Abstract
New psychoactive substances (NPS) have completely modified the drug scene and the current landscape of addiction. Synthetic substances, such as substituted or synthetic cathinones, also known as « legal highs », are often produced and used to mimic the effects of controlled drugs such as cocaine, methylenedioxymethamphetamine (MDMA, ecstasy), and methamphetamine. The overwhelming majority of synthetic cathinones are produced in China and South East Asian countries. The Internet has emerged as the new marketplace for NPS, playing a major role in providing information on acquisition, synthesis, extraction, identification, and substance use. All these compounds are intentionally mislabeled and sold on-line under slang terms such as bath salts, plant food, plant feeders and research chemicals. They are sometimes labeled « not for human use » or « not tested for hazards or toxicity ». The rapid spread of NPS forces member countries of the European Union to adapt their response to the potential new dangers that may cause. To date, not only health actors but also the general public need to be clearly informed and aware of dangers resulting from NPS spread and use. Here, we review the major clinical effects of synthetic cathinones to highlight their impact on public health. A literature search was conducted from 2009 to 2014 based on PubMed, Google Scholar, Erowid, and governmental websites, using the following keywords alone or in combination: “new psychoactive substances”, “synthetic cathinones”, “substituted cathinones”, “mephedrone”, “methylone”, “MDPV”, “4-MEC”, “addiction”, and “substance use disorder”.
Keywords: Addiction, MDPV, 4-MEC, mephedrone, methylone, new psychoactive substances, substance use disorder, substituted cathinones, synthetic cathinones
INTRODUCTION
New psychoactive substances (NPS) have completely modified the drug scene and the current landscape of addiction [1]. Synthetic substances, such as substituted or synthetic cathinones, also known as « legal highs », are a group of β-ketone amphetamine compounds derived from cathinone, the active stimulant in the khat plant. These drugs, still not controlled by international laws, are often produced and used to mimic the effects of controlled drugs such as cocaine, methylenedioxymethamphetamine (MDMA, ecstasy), and methamphetamine. NPS can appear on the market either under the guise of a controlled drug or as an alternative to a controlled drug [2]. In this way, 4-methylamphetamine was sold directly on the illicit drug market as amphetamine.
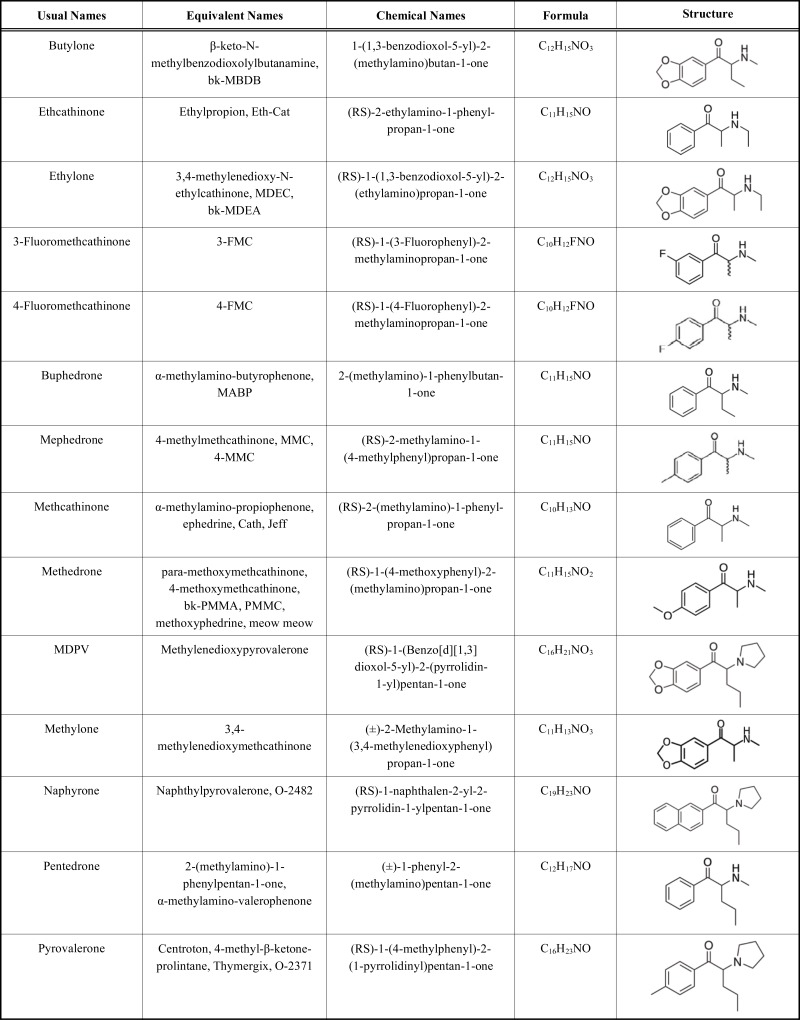
Synthetic cathinones and synthetic cannabinoids represent more than two thirds of the NPS available in this new drug market. The overwhelming majority of synthetic cathinones is produced in China and South East Asian countries (3). Mephedrone (4-methylmethcathinone), methylone (3,4-methylenedioxymethcathinone), 3,4- methylenedioxypyrovalerone (MDPV), methylethcathinone (4-MEC), 3-fluoromethcathinone (3-FMC), 4-fluoromethcathinone (4-FMC), buphedrone (alpha-methylamino-butyrophenone), butylone (beta-keto-N-methyl-3,4-benzodioxyolybutanamine), methedrone (4-methoxymethcathinone), pentedrone (α-methylaminovalerophenone) and naphyrone (naphthylpyrovalerone) are some of the most well-known synthetic cathinones (Table 1) [4].
Table 1.
Molecular structure of the most spread synthetic cathinones.
 |
The Drug Enforcement Administration (DEA) has noted that the terminology of synthetic cathinones tends to have a glamorous aura including « Meow Meow », « Bliss », « Energy-1 », « Hurricane Charlie », « White rush », « Bloom », « Blue magic », « Blue silk », « Cloud 9 », « Cloud 10 », « Mind Candy », « Rocket Fuel », « Sextasy », and « Torpedo ». All these compounds are intentionally mislabeled and sold on-line under slang terms such as bath salts, plant food, plant feeders and research chemicals. They are sometimes labeled « not for human use » or « not tested for hazards or toxicity » [3]. Their street price in the US is approximately $25–35 per half gram package, while in Europe prices range between 18 and 25 € per gram [1].
Constant modification of the chemical structure by covert laboratories allows manufacturers to stay one step ahead of the legal process. The Internet has emerged as the new marketplace for NPS, playing a major role in providing information on acquisition, synthesis, extraction, identification, and substance use. Manufacturers, suppliers, retailers, website-hosting and payment processing services may all be based on internet in different countries [5], making this new drug business particularly difficult to be controlled. Furthermore, the growing use of anonymised illegal networks (« Darknet ») for the sale of drugs to dealers and users adds to these challenges. In 2013, 651 websites selling « legal highs » to Europeans were identified [1].
The main sources providing information for the study of NPS are the European Monitoring Center for Drugs and Drug Abuse (EMCDDA) (reporting form, risk assessment report, drug profile), the European Union Early Warning System (EU-EWS) reports, the ESPAD School Survey Project, and the National Reitox reports as well as official governmental and international organizations documents, the Internet underground and governmental websites and the discussion groups [1]. The Psychonaut Web Mapping Project is a 2-year European Union-funded project aiming at developing a web scanning system to identify and categorize novel recreational drugs/psychoactive compounds and new trends in drug use based on information available on the Internet [6]. The draft European Drug Emergencies Network (EuroDEN) is also funded by Europe to identify the consumption of NPS among patients visiting emergency departments and analyze any differences between countries [7].
The rapid spread of NPS forces member countries of the European Union to adapt their response to the potential new dangers that may cause. In October 2011, mephedrone, MDPV and methylone were temporarily classified in the US as Schedule I controlled substances [8]. Additionally, a permanent Schedule I distinction was attributed in July 2012 to mephedrone and MDPV and further in 2013 to methylone [9,10]. Similarly, in July 2012, synthetic cathinones and derivatives were classified as illicit substances by the Agence Nationale de Sécurité du Médicament (ANSM) in France [11].
To date, not only health actors but also the general public need to be clearly informed and aware of dangers resulting from NPS spread and use [12]. Here, we review the major clinical effects of synthetic cathinones to highlight their impact on public health. A literature search was conducted from 2009 to 2014 based on PubMed, Google Scholar, Erowid, and governmental websites, using the following keywords alone or in combination: “new psychoactive substances”, “synthetic cathinones”, “substituted cathinones”, “mephedrone”, “methylone”, “MDPV”, “4-MEC”, “addiction”, and “substance use disorder”.
HISTORY OF SYNTHETIC CATHINONES
Catha edulis was discovered in Yemen by an eighteenth century botanist called Peter Forskal [13]. The fresh leaves of the shrub Catha edulis are chewed and occasionally brewed as tea in the Arabian Peninsula and in certain regions of Eastern Africa due to their stimulant effects [14]. Cathinone is the stimulant alkaloid found in the leaves of the khat bush. Cathinone was found to be chemically similar to ephedrine, cathine and other amphetamines. It produces amphetamine-like psychostimulatory effects, including euphoria, alertness and psychomotor hyperactivity, but its potency appears to be lower than that of amphetamine [15]. Many designer drugs were first synthesized by chemists in the pharmaceutical industry or in academia who were investigating potential new pharmaceuticals [16]. Methcathinone, synthesized in the first half of the 20th century [17], was the first synthetic cathinone with reports of abuse beginning in the early nineties [18]. Mephedrone and MDPV were first described in 1929 in the Bulletin de la Société Chimique de France [17] and in 1967 [19], respectively. Their abuse was not reported until the early 21st century, when they were claimed to be legal alternatives to MDMA on Internet drug websites [20]. Mephedrone had been the first synthetic cathinone detected by European authorities in November 2007 and notified via the EU-EWS in March 2008 [21]. By 2010, mephedrone was detected and seized in 28 European countries [22]. According to the 2010 Europol–EMCDDA Joint Report, mephedrone was usually found in combination with other synthetic cathinones including methylone, butylone, ethylcathinone, methoxymethcathinone, and fluoromethcathinone [22]. In addition, other associated substances were found including MDMA, mCPP, lactose, and caffeine [21]. Methylone was first reported in 2004 as a liquid solution sold as vanilla-scented room odoriser [23]. Furthermore, methylone was sold via the Internet and in headshops using plastic tubes containing 5 ml liquid called “Explosion” [24]. MDPV was first detected in Japan in 2006, in Germany in 2007, in Finland in 2008 and in the UK and Poland after the ban on mephedrone in 2010. Butylone, also known as bk-MBDB, was first synthesized by Koeppe and collaborators in 1967. It has been available at least since 1994 but its popularity on the synthetic drug market remains marginal [24].
EPIDEMIOLOGICAL DATA
Data on synthetic cathinones use are still limited. Available evidence suggests that in most countries, use of NPS including cathinones use remains low in the general population. The limited number of countries that have included synthetic cathinones in recent general population surveys indicated prevalence rates of 1 % or less among adults. Repeated surveys about NPS are only available in England and Wales [1]. By 2010, mephedrone was the third most commonly abused drug in the UK. During 2013, eighty-one NPS including seven synthetic cathinones were notified for the first time to the EU-EWS by one state member [1]. In France, MDPV and 4-MEC were among the 10 most popular NPS sold online during 2010-2011, [25]. According to the UK National Poisons Information Service, reported cases of cathinone-related toxicity increase from none in 2009 to over 600 cases in 2010 [26]. The Drug Abuse Warning Network (DAWN) reported that 22904 emergency department visits were due to bath salt exposures in 2011 [27]. Five per cent of the young Europeans declared that they have used NPS at least once, with higher prevalence observed in the UK, Ireland, Latvia, and Poland [22]. In a 2010 national survey, 0.7% of the Spanish students declared the use of “legal highs” during their lifetime and 0.4% mephedrone. In the same year, about 20% of 14-20 year-old UK individuals reported mephedrone use [28].
Based on different surveys, the use of mephedrone rapidly increased to reach the level of ecstasy use (1.4%) and even the level of cocaine use (4.4%) among the participants to the festive scene [1]. For both mephedrone and “legal highs”, prevalence of use in Northern Ireland was estimated at 2 % during lifetime and at 1 % during the previous year. Lifetime prevalence levels were higher among the 15 to 24-year aged group (6 %). NPS (previous year use, 4%) were the second most frequently reported illicit drugs after cannabis (previous year use, 6 %). In 2012-2013, last year use of mephedrone among adults (16–59 years old) was estimated at 0.5 %. A survey of individuals attending “gay friendly” nightclubs in South-East London in 2011 found that, among the 313 participants, lifetime use of a “legal high” and mephedrone was 65.8 % and 63.8 %, respectively [29]. A survey of UK regular clubbers showed a decrease in the use of mephedrone in the previous year (from 19.5 % in 2011 to 13.8 % in 2012). By 2012, 128 mephedrone-associated fatalities had been reported [30].
The injection of synthetic cathinones is reported rarely and is restricted to specific groups of high-risk drug users (Austria, Belgium, the Czech Republic, France, Germany, Ireland, Poland, Spain and the UK). However, in Hungary and Romania, a more significant prevalence of synthetic cathinones injection was identified among large cohorts of drug users [31].
PATTERNS OF USE
Populations of Users
Various populations of psychoactive drug users use NPS [1]. Profiles of synthetic cathinone addicts and abusers are limited to surveys of UK mephedrone users and individual case reports of toxicity [15]. There are increasing concern about some sub-groups of gay men involved in « Chem Sex Parties » [32]. Synthetic cathinones are typically used in parallel or in combination with other drugs such as methamphetamine (UK), GBL/GHB, cocaine (France) and sildenafil to enhance sexual experiences. Parties can last from few hours up to many days with the subjects frequently engaging in risky sexual practices (i.e. not using condoms, sharing multiple partners) [31]. There is an increased risk of transmission of hepatitis C, human immunodeficiency virus (HIV) and other sexually transmitted diseases.
Analysis of the current data also indicates the emergence of a population of young adults attending techno-alternative parties. Occasional drug users may use the Internet to acquire some NPS. Opioid and cocaine users may switch their drug consumption to cathinones or include them in their drug-using repertoire. E-trip reports should be considered by clinicians because of the shared experiences, mostly on private places between users or via dedicated Internet forums. These provide valuable information on consumption patterns.
Routes of Administration
Synthetic cathinones are not only frequently used as white powder or crystalline mixtures (bath salts) but are also taken orally as tablets [33]. Users frequently practice « keying » (snorting powder via a key) and « bombing » (ingesting powder rolled up in cigarette paper) [15]. The rapid onset of action from insufflation combined with the prolonged effects of ingestion results in immediate and sustained intoxication [15]. Rectal administration, gingival delivery, inhalation, intramuscular injection are less common routes [34]. There have been occasional reports of synthetic cathinones inserted into the eye (« eyeballing ») [35].
The intravenous route for cathinones or « slamming », (including mephedrone, MDPV, 4-MEC, and pentedrone) represents a major health concern [36]. Mixing mephedrone with heroin in a « speedball » type of use was also documented. Young drug users have been reported to start their injecting career with cathinones in some recreational settings. Similarly, long-term abstinent ex-opiate and ex-cocaine users, drug treatment clients, prisoners and men who have sex with men seem to be keen to inject cathinones [31].
EXPERIMENTAL DATA ON THE PHARMACOLOGY OF SYNTHETIC CATHINONES
Studies investigating mechanisms of action and toxicity of cathinones are sparse. Case reports or small case series describe features of toxicity, but confounding factors limit any definitive conclusions. Patients have usually consumed several drugs simultaneously. Features attributed to cathinones could be influenced and even mimicked by their underlying psychiatric and medical diseases. None of these reports have investigated the dose-effect relationships in the absence of human volunteer studies. To fill this gap, experimental studies, even though still rare, are helpful.
Synthetic cathinones-related effects rely on two major mechanisms, i.e. monoamine uptake blockade resulting from transporter inhibition and increased monoamine release as well as the combination of these two mechanisms (Table 2) [40]. Cathinones are all potent inhibitors of norepinephrine reuptake transporter, but marked differences exist in their inhibition profiles regarding dopamine and serotonin reuptake transporters as well as their ability to release monoamines, possibly explaining clinical differences reported in their effects and toxicities. MDPV acts as a potent and selective monoamine uptake blockers but has less impact on monoamine release than cocaine. Mephedrone and methylone act not only as nonselective monoamine uptake inhibitors like cocaine but also increase serotonin release similarly to MDMA. Cathinone highly inhibits dopamine but is a less potent serotonine reuptake transporter.
Table 2.
Classification of the different cathinones according to their relative potential of monoamine reuptake inhibition and release, in comparison to methylenedioxymethamphetamine (MDMA), methamphetamine, and cocaine.
| Monamine Reuptake Inhibition | Monoamine Release | |||||
|---|---|---|---|---|---|---|
| Dopamine | Norepinephrine | Serotonine | Dopamine | Norepinephrine | Serotonine | |
| MDMA-like cathinones | ||||||
| Mephedrone | +++ | +++ | ++ | ++ | ++ | ++ |
| Methylone | +++ | +++ | ++ | ++ | ++ | ++ |
| Methamphetamine-like cathinones | ||||||
| Cathinone | ++ | +++ | + | +++ | +++ | - |
| Methcathinone | ++ | +++ | + | +++ | +++ | - |
| Cocaine-like cathinones | ||||||
| Pyrovalerone | ++ | +++ | ++ | - | - | - |
| MDPV | ++ | +++ | ++ | - | - | - |
Synthetic cathinones constantly increased locomotor activity. MDPV is at least 10 times more potent than cocaine at producing locomotor activation and stereotypies [41], while mephedrone-induced effects on locomotor activity are similar to MDMA but lower than amphetamine [42]. Interestingly, response to MDPV is biphasic with increased activity at lower doses and suppression of activity at higher doses. Stereotypes, observed at elevated doses, are present with MDPV and mephedrone but not methylone. Effects on memory and learning behavior in rodents are imperfectly studied, with demonstrated long-term reduction in working memory by mephedrone but no significant effects assessed with methylone [43]. Mephedrone is an entactogenic drug with similar effects to MDMA, able to increase social interaction among rats [44].
Drug discriminative studies represent an effective model to investigate subjective drug effects in humans. Mice reliably discriminate MDPV from saline and cumulative doses of MDPV, MDMA and methamphetamine fully substituted for MDPV training stimulus [45]. In rats, MDPV, methylone, mephedrone, naphyrone, and butylone can fully substitute for the discriminative stimulus effects of cocaine and methamphetamine. Mephedrone effects are comparable to those of MDMA, cocaine and methamphetamine.
Acquisition of self-administration with MDPV is obtained with a greater potency and efficacy than with methamphetamine [46]. Escalation of intake is also observed at higher rather than lower MDPV doses [47]. These data highly suggest that MDPV may possess a unique reinforcing profile among psychostimulants.
CLINICAL TOXICOLOGY
General Data on Toxicity
Synthetic cathinone causes amphetamine-like psychoactive and sympathomimetic effects. They are mainly used for social and economic reasons in addition to their stimulant properties, often serving as replacement for others illicit stimulant drugs [37]. Psychotropic effects of substituted cathinones are individual-, dose- and route of administration-dependent [34]. The primary effects sought by users include increased alertness, empathy, euphoria, openness in communication, talkativeness, intensification of sensory experiences, music sensitivity, reduced appetite, insomnia, sexual performance, increased sociability and capacity to work [38].
Users report a number of negative physical and psychiatric effects associated with synthetic cathinones [3]. The main NPS adverse effects are summarized in Table 3. Cardiac, psychiatric, and neurological adverse effects are the most common reported ones requiring medical care. NPS use may lead to violence, homicidal combative behavior, self-mutilation, coma, and death [39]. Acute toxicity is the leading cause of NPS-induced fatalities. However, concomitant synthetic cathinones and other drugs use have been reported in numerous fatalities, limiting any definitive conclusion regarding their implication in the onset of death.
Table 3.
Adverse and toxic effects of synthetic cathinones
| Somatic Adverse Effects | Psychiatric Adverse Effects |
|---|---|
|
|
In 2012, 2.5% of telephone enquiries to the UK National Poisons Information Service and 2.4% of enquiries to US poisons centres are related to recreational drugs [50]. The retrospective telephone call data obtained from the UK Poisons information service showed that 28% of the presumed NPS-intoxicated persons presented agitation [29]. Interestingly, agitation is the most common symptom reported in the retrospective review of self-reported exposures in the Scottish emergency department (prevalence: 26%) [51], in the retrospective study from two US Poison centers (prevalence: 82%) [52], as well as in the prospective US series (prevalence: 66%) [53]. Detailed physical examination at the bedside allows recognizing the sympathomimetic toxidrome including psychosis, significant tachycardia, hypertension, and seizures. Signs attributed to serotonin toxicity are also not rare. Cardiovascular complications are commonly reported including chest pain, palpitation, and collapse. The majority of cathinon-exposed patients is tachycardic with increased systolic and diastolic blood pressure. Cases of cathinone-induced hyponatremia have been reported, questioning the possibility of over hydration in the setting of drug-induced secretion of vasopressin like with MDMA [54]. In a large series of 236 patients exposed to "bath salts" and "legal highs", bought under 37 separate "brand" names in the US and identified as MDPV by gas chromatography/mass spectrometry when performed, the following clinical consequences were described: agitation (82%), combative behavior (57%), tachycardia (56%), hallucinations (40%), paranoia (36%), confusion (35%), chest pain (17%), myoclonus (19%), hypertension (17%), mydriasis (13%), CPK elevations (9%), hypokalemia (4%), and blurred vision (3%) [52].
Management of synthetic cathinone-induced toxicity is primarily supportive and may necessitate admission to the intensive care unit. Intravenous fluids, seizure-prevention measures, close monitoring and restraints are generally recommended to prevent harm to self or others [37, 55, 56]. Other key-measures include benzodiazepines to treat agitation seizures, and sympathomimetic symptoms; aggressive cooling to treat hyperthermia; water restriction or hypertonic saline to treat hyponatremia; mechanical ventilation to protect airways and treat respiratory failure; fluids and catecholamine infusion to treat shock; and dialysis to treat severe metabolic disturbances and acute renal failure. In the series from the US poison centers, patients were treated and released from the emergency department (49%), admitted to the intensive care unit (21%), admitted to the psychiatry ward (12%) and lost to follow up (12%); additionally, one fatality was reported [52].
Specific Considerations on the Most Popular Synthetic Cathinones
Mephedrone-related psychoactive effects last from 1 to 4 h and resemble those of methamphetamine, including euphoria, elevated mood, alertness, increased concentration, talkativeness, empathy, an “urge to move”, pleasurable rushing, sense of being sped up, enhanced music appreciation, elevated mood, and mild sexual stimulation [40]. The higher the dose or the more prolonged the mephedrone use is and, the more severe unwanted effects appear. The first reported death related solely to mephedrone was a Swedish decedent with hyponatremia and brain edema [61]. Several additional cases were attributed to mephedrone, at least as adjunctive causative agent [62, 63].
MDPV-related psychoactive effects resembling those of cocaine, which lasts from 3 to 4 hours [41]. Its short duration of action leads users to consume numerous doses in succession [15], to counteract the unpleasant comedown effects. MDPV use may result in tolerance and consequently in overdose (42). Recent findings indicated that MDPV has reinforcing properties and activates brain reward circuitry, suggesting a potential for abuse and addiction in humans [43]. Patients using MDPV are prone to development of bizarre behaviors, hallucinations suicidality, and excited delirium syndrome, a condition described with phencyclidine at considerable risk for serious medical morbidity like hyperthermia, rhabdomyolysis, kidney failure and even fatality [67-69]. MDPV has been detected in up to 107 non-fatal intoxications and 99 deaths, particularly in Finland and in the UK [34, 44].
Methylone, a close structural analogue of MDMA and the second most popular designer drug used in 2010 and combined to mephedrone [21], is responsible for psychoactive effects resembling those of MDMA [45]. At average doses of 100 to 200 mg, effects of methylone include calmer euphoria, alertness, restlessness, strong feeling of empathy, with milder stimulation. Antidepressant effects, number of significant adverse effects and even fatalities [74] were attributed to methylone use, mainly in association with other illicit drugs.
4-MEC, a synthetic stimulant chemically similar to methcathinone with empathogenic effects, is marketed alone and in mixtures containing other synthetic cathinones under the names NRG-1 or NRG-2 [15]. Users report that 4-MEC involves multiple time redosing (“boosting”, “bumping”), with difficulty not redosing after using a strong dose if more 4-MEC is available when they are starting to come down. Multidrug poisoning involving 4-MEC as well as acute tolerance to this synthetic cathinone was reported [46].
Pentedrone, identified in 2010 as one of the varieties of bath salts throughout the US and UK. Administered by a variety of different routes including intravenously, pentedrone has stimulant effects and a number of adverse effects.
Naphyrone, structurally similar to mephedrone and MDPV, is genrally found in mixtures of cathinones, called NRG-1 and NRG-3, mainly including 4-fluoromethcathinone, MDPV, 3',4'-methylenedioxy-α-pyrrolidinobutyrophenone (MDPBP), 4'-methyl-α-pyrrolidinopropiophenone (4-MePPP, MPPP or MαPPP), and β-keto-methylbenzodioxolylpentanamine (pentylone, bk-methyl-K, bk-MBDP). Naphyrone’s psychoactive and side-effects are thus rather difficult to distinguish from those of the other NPS present in the mixture. However, one 27-year old male who ingested 1 g of naphyrone was reported to have developed a prolonged high associated with palpitations, sweating and insomnia [77].
CONCLUSION
In the last 8 years, NPS, including synthetic cathinones, has extensively dominated the drug scene in Europe and the US. Many drug users have switched from their traditional drugs to NPS use. Several factors have contributed to their increasing popularity including their falsely legal image, their more reasonable costs, and their distribution based on the new technologies. However, major health issues have emerged in relation to the somatic, mental, and addictive consequences of their use with persistent unknowns for the future. It is mandatory to develop clinical research and improve the management of addiction and poisonings attributed to these NPS.
CONFLICT OF INTEREST
Dr Laurent Karila receives consulting fees from Sanofi Aventis, BMS Otsuka, Lundbeck, Gillead, Shering Plough, Eutherapie, Merck/Serono, Astra Zeneca Pharmaceuticals, Bouchara-Recordati, Jansse-Cilag, DA Pharma Phar-maceuticals.
Prof Olivier Cottencin receives consulting fees from Bouchara-Recordati, Reckitt, Lundbeck, Janssen-cilag.
Prof Michel Lejoyeux receives consulting fees from Merck/Serono, Lundbeck.
ACKNOWLEDGEMENTS
Declared none.
References
- 1.E.M.C.D.D.A. European Monitoring Centre for Drugs and Drug Addiction European Drug Report 2014; Trends and developments; Publications Office of the European Union:; Luxembourg. 2014. pp. 1–80. [Google Scholar]
- 2.Karila L. Emergency of synthetic drugs in the general landscape of addiction. Rev. Prat. 2012;62(5):661–663. [PubMed] [Google Scholar]
- 3.Valente M.J., Guedes de Pinho P., de Lourdes Bastos M., Carvalho F., Carvalho M. Khat and synthetic cathinones: a review. Arch. Toxicol. 2014;88(1):15–45. doi: 10.1007/s00204-013-1163-9. [DOI] [PubMed] [Google Scholar]
- 4.Cottencin O., Rolland B., Karila L. New designer drugs (synthetic cannabinoids and synthetic cathinones): review of literature. Curr. Pharm. Des. 2014;20(25):4106–4111. doi: 10.2174/13816128113199990622. [DOI] [PubMed] [Google Scholar]
- 5.Karila L., Mégarbane B., Chevillard L., Benturquia N., Laplanche J.L., Lejoyeux M. Nov el Psychoactive Substances: A review. Press. Med. 2011. pp. pii: S0755-4982–(15)00003-2. Epub a head of print. [DOI] [PubMed]
- 6.Karila L., Reynaud M. GHB and synthetic cathinones: clinical effects and potential consequences. Drug Test. Anal. 2011;3(9):552–559. doi: 10.1002/dta.210. [DOI] [PubMed] [Google Scholar]
- 7.Randolph S.A. Synthetic drugs: bath salts and spice. Workplace Health Saf. 2014;62(2):88. doi: 10.3928/21650799-20140121-06. [DOI] [PubMed] [Google Scholar]
- 8.Deluca P., Davey Z., Corazza O., Di Furia L., Farre M., Flesland L.H., Mannonen M., Majava A., Peltoniemi T., Pasinetti M., Pezzolesi C., Scherbaum N., Siemann H., Skutle A., Torrens M., van der Kreeft P., Iversen E., Schifano F. Identifying emerging trends in recreational drug use; outcomes from the Psychonaut Web Mapping Project. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):221–226. doi: 10.1016/j.pnpbp.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Wood D.M., Heyerdahl F., Yates C.B., Dines A.M., Giraudon I., Hovda K.E., Dargan P.I. The European Drug Emergencies Network (Euro-DEN) Clin. Toxicol. (Phila.) 2014;52(4):239–241. doi: 10.3109/15563650.2014.898771. [DOI] [PubMed] [Google Scholar]
- 10.Drug Enforcement Administration, Department of Justice Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed. Regist. 2011;76(204):65371–65375. [PubMed] [Google Scholar]
- 11.U.S. Drug Enforcement Administration, Office of Diversion Control. National Forensic Laboratory Information System Midyear Report. U.S. Drug Enforcement Administration. http://www.deadiversion.usdoj.gov/nflis/2012midyear.pdf . [Accessed on: 4th October, 2014].
- 12.American Association of Poison Control Centers. Bath salts data. http://www.aapcc.org/alerts/bath-salts/ 2014. [Accessed on: 4th October].
- 13.Journal Officiel de la République Française. Arrêté du 27 juillet 2012 modifiant les arrêtés du 22 février 1990 fixant la liste des substances classées comme stupéfiants et la liste des substances psychotropes. http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000026246525&dateTexte=&categorieLien=id . [Accessed on: 4th October, 2014].
- 14.Karila L., Petit A., Cottencin O., Coscas S., Reynaud M. Synthetic drugs: the new low-cost landscape of drugs. Rev. Prat. 2012;62(5):664–666. [PubMed] [Google Scholar]
- 15.Krikorian A.D. Kat and its use: an historical perspective. J. Ethnopharmacol. 1984;12:115–178. doi: 10.1016/0378-8741(84)90047-3. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths P., Lopez D., Sedefov R., Gallegos A., Hughes B., Noor A., Royuela L. Khat use and monitoring drug use in Europe: the current situation and issues for the future. J. Ethnopharmacol. 2010;132(3):578–583. doi: 10.1016/j.jep.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Zawilska J.B., Wojcieszak J. Designer cathinones--an emerging class of novel recreational drugs. Forensic Sci. Int. 2013;231(1-3):42–53. doi: 10.1016/j.forsciint.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 18.King L.A., Kicman A.T. A brief history of ‘new psychoactive substances’. Drug Test. Anal. 2011;3(7-8):401–403. doi: 10.1002/dta.319. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez S. Sur un homologue de l'éphédrine. Bull. Soc. Chim. Fr. 1929;45:284–286. [Google Scholar]
- 20.Emerson T.S., Cisek J.E. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann. Emerg. Med. 1993;22(12):1897–1903. doi: 10.1016/S0196-0644(05)80419-6. [DOI] [PubMed] [Google Scholar]
- 21.German C.L., Fleckenstein A.E., Hanson G.R. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97(1):2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris H. Mephedrone: the phantom menace Vice Magazine, [Online] 2010. http://www.vice.com/en_ca/read/hamilton-s-pharmacopeia-455-v17n6 . [Accessed on: 4th October, 2014]. pp. 98–100.
- 23.E.M.C.D.D.A. EMCDDA and Europol step up information collection on mephedrone. http://www.emcdda.europa.eu/publications/drugnet/online/2010/69/article3 . [Accessed on: 4th October, 2014].
- 24. E.M.C.D.D.A. European Monitoring Center for Drugs and Drug Abuse: Annual report 2011. The state of the drug problem in Europe. http://www.emcdda.europa.eu/publications/annual-report/ . [Accessed on: 4th October, 2014].
- 25.Erowid. http://www.erowid.org/chemicals/methylone/methylone_info1.shtml . [Accessed on: 4th October, 2014].
- 26.Meyer M.R., Wilhelm J., Peters F.T., Maurer H.H. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2010;397(3):1225–1233. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- 27.Katz D.P., Bhattacharya D., Bhattacharya S., Deruiter J., Clark C.R., Suppiramaniam V., Dhanasekaran M. Synthetic cathinones: “a khat and mouse game”. Toxicol. Lett. 2014;229(2):349–356. doi: 10.1016/j.toxlet.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Lahaie E., Martinez M., Cadet-Taïrou A. Nouveaux produits de synthèse et Internet. 2013;84:8–8. [Google Scholar]
- 29.James D., Adams R., Spears R., Cooper G., Lupton D.J., Thompson J.P., Thomas S.H. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg. Med. J. 2011;28:686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The DAWN report. http://www.samhsa.gov/data/spotlight/spot117- bath-salts-2013.pdf . [Accessed on: 4th October, 2014].
- 31.Dargan P.I., Albert S., Wood D.M. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM. 2010;103(11):875–879. doi: 10.1093/qjmed/hcq134. [DOI] [PubMed] [Google Scholar]
- 32.EMCDDA–Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA. http://www.samhsa.gov/data/spotlight/spot117- bath-salts-2013.pdf . [Accessed on: 4th October, 2014].
- 33.Schifano F., Corkery J., Ghodse A.H. Suspected and confirmed fatalities associated with mephedrone (4-methylmethcathinone, “meow meow”) in the United Kingdom. J. Clin. Psychopharmacol. 2012;32(5):710–714. doi: 10.1097/JCP.0b013e318266c70c. [DOI] [PubMed] [Google Scholar]
- 34.E.M.C.D.D.A. Perspectives on drugs. Injection of synthetic cathinones. http://www.emcdda.europa.eu/topics/pods/syntheticcathinones-injection . [Accessed on: 4th October, 2014].
- 35.Bourne A., Reid D., Hickson F., Torres Rueda S., Weatherburn P. The chemsexstudy: drug use in sexual settings among gay and bisexual men in Lambeth, Southwark and Lewisham. Sigma Research, London School of Hygiene and Tropical Medicine, London. http://www.sigmaresearch.org.uk/chemsex . [Accessed on: 4th October, 2014].
- 36.Wood D.M., Hunter L., Measham F., Dargan P.I. Limited use of novel psychoactive substances in South London nightclubs. QJM. 2012;105(10):959–964. doi: 10.1093/qjmed/hcs107. [DOI] [PubMed] [Google Scholar]
- 37.Prosser J.M., Nelson L.S. The toxicology of bath salts: a review of synthetic cathinones. J. Med. Toxicol. 2012;8(1):33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CREW. Info sheet: Naphyrone. http://crew2000.org.uk . [Accessed on: 4th October, 2014].
- 39.Foureur N., Fournier S., Jauffret-Roustide M., et al. SLAM, première enquête qualitative en France. http://www.aides.org/download.php?filepath=/sites/default/files/doc/Rapport_SLAM.pdf . [Accessed on: 4th October, 2014].
- 40.Simmler L.D., Buser T.A., Donzelli M., Schramm Y., Dieu L.H., Huwyler J., Chaboz S., Hoener M.C., Liechti M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumann M.H., Partilla J.S., Lehner K.R., Thorndike E.B., Hoffman A.F., Holy M., Rothman R.B., Goldberg S.R., Lupica C.R., Sitte H.H., Brandt S.D., Tella S.R., Cozzi N.V., Schindler C.W. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine 3.-methylenedioxymethamphetamine 3.4-methylenedioxypyrovalerone and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López-Arnau R., Martínez-Clemente J., Pubill D., Escubedo E., Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br. J. Pharmacol. 2012;126:168–175. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motbey CP, Hunt GE, Bowen MT, Artiss S. Mephedrone (4-methylmethcathinone, 'meow'): acute behavioural effects and distribution of Fos expression in adolescent rats.. Addict. Biol. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- 45.Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of 'bath salt' cathinones. Behav. Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aarde S.M., Huang P.K., Creehan K.M., Dickerson T.J., Taffe M.A. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watterson L.R., Kufahl P.R., Nemirovsky N.E., Sewalia K., Grabenauer M., Thomas B.F., Marusich J.A., Wegner S., Olive M.F. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict. Biol. 2014;19(2):165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baumann M.H., Partilla J.S., Lehner K.R. Psychoactive “bath salts”: not so soothing. Eur. J. Pharmacol. 2013;698(1-3):1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenbaum C.D., Carreiro S.P., Babu K.M. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J. Med. Toxicol. 2012;8(1):15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood D.M., Hill S.L., Thomas S.H., Dargan P.I. Using poisons information service data to assess the acute harms associated with novel psychoactive substances. Drug Test. Anal. 2014;6(7-8):850–860. doi: 10.1002/dta.1671. [DOI] [PubMed] [Google Scholar]
- 51.Regan L., Mitchelson M., Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg. Med. J. 2011;28(12):1055–1058. doi: 10.1136/emj.2010.103093. [DOI] [PubMed] [Google Scholar]
- 52.Spiller H.A., Ryan M.L., Weston R.G., Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin. Toxicol. (Phila.) 2011;49(6):499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (CDC) Emergency department visits after use of a drug sold as “bath salts”--Michigan, November 13, 2010-March 31, 2011. MMWR Morb. Mortal. Wkly. Rep. 2011;60(19):624–627. [PubMed] [Google Scholar]
- 54.Boulanger-Gobeil C., St-Onge M., Laliberté M., Auger P.L. Seizures and hyponatremia related to ethcathinone and methylone poisoning. J. Med. Toxicol. 2012;8(1):59–61. doi: 10.1007/s13181-011-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross E.A., Reisfield G.M., Watson M.C., Chronister C.W., Goldberger B.A. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. Am. J. Med. 2012;125(9):854–858. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 56.Regunath H., Ariyamuthu V.K., Dalal P., Misra M. Bath salt intoxication causing acute kidney injury requiring hemodialysis. Hemodial. Int. 2012;16(Suppl. 1):S47–S49. doi: 10.1111/j.1542-4758.2012.00750.x. [DOI] [PubMed] [Google Scholar]
- 57.Gibbons S., Zloh M. An analysis of the ‘legal high’ mephedrone. Bioorg. Med. Chem. Lett. 2010;20(14):4135–4139. doi: 10.1016/j.bmcl.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 58.Petit A., Karila L., Sananes M., Lejoyeux M. Mephedrone: a new synthetic drug. Presse Med. 2013;42(10):1310–1316. doi: 10.1016/j.lpm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Karila L., Reynaud M. Mephedrone: A designer drug legally available on the Web. Presse Med. 2010;39(7-8):834–835. doi: 10.1016/j.lpm.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Wood D.M., Dargan P.I. Mephedrone (4-methylmethcathinone): what is new in our understanding of its use and toxicity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):227–233. doi: 10.1016/j.pnpbp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Gustavsson D., Escher C. Mephedrone--Internet drug which seems to have come and stay. Fatal cases in Sweden have drawn attention to previously unknown substance. Lakartidningen. 2009;106(43):2769–2771. [PubMed] [Google Scholar]
- 62.Dickson A.J., Vorce S.P., Levine B., Past M.R. Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin. J. Anal. Toxicol. 2010;34(3):162–168. doi: 10.1093/jat/34.3.162. [DOI] [PubMed] [Google Scholar]
- 63.Maskell P.D., De Paoli G., Seneviratne C., Pounder D.J. Mephedrone (4-methylmethcathinone)-related deaths. J. Anal. Toxicol. 2011;35(3):188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- 64.Cameron K., Kolanos R., Vekariya R., De Felice L., Glennon R.A. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl.) 2013;227(3):493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coppola M., Mondola R. 3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol. Lett. 2012;208(1):12–15. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Watterson L.R., Kufahl P.R., Nemirovsky N.E., Sewalia K., Grabenauer M., Thomas B.F., Marusich J.A., Wegner S., Olive M.F. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict. Biol. 2014;19(2):165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Penders T.M., Gestring R.E., Vilensky D.A. Intoxication delirium following use of synthetic cathinone derivatives. Am. J. Drug Alcohol Abuse. 2012;38(3):616–617. doi: 10.3109/00952990.2012.694535. [DOI] [PubMed] [Google Scholar]
- 68.Thornton S.L., Gerona R.R., Tomaszewski C.A. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J. Med. Toxicol. 2012;8(3):310–313. doi: 10.1007/s13181-012-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kesha K., Boggs C.L., Ripple M.G., Allan C.H., Levine B., Jufer-Phipps R., Doyon S., Chi P., Fowler D.R. Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature. J. Forensic Sci. 2013;58(6):1654–1659. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- 70.Meyer M.R., Maurer H.H. Metabolism of designer drugs of abuse: an updated review. Curr. Drug Metab. 2010;11(5):468–482. doi: 10.2174/138920010791526042. [DOI] [PubMed] [Google Scholar]
- 71.Dal Cason T.A., Young R., Glennon R.A. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol. Biochem. Behav. 1997;58(4):1109–1116. doi: 10.1016/S0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- 72.Bossong M.G., Van Dijk J.P., Niesink R.J. Methylone and mCPP, two new drugs of abuse? Addict. Biol. 2005;10(4):321–323. doi: 10.1080/13556210500350794. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu E., Watanabe H., Kojima T., Hagiwara H., Fujisaki M., Miyatake R., Hashimoto K., Iyo M. Combined intoxication with methylone and 5-MeO-MIPT. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(1):288–291. doi: 10.1016/j.pnpbp.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 74.McIntyre I.M., Hamm C.E., Aldridge L., Nelson C.L. Acute methylone intoxication in an accidental drowning--a case report. Forensic Sci. Int. 2013;231(1-3):e1–e3. doi: 10.1016/j.forsciint.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Araujo A.M., Valente M.J., Carvalho M., Dias da Silva D., Gaspar H., Carvalho F., de Lourdes B.M., Guedes de Pinho P. Raising awareness of new psychoactive substances: chemical analysis and in vitro toxicity screening of 'legal high' packages containing synthetic cathinones. Arch. Toxicol. doi: 10.1007/s00204-014-1278-7. in press. [DOI] [PubMed] [Google Scholar]
- 76.Belhadj-Tahar H., Sadeg N. Methcathinone: a new postindustrial drug. Forensic Sci. Int. 2005;153:99–101. doi: 10.1016/j.forsciint.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 77.Wood D.M., Davies S., Cummins A., Button J., Holt D.W., Ramsey J., Dargan P.I. Energy-1 (‘NRG-1’): don’t believe what the newspapers say about it being legal. Emerg. Med. J. 2011;28(12):1068–1070. doi: 10.1136/emj.07.2010.3184rep. [DOI] [PubMed] [Google Scholar]


