yet to be tested in the context of cardiovascular disorders.23
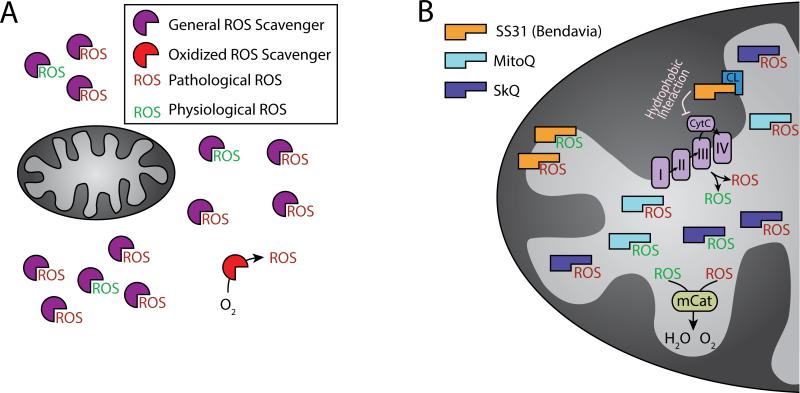
Treatment of cardiovascular diseases with general ROS scavengers has multiple theoretical limitations that may play a role in their apparent failure in the clinic (Figure 1A). One limitation of ROS scavengers is that they must be provided in stoichiometric (greater than 1:1 ratio) levels relative to cellular ROS. In addition, ROS scavengers, such as NAC, are unstable; they can undergo auto-oxidation prior to treatment, effectively rendering them as oxidants.24 As a result, anti-oxidants may exert an opposite effect to the one intended. Finally, ROS also have physiological functions at lower amounts as regulators of autophagy, immunity, differentiation, and longevity (these pathways are described in detail by Sena and Chandel.25) For example, in the context of development, overexpression of the anti-oxidant GTPx-1 suppressed differentiation of multipotent hematopoietic progenitors in Drosophila.26 In addition, ROS activate signaling cascades that enable responses to stress conditions (more details are provided later in the review). In this review, we define lower levels of ROS involved in signaling pathways as physiological ROS and excessive levels of ROS that induce cell damage as pathological ROS. Therefore, targeting ROS elimination at a particular source, i.e., the mitochondria, may overcome the limitations of generalized ROS scavengers. This is of particular importance in the context of cardiovascular disorders because mitochondria are a significant source of pathological ROS production in the heart as well as the sites in which ROS-activated signaling pathways converge.
Figure 1.
Targeting ROS using scavengers. A. General ROS scavengers (purple symbol) target and reduce both pathological (in red) and physiological (in green) ROS. These ROS scavengers do not have localization specificity and must be administered at stoichiometric (at least 1:1) amounts. In addition, ROS scavengers may get oxidized (red symbol) and instead exert an opposite effect than intended. B. Mitochondria-targeted anti-oxidants (e.g., SS31, MitoQ, SkQ, and mCat; see text for details) aim at ROS-producing sites. Whereas some, such as SS31 (orange symbol) do not have to be administered at stoichiometric doses, they may still reduce both pathological and pathologic ROS. The electron transport machinery (complexes I-IV) and cytochrome c are depicted in the inner mitochondrial membrane/matrix interface. CL = Cardiolipin.
SECTION II – Targeting Defective Mitochondria to Prevent Production of Excessive ROS
Targeting ROS scavengers to the mitochondria eliminates some of the localization and stoichiometric difficulties that general ROS scavengers must overcome (Figure 1B). An example of such ROS scavenger is MitoQ. MitoQ is an ubiquinol with a lipophilic triphenylphosphonium cation (TPP+) modification, which enables mitochondrial delivery of the compound. In a rat heart Langendorff model of IR damage, MitoQ protects against cardiac function loss as well as tissue and mitochondrial damage.27 An additional TPP+-modified ROS scavenger, SkQ, increases the life-span of male versus female mice.28 In addition to TPP+-modified ROS scavengers, SS31 (Bendavia), a four amino acid synthetic peptide (phenylalanine-darginine-phenylalanine-lysine), selectively reaches the mitochondrial inner membrane, scavenges ROS through a dimethyltyrosine group, reduces mitochondrial ROS production, and prevents mPTP opening.29 SS31 inhibits hydrophobic cytochrome c interactions with cardiolipin [1,3-bis(sn-3'-phosphatidyl)-snglycerol; a component of the inner mitochondrial membrane], freeing cytochrome c to function as an electron carrier.30 SS31 has a cardioprotective effect in response to IR damage in multiple animal models of MI and heart failure, including in mice, rats, guinea pigs, rabbits, and sheep.31–33 Furthermore, SS31 selectively improves multiple functional parameters in mitochondria isolated from old versus young mouse skeletal muscle as measured in vivo.34 In addition, SS31's selective effect on old over young mice suggests that SS31 may better target pathological ROS production that occurs in aged mitochondria. SS31 is currently in a phase IIa clinical trial for patients with ST-segment elevation MI.35 Finally, mitochondria-targeted enzymatic anti-oxidants have cardioprotective effects as well. For example, overexpression of catalase specifically in the mitochondria (mCat) reduces mitochondrial oxidative damage, increases lifespan of mice, and protects against angiotensin II-induced cardiac hypertrophy, fibrosis, and heart failure.36,37 Anti-oxidants that directly target the mitochondria seem to be more efficacious than generalized ROS scavengers in animal models, yet their benefit in humans is unknown. Whereas mitochondria-targeted anti-oxidants solve the localization limitation of ROS scavengers, they may still eliminate physiologically-relevant ROS as well (Figure 1B).
How can pathological and physiological ROS be distinguished and can pathological ROS generation be specifically targeted? One mechanism focuses on the removal of damaged mitochondria, which cause further damage by producing excessive or pathological ROS.38 Perhaps specifically targeting damaged mitochondria or mechanisms that sequester and remove damaged mitochondria is an effective strategy to eliminate the production of pathological ROS.
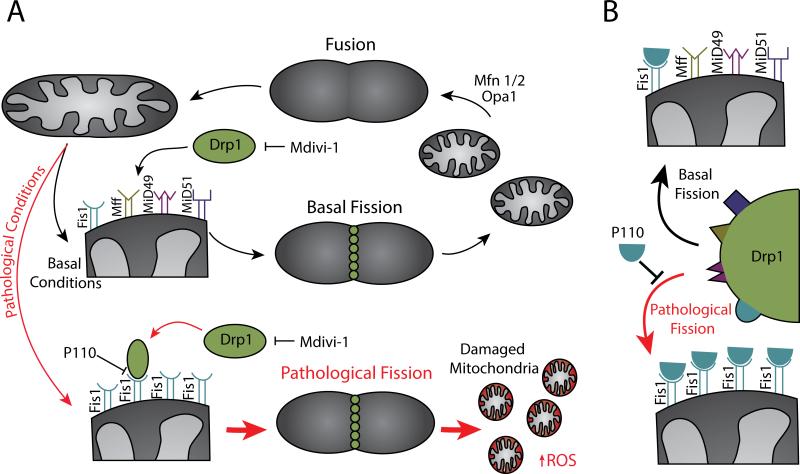
Before considering elimination of damaged mitochondria, we should discuss normal mitochondrial fate. Mitochondria continuously undergo changes in morphology and size through processes including fission and fusion that are collectively referred to as “mitochondrial dynamics.” Mitochondrial fission is the division of a single mitochondrion into two or more mitochondria, whereas mitochondrial fusion is the joining of two or more mitochondria to form a single mitochondrion. Mitochondrial fission is mediated by a large GTPase called Drp1 (dynamin-related protein 1) that is recruited to the outer mitochondrial membrane (OMM) by multiple protein adaptors, including Fis1 (fission 1), Mff (mitochondrial fission factor), as well as MiD49 and MiD51 (mitochondrial dynamics protein of 49 and 51 kDa). Once on the OMM, Drp1 polymerizes and constricts the mitochondria to induce division. During mitochondrial fusion, joining of the OMM is mediated by two GTPases, Mfn1 (mitofusin) and Mfn2, and stitching of the inner mitochondrial membrane (IMM) is mediated by Opa1 (optic atrophy 1), another large GTPase and a homolog of dynamin.38–40 A balance between fission and fusion must be kept to maintain healthy mitochondria and cellular homeostasis (Figure 2A).
Figure 2.
Targeting defective mitochondria to prevent production of excessive ROS. A. Mitochondrial fusion opposed by either basal or pathological fission including the relevant players. Fission inhibitors Mdivi-1 and P110 inhibit either Drp1 directly or Drp1 recruitment to the mitochondrial membrane, respectively. B. Mechanism of pathological fission inhibition by P110. Depicted are the four mitochondrial adaptors of Drp1 in mammalian cells including Fis1, Mff, Mid49 and MiD51. To illustrate the specific inhibition of Fis1-dependent pathological fission, the mitochondria at the bottom left and right show only Fis1 on the outer mitochondrial membrane. P110, a selective inhibitor of only Drp1 binding to Fis1, acts as a separation of function inhibitor, separating the Fis1-dependent function (likely pathological) from the other Drp1 functions (including physiological fission).
Mitochondrial fission/fusion does not seem to occur in isolated cardiomyocytes to as great of an extent as in cultured cells.41 Nevertheless, an imbalance between fission and fusion is observed in multiple cardiovascular disorders as thoroughly reviewed by Dorn.42 One example of particular interest is the observation that Opa1 is down-regulated in hearts from both humans with heart failure (HF) and a rat model of HF. In addition, mitochondria in HF are fragmented, suggesting the occurrence of excessive, pathological, and unopposed fission.43 Mitochondrial fragmentation is found in other pathologies as well, including pulmonary arterial hypertension, which is associated with both reduced Mfn2 expression and up-regulation of Drp1.44,45 Furthermore, excessive fragmentation occurs in other non-cardiac disorders, such as cancer and in a number of neurodegenerative diseases. Therefore, fission and fusion proteins are attractive therapeutic targets. The ability to maintain a balanced fission/fusion cycle can prevent the accumulation of damaged and fragmented mitochondria and thus eliminate the production of pathological ROS (Figure 2A).
Recent work on pharmacological intervention of mitochondrial dynamics focused on the ability to inhibit mitochondrial fission though targeting Drp1. Cassidy-Stone et al. screened for inhibitors of mitochondrial fission using a yeast fzo1-1 mutant that have excessive fragmentation and failure to grow on glycerol. From this screen, mdivi-1 was identified as an inhibitor of Drp1.46 Mdivi-1 treatment in mice undergoing IR reduces myocardial infarct size. In addition, mdivi-1 decreases cell death, normalizes mitochondrial membrane potential, and reduces excessive fragmentation in adult rat cardiomyocytes exposed to simulated IR injury.47 However, Drp1 may have multiple functions, depending on its interaction with each of its adaptor proteins, Fis1, Mff, MiD49 and MiD51 (Figure 2A), as well as other functions independent of mitochondrial fission; inhibition of all these functions may have undesired effects. Recent work on mdivi-1 in porcine embryos and primary cells showed that mdivi-1 reduces cell growth, blastocyst production, and mitochondrial membrane potential while increasing ROS production.48 Whereas mdivi-1 inhibits excessive fission under stress conditions as with the IR model, mdivi-1 may also inhibit other functions of Drp1, including healthy physiological fission (Figure 2A). This is consistent with the observation that a Drp1 knockout interferes with mitochondrial clearance and is lethal.49,50 Therefore, a more specific inhibitor of Drp1, a so-called ‘separation of function’ inhibitor,51 that is selective for pathological fission and spares physiological fission is needed.
To overcome the potential toxic effects of mdivi-1 through the complete inhibition of Drp1 function, our lab identified a small separation of function peptide inhibitor that selectively prevents recruitment of Drp1 to the mitochondria only under pathological conditions. The peptide, P110, hinders the interaction between Drp1 and Fis1 only and does not affect Drp1 interaction with Mff and MiD51. This separation of function inhibitor was derived using a rational approach that examines sequences of homology between Fis1 and Drp1.52 P110 treatment diminishes excessive mitochondrial fission and reduces neurotoxicity in cells derived from Parkinson's patients but does not affect the recruitment of Drp1 to the mitochondria under physiological conditions.52 It is therefore hypothesized that Fis1-mediated Drp1 recruitment to the mitochondrial membrane occurs under stress conditions and that treatment with P110 selectively inhibits pathological fragmentation without interfering with normal Drp1 functions (Figure 2B). Relevant to this review, treatment with P110 decreases mitochondrial fragmentation, improves mitochondrial function, and reduces ROS production in three models of IR in rats, including primary cardiomyocytes, ex vivo heart model, and an in vivo MI model.53 In primary cardiomyocytes that were not exposed to IR stress, P110 does not affect ROS production and mitochondrial function nor does it induce cell death. Importantly, P110 treatment reduced infarct size and increased ATP production in the MI in vivo model.53 Further, eight weeks of P110 treatment shows no adverse effects in wild-type mice, but improves behavior and reduced mortality in Huntington's mice.54 Thus, targeting of mitochondrial fragmentation by selectively inhibiting pathological fission may be an effective strategy for reducing pathological ROS release and inducing a cardioprotective effect.
Another mitochondrial dynamics mechanism that prevents the production of pathological ROS occurs through a process of eliminating damaged mitochondria. Autophagy is a vital catabolic mechanism degrading unnecessary, excessive, or dysfunctional cellular components by the lysosome and recycling them to sustain cellular metabolism.55 Autophagy has long been thought to non-specifically mediate the bulk degradation of cytosolic components in response to acute stress, such as energy deprivation, to meet energy demand and maintain homeostasis and viability.56,57 However, multiple lines of evidence suggest that autophagy selectively mediates removal of specific targets. Under physiological conditions (in which mitochondrial ROS is balanced with the anti-oxidant capacity), autophagy provides limited mitochondrial pruning, thus promoting mitochondrial quality control to meet the cell's energy requirement.58 When exposed to cellular insults, such as oxidative stress caused by IR, mitochondrial pruning through autophagy (mitophagy) is up-regulated by diverse molecular machinery.59,60
Depending on the way mitochondria are delivered to lysosomes, two distinct molecular pathways contribute to mitochondrial elimination: macro- and micro-mitophagy. Macro-mitophagy, the better-studied form of mitophagy, is characterized by encapsulation of mitochondria into unique, double-membrane vesicles known as autophagosomes (Figure 3A). The subsequent fusion of autophagosomes with lysosomes leads to degradation of their content.56 A number of molecular pathways regulate the formation of the autophagosome and lead to mitochondrial clearance. Beclin 1 (mammalian ortholog of the yeast autophagy-related gene, Atg 6, and BEC-1 in C. elegans) is involved in initiation of autophagosome formation, which is primed by ULK1 (unc-51 like autophagy activating kinase 1) and AMBRA1 (activating molecule in beclin 1-regulated autophagy).61–63 A group of Atg proteins and LC3 (microtubule-associated protein 1 light chain) then promote elongation and maturation of the autophagosome.64 Damaged and depolarized mitochondria, previously segregated from healthy mitochondria by Drp1-mediated fission (a physiological role of Drp1), are recognized and flagged by sensing molecules, including phosphatase and tensin homolog-induced putative kinase protein 1, PINK1 (PTEN-induced putative kinase 1), Parkin (a component of an E3 ubiquitin ligase complex), and NIP1-like protein X (NIX, a pro-apoptotic protein).65,66 PINK1, initially implicated in the pathogenesis of Parkinson's disease, is a molecular sensor to induce mitophagy in heart.60,67 Upon depolarization, PINK1 accumulates on the outer mitochondrial membrane and recruits the cytosolic E3 ubiquitin ligase Parkin. Parkin recruitment leads to increased ubiquitination of mitochondrial proteins, which induces removal of impaired mitochondria.56,66,68 Although the exact process has not been clearly elucidated, a number of molecular factors promoting mitophagy have been discovered. In addition to Parkin, several other substrates of PINK1 have recently been identified. For example, Mfn2 phosphorylation by PINK1 mediates Parkin recruitment to damaged mitochondria in mouse cardiac myocytes, suggesting that mitochondrial fusion, in addition to fission, is also connected to mitophagy.69,70 Phosphorylation of MIRO (mitochondrial membrane rho GTPase protein mediating mitochondrial motility)71 by PINK1 and subsequent degradation in a Parkin-dependent manner helps quarantine damaged mitochondria by arresting their motility and thus preventing their re-fusion with healthy mitochondria.72 VDAC (voltage-dependent anion channel), controlling mitochondrial membrane permeability, functions as a molecular receptor that aids in Parkin recruitment to mitochondria, albeit it is not clear whether ubiquitination of VDAC is necessary for a mitophagic process.73,74 Following ubiquitination of these and other mitochondrial proteins, molecular linkers, such as p62 (also called sequestosome 1 or SQSTM1), are recruited to the mitochondria and link ubiquitinated proteins on damaged mitochondria to autophagosomes via LC3.73
Figure 3.
Targeting damaged mitochondrial clearance to prevent production of excessive ROS. A. Parkin- and NIX-dependent macro-mitophagy. B. GAPDH-dependent micro-mitophagy. See text for details.
Along with PINK1/Parkin-mediated mitophagy, NIX (a BH3-only member of the Bcl-2 family), originally identified for its role in apoptotic cell death, induces mitophagy during erythrocyte maturation (Figure 3A).65,75 NIX localizes to the OMM and directly binds autophagy machinery components LC3/GABARAP (γ-aminobutyric acid receptor-associated protein) and targets mitochondria to autophagosomes for degradation under a stressed condition (induced by mitochondrial uncoupler CCCP).76 In addition, NIX exerts multiple functions on mitochondria, such as stimulating cardiomyocyte apoptosis, inducing clearance of damaged mitochondria, and regulating the mPTP.77 Similar to NIX-mediated mitophagy, another mitochondrial membrane protein FUNDC1 (FUN14 domain containing 1) also mediates hypoxia-induced mitophagy by interacting with LC3.78
Independently of macro-mitophagy, micro-mitophagy, whereby damaged mitochondria are sequestered and degraded by direct invagination of lysosomal membranes, also contributes to the maintenance of mitochondrial quality control. Only photodamage-induced micro-mitophagy in hepatocytes occurring in the presence of 3-methyladenine, an inhibitor of autophagosome formation, has been reported.79 Following that study, our lab recently identified a new role for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), one of the common housekeeping metabolic enzymes, in micro-mitophagy (Figure 3B)80. Proteins readily undergo oxidation, such as S-nitrosylation, in response to ROS, thus changing their conformation and functions.81 GAPDH is one such protein that is sensitive to oxidative post-translational modifications; particularly, oxidation of a cysteine residue (C152) in its active site results in the inactivation of the enzyme.82 This metabolically inactive GAPDH (iGAPDH) confers a novel role for the enzyme: inducing mitophagy. It is possible that high levels of ROS released by mitochondria inactivate nearby GAPDH, which, in turn, triggers its association with damaged mitochondria. Therefore, iGAPDH serves as a molecular sensor for detecting and tagging damaged mitochondria. Furthermore, mitochondrial fractions isolated from hearts subjected to myocardial ischemic preconditioning (IPC) has higher levels of oxidized GAPDH.83 Mitochondria-associated iGAPDH promotes direct uptake of damaged mitochondria into a lysosomal-like (LL) structure, a hybrid organelle of late endosome and lysosome.80 Exogenously-expressed, catalytically-dead iGAPDH is sufficient to induce LL structures to engulf damaged mitochondria for degradation.80 The mechanism by which mitochondria-associated iGAPDH promotes mitochondrial uptake into the LL is still unknown, but may be triggered by the fusogenic property of GAPDH.84,85 iGAPDH-bound mitochondria may also be eliminated by chaperone-mediated autophagy (CMA), whereby oxidized GAPDH is recognized by chaperone heat shock cognate protein and subsequently targeted to lysosome.86,87 Removing damaged or excessively ROS-producing mitochondria by modulating mitochondrial GAPDH may be key in protecting cells from damage. Together, it appears that activators of macro- and micro-mitophagy that enhance removal of damaged mitochondria and spare healthy mitochondria may be beneficial in preventing ROS-mediated damages in cardiac diseases.
In addition to mitochondrial dynamics, other mechanisms such as the unfolded protein response (UPR) and the upregulation of the proteasome machinery maintain mitochondrial quality control. These mechanisms are discussed in greater detail in the referenced reviews.88,89 In particular, inhibition of the proteasome using bortezomib and overexpression of K48R mutated ubiquitin induces mitochondrial damage, which consequently leads to accumulation of pathological ROS.90 Therefore, components of the UPR and the proteasome machinery pose as attractive therapeutics targets, as well.
SECTION III – Targeting Mitochondria to Promote Beneficial ROS Signaling Through Protein Kinase C ε and its Downstream Substrates
In addition to the challenges related to providing stoichiometric amounts of scavengers to quench each ROS and the inherit instability of ROS scavengers, not all amounts of ROS are damaging; ROS production may induce beneficial effects as well. The best example is ischemic preconditioning (IPC) of the heart, in which sublethal cycles of IR bursts prior to a prolonged ischemic event protect the heart from reperfusion-mediated injury, suggesting a beneficial 91. IPC and multiple pharmacological preconditioning tools trigger a cascade of signaling events that are at least partially mediated by the release of mitochondrial ROS.92–95
One cardioprotective mechanism triggered by ROS involves protein kinase C epsilon (PKCε) activation. Antimycin A, which triggers preconditioning, induces ROS production and results in PKCε translocation to the mitochondria and subsequent reduction in cardiac infarct size; this ROS-induced cardioprotective effect is lost in PKCε knockout mice.95 Furthermore, treatment of isolated cardiomyocytes with the PKCε agonist, ψεRACK, as well as the expression of this octapeptide in postnatal transgenic mice increases PKCε translocation to the mitochondria and promotes cardioprotection following IR injury.96,97 The finding that PKCε translocation to the mitochondria underlies a mechanism behind ROS-induced cardioprotection during IPC suggests that downstream mitochondrial PKCε substrates could be powerful therapeutic targets (Figure 4A).
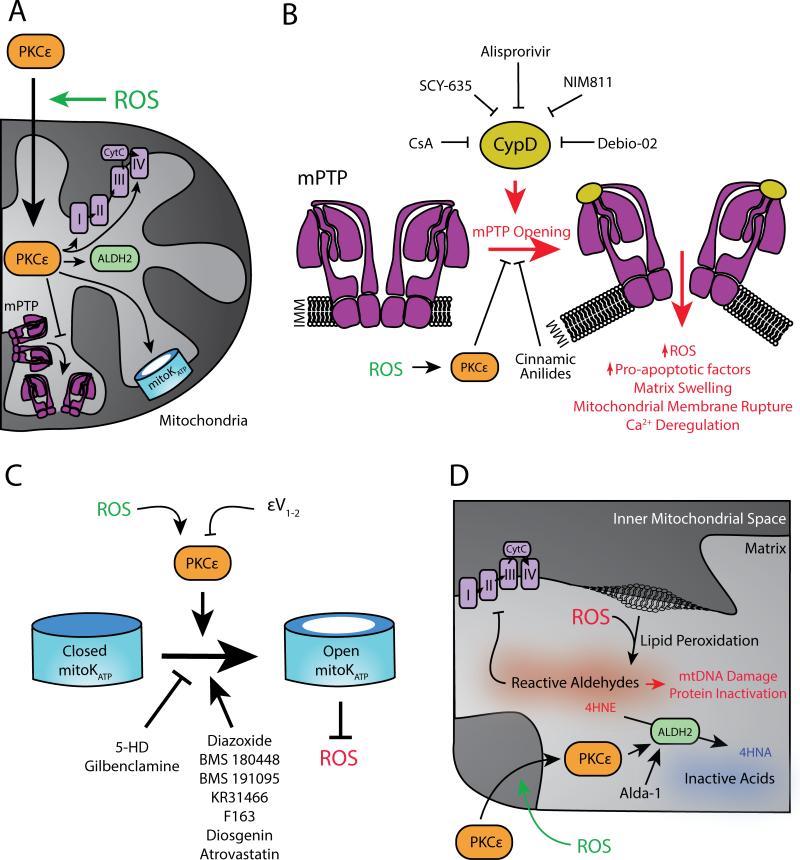
Figure 4.
Targeting downstream ROS signaling pathways that are beneficial for the cell. A. Summary of physiological ROS-mediated PKCε activation and translocation to the mitochondria and its mitochondrial substrates. B. Pharmacological inhibition of mPTP opening as a therapeutic strategy. C. Activation of the mitoKATP channel as a therapeutic strategy. D. Activation of the mitochondrial ALDH2 as a therapeutic strategy and the molecular events affected by it. See text for details. IMM = inner mitochondrial membrane.
Multiple downstream substrates of PKCε that reside in the mitochondria have been identified. These include proteins involved in glycolysis, lipid metabolism, and the electron transport chain as well as heat shock proteins.98 It is not surprising that PKCε phosphorylates members of the electron transport chain, in particular members of complexes I,II, and III, as these sites have been previously implicated as locations of ROS production through superoxide leakage.99 PKCε co-immunoprecipitates with cytochrome oxidase subunit IV (COIV) and this interaction contributes to ROS-induced preconditioning.100 In addition, IR damage is associated with a loss of cytochrome oxidase subunit I (COI). Whereas preconditioning prevents the loss of COI, treatment with the PKCε translocation inhibitor, εV1-2, exacerbates COI loss.101 The underlying hypothesis stemming from these two studies suggests that ROS-mediated preconditioning is dependent upon PKCε activation and translocation to the mitochondria. Once at the mitochondria, stabilization of cytochrome oxidase subunits by PKCε elicits a cardioprotective effect, potentially through restoring ATP production or preventing pathological ROS release through electron leakage (Figure 4A). Alongside phosphorylation of electron transport chain components, other post-translational modifications elicit cardioprotective effects as well. For example, selective S-nitrosation of Cys39 on the ND3 subunit of complex I by MitoSNO reduces infarct size in mice exposed to IR damage.102 Therefore, targeting PKCε's mitochondrial substrates to mimic ROS-induced preconditioning may serve as an effective therapeutic strategy for cardiovascular disorders.
One of PKCε's substrates is the mPTP Initial co-immunoprecipitation studies in mitochondria isolated from mouse heart suggested that mPTP components, including the voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT), and hexokinase II (HKII), are PKCε targets during IPC.103 Since these studies, work by the Bernardi group has elucidated a more plausible structure of the mPTP, which is composed of an F0F1 ATP synthase dimer.104 Interestingly, ATP synthase is also a PKCε substrate.98 Identifying the mPTP as a phosphorylation target of PKCε suggests that PKCε-mediated cardioprotection occurs by preventing the opening of the mPTP (Figure 4B). Pathological opening of this pore leads to matrix expansion and OMM rupturing, which releases pro-apoptotic factors (such as cytochrome c, Smac/DIABLO [second mitochondria-derived activator of caspases /direct IAP binding protein with low pI], AIF [Apoptosis-inducing factor], and Omi/HtrA2 [mitochondrial serine protease]), dissipates the mitochondrial membrane potential, deregulates Ca2+ homeostasis, and releases pathological ROS.105 Still, the mechanism by which ROS-induced PKCε translocation to the mitochondria and phosphorylation of mPTP components contributes to mPTP closing remains unclear.
Targeting the mPTP to inhibit pore opening and mimic the effects observed with IPC serves as a valuable therapeutic approach for cardioprotection against IR-induced injury (Figure 4B). Multiple mPTP inhibitors have been tested in the context of MI, including cyclosporin A (CsA), which was initially discovered as part of a screen for anti-fungal antibiotics.106 CsA is an effective inhibitor of cyclophilin D (CypD), a central regulator of mPTP dynamics that resides in the mitochondria.107 CsA treatment prevents myocardial necrosis during reperfusion (initially demonstrated by Di Lisa and co-workers).106,108 Although CsA successfully prevents mPTP opening through inhibiting CypD, its off-target effects make it a less ideal drug candidate. Most notably, CsA forms a complex with cyclophilin that binds and inhibits calcineurin, resulting in immunosuppressive effects.109 CsA's off-target effects highlight the need to develop more specific inhibitors of CypD that inhibit mPTP opening.
Multiple strategies have been employed to harness CsA's mPTP closing effects while minimizing its off-target effects. Additional screens for inhibitors of CypD that do not have corresponding immunosuppressive effects yielded compounds such as alisporivir, SCY-635, and NIM811 (as reviewed elsewhere).110 NIM811, for example, is as effective as CsA in preventing mitochondrial swelling and loss of mitochondrial membrane potential due to CypD inhibition, yet it lacks CsA's immunosuppressive effects.111,112 Treatment of streptozotocin-induced diabetic and control rat hearts with NIM811 at post-ischemia reduces infarct size.113 Another inhibitor of CypD, Debio-025, potently blocks mPTP opening, reduces infarct size and left-ventricle dilation, and improves survival following IR injury.114,115 More recently, a new class of cinnamic anilides were found to inhibit mPTP opening of isolated mitochondria in response to calcium overload, to electron transport chain uncoupling, and to oxidative stress.116 In addition, one of the lead compounds in the class is cardioprotective in a rabbit heart model of myocardial infarction. Interestingly, using a calcium retention capacity assay, this lead compound has additive effect to CsA, suggesting that cinnamic anilides inhibit mPTP opening using an alternative target to CypD.116 However, it is also possible that CsA does not completely inhibit CypD, due to its poor selectivity, and so treatment with an additional CypD inhibitor would have an additive effect. Furthermore, mitochondrial pore opening has been observed in the presence of CsA and the absence of CypD due to excess Ca2+ in the matrix, posing a limitation on CypD as a therapeutic target.104 By identifying the proteins that bind this novel class of inhibitors, we may find alternative protein targets to prevent mPTP opening and the corresponding pathological ROS release to benefit the heart.
The mitochondrial adenosine triphosphate-dependent potassium, mitoKATP, channel is also a potential target of PKCε phosphorylation upon ischemic reperfusion injury (Figure 4C). Initial work suggesting the mitoKATP channel's cardioprotective role showed that inhibitors of mitoKATP channel opening, such as glibenclamide and sodium 5-hydroxydecanoate (5-HD), prevent the effect observed during ischemic pre-conditioning in a canine heart model.117,118 The mitoKATP channel is a downstream of PKCε-mediated preconditioning; pharmacological inhibition of mitoKATP channel opening does not affect PKCε translocation to the mitochondria.119 In addition, using a reconstituted liposome system, PKCε associates with activated mitoKATP. In this reconstituted system, addition of ROS in the form of H2O2 induces potassium flux through the channel, which is abolished in the presence of the selective PKCε inhibitor εV1-2 and the mitoKATP inhibitor 5-HD.120 In isolated heart mitochondria, treatment with H2O2 but not superoxide activates the mitoKATP channel in a PKCε-dependent manner, as treatment with εV1-2 reduces the potassium flux.121 These results suggest that physiological ROS induce PKCε phosphorylation and open the mitoKATP channel, which contributes to cardioprotection during (IPC). Although the mechanism by which mitoKATP opening contributes to cardioprotection is not completely understood, mitoKATP activation is associated with a reduction of pathological ROS, demonstrated by using 2’,7’-dichlorofluorescein as a fluorescent ROS indicator in isolated rat myocytes.122 Thus, therapeutics that specifically activate the mitoKATP channel can trigger the downstream cardioprotective preconditioning effects of physiological ROS and prevent the overproduction of pathological ROS.
Several therapeutics aimed at the mitoKATP channel activation have been tested. Diazoxide is both an opener of mitoKATP and sarcolemmal KATP channels, with a much stronger preference for mitoKATP. Diazoxide also elicits a cardioprotective effect, measured by the reduction of cytosolic LDH (lactate dehydrogenase) release (a marker of cell necrosis) in an isolated rat heart model of MI; diazoxide's effect is abolished by the mitoKATP inhibitor 5-HD.123 Interestingly, it was suggested that diazoxide activates mitoKATP channel opening by generating ROS in a connexin 43-dependent manner, which further solidifies an important physiological role for ROS during preconditioning (Connexin-43 is a gap junction protein, providing electrical coupling between cardiac cells).124 However, because diazoxide also has vasodilator and hyperglycemic effects, it is not an ideal pharmacological preconditioning mimetic.125 A recent review discusses the benefits of additional mitoKATP activators, such as BMS 180448, BMS 191095, KR31466, and F163.125 Since the review's publication, two other drugs proposed to target the mitoKATP channel have been tested in the context of IR damage: diosgenin and atorvastatin. Two independent studies showed that diosgenin and atorvastatin induce a cardioprotective preconditioning effect in an IR rat heart model, measured either by the infarct area or by LDH release. The effect of either drug is abolished in the presence of 5-HD.126,127 However, in both cases, it is unclear whether the mitoKATP channel is the direct target of these drugs. Lonidamine, a mPTP opener, abolishes atorvastatin's cardioprotective effect,127 whereas treatment with an NO synthase blocker, L-nitro-arginine methyl ester (L-NAME), abolished diosgenin's cardioprotective effect.126 Although both diosgenin and atorvastatin induce pharmacological pre-conditioning that results in mitoKATP channel opening, neither have been shown to specifically bind and open the mitoKATP channel.
Another mitochondrial PKCε substrate is the mitochondrial aldehyde dehydrogenase 2 (ALDH2). The major function of ALDH2 is to detoxify reactive aldehyde substrates to their corresponding acids.128 One of the deleterious effects of pathological ROS is the generation of toxic and reactive aldehydes, such as unsaturated alkynals, 4-hydroxy-2-nonenal (4HNE) and malondialdehyde (MDA), by peroxidation of the mitochondrial lipid membrane.129 These reactive aldehydes can lead to mitochondrial dysfunction through covalently binding to and inactivating proteins, lipids and DNA.130 Notably, reactive aldehyde-mediated mitochondrial dysfunction has long been suspected to be a culprit of many human cardiovascular diseases, such as atherosclerosis, hypertension, peripheral artery disease, cardiomyopathy, MI and heart failure.131 Our group showed that PKCε phosphorylation of ALDH2 leads to enhanced aldehyde dehydrogenase activity and reduction of toxic aldehydes under oxidative stress.132 Physiological ROS-mediated activation of PKCε can lead to a protective effect through ALDH2 activation. Therefore, increasing ALDH2's aldehyde detoxification activity directly should also mimic the function of physiological and protective ROS (Figure 4D).
ALDH2 deficiency is one of the most common genetic mutations in humans. About 560 million East Asians or 8% of the world population are deficient in ALDH2 enzyme activity due to a single point mutation, resulting in an amino acid substitution of glutamic acid for lysine, in ALDH2 (ALDH2*2).133 The mutation behaves in an over-dominant fashion so that ALDH2 enzymatic activity is reduced to 20-35% of wild type enzyme in heterozygous individuals. Recent genome-wide association and epidemiological studies provide strong evidence linking ALDH2*2 genotype with the risk of coronary artery disease (CAD),134,135 MI,136 hypertension,137 dyslipidemia,138 and hyperglycemia.139 The elevated health risk of cardiovascular diseases that many East Asians may face due to the ALDH2*2 further demonstrates the relevance of ALDH2 as a cardiovascular therapeutic target.
The use of an isozyme-selective small molecule activator of ALDH2 is particularly attractive for its ability to accelerate the removal of toxic aldehyde in the mitochondria, where pathological ROS-induced lipid peroxidation is damaging under stressed conditions. Our group first discovered a class of ALDH2 selective activators, represented by Alda-1 (for Aldehyde dehydrogenase activator).140 Alda-1 enhances ALDH2 enzymatic activity by binding to and accelerating the clearance of its aldehyde substrates, such as acetaldehyde and 4HNE.140–142 Administration of Alda-1 or its analog causes significant cardioprotection against IR injury.132,140 Targeting ALDH2 by an Alda-like compound is therefore pursued as a potential therapeutic target. In this review, we focus only on more recent advances in the application of ALDH2 activators as a potential mitochondria-based therapeutics for cardiovascular diseases.
The efficacy of Alda-1 has been demonstrated in many oxidative stress-related cardiovascular conditions or models. Related to IR injury during MI, activation of ALDH2 by Alda-1 in a cardioplegia solution, which is commonly used for human open-heart surgeries, results in better cardiac function preservation after IR.143 Alda-1 treatment is also effective in preventing MI-induced heart failure in rats. Alda-1 given continuously for 4 weeks at 24 hours immediately following the LAD occlusion surgery significantly improves mitochondrial and left ventricular function with concomitant reduction of aldehydic load in cardiomyocytes.144 Separately, a 6-week delivery of Alda-1 to rats during the heart failure stage results in a significant improvement in heart failure outcome.145 In both cases, long-term, systemic treatment of Alda-1 has no observable adverse effect. Furthermore, prolonged oral ingestion of Alda-1 together with a high fat-diet for 4 months reduces atherosclerotic lesion by 25% and alleviates liver steatosis, as compared with high-fat diet alone in an Apo-E knockout mice model.146 Similar to MI-induced IR injury, in a rat stroke model employing middle cerebral artery occlusion, Alda-1 enhances ALDH2-mediated clearance of 4HNE, MDA and reduces infarct volume, adverse neurological score, and mortality rate.147 In type I diabetes-induced myocardial injuries and suppression of autophagy, administration of Alda-1 restores cardiac dysfunction and high-glucose-induced cardiotoxicity, most likely through 5' AMP-activated protein kinase (AMPK)-dependent autophagy regulation.148 Alda-1 also improves the viability of induced human pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) after ischemia.149 In this study, we showed that after an ischemic insult in culture, iPSC-CMs derived from the fibroblasts of ALDH2-deficient human subjects (carrying the inactive mutant ALDH2*2 allele) generate more toxic aldehydes and exhibit increased apoptosis as well as cell cycle arrest as compared to iPSC-CMs derived from normal ALDH2 human subjects (carrying the ALDH2*2 wild type allele). Alda-1 treatment reduces toxic aldehyde production and rescues ischemic damage in the mutant iPSC-CMs. These results indicate that ROS-induced ADLH2 activation can be mimicked by direct activation of ALDH2 by a small molecule (such as Alda-1) to produce not only increased cell survival, but also to enhance cardiac repair and regeneration after ischemia.
The case study of ROS-mediated PKCε activation to promote signaling pathways that protect the cell from stress reveals multiple mitochondrial therapeutic targets for cardiovascular disorders, including the mPTP, mitoKATP, and ALDH2. Pharmacological interventions that mimic ROS-dependent PKCε activation by closing the mPTP, opening the mitoKATP, and activating ALDH2 are effective therapeutic strategies in the pre-clinical setting. Targeting these mitochondrial proteins directly not only preserves physiological ROS signaling pathways, but also enhances them to aid the cell in its response to stress.
SECTION IV – Targeting Mitochondria to Prevent Harmful ROS Signaling Through Protein Kinase Cδ and its Downstream Effectors
TPPPKCδ, another member of the PKC family, is implicated in a number of pathological conditions including cancer, stroke, diabetes, neurodegenerative diseases and ischemic heart disease.150–153 ROS can directly activate PKCδ via oxidative modification or/and tyrosine phosphorylation154 as well as by inducing caspase 3-mediated PKCδ proteolysis.155,156 For example, glutathione depletion-induced ROS generation of heart-derived H9C2 cells is triggered by caspase 3 and PKCδ activation.157 Following ischemia, right at the onset of reperfusion, the rise in ROS158 triggers PKCδ translocation to the mitochondria,159 and enhances superoxide anion production, which induces mitochondrial dysfunction and subsequent potentiation of oxidative stress. This PKCδ activation can be blocked by a specific PKCδ translocation peptide inhibitor called δV1-1.160,161 PKCδ inhibition also reduces endothelial vascular dysfunction, due to eNOS-mediated ROS levels, by increasing survival of coronary endothelial cells.162 Endoplasmic reticulum (ER) oxidative stress, induced by tunicamycin or by IR in cardiac myocytes, triggers PKCδ localization to the ER and causes cell death that is inhibited by δV1-1.163 Therefore, in addition to its negative effect at the mitochondria, ROS-induced PKCδ activation disrupts ER homeostasis causing cell death. Together, these results suggest that a selective PKCδ inhibitor might be a useful therapeutic agent against cell injury due to ROS elevation.
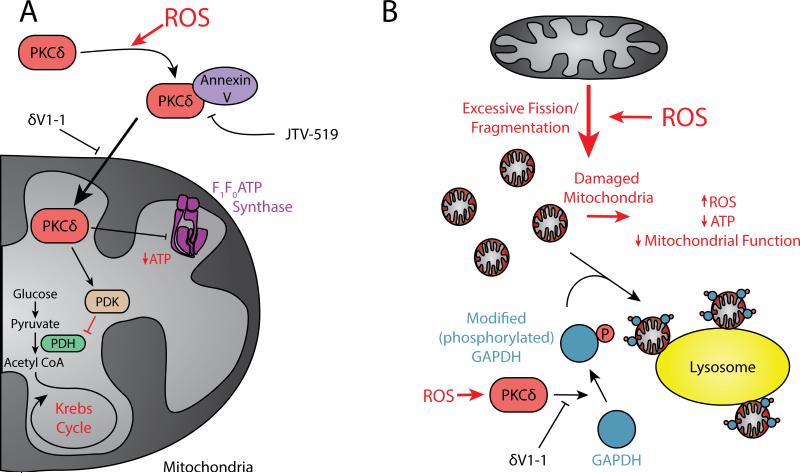
The mechanisms involved in PKCδ translocation to the mitochondria and by which proteins shuttle the enzyme into mitochondrial matrix are unknown. PKCδ does not have a mitochondrial targeting sequence.164 However, we provided evidence for an interaction between activated PKCδ and annexin V, a calcium-dependent phospholipid binding protein, which promotes the translocation of PKCδ from the cytosol to the cell membranes (a cell fraction composed with heavy membranes as well as organelles). The binding between those two proteins regulates PKCδ translocation and inhibiting this interaction with a small molecule, JTV519,165 leads to cardioprotection in a model of MI.166 This suggests that annexin V might serve as a shuttle protein moving PKCδ on the microtubules to its subcellular compartments. Treatment with JTV519, an inhibitor of annexin V improves LV function and reduces calcium overload following IR damage when administered at the time of reperfusion in an isolated rat heart model.165 Therefore, annexin V may serve as an additional therapeutic target to inhibit pathological ROS-induced translocation of PKCδ to the mitochondria.
What are the downstream targets of PKCδ as mediators of apoptosis/necrosis at the mitochondria and can these be therapeutic targets to provide additional selective inhibition of ROS-induced cardiac injury? Electron microscopy studies demonstrated that activated PKCδ crosses the mitochondrial membrane to interact with specific substrates and mediates cardiac injury, at least in part, by phosphorylating the mitochondrial matrix enzyme pyruvate dehydrogenase kinase (PDK, a key enzyme leading to the Krebs cycle).164 Thirty years ago, Patel et al. reported a decline in mitochondrial pyruvate dehydrogenase (PDH) activity in ischemic rat heart.167 Subsequent work demonstrated that once phosphorylated by PKCδ, activated PDK phosphorylates and inhibits PDH thus blocking glycolytic flux and ATP production by the mitochondria (Figure 5A). Thus, cells must rely on alternative mechanisms for ATP production such as fatty acid oxidation. Inhibiting PKCδ with δV1-1 at reperfusion increases ATP levels and mitochondrial function as well as reduces MI.164 Therefore, specific inhibitors of PKCδ-mediated PDK activation will likely reduce pathological ROS-induced abrogation of metabolism through the Krebs cycle and subsequent cardiac injury.
Figure 5.
Targeting downstream ROS signaling pathways that are harmful for the cell. A. Pathological ROS-mediated PKCδ activation and translocation to the mitochondria. Once at the mitochondria, PKCδ activates PDK, which in turn inhibits PDH and entrance to the Krebs cycle. In addition, PKCδ reduces ATP production by targeting F1F0 ATP synthase. B. Pathological ROS-mediated PKCδ disruption of micro-mitophagy. See text for details.
ATP levels in the cell are crucial for survival. The big majority of cellular ATP is produced by the mitochondrial F1F0ATP synthase.168 However, cardiac ATP levels do not recover for a long time after IR, resulting in tissue injury,169 and it is still unclear whether PKC signaling directly affects ATP production. Evidence about the modulation of the mitochondrial ATP synthase was discussed earlier in the context of PKCε and the closing of the mPTP. PKCδ activation was also reported to regulate ATP synthase.170 The study demonstrated that PKCδ's direct interaction with F1F0 ATP synthase during IR prolongs hypoxia in cardiomyocytes, inhibits ATP synthesis, and contributes to cardiac injury. However, no phosphorylation of the complex by PKCδ has been reported. Thus, it is not clear whether F1F0ATP synthase is an additional PKCδ substrate at the mitochondria that modulates cardiac injury (Figure 5A).
As discussed earlier, we recently found that GAPDH associates with the mitochondria following IR injury, where it mediates mitochondrial elimination by the lysosomal machinery.80 In addition to oxidation modification, we demonstrated that in a rat IR model and in cardiomyocytes, PKCδ induces GAPDH phosphorylation, predominantly on threonine 246, which results in cardiac damage by inhibiting the GAPDH-mediated mitophagy process for damaged mitochondrial elimination (Figure 5B). Therefore, targeting the interaction between PKCδ and GAPDH may reduce cardiac damage due to pathological-ROS disruption of micro-mitophagy.
CONCLUSION
Beyond their canonical function as “powerhouses” of the cell, mitochondria act as integral hubs of signaling pathways that determine cell fate. Their role in regulating cell death as well as their abundance in heart tissue make mitochondria an attractive therapeutic target for cardiovascular disorders. Among the various signaling pathways that converge on the mitochondria, ROS-mediated cellular responses have been of particular interest due to their association with age- and cardiovascular-related diseases. Whereas traditional notion suggests that ROS is exclusively pathological, through its contribution to oxidative stress, the use of ROS scavengers in the clinic to treat cardiovascular disorders has not been successful. Emerging research suggests that ROS also hold physiological and protective functions that gross treatment with anti-oxidants may eliminate. Therefore, an effective therapeutic approach must take ROS's pathological and physiological roles into account. Mitochondria are attractive targets for cardiovascular diseases because they are both a significant source of ROS and also the downstream ends of ROS-induced signaling pathways. Rather than eliminating all ROS directly, it is possible to pharmacologically target specific proteins in the mitochondria to selectively prevent the production of pathological ROS. In addition, inhibiting select downstream ROS pathways that result in cell damage and promoting ROS-induced cell survival pathways can involve the mitochondria.
A distinction between pathological and physiological mitochondrial ROS production can be achieved by selectively targeting damaged mitochondria. Selective inhibition of pathological fission prevents excessive fragmentation of mitochondria and reduces the production of ROS. Similarly, activating elimination of damaged mitochondria by macro- or micro-mitophagy will prune the dysfunctional mitochondria and avoid healthy nearby mitochondria to selectively reduce pathological ROS. Elucidation of the mechanisms that lead to enrichment of functional mitochondria, therefore, identifies important therapeutic targets to selectively reduce the production of cell-damaging ROS.
We also discussed additional therapeutic targets for heart disease that are downstream of ROS. These targets specifically activate cardioprotective pathways and inhibit damaging pathways. ROS activates PKCε signaling which in turn phosphorylates multiple mitochondrial substrates that elicit cardioprotective effects. Pharmacological agents that target these substrates directly mimic these protective effects in multiple cardiovascular disorders. Finally, there is a therapeutic opportunity in opposing ROS-mediated PKCδ activation and translocation to the mitochondria, which results in a harmful molecular cascade for the cell. Pharmacological inhibition of PKCδ translocation to the mitochondria is cardioprotective. Furthermore, inhibitors of mitochondrial substrates of ROS-activated PKCδ, including PDK and GAPDH, are also attractive targets for cardiovascular disease intervention. In culmination, mitochondria-based therapeutics that modulate molecular events upstream and downstream of ROS production provide a new approach for diseases of the old in general, and of ischemic cardiovascular disease in particular.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH grants HL52141 and NIAAA11147 to DMR.
Footnotes
Nonstandard Abbreviation and Acronyms:
None.
DISCLOSURES
DM-R and C-HC are founders of ALDEA pharmaceuticals. However none of the work in the DM-R lab is in collaboration with or supported by the company. The other authors have no potential conflict related to this work.
REFERENCES
- 1.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. Mol. Cell Cardiol. 1992;24(7):669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 2.Dromparis P, Michelakis ED. Mitochondria in vascular health and disease. Annu. Rev. Physiol. 2013;75:95–126. doi: 10.1146/annurev-physiol-030212-183804. [DOI] [PubMed] [Google Scholar]
- 3.Dedkova EN, Blatter LA. Mitochondrial Ca2+ and the heart. Cell Calcium. 2008;44(1):77–91. doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem. J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DI, Griendling KK. Regulation of Signal Transduction by Reactive Oxygen Species in the Cardiovascular System. Circ. Res. 2015;116:531–550. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011;51(5):978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahara EB, Navarete FDT, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic. Biol. Med. 2009;46(9):1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Goncalves RLS, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Brand MD. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015;290(16):209–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann PJG, Quastel JH. Toxic effects of oxygen and of hydrogen peroxide on brain metabolism. Biochem. J. 1946;40:139–144. doi: 10.1042/bj0400139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 12.Jaffery Z, Verma A, White CJ, Grant AG, Collins TJ, Grise MA, Jenkins JS, McMullan PW, Patel RA, Reilly JP, Thornton SN, Ramee SR. A randomized trial of intravenous N-acetylcysteine to prevent contrast induced nephropathy in acute coronary syndromes. Catheter. Cardiovasc. Interv. 2012;79:921–926. doi: 10.1002/ccd.23157. [DOI] [PubMed] [Google Scholar]
- 13.Lauri G, Campodonico J, Grazi M, Metrio M De, Galli S, Fabbiocchi F, Montorsi P, Veglia F, Bartorelli AL. N-Acetylcysteine and Contrast-Induced Nephropathy in Primary Angioplasty. N Engl J Med. 2006;354(26):2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 14.Camuglia a, Maeder M, Starr J, Farrington C, Kaye D. Impact of N-Acetylcysteine on Endothelial Function, B-type Natriuretic Peptide, and Renal Function in Patients with the Cardiorenal Syndrome: A Pilot Cross-over Randomised Controlled Trial. Hear. Lung Circ. 2010;19(4):S72–S73. doi: 10.1016/j.hlc.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Weisbord SD, Gallagher M, Kaufman J, et al. Prevention of contrast-induced AKI: A review of published trials and the design of the prevention of serious adverse events following angiography (PRESERVE) trial. Clin. J. Am. Soc. Nephrol. 2013;8:1618–1631. doi: 10.2215/CJN.11161012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee B-J, Lin J-S, Lin Y-C, Lin P-T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: a randomized, placebo-controlled trial. Nutr. J. 2014;13(79):1–7. doi: 10.1186/1475-2891-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue Y-Z, Wang L-X, Liu H-Z, Qi X-W, Wang X-H, Ren H-Z. L-carnitine as an adjunct therapy to percutaneous coronary intervention for non-ST elevation myocardial infarction. Cardiovasc. drugs Ther. 2007;21:445–448. doi: 10.1007/s10557-007-6056-9. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Karunakaran U, Jeoung NH, Jeon JH LI. Physiologicaleffect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014;21:3636–3645. doi: 10.2174/0929867321666140706141806. [DOI] [PubMed] [Google Scholar]
- 19.Tresguerres IF, Tamimi F, Eimar H, Barralet J, Prieto S, Torres J, Calvo-Guirado JL, Fernández-Tresguerres JA. Melatonin dietary supplement as an anti-aging therapy for age-related bone loss. Rejuvenation Res. 2014;17(4):1–26. doi: 10.1089/rej.2013.1542. [DOI] [PubMed] [Google Scholar]
- 20.Xiang G, Pu J, Yue L, Hou J, Sun H. Alpha-lipoic acid can improve endothelial dysfunction in subjects with impaired fasting glucose. Metabolism. 2011;60(4):480–485. doi: 10.1016/j.metabol.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B. Dietary α-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice. Circulation. 2008;117:421–428. doi: 10.1161/CIRCULATIONAHA.107.725275. [DOI] [PubMed] [Google Scholar]
- 22.Halladin NL, Busch SE, Jensen SE, Hansen HS, Zaremba T, Aarøe J, Rosenberg J, Gögenur I. Intracoronary and systemic melatonin to patients with acute myocardial infarction: Protocol for the IMPACT trial. Dan. Med. J. 2014;61:1–5. [PubMed] [Google Scholar]
- 23.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener H-C, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 24.Chan ED, Riches DW, White CW. Redox paradox: effect of N-acetylcysteine and serum on oxidation reduction-sensitive mitogen-activated protein kinase signaling pathways. Am. J. Respir. Cell Mol. Biol. 2001;24:627–632. doi: 10.1165/ajrcmb.24.5.4280. [DOI] [PubMed] [Google Scholar]
- 25.Sena LA, Chandel NS. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell. 2015;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461(7263):537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RAJ, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19(12):1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 28.Anisimov VN, Egorov MV, Krasilshchikova MS, et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging. 2011;3(11):1110–1119. doi: 10.18632/aging.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br. J. Pharmacol. 2014;171(8):2017–2028. doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, Gorman JH, Sloan RC, Frasier CR, Watson CA, Bostian PA, Kypson a P, Brown DA. Reduction of ischemia/reperfusion injury with Bendavia, a mitochondria-targeting cytoprotective peptide. J. Am. Heart Assoc. 2012;1:e001644–e001644. doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron. Artery Dis. 2007;18:215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- 33.Dai D-F, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH, Tian R, MacCoss MJ, Rabinovitch PS. Global Proteomics and Pathway Analysis of Pressure-Overload–Induced Heart Failure and Its Attenuation by Mitochondrial-Targeted Peptides. Circ. Hear. Fail. 2013;6(5):1067–1076. doi: 10.1161/CIRCHEARTFAILURE.113.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS, Marcinek DJ. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell. 2013;12:763–771. doi: 10.1111/acel.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakrabarti AK, Feeney K, Abueg C, Brown DA, Czyz E, Tendera M, Janosi A, Giugliano RP, Kloner RA, Weaver WD, Bode C, Godlewski J, Merkely B, Gibson CM. Rationale and design of the EMBRACE STEMI study: a phase 2a, randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of intravenous Bendavia on reperfusion injury in patients treated with standard therapy inclu. Am. Heart J. 2013;165(4):509–514. doi: 10.1016/j.ahj.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Dai D-F, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintrón M, Chen T, Marcinek DJ, Dorn GW, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial Oxidative Stress Mediates Angiotensin II–Induced Cardiac Hypertrophy and Gαq Overexpression–Induced Heart Failure. Circ. Res. 2011;108(7):837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 38.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–5. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Disatnik M-H, Hwang S, Ferreira JCB, Mochly-Rosen D. New therapeutics to modulate mitochondrial dynamics and mitophagy in cardiac diseases. J. Mol. Med. 2015;93:279–287. doi: 10.1007/s00109-015-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorn II GW. Mitochondrial Dynamism and Cardiac Fate. Circ. J. 2013;77(6):1370–1379. doi: 10.1253/circj.cj-13-0453. [DOI] [PubMed] [Google Scholar]
- 41.Beraud N, Pelloux S, Usson Y, Kuznetsov AV, Ronot X, Tourneur Y, Saks V. Mitochondrial dynamics in heart cells: Very low amplitude high frequency fluctuations in adult cardiomyocytes and flow motion in non beating Hl-1 cells. J. Bioenerg. Biomembr. 2009;41:195–214. doi: 10.1007/s10863-009-9214-x. [DOI] [PubMed] [Google Scholar]
- 42.Dorn GW. Mitochondrial dynamics in heart disease. Biochim. Biophys. Acta. 2013;1833(1):233–41. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc. Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan JJ, Marsboom G, Fang YH, et al. PGC1α-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2013;187(9):865–878. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsboom G, Toth PT, Ryan JJ, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization. Dev. Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 48.Yeon J-Y, Min S-H, Park H-J, Kim J, Lee Y-H, Park S-Y, Jeong P-S, Park H, Lee D-S, Kim S-U, Chang K-T, Koo D-B. Mdivi-1, mitochondrial fission inhibitor, impairs developmental competence and mitochondrial function of embryos and cells in pigs. J. Reprod. Dev. 2014 doi: 10.1262/jrd.2014-070. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorn GW. Gone fission...: diverse consequences of cardiac Drp1 deficiency. Circ. Res. 2015;116:225–228. doi: 10.1161/CIRCRESAHA.114.305672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW. Mitochondrial Fission and Fusion Factors Reciprocally Orchestrate Mitophagic Culling in Mouse Hearts and Cultured Fibroblasts. Cell Metab. 2015;21(2):273–285. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qvit N, Mochly-Rosen D. The many hats of protein kinase Cδ: one enzyme with many functions. Biochem. Soc. Trans. 2014;42:1529–1533. doi: 10.1042/BST20140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi X, Qvit N, Su Y-C, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 2013;126(3):789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Disatnik M-H, Ferreira JCB, Campos JC, Gomes KS, Dourado PMM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J. Am. Heart Assoc. 2013;2(5):e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo X, Disatnik MH, Monbureau M, Shamloo M, Mochly-Rosen D, Qi X. Inhibition of mitochondrial fragmentation diminishes Huntington's disease-associated neurodegeneration. J. Clin. Invest. 2013;123:5371–5388. doi: 10.1172/JCI70911. doi:10.1172/JCI70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat. Cell Biol. 2010;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: The yin and yang of cell death control. Circ. Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurihara Y, Kanki T, Aoki Y, Hirota Y, Saigusa T, Uchiumi T, Kang D. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J. Biol. Chem. 2012;287(5):3265–3272. doi: 10.1074/jbc.M111.280156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andres AM, Hernandez G, Lee P, Huang C, Ratliff EP, Sin J, Thornton C a, Damasco MV, Gottlieb R a. Mitophagy is required for acute cardioprotection by Simvastatin. Antioxid. Redox Signal. 2013;11(14):1960–1973. doi: 10.1089/ars.2013.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–80. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell RC, Tian Y, Yuan H, Park HW, Chang Y-Y, Kim J, Kim H, Neufeld TP, Dillin A, Guan K-L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013;15(7):741–50. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Bartolomeo S, Corazzari M, Nazio F, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J. Cell Biol. 2010;191(1):155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. USA. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuda N, Sato S, Shiba K, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010;189(2):211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Billia F, Hauck L, Konecny F, Rao V, Shen J, Wah T. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc. Natl. Acad. Sci. USA. 2011;108(23):9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 2012;125:795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Dorn GW. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340(6131):471–5. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song M, Chen Y, Gong G, Murphy E, Rabinovitch P, Dorn GW. Super-suppression of mitochondrial ROS signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ. Res. 2014;115(3):348–53. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ong S-B, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid. Redox Signal. 2013;19:400–14. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, Lavoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147(4):893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 74.Sun Y, Vashisht AA, Tchieu J, Wohlschlegel JA, Dreier L. Voltage-dependent anion channels (VDACs) recruit parkin to defective mitochondria to promote mitochondrial autophagy. J. Biol. Chem. 2012;287(48):40652–40660. doi: 10.1074/jbc.M112.419721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, Yin XM. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkinubiquitin-p62-mediated mitochondrial priming. J. Biol. Chem. 2010;285(36):27879–27890. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dorn GW. Mitochondrial pruning by nix and BNip3: An essential function for cardiac-expressed death factors. J. Cardiovasc. Transl. Res. 2010;3:374–383. doi: 10.1007/s12265-010-9174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu L, Feng D, Chen G, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012;14(2):177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 79.Kim I, Lemasters JJ. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid. Redox Signal. 2011;14(10):1919–1928. doi: 10.1089/ars.2010.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yogalingam G, Hwang S, Ferreira JCB, Mochly-Rosen D. Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH) Phosphorylation by Protein Kinase Cδ (PKCδ) Inhibits Mitochondria Elimination by Lysosomal-like Structures following Ischemia and Reoxygenation-induced Injury. J. Biol. Chem. 2013;288(26):18947–18960. doi: 10.1074/jbc.M113.466870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai Z, Yan L-J. Protein oxidative modifications: beneficial roles in disease and health. J. Biochem. Pharmacol. Res. 2013;1(1):15–26. [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang NR, Yim S-H, Kim YM, Jeong J, Song EJ, Lee Y, Lee JH, Choi S, Lee K-J. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem. J. 2009;423:253–264. doi: 10.1042/BJ20090854. [DOI] [PubMed] [Google Scholar]
- 83.Kohr MJ, Murphy E, Steenbergen C. Glyceraldehyde-3-phosphate dehydrogenase acts as a mitochondrial trans-S-nitrosylase in the heart. PLoS One. 2014;9(10):e111448. doi: 10.1371/journal.pone.0111448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morero RD, Viñals AL, Bloj B, Farías RN. Fusion of phospholipid vesicles induced by muscle glyceraldehyde-3-phosphate dehydrogenase in the absence of calcium. Biochemistry. 1985;24:1904–1909. doi: 10.1021/bi00329a015. [DOI] [PubMed] [Google Scholar]
- 85.Glaser PE, Gross RW. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry. 1995;34:12193–12203. doi: 10.1021/bi00038a013. [DOI] [PubMed] [Google Scholar]
- 86.Seidler N. GAPDH: Biological Properties and Diversity SE - 4.Vol 985. Advances in Experimental Medicine and Biology. Springer; Netherlands: 2013. Functional Diversity. pp. 103–147. [DOI] [PubMed] [Google Scholar]
- 87.Kaushik S, Bandyopadhyay U, Sridhar S, Kiffin R, Martinez-Vicente M, Kon M, Orenstein SJ, Wong E, Cuervo AM. Chaperone-mediated autophagy at a glance. J. Cell Sci. 2011;124:495–499. doi: 10.1242/jcs.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta - Mol. Cell Res. 2013;1833(2):410–416. doi: 10.1016/j.bbamcr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen MB, Jasper H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 2014;20(2):214–225. doi: 10.1016/j.cmet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maharjan S, Oku M, Tsuda M, Hoseki J, Sakai Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci. Rep. 2014;4:5896. doi: 10.1038/srep05896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 92.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J. Mol. Cell. Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 93.Das DK, Engelman RM, Maulik N. Oxygen free radical signaling in ischemic preconditioning. Ann. N. Y. Acad. Sci. 1999;874:49–65. doi: 10.1111/j.1749-6632.1999.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka M, Fujiwara H, Yamasaki K, Sasayama S. Superoxide dismutase and N-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischaemic preconditioning in the rabbit. Cardiovasc. Res. 1994;28:980–986. doi: 10.1093/cvr/28.7.980. [DOI] [PubMed] [Google Scholar]
- 95.Kabir AMN, Clark JE, Tanno M, Cao X, Hothersall JS, Dashnyam S, Gorog DA, Bellahcene M, Shattock MJ, Marber MS. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKCepsilon. Am. J. Physiol. 2006;291:H1893–H1899. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- 96.Dorn GW, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc. Natl. Acad. Sci. USA. 1999;96(22):12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Budas GR, Churchill EN, Disatnik M-H, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc. Res. 2010;88(1):83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Budas G, Costa HM, Ferreira JCB, Teixeira da Silva Ferreira ATS, Perales J, Krieger JE, Mochly-Rosen D, Schechtman D. Identification of εPKC targets during cardiac ischemic injury. Circ. J. 2012;76(6):1476–1485. doi: 10.1253/circj.cj-11-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, Caldwell RW, Johnson JA. Protein kinase C-epsilon coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am. J. Physiol. 2007;293:H2219–H2230. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- 101.Yu Q, Nguyen T, Ogbi M, Caldwell RW, Johnson JA. Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: exacerbation of COI subunit loss by PKC-epsilon inhibition. Am. J. Physiol. 2008;294:H2637–H2645. doi: 10.1152/ajpheart.91476.2007. [DOI] [PubMed] [Google Scholar]
- 102.Chouchani ET, Methner C, Nadtochiy SM, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19(6):753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cε interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bernardi P. The mitochondrial permeability transition pore: A mystery solved? Front. Physiol. 2013;4:1–12. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P. Identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:142–153. doi: 10.1016/j.yjmcc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Hausenloy DJ, Boston-Griffiths EA, Yellon DM. Cyclosporin A and cardioprotection: From investigative tool to therapeutic agent. Br. J. Pharmacol. 2012;165:1235–1245. doi: 10.1111/j.1476-5381.2011.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alam MR, Baetz D, Ovize M. Cyclophilin D and myocardial ischemia–reperfusion injury: A fresh perspective. J. Mol. Cell. Cardiol. 2015;78:80–89. doi: 10.1016/j.yjmcc.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 108.Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and Is a causative event in the death of myocytes in postischemic reperfusion of the heart. J. Biol. Chem. 2001;276(4):2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 110.Sweeney ZK, Fu J, Wiedmann B. From chemical tools to clinical medicines: nonimmunosuppressive cyclophilin inhibitors derived from the cyclosporin and sanglifehrin scaffolds. J. Med. Chem. 2014;57:7145–7159. doi: 10.1021/jm500223x. [DOI] [PubMed] [Google Scholar]
- 111.Waldmeier PC, Feldtrauer J-J, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 2002;62(1):22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- 112.Rosenwirth B, Billich A, Datema R, et al. Inhibition of human immunodeficiency virus type 1 replication by SDZ NIM 811, a nonimmunosuppressive cyclosporine analog. Antimicrob. Agents Chemother. 1994;38(8):1763–1772. doi: 10.1128/aac.38.8.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, Brown DA. Mitochondrial permeability transition in the diabetic heart: Contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J. Mol. Cell. Cardiol. 2012;52(5):1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 114.Hansson MJ, Mattiasson G, Månsson R, Karlsson J, Keep MF, Waldmeier P, Ruegg UT, Dumont JM, Besseghir K, Elmér E. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J. Bioenerg. Biomembr. 2004;36(4):407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- 115.Gomez L, Thibault H, Gharib A, Dumont J-M, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am. J. Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- 116.Fancelli D, Abate A, Amici R, et al. Cinnamic anilides as new mitochondrial permeability transition pore inhibitors endowed with ischemia-reperfusion injury protective effect in vivo. J. Med. Chem. 2014;57:5333–5347. doi: 10.1021/jm500547c. [DOI] [PubMed] [Google Scholar]
- 117.Auchampach JA, Grover GJ, Gross GJ. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc. Res. 1992;26:1054–1062. doi: 10.1093/cvr/26.11.1054. [DOI] [PubMed] [Google Scholar]
- 118.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ. Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 119.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, Shimamoto K. Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. Am. J. Physiol. 2002;283:H440–H447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 120.Jabůrek M, Costa ADT, Burton JR, Costa CL, Garlid KD. Mitochondrial PKCε and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ. Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 121.Costa ADT, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008;295(2):H874–82. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin C, Wu J, Watanabe M, Okada T, Iesaki T. Mitochondrial K+ channels are involved in ischemic postconditioning in rat hearts. J. Physiol. Sci. 2012;62:325–332. doi: 10.1007/s12576-012-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective Effect of Diazoxide and Its Interaction With Mitochondrial ATP-Sensitive K+ Channels: Possible Mechanism of Cardioprotection. Circ. Res. 1997;81(6):1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 124.Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, García-Dorado D, Di Lisa F, Schulz R, Heusch G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ. Res. 2005;97:583–586. doi: 10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
- 125.Testai L, Rapposelli S, Martelli A, Breschi MC, Calderone V. Mitochondrial Potassium Channels as Pharmacological Target for Cardioprotective Drugs. Med. Res. Rev. 2014;0(00):1–34. doi: 10.1002/med.21332. [DOI] [PubMed] [Google Scholar]
- 126.Badalzadeh R, Yousefi B, Tajaddini A, Ahmadian N. Diosgenin-induced protection against myocardial ischaemia-reperfusion injury is mediated by mitochondrial KATP channels in a rat model. Perfusion. 2015 doi: 10.1177/0267659114566064. In Press. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Z, Cui W, Zhang H, Gao H, Li X. Pre-treatment of a single high-dose of atorvastatin provided cardioprotection in different ischaemia / reperfusion models via activating mitochondrial KATP channel. Eur. J. Pharmacol. 2015:1–11. doi: 10.1016/j.ejphar.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 128.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin. Drug Metab. Toxicol. 2008;4(6):697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 130.Anderson EJ, Katunga LA, Willis MS. Mitochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin. Exp. Pharmacol. Physiol. 2012;39(2):179–193. doi: 10.1111/j.1440-1681.2011.05641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mali VR, Palaniyandi SS. Regulation and therapeutic strategies of 4-hydroxy-2-nonenal metabolism in heart disease. Free Radic. Res. 2013;48(3):251–263. doi: 10.3109/10715762.2013.864761. [DOI] [PubMed] [Google Scholar]
- 132.Budas GR, Disatnik M-H, Chen C-H, Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCε) knockout mice. J. Mol. Cell. Cardiol. 2010;48(4):757–764. doi: 10.1016/j.yjmcc.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H, Borinskaya S, Yoshimura K, Kal'ina N, et al. Refined geographic distribution of the oriental ALDH2* 504Lys (nee 487Lys) variant. Ann. Hum. Genet. 2009;73:335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo YJ, Chen L, Bai YP, Li L, Sun J, Zhang GG, Yang TL, Xia J, Li YJ, Chen XP. The ALDH2 Glu504Lys polymorphism is associated with coronary artery disease in Han Chinese: Relation with endothelial ADMA levels. Atherosclerosis. 2010;211(2):545–550. doi: 10.1016/j.atherosclerosis.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 135.Takeuchi F, Yokota M, Yamamoto K, et al. Genome-wide association study of coronary artery disease in the Japanese. Eur. J. Hum. Genet. 2012;20(3):333–340. doi: 10.1038/ejhg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takagi S, Iwai N, Yamauchi R, et al. Aldehyde dehydrogenase 2 gene is a risk factor for myocardial infarction in Japanese men. Hypertens. Res. 2002;25(5):677–681. doi: 10.1291/hypres.25.677. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Zhang Y, Zhang J, Tang X, Qian Y, Gao P, Zhu D. Association of a functional single-nucleotide polymorphism in the ALDH2 gene with essential hypertension depends on drinking behavior in a Chinese Han population. J. Hum. Hypertens. 2012;27(3):181–186. doi: 10.1038/jhh.2012.15. [DOI] [PubMed] [Google Scholar]
- 138.Hao PP, Xue L, Wang XL, Chen YG, Wang JL, Ji WQ, Xu F, Wei SJ, Zhang Y. Association between aldehyde dehydrogenase 2 genetic polymorphism and serum lipids or lipoproteins: A meta-analysis of seven East Asian populations. Atherosclerosis. 2010;212:213–216. doi: 10.1016/j.atherosclerosis.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 139.Xu F, Chen Y, Lv R, Zhang H, Tian H, Bian Y, Feng J, Sun Y, Li R, Wang R, Zhang Y. ALDH2 genetic polymorphism and the risk of type II diabetes mellitus in CAD patients. Hypertens. Res. 2010;33(1):49–55. doi: 10.1038/hr.2009.178. [DOI] [PubMed] [Google Scholar]
- 140.Chen C-H, Budas GR, Churchill EN, Disatnik M-H, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321(5895):1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perez-Miller S, Younus H, Vanam R, Chen C-H, Mochly-Rosen D, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat. Struct. Mol. Biol. 2010;17(2):159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beretta M, Gorren ACF, Wenzl MV, Weis R, Russwurm M, Koesling D, Schmidt K, Mayer B. Characterization of the East Asian variant of aldehyde dehydrogenase-2: Bioactivation of nitroglycerin and effects of Alda-1. J. Biol. Chem. 2010;285(2):943–952. doi: 10.1074/jbc.M109.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gong D, Zhang Y, Zhang H, Gu H, Jiang Q, Hu S. Aldehyde dehydrogenase-2 activation during cardioplegic arrest enhances the cardioprotection against myocardial ischemia-reperfusion injury. Cardiovasc. Toxicol. 2012;12:350–358. doi: 10.1007/s12012-012-9179-6. [DOI] [PubMed] [Google Scholar]
- 144.Gomes KMS, Bechara LRG, Lima VM, Ribeiro MAC, Campos JC, Dourado PM, Kowaltowski AJ, Mochly-Rosen D, Ferreira JCB. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post-myocardial infarction cardiomyopathy: Benefits of Alda-1. Int. J. Cardiol. 2015;179:129–138. doi: 10.1016/j.ijcard.2014.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gomes KMS, Campos JC, Bechara LRG, Queliconi B, Lima VM, Disatnik M-H, Magno P, Chen C-H, Brum PC, Kowaltowski AJ, Mochly-Rosen D, Ferreira JCB. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodeling. Cardiovasc. Res. 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stachowicz A, Olszanecki R, Suski M, Wisniewska A, Toton-Zuranska J, Madej J, Jawien J, Bialas M, Okon K, Gajda M, Gombik K, Basta-Kaim A, Korbut R. Mitochondrial aldehyde dehydrogenase activation by Alda-1 inhibits atherosclerosis and attenuates hepatic steatosis in apolipoprotein E-knockout mice. J. Am. Heart Assoc. 2014;3:e001329–e001329. doi: 10.1161/JAHA.114.001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo J-M, Liu A-J, Zang P, Dong W-Z, Ying L, Wang W, Xu P, Song X-R, Cai J, Zhang S-Q, Duan J-L, Mehta JL, Su D-F. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013;23:915–30. doi: 10.1038/cr.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guo Y, Yu W, Sun D, et al. A novel protective mechanism for mitochondrial aldehyde dehydrogenase (ALDH2) in type i diabetes-induced cardiac dysfunction: Role of AMPK-regulated autophagy. Biochim. Biophys. Acta - Mol. Basis Dis. 2014;1852:319–331. doi: 10.1016/j.bbadis.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 149.Ebert AD, Kodo K, Liang P, et al. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci. Transl. Med. 2014;6(255):255ra130–255ra130. doi: 10.1126/scitranslmed.3009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bright R, Raval AP, Dembner JM, Pérez-Pinzón MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J. Neurosci. 2004;24(31):6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009;15(11):1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qi X, Inagaki K, Sobel RA, Mochly-Rosen D. Sustained pharmacological inhibition of deltaPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J. Clin. Invest. 2008;118(1):173–182. doi: 10.1172/JCI32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108(19):2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 154.Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J. Biol. Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- 155.Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lord JM. PKC-delta is an apoptotic lamin kinase. Oncogene. 2000;19:2331–2337. doi: 10.1038/sj.onc.1203555. [DOI] [PubMed] [Google Scholar]
- 156.Raval AP, Dave KR, Prado R, Katz LM, Busto R, Sick TJ, Ginsberg MD, Mochly-Rosen D, Pérez-Pinzón MA. Protein kinase C delta cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. J. Cereb. Blood Flow Metab. 2005;25(6):730–741. doi: 10.1038/sj.jcbfm.9600071. [DOI] [PubMed] [Google Scholar]
- 157.Kim Y-A, Kim M-Y, Jung Y-S. Glutathione Depletion by L-Buthionine-S,R-Sulfoximine Induces Apoptosis of Cardiomyocytes through Activation of PKC-δ. Biomol. Ther. 2013;21(5):358–363. doi: 10.4062/biomolther.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bolli R, Jeroudi MO, Patel BS, DuBose CM, Lai EK, Roberts R, McCay PB. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc. Natl. Acad. Sci. USA. 1989;86(June):4695–4699. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ. Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 160.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc. Natl. Acad. Sci. USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Churchill EN, Szweda LI. Translocation of deltaPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch. Biochem. Biophys. 2005;439:194–199. doi: 10.1016/j.abb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 162.Monti M, Donnini S, Giachetti A, Mochly-Rosen D, Ziche M. δPKC inhibition or ε PKC activation repairs endothelial vascular dysfunction by regulating eNOS post-translational modification. J. Mol. Cell. Cardiol. 2010;48(4):746–756. doi: 10.1016/j.yjmcc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qi X, Vallentin A, Churchill E, Mochly-Rosen D. DeltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart. J. Mol. Cell. Cardiol. 2007;43(4):420–428. doi: 10.1016/j.yjmcc.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Churchill EN, Murriel CL, Chen C-H, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ. Res. 2005;97(1):78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 165.Inagaki K, Kihara Y, Izumi T, Sasayama S. The cardioprotective effects of a new 1,4-benzothiazepine derivative, JTV519, on ischemia/reperfusion-induced Ca2+ overload in isolated rat hearts. Cardiovasc. Drugs Ther. 2000;14:489–495. doi: 10.1023/a:1007884905461. [DOI] [PubMed] [Google Scholar]
- 166.Kheifets V, Bright R, Inagaki K, Schechtman D, Mochly-Rosen D. Protein kinase C delta (deltaPKC)-annexin V interaction: a required step in deltaPKC translocation and function. J. Biol. Chem. 2006;281(32):23218–26. doi: 10.1074/jbc.M602075200. [DOI] [PubMed] [Google Scholar]
- 167.Patel TB, Olson MS. Regulation of pyruvate dehydrogenase complex in ischemic rat heart. Am. J. Physiol. 1984;246(14):H858–H864. doi: 10.1152/ajpheart.1984.246.6.H858. [DOI] [PubMed] [Google Scholar]
- 168.Di Pancrazio F, Mavelli I, Isola M, Losano G, Pagliaro P, Harris DA, Lippe G. In vitro and in vivo studies of F(0)F(1)ATP synthase regulation by inhibitor protein IF(1) in goat heart. Biochim. Biophys. Acta. 2004;1659:52–62. doi: 10.1016/j.bbabio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 169.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemiareperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nguyen T, Ogbi M, Johnson JA. Delta protein kinase C interacts with the D subunit of the F1F0 ATPase in neonatal cardiac myocytes exposed to hypoxia or phorbol ester: Implications for F1F0 ATPase regulation. J. Biol. Chem. 2008;283:29831–29840. doi: 10.1074/jbc.M801642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kocharian A, Shabanian R, Rafiei-Khorgami M, Kiani A, Heidari-Bateni G. Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiol Young. 2009;19:501–506. doi: 10.1017/S1047951109990795. [DOI] [PubMed] [Google Scholar]
- 172.Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez MJ, Kaski JC, Reiter RJ, Jimenez-Sosa A. A unicenter, randomized, double-blind, parallel-group, placebo-controlled study of Melatonin as an Adjunct in patients with acute myocardial Infarction undergoing primary Angioplasty. The Melatonin Adjunct in the acute myocaRdial Infarction treated with A. Contemp. Clin. Trials. 2007;28:532–539. doi: 10.1016/j.cct.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 173.Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 2004;44(9):1780–1785. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.