Abstract
Heart and kidney are closely related in the clinical syndrome of heart failure (HF). It is now sufficiently clear that renal dysfunction occurs frequently in all phenotypes of HF, and when present, it is associated with higher mortality and morbidity. While the pathophysiology is multifactorial, the most important factors are a reduced renal perfusion and venous congestion. Recent interest has focused on worsening renal function (WRF), a situation strongly related to mortality, but seemingly only when HF status deteriorates. Unfortunately, to date clinicians are unable to identify specifically those patients with a grim prognosis following WRF. Although much has been learned on cardiorenal interaction in HF, still more questions have been left unanswered. The coming decade should provide us with more dedicated epidemiologic, mechanistic, and controlled trials in HF patients with reduced renal function. An updated classification of the cardiorenal syndrome that incorporates recent evidence and points towards areas of interest and uncertainties, and areas where progress is needed could facilitate this process. Ultimately, this should lead to preventive and treatment strategies that can preserve renal function and associated outcome in patients with HF.
Keywords: Heart failure, Renal dysfunction, Cardiorenal interaction
Introduction
The marriage between heart and kidney is like any other relationship; it resembles a rollercoaster ride with frequent ups and downs, and in some cases, an unexpected early ending. In health, they both contribute to the wellbeing of the whole body. However, once either falls ill, the other organ frequently suffers as well. Although the heart has intense relationships with other organs, the marriage to the kidneys is particularly special. The heart is directly dependent of the regulation of salt and water content of the body by the kidneys, and vise versa, the kidneys are directly dependent of blood flow and pressure generated by the heart. This is especially true in conditions of increased congestion and extracellular water content, such as heart failure (HF), this interdependency of both organs can result in a vicious circle where deterioration of either organ results in a severe, potentially self-perpetuating, high-mortality condition. We have come to know this relationship as the cardiorenal syndrome, a term highlighting the fact that it represents a multitude of often overlapping disease states that all together are part of the same condition.1 The last decade has seen a remarkable re-appraisal of the interaction between heart and kidney disease, especially in HF, and progress has been made in the recognition, risk stratification, and public awareness of the syndrome. Unfortunately, as we will discuss, there is no specific evidence-based effective treatment of patients with HF experiencing deterioration of renal function, although currently available HF treatment is not always insufficient. In the present review, we will highlight insights from the last 5 to 10 years in the terminology, pathophysiology, prognosis, and possible treatment of HF patients with concomitant renal dysfunction.
Classification and terminology
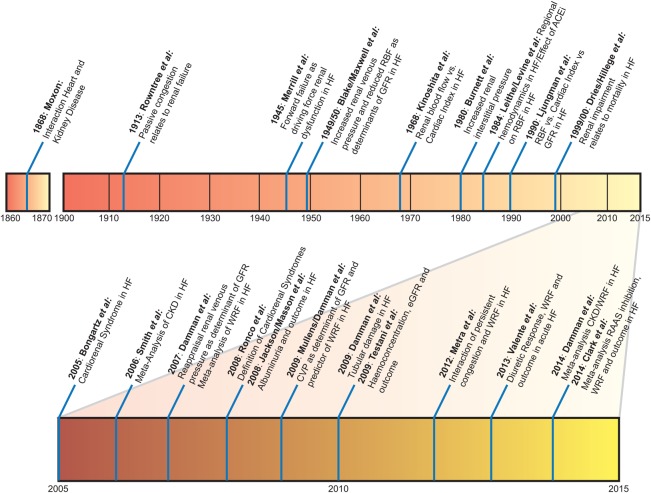
Although the term ‘cardiorenal syndrome’ is now in use for little over a decade, this does not mean that the interaction between heart and kidney was unrecognized before. In fact, the finding of renal dysfunction in the presence of heart disease has been widely studied, especially in the first part of the 20th century (Figure 1).2–4 The cardiorenal syndrome is also not limited to patients with HF, as cardiovascular disease (including HF) frequently develops in patients with chronic and acute kidney disease, and signifies a poor outcome.5 Significant recognition of cardiorenal interactions as a syndrome occurred around 2004 with multiple publications, followed by a description of the condition as a distinct entity by Ronco and colleagues, suggesting that at least five conceptual subtypes may exist.1,6,7 This classification has been of great value for awareness among researchers and clinicians, as well as the identification of patients. However, it is based largely on expert opinion and data to support the distinction based on pathophysiology, treatment, and prognosis is limited. In fact, one could even argue that there is only scarce evidence to classify the cardiorenal syndrome as a true distinct entity as it could merely be regarded as a physiological (and passive) response of the kidney to a failing heart. With new data and evidence from the last 10 years which will be discussed in this review, it may be necessary to update and change this classification.
Figure 1.
History of research in cardiorenal interaction. Overview of some key investigations in cardiorenal research. For reference list, see Supplementary material online, files.
Epidemiology
Baseline renal function
Around 4.5% of people in the general population have an eGFR <60 mL/min/1.73 m2 (normally regarded as CKD), while over 50% of patients with acute and chronic HF (both preserved and reduced) have a similar reduction in eGFR.8
The prognostic importance of a reduction in GFR has only relatively recently been recognized. Two landmark retrospective analyses from randomized controlled trials showed that any reduction in eGFR was strongly associated with higher mortality rates.9,10 Since then, over 50 studies have been published on the association between renal dysfunction and mortality.8 Overall, the risk associated with concomitant renal dysfunction is around twice that of patients without evidence of renal dysfunction; an association that was independent of chronicity or phenotype of HF (Table 1).
Table 1.
Overview of important meta-analyses of renal impairment in HF
| Author | Year | Population | Total n | Main results |
|---|---|---|---|---|
| Smith12 | 2006 | Acute and chronic HF | CKD: 80 098 WRF: 12 634 |
|
| Tonelli66 | 2006 | CV disease, including chronic HF | Total: 1 371 990 HF: 78 272 |
|
| Damman13 | 2007 | Acute and chronic HF | HF: 18 634 |
|
| Clark24 | 2014 | Chronic HF patients included in RAAS-inhibitor trials | HF: 20 573 |
|
| Damman8 | 2014 | Acute and chronic HF | CKD: 1 076 104 WRF: 49 890 |
|
Worsening renal function
Against this background of renal dysfunction, worsening renal function (WRF) has been recognized as a distinct identity. Especially during hospitalization, it was observed that even a small, as low as 17 µmol/L (0.2 mg/dL) increase in serum creatinine was associated with poor outcomes.11 Several meta-analyses have now demonstrated, on average, WRF is associated with increased mortality in both inpatients and outpatients with larger increases in serum creatinine predicting worse outcomes.8,12,13 An ongoing debate continues for the optimal definition for WRF. Adapted from the nephrology acute kidney injury (AKI) literature, most early reports used an increase in creatinine ≥26.5 µmol/L (0.3 mg/dL) to define WRF. However, this definition fails to acknowledge the exponential relationship between serum creatinine and eGFR such that depending on the absolute level of baseline creatinine, either small or large changes in actual renal function can accompany a 26.5 µmol/L (0.3 mg/dL) change in serum creatinine. Therefore, consideration of a relative increase in serum creatinine as well is critically important.14 Sheerin et al.15 recently recommended changes in the definition of WRF and argued that WRF in acute HF should be evaluated over the entire inhospital period, and during 3 months after discharge, to evaluate possible transient WRF. In the latest meta-analysis, WRF of varying definitions was associated with increased mortality risk.8 However, there was also evidence of publication bias, suggesting that we might be overestimating the true association between WRF and outcome. This is further supported by recent observations where WRF was only associated with poor outcome if the clinical status of a patient simultaneously deteriorated.16 In other words, if the clinical status of a patient improves or stays equal and serum creatinine increases, this WRF which we have recently called ‘pseudo-WRF’ may not translate into a poor prognosis.14 Figure 2 shows this proposed association between HF status and changes in renal function. For instance, WRF that occurs in the setting of haemoconcentration, complete decongestion, or a reduction in blood pressure had a much better outcome compared with those patients who had WRF that appeared to be unprovoked.17–19 Recently, diuretic response or efficiency was proposed as an easy tool to monitor patients, and in one study it was shown that although patients who had the best diuretic response/efficiency also more commonly showed increases in serum creatinine, these patients still had the best clinical outcome.16 So, at least in acute HF, some increase in serum creatinine may be acceptable, as long as the overall clinical status does not deteriorate.14
Figure 2.
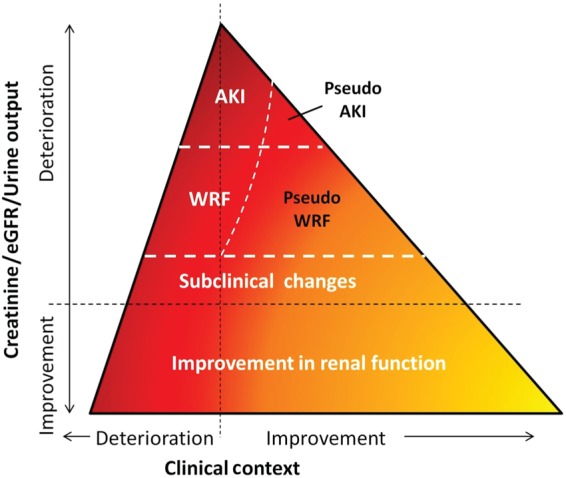
Visual depiction of association between changes in renal function, clinical condition, and mortality risk. AKI, acute kidney injury; GFR, glomerular filtration rate; WRF, worsening renal function. Darker colours indicate higher mortality risk. Suggested cut-off values for WRF (chronic HF): ≥26.5 µmol/L and ≥25% increase in creatinine OR ≥ 20% decrease in eGFR over 1–26 weeks, and AKI (acute HF): increase of 1.5–1.9 times baseline creatinine within 1–7 days before or during hospitalization OR ≥ 26.5 µmol/L increase in creatinine within 48 h OR urine output < 0.5 mL/kg/h for 6–12 h (based on Damman et al.14)
For chronic HF, the overall advice is similar. A small increase in serum creatinine is probably acceptable when the clinical status is stable or improves. There is however a special circumstance: the rise in serum creatinine that occurs in the setting of the initiation and uptitration of renin angiotensin aldosterone system (RAAS) inhibitors.20 Several retrospective analyses of large randomized controlled RAAS-inhibitor trials have now re-evaluated these compounds in the light of the findings on WRF in the general HF population.21–24 Most, if not all of these analyses have shown that if WRF occurs with the initiation of these therapies (including ACE-inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists), the beneficial effect of these therapies is maintained, and in some cases, this RAAS inhibitor induced WRF is not even associated with poor outcome. This is probably the net results of the strong protective effects of these agents balanced by the negative effects of WRF, or that these haemodynamic changes in filtration simply are not important. Importantly, the deterioration in eGFR in most of these studies was modest and thus these data provide limited evidence to indicate that it is safe (or unsafe) to continue these therapies if creatinine rises extensively. However, these data do clearly show that the beneficial effects of the treatment are maintained even in the setting of a modest rise in creatinine and thus some increase should be accepted with the caveat that frequent assessment of renal function and potassium should occur and are incorporated into good clinical judgment, as also indicated in the most recent ESC HF guidelines.20
Pathophysiology of renal impairment in heart failure
Haemodynamics
Early in the 20th century, the importance of reduced RBF and increased central venous pressure (CVP) as primary effector mechanisms for renal impairment has been established.2,3,25 Landmark papers that further established the relationship between renal haemodynamics, GFR and the severity of HF were published by Cody and colleagues.26 They demonstrated in ACEi naïve patients that the reduction in RBF was out of proportion to the reduction in cardiac index, while GFR was relatively maintained; a phenomenon now easily explained by renal autoregulation. Then, when RBF drops further, GFR declines as autoregulatory capacity is exhausted. These findings have been reproduced in patients on ACEi, with the difference that RBF and GFR declined in parallel since compensatory efferent arteriolar vasoconstriction is reduced by ACEi.27
In the last few years, focused has shifted to venous congestion as another important determinant of reduced GFR. It should be noted that this is a re-appraisal of this relationship rather than a new discovery (Figure 1). It has now been convincingly shown in modern HF patients that, independent of a reduction in RBF, there is an epidemiologic association between increased CVP or venous congestion and reduced GFR.28,29 In the chronic setting, a significant association between increasing CVP and lower eGFR was found in over 2500 patients, but not necessarily HF.30 It must be acknowledged that the magnitude of these associations was small, although significant. In acute HF, Mullens et al.29 have shown that higher CVP predetermines the risk of WRF inhospital and does this to a greater extent compared with low cardiac index. The latter was inversely related to WRF, although there was no association with baseline GFR.29 The relationship between high CVP and GFR in acute HF appears to be complex31; it has now been found in multiple studies, although not all, and there have even been reports that lower CVP predisposes to WRF.32–35
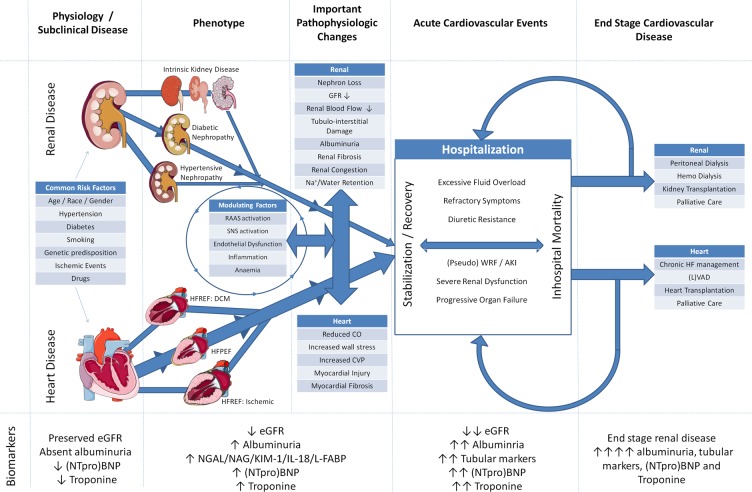
More importantly, the overall assumption in most contemporary studies has shifted from a RBF to a more CVP or venous congestion dependent explanation for GFR. However, this fails to acknowledge the fact that RBF remains—by far—the most important determinant of GFR in HF. GFR and RBF are by definition inexorably linked and under almost all circumstances, the RBF will be the primary driver of GFR. This relationship exists as GFR is simply the product of renal plasma flow times the filtration fraction. As a result, by definition renal plasma flow is an important determinant of GFR. Although there is a modest dynamic range of filtration fraction, a high value for filtration fraction multiplied by a very low RBF will still result in a low GFR, as is true of the opposite analogy. The relative contribution of venous congestion in these circumstances is marginal at best, and mostly seen in patients with compromised RBF. In acute HF, the importance of venous congestion in determining GFR is probably much greater, but we do not have data on RBF, venous congestion, and GFR in patients with acute HF. The relative contributions of these components are therefore unknown. Figure 3 summarizes the pathophysiologic pathways of cardiorenal interaction.
Figure 3.
Pathophysiologic pathways of cardiorenal interaction. AKI, acute kidney injury; CO, cardiac output; CVP, central venous pressure; DCM, dilated cardiomyopathy; GFR, glomerular filtration rate; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver type fatty acid binding protein; LVAD, left ventricular assist device; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; NTproBNP, N-terminal pro brain natriuretic peptide; RAAS, renin angiotensin aldosterone system; SNS, sympathetic nervous system; WRF, worsening renal function. The diagram illustrates predisposing factors that can cause both cardiac and renal disease. From both ends of the spectrum, disease of one organ can lead to progressive dysfunction leading to heart and renal failure. Both interact with each other through haemodynamic and (neurohormonal) (mal)adaptive processes, and modulating factors further affect these associations. Further progression of disease is caused by (re)hospitalizations. Eventually, patients enter a vicious circle of mutual organ dysfunction, resulting either in end stage renal disease, end stage heart failure, or a combination of both. Illustrations (adapted from) Servier Medical Art (http://www.servier.com/Powerpoint-image-bank), under the Creative Commons Attribution 3.0 Unported License (http://creativecommons.org/licenses/by/3.0/).
Non-haemodynamic factors
It must be emphasized again that, the main determinants of GFR in HF are renal haemodynamics and non-haemodynamic factors directly only account for a fraction of the pathophysiology. Having said that, these so-called cardiorenal connectors can shift the balance of susceptibility, severity, and mortality risk.6 Also, the mechanisms by which these non-renal factors influence GFR are primarily through haemodynamic changes, and therefore, these factors are more mediators than direct effectors. A multitude of factors influence the association between haemodynamics and GFR. Of particular interest are (modulation of) the RAAS, sympathetic nervous system (SNS) activation, inflammation, endothelial dysfunction, and anaemia. Next to the direct effect on renal perfusion, angiotensin II promotes renal fibrosis, directly affects GFR, induces hyporesponsiveness to natriuretic peptide and mediates SNS activation.36–40 The latter in turn can alter the ultrafiltration coefficient, and SNS activation is associated with tubular injury and the formation of reactive oxygen species (as well as RAAS activation).6,36,40 The effect of oxidative stress and endothelial dysfunction seems to be modulated by angiotensin II as well. Through NADP(H) activation, angiotensin II promotes the formation of reactive oxygen species, which can cause intrarenal (proximal tubular) damage.6 Finally, anaemia is an important factor in HF patients with renal impairment. Anaemia has diverse causes in HF, including reduced renal function with lower erythropoietin production and blunted response, bone marrow suppression in HF, iron deficiency, and not unimportantly, haemodilution due to excessive venous congestion which in some series is the most prevalent cause.41 In acute HF, improvement of haemodiluted anaemia assessed by haemoconcentration does relate to better outcome and could even potentially be a target for therapy in these patients.17
Renal function: factors beyond glomerular filtration rate
Impaired renal function in HF represents much more than simply a reduction in GFR. Albuminuria is frequently observed in patients with chronic HF as was observed in retrospective analyses of CHARM and GISSI-HF.42,43 Around 30% of patients have albuminuria, many of which have microalbuminuria. When present there is a stepwise increase in the risk of HF hospitalizations and mortality from normo-, to micro- and macro-albuminuria. In addition to increased glomerular permeability, decreased re-absorption in the tubules due to tubular damage likely further contributes to the development of albuminuria. Tubular damage is now increasingly recognized in patients with acute and chronic HF.44,45 Probably because of the fact that the kidney is one of the few organs that will simultaneously decrease oxygen delivery (reduced renal blood flow) while increasing relative oxygen requirement (since sodium reabsorption is highly energetically demanding), tubular damage may develop. In addition, increased congestion may be associated with tubular damage. In a retrospective analysis of GISSI-HF, tubular damage assessed by urinary markers such as N-acetyl-β-d-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule 1 (KIM-1) was frequently present among patients with chronic HF and strongly associated with mortality.44 In acute HF, multiple studies have assessed the prevalence of tubular injury. Most of the research focusing on tubular damage markers in acute HF has been focused on the identification of patients at risk of WRF. In non-HF patient populations, tubular damage markers are sensitive and specific markers of severe AKI.46 Unfortunately, studies in acute HF that have been conducted thus far have failed to demonstrate clinical usefulness of NGAL to identify patients at risk of clinical significant WRF, and notably in patients that do develop WRF urine NGAL levels do not meaningfully increase.47,48 In chronic HF, urinary KIM-1 levels were the best predictors of WRF.49 With respect to therapy, loop diuretics that seem to reduce urinary NAG and KIM-1 levels in stable HF patients and reducing congestion has been shown to improve albuminuria in acute HF.50,51 Until we have more information on the clinical applicability of these novel (tubular) markers, their routine use in patients with HF does not seem justified yet.
Patient identification and prognostication
Clearly, identification of patients at high risk of mortality and/or HF hospitalizations should include some measure of ‘renal function’: a GFR, and possibly, albuminuria or a marker of tubular damage. Recent reports have indicated that blood urea nitrogen (BUN) could be an even better prognosticator that resembles (some form) of GFR. However, BUN has been associated with factors beyond glomerular filtration, such as neurohormonal activation and haemodynamic status, which could be the reason for the fact that it retains powerful prognostic information even after controlling for GFR.52
Since renal dysfunction in patients with HF is a mechanistically heterogeneous disorder, it is logical to assume that prognosis and treatment may also differ. Unfortunately, phenotyping patients with renal dysfunction has proved a challenging endeavour since no gold standard exists by which HF-induced renal dysfunction can be differentiated from intrinsic renal parenchymal disease. However, it has been described that the majority of risk associated with renal dysfunction is restricted to patients with either an elevated NT-proBNP or an elevated BUN to creatinine ratio (BUN/Creat), markers which may help to identify HF-induced renal dysfunction.53,54 Combination of these markers produces even more striking results.55 Notably, patients with a low eGFR in the setting of an elevated BNP and BUN/Creat have multiple parameters consistent with HF-induced renal dysfunction including very poor prognosis but those patients with a low eGFR but normal BNP and BUN/Creat have a cardiorenal clinical profile and prognosis similar to patients without renal dysfunction. However, in the absence of a gold standard, it is impossible to determine if these markers are actually identifying mechanistically distinct types of renal dysfunction. Additional research within this domain is warranted.
Treatment of heart failure patients with renal dysfunction
HF patients with severe renal dysfunction have been excluded from randomized clinical trials of current evidence-based treatments. It must however be acknowledged that the benefit observed in most of these trials was also similar if not greater in patients with eGFR < 60 (or even 45 mL/min/1.73 m2).56
Recently, the effect of evidence-based treatments on the slope of eGFR was reported for most evidence-based therapies. RAAS inhibitors are associated with a decline in eGFR with initiation, after which eGFR declines in parallel with placebo.22,23 For beta-blockers, similar observations have been reported, although the magnitude of the effect was smaller. For device therapy, such as biventricular pacing or left ventricular assist devices, two devices that improve cardiac output and possibly RBF, eGFR has been reported to increase at least transiently in responders.57–59
In acute HF, trials have been designed to specifically treat or prevent WRF in acute HF. In PROTECT, the adenosine receptor antagonist rolofylline did not result in the expected benefit on renal function in acute HF.60 In the ROSE-AHF study, low-dose nesiritide and dopamine were evaluated in patients with renal impairment. However, both treatments failed to provide renal benefit.61
Finally, therapies that modulate congestion may also influence renal function. Loop diuretics are the cornerstone of the treatment of symptoms and signs of congestion in acute and chronic HF. However, their effect on renal function is poorly understood and studied, and observational data are strongly confounded by indication, since patients with more severe HF (and renal dysfunction) are prescribed more loop diuretics.62 The DOSE trial studied different dosages and intermittent vs. continuous prescription of loop diuretics in acute HF. The study did show that, although post-discharge outcomes were similar, higher loop diuretics dosages were associated with more fluid and weight loss but a higher incidence of WRF.63 However, as we have indicated earlier, this does not directly indicate that this particular WRF would be associated with poorer outcome and could be regarded as pseudo-WRF (Figures 2 and 4).
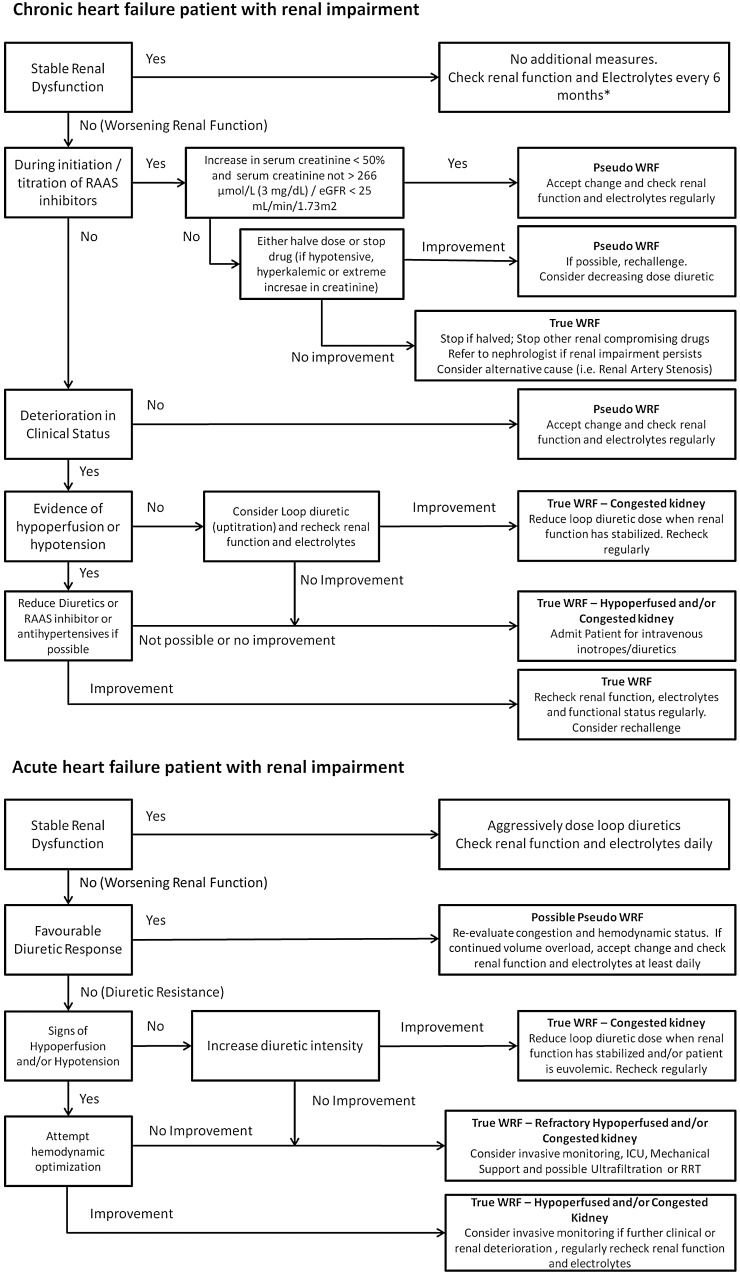
Figure 4.
Approach to the heart failure patients with renal dysfunction. GFR, glomerular filtration rate; RAAS, renin angiotensin aldosterone system; WRF, worsening renal function. *At least every 6 months, can be individually determined.
Since ultrafiltration directly reduces venous congestion, it could directly influence renal function by reducing renal venous pressure. However, in the UNLOAD and RAPID-CHF studies there was no evidence of improvement in renal function compared with loop diuretics.57,64 In the latest CARRESS-HF study in patients with WRF and persistent congestion admitted for acute HF, ultrafiltration was actually inferior to stepped pharmacologic therapy with respect to changes in creatinine, and this was sustained after discharge.65
Until we know more, with the current available evidence, we have highlighted possible treatment decisions based on changes in renal function and response to diuretics in Figure 4. By no means should this figure serve as guideline, but it might serve to suggest a possible course of action in response to deterioration of renal function in different situations.
Future outlook: what is needed in the next decade
In the next 10 years, research will need to focus on further characterizing why some patients with impaired renal function and WRF fair pretty well, while others struggle to survive. Studies should be conducted that differentiate between true and pseudo-WRF, and how we can possibly (early) distinguish between both, possibly via markers of tubular or glomerular damage, or yet to be discovered markers or imaging modalities. It is clear that renal dysfunction does not mean the same thing in each patient, and we need strategies to determine the individual response. If possible, we need treatment options that can prevent significant deteriorations in renal function, since more severe renal dysfunction is associated with persistent reduction in GFR and structural renal damage. Furthermore, in acute HF we need strategies that improve diuretic response in patients that are most likely to benefit from the therapy, without compromising renal function. To do so, we need more information on the changes in haemodynamics, cardiorenal connectors, renal function and structure during and possibly before hospitalization. Additionally, in both acute and chronic HF, we need more information on whether specifically targeting renal function with therapies alters prognosis. In chronic HF, where the incidence of severe renal dysfunction is increasing, we need evidence-based treatments or strategies that are specifically designed and executed in HF patients with low GFR, an area now underdeveloped. We also need more information on how modulation of congestion in patients with chronic HF may alter renal function and structure, since the importance of venous congestion in the chronic situation remains poorly understood. Finally, to help determine where progress is made or needed, researchers should embark on a voyage to redesign and define the cardiorenal syndrome in HF with evidence of the last 10 years. It should highlight possible pathophysiologic patient trajectories and treatment options, and also highlight dynamics in cardiac and renal function once simultaneous deterioration in heart and renal function has been diagnosed. It could also include specific research questions and areas of interest and uncertainties, and look forward to what is needed in the next 10 years.
Conclusion
It is now sufficiently clear that renal dysfunction occurs frequently in all phenotypes of HF, and when present, it is associated with higher mortality and morbidity. The cause of renal dysfunction is multifactorial, but reduced renal perfusion and venous congestion are prominent factors, which are probably mediated and modified by a multitude of cardiorenal connectors. New evidence suggests that not all deteriorations in renal function during treatment are a bad sign, but we are still unable to identify beforehand which patients will respond and this is a challenge for the near future. Finally, although much has been learned on the interaction between heart and kidney in HF, we need more dedicated epidemiologic, mechanistic, and controlled trials in HF patients with reduced renal function. To facilitate this, a new, updated classification of cardiorenal syndromes is needed which incorporates recent evidence and highlights areas of interest and areas of uncertainties where progress is wanted. Ultimately, this should lead to preventive and treatment strategies that can preserve renal function in patients with HF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
K.D. is supported by the Netherlands Heart Institute (ICIN) and an European Society of Cardiology Heart Failure Association Research Grant. J.T. is supported by grants from the National Institutes of Health (Grant numbers K23HL114868 and L30HL115790) and has received consulting fees for Novartis and Amgen.
Conflict of interest: none declared.
References
- 1.Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation 2004;110:1514–1517. [DOI] [PubMed] [Google Scholar]
- 2.Blake WD, Wegria R, Keating RP, Ward HP. Effect of increased renal venous pressure on renal function. Am J Physiol 1949;157:1–13. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell MH, Breed ES, Schwartz IL. Renal venous pressure in chronic congestive heart failure. J Clin Invest 1950;29:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leithe ME, Margorien RD, Hermiller JB, Unverferth DV, Leier CV. Relationship between central hemodynamics and regional blood-flow in normal subjects and in patients with congestive heart-failure. Circulation 1984;69:57–64. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Kellum JA, Haase M, Muller C, Damman K, Murray PT, Cruz D, House AA, Schmidt-Ott KM, Vescovo G, Bagshaw SM, Hoste EA, Briguori C, Braam B, Chawla LS, Costanzo MR, Tumlin JA, Herzog CA, Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C; Acute Dialysis Quality Initiative (ADQI) Consensus Group. Pathophysiology of the cardiorenal syndromes: executive summary from the Eleventh Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Blood Purif 2014;37:2–13.24457487 [Google Scholar]
- 6.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J 2005;26:11–17. [DOI] [PubMed] [Google Scholar]
- 7.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 8.Damman K, Valente MA, Voors AA, O'Connor CM, Van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 9.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2000;35:681–689. [DOI] [PubMed] [Google Scholar]
- 10.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, De ZD, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000;102:203–210. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, O'Connor CM, Rich MW, Stevenson LW, Young J, Krumholz HM. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail 2002;8:136–141. [DOI] [PubMed] [Google Scholar]
- 12.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 2006;47:1987–1996. [DOI] [PubMed] [Google Scholar]
- 13.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, Van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 2007;13:599–608. [DOI] [PubMed] [Google Scholar]
- 14.Damman K, Tang WH, Testani JM, McMurray JJ. Terminology and definition of changes renal function in heart failure. Eur Heart J 2014;35:3413–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheerin NJ, Newton PJ, Macdonald PS, Leung DY, Sibbritt D, Spicer ST, Johnson K, Krum H, Davidson PM. Worsening renal function in heart failure: the need for a consensus definition. Int J Cardiol 2014;174:484–491. [DOI] [PubMed] [Google Scholar]
- 16.Valente MA, Voors AA, Damman K, van Veldhuisen DJ, Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284–1293. [DOI] [PubMed] [Google Scholar]
- 17.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol 2013;62:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei CL. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 2012;5:54–62. [DOI] [PubMed] [Google Scholar]
- 19.Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011;13:877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 21.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011;4:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesogor A, Cohn JN, Latini R, Tognoni G, Krum H, Massie B, Zalewski A, Kandra A, Hua TA, Gimpelewicz C. Interaction between baseline and early worsening of renal function and efficacy of renin-angiotensin-aldosterone system blockade in patients with heart failure: insights from the Val-HeFT study. Eur J Heart Fail 2013;15:1236–1244. [DOI] [PubMed] [Google Scholar]
- 23.Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012;60:2082–2089. [DOI] [PubMed] [Google Scholar]
- 24.Clark H, Krum H, Hopper I. Worsening renal function during renin–angiotensin–aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail 2014;16:41–48. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita M. Studies on cardiac output to blood volume, and renal circulation in chronic congestive heart failure. Jpn Circ J 1968;32:249–270. [PubMed] [Google Scholar]
- 26.Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990;39:10–21. [DOI] [PubMed] [Google Scholar]
- 27.Smilde TD, Damman K, van der Harst P, Navis G, Daan Westenbrink B, Voors AA, Boomsma F, van Veldhuisen DJ, Hillege HL. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol 2009;98:121–129. [DOI] [PubMed] [Google Scholar]
- 28.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007;9:872–878. [DOI] [PubMed] [Google Scholar]
- 29.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damman K, van Deursen VM, Navis G, Voors AA, Van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 31.Testani JM, Damman K. Venous congestion and renal function in heart failure…it's complicated. Eur J Heart Fail 2013;15:599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, Socrates T, Heinisch C, Noveanu M, Frischknecht B, Baumann U, Jaeger KA, Mueller C. Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail 2011;13:432–439. [DOI] [PubMed] [Google Scholar]
- 33.Dupont M, Mullens W, Finucan M, Taylor DO, Starling RC, Tang WH. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013;15:433–440. [DOI] [PubMed] [Google Scholar]
- 34.Aronson D, Abassi Z, Allon E, Burger AJ. Fluid loss, venous congestion, and worsening renal function in acute decompensated heart failure. Eur J Heart Fail 2013;15:637–643. [DOI] [PubMed] [Google Scholar]
- 35.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 2008;51:1268–1274. [DOI] [PubMed] [Google Scholar]
- 36.Kon V, Yared A, Ichikawa I. Role of renal sympathetic nerves in mediating hypoperfusion of renal cortical microcirculation in experimental congestive heart failure and acute extracellular fluid volume depletion. J Clin Invest 1985;76:1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiksen-Olsen MJ, Strick DM, Hawley H, Romero JC. Renal effects of angiotensin II inhibition during increases in renal venous pressure. Hypertension 1992;19:137–141. [DOI] [PubMed] [Google Scholar]
- 38.DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin–angiotensin system in the control of renal function. Hypertension 2000;36:1083–1088. [DOI] [PubMed] [Google Scholar]
- 39.Charloux A, Piquard F, Doutreleau S, Brandenberger G, Geny B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur J Clin Invest 2003;33:769–778. [DOI] [PubMed] [Google Scholar]
- 40.Blantz RC, Konnen KS, Tucker BJ. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest 1976;57:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westenbrink BD, Visser FW, Voors AA, Smilde TD, Lipsic E, Navis G, Hillege HL, van Gilst WH, van Veldhuisen DJ. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. Eur Heart J 2007;28:166–171. [DOI] [PubMed] [Google Scholar]
- 42.Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, Frisinghelli A, Minneci C, Valisi M, Maggioni AP, Marchioli R, Tognoni G, Tavazzi L. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail 2010;3:65–72. [DOI] [PubMed] [Google Scholar]
- 43.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009;374:543–550. [DOI] [PubMed] [Google Scholar]
- 44.Damman K, Masson S, Hillege HL, Maggioni AP, Voors AA, Opasich C, van Veldhuisen DJ, Montagna L, Cosmi F, Tognoni G, Tavazzi L, Latini R. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J 2011;32:2705–2712. [DOI] [PubMed] [Google Scholar]
- 45.Collins SP, Hart KW, Lindsell CJ, Fermann GJ, Weintraub NL, Miller KF, Roll SN, Sperling MI, Sawyer DB, Storrow AB. Elevated urinary neutrophil gelatinase-associated lipocalcin after acute heart failure treatment is associated with worsening renal function and adverse events. Eur J Heart Fail 2012;14:1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012–1024. [DOI] [PubMed] [Google Scholar]
- 47.Dupont M, Shrestha K, Singh D, Awad A, Kovach C, Scarcipino M, Maroo AP, Tang WH. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail 2012;14:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verbrugge FH, Dupont M, Shao Z, Shrestha K, Singh D, Finucan M, Mullens W, Tang WH. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damman K, Masson S, Hillege HL, Voors AA, van Veldhuisen DJ, Rossignol P, Proietti G, Barbuzzi S, Nicolosi GL, Tavazzi L, Maggioni AP, Latini R. Tubular damage and worsening renal function in chronic heart failure. JACC Heart Fail 2013;1:417–424. [DOI] [PubMed] [Google Scholar]
- 50.Damman K, Ng Kam Chuen MJ, MacFadyen RJ, Lip GY, Gaze D, Collinson PO, Hillege HL, van Oeveren W, Voors AA, Van Veldhuisen DJ. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol 2011;57:2233–2241. [DOI] [PubMed] [Google Scholar]
- 51.Koyama S, Sato Y, Tanada Y, Fujiwara H, Takatsu Y. Early evolution and correlates of urine albumin excretion in patients presenting with acutely decompensated heart failure. Circ Heart Fail 2013;6:227–232. [DOI] [PubMed] [Google Scholar]
- 52.Schrier RW. Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail 2008;1:2–5. [DOI] [PubMed] [Google Scholar]
- 53.van Kimmenade RR, Januzzi JL, Jr, Baggish AL, Lainchbury JG, Bayes-Genis A, Richards AM, Pinto YM. Amino-terminal pro-brain natriuretic Peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol 2006;48:1621–1627. [DOI] [PubMed] [Google Scholar]
- 54.Testani JM, Coca SG, Shannon RP, Kimmel SE, Cappola TP. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: analysis of three randomized controlled trials. Eur J Heart Fail 2011;13:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Testani JM, Damman K, Brisco MA, Chen S, Laur O, Kula AJ, Wilson Tang WH, Parikh C. A combined-biomarker approach to clinical phenotyping renal dysfunction in heart failure. J Card Fail 2014;20:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray JJ. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol 2014;63:853–871. [DOI] [PubMed] [Google Scholar]
- 57.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–683. [DOI] [PubMed] [Google Scholar]
- 58.Boerrigter G, Costello-Boerrigter LC, Abraham WT, Sutton MG, Heublein DM, Kruger KM, Hill MR, McCullough PA, Burnett JC., Jr Cardiac resynchronization therapy improves renal function in human heart failure with reduced glomerular filtration rate. J Card Fail 2008;14:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, Parikh CR, Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail 2014;7:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, Delucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–1428. [DOI] [PubMed] [Google Scholar]
- 61.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, vila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yilmaz MB, Gayat E, Salem R, Lassus J, Nikolaou M, Laribi S, Parissis J, Follath F, Peacock WF, Mebazaa A. Impact of diuretic dosing on mortality in acute heart failure using a propensity-matched analysis. Eur J Heart Fail 2011;13:1244–1252. [DOI] [PubMed] [Google Scholar]
- 63.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, Kraemer M, Mackedanz S, Sobotka PA, Schollmeyer M, Goldsmith SR. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients with Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol 2005;46:2043–2046. [DOI] [PubMed] [Google Scholar]
- 65.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17:2034–2047. [DOI] [PubMed] [Google Scholar]