Summary
Primary sclerosing cholangitis (PSC) is a chronic cholangiopathy characterized by biliary fibrosis, development of cholestasis and end stage liver disease, high risk of malignancy, and frequent need for liver transplantation. The poor understanding of its pathogenesis is also reflected in the lack of effective medical treatment. Well-characterized animal models are utterly needed to develop novel pathogenetic concepts and study new treatment strategies. Currently there is no consensus on how to evaluate and characterize potential PSC models, which makes direct comparison of experimental results and effective exchange of study material between research groups difficult. The International Primary Sclerosing Cholangitis Study Group (IPSCSG) has therefore summarized these key issues in a position paper proposing standard requirements for the study of animal models of PSC.
Keywords: Animal model, Biliary fibrosis, Bile acids, Cholangiopathies, Cholestatic liver disease, Primary sclerosing cholangitis
Introduction
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown etiology characterized by inflammation, fibrosis, and strictures of the intra- and extrahepatic bile ducts. PSC is more prevalent in men and frequently associated with inflammatory bowel disease (IBD), predominantly ulcerative colitis (UC) with a specific PSC-IBD phenotype [1–5]. As a chronic progressive disease it may ultimately lead to biliary cirrhosis and end-stage liver disease [6,7]. Moreover, PSC carries a high risk for malignancies of the biliary tract and colon, as well as of the liver and probably the pancreas. These neoplastic complications have come to represent the principal cause of deaths since liver transplantation became the standard of care for patients progressing towards end stage biliary cirrhosis [6–9]. Disease modifying pharmacologic treatments unfortunately are lacking.
Longitudinal studies in PSC have been restricted by the lack of early disease markers, low incidence of the disease, and the limited accessibility of the human biliary tract, all of which may also represent major reasons for the diminutive progress made in the understanding of the disease. The pathogenesis of PSC can be considered as enigmatic [10,11]. A number of different concepts have recently been reviewed on the potential role of genetic factors, aberrant lymphocyte homing, leaky gut, vascular defects, and altered bile composition in PSC [7,10–12]. Moreover, PSC may still represent a mixed container of yet unrecognized etiologies and the general perception is that it may fragment into several clinical subentities in the near future. Therefore, PSC might well be designated as a “syndrome” rather than a “disease entity”. Although at first glance perhaps semantic, such a re-classification (i) may better reflect our uncertainties in understanding the pathogenesis, natural history, and response to currently available treatment of this syndrome and (ii) may provide more space for future research developments. These developments will be difficult to come if animal models of PSC will not become available. The major attributes of an “ideal PSC model” have been summarized thoughtfully by John Vierling [14] and currently available animal models for sclerosing cholangitis (SC) have recently been reviewed in detail elsewhere [15]. In brief, immunogenetically predisposed animals may develop fibrous-obliterative cholangitis of the intra- and extrahepatic bile ducts at best in association with inflammation of the gut (especially colitis with a specific distribution pattern and clinical presentation recently referred to as PSC-IBD phenotype with predominant right-sided colitis) and the development of cholangiocellular carcinoma. In addition, special immunological phenotypes of inflammatory cells infiltrating portal tracts (similar to the human situation) as well as atrophy of cholangiocytes should be present. In an ideal world such a model would also reflect gender aspects such as the male predominance of PSC (Fig. 1A). As no animal model yet exists with all these attributes, there is a need for new, well-characterized and highly reproducible PSC animal models to better understand the pathobiology of PSC and test novel treatment modalities. Although some of the currently available animal models show some individual characteristics of PSC, allowing longitudinal studies and testing of innovative medical treatment strategies, it is obvious that all of them have substantial limitations in regard to their construct and/or their face validity (Table 1) [15]. In addition, some of the models may be useful to study certain pathophysiological aspects of sclerosing cholangitis, but may not fit well for drug testing (e.g., common bile duct ligated rodents) [16,17]. It is also clear, that, due to the syndromic nature of the disease it is very possible that no single ideal model will ever be generated, and that the study of combinations of specific biliary models will turn-out to be the most productive and doable approach. Furthermore, appropriate comparison of different experimental findings is significantly limited due to the lack of guidelines in the characterization using well-defined and generally accepted standard methods and tests.
Fig. 1. PSC characteristics and attributes of an ideal PSC animal model.

(A) Attributes of an ideal PSC animal model. Ideally onionskin-type periductal fibrosis of intrahepatic bile ducts, bile duct replacement by a scar formation (as illustrated by the asterisk on SR stained section) and development of bile duct proliferation (visualized with K19 staining) as shown in human PSC in the upper panel would be mirrored in such a model. Typical macroscopic appearance of the biliary tree with strictures and dilatations of large and medium-sized bile ducts shown with bile duct plastination of an Abcb4−/− mouse (left) as well as via MRCP in a PSC patient (central panel). In addition, special immunological phenotypes of inflammatory cells infiltrating portal tracts (similar to the human situation) as well as atrophy of cholangiocytes should be present as illustrated by the cartoon (lower panel, left). An association with inflammatory bowel disease together with male predominance (lower panel) would round off the ideal PSC model. Original magnification for the upper panel 100× and 200×. bd, bile duct; cv, central vein; pv, portal vein. (B) Histological changes in human PSC. The characteristics of early stage (stage I and II) include a diffuse mixed cell inflammatory infiltrate around the bile ducts, portal edema, ductular reaction and invading neutrophilic granulocytes (biliary interphase activity) as illustrated by the cartoons (upper panel) and by the HE- and SR-stained sections of human PSC livers (lower panel). Further progression of the disease is accompanied by increasing portal fibrosis, ductular reaction (indicated by the arrows), bile duct replacement by a scar formation (as illustrated by the asterisks) with the formation of portal-portal linking septa (biliary fibrosis) (stage III) and finally the development of cirrhosis (stage IV). Original magnification for the lower panel 100× and 40×. bd, bile duct; cv, central vein; dr, ductular reaction; pv, portal vein.
Table 1. Animal models of sclerosing cholangitis.
| Animal model | Species | Limitations | [Ref.] |
|---|---|---|---|
| Chemically induced cholangitis | |||
| TNBS | Sprague-Dawley, Lewis rats | High mortality rate | [101-103] |
| ANIT | Sprague-Dawley rats | No large duct involvement | [104,105] |
| DDC | Swiss albino mice PDX-1 knockout mice | No typical findings on BD plastination | [106,107] |
| LCA | Swiss albino mice | No tolerable long-term protocol | [108] |
| Knockout mouse models | |||
| Abcb4-/- | FVB/N | No development of IBD or CCC (but HCC) | [86,109] |
| Cftr-/- | C57BL/6J | High risk for intestinal obstruction, weak spontaneous phenotype (without DSS) | [110,111] |
| fch/fch | BALB/c | Extrahepatic BD not studied so far | [112,113] |
| Infectious agents | |||
| Cryptosporidium parvum | BALB/c nu/nu, BALB/c SCID, C57BL/6- SCID, NIH-III nu/nu CD40-/-, IFNγ-/-, CD154-/-, CD40- CD154-/-, Tnfsf5-/-, Tnfrsf1a-/-, Tnfrsf1b-/-, Tnfrsf1a/1b-/-, Tnfsf5-Tnfrsf1a-/-, Tnfsf5-Tnfrsf1b-/-, Tnfsf5-Tnfrsf1a/1b-/-, CD40-Tnfrsf1a/1b-/- | Complex models, phenotype so far not well characterized | [114-116] |
| Helicobacter hepaticus | A/JCr, C3H/HeNCr, C57BL/6NCr, A/J | Complex models | [118,119] |
| Experimental biliary obstruction | C57BL/6J | Technical pitfalls | [120] |
| Models involving enteric bacterial cell-wall components or colitis | |||
| SBBO | Lewis and Wistar rats | No development of fibrosis; no large duct involvement | [121] |
| PG-PS | Lewis rats | No development of fibrosis; no large duct involvement | [121] |
| fMLT | Wistar rats | No development of fibrosis; high mortality rate | [122,123] |
| DSS | CD-1 mice | No development of fibrosis; no large duct involvement | [124] |
| TNBS + ANIT | Spraque-Dawley rats | No development of fibrosis | [125] |
| Models of biliary epithelial and endothelial cell injury | |||
| Experimental GVHD | BALB/c | Low fibrotic response | [126] |
| TNBS | Lewis rats | Mild phenotype | [127] |
| Complete hepatic arterial deprivation | Wistar rats | Low fibrotic response | [128] |
| Antigen driven models of biliary injury | |||
| Ova-Bil model | C57BL/6 mice | Lack of colitis, no fibrosis | [129] |
| Ova-Bil- iFABP-OVA T cell transfer mode | l C57BL/6 mice | Low level biliary inflammation, no fibrosis | [130] |
ANIT, alpha-naphthylisothiocyanate; CCC, cholangiocellular carcinoma; Cftr, cystic fibrosis transmembrane conductance regulator; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; DSS, dextrane sodium sulfate; fch, ferrochelatase; fMLT, N-formyl L-methionine L-leucin L-tyrosine; GVHD, graft-vs.-host disease; LCA, lithocholic acid; PG-PS, peptidoglycan-polysaccharide; SBBO, small bowel bacterial overgrowth; TNBS, 2,4,5-trinitrobenzene sulfonic acid.
In group and individual meetings, the IPSCSG discussed the need for methodological standards to characterize potential animal models for PSC. We performed a systematic Medline search using the combined search terms “cholangitis” and “animal model” for suitable animal models of SC with biliary fibrosis, with focus on rodent models since these are currently the most common. The aim of this position paper is to provide an up-to-date standardized evaluation and characterization of potential PSC models to enable better future comparison of data and exchange of relevant reagents, study specimens, and material including liver tissue, bile, and serum samples, thus increasing the effectiveness and accelerating the scientific output of experimental animal research in the PSC field. In keeping with the clinical (IBD/PSC phenotype), and laboratory characteristics radiological findings, and histomorphological hallmarks of PSC [6,18], the study group proposed a standardized work-up for prospective mouse models (Table 2). The proposed complete workup however may not necessarily be performed in studies, which may well focus on single aspects of PSC.
Table 2. Standardized work-up for mouse models.
| Biometric (“clinical”) data | Serum tests | Visualization of the bile duct system | Conventional histological evaluation liver and extrahepatic BDs (eBDs) | Histochemical stains (liver and eBDs; FFPE) | IHC (liver and eBDs) | IF (liver and eBDs) | EM | Bile | Histological evaluation (ileum and colon)§ | Additional/back-up material |
|---|---|---|---|---|---|---|---|---|---|---|
| Animal activity and appearance (e.g. pilorection) scoring for diarrhoea, body weight, food intake liver weight, spleen weight, colon length | ALT, AP, SBA, Bilirubin | Plastination of BDs with maceration of the remaining liver Cholangiography MRI, CT |
Liver: Lobular architecture and type of fibrosis, portal inflammation iBDs: Ductular reaction, lobular inflammation, cholestasis eBDs: Caliber, epithelial membrane integrity, peribiliary glands (proliferation, metaplasia, mucin composition) |
H&E Sirius red PAS Hall's stain Oil red O (frozen section) |
K19 CD11b F4/80 CD4 CD8 VCAM-1 LFA-1 ICAM-1 PECAM-1 CX3CL-1 CX3CR-1 α-SMA |
ZO-1 Laminin Cadherin |
Tight junction alterations, features of autophagy, cellular inclusions | Bile flow, pH composition (BA, PL, cholesterol, GSH, bicarbonate concentrations) |
Ileum: Mucosal architecture, inflammation Colon:§,§§ Mucosal architecture, inflammation inflammation goblet cells |
Serum sample back-up Tissue: Ileum/colon spleen, kidney, white adipose tissue, brown adipose tissue stool-microbial studies urine |
The use of a histopathological scoring system is recommended [92].
The use of a clinical scoring system is recommended [94].
ALT, alanin-aminotransferase; α-SMA, alpha-smooth muscle antigen; AP, alkaline phosphatase; BA, bile acids; CD, cluster of differentiation; CX3CL1, fractalkine; CX3CR-1, CXC3 chemokine receptor; eBDs, extrahepatic BDs; EM, electron microscopy; FFPE, formalin-fixed paraffin-embedded; GSH, glutathione; iBDs, intrahepatic BDs; ICAM-1, intercellular adhesion molecule-1; K19, keratin 19; LFA-1, lymphocyte function-associated antigen-1; MRI, magnetic resonance imaging; PAS, periodic acid-Schiff; PECAM-1, platelet endothelial cell adhesion molecule-1; PL, phospholipids; SBA, serum bile acids; VCAM-1, vascular cell adhesion molecule-1; ZO-1, zonula occludens-1.
Housing conditions and diet
In the light of recent advancements with rather sophisticated metabolomics and microbiome analysis, it is essential to formulate a standardized diet. Since most biological processes have a pronounced circadian rhythm, tissue and biological fluids should preferentially be harvested between 8:00 a.m. and 12:00 noon. Mice should have free access to water and diet unless specific research questions require overnight fasting (which should be clearly stated). Experiments and tests should be compared in male and female mice since they may significantly differ in regard to every single aspect discussed below.
Standard laboratory testing
Sera should be analyzed for ALT, AP, serum bilirubin, and serum bile acid (BA) levels, and aliquots kept frozen at −80 °C for subsequent analyses at the time of sacrifice. Longitudinal studies and estimation of individual changes may be managed via repeated by retro-orbital blood-sampling, which is however not accomplishable in each country due to legal issues and repeated general anesthesia may also affect liver tests. Upon harvesting, blood samples can be taken by either cardiac puncture or decapitation. ALT, AP, and serum bilirubin levels may be determined with standard clinical measurement techniques. Total murine serum BAs are commonly measured photometrically using the 3α-hydroxy-steroid dehydrogenase (HSDH) reaction; however, available HSDH kits are adjusted for automated multisampler analysis of primary human, unconjugated, and glycine-conjugated BAs. Thus, careful standard curve evaluations with unconjugated and taurine-conjugated cholic acid are to be made for manual photometric measurements of murine BAs. This is of particular importance for biliary BAs with 1000-fold higher concentration. Interference with sulfated or glucuronidated BAs is of no concern, since these conjugates are not formed in significant amounts in rodents [20]. For specific questions, ultra performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry is considered state-of-the-art for detailed analysis of the composition of serum BAs [21–24].
Handling of tissue and organs
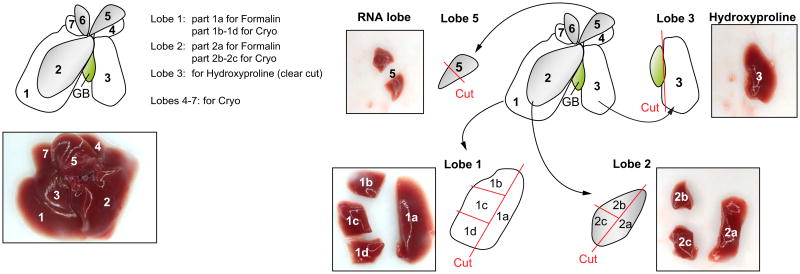
Body, liver, and spleen weight should be recorded upon harvesting. We suggest a standardized work-up for mouse liver tissue since there are significant morphological, physiological, and structural differences between the liver lobes, such as the degree of ductular reaction, size of bile ducts, degree of steatosis and hydroxyproline content (Fig. 2). As illustrated in Fig. 2, the liver lobes are numbered consecutively according to their size from 1 to 7. A central part should be excised from lobes 1 and 2 that is further fixed in 4% neutral-buffered formaldehyde solution (parts 1a and 2a), embedded in paraffin and processed for histological work-up (i.e., H&E, immunhistochemical staining) and a peripheral part excised for cryopreservation. We suggest consistent use of lobe 3 for hydroxyproline measurement and lobe 5 for RNA isolation. In addition, gallbladder and the extrahepatic bile ducts should be collected since PSC can affect the entire biliary tree.
Fig. 2. Liver preparation and processing.
Liver lobes are numbered consecutively according to their size from 1 to 7. A central part should be excised from lobes 1 and 2 that is further fixed in 4% neutral-buffered formaldehyde solution (parts 1a and 2a), embedded in paraffin and processed for histological work-up (i.e., H&E, immunohistochemical staining) and a peripheral part excised for cryopreservation. Lobe 3 is used for hydroxyproline measurement and lobe 5 for RNA isolation.
A standardized assessment of the small and large intestine should form an integral part of the work-up for potential PSC models in the light of the clinical association with PSC-IBD [5]. This should include both small intestinal (in particular ileal) as well as colonic assessment. Spontaneous signs of intestinal alterations that might not be apparent upon macroscopic examination need detailed histopathological evaluation including evaluation of cell proliferation by a blinded specialist histopathologist (see below, section on IBD). Pancreatic tissue should also be collected and stored.
Standard histomorphological characterization
One major problem in modeling PSC is its longstanding, chronically progressive, and variable clinical course, which is also mirrored in the human situation at least in part in the histopathological grading system. The histological changes in human PSC may be divided arbitrarily into four stages (as illustrated in Fig. 1B) [25,26]. In stage I, the typical histological changes are limited to the portal tracts with a diffuse mixed cell inflammatory infiltrate around the bile ducts and occasionally lymphoid follicles or aggregates with some mild fibrosis [25,27]. The biliary epithelium shows vacuolated cholangiocytes or atrophic changes (Fig. 1B) [25]. Stage II shows portal tract edema with disruption of the parenchymal limiting plate, ductular reaction and invading neutrophilic granulocytes (biliary interphase activity) [28]. The characteristic bile duct lesion of PSC is a fibro-obliterative cholangitis with an “onion skin” type of periductal fibrosis around medium sized and/or larger bile ducts with degeneration and atrophy of the biliary epithelium. Occasionally, bile ducts are replaced by fibrotic cords (scars), which may be found in all stages of PSC [29,30]. Further progression of the disease is accompanied by increasing portal fibrosis with the formation of portal-portal linking septa (biliary fibrosis) (stage III) and finally the development of cirrhosis (stage IV) [29]. In later stages of the disease the inflammation has a tendency to subside, followed by cholate stasis with feathery degeneration of periportal hepatocytes and Mallory-Denk body formation [31].
Accordingly, longitudinal histomorphological studies are required in potential PSC models to follow up the development, time course, and progression of sclerosing cholangitis, ductular reaction, fibrosis, and potential tumor development. In new genetic mouse models, routine histological examination of liver lobes 1 and 2 using H&E and Sirius red (SR) staining should be performed at least in 2-, 4- and 8-week as well as 6- and 12-month-old mice. However, this may just be a general policy/guideline, since different time points for histomorphological analyses will vary widely, depending on the specificities of the model used with significantly differing time course, dynamics, and disease progression.
Immunological characterization
PSC patients show a relatively high prevalence of atypical perinuclear antineutrophil cytoplasmic antibodies (pANCA) [7,32,33]. However, due to their low specificity [34], the missing correlation with PSC activity [35], pANCA are of limited clinical value in PSC patients [6,15]. In addition, low specificity was reported for anti-nuclear (ANA) and anti-smooth muscle antigen (SMA) antibodies, anti-endothelial cell antibodies, anti-cardiolipin antibodies, thyroperoxidase, thyroglobulin and rheumatoid factor in PSC [7]. In addition, the value of AMA testing in murine models of autoimmune biliary diseases mice has recently been questioned due to its low specificity [19]. Consequently, serum autoantibody testing in animal models for PSC is of limited interest.
The inflammatory infiltrate in PSC suggests that initiation of the hepatic innate immune response by exogenous triggers such as pathogen-associated molecular patterns (PAMPs) entering the portal circulation via a permeable intestinal mucosa might be a primary inciting event in the pathogenesis of PSC. Accordingly, inflammatory cells, including macrophages, neutrophils, dendritic cells (DCs), lymphocytes, and NK cells are activated through pattern recognition receptors, secrete cytokines and chemokines, and perpetuate inflammatory reaction by activation of NK cells through IL-12 and recruitment of lymphocytes via TNF-α, IL-1β, and CXCL8 [36]. In addition, proinflammatory cytokines directly affect the secretory function of cholangiocytes [37–39]. In PSC, a predominant T cell infiltrate can be found in the portal area [40,41]. The T cell CD4/CD8 ratio shows considerable inconsistencies in different studies in PSC patients [40,41], also reflecting the distribution of T cell subsets within the liver, in which CD4 cells are seen more commonly in the portal tracts and CD8 cells predominately in areas of lobular hepatitis [42]. Consequently, potential PSC models can be characterized using specific antibodies for CD4 and CD8 lymphocytes, antibodies against neutrophils and macrophages (e.g., F4/80, CD11b). The liver lymphocyte population should also be examined and characterized with flow cytometry. Standardized methods for extracting liver lymphocytes [43] are recommended. Accordingly, the portal vein is perfused in situ followed by dissection, homogenization of the liver tissue, and density centrifugation to separate out the lymphocytes. The lymphocytes should be characterized with monoclonal antibodies and multi-colour flow cytometry. Lymphocytes from spleen, thymus and blood should be examined at the same time to distinguish liver specific phenomena from general attributes. The standard examination should include antibodies against CD4, CD8, CD45R/B220, CD25, and CD69, while more specific studies should also include other subset markers, maturation markers, and further activation markers [44]. Since flow cytometric examination allows quantification of different subsets of lymphocytes and their characteristics but does not give any information on their microanatomical localization, immunohistochemical staining should be added [45]. For immunophenotyping, we recommend that at least five animals to be included in each group to allow sufficient power to detect statistical differences. Ideally, the immunophenotyping should be performed before disease is histologically evident to detect initiating events and then later at a time point with full-blown histological phenotype.
Potential animal models for PSC should be studied for their hepatic and predominately cholangiocellular expression of ICAM-1, VCAM-1, MadCAM-1, since these markers are upregulated on bile ducts in PSC, which seems to be quite specific for PSC [46–56]. This reactive cholangiocyte phenotype plays an active role in propagating inflammation and fibrosis in PSC by aberrant expression of HLA class molecules and adhesion molecules [44–46]. In line with these data, increased numbers of LFA1-positive lymphocytes are frequently observed near damaged bile ducts and ICAM-1 expressing cholangiocytes in PSC [49], suggesting a major pathogenetic role for these mechanisms [49]. The strong association of PSC and IBD but the frequently independent clinical course of both prompted Grant and colleagues to postulate the “gut lymphocyte homing hypothesis” [52,55]. Several lines of evidence support this elegant hypothesis: (i) MAdCAM-1 expression, while not detected in normal liver, can be expressed aberrantly by hepatic endothelium of IBD patients, especially with concomitant PSC [54,55]. (ii) The intestinal expression of the vascular adhesion protein-1 (VAP-1) is significantly increased in IBD [54] and hepatic expression as well as serum activity is increased in PSC (iii). The imprinting and plasticity of gut-homing human T cells requires primary activation or reactivation by gut DCs. The inability of liver DCs to imprint gut tropism implies that α4β7+ CCR9+ T cells that infiltrate the liver in PSC are primed in the gut [55]. In addition, over-expression of CCL25 and its receptor CCR9 is highly specific for PSC [55]. It so is reasonable to assume that a MAdCAM-1/α4β7/CCL25/CCR9 axis plays a crucial role in PSC pathogenesis. Alternative chemokines that might be involved in PSC pathogenesis include CCL21 and CCL28, which are implicated in activating α4β7-integrins and thereby mediate lymphocyte binding to MAdCAM-1 [51]. The integrin αυβ6 is overexpressed in biliary epithelial cells of the ductular reaction and triggers the activation of TGFβ, which is of particular relevance to biliary-type fibrogenesis [57,58]. Accordingly, potential animal models for PSC should be studied for their hepatic expression of ICAM-1, VCAM-1, Mad-CAM-1, and integrin αυβ6. Since there is rapid development in novel research tools for the detection of different chemokines, cytokines and neuropeptides, investigators should ensure sufficient back-up of liver tissue for cross validation with other models and human tissue samples.
Characterization of ductular reaction and the reactive cholangiocyte phenotype
This reaction is characterized by atypical thin ductules with elongated structures, lined by flattened cells that are probably the progeny of the hepatic progenitor/stem cell compartment localized in the canals of Hering. Ductular reaction is frequently observed in cholestatic liver diseases and may, at least in part, reflect a regenerative response of the liver to cholestatic liver injury; it also represents a potential trigger for liver fibrosis of the biliary type, which has to be elucidated in more detail [59– 61].
Typically the ductular reaction is most pronounced at the periphery of the portal tracts and expands from portal tract to portal tract [59]. The functional significance of this characteristic histological finding in cholestatic liver disease is still not entirely clear and speculation surrounds its potential role in bile formation, liver regeneration, formation of a kind of bile reservoir, and as a trigger for liver fibrosis of the biliary type [11]. Studies should address the dynamics, mechanisms, and impact of this interesting phenomenon in animal models for PSC. Most importantly, well characterized animal models should allow detailed in vivo studies on the important cell-to-cell interactions between activated proliferating cholangiocytes, inflammatory cells, and portal myofibroblasts with many remaining open questions, since most of these concepts so far have only been studied using in vitro systems [62–65].
Ductular proliferation should be quantified with the aid of specific cholangiocellular markers such as the intermediate filament keratin 19, which is specifically expressed in normal rodent cholangiocytes. Since the phenomenon of ductal metaplasia (i.e., the de novo expression of keratin 7/19 in cholestatic hepatocytes) does not seem to represent a prominent feature in cholestatic rodent models, assessment of ductular reaction by digital image analysis (morphometry) of K7/19 immunohistochemistry as well as western blotting for keratin 7 or 19 in the same liver lobes represent well suited tools for quantification of ductular reaction/proliferation. In addition, morphometric analysis of liver tissue sections of the same liver lobes may be useful. Furthermore, progenitor cells destined to be of biliary lineage should be SOX9-, A6-, EpCam-positive [65–70]. However, little is known so far about the expression pattern of these interesting markers in different PSC animal models.
Characterization and quantification of liver fibrosis
PSC patients develop liver fibrosis of the biliary type with predominant portal fibrosis and porto-portal septa, which may progress to liver cirrhosis (Fig. 1B). In addition, there is typically onionskin type periductal fibrosis, particularly around mediumsized and large bile ducts (Fig. 1A). Comparable to other forms of liver fibrosis, collagen represents a major extracellular matrix protein in biliary fibrosis, which is typically found in broadened portal tracts and porto-portal septa (Fig. 1A). Myofibroblasts located in portal tracts originate either from hepatic stellate cells (HSCs) or portal fibroblasts and may represent the main cellular source of fibrosis in cholestatic liver disease; their proliferation is mediated by fibroblast growth factor-2 (FGF-2) and inhibited by TGFβ1 and TGFβ2 [71–73]. Immunhistochemical studies have shown that in fibrotic human and rat liver, portal and septal myofibroblasts displayed expression profiles that were distinct from those of interface myofibroblasts or sinusoidally located HSCs, suggesting that at least two subpopulations of myofibroblasts, HSC-derived myofibroblasts and portal mesenchymal cell-derived myofibroblasts, populated the injured liver [74]. To further distinguish HSCs from myofibroblasts, specific stellate cells markers, including cytoglobin (also known as stellate cell activation-associated protein – STAP), desmin, cellular retinol-binding proteins (CRBP) or lecithin-retinol acyltransferase (LRAT), might be used [75]. However, so far no reliable markers have been identified that allowed investigators to fully distinguish HSCs from portal mesenchymal cells at the stage of myofibroblasts. The relative contribution of HSCs to biliary fibrosis is in addition receiving increasing attention. Interestingly, there seems to be an extensive paracrine interaction between hepatic stellate cells, portal fibroblasts and activated cholangiocytes involving several cytokine-, chemokine-, and heat shock protein pathways, which may be critical for the development and progress of biliary fibrosis [76,77]. The relative contribution of HSCs to biliary fibrosis is in addition receiving increasing attention. However, so far no reliable markers have been identified that allowed investigators to fully distinguish HSCs from portal mesenchymal cells at the stage of myofibroblasts.
In animal models for PSC, PAS stain will demonstrate the thickening of the basal membrane of cholangiocytes and periductal condensation of fibrosis [78]. SR staining will show periductal onionskin type fibrosis and porto-portal septa. In general, it is again critical to compare the same liver lobes (e.g., lobe 3 in mouse livers) to quantify fibrosis, since in the biliary type there is enormous variability in fibrosis among the different liver lobes, also depending on the model studied. Measurement of the proportion of the collagen area seems to represent a useful tool in human liver tissue samples [79–81] and attempts should be made to establish this interesting method for use in animal models as well. Since morphometric analysis of SR-stained liver sections for quantification of liver fibrosis in rodents may present methodological problems depending on section thickness [82], direction of cutting, intensity of staining techniques, and lobular region investigated, it may be best to combine it with measurement of hydroxyproline content normalized to gram of liver (e.g., from liver lobe 3 in the case of mouse liver, Fig. 2). In analyses of gene expression reflecting fibrogenesis, we suggest considering collagen 4 and components of the basement membrane such as laminin, heparan sulphate proteoglycan, and fibronectin, which may be preferentially overexpressed in the biliary type of fibrosis [83]. Moreover, activated myofibroblast markers such as α-SMA and collagen-α1 may be stained immunohistochemically. Since therapeutic approaches may aim at apoptosis or silencing of myofibroblasts, specific staining for activation (e.g., α-SMA, collagenα1, fibronectin, lack of CD34/CD45) or silencing may be appropriate for specific research questions [84].
Characterization of bile duct tight junction (TJ) alterations
Bile regurgitation is considered to develop at least in late stage cholangiopathies through leaky bile ducts, bile duct ulceration, and damaged hepatocytes [85]. A decrease or disappearance of the TJ protein 7H6 was detected selectively at the hepatocyte level in PSC patients with immunofluorescence [85]. The lack of evidence for TJ alterations on the bile duct level in PSC patients may be related to sampling errors and the inaccessibility of the bile duct system for systematic studies in a disease primarily affecting large and medium-sized bile ducts. In order to uncover the functional significance of TJ integrity in PSC, future studies are needed to determine whether TJ alterations of bile ducts could play a role. It will be critical to determine whether such TJ alterations are causative or represent the consequence of bile duct alterations.
Models for PSC should therefore be explored for potential tight junction alterations in bile ducts and hepatocytes using double labeling fluorescence microscopy combining cell specific markers (e.g., K8/18 for hepatocytes and K7/19 for cholangiocytes) with tight junction protein markers such as E-cadherin and ZO-1 [86]. In addition, secreted fluorescent biliary compounds (e.g., fluorescent bile acids) or alternative tracers might help to characterize potential hepatocyte and cholangiocellular tight junction alterations [86]; transelectron microscopy could also be useful to this end. Transepithelial potential as well as permeation of opportunely sized fluorescent dextrans could be another option for functional characterization of a monolayer of isolated cholangiocytes [87].
Large bile duct imaging – Characterization of strictures and dilatations
Magnetic resonance cholangiography (MRC) represents a standard technique for examining PSC patients for strictures and dilatations of large and medium-sized bile ducts that eventually come to resemble a prune tree. Modern ECG-triggered MRI imaging on anesthetized mice using gadoxetate disodium as biliary contrast medium achieves high resolution MRC, but availability and experience with this method are currently limited [88]. However, MRC is an extremely attractive approach to study large duct morphology in PSC models and has the additional advantage of allowing repeated imaging for longitudinal studies in individual animals (Fig. 3). Alternatively, large duct disease and characteristic PSC-like alterations may be assessed using plastination of the bile duct system followed by chemical maceration of the remaining liver [89]. The term plastination refers to procedures that turn degradable biological tissues into quite stable specimens by replacing intra- and/or extracellular tissue fluids with curable polymers that are infiltrated or injected [89]. This technique gives highly reproducible results and it may also allow future studies to explore potential alterations of the vascular architecture including the peribiliary plexus by parallel plastination of hepatic arteries and portal veins using different colors for bile ducts and vessels. For reproducible high quality results the following steps should be followed: the filling of the investigated system depends on the viscosity of the injected resin and the pressure applied to the system. The viscosity of the polymer is reduced by adding 15% acetone, which will shrink the specimens by 15%. For comparable results it is crucial to use the same solution for all specimens. The pressure applied by the system should not exceed the normal system pressure (i.e., mean intrabiliary pressure 10 mmHg in mice) [90]. It is important to keep the pressure stable until the polymer is cured. An air cushion chamber should be used to keep the pressure stable, it should not be reduced or stopped until the resin has hardened. Finally, maceration in potassium hydroxide requires gentle treatment of the specimens to avoid fragmentation of the biliary tree. Each specimen should be treated in an individual receptacle. To eliminate dissolved tissue, the specimen should be washed gently in lukewarm water, since hot water will destroy the 3-D appearance by softening the cured polymer; water and the potassium chloride should be changed every 24–48 h. The specimen is studied under a stereomicroscope.
Fig. 3. Magnetic resonance imaging of the biliary system of a female Abcb4−/− mouse aged 10 weeks.

(A) Coronal view of the liver for anatomic reference. Bile is displayed hyperintense. (B and C) Maximum intensity projections (MIP) of the ventral section of the left liver lobe extracted from a 3D dataset in coronal (B) and transversal (C) orientation. The intrahepatic bile ducts clearly display a stricture (arrow) and an adjacent duct dilatation. Images were acquired with heavily T2-weighted respiratory-triggered 3D fast recovery fast spin echo sequence at 7 T field strength.
Characterization of biliary physiology
As the composition of bile may critically affect the biliary phenotype in certain mouse models (e.g., Abcb4−/− mice, lithocholic acid-fed mice, DDC-fed mice) [13,91], biliary physiology and bile composition should be studied in detail in potential PSC models. Bile should be sampled under general anesthesia after ligation of the common bile duct, cannulation of the gall bladder, and at least five min' equilibration time. Animals should be placed on a heater plate and kept on 38–39.5 °C. Bile volume should be determined gravimetrically to calculate bile flow following normalization to liver weight. Biliary concentration of bile acids, cholesterol, phospholipids, glutathione, and bicarbonate should be determined to calculate biliary output of each component. Biliary bile acid concentrations are analyzed with a HSDH assay. Biliary cholesterol concentrations are measured photometrically at 546 nm after enzymatic hydrolysis and oxidation; the indicator quinoneimine is formed from hydrogen peroxide and 4-amino-phenazone in the presence of phenol and peroxidase. Biliary phospholipid concentrations are analyzed utilizing N-ethyl-N(2-hydroxy-3-sulofopropyl)-3,5-domethoxyaniline, resulting in a blue pigment that is measured spectrophotometrically at 600 nm. After protein precipitation in 5% metaphosphoric acid, biliary glutathione (GSH) concentrations are determined spectrophotometrically at 356 to 400 nm. To determine biliary bicarbonate, total carbon dioxide, and pH concentration, bile is collected under mineral oil for 30 min and measured with an automatic blood gas analyzer. Tissue analyses or serum biochemical testing of mice used for bile sampling should be avoided due to the long-term surgical manipulation and resulting artifacts.
Characterization of the “IBD/PSC phenotype”
There is a well-known coincidence of PSC and inflammatory bowel diseases (IBD) with a special PSC-IBD phenotype [2–5]. Accordingly, we search for immunogenetically predisposed animals with fibrous-obliterative cholangitis of the intra- and extra-hepatic bile ducts in association with inflammation of the gut (especially right-sided colitis). Models with sclerosing cholangitis, or more generally cholangiopathy models, thus should be screened for gut inflammation and vice versa; potential IBD models should be tested for the development of sclerosing cholangitis over time. Consequently, we propose that an experienced pathologist screens the small and large intestine for inflammation using H&E-stained tissue sections of the ileum, jejunum, caecum, and left-sided colon in experimental animals. Histopathological features are alterations in the crypt-villus ratio (in the small intestine), crypt distortion, assessment of goblet cell numbers and configuration, neutrophil infiltration, and crypt abscess formation. A histopathological scoring system might be used [92,93]. In the case of clinically apparent colitis, a standardized clinical scoring system [94], and determination of colon length and weight should complement this analysis. A critical element of IBD and experimental models of IBD is the gut microbiome [95]. This should be assessed on colonic stool samples by next generation sequencing to detect changes in microbial communities that drive disease or are altered as a consequence of host changes e.g., gene deletions or therapies. To correct for microbial changes related to sourcing and husbandry, animals should - where possible – be purchased from the same supplier and co-housed.
How should we test therapies in PSC models?
Detailed long-term studies to describe a PSC model are indispensible for meaningful testing of potential therapies and drugs for PSC. It is essential to determine the times of high disease activity in relation to inflammation and fibrogenesis and those of stable disease for each particular model to allow firm conclusions as whether a tested drug or antibody inhibits or even heals the biliary disease. Best of all, of course, would be prevention vs. rescue approach. Ideally, a spontaneous genetic model with high construct and face validity without the need of additional surgical or toxic/infectious manipulations would be available to test potential drugs.
How to test tumor development in PSC models?
To our knowledge there is as yet no murine model with PSC features that develops cholangiocellular carcinoma. There are a few rodent models of hepatocellular cancer (HCC) that arise spontaneously within the context of cirrhosis and most of them require the administration of hepatotoxic and/or carcinogenic agents [96]. Abcb4−/− mice develop nodules and HCC at 6–12 months; however, this may differ significantly depending on the genetic background [97]. For current and future PSC models, systematic analysis using MRI techniques combined with careful histological analysis is recommended. Depending on the frequently varying life span of the animal models used, livers should be studied several times within the last third of the animals' life (e.g., at 9, 12, and 15 months). Investigators should also carefully monitor pathologically enlarged lymph nodes or extrahepatic spread of tumors (e.g., lungs), which, however, may be an unusual feature of malignancy in mice in general. Tumor specimens should be investigated by a specialized pathologist using a combination of routine histological and special immunohistochemical techniques, possibly supported by digital image analysis.
Could there be already some “undiscovered” PSC mouse models out there?
There are numerous publications on different mouse models including genetically modified inbred mouse strains with a more or less cholestatic phenotype [13]. Currently, however, we have no systematic catalogue of all those interesting models including all the readouts outlined above that should be available in an open database. As an example, there are no data on bile duct imaging or bile duct plastination in NOD.c3c4 mice, which also have large duct disease on liver histology and so could represent an interesting candidate studies on certain aspects of PSC [13]. Notably, all potential IBD models should be systematically screened for a PSC-like liver phenotype. Future research activities should therefore aim for a detailed phenotypic catalogue of potential PSC models to allow easy and correct selection of a model for specific research tools.
It is not all about mice: Non-rodent models of PSC
With the advances in genetic engineering with generation of knockout and transgenic models, mice belong to the most favored animal species in modern experimental biology and frequently have to take over the research baton from flies and worms. But using a very limited number of species to model an obviously complex disease such as PSC harbors the potential danger that we limit the possible answers to those that such organisms can provide [98]. Undoubtedly, mice have numerous advantages when used as experimental animals including the possibility of genetic manipulations, well characterized strains, easy handling, and relatively low costs. Currently, however, there is no ideal mouse PSC model and there are substantial limitations when we try to transfer experimental mouse data to the human situation, mainly due to the obvious disparities between mice and humans. In addition, there are numerous discouraging examples of initially promising therapeutic approaches in mouse models in a diversity of diseases [98,99]. Moreover, mouse models of IBD must be viewed very cautiously when it comes to advancement of new therapeutic concepts. We also should bear in mind that there are very exciting diseases in different species such as sclerosing cholangitis with inflammatory bowel disease in cats and a PSC-like phenotype recently described in baboons [100]. This leads to the question of whether we should increase our range of experimental animal species and discuss our urgent questions in regard to human PSC in more detail with specialized veterinarians to speed up and improve our search for a better PSC model.
Conclusion
We herein provide a practice guideline for standardized evaluation of potential PSC models that should encourage and facilitate systematic work-up of different mouse models with a clear cholangiopathy phenotype. Importantly, such a systematic approach is also recommended for novel models with suggestive signs of a cholestatic phenotype such as increased AP or SBA levels or ductular reaction. In addition this position paper could represent the starting point for a common database for potential PSC models to speed up our PSC research agenda.
Key Points.
Well-defined animal models for PSC are the basis for development of novel pathogenetic concepts and new treatment strategies
Standardized work-up of animal models for PSC for optimized comparison of obtained findings between research groups requires definition of housing conditions, diet, laboratory testing, tissue harvesting and processing, large bile duct and liver imaging, as well as biliary physiology
Histomorphological characterization of the liver should include standard staining techniques, such as H&E, Sirius red, and PAS staining
Immunological characterization should include characterization of lymphocyte subpopulations by flow cytometry and immunohistochemical evaluation of adhesion molecules
Longitudinal studies allow monitoring of progression of sclerosing cholangitis, ductular reaction, fibrosis, and tumor development
Detailed histopathological evaluation of the small and large intestine should be performed using standardized clinical and histopathological scoring systems
Large bile duct morphology should be assessed via plastination of the bile duct system and/or MRC, the latter enabling longitudinal studies
Biliary physiology and bile composition should be analyzed to explore potential alterations as factors determining disease progression and representing therapeutic targets
Abbreviations
- ANA
anti-nuclear antibody
- ANCA
anti-neutrophil cytoplasmic antibody
- ANIT
alpha-naphthylisothiocyanate
- BA
bile acids
- BDL
bile duct ligation
- BEC
biliary epithelial cell
- CCC
cholangiocellular carcinoma
- Cftr
cystic fibrosis transmembrane conductance regulator
- DC
dendritic cell
- DDC
3,5-die-thoxycarbonyl-1,4-dihydrocollidine
- DSS
dextrane sodium sulfate
- fch
ferroche-latase
- fMLT
N-formyl L-methionine L-leucin L-tyrosine
- GVHD
graft-vs.-host disease
- h
hours
- HSDH
3α-hydroxysteroid dehydrogenase
- IBD
inflammatory bowel disease
- IPSCSG
international primary sclerosing cholangitis study group
- LCA
lithocholic acid
- m
months
- Mdr2
multidrug resistance protein-2
- n.d.
not determined
- PG-PS
peptidoglycan-polysaccharide
- PSC
primary sclerosing cholangitis
- SBBO
small bowel bacterial overgrowth
- SFBL
self-filling blind loop
- SMA
smooth muscle-antigen
- SR
Sirius red
- TNBS
2,4,5-trinitrobenzene sulfonic acid
- UC
ulcerative colitis
Footnotes
Conflict of interest: The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Loftus EV, Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broome U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610–615. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, et al. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870–877. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430–436. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 5.Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31–39. doi: 10.1055/s-2008-1040420. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Krones E, Graziadei I, Trauner M, Fickert P. Evolving concepts in primary sclerosing cholangitis. Liver Int. 2012;32:352–369. doi: 10.1111/j.1478-3231.2011.02607.x. [DOI] [PubMed] [Google Scholar]
- 8.Fevery J, Henckaerts L, Van Oirbeek R, Vermeire S, Rutgeerts P, Nevens F, et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int. 2012;32:214–222. doi: 10.1111/j.1478-3231.2011.02575.x. [DOI] [PubMed] [Google Scholar]
- 9.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 10.Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis–aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48:S38–S57. doi: 10.1016/j.jhep.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 12.Karlsen TH, Franke A, Melum E, Kaser A, Hov JR, Balschun T, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther. 2012;36:517–533. doi: 10.1111/j.1365-2036.2012.05223.x. [DOI] [PubMed] [Google Scholar]
- 14.Vierling JM. Animal models for primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:591–610. doi: 10.1053/bega.2001.0207. [DOI] [PubMed] [Google Scholar]
- 15.Pollheimer MJ, Trauner M, Fickert P. Will we ever model PSC?– “It's hard to be a PSC model!”. Clin Res Hepatol Gastroenterol. 2011;35:792–804. doi: 10.1016/j.clinre.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Fickert P. Time to say goodbye to the drug or the model? – Why do drugs fail to live up to their promise in bile duct ligated mice? J Hepatol. 2014;60:12–15. doi: 10.1016/j.jhep.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Fickert P, Pollheimer MJ, Österreicher CH, Trauner M. Animal models of cholestasis in animal models for the study of human diseases. Academic Press; 2013. pp. 331–410. [Google Scholar]
- 18.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 19.Hohenester S, Beuers U, Medina JF, Elferink RP. Antimitochondrial antibodies may be insufficiently specific to define primary biliary cirrhosis-like disease in mouse models. Hepatology. 2013;58:828–830. doi: 10.1002/hep.26243. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51:3230–3242. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:209–217. doi: 10.1016/j.jchromb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Bathena SP, Csanaky IL, Alnouti Y. Simultaneous characterization of bile acids and their sulfate metabolites in mouse liver, plasma, bile, and urine using LC-MS/MS. J Pharm Biomed Anal. 2011;55:1111–1119. doi: 10.1016/j.jpba.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Humbert L, Maubert MA, Wolf C, Duboc H, Mahé M, Farabos D, et al. Bile acid profiling in human biological samples: comparison of extraction procedures and application to normal and cholestatic patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;15:135–145. doi: 10.1016/j.jchromb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Goto T, Myint KT, Sato K, Wada O, Kakiyama G, Iida T, et al. LC/ESI-tandem mass spectrometric determination of bile acid 3-sulfates in human urine 3beta-Sulfooxy-12alpha-hydroxy-5beta-cholanoic acid is an abundant nonamidated sulfate. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:69–77. doi: 10.1016/j.jchromb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Portmann BC, Nakamura Y. Diseases of the bile ducts. In: Burt AD, Portmann BC, Ferrell LD, editors. MacSween's pathology of the liver. Philadelphia: Churchill Livingstone Elsevier; 2007. pp. 517–581. [Google Scholar]
- 26.Portmann B, Zen Y. Inflammatory disease of the bile ducts-cholangiopathies: liver biopsy challenge and clinicopathological correlation. Histopathology. 2012;60:236–248. doi: 10.1111/j.1365-2559.2011.03853.x. [DOI] [PubMed] [Google Scholar]
- 27.Thorpe ME, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis, the biliary tree, and ulcerative colitis. Gut. 1967;8:435–448. doi: 10.1136/gut.8.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefkowitch JH. Primary sclerosing cholangitis. Arch Intern Med. 1982;142:1157–1160. [PubMed] [Google Scholar]
- 29.Desmet VJ. Histopathology of chronic cholestasis and adult ductopenic syndrome. Clin Liver Dis. 1998;2:249–264. viii. doi: 10.1016/s1089-3261(05)70006-4. [DOI] [PubMed] [Google Scholar]
- 30.Lefkowitch JH. Scheuer's liver biopsy interpretation. 8th. Edinburgh: Saunders/Elsevier; 2010. pp. 47–74. [Google Scholar]
- 31.Desmet VJ. Histopathology of cholestasis. Verh Dtsch Ges Pathol. 1995;79:233–240. [PubMed] [Google Scholar]
- 32.Seibold F, Weber P, Klein R, Berg PA, Wiedmann KH. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992;33:657–662. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansi DS, Fleming KA, Chapman RW. Importance of antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and ulcerative colitis: prevalence, titre, and IgG subclass. Gut. 1996;38:384–389. doi: 10.1136/gut.38.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terjung B, Worman HJ. Anti-neutrophil antibodies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:629–642. doi: 10.1053/bega.2001.0209. [DOI] [PubMed] [Google Scholar]
- 35.Schwarze C, Terjung B, Lilienweiss P, Beuers U, Herzog V, Sauerbruch T, et al. IgA class antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and autoimmune hepatitis. Clin Exp Immunol. 2003;133:283–289. doi: 10.1046/j.1365-2249.2003.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol. 2009;31:383–397. doi: 10.1007/s00281-009-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spirlì C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, et al. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology. 2003;124:737–753. doi: 10.1053/gast.2003.50100. [DOI] [PubMed] [Google Scholar]
- 39.Spirlì C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, et al. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 40.Bo X, Broome U, Remberger M, Sumitran-Holgersson S. Tumour necrosis factor alpha impairs function of liver derived T lymphocytes and natural killer cells in patients with primary sclerosing cholangitis. Gut. 2001;49:131–141. doi: 10.1136/gut.49.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteside TL, Lasky S, Si L, Van Thiel DH. Immunologic analysis of mononuclear cells in liver tissues and blood of patients with primary sclerosing cholangitis. Hepatology. 1985;5:468–474. doi: 10.1002/hep.1840050321. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto E, Lindor KD, Homburger HA, Dickson ER, Czaja AJ, Wiesner RH, et al. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc. 1993;68:1049–1055. doi: 10.1016/s0025-6196(12)60897-0. [DOI] [PubMed] [Google Scholar]
- 43.Zeissig S, Olszak T, Melum E, Blumberg RS, Hov JR, Lleo A, et al. Analyzing antigen recognition by natural killer T cells. Methods Mol Biol. 2013;960:557–572. doi: 10.1007/978-1-62703-218-6_41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollheimer MJ, Halilbasic E, Fickert P, Trauner M. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2011;25:727–739. doi: 10.1016/j.bpg.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasoshima M, Nakanuma Y, Tsuneyama K, Van de Water J, Gershwin ME. Immunohistochemical analysis of adhesion molecules in the micro-environment of portal tracts in relation to aberrant expression of PDC-E2 and HLA-DR on the bile ducts in primary biliary cirrhosis. J Pathol. 1995;175:319–325. doi: 10.1002/path.1711750310. [DOI] [PubMed] [Google Scholar]
- 47.Bloom S, Fleming K, Chapman R. Adhesion molecule expression in primary sclerosing cholangitis and primary biliary cirrhosis. Gut. 1995;36:604–609. doi: 10.1136/gut.36.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borchers AT, Shimoda S, Bowlus C, Keen CL, Gershwin ME. Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Semin Immunopathol. 2009;31:309–322. doi: 10.1007/s00281-009-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams DH, Hubscher SG, Shaw J, Johnson GD, Babbs C, Rothlein R, et al. Increased expression of intercellular adhesion molecule 1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 1991;14:426–431. [PubMed] [Google Scholar]
- 50.Dillon P, Belchis D, Tracy T, Cilley R, Hafer L, Krummel T. Increased expression of intercellular adhesion molecules in biliary atresia. Am J Pathol. 1994;145:263–267. [PMC free article] [PubMed] [Google Scholar]
- 51.Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 53.Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 54.Hillan KJ, Hagler KE, MacSween RN, Ryan AM, Renz ME, Chiu HH, et al. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver. 1999;19:509–518. doi: 10.1111/j.1478-3231.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 55.Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hubscher SG, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee-Turner L, Villablanca EJ, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137:320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications III. Implications for liver pathology. Virchows Arch. 2011;458:271–279. doi: 10.1007/s00428-011-1050-9. [DOI] [PubMed] [Google Scholar]
- 60.Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis. 2011;31:11–32. doi: 10.1055/s-0031-1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Eischeid AN, Chen XM. Col1A1 production and apoptotic resistance in TGF-β1-induced epithelial-to-mesenchymal transition-like phenotype of 603B cells. PLoS One. 2012;7:e51371. doi: 10.1371/journal.pone.0051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alabraba EB, Lai V, Boon L, Wigmore SJ, Adams DH, Afford SC. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2008;47:552–562. doi: 10.1002/hep.22011. [DOI] [PubMed] [Google Scholar]
- 64.Alvaro D, Onori P, Metalli VD, Svegliati-Baroni G, Folli F, Franchitto A, et al. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology. 2002;36:297–304. doi: 10.1053/jhep.2002.34741. [DOI] [PubMed] [Google Scholar]
- 65.Cruickshank SM, Southgate J, Selby PJ, Trejdosiewicz LK. Expression and cytokine regulation of immune recognition elements by normal human biliary epithelial and established liver cell lines in vitro. J Hepatol. 1998;29:550–558. doi: 10.1016/s0168-8278(98)80149-9. [DOI] [PubMed] [Google Scholar]
- 66.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 67.Chapman R, Cullen S. Etiopathogenesis of primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3350–3359. doi: 10.3748/wjg.14.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van den Oord JJ, Sciot R, Desmet VJ. Expression of MHC products by normal and abnormal bile duct epithelium. J Hepatol. 1986;3:310–317. doi: 10.1016/s0168-8278(86)80483-4. [DOI] [PubMed] [Google Scholar]
- 69.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, et al. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39:158–161. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 72.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832:948–954. doi: 10.1016/j.bbadis.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 75.Penz-Österreicher M, Österreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:245–258. doi: 10.1016/j.bpg.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Österreicher CH, Penz-Österreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinzani M. Epithelial-mesenchymal transition in chronic liver disease: fibrogenesis or escape from death? J Hepatol. 2011;55:459–465. doi: 10.1016/j.jhep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Lefkowitch JH. Special stains in diagnostic liver pathology. Semin Diagn Pathol. 2006;23:190–198. doi: 10.1053/j.semdp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 80.Jimenez W, Parés A, Caballería J, Heredia D, Bruguera M, Torres M, et al. Measurement of fibrosis in needle liver biopsies: evaluation of a colorimetric method. Hepatology. 1985;5:815–818. doi: 10.1002/hep.1840050517. [DOI] [PubMed] [Google Scholar]
- 81.Malkusch W, Rehn B, Bruch J. Advantages of Sirius Red staining for quantitative morphometric collagen measurements in lungs. Exp Lung Res. 1995;21:67–77. doi: 10.3109/01902149509031745. [DOI] [PubMed] [Google Scholar]
- 82.Junqueira LC, Montes GS, Sanchez EM. The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry. 1982;74:153–156. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- 83.Bolarin DM, Azinge EC. Biochemical markers, extracellular components in liver fibrosis and cirrhosis. Niger Q J Hosp Med. 2007;17:42–52. doi: 10.4314/nqjhm.v17i1.12541. [DOI] [PubMed] [Google Scholar]
- 84.Kisseleva T, Brenner DA. Inactivation of myofibroblasts during regression of liver fibrosis. Cell Cycle. 2013;12:381–382. doi: 10.4161/cc.23549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakisaka S, Kawaguchi T, Taniguchi E, Hanada S, Sasatomi K, Koga H, et al. Alterations in tight junctions differ between primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2001;33:1460–1468. doi: 10.1053/jhep.2001.25086. [DOI] [PubMed] [Google Scholar]
- 86.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Muendoerfer M, Schaefer UF, Koenig P, Walk JS, Loos P, Balbach S, et al. Online monitoring of transepithelial electrical resistance (TEER) in an apparatus for combined dissolution and permeation testing. Int J Pharm. 2010;392:134–140. doi: 10.1016/j.ijpharm.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 88.Tabibian JH, Macura SI, O'Hara SP, Fidler JL, Glockner JF, Takahashi N, et al. Micro-computed tomography and nuclear magnetic resonance imaging for noninvasive, live-mouse cholangiography. Lab Invest. 2013;93:733–743. doi: 10.1038/labinvest.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 90.Wiener SM, Hoyt RF, Jr, Deleonardis JR, Clevenger RR, Jeffries KR, Nagashima K, et al. Manometric changes during retrograde biliary infusion in mice. Am J Physiol Gastrointest Liver Physiol. 2000;279:G49–G66. doi: 10.1152/ajpgi.2000.279.1.G49. [DOI] [PubMed] [Google Scholar]
- 91.Lyoumi S, Abitbol M, Rainteau D, Karim Z, Bernex F, Oustric V, et al. Protoporphyrin retention in hepatocytes and Kupffer cells prevents sclerosing cholangitis in erythropoietic protoporphyria mouse model. Gastroenterology. 2011;141:1509–1519. doi: 10.1053/j.gastro.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 92.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, et al. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174–2185. [PubMed] [Google Scholar]
- 94.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;49:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM. Experimental models of hepatocellular carcinoma. J Hepatol. 2008;48:858–879. doi: 10.1016/j.jhep.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Katzenellenbogen M, Pappo O, Barash H, Klopstock N, Mizrahi L, Olam D, et al. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66:4001–4010. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- 98.Bolker J. Model organisms: there's more to life than rats and flies. Nature. 2012;491:31–33. doi: 10.1038/491031a. [DOI] [PubMed] [Google Scholar]
- 99.Geerts H. Of mice and men: bridging the translational disconnect in CNS drug discovery. CNS Drugs. 2009;23:915–926. doi: 10.2165/11310890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 100.Arenas-Gamboa AM, Bearss JJ, Hubbard GB, Porter BF, Owston MA, Dick EJ, Jr, et al. Sclerosing cholangitis in baboons (Papio spp) resembling primary sclerosing cholangitis of humans. Vet Pathol. 2012;49:524–527. doi: 10.1177/0300985811419532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mourelle M, Salas A, Vilaseca J, Guarner F, Malagelada JR. Induction of chronic cholangitis in the rat by trinitrobenzene sulfonic acid. J Hepatol. 1995;22:219–225. doi: 10.1016/0168-8278(95)80432-3. [DOI] [PubMed] [Google Scholar]
- 102.Orth T, Neurath M, Schirmacher P, Galle PR, Mayet WJ. A novel rat model of chronic fibrosing cholangitis induced by local administration of a hapten reagent into the dilated BD is associated with increased TNF-alpha production and autoantibodies. J Hepatol. 2000;33:862–872. doi: 10.1016/s0168-8278(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 103.Goetz M, Lehr HA, Neurath MF, Galle PR, Orth T. Long-term evaluation of a rat model of chronic cholangitis resembling human primary sclerosing cholangitis. Scand J Immunol. 2003;58:533–540. doi: 10.1046/j.1365-3083.2003.01335.x. [DOI] [PubMed] [Google Scholar]
- 104.Tjandra K, Sharkey KA, Swain MG. Progressive development of a Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology. 2000;31:280–290. doi: 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- 105.Lichtman SN, Wang J, Clark RL. A microcholangiographic study of liver disease models in rats. Acad Radiol. 1995;2:515–521. doi: 10.1016/s1076-6332(05)80410-6. [DOI] [PubMed] [Google Scholar]
- 106.Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marzioni M, Saccomanno S, Agostinelli L, Rychlicki C, De Minicis S, Pierantonelli I, et al. PDX-1/Hes-1 interactions determine cholangiocyte proliferative response to injury in rodents: possible implications for sclerosing cholangitis. J Hepatol. 2013;58:750–756. doi: 10.1016/j.jhep.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 108.Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, et al. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168:410–422. doi: 10.2353/ajpath.2006.050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 110.Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blanco PG, Zaman MM, Junaidi O, Sheth S, Yantiss RK, Nasser IA, et al. Induction of colitis in cftr−/− mice results in bile duct injury. Am J Physiol Gastrointest Liver Physiol. 2004;287:G491–G496. doi: 10.1152/ajpgi.00452.2003. [DOI] [PubMed] [Google Scholar]
- 112.Meerman L, Koopen NR, Bloks V, Van Goor H, Havinga R, Wolthers BG, et al. Biliary fibrosis associated with altered bile composition in a mouse model of erythropoietic protoporphyria. Gastroenterology. 1999;117:696–705. doi: 10.1016/s0016-5085(99)70464-6. [DOI] [PubMed] [Google Scholar]
- 113.Libbrecht L, Meerman L, Kuipers F, Roskams T, Desmet V, Jansen P. Liver pathology and hepatocarcinogenesis in a long-term mouse model of erythropoietic protoporphyria. J Pathol. 2003;199:191–200. doi: 10.1002/path.1257. [DOI] [PubMed] [Google Scholar]
- 114.Stephens J, Cosyns M, Jones M, Hayward A. Liver and bile duct pathology following Cryptosporidium parvum infection of immunodeficient mice. Hepatology. 1999;30:27–35. doi: 10.1002/hep.510300138. [DOI] [PubMed] [Google Scholar]
- 115.Ungar BL, Burris JA, Quinn CA, Finkelman FD. New mouse models for chronic Cryptosporidium infection in immunodeficient hosts. Infect Immun. 1990;58:961–969. doi: 10.1128/iai.58.4.961-969.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mead JR, Arrowood MJ, Sidwell RW, Healey MC. Chronic Cryptosporidium parvum infections in congenitally immunodeficient SCID and nude mice. J Infect Dis. 1991;163:1297–1304. doi: 10.1093/infdis/163.6.1297. [DOI] [PubMed] [Google Scholar]
- 117.Ponnuraj EM, Hayward AR. Requirement for TNF-Tnfrsf1 signalling for sclerosing cholangitis in mice chronically infected by Cryptosporidium parvum. Clin Exp Immunol. 2002;128:416–420. doi: 10.1046/j.1365-2249.2002.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ward JM, Anver MR, Haines DC, Benveniste RE. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 119.Avenaud P, Le Bail B, Mayo K, Marais A, Fawaz R, Bioulac-Sage P, et al. Natural history of Helicobacter hepaticus infection in conventional A/J mice, with special reference to liver involvement. Infect Immun. 2003;71:3667–3672. doi: 10.1128/IAI.71.6.3667-3672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Georgiev P, Jochum W, Heinrich S, Jang JH, Nocito A, Dahm F, et al. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008;95:646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 121.Lichtman SN, Sartor RB. Hepatobiliary injury associated with experimental small-bowel bacterial overgrowth in rats. Immunol Res. 1991;10:528–531. doi: 10.1007/BF02919752. [DOI] [PubMed] [Google Scholar]
- 122.Yamada S, Ishii M, Liang LS, Yamamoto T, Toyota T. Small duct cholangitis induced by N-formyl L-methionine L-leucin L-tyrosine in rats. J Gastroenterol. 1994;29:631–636. doi: 10.1007/BF02365447. [DOI] [PubMed] [Google Scholar]
- 123.Yamada S, Ishii M, Kisara N, Nagatomi R, Toyota T. Macrophages are essential for lymphocyte infiltration in formyl peptide-induced cholangitis in rat liver. Liver. 1999;19:253–258. doi: 10.1111/j.1478-3231.1999.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 124.Numata Y, Tazuma S, Nishioka T, Ueno Y, Chayama K. Immune response in mouse experimental cholangitis associated with colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol. 2004;19:910–915. doi: 10.1111/j.1440-1746.2003.03333.x. [DOI] [PubMed] [Google Scholar]
- 125.Tjandra K, Le T, Swain MG. Experimental colitis attenuates development of toxin-induced cholangitis in rats. Dig Dis Sci. 2002;47:1216–1223. doi: 10.1023/a:1015330809095. [DOI] [PubMed] [Google Scholar]
- 126.Nonomura A, Kono N, Minato H, Nakanuma Y. Diffuse biliary tract involvement mimicking primary sclerosing cholangitis in an experimental model of chronic graft-vs.-host disease in mice. Pathol Int. 1998;48:421–427. doi: 10.1111/j.1440-1827.1998.tb03927.x. [DOI] [PubMed] [Google Scholar]
- 127.Orth T, Neurath M, Schirmacher P, Treichel U, zum Büschenfelde Meyer, Mayet W. Anti-neutrophil cytoplasmic antibodies in a rat model of trinitrobenzenesulphonic acid-induced liver injury. Eur J Clin Invest. 1999;29:929–939. doi: 10.1046/j.1365-2362.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 128.Beaussier M, Wendum D, Fouassier L, Rey C, Barbu V, Lasnier E, et al. Adaptive bile duct proliferative response in experimental bile duct ischemia. J Hepatol. 2005;42:257–265. doi: 10.1016/j.jhep.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 129.Buxbaum J, Qian P, Khuu C, Shneider BL, Daikh DI, Gershwin ME, et al. Novel model of antigen-specific induction of bile duct injury. Gastroenterology. 2006;131:1899–1906. doi: 10.1053/j.gastro.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seidel D, Eickmeier I, Kühl AA, Hamann A, Loddenkemper C, Schott E. CD8 T cells primed in the gut-associated lymphoid tissue induce immune-mediated cholangitis in mice. Hepatology. 2013 doi: 10.1002/hep.26702. http://dx.doi.org/10.1002/hep.26702. [DOI] [PubMed]