ABSTRACT
Bacterial spore germination is a process whereby a dormant spore returns to active, vegetative growth, and this process has largely been studied in the model organism Bacillus subtilis. In B. subtilis, the initiation of germinant receptor-mediated spore germination is divided into two genetically separable stages. Stage I is characterized by the release of dipicolinic acid (DPA) from the spore core. Stage II is characterized by cortex degradation, and stage II is activated by the DPA released during stage I. Thus, DPA release precedes cortex hydrolysis during B. subtilis spore germination. Here, we investigated the timing of DPA release and cortex hydrolysis during Clostridium difficile spore germination and found that cortex hydrolysis precedes DPA release. Inactivation of either the bile acid germinant receptor, cspC, or the cortex hydrolase, sleC, prevented both cortex hydrolysis and DPA release. Because both cortex hydrolysis and DPA release during C. difficile spore germination are dependent on the presence of the germinant receptor and the cortex hydrolase, the release of DPA from the core may rely on the osmotic swelling of the core upon cortex hydrolysis. These results have implications for the hypothesized glycine receptor and suggest that the initiation of germinant receptor-mediated C. difficile spore germination proceeds through a novel germination pathway.
IMPORTANCE Clostridium difficile infects antibiotic-treated hosts and spreads between hosts as a dormant spore. In a host, spores germinate to the vegetative form that produces the toxins necessary for disease. C. difficile spore germination is stimulated by certain bile acids and glycine. We recently identified the bile acid germinant receptor as the germination-specific, protease-like CspC. CspC is likely cortex localized, where it can transmit the bile acid signal to the cortex hydrolase, SleC. Due to the differences in location of CspC compared to the Bacillus subtilis germinant receptors, we hypothesized that there are fundamental differences in the germination processes between the model organism and C. difficile. We found that C. difficile spore germination proceeds through a novel pathway.
INTRODUCTION
Clostridium difficile (a Gram-positive, spore-forming, strict anaerobe) has become a significant threat to antibiotic-treated or immunocompromised hosts. Antibiotics are known to disrupt the colonic microbiota, and this perturbation permits C. difficile colonization (1, 2). Due to the strict anaerobic nature of C. difficile cells, spores are generally thought to be the infectious agent (only the spore can survive for extended periods of time in the aerobic environment outside a host) (3, 4). Because the spore form is noninfectious, spores must germinate to actively growing bacteria which initiate infection (5, 6). Thus, germination by C. difficile spores represents one of earliest steps in the pathogenesis of this organism.
Endospore germination has been extensively studied in Bacillus sp. and, more recently, in clostridia (7, 8). In the spore core, small acid soluble proteins help protect the chromosomal DNA, and much of the water is replaced by pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) as a 1:1 chelate with calcium (CaDPA), accounting for ca. 10% of the dry weight of the spore (8). Surrounding the spore core, is an inner spore membrane, a thin layer of cell wall peptidoglycan, a thick layer of specialized cortex peptidoglycan, an outer spore membrane, and spore coat proteins. These features help protect the spore from environmental hardship and help the spore remain in a metabolically dormant state (8). Even though spores are metabolically dormant, they interact with the environment and germinate when conditions become favorable for vegetative growth.
In Bacillus subtilis, germinant receptor-mediated germination can be divided into two stages. Stage I is triggered when germinant receptors embedded within the inner spore membrane respond to the presence of small molecule germinants (8). The most often described germinants for B. subtilis spores are l-alanine (or l-valine) or a mixture of l-asparagine, glucose, fructose, and potassium ions (AGFK) (8). The interaction of l-alanine/valine with the GerAA-AB-AC germinant receptor or AGFK with the GerB/GerK germinant receptor leads to the release of CaDPA from the core, likely through the SpoVA channel, in exchange for water (8). The release of CaDPA from the core completes stage I.
Stage II is activated by the release of CaDPA from the core during stage I, and stage II can be directly activated by an abundance of exogenous CaDPA (non-nutrient-mediated spore germination) (8). During stage II, cortex is degraded by the spore cortex lytic enzymes (SCLEs) CwlJ and SleB (8). While the mechanism of activation of SleB is unknown, CwlJ activity is activated by DPA (8). Thus, CaDPA release from the core stimulates cortex hydrolysis, which leads to the swelling of the germ cell wall and core expansion. The expansion of the core results in further hydration of the core and complete CaDPA release (8). Upon completing stage II, spores have lost most of their resistances and are no longer considered dormant. Then, in what has been described as “ripening,” the germinated spore prepares for the outgrowth of a vegetative cell (9).
C. difficile spore germination is stimulated by a combination of cholic acid derivatives and glycine (10, 11) and inhibited by chenodeoxycholic acid derivatives (10, 12–15). Although many of the ultrastructural features of the spore are conserved between B. subtilis and C. difficile, there are many differences (5, 16). Significantly, C. difficile does not encode the classical ger-type germinant receptor (17). Also, C. difficile encodes a single SCLE, SleC (17, 18). C. difficile SleC is synthesized in the mother cell during spore formation as a preproprotein and sleC is required for colony formation by C. difficile spores (18, 19). The presequence is cleaved off, presumably during transport across the spore outer membrane. The proprotein remains inactive in the dormant spore until it is cleaved by a germination-specific protease, CspB (20). In C. difficile, cspB is encoded as a fusion to cspA (17, 20). Upon translation of the cspBA mRNA, CspBA undergoes interdomain cleavage to generate both CspB and CspA proteins (20). A third protein, CspC, is encoded downstream of cspBA (17, 20). In C. perfringens, all three Csp proteins have predicted catalytic activity (all three possess intact catalytic triads) (21, 22). In C. difficile, only CspB is predicted to have catalytic activity because the residues important for catalysis are mutated in cspA and cspC (6, 17, 20). Recently, we identified C. difficile CspC as the bile acid germinant receptor (6). Certain SNPs in C. difficile cspC can abrogate spore germination, while other SNPs alter germinant specificity (6). We proposed a model where CspC activates CspB proteolytic activity and CspB cleaves pro-SleC to an active form. Activated SleC then begins to degrade the C. difficile spore cortex (6). Because the C. difficile germinant receptor complex (CspA, CspB, CspC, and SleC) is likely located in or near the spore cortex, while the B. subtilis germinant receptor complex is located in the spore's inner membrane, we hypothesized that there may be fundamental differences between the mechanisms of germinant receptor-mediated C. difficile spore germination and B. subtilis spore germination.
We investigated how C. difficile spores germinate with respect to the proposed stages of germination, as described for B. subtilis. In contrast to what is observed for B. subtilis spore germination (and C. perfringens [23]), we found that cortex hydrolysis preceded DPA release during C. difficile spore germination. Significantly, mutations in either the C. difficile bile acid germinant receptor, CspC, or the cortex hydrolase, SleC, prevented both cortex hydrolysis and DPA release by germinating C. difficile spores. These results suggest that DPA release during C. difficile spore germination may be entirely dependent on core swelling or changes to cortex peptidoglycan and that the hypothesized glycine germinant receptor is likely not located in the spore inner membrane.
MATERIALS AND METHODS
Bacteria and strains.
Wild-type C. difficile UK1 (6, 12, 15) and C. difficile M68 (15, 24, 25), C. difficile JSC10 (cspC::ermB) (6), and C. difficile CAA5 (sleC::ermB) were routinely grown in an anaerobic atmosphere (10% H2, 5% CO2, 85% N2) at 37°C in brain heart infusion agar supplemented with 5 g/liter yeast extract and 0.1% l-cysteine (BHIS). B. subtilis PS533 and B. subtilis FB113 (cwlJ::tet sleB::spc) (26) were a generous gift from Peter Setlow and were routinely grown on Difco sporulation medium (DSM). E. coli DH5α was grown on Luria-Bertani (LB) medium. Chloramphenicol (20 μg/ml), thiamphenicol (10 μg/ml), lincomycin (10 μg/ml), kanamycin (50 μg/ml for C. difficile, 20 μg/ml for E. coli, and 7 μg/ml for B. subtilis), spectinomycin (100 μg/ml), or tetracycline (5 μg/ml for C. difficile and 20 μg/ml for B. subtilis) were added where indicated.
Molecular biology.
To generate the TargeTron insertion into C. difficile UK1 sleC, we took advantage of a previously described primer set (18). The intron retargeting fragment was generated using the following primers: sleC(128a) IBS (AAAAAAGCTTATAATTATCCTTACATTACTTCTTAGTGCGCCCAGATAGGGTG), sleC(128a)EBS1d (CAGATTGTACAAATGTGGTGATAACAGATAAGTCTTCTTAGGTAACTTACCTTTCTTTGT), sleC(128a)EBS2 (TGAACGCAAGTTTCTAATTTCGGTTTAATGTCGATAGAGGAAAGTGTCT), and EBS universal (CGAAATTAGAAACTTGCGTTCAGTAAAC) using SOE-PCR as describe in the TargeTron manual (Sigma-Aldrich, St. Louis, MO). The 350-bp fragment was cloned into pCR2.1-TOPO (Life Technologies, Carlsbad, CA) to yield pCA2, and the sequence of the insert was verified. The 350-bp fragment was subcloned into the HindIII/BsrGI sites of the pJS107 TargeTron shuttle vector (15) to yield pJS113. The pJS113 plasmid was introduced into B. subtilis Bs49 using standard techniques. The pJS113 shuttle vector was introduced into C. difficile UK1 via conjugal transfer from B. subtilis Bs49, as described previously (6). Tetracycline-sensitive, thiamphenicol-resistant (Tn916 transposon-negative, plasmid-positive) strains were identified. These isolates were then spread on BHIS medium supplemented with lincomycin to select for the TargeTron insertion into sleC. Lincomycin-resistant colonies were screened by PCR for the presence of the TargeTron insertion into sleC, as described previously (18). Isolates with the insertion were frozen down as C. difficile CAA5 and have the expected phenotype of an sleC mutant (inability of spores to form colonies on BHIS agar supplemented with taurocholic acid) (18).
C. difficile CAA5 was complemented by expressing sleC in trans from the pJS116 shuttle vector (a pMTL84151 derivative) (6, 27). The C. difficile UK1 sleC gene and promoter region were amplified using the primers 5′sleC_Gibson (TACGAATTCGAGCTCGGTACCCGGGGATCCGATTATTTTCCTTTCAAAATTTTTGATTTATTTATGATTTATATC) and 3′sleC_Gibson (AGTGCCAAGCTTGCATGTCTGCAGGCCTCGAGTTAAATTAAAGGATTTAAAGAAGCTATTCTAGTTGTAG) and Phusion DNA polymerase (New England BioLabs, Beverly, MA). The resulting fragment was introduced into pJS116 between the BamHI and XhoI restrictions sites using Gibson assembly (28). The resulting plasmid, pMF02, was introduced into C. difficile CAA5 as described above.
Spore formation.
C. difficile spores were generated as described previously (6, 12, 15, 29). B. subtilis vegetative cells were spread on DSM agar medium for spore production (30). After 2 days, growth was harvested by scraping the plates and suspending the samples in water. This suspension (containing vegetative cells, cell debris, and spores) was then heated to 75°C for 1 h to melt any agar that was scraped with spores. The suspension was centrifuged for 10 min at room temperature and 3,000 × g. The supernatant was removed, and the pellet was resuspended in 10 ml of sterile water. To purify the spores from the vegetative cells and cell debris, the resuspended samples were layered onto a gradient of 10 ml of 20% HistoDenz (wt/vol), and 10 ml of 50% HistoDenz (wt/vol) and centrifuged for 1 h at 4°C and 18,900 × g. The supernatant was then removed, and the spore pellet was resuspended in 1 ml of water. The purified spores were then washed five times in water by centrifuging for 1 min at room temperature and 14,000 × g.
Monitoring the initiation of spore germination.
The initiation of spore germination was monitored aerobically at 600 nm (the initiation of C. difficile spore germination is unaffected by the presence of oxygen). To initiate B. subtilis spore germination, purified spores were suspended in 10 mM Tris (pH 8.4) and 100 mM l-valine. C. difficile spore germination was initiated by suspending spores in 10 mM Tris (pH 7.5), 150 mM NaCl, 100 mM glycine, and 10 mM taurocholic acid. Spores were heat shocked at either 80°C for B. subtilis or 65°C for C. difficile for 30 min and then placed on ice. Then, 5 μl of spores was diluted into 995 μl of buffer with or without germinant and mixed, and the change in optical density at 600 nm (OD600) was measured.
Monitoring CaDPA release.
CaDPA release was monitored in real time using terbium fluorescence (31). An opaque, 96-well plate was prepared with the 125 μl of the germination solutions (see above) supplemented with 800 μM TbCl3. Heat-activated spores were then sedimented for 1 min at 14,000 × g and resuspended in an equal volume of water to remove any CaDPA that may have released due to autogerminating spores. A 5-μl sample of a spore suspension (OD600 of 60) was added to each well, and the CaDPA release was monitored by using a Molecular Devices Spectramax M3 fluorescence plate reader (Molecular Devices, Sunnyvale, CA) (excitation, 270 nm; emission, 545 nm; cutoff, 420 nm [appropriate wavelengths for the DPA-Tb3+ complex]). For experiments involving mutations in the germination pathway (i.e., B. subtilis cwlJ::tet sleB::spc or sleB, C. difficile cspC::ermB, or C. difficile sleC::ermB), the amount of CaDPA released was compared to that of the wild-type strain.
Assaying cortex fragment release by germinating spores.
Cortex fragments were detected using an assay based on the presence of reducing sugars in the germination medium, as described previously (32, 33). Briefly, B. subtilis spores or C. difficile spores were heat activated, as described above, and stored on ice until use. An 11-ml germination solution (see above) was prepared. Before beginning the assay, a 1.0-ml sample was drawn to serve as a blank for cortex fragment detection, and a separate 100-μl sample was taken as a blank for measuring DPA release. A target spore density (OD600) of ∼3.0 yielded the best results for detecting cortex fragments. A zero time point sample was taken immediately after the addition of spores and centrifuged for 1 min at 14,000 × g. Then, 1.0 ml of this sample was transferred to a fresh tube for cortex fragment analysis (see below), and 100 μl was taken to monitor the amount of CaDPA released. This procedure was repeated at selected time points until the experiment was completed. After all time point samples were collected, the samples were frozen at −80°C and lyophilized.
Lyophilized samples were resuspended in 120 μl of 3N HCl supplemented with 1% phenol and 0.5% β-mercaptoethanol and then transferred to 2-ml screw-cap tubes. Samples were then placed in a 95°C recirculating water bath for 4 h. After incubation, the samples were placed on ice until cool and neutralized with 120 μl of 3 M NaOH. To these samples 80 μl of a saturated sodium bicarbonate solution and 80 μl of a 5% acetic anhydride solution were added, and the samples were mixed. The samples were incubated at room temperature for 10 min and then transferred back to the 95°C water bath for 3 min. The samples were removed from the water bath and cooled on ice, and then 400 μl of 6.54% K2B4O7·4H2O was added to each tube, followed by mixing. The resulting solution was then heated for 7 min in the 95°C water bath and placed on ice for 5 min, during which the color reagent was made. This color reagent was made by dissolving 0.320 g of p-dimethylaminobenzaldehyde in 1.9 ml of glacial acetic acid. After the p-dimethylaminobenzaldehyde was completely dissolved, 100 μl of 10 N HCl was added, the solution was mixed, and then 5 ml of glacial acetic acid was added. Next, 100 μl of each cooled cortex sample was transferred to a new 1.5-ml microcentrifuge tube, and 700 μl of the color solution was added. Samples were incubated in a 37°C water bath for 20 min. After incubation, 200 μl of each sample was transferred to a clear 96-well plate and quantified at 585 nm using a Molecular Devices Spectramax M3 fluorescence plate reader. As a positive control for reducing sugar detection, a standard curve was generated in each experiment using 0, 12.5, 25, 50, 100, 250, 500, and 5,000 nmol of N-acetylglucosamine. For experiments involving mutations in the germination pathway (i.e., B. subtilis cwlJ::tet sleB::spc, C. difficile cspC::ermB, or C. difficile sleC::ermB), the amount of cortex released was compared to that of the wild-type strain.
Statistical analysis.
Data points represent the mean from three independent experiments, and error bars represent the standard deviations from the mean. Statistical analysis between time points, where indicated, was performed using a two-tailed Student t test.
RESULTS
Comparison of the initiation of C. difficile and B. subtilis spore germination.
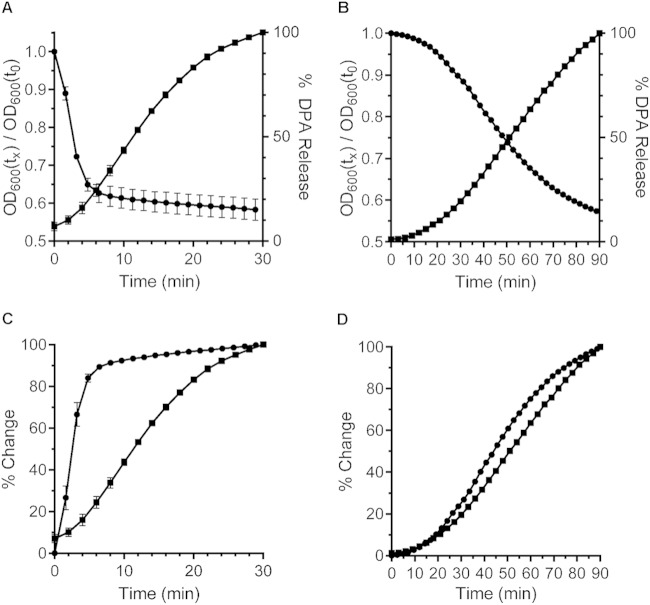
B. subtilis spore germination can be triggered via several pathways (e.g., nutrient-mediated activation of the ger-type germinant receptors or direct activation of cortex hydrolysis by CaDPA). To begin to understand the events that occur during C. difficile spore germination, we compared C. difficile spore germination to that of B. subtilis spore germination via activation of their respective germinant receptors. During spore germination, spores transition from a phase-bright state (dormant) to a phase-dark state (loss of dormancy). This transition can be monitored spectrophotometrically by measuring the OD600 of pure spore suspensions incubated under different conditions. When spores respond to germinant, they release their large depot of CaDPA from the core. This action results in a large and rapid decrease in the OD600 of the spore suspension (34). C. difficile UK1 spores were suspended in buffer supplemented with either taurocholic acid and glycine or taurocholic acid alone, and germination was monitored at 600 nm. As described previously, spores rapidly germinated upon exposure to both taurocholic acid and glycine but not when exposed to taurocholic acid alone (Fig. 1A and data not shown) (10). Although the use of absorbance to monitor germination is convenient, it is not a quantitative measure of CaDPA release and can include cortex hydrolysis at later time points (34). Thus, to provide a quantitative measure of DPA release, we monitored CaDPA release in real-time using an assay based on terbium fluorescence (31, 35). C. difficile spores released CaDPA in the presence of taurocholic acid and glycine but not in response to taurocholic acid alone (Fig. 1A and data not shown) and completed DPA release in ∼30 min (no further increase in DPA occurred after 30 min).
FIG 1.
Comparison of the initiation of C. difficile and B. subtilis spore germination. (A) Purified C. difficile UK1 spores were suspended in buffer supplemented with taurocholic acid and glycine. Germination was monitored by plotting the ratio of the OD600 at a given time to the OD600 at time zero (●) and DPA release from germinating C. difficile spores was monitored using Tb3+ fluorescence and normalized to the maximum amount of DPA released in the indicated time frame (■). (B) Purified B. subtilis PS533 spores were suspended in buffer supplemented with l-valine and germination was monitored as described above. The data from the OD600 in panels A and B were converted to the percent change so that the curves could be directly compared. The converted OD600 data were plotted with the DPA release data in panels C and D, respectively. The data represent the averages from three independent experiments, and error bars represent the standard deviations.
To compare C. difficile spore germination to that of B. subtilis, we incubated purified B. subtilis spores in buffer supplemented with l-valine. As described previously, the absorbance of the spore suspension decreased when incubated in the presence of l-valine and not in the absence of l-valine (Fig. 1B and data not shown) (36). Similarly, Tb3+ fluorescence increased when B. subtilis spores were suspended in buffered l-valine (indicating CaDPA release) but not in buffer alone (Fig. 1B and data not shown).
So that we could directly compare the absorbance assay to the terbium fluorescence assay, we plotted the percent change from the absorbance assay and the percent maximum Tb3+ fluorescence on the same graph. When analyzed in this manner, changes in the absorbance of germinating C. difficile spores occurred much earlier than changes observed in Tb3+ fluorescence (CaDPA release) (Fig. 1C). During B. subtilis spore germination, the CaDPA release curve closely followed that of the absorbance curve, confirming a previous study which demonstrated that much of the absorbance change is due to the released CaDPA (Fig. 1D) (34). Because CaDPA release is one of the first measurable events during germinant receptor-activation of B. subtilis spore germination, these results suggest that there may be events occurring during C. difficile spore germination before CaDPA is released.
CaDPA release precedes cortex hydrolysis release during B. subtilis spore germination.
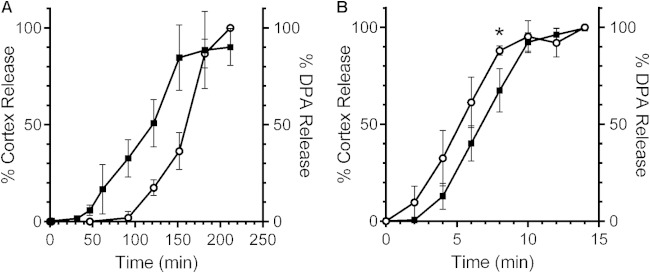
When CaDPA is released from the spore core, it transits through the spore cortex and activates the spore cortex hydrolase, CwlJ. CwlJ activity (and SleB activity) leads to the release of cortex fragments into the surrounding germination medium. B. subtilis spores suspended in germination buffer supplemented with l-valine released most of their CaDPA within 2.5 h (Fig. 2A). Spores suspended in buffer alone did not release CaDPA (data not shown). When we monitored for the presence of cortex fragments (as measured by the abundance of reducing sugars in the germination solution), we observed that these cortex fragments appeared after CaDPA is released (Fig. 2A) and that their presence was dependent on l-valine (data not shown). These results confirm the previous observations that CaDPA release precedes cortex hydrolysis in B. subtilis and, importantly, that we can detect cortex fragments during spore germination (34).
FIG 2.
Comparing the release of cortex fragments and CaDPA from germinating B. subtilis and C. difficile spores. (A) Purified B. subtilis PS533 spores were suspended in buffer with l-valine. (B) Purified C. difficile UK1 spores were suspended in buffer with taurocholic acid and glycine. At the indicated time points, a sample was taken, and the amounts of cortex fragments (○) and CaDPA (■) in the germination solutions were determined. The data represent the averages from three independent experiments, and error bars represent the standard deviations. *, P < 0.04.
Cortex hydrolysis precedes CaDPA release during C. difficile spore germination.
Based on our observations that the optical density of germinating C. difficile spores decreased before the appearance of CaDPA in solution (Fig. 1C) and that the newly identified bile acid germinant receptor is likely localized in the cortex (6), we hypothesized that cortex hydrolysis may precede CaDPA release during C. difficile spore germination. C. difficile UK1 spores were suspended in germination buffer supplemented with taurocholic acid only or both taurocholic acid and glycine and assayed for the presence of both cortex fragments and DPA in the germination solution. Interestingly, we detected cortex fragment release from the germinating spores within 2 min of germination (the earliest time point we can measure). At this time point, CaDPA is either not released or below the limit of detection (Fig. 2B). At 4 min, the rate of cortex fragment release increased. During this time CaDPA begins to be released and followed closely the curve for the cortex fragments, which remained steady until 8 min after the initiation of germination (Fig. 2B). At 8 min, there was a statistically significant difference between the cortex fragment curve and the CaDPA curve (P < 0.04). Taken together, these results suggest that C. difficile spore cortex hydrolysis precedes CaDPA release during germination.
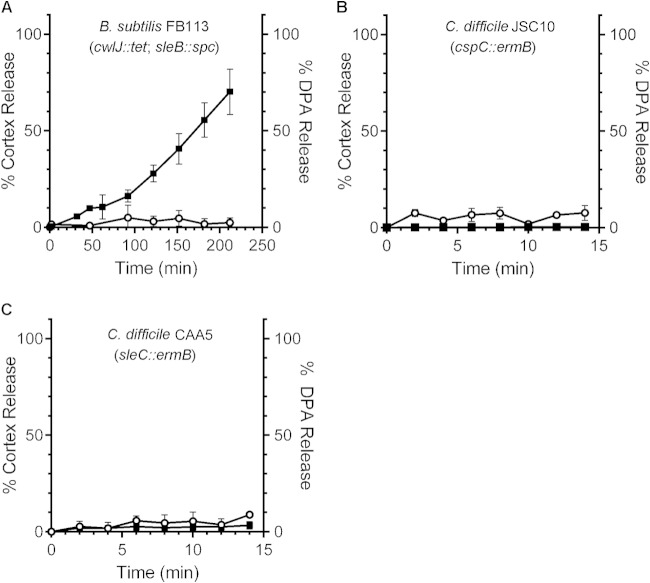
To control for the observed differences in cortex hydrolysis and CaDPA release, we analyzed cortex hydrolysis in B. subtilis FB113, a strain with engineered mutations in both cortex hydrolases (sleB and cwlJ). When B. subtilis FB113 spores were suspended in buffer supplemented with l-valine, spores released CaDPA (Fig. 3A). However, inactivating both sleB and cwlJ cortex hydrolases resulted in the inability of these B. subtilis spores to hydrolyze cortex in response to l-valine (Fig. 3A).
FIG 3.
Genetic analysis of cortex hydrolysis and CaDPA release from germinating B. subtilis and C. difficile spores. (A) Purified B. subtilis FB113 (cwlJ::tet sleB::spc) spores were suspended in buffer supplemented with l-valine. (B and C) C. difficile JSC10 (cspC::ermB) spores (B) and C. difficile CAA5 (sleC::ermB) spores (C) were suspended in buffer supplemented with taurocholic acid and glycine. At the indicated time points, a sample was taken, and the amounts of cortex fragments (○) and CaDPA (■) in the germination solution were determined. The data represent the averages from three independent experiments, and error bars represent the standard deviations.
Conversely, when C. difficile cspC spores are suspended in buffer supplemented with taurocholic acid and glycine, neither cortex fragments nor CaDPA are released (Fig. 3B); CaDPA release was restored by expressing cspBAC in trans (6). Further, inactivating the lone C. difficile SCLE, sleC, also prevented cortex hydrolysis and CaDPA release (Fig. 3C); germination (measured both by OD600 level and CaDPA release) was restored by expressing in trans a copy of C. difficile sleC (see Fig. S1 in the supplemental material). Because C. difficile cspC still expresses the SleC cortex hydrolase, but CaDPA and cortex fragments are not released, these results suggest that cortex hydrolysis and CaDPA release during C. difficile spore germination are coupled.
Analyzing spore germination in another C. difficile strain.
It was previously reported that there may be heterogeneity among C. difficile isolates in terms of their germination responses (37, 38). Therefore, we analyzed how cortex hydrolysis and CaDPA release occurs in another C. difficile ribotype. As described above for C. difficile UK1, when C. difficile M68 spores are suspended in buffer containing taurocholic acid and glycine (but not taurocholic acid only [data not shown]), cortex fragments appeared in the germination solution before CaDPA is detected (Fig. 4). During C. difficile M68 spore germination, at the earliest time point of 2 min, there was a difference between cortex fragments and CaDPA (P < 0.001) (Fig. 4). These results support the idea that cortex hydrolysis preceding CaDPA release is a general phenomenon during C. difficile spore germination and not specific to one isolate. Our results clearly show that the initiation of germinant receptor-mediated C. difficile spore germination occurs through a novel pathway.
FIG 4.
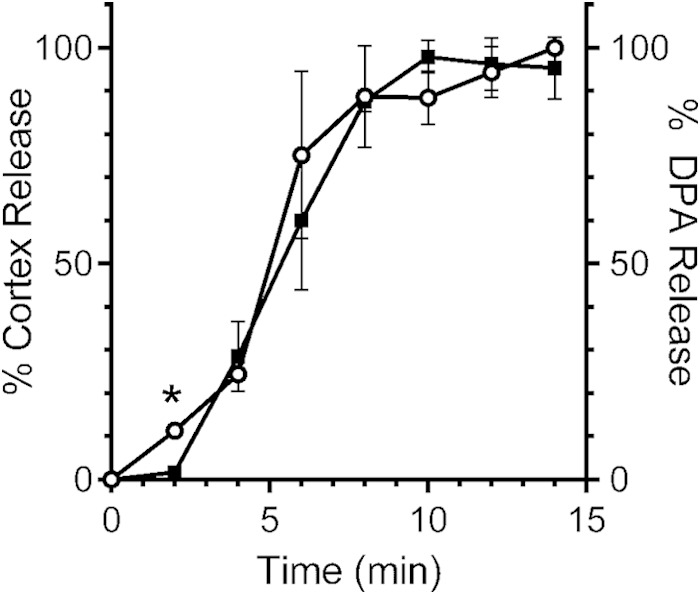
Cortex hydrolysis precedes CaDPA release during C. difficile M68 spore germination. Purified C. difficile M68 spores were suspended in buffer with taurocholic acid and glycine. At the indicated time points, a sample was taken, and the amounts of cortex fragments (○) and CaDPA (■) in the germination solution were determined. The data represent the averages from three independent experiments, and error bars represent the standard deviations. *, P < 0.001.
DISCUSSION
Germination by C. difficile spores seems to occur differently than for other spore-forming bacteria. Upon sequencing and analysis of the C. difficile genome, it was apparent that C. difficile did not encode orthologues of the ger-type germinant receptors found in other spore-forming bacteria. This suggested that C. difficile spores may germinate in response to unique germinants or use novel mechanisms to initiate spore germination or both (17).
It has been known for approximately 30 years that certain bile acids stimulate C. difficile spore germination (39, 40). Although much work has focused on the signals that can stimulate or inhibit C. difficile spore germination, the proteins that responded to these signals had remained elusive. In a genetic screen to select for C. difficile mutants whose spores do not respond to taurocholic acid as a germinant, we identified CspC as the bile acid germinant receptor (6). Due to the differences between the predicted locations of the C. difficile germinant receptor complex (CspC, CspB, and SleC) and the locations of the B. subtilis spore germinants receptors (GerAA-AB-AC), we hypothesized that C. difficile spore germination may occur differently than that observed in the model organism.
We observed that C. difficile spore germination is not initiated in the same manner, as observed for B. subtilis. We found that cortex hydrolysis precedes CaDPA release during germination by C. difficile spores and that this seems to be a general phenomenon among C. difficile isolates; the C. difficile M68 strain, a different ribotype, also released cortex fragments before CaDPA. Unlike what is observed for B. subtilis spore germination, we could not genetically separate cortex hydrolysis from CaDPA release by inactivating either the bile acid germinant receptor or the SCLE. Both C. difficile cspC and C. difficile sleC are required to hydrolyze cortex and, without cortex hydrolysis, the release of CaDPA from the core in is not observed (Fig. 3B and C, respectively).
Interestingly, C. perfringens encodes orthologues of both the classical ger-type germinant receptor, the Csp proteases and SleC. However, C. perfringens does not germinate in response to bile acids (41, 42). Although there seems to be conservation in the Csp proteases, C. perfringens CspA, CspB, and CspC are catalytically active proteases that could activate SleC to stimulate cortex hydrolysis (21, 22, 32). Mutations in C. perfringens sleC result in strains that still release CaDPA but do not hydrolyze cortex (23). Thus, our observation that cortex hydrolysis precedes CaDPA release during C. difficile spore germination is not a general phenomenon among all clostridia. However, this may be a novel mechanism for stimulating germination in spore-forming bacteria that do not encode the classical ger-type germinant receptor.
If mutations in the bile acid germinant receptor prevent both cortex hydrolysis and CaDPA release from the core, how is CaDPA release mediated during C. difficile spore germination? In our working model (Fig. 5), taurocholic acid interacts with CspC, which transmits the bile acid signal to CspB. Activated CspB, in turn, cleaves pro-SleC to an active hydrolase, which begins to hydrolyze cortex, releasing cortex fragments into the surrounding milieu. Cortex hydrolysis allows the germ cell wall to expand and, with it, the inner spore membrane. In our model, either an unidentified protein responds to the cell wall expansion and triggers CaDPA release, or the expansion of the inner spore membrane alone triggers CaDPA release. This suggests that a mechanosensitive channel is responsible for the release of CaDPA during C. difficile spore germination. C. difficile encodes orthologues of several mechanosensitive proteins (e.g., mscL and mscS), and most of these are likely to be involved in maintaining osmotic homeostasis during vegetative growth and probably have no role in spore germination (17, 43–45).
FIG 5.
Models for spore germination. (A) During the initiation of B. subtilis spore germination, l-alanine (or l-valine) interacts with the GerA germinant receptor complex (location 1). The SpoVA channel (which includes the SpoVAD DPA-binding protein) is then activated (location 2), and it releases CaDPA from the spore core (3). Released CaDPA activates the CwlJ cortex hydrolase (location 4) triggering cortex hydrolysis. (B) The initiation of C. difficile spore germination is triggered when the bile acid germinant receptor, CspC, interacts with taurocholic acid (location 1). Activated CspC then activates the germination-specific protease, CspB (2), which processes pro-SleC to an active form (location 3), and cortex hydrolysis begins (location 4). Then, due to either core swelling (location 5a) or through the action of an unknown protein (location 5b), SpoVAC releases CaDPA (location 6).
In B. subtilis, germination is triggered through the interaction of germinants with the germinant receptors imbedded in the inner spore membrane (Fig. 5). The interaction of these germinants with their cognate receptors triggers CaDPA release, likely through the SpoVA channel (Fig. 5) (46). Then, as described above, CaDPA activates cortex hydrolysis (Fig. 5). The B. subtilis SpoVA complex is composed of seven different proteins: SpoVAA, SpoVAB, SpoVAC, SpoVAD, SpoVAEa, SpoVAEb, and SpoVAF. Most of these proteins are important for both CaDPA import into the developing spore during sporulation and CaDPA release during germination (e.g., SpoVAD binds DPA [47]). C. difficile does not encode orthologues of many of these proteins. However, C. difficile does encode spoVAC, spoVAD and spoVAE. Recently, Velasquez et al. reported a function for SpoVAC (48). These authors determined that SpoVAC is a mechanosensitive channel (48). How this protein functions during B. subtilis spore germination is unclear. For C. difficile spore germination, we propose that SpoVAC responds to the change in osmolarity that occurs upon cortex hydrolysis, and then either it alone provides a channel for CaDPA release or it is part of a larger channel that is mechanically gated.
Although the C. difficile bile acid germinant receptor is known, the receptor with which glycine interacts is not. We speculated that the unidentified glycine germinant receptor may be localized to the inner membrane of the spore core, thus performing a function similar to that of the B. subtilis germinant receptors (6). However, because a mutation in the bile acid germinant receptor prevented both cortex hydrolysis and CaDPA release, we propose that the hypothesized glycine receptor is either (i) part of the known C. difficile germinant receptor complex (CspB, CspA, CspC, and SleC) or (ii) located in the inner spore membrane but whose activity is dependent on CspC activity.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the American Heart Association National Scientist Development grant (11SDG7160013) to J.A.S. Research reported in this publication was also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award R56AI108987.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Leif Smith (Texas A&M University) and members of the Sorg and Smith labs for helpful comments during the preparation of the manuscript. We also thank Peter Setlow for the gift of the B. subtilis strains used in the present study.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02575-14.
REFERENCES
- 1.Theriot CM, Koenigsknecht MJ, Carlson PE Jr, Hatton GE, Nelson AM, Li B, Huffnagle GB, Li J, Young VB. 2014. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross J, Toussaint NC, Xavier JB, Pamer EG. 2014. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, Wren BW, Fairweather NF, Dougan G, Lawley TD. 2012. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun 80:2704–2711. doi: 10.1128/IAI.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jump RLP, Pultz MJ, Donskey CJ. 2007. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother 51:2883–2887. doi: 10.1128/AAC.01443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawley TD, Croucher NJ, Yu L, Clare S, Sebaihia M, Goulding D, Pickard DJ, Parkhill J, Choudhary J, Dougan G. 2009. Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J Bacteriol 191:5377–5386. doi: 10.1128/JB.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis MB, Allen CA, Shrestha R, Sorg JA. 2013. Bile acid recognition by the Clostridium difficile Germinant Receptor, CspC, is important for establishing infection. PLoS Pathog 9:e1003356. doi: 10.1371/journal.ppat.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paredes-Sabja D, Shen A, Sorg JA. 2014. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol 22:406–416. doi: 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setlow P. 2014. Germination of spores of Bacillus species: what we know and do not know. J Bacteriol 196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segev E, Rosenberg A, Mamou G, Sinai L, Ben-Yehuda S. 2013. Molecular kinetics of reviving bacterial spores. J Bacteriol 195:1875–1882. doi: 10.1128/JB.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorg JA, Sonenshein AL. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridlon JM, Kang D, Hylemon PB. 2006. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Sorg JA, Sonenshein AL. 2010. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol 192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorg JA, Sonenshein AL. 2009. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J Bacteriol 191:1115–1117. doi: 10.1128/JB.01260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez N, Liggins M, Abel-Santos E. 2010. Kinetic evidence for the presence of putative germination receptors in Clostridium difficile spores. J Bacteriol 192:4215–4222. doi: 10.1128/JB.00488-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis MB, Allen CA, Sorg JA. 2013. Muricholic acids inhibit Clostridium difficile spore germination and growth. PLoS One 8:e73653. doi: 10.1371/journal.pone.0073653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriques AO, Moran CPJ. 2007. Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- 17.Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, Stabler R, Thomson NR, Roberts AP, Cerdeno-Tarraga AM, Wang H, Holden MTG, Wright A, Churcher C, Quail MA, Baker S, Bason N, Brooks K, Chillingworth T, Cronin A, Davis P, Dowd L, Fraser A, Feltwell T, Hance Z, Holroyd S, Jagels K, Moule S, Mungall K, Price C, Rabbinowitsch E, Sharp S, Simmonds M, Stevens K, Unwin L, Whithead S, Dupuy B, Dougan G, Barrell B, Parkhill J. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet 38:779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 18.Burns DA, Heap JT, Minton NP. 2010. SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J Bacteriol 192:657–664. doi: 10.1128/JB.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutelius D, Hokeness K, Logan SM, Reid CW. 2014. Functional analysis of SleC from Clostridium difficile: an essential lytic transglycosylase involved in spore germination. Microbiology 160:209–216. doi: 10.1099/mic.0.072454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams CM, Eckenroth BE, Putnam EE, Doublie S, Shen A. 2013. Structural and functional analysis of the CspB protease required for Clostridium spore germination. PLoS Pathog 9:e1003165. doi: 10.1371/journal.ppat.1003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masayama A, Hamasaki K, Urakami K, Shimamoto S, Kato S, Makino S, Yoshimura T, Moriyama M, Moriyama R. 2006. Expression of germination-related enzymes, CspA, CspB, CspC, SleC, and SleM, of Clostridium perfringens S40 in the mother cell compartment of sporulating cells. Genes Genet Syst 81:227–234. doi: 10.1266/ggs.81.227. [DOI] [PubMed] [Google Scholar]
- 22.Shimamoto S, Moriyama R, Sugimoto K, Miyata S, Makino S. 2001. Partial characterization of an enzyme fraction with protease activity which converts the spore peptidoglycan hydrolase (SleC) precursor to an active enzyme during germination of Clostridium perfringens S40 spores and analysis of a gene cluster involved in the activity. J Bacteriol 183:3742–3751. doi: 10.1128/JB.183.12.3742-3751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paredes-Sabja D, Setlow P, Sarker MR. 2009. SleC is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens. J Bacteriol 191:2711–2720. doi: 10.1128/JB.01832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, Mastroeni P, Scott P, Raisen C, Mottram L, Fairweather NF, Wren BW, Parkhill J, Dougan G. 2009. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immun 77:3661–3669. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drudy D, Harnedy N, Fanning S, O'Mahony R, Kyne L. 2007. Isolation and characterisation of toxin A-negative, toxin B-positive Clostridium difficile in Dublin, Ireland. Clin Microbiol Infect 13:298–304. doi: 10.1111/j.1469-0691.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 26.Paidhungat M, Ragkousi K, Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol 183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heap JT, Pennington OJ, Cartman ST, Minton NP. 2009. A modular system for Clostridium shuttle plasmids. J Microbiol Methods 78:79–85. doi: 10.1016/j.mimet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 29.Allen CA, Babakhani F, Sears P, Nguyen L, Sorg JA. 2013. Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob Agents Chemother 57:664–667. doi: 10.1128/AAC.01611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Molecular biological methods for Bacillus. John Wiley, Chichester, United Kingdom. [Google Scholar]
- 31.Hindle AA, Hall EA. 1999. Dipicolinic acid (DPA) assay revisited and appraised for spore detection. Analyst 124:1599–1604. doi: 10.1039/a906846e. [DOI] [PubMed] [Google Scholar]
- 32.Paredes-Sabja D, Setlow P, Sarker MR. 2009. The protease CspB is essential for initiation of cortex hydrolysis and dipicolinic acid (DPA) release during germination of spores of Clostridium perfringens type A food poisoning isolates. Microbiology 155:3464–3472. doi: 10.1099/mic.0.030965-0. [DOI] [PubMed] [Google Scholar]
- 33.Ghuysen J-M, Tipper DJ, Strominger JL. 1966. Enzymes that degrade bacterial cell walls. Methods Enzymol 8:685–699. doi: 10.1016/0076-6879(66)08124-2. [DOI] [Google Scholar]
- 34.Zhang P, Kong L, Wang G, Setlow P, Li YQ. 2010. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J Biomed Optics 15:056010. doi: 10.1117/1.3494567. [DOI] [PubMed] [Google Scholar]
- 35.Kort R, O'Brien AC, van Stokkum IH, Oomes SJ, Crielaard W, Hellingwerf KJ, Brul S. 2005. Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl Environ Microbiol 71:3556–3564. doi: 10.1128/AEM.71.7.3556-3564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh S, Zhang P, Li YQ, Setlow P. 2009. Superdormant spores of Bacillus species have elevated wet-heat resistance and temperature requirements for heat activation. J Bacteriol 191:5584–5591. doi: 10.1128/JB.00736-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heeg D, Burns DA, Cartman ST, Minton NP. 2012. Spores of Clostridium difficile clinical isolates display a diverse germination response to bile salts. PLoS One 7:e32381. doi: 10.1371/journal.pone.0032381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson PE Jr, Kaiser AM, McColm SA, Bauer JM, Young VB, Aronoff DM, Hanna PC. 2015. Variation in germination of Clostridium difficile clinical isolates correlates to disease severity. Anaerobe 33:64–70. doi: 10.1016/j.anaerobe.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson KH, Kennedy MJ, Fekety FR. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol 15:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Railbaud P, Ducluzeau R, Muller MC, Sacquet E. 1974. (Sodium taurocholate, a germination factor for anaerobic bacterial spores “in vitro” and “in vivo.” Ann Microbiol 125B:381–391. (Author's translation.) [PubMed] [Google Scholar]
- 41.Paredes-Sabja D, Torres JA, Setlow P, Sarker MR. 2008. Clostridium perfringens spore germination: characterization of germinants and their receptors. J Bacteriol 190:1190–1201. doi: 10.1128/JB.01748-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paredes-Sabja D, Udompijitkul P, Sarker MR. 2009. Inorganic phosphate and sodium ions are cogerminants for spores of Clostridium perfringens type A food poisoning-related isolates. Appl Environ Microbiol 75:6299–6305. doi: 10.1128/AEM.00822-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahome PG, Cowan AE, Setlow B, Setlow P. 2009. Levels and localization of mechanosensitive channel proteins in Bacillus subtilis. Arch Microbiol 191:403–414. doi: 10.1007/s00203-009-0465-z. [DOI] [PubMed] [Google Scholar]
- 44.Wahome PG, Setlow P. 2006. The synthesis and role of the mechanosensitive channel of large conductance in growth and differentiation of Bacillus subtilis. Arch Microbiol 186:377–383. doi: 10.1007/s00203-006-0152-2. [DOI] [PubMed] [Google Scholar]
- 45.Wahome PG, Setlow P. 2008. Growth, osmotic downshock resistance and differentiation of Bacillus subtilis strains lacking mechanosensitive channels. Arch Microbiol 189:49–58. [DOI] [PubMed] [Google Scholar]
- 46.Vepachedu VR, Setlow P. 2007. Role of SpoVA proteins in release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J Bacteriol 189:1565–1572. doi: 10.1128/JB.01613-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Davis A, Korza G, Zhang P, Li YQ, Setlow B, Setlow P, Hao B. 2012. Role of a SpoVA protein in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 194:1875–1884. doi: 10.1128/JB.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velasquez J, Schuurman-Wolters G, Birkner JP, Abee T, Poolman B. 2014. Bacillus subtilis spore protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol 92:813–823. doi: 10.1111/mmi.12591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.