Abstract
The rapidly growing nontuberculous mycobacterial species Mycobacterium abscessus has recently emerged as an important pathogen in patients with cystic fibrosis (CF). Treatment options are limited because of the organism's innate resistance to standard antituberculous antibiotics, as well as other currently available antibiotics. New antibiotic approaches to the treatment of M. abscessus are urgently needed. The goal of the present study was to assess the growth-inhibitory activity of different Ga compounds against an American Type Culture Collection (ATCC) strain and clinical isolates of M. abscessus obtained from CF and other patients. In our results, using Ga(NO3)3 and all of the other Ga compounds tested inhibited the growth of ATCC 19977 and clinical isolates of M. abscessus. Inhibition was mediated by disrupting iron uptake, as the addition of exogenous iron (Fe) restored basal growth. There were modest differences in inhibition among the isolates for the same Ga chelates, and for most Ga chelates there was only a slight difference in potency from Ga(NO3)3. In contrast, Ga-protoporphyrin completely and significantly inhibited the ATCC strain and clinical isolates of M. abscessus at much lower concentrations than Ga(NO3)3. In in vitro broth culture, Ga-protoporphyrin was more potent than Ga(NO3)3. When M. abscessus growth inside the human macrophage THP-1 cell line was assessed, Ga-protoporphyrin was >20 times more active than Ga(NO3)3. The present work suggests that Ga exhibits potent growth-inhibitory capacity against the ATCC strain, as well as against antibiotic-resistant clinical isolates of M. abscessus, including the highly antibiotic-resistant strain MC2638. Ga-based therapy offers the potential for further development as a novel therapy against M. abscessus.
INTRODUCTION
Individuals with cystic fibrosis (CF) suffer from chronic and recurrent bacterial infections of the lung that are, in turn, associated with progressive deterioration of respiratory function (1). Staphylococcus aureus is the most commonly isolated pathogen in young children (2), with the proportion of patients colonized with Pseudomonas aeruginosa increasing with age (1). Among the organisms of growing importance in recent years is the rapidly growing nontuberculous mycobacterial (NTM) species Mycobacterium abscessus.
M. abscessus infects macrophages of the lungs and skin and causes a variety of clinical syndromes in humans (3, 4). It has recently emerged as an important pathogen in patients with CF, causing severe lung disease (5, 6), and infection with M. abscessus is considered a relative contraindication to lung transplantation (7). Moreover, despite cross-infection prevention measures, transmission of multidrug-resistant NTM between patients with CF still occurs (8).
Treatment options are limited because first, M. abscessus has a complex cell wall that produces intrinsic resistance to a variety of antibiotics (9, 10); second, antibiotics need to penetrate the macrophage reservoir of the organism; and third, because M. abscessus can form biofilms (1, 11). M. abscessus is resistant to standard antituberculous antibiotics. Cefoxitin, clarithromycin, and amikacin appear to be active in vitro, but these agents show relatively poor efficacy in treating clinical disease (1, 11). New antibiotic approaches to the treatment of M. abscessus are urgently needed.
Iron (Fe) is essential for the growth of most microorganisms, including M. abscessus (12). Our lab and others have shown that M. tuberculosis replicating in human macrophages can acquire Fe bound to transferrin or lactoferrin and from exogenous sources (13–16). Fe is an important component of enzymes involved in critical cellular functions such as DNA synthesis (17), general metabolism, and oxidative stress resistance. Furthermore, Fe availability can mediate bacterial virulence and pathogenesis functions (18, 19). Our laboratory and others have shown that control of Fe availability or interference with Fe uptake could inhibit the growth of M. tuberculosis and other bacteria, regardless of whether they are growing extracellularly or within human macrophages (18–21). In addition, we and others have recently reported that the virulence of some bacteria, including mycobacteria, is increased by greater availability of Fe in animal models (21–24). Therefore, targeting of M. abscessus Fe metabolism is a promising approach for novel therapy.
Gallium (Ga) shows many similarities to Fe (17), and Ga in the form of Ga(NO3)3 is an FDA-approved drug for the treatment of hypercalcemia of malignancy (25). In biological systems, Fe3+ can be reduced to Fe2+, making it useful as a metal cofactor to facilitate electron transfer by a variety of enzymes. In spite of its similarity to Fe3+, Ga3+ does not undergo reduction under physiologic conditions (17). These characteristic makes Ga3+ an attractive “Trojan-horse” metabolic inhibitor since it can both compete with Fe for acquisition and inhibit Fe-dependent enzymes if it is substituted for Fe3+ in their active sites. For example, we recently demonstrated that Ga disrupts Fe metabolism and inhibits key Fe-containing regulatory enzymes such as ribonucleotide reductases and aconitase (21). Our lab and others showed the ability of Ga to inhibit the growth of a variety of human pathogens, including several mycobacterial species (17, 19–21, 26–28). In addition, Ga has been active in murine models of several bacterial infections, including M. tuberculosis (21).
In the present work, we show that Ga exhibits potent M. abscessus growth-inhibitory capacity. We also extended our research to show the effectiveness of different Ga compounds against antibiotic-resistant clinical isolates obtained from CF patients.
MATERIALS AND METHODS
Materials.
A reference strain of M. abscessus (ATCC 19977) was purchased from the American Type Culture Collection. Clinical isolates of M. abscessus subsp. massiliense from the University of Washington (MC6067, MC5260, MC5315-1, MC5315-2, MC2638, and MC5597) were acquired by Moira Aitken (University of Washington), characterized, and transferred to our laboratory by material transfer agreement from the Mycobacteria/Nocardia Laboratory at the University of Texas Health Science Center, Tyler, TX (6, 29). Additional unrelated clinical isolates of M. abscessus were provided by Paul Fey, associate director of the Clinical Pathology/Microbiology Laboratory at Nebraska Medicine, Omaha, NE.
Sodium citrate, dehydrate, citric acid monohydrate, and ferric nitrate Fe(NO3)3 were purchased from Fisher Scientific, Fair Lawn, NJ. d-Malic acid, l-malic acid, sodium succinate (dibasic) hexahydrate, oxalic acid, and deferoxamine mesylate salt were purchased from Sigma-Aldrich, St. Louis, MO. Gallium nitrate hydrate [Ga (NO3)3] was obtained from Acros Organics, Pittsburgh, PA; and pyridoxal isonicotinoyl hydrazine (PIH) was obtained from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Chelex 100 resin was purchased from Bio-Rad Laboratories, Richmond, CA. Gallium maltolate was provided by Aridis Pharmaceuticals, San Jose, CA; and gallium protoporphyrin was purchased from Frontier Scientific, Logan, UT. Apotransferrin was purchased from Athens Research, and RPMI 1640, l-glutamine, HEPES, sodium pyruvate, and fetal bovine serum were obtained from HyClone, Logan, UT.
Gallium chelates.
Gallium chelates (sodium citrate, citric acid, d- and l-malic acid, sodium succinate, and oxalic acid) were prepared with a 1.0:1.5 molar ratio of gallium nitrate plus chelate in Fe-free water and mixed for at least 24 h at 4°C as described previously (30). Gallium maltolate, gallium protoporphyrin, and gallium PIH were prepared in a 1:1 molar ratio as described previously (31). All solutions were prepared with water treated with BT Chelex 100 resin at 10 g/liter of water. Gallium transferrin was prepared as previously described (31, 32). All reagents were verified spectroscopically. Briefly, apotransferrin was combined at a 1:3 molar ratio with gallium nitrate in an acetic acid sodium chloride solution, buffered to pH 7.4 with NaHCO3, and mixed for 6 h at 4°C. The compound was dialyzed in phosphate-buffered saline (PBS)–1 mM NaHCO3 with Chelex gel to capture nonbound gallium.
Fe-free 7H9 medium (32) was made in our laboratory by using the standard formulation but without ferric ammonium citrate. It has a final concentration of 0.005% (vol/vol) glycerol, no bovine albumin fraction V-dextrose-catalase, and 0.5% (vol/vol) Tween 80. The Fe content was determined to be 2 μM with the standard ferrozine assay (33).
In vitro growth inhibition in broth culture.
Bacteria were grown at 35°C with shaking for 3 days in Fe-free 7H9 medium. The bacterial suspensions were diluted to yield an optical density at 600 nm (OD600) between 0.020 and 0.200. This value was multiplied by a conversion factor based on the CFU counts of bacterial suspensions with an OD600 of 1.0, to give the concentration of bacteria at 106/ml. A final bacterial concentration of 0.25 × 106/ml was used for all experiments. Outside wells were filled with deionized water to maintain moisture and growth conditions. Gallium chelates were added to the wells to achieved the desired concentrations. Plates were incubated statically at 37°C for up to 7 days, with OD600 readings taken on a daily basis with a microplate reader (Synergy H1; BioTek, Winooski, VT) to measure turbidity, indicating growth. Blank values were subtracted and adjusted “to medium.” OD values of growth in wells containing each gallium compound were divided by the OD values of the drug-free control. Controls included non-gallium-bound chelates (showed no effect on bacterial growth), gallium nitrate as an internal control, and Fe-free 7H9 medium as reagent blanks. Gallium protoporphyrin assays included a nonbacterial control for color at each concentration tested.
In vitro inhibition of M. abscessus growth in the THP-1 macrophage cell line.
THP-1 cells (human monocytic cell line; ATCC TIB-202) were maintained in RPMI 1640–10 mM HEPES–2 mM l-glutamine–1 mM sodium pyruvate–10% fetal bovine serum. Cells were cultured and transformed into functionally mature macrophages with phagocytic activity in the presence of 15 ng/ml TPA (12-O-tetradecanoylphorbol-13 acetate; Cell Signaling) overnight at 37°C in 5% CO2 as described elsewhere (34). Cells were then washed, infected with bacteria at a multiplicity of infection of 10 THP-1 cells to 1 bacterium for 1 h at 37°C under 5% CO2. The desired concentrations of gallium chelates were then added to the cell cultures, following which they were incubated at 37°C under 5% CO2. At defined times, the cells were washed and harvested for of M. abscessus CFU counting. Potential toxicity of compounds for the THP-1 cells was determined by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (35).
CFU counting.
All supernatants and first washes were collected from the plates and combined for CFU counting. After centrifugation at 250 × g for 7 min, pellets were suspended in chilled, Chelex-treated, deionized, sterile water and vortexed. Adherent cells were washed, and resuspended pellets (in water) were added to the adherent cell wells and incubated on ice for 10 min. Lysis buffer was then added as described previously (20). Contents were collected and centrifuged at 14,000 × g for 15 min. Pellets were resuspended in sterile PBS and plated on tryptic soy agar plates. Colonies were counted 3 to 5 days after plating. Accepted CFU counts were in the range of 15 to 200 colonies in the plating area.
Statistical analysis of data.
Means and standard deviations were calculated from independent experiments. Statistical analysis was done with GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, CA). Differences between three or more means were determined by one- and two-way analyses of variance with Bonferroni post hoc tests. Error bars represent standard errors of the means (SEM). All statistical analyses were considered significant at a P value of <0.05.
RESULTS
Gallium inhibits growth of ATCC 19977 and clinical isolates of M. abscessus.
Like most bacteria, including other mycobacterial species, M. abscessus requires Fe for growth and metabolism (12). In the present work, we tested the in vitro efficacy of Ga against an M. abscessus strain obtained from ATCC, as well as clinical isolates obtained from two different institutions.
As shown in Table 1, when M. abscessus ATCC 19977 and the clinical isolates were grown in the presence of increasing concentrations of Ga(NO3)3, significant inhibition (P < 0.05) of the growth of the M. abscessus strains was observed (Table 1). Each of the clinical isolates from an outbreak among CF patients at the University of Washington varied somewhat in their level of inhibition by Ga. For example, growth of M. abscessus MC5597 was completely inhibited by Ga at 4 μM (1.02 μg/ml), whereas M. abscessus MC5260 was still able to grow somewhat at Ga concentrations of up to 12 μM (3.06 μg/ml) (Table 1). Clinical isolates obtained from the University of Nebraska Medical Center (UNMC) also demonstrated susceptibility to Ga, with somewhat less variation in their dose-response curves than the Washington isolates (Table 1). Almost 70% of the clinical isolates were inhibited more by Ga than was the reference lab strain (ATCC 19977).
TABLE 1.
Effects of different concentrations of Ga(NO3)3 on in vitro growth of M. abscessus ATCC 19977 and clinical isolates
| Ga(NO3)3 concn (mM) | Normalized mean OD600 ± SEM |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 19977 | MC6067a | MC2638 | MC5260 | MC5315-1 | MC5315-2a | MC5597a | UNMC-1362 | UNMC-1374 | UNMC-1423 | UNMC-1477 | |
| 2 | 0.90 ± 0.08 | 1.08 ± 0.00 | 0.80 ± 0.03 | 1.01 ± 0.00 | 0.79 ± 0.01 | 1.27 ± 0.00 | 0.90 ± 0.02 | 0.55 ± 0.04 | 0.44 ± 0.05 | 1.06 ± 0.10 | 0.96 ± 0.11 |
| 10 | 0.76 ± 0.06 | 0.70 ± 0.04 | 0.70 ± 0.01 | 1.04 ± 0.01 | 0.71 ± 0.02 | 0.63 ± 0.02 | 0.28 ± 0.01 | 0.27 ± 0.02 | 0.37 ± 0.13 | 0.65 ± 0.02 | 0.78 ± 0.04 |
| 15 | 0.59 ± 0.02 | 0.49 ± 0.03 | 0.66 ± 0.00 | 0.90 ± 0.01 | 0.69 ± 0.05 | 0.43 ± 0.02 | 0.01 ± 0.01 | 0.28 ± 0.01 | 0.41 ± 0.18 | 0.58 ± 0.01 | 0.67 ± 0.08 |
| 20 | 0.53 ± 0.02 | 0.30 ± 0.04 | 0.67 ± 0.02 | 0.84 ± 0.06 | 0.62 ± 0.04 | 0.05 ± 0.01 | 0.01 ± 0.01 | 0.25 ± 0.05 | 0.22 ± 0.05 | 0.56 ± 0.19 | 0.57 ± 0.05 |
| 25 | 0.69 ± 0.05 | 0.10 ± 0.00 | 0.52 ± 0.01 | 0.56 ± 0.02 | 0.63 ± 0.03 | 0.04 ± 0.03 | 0.02 ± 0.01 | 0.21 ± 0.01 | 0.28 ± 0.02 | 0.40 ± 0.03 | 0.43 ± 0.03 |
| 30 | 0.35 ± 0.02 | 0.02 ± 0.00 | 0.51 ± 0.01 | 0.62 ± 0.06 | 0.48 ± 0.03 | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.25 ± 0.05 | 0.22 ± 0.02 | 0.34 ± 0.01 | 0.41 ± 0.03 |
| 40 | 0.35 ± 0.03 | 0.00 ± 0.01 | 0.51 ± 0.01 | 0.52 ± 0.00 | 0.44 ± 0.06 | 0.00 ± 0.01 | 0.01 ± 0.00 | 0.28 ± 0.01 | 0.23 ± 0.03 | 0.29 ± 0.01 | 0.29 ± 0.01 |
| 50 | 0.45 ± 0.03 | 0.02 ± 0.01 | 0.48 ± 0.03 | 0.61 ± 0.03 | 0.17 ± 0.02 | 0.00 ± 0.00 | 0.02 ± 0.02 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.14 ± 0.01 | 0.42 ± 0.06 |
P < 0.05.
The growth-inhibitory effect of Ga is reversed by exogenous Fe.
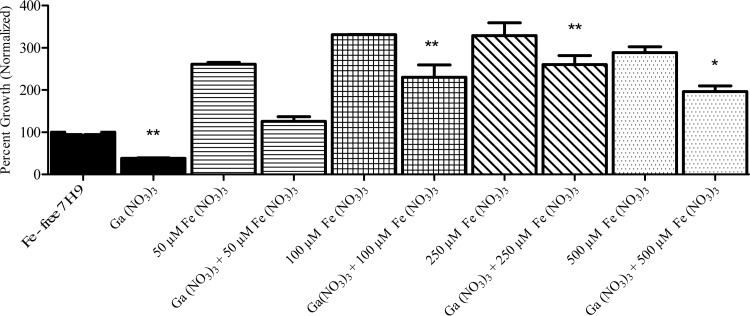
Previous work with other bacterial species found that the growth-inhibitory effect of Ga relates to disruption of bacterial Fe uptake/utilization, as evidenced by reversal of Ga-mediated growth inhibition by increasing concentrations of extracellular Fe (20). We hypothesized that this would be the case with M. abscessus as well. We measured growth inhibition of M. abscessus in the presence of increasing concentrations of Fe (NO3)3 (Fig. 1) and found that addition of Fe to the medium reversed the growth-inhibitory effects of Ga. The addition of an equimolar or greater ratio of Fe(NO3)3 to Ga was required to restore growth to control levels (Fig. 1). Doubling the molar concentration of Fe further enhanced M. abscessus growth. However, the inhibitory effect of Ga was still noticeable even after the addition of a 10× molar concentration of Fe (Fig. 1). This observation is similar to our previous findings with P. aeruginosa (26). These data are consistent with the idea that the growth-inhibitory effect of Ga on M. abscessus is mediated by interference with M. abscessus Fe metabolism, as we and other have seen with other bacterial species (19–21).
FIG 1.
Ga(NO3)3-mediated growth inhibition is reversed by Fe. Strain ATCC 19977 was cultured in the presence of 50 μM Ga(NO3)3 and increasing concentrations of Fe(NO3)3 for up to 7 days. Addition of equimolar or greater amounts of Fe relative to Ga increased M. abscessus growth significantly. *, P < 0.05; **, P < 0.01; n = 3 replicates.
Inhibition exhibited by different Ga compounds.
We sought to determine if other forms of Ga might possess a greater ability than Ga(NO3)3 to inhibit M. abscessus growth. A concentration of 10 μM Ga was used, which does not result in maximal growth inhibition for Ga(NO3)3 to more readily allow identification of Ga formulations with greater activity than Ga(NO3)3. The other Ga salts tested included Ga-citrate, Ga-citric acid, Ga–d-malic acid, Ga–l-malic acid, Ga-maltolate, Ga-oxalic acid, and Ga-succinic acid (Table 2), and they were tested against ATCC 19977 and clinical isolates of M. abscessus. With the exception of Ga-citrate, most of the Ga compounds exhibited approximately equivalent activity against M. abscessus relative to that of Ga(NO3)3 over 7 days of culture (Table 2). Ga-maltolate exhibited a somewhat higher inhibitory effect (Table 2). Certain clinical isolates (MC5597) were inhibited more by some Ga compounds than others (Table 2). Thus, although some modest differences were observed, none of these chelates demonstrated a major difference in potency from that of Ga(NO3)3.
TABLE 2.
Effects of different Ga chelates at 10 μM on in vitro growth of M. abscessus ATCC 19977 and clinical isolates
| Gallium chelate | Normalized mean OD600 ± SEM |
||||||
|---|---|---|---|---|---|---|---|
| ATCC 19977 | MC6067 | MC2638 | MC5260 | MC5315-1 | MC5315-2 | MC5597 | |
| Gallium nitrate | 0.72 ± 0.06 | 0.89 ± 0.05 | 0.76 ± 0.03 | 0.80 ± 0.05 | 0.63 ± 0.05 | 0.79 ± 0.07 | 0.58 ± 0.08 |
| Gallium citrate | 0.99 ± 0.06 | 1.35 ± 0.18 | 0.99 ± 0.07 | 1.06 ± 0.04 | 1.31 ± 0.04 | 1.08 ± 0.05 | 1.09 ± 0.01 |
| Gallium citric acid | 1.00 ± 0.01 | 0.58 ± 0.03 | 0.45 ± 0.00 | 0.49 ± 0.02a | 0.76 ± 0.00 | 0.15 ± 0.029a | 0.01 ± 0.00a |
| Gallium d-malic | 0.92 ± 0.04 | 0.79 ± 0.02 | 0.76 ± 0.02 | 0.86 ± 0.02 | 0.78 ± 0.02 | 0.04 ± 0.00a | 0.84 ± 0.17 |
| Gallium l-malic | 1.21 ± 0.14 | 0.72 ± 0.01 | 0.81 ± 0.01 | 0.79 ± 0.01 | 1.09 ± 0.07 | 0.13 ± 0.02a | 0.81 ± 0.16 |
| Gallium maltolate | 0.69 ± 0.00 | 0.35 ± 0.01 | 0.32 ± 0.03 | 0.35 ± 0.02 | 0.43 ± 0.00 | 0.56 ± 0.02 | 1.42 ± 0.08 |
| Gallium oxalic | 1.12 ± 0.01 | 0.79 ± 0.04 | 0.99 ± 0.02 | 0.82 ± 0.01 | 0.78 ± 0.06 | 0.01 ± 0.00a | 0.91 ± 0.19 |
| Gallium succinic | 0.87 ± 0.07 | 0.86 ± 0.04 | 0.78 ± 0.01 | 0.85 ± 0.02 | 0.53 ± 0.06 | 0.63 ± 0.05 | 1.16 ± 0.04 |
| Gallium transferrin | 0.84 ± 0.02 | 0.66 ± 0.02 | 0.51 ± 0.01 | 0.79 ± 0.09 | 0.57 ± 0.03 | 0.60 ± 0.05a | 0.02 ± 0.01a |
| Gallium PIH | 0.73 ± 0.06 | 0.89 ± 0.08 | 0.82 ± 0.02 | 0.93 ± 0.03 | 0.59 ± 0.03 | 1.06 ± 0.04 | 1.27 ± 0.02 |
P < 0.01 compared to gallium nitrate.
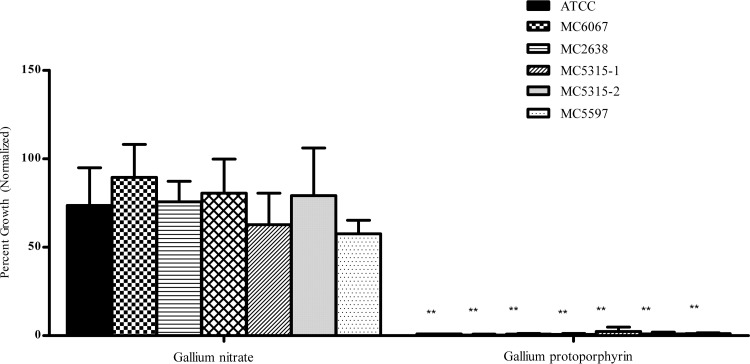
We also tested both ATCC 19977 and clinical isolates against a set of Ga compounds in which the Ga was chelated to larger molecule, protoporphyrin (Fig. 2). As shown in Fig. 2, Ga-protoporphyrin was more active. It completely and significantly (P < 0.001) inhibited all strains of M. abscessus at 10 μM Ga (Fig. 2).
FIG 2.
Ga-protoporphyrin shows the greatest inhibition among Ga chelates. M. abscessus strains were cultured in the presence of 10 μM Ga-protoporphyrin for up to 7 days. Results show inhibition of all strains normalized to the control. Statistical analysis was done by comparing growth inhibition to that observed with Ga(NO3)3. **, P < 0.001; n = 3 replicates.
Ga-protoporphyrin inhibits clinical isolates of M. abscessus.
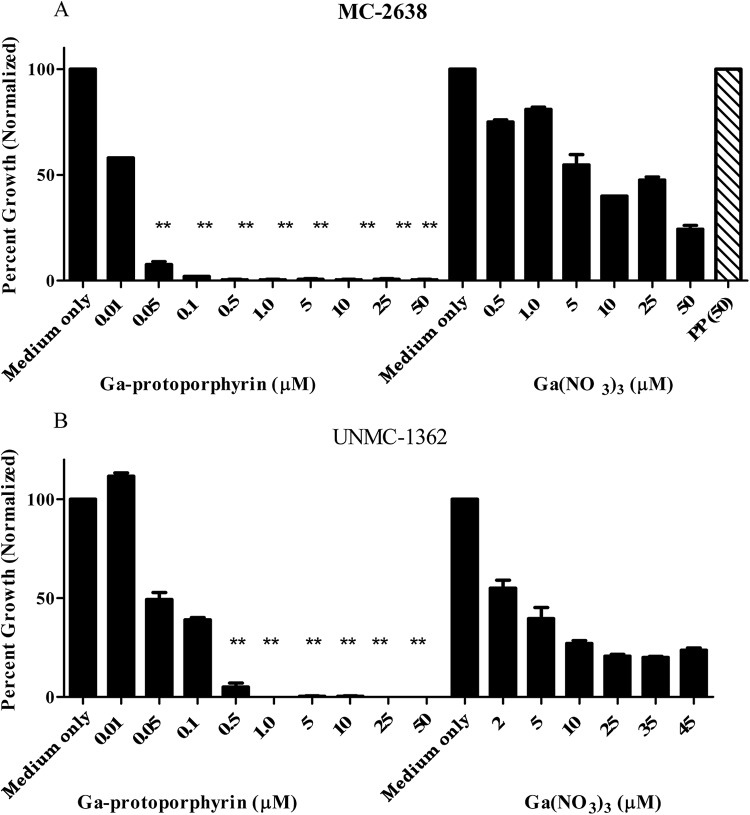
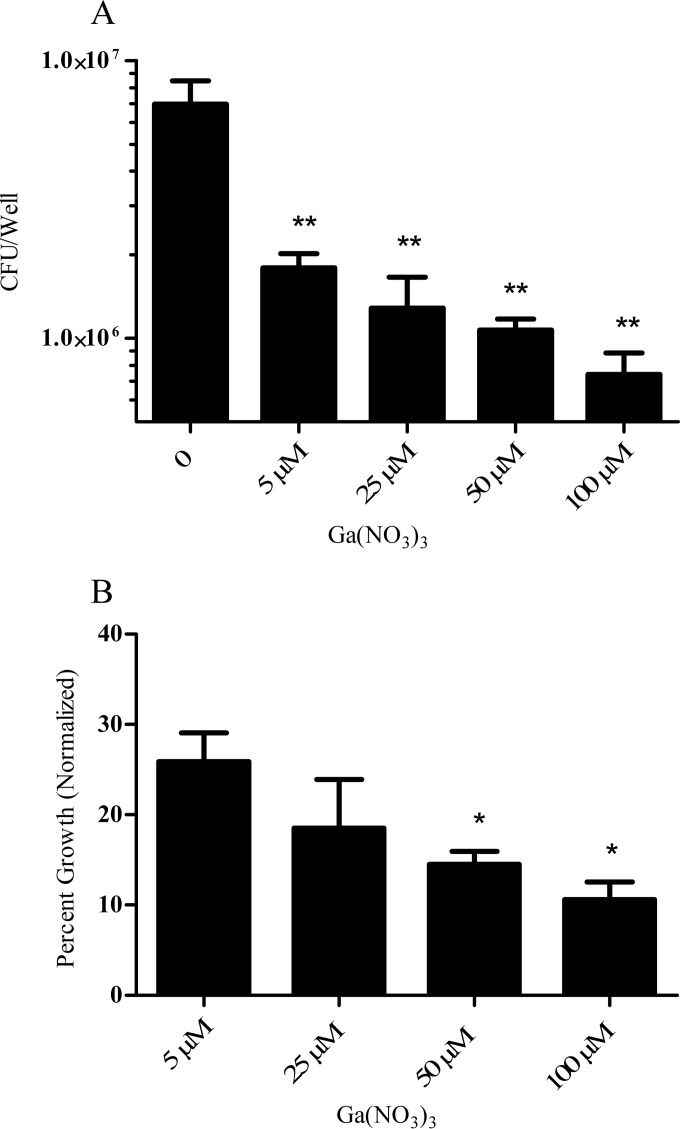
To further define the pronounced growth-inhibitory action of Ga-protoporphyrin, we titrated Ga-protoporphyrin to lower concentrations with clinical isolate MC2638 (Fig. 3A). Ga-protoporphyrin completely inhibited M. abscessus at lower concentrations (1 μM) and showed growth-inhibitory activity at a concentration as low as 0.01 μM. Ga-protoporphyrin was about eight times as potent as Ga(NO3)3 (Fig. 3A). Similarly, Ga-protoporphyrin inhibited the UNMC-1362 M. abscessus clinical isolate, as shown in Fig. 3B. Although not shown, similar inhibitory effects were seen with other UNMC M. abscessus clinical isolates. In addition, protoporphyrin alone did not show any inhibitory effect on bacteria (Fig. 3A). This demonstrates that the effect is specific for the Ga chelate and not due to the additive effects of Ga and protoporphyrin.
FIG 3.
Ga-protoporphyrin inhibits clinical isolates of M. abscessus. Clinical isolates MC2638 (A) and UNMC-1362 (B) were cultured in the presence of different concentrations of Ga-protoporphyrin and Ga(NO3)3 for up to 7 days. Results show inhibition by each compound normalized to the control. PP(50) shows bacterial growth in the presence of protoporphyrin only at 50 μM. Statistical analysis was done by comparing Ga-protoporphyrin to Ga(NO3)3. **, P < 0.001; n = 3 replicates.
Ga inhibits M. abscessus growth inside human macrophages.
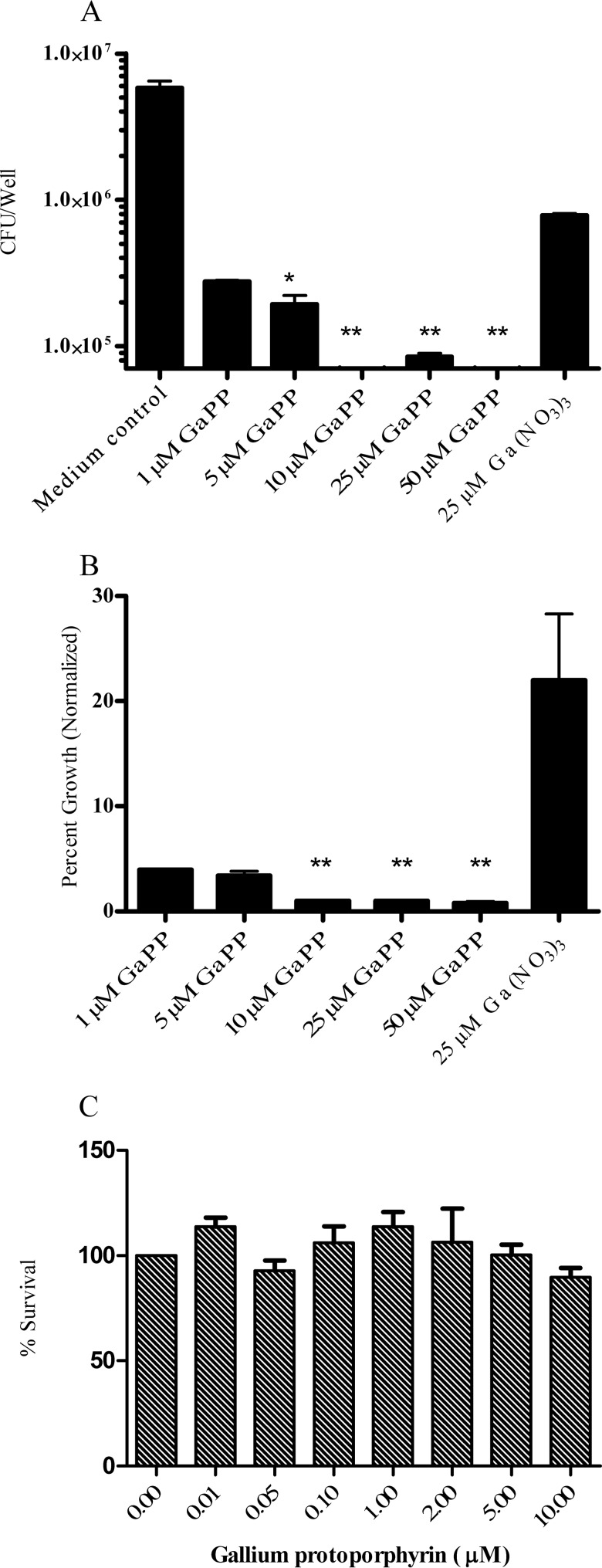
One of the challenges in the treatment of M. abscessus is that the antibiotic must possess the ability to effectively penetrate and inhibit the bacteria growing within host macrophages. Therefore, the effectiveness of a drug against M. abscessus in broth medium does not necessarily correlate with its efficacy against intracellular M. abscessus (36). Accordingly, we investigated whether a similar pattern of growth inhibition would be observed with Ga against M. abscessus growing within human macrophages. In order to eliminate the potential confounding effect of different human donors that would occur with primary macrophages, we used TPA-activated THP-1 cells as a model for macrophages. As shown in Fig. 4, Ga(NO3)3 significantly (P < 0.001) inhibited M. abscessus growth inside THP-1 cells for up to 6 days, as determined by CFU counting (Fig. 4A). When normalized to the control, Ga(NO3)3 inhibited more than 90% of M. abscessus intracellular growth over 6 days of culture (Fig. 4B).
FIG 4.
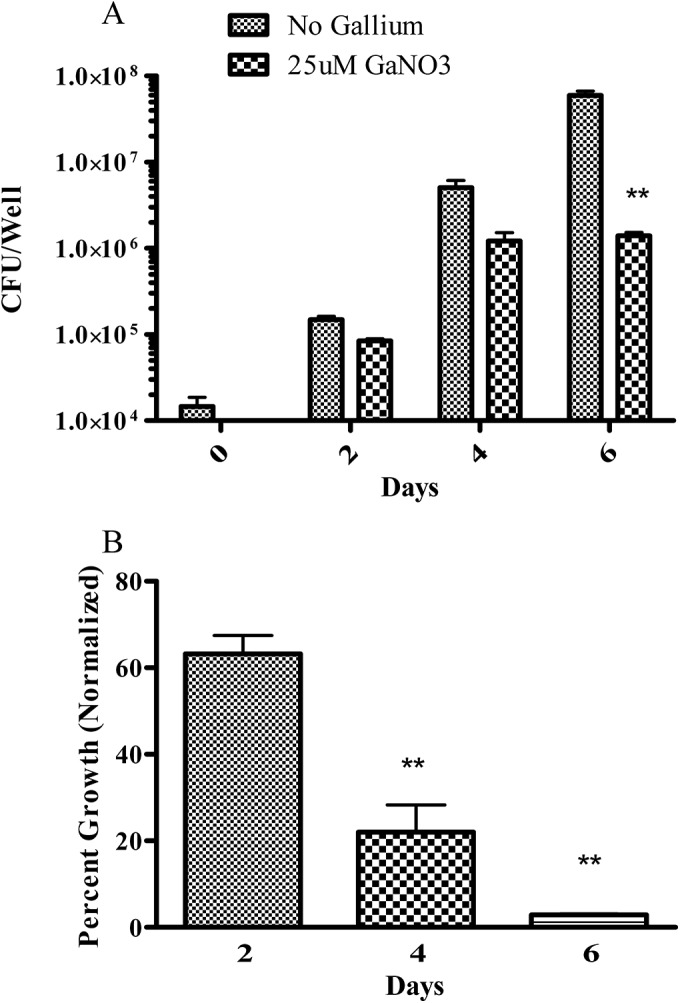
Ga(NO3)3 inhibits intracellular M. abscessus growth. THP-1 cells were TPA activated into macrophage-like cells. Cells were then infected with M. abscessus ATCC 19977 and cultured for up to 6 days with or without 25 μM Ga(NO3)3. Panel A shows results expressed in CFU counts, and panel B shows results normalized to the control (no Ga). Growth of intracellular M. abscessus was significantly inhibited in the presence of 25 μM Ga(NO3)3. **, P < 0.01; n = 3 replicates.
Ga-protoporphyrin inhibits M. abscessus growth inside human macrophages.
Similarly, we investigated the inhibitory effect of Ga-protoporphyrin against M. abscessus-infected THP-1 cells. As shown in Fig. 5, Ga-protoporphyrin significantly inhibited M. abscessus growth inside infected human THP-1 cells compared to the untreated control and to Ga(NO3)3-treated cells (Fig. 5A). When normalized to the control, 1 μM Ga-protoporphyrin inhibited intracellular M. abscessus growth to a level significantly greater than 25 μM Ga(NO3)3 (Fig. 5B). No significant toxic effects of Ga-protoporphyrin against THP-1 cells were shown at the concentrations used in this experiment (Fig. 5C).
FIG 5.
Ga-protoporphyrin inhibits intracellular M. abscessus growth. THP-1 cells were transformed and infected with M. abscessus ATCC 19977 for 1 h, washed, and cultured for up to 4 days in the presence of different concentrations of Ga-protoporphyrin as described in Materials and Methods. Ga-protoporphyrin significantly inhibited intracellular M. abscessus relative to the control (no Ga) or Ga(NO3)3 treated when expressed as the number of CFU/well (panel A) (P < 0.001) or when the data were expressed normalized to the control (no Ga). The time zero CFU count was 2.9 × 104. No toxic effects of Ga-protoporphyrin against THP-1 cells were seen (panel C), as determined by MTT assay. *, P < 0.01; **, P < 0.001; n = 4 replicates.
We next expanded the study to include one of the more resistant (Table 2) clinical isolates of M. abscessus, MC2638. As shown in Fig. 6A, consistent with observations in broth culture, MC2638 required higher concentrations of Ga(NO3)3 to inhibit intracellular growth than other M. abscessus strains. Nevertheless, when the values were normalized to those of the control, gradual and concentration-dependent inhibition of MC2638 was seen (Fig. 6B).
FIG 6.
Ga(NO3)3 inhibits intracellular growth of clinical isolate MC2638 at higher concentrations. THP-1 cells were transformed and infected with clinical isolate MC2638 for 1 h, washed, and cultured for up to 6 days in the presence of different concentrations of Ga(NO3)3 as described in Materials and Methods. Ga(NO3)3 significantly inhibited intracellular MC2638 (**, P < 0.001) as measured by CFU counting (A) or when results were normalized to the control (B) (*, P < 0.05; n = 4 replicates). The time zero CFU count was 2.2 × 104.
When MC2638 was cultured in Ga-protoporphyrin for up to 6 days, significant inhibition was seen at 50 μM compared to that obtained with the control and Ga(NO3)3 treatments (Fig. 7A). When normalized to the control, Ga-protoporphyrin exhibited about 90% inhibition of MC2638 at 6 days of culture and was significantly more effective than Ga(NO3)3 (Fig. 7B).
FIG 7.
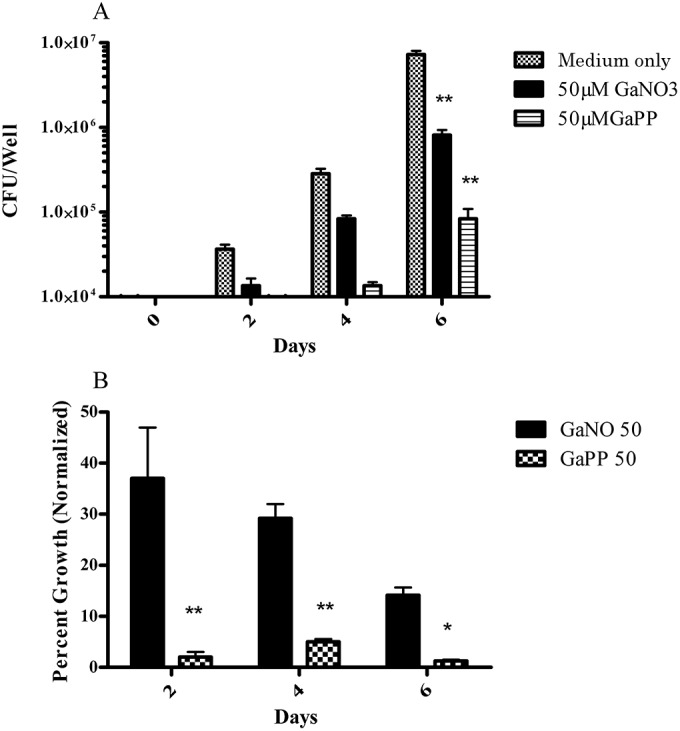
Ga-protoporphyrin inhibits intracellular growth of clinical isolate MC2638. THP-1 cells were transformed and infected with clinical isolate MC2638 for 1 h, washed, and cultured for up to 6 days in the presence of different concentrations of Ga-protoporphyrin as described in Materials and Methods. Ga-protoporphyrin significantly inhibited intracellular MC2638 (**, P < 0.001) as determined by CFU counting (A) or when data were normalized to the control (B) (*, P < 0.05; n = 4 replicates).
DISCUSSION
Most recent antibacterial drugs advances have been achieved by modify existing classes of drugs that inhibit critical bacterial targets. Although this is a valid approach, increasing resistance of clinically important pathogens to known classes of antibiotics has made this approach less productive. Therefore, the search for new antibacterial agents and targets is becoming an urgent necessity for the health care community.
M. abscessus is a rapidly growing mycobacterium that is capable of causing human disease, particularly involving the lungs or skin (3, 4). It has recently emerged as an important pathogen in patients with CF, resulting in progressive lung destruction and death (7). M. abscessus has a complex cell wall that makes it resistant to many antibiotics. In addition, since M. abscessus grows and replicates in macrophages, antibiotics must successfully penetrate this niche to be effective. M. abscessus infections respond poorly to currently available antibiotics, resulting in poor clinical outcomes (10, 36). Therefore, new antimicrobial agents and new targets for this organism are needed.
Iron is essential for the growth of most bacteria, including M. abscessus, because of the role of Fe in enzymes involved in bacterial DNA synthesis and other critical biochemical functions (17). Iron is also involved in bacterial virulence and pathogenesis (18, 19). Therefore, Fe metabolism and the Fe uptake pathway are potential therapeutic targets for new antibiotics (37–39).
Our group and others have previously shown that Ga disrupts bacterial Fe metabolism and inhibits the growth multiple species of bacteria, regardless of whether they are growing extracellularly (e.g., P. aeruginosa) or within human macrophages (e.g., M. tuberculosis) (20, 21, 26). Ga has proven to be an effective antimicrobial agent both in vitro and in animal models (21). Adding to that, it has been shown to be safe in humans and generally well tolerated. Ga is already a FDA-approved drug for the treatment of hypercalcemia of malignancy.
We have previously shown that Ga-nitrate exhibits potent antimicrobial activity against M. tuberculosis and other bacteria that grow intra- or extracellularly (20, 26, 27). This appears to be related to the ability of Ga to disrupt various aspects of bacterial Fe metabolism. The CF pathogen, P. aeruginosa, has been found to extensively evolve during infection, and evolution can affect Fe uptake systems (40–42). Despite this, most CF P. aeruginosa isolates retain sensitivity to Ga (26, 43–45). The fact that even highly evolved clinical isolates remain Ga-sensitive led us to hypothesize that CF M. abscessus isolates would also be inhibited by Gallium compounds.
In this work, we sought to test the potential of Ga-based therapy against M. abscessus. Different Ga compounds and chelates were tested against ATCC 19977, as well as clinical isolates of M. abscessus. Initial work found that Ga(NO3)3, the FDA approved form of the Ga, showed the ability to inhibit both lab strains and clinical isolates of M. abscessus. The susceptibility to Ga(NO3)3 varied among the M. abscessus strains. Nevertheless, growth inhibition was detected at Ga concentrations well within those achievable in serum and tissues with administration of Ga(NO3)3 (21). Intravenous Ga(NO3)3 for treating hypercalcemia leads to steady-state plasma levels of 12 to 32 μM Ga (46, 47). Bolus intravenous infusions yield peak serum levels of 140 to 700 μM (48, 49).
Consistent with work in other bacterial species (21), the Ga effect on M. abscessus appears to be mediated through disruption of the bacteria's Fe metabolism. The addition of increasing concentrations of Fe negated the inhibitory effect of Ga, restoring M. abscessus growth to control levels.
Although Ga(NO3)3 is the formulation of Ga currently commercially available, other forms of Ga could prove more active against M. abscessus. Therefore, we tested a variety of Ga salts and small-molecule complexes against ATCC 19977 and the clinical M. abscessus isolates. A variation in potency was observed, but the modest magnitude is unlikely to be of significance from the standpoint of new drug development. Nevertheless, the result demonstrates that Ga compounds with different solubility properties and chemical characteristics can be effective.
In contrast to results obtained with Ga small-molecule complexes, when Ga compounds in which the Ga was chelated to larger molecules were tested, Ga-protoporphyrin showed a significantly increased ability to inhibit M. abscessus growth in vitro. Ga-protoporphyrin was previously shown to exhibit antimicrobial activity against Yersinia enterocolitica, Neisseria gonorrhoeae, Haemophilus ducreyi, Staphylococcus aureus, Porphyromonas gingivalis, and some species of mycobacteria (28, 50, 51). When examined, this activity was in excess of that seen with other Ga chelates, consistent with our results with M. abscessus.
M. abscessus grows and replicates within human macrophages during infection (11). In order for an antibiotic to be effective against mycobacterial species in vivo, it must be able to enter the macrophage in sufficient quantities to inhibit mycobacterial growth. As we had previously reported with M. tuberculosis, Ga(NO3)3 inhibited intracellular M. abscessus growth with THP-1 cells as the macrophage model. As was observed in in vitro growth in bacteriologic medium, Ga-protoporphyrin demonstrated greater potency against M. abscessus growing within THP-1 cells than Ga(NO3)3 did.
In summary, we and others have previously reported that Ga(NO3)3 possesses in vitro antimicrobial activity against M. tuberculosis and other mycobacterial species (21, 51). Ga(NO3)3 was also shown to exhibit efficacy in a murine model of tuberculosis. We found that Ga(NO3)3 exhibits similar activity in vitro against M. abscessus, a pathogen of growing importance in patients with CF. Our studies confirm that Ga-protoporphyrin appears to possess greater in vitro antimicrobial against M. abscessus, consistent with findings on other mycobacteria. Although Ga-protoporphyrin exhibited greater in vitro activity than Ga(NO3)3, the potential toxicity of Ga-protoporphyrin has not been extensively studied, whereas the toxicity profile of Ga(NO3)3 has been more clearly defined, at least in the dosing regimen used in the use of the drug for hypercalcemia of malignancy. Interestingly, a recent small phase 1 trial of parenteral Ga(NO3)3 in CF patients infected with P. aeruginosa has been recently completed that showed significant improvement in lung function (43–45). The drug was well tolerated. The current work suggests that Ga-based therapy offers the potential for development as a novel therapy against M. abscessus. The optimal dosing formulation remains to be defined, and in vivo efficacy has yet to be demonstrated.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Cystic Fibrosis Foundation and by the Department of Veterans Affairs though a merit review grant to B.E.B. The contents of this report do not necessarily represent the view of the Department of Veterans Affairs or the U.S. Government.
REFERENCES
- 1.Hauser AR, Jain M, Bar-Meir M, McColley SA. 2011. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev 24:29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, Saiman L. 2009. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 136:1554–1560. doi: 10.1378/chest.09-0132. [DOI] [PubMed] [Google Scholar]
- 3.Petrini B. 2006. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS 114:319–328. doi: 10.1111/j.1600-0463.2006.apm_390.x. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DE, Girard WM, Wallace RJ. 1993. Clinical features of pulmonary disease caused by rapidly growing mycobacteria: an analysis of 154 patients. Am Rev Respir Dis 147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 5.Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. 2010. Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. Am J Respir Cell Mol Biol 43:387–393. doi: 10.1165/rcmb.2009-0276TR. [DOI] [PubMed] [Google Scholar]
- 6.Aitken ML, Limaye A, Pottinger P, Whimbey E, Goss CH, Tonelli MR, Cangelosi GA, Dirac MA, Olivier KN, Brown-Elliott BA, McNulty S, Wallace RJ Jr. 2012. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 185:231–232. doi: 10.1164/ajrccm.185.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Román A, Ussetti P, Sole A, Zurbano F, Borro JM, Vaquero JM, de Pablo A, Morales P, Blanco M, Bravo C, Cifrian J, de la Torre M, Gemez P, Laporta R, Monforte V, Mons R, Salvatierra A, Santos F, Sole J, Varela A. 2011. Guidelines for the selection of lung transplantation candidates. Arch Bronconeumol 47:303–309. doi: 10.1016/j.arbres.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 10.Broda A, Jebbari H, Beaton K, Mitchell S, Drobniewski F. 2013. Comparative drug resistance of Mycobacterium abscessus and M. chelonae isolates from patients with and without cystic fibrosis in the United Kingdom. J Clin Microbiol 51:217–223. doi: 10.1128/JCM.02260-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medjahed H, Gaillard J-L, Reyrat J-M. 2010. Mycobacterium abscessus: a new player in the mycobacterial field. Trends Microbiol 18:117–123. doi: 10.1016/j.tim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 12.De Voss JJ, Rutter K, Schroeder BG, Barry CE. 1999. Iron acquisition and metabolism by mycobacteria. J Bacteriol 181:4443–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olakanmi O, Kesavalu B, Abdalla MY, Britigan BE. 2013. Iron acquisition by Mycobacterium tuberculosis residing within myeloid dendritic cells. Microb Pathog 65:21–28. doi: 10.1016/j.micpath.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Boradia VM, Malhotra H, Thakkar JS, Tillu VA, Vuppala B, Patil P, Sheokand N, Sharma P, Chauhan AS, Raje M, Raje CI. 2014. Mycobacterium tuberculosis acquires iron by cell-surface sequestration and internalization of human holo-transferrin. Nat Commun 5:4730. doi: 10.1038/ncomms5730. [DOI] [PubMed] [Google Scholar]
- 15.Tullius MV, Harmston CA, Owens CP, Chim N, Morse RP, McMath LM, Iniguez A, Kimmey JM, Sawaya MR, Whitelegge JP, Horwitz MA, Goulding CW. 2011. Discovery and characterization of a unique mycobacterial heme acquisition system. Proc Natl Acad Sci U S A 108:5051–5056. doi: 10.1073/pnas.1009516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones CM, Niederweis M. 2011. Mycobacterium tuberculosis can utilize heme as an iron source. J Bacteriol 193:1767–1770. doi: 10.1128/JB.01312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein LR. 1998. Mechanisms of therapeutic activity for gallium. Pharmacol Rev 50:665–682. [PubMed] [Google Scholar]
- 18.Gobin J, Moore CH, Reeve JR, Wong DK, Gibson BW, Horwitz MA. 1995. Iron acquisition by Mycobacterium tuberculosis: isolation and characterization of a family of iron-binding exochelins. Proc Natl Acad Sci U S A 92:5189–5193. doi: 10.1073/pnas.92.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antunes LCS, Imperi F, Minandri F, Visca P. 2012. In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 56:5961–5970. doi: 10.1128/AAC.01519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olakanmi O, Britigan BE, Schlesinger LS. 2000. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect Immun 68:5619–5627. doi: 10.1128/IAI.68.10.5619-5627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olakanmi O, Kesavalu B, Pasula R, Abdalla MY, Schlesinger LS, Britigan BE. 2013. Gallium nitrate is efficacious in murine models of tuberculosis and inhibits key bacterial Fe-dependent enzymes. Antimicrob Agents Chemother 57:6074–6080. doi: 10.1128/AAC.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes MS, Boelaert JR, Appelberg R. 2001. Role of iron in experimental Mycobacterium avium infection. J Clin Virol 20:117–122. doi: 10.1016/S1386-6532(00)00135-9. [DOI] [PubMed] [Google Scholar]
- 23.Gobin J, Horwitz MA. 1996. Exochelins of Mycobacterium tuberculosis remove iron from human iron-binding proteins and donate iron to mycobactins in the M. tuberculosis cell wall. J Exp Med 183:1527–1532. doi: 10.1084/jem.183.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lounis N, Truffot-Pernot C, Grosset J, Gordeuk VR, Boelaert JR. 2001. Iron and Mycobacterium tuberculosis infection. J Clin Virol 20:123–126. doi: 10.1016/S1386-6532(00)00136-0. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein LR. 2005. 31Ga therapeutic gallium compounds, p 259–277. In Gielen M, Tiekink ERT (ed), Metallotherapeutic drugs and metal-based diagnostic agents. John Wiley & Sons, Inc., New York, NY. [Google Scholar]
- 26.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 117:877–888. doi: 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olakanmi O, Gunn JS, Su S, Soni S, Hassett DJ, Britigan BE. 2010. Gallium disrupts iron uptake by intracellular and extracellular Francisella strains and exhibits therapeutic efficacy in a murine pulmonary infection model. Antimicrob Agents Chemother 54:244–253. doi: 10.1128/AAC.00655-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stojiljkovic I, Kumar V, Srinivasan N. 1999. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol Microbiol 31:429–442. doi: 10.1046/j.1365-2958.1999.01175.x. [DOI] [PubMed] [Google Scholar]
- 29.Tettelin H, Davidson RM, Agrawal S, Aitken ML, Shallom S, Hasan NA, Strong M, de Moura VC, De Groote MA, Duarte RS, Hine E, Parankush S, Su Q, Daugherty SC, Fraser CM, Brown-Elliott BA, Wallace RJ Jr, Holland SM, Sampaio EP, Olivier KN, Jackson M, Zelazny AM. 2014. High-level relatedness among Mycobacterium abscessus subsp. massiliense strains from widely separated outbreaks. Emerg Infect Dis 20:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olakanmi O, Stokes JB, Britigan BE. 1994. Acquisition of iron bound to low molecular weight chelates by human monocyte-derived macrophages. J Immunol 153:2691–2703. [PubMed] [Google Scholar]
- 31.Chitambar CR, Boon P, Wereley JP. 1996. Evaluation of transferrin and gallium-pyridoxal isonicotinoyl hydrazone as potential therapeutic agents to overcome lymphoid leukemic cell resistance to gallium nitrate. Clin Cancer Res 2:1009–1015. [PubMed] [Google Scholar]
- 32.Cortes MA, Nessar R, Singh AK. 2010. Laboratory maintenance of Mycobacterium abscessus. Curr Protoc Microbiol Chapter 10:Unit 10D.1. doi: 10.1002/9780471729259.mc10d01s18. [DOI] [PubMed] [Google Scholar]
- 33.Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. 2004. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331:370–375. doi: 10.1016/j.ab.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. 1982. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res 42:1530–1536. [PubMed] [Google Scholar]
- 35.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 36.Rastogi N, Potar M-C, David HL. 1987. Intracellular growth of pathogenic mycobacteria in the continuous murine macrophage cell line J-774: ultrastructure and drug-susceptibility studies. Curr Microbiol 16:79–92. doi: 10.1007/BF01588176. [DOI] [Google Scholar]
- 37.Owens CP, Chim N, Goulding CW. 2013. Insights on how the Mycobacterium tuberculosis heme uptake pathway can be used as a drug target. Future Med Chem 5:1391–1403. doi: 10.4155/fmc.13.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horwitz LD, Horwitz MA. 2014. The exochelins of pathogenic mycobacteria: unique, highly potent, lipid- and water-soluble hexadentate iron chelators with multiple potential therapeutic uses. Antioxid Redox Signal 21:2246–2261. doi: 10.1089/ars.2013.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neyrolles O, Wolschendorf F, Mitra A, Niederweis M. 2015. Mycobacteria, metals, and the macrophage. Immunol Rev 264:249–263. doi: 10.1111/imr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong A, Rodrigue N, Kassen R. 2012. Genomics of adaptation during experimental evolution of the opportunistic pathogen Pseudomonas aeruginosa. PLoS Genet 8:e1002928. doi: 10.1371/journal.pgen.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penterman J, Nguyen D, Anderson E, Staudinger BJ, Greenberg EP, Lam JS, Singh PK. 2014. Rapid evolution of culture-impaired bacteria during adaptation to biofilm growth. Cell Rep 6:293–300. doi: 10.1016/j.celrep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen D, Singh PK. 2006. Evolving stealth: genetic adaptation of Pseudomonas aeruginosa during cystic fibrosis infections. Proc Natl Acad Sci U S A 103:8305–8306. doi: 10.1073/pnas.0602526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goss CH, Hornick DB, Aitken ML, Anderson G, Caldwell E, Lechtzin N, Wilhelm E, Wolfstone A, Allgood S, Teresi M, Singh PK. 2012. Phase 1 pharmacokinetic and safety study of intravenous Ganite (gallium nitrate) in CF. Pediatr Pulmonol 47:A226. [Google Scholar]
- 44.Goss C, Singh P. 2012. Gallium, a novel approach to treating chronic pseudomonal lung infections in CF. Pediatr Pulmonol 3:174–175. [Google Scholar]
- 45.Goss CH, Hornick DB, Aitken ML, Caldwell E, Wilhelm E, Wolfstone A, Teresi M, Singh PK. 2011. Phase 1 pharmacokinetic and safety study of intravenous Ganite™ (gallium nitrate) in cystic fibrosis. Pediatr Pulmonol 46:A230. [Google Scholar]
- 46.Todd PA, Fitton A. 1991. Gallium nitrate. A review of its pharmacological properties and therapeutic potential in cancer-related hypercalcaemia. Drugs 42:261–273. [DOI] [PubMed] [Google Scholar]
- 47.Seligman PA, Moran PL, Schleicher RB, Crawford ED. 1992. Treatment with gallium nitrate: evidence for interference with iron metabolism in vivo. Am J Hematol 41:232–240. doi: 10.1002/ajh.2830410403. [DOI] [PubMed] [Google Scholar]
- 48.Kelsen DP, Alcock N, Yeh S, Brown J, Young C. 1980. Pharmacokinetics of gallium nitrate in man. Cancer 46:2009–2013. doi:. [DOI] [PubMed] [Google Scholar]
- 49.Krakoff IH, Newman RA, Goldberg RS. 1979. Clinical, toxicologic and pharmacologic studies of gallium nitrate. Cancer 44:1722–1727. doi:. [DOI] [PubMed] [Google Scholar]
- 50.Bozja J, Yi K, Shafer WM, Stojiljkovic I. 2004. Porphyrin-based compounds exert antibacterial action against the sexually transmitted pathogens Neisseria gonorrhoeae and Haemophilus ducreyi. Int J Antimicrob Agents 24:578–584. doi: 10.1016/j.ijantimicag.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Olczak T, Maszczak-Seneczko D, Smalley J, Olczak M. 2012. Gallium(III), cobalt(III) and copper(II) protoporphyrin IX exhibit antimicrobial activity against Porphyromonas gingivalis by reducing planktonic and biofilm growth and invasion of host epithelial cells. Arch Microbiol 194:719–724. doi: 10.1007/s00203-012-0804-3. [DOI] [PubMed] [Google Scholar]