Abstract
Successful combination therapy for human immunodeficiency virus (HIV) has transformed this disease from a short-lived infection with high mortality to a chronic disease associated with increasing life expectancy. This is true for high- as well as low- and middle-income countries. As a result of this increased life expectancy, people living with HIV are now at risk of developing other chronic diseases associated with aging. Heart failure has been common among people living with HIV in the eras of pre- and post- availability of antiretroviral therapy; however, our current understanding of the pathogenesis and approaches to management have not been systematically addressed. HIV may cause heart failure through direct (e.g., viral replication, mitochondrial dysfunction, cardiac autoimmunity, autonomic dysfunction) and indirect (e.g., opportunistic infections, antiretroviral therapy, alcohol abuse, micronutrient deficiency, tobacco use) pathways. In low- and middle-income countries, 2 large observational studies have recently reported clinical characteristics and outcomes in these patients. HIV-associated heart failure remains a common cardiac diagnosis in people living with heart failure, yet a unifying set of diagnostic criteria is lacking. Treatment patterns for heart failure fall short of society guidelines. Although there may be promise in cardiac glycosides for treating heart failure in people living with HIV, clinical studies are needed to validate in vitro findings. Owing to the burden of HIV in low- and middle-income countries and the concurrent rise of traditional cardiovascular risk factors, strategic and concerted efforts in this area are likely to impact the care of people living with HIV around the globe.
Keywords: developing countries, heart failure, human immunodeficiency virus
People living with human immunodeficiency virus (PLHIV) around the globe and taking antiretroviral therapy (ART) now achieve a near-normal life expectancy (1,2). As a consequence, PLHIV increasingly experience the chronic diseases of aging. Of particular concern is the risk of coronary artery disease, myocardial infarction, and heart failure (HF) among PLHIV, as observed in North American, European, and Australian HIV cohorts (3–9). Sub-Saharan Africa (SSA), which accounts for 12% of the global population, is disproportionately affected by HIV, with 69% of all adults and 90% of all children living with HIV residing here (10). Cardiovascular manifestations of HIV infection in SSA have been reported for more than 25 years, but most of the reports are prior to 2004 when ART became widely available (11).
Prior to the advent of ART, studies supported a relationship between HIV and left ventricular (LV) dysfunction. Numerous terms were used to describe the syndrome, including HIV-associated cardiomyopathy, HIV-associated HF, and HIV-associated LV dysfunction. As initially described, HIV-associated HF was related to severe immune system compromise, had no specific therapy, and had a median survival of 101 days after diagnosis (12–15). Concurrent shifts in epidemiological patterns of HIV treatment and cardiovascular diseases produced a complex and evolving relationship between HF and HIV that merits a contemporary review. Most reports related to HF are focused on high-income countries (16) and are misaligned with the global predominance of HIV in low- and middle-income countries (LMICs) (Figure 1) (11,17–21). In this review, we summarize the epidemiology, approach to diagnosis, therapy, and prognosis of HIV-associated HF in LMICs in the ART era. Our review of the publications was accomplished by searching PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials for studies published through 2014. We used the following terms as keywords and subject headings: human immunodeficiency virus, heart failure, cardiomyopathy, echocardiography, antiretroviral therapy, low- and middle-income countries, developing countries, and each of the countries in the World Bank low- and middle-income groups. We included studies that report HF characteristic of HIV-infected individuals in LMICs receiving ART. Studies were excluded if the abstract was not available in English. Data extraction (e.g., year of study, location, study description, and findings) was performed and confirmed by 2 investigators (F.A., G.S.B.). In the present discussion we use the terms “HIV-associated HF” or “HF in PLHIV” to describe the syndrome of HF, including diastolic dysfunction, and use the term “HIV-associated LV systolic dysfunction” (LVSD) only when discussing asymptomatic ventricular dysfunction or to describe heart muscle disease. Owing to the heterogeneity of definitions of HF in HIV, we defined HF according to the criteria proposed in each study. This represents the state of published reports. We ultimately propose a framework to invigorate research that will inform best-care practices for patients with HIV-associated HF in LMICs and the rest of the world.
Figure 1. Worldwide Distribution of Studies of HF and Burden of HIV.
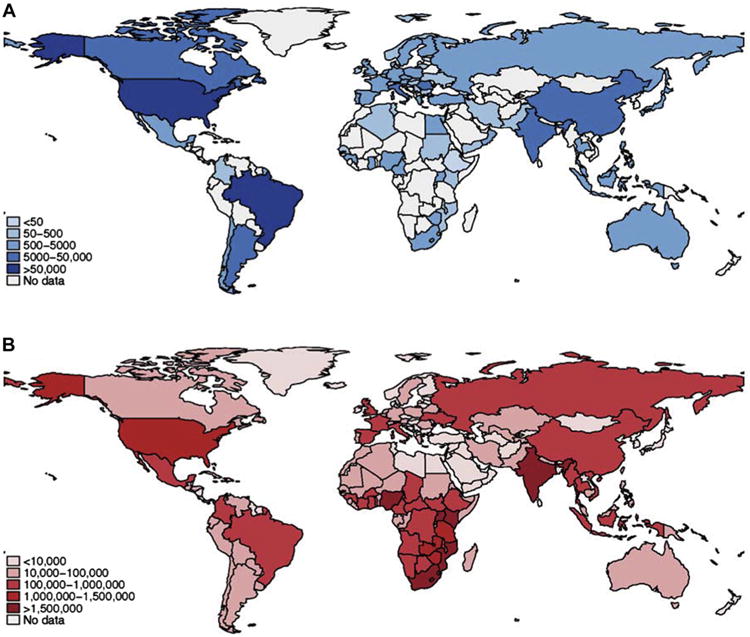
Country-level data for (A) the number of individuals included in clinical studies and registries for HF, and (B) the number of adults living with HIV Data from the World Health Organization Global Health Observatory Data Repository; U.S. Central Intelligence Agenct World Factbook, and the Centers for Disaese Control, Taiwan (ROC). (14,22-25,99-101). HF = heart failure; HIV = human immunodeficiency virus.
Causes of HIV-associated HF
There is no unifying definition of HIV-associated HF, and it is often a diagnosis of exclusion (22-25). This is due to the knowledge gaps regarding the causes of HF in PLHIV; however, there are a number of prevailing hypotheses (Figure 2) (21,26-29). Reports from high-, low-, and middle-income countries have shed light on the probable causes.
Figure 2. Pathways of HIV-Associated HF.

HIV may cause HF through direct and indirect pathways. Direct pathways involve HIV viral replication, an autoimmune milieu, and autonomic dysfunction. Viral replication also affects mitochondrial function, which is associated with cardiac myocyte dysfunction. Indirect pathways leading to HF include factors that are common among PLHIV such as opportunistic infections, antiretroviral therapy, alcohol (EtOH) abuse, micronutrient deficiency, and tobacco use. HF = heart failure; HIV = human immunodeficiency virus; PLHIV = people living with HIV.
HIV Viral Replication
One hypothesis implicates direct myocardial viral toxicity. HIV ribonucleic acid (RNA) concentrations ≥500 copies/ml are associated with a nearly 2.5-fold increased risk of developing HF compared with HIV-uninfected individuals (8). Targeted myocardial transgenic expression of HIV transactivator of transcription (Tat) protein activates endothelial cells, causing LV systolic dysfunction (LVSD), increased LV mass, and expression of natriuretic peptides in mice that may lead to hemodynamic compromise (30). How and whether HIV directly damages cardiac myocytes, which do not possess CD4 receptors, have been hotly debated (31-33). In vitro studies of human and rat cardiac myocytes show that HIV can enter cardiac myocytes through pathways independent of CCR5 or CXCR4 receptors (32,34).
Mitochondrial Injury
HIV may impair cardiac function through mitochondrial pathways. HIV infection initiates a mitochondrion-mediated cascade, releasing proteases that lead to cardiac myocyte damage and apoptosis (35). Tat protein also disrupts mitochondrial membrane permeability (36). The complete pathway between HIV infection and LVSD remains unclear. To date, a clear genetic predisposition does not appear to play a major role, as 1 specific mitochondrial deoxyribonucleic acid (DNA) polymorphism, mtDNA T16189C, is not more common in patients with HIV and HF than in HIV-infected controls without HF in South Africa (37).
Opportunistic Infections
In SSA, there have been 2 studies examining myocardial biopsy specimens among patients with HIV-associated LVSD. In a 1998 postmortem study of 16 patients with HIV/acquired immune deficiency syndrome (AIDS), myocarditis was attributed to HIV (50%) and infection by Toxoplasma gondii (19%), Cryptococcus neoformans (19%), and Mycobacterium avium intracellulare (13%) (38). An antemortem study of patients compared analyses of endomyocardial biopsy specimens among HIV-associated HF patients (n = 14), HIV-uninfected patients with idiopathic dilated cardiomyopathy (n = 8), and heart transplant recipients in South Africa (n = 11) (39). In contrast to the earlier study (38), infection with multiple cardiotropic viruses (average 2.5 viruses per individual) was common among those with HIV-associated HF. The most common viruses were Epstein-Barr virus (64%), herpes simplex virus (50%), and parvovirus B19 (14%). Whether viral suppression and restoration of immune function alter the role played by opportunistic infections in HIV-associated HF in LMICs is unknown.
Tobacco and Alcohol Use
Unhealthy behaviors have been implicated in the development of HF among PLHIV, specifically, heavy alcohol consumption and tobacco use (40). A systematic review and meta-analysis investigating LVSD in paucisymptomatic HIV-infected patients in the ART era found that active tobacco smoking was associated with a 1.57-fold higher odds of having HF and was second only to age as the univariate risk factor for HF (41). Only 1 of the included studies, however, was from an LMIC (42). A study from Rwanda has shown that the relationship among alcohol intake, cigarette smoking, and dilated cardiomyopathy in PLHIV is attenuated after adjusting for HIV stage, socioeconomic status, duration of HIV infection, and plasma selenium levels (43).
Micronutrient Deficiency
Of the micronutrient deficiencies, selenium has been the most extensively studied, related to HIV-associated HF. Selenium's antioxidant properties protect against endothelial dysfunction, and its deficiency has been associated with cardiac dysfunction. Due to soil composition and agricultural practices in SSA, 28% of the population is at risk for selenium deficiency (44). Selenium deficiency is common in PLHIV with and without LVSD (43,45). Selenium supplementation improves cardiac function in PLHIV in small studies (46,47), but the debate as to whether HIV-associated LVSD responds to selenium supplementation is unresolved. Nonetheless, there is evidence of association between lower body mass index and LVSD in PLHIV, suggesting that overall nutrition may be a factor (48).
Antiretroviral Therapy Toxicity
Although the introduction of ART in high-income countries has been associated with a reduction in the incidence of HIV-associated HF of 30% to 50% (49,50), ART has also been implicated in causing LVSD (51). Zidovudine (AZT), a nucleoside reverse transcriptase inhibitor, has been associated with reversible, dose-dependent skeletal and cardiac myopathy attributed to mitochondrial damage (52,53). Studies pre- and post-widespread availability of combination ART link AZT to cardiomyopathy (33,54). There is also contemporary evidence linking AZT to diastolic dysfunction (55). These findings are especially important for LMICs, where AZT is still a first-line option for HIV treatment for adults, adolescents, and children.
Cardiac Autoimmunity
Circulating cardiac autoantibodies are detected more frequently in PLHIV with LVSD (43%) than in HIV-infected patients without LVSD (19%) or in HIV-uninfected controls (3%) (56). Autoantibody concentrations in PLHIV correlate with mortality, but biopsy-proven autoimmune myocarditis in PLHIV with LVSD responds well to steroid therapy (56,57). Together with findings of increased myocardial expression of human leukocyte antigen (HLA) class I antigen in PLHIV with HF (58), these observations support a role for cardiac autoimmunity in the pathogenesis of HIV-associated LVSD.
In the ART era, the causes of HIV-associated LVSD appear to depend on the degree of viral suppression. When viral suppression is adequate and immune function returns to normal, other cardiovascular risk factors or ART may be the main causative factor. On the other hand, when viral suppression has not been achieved and at worse stages of HIV, it is more likely that HIV, ART, opportunistic pathogens, autoimmunity, or nutritional deficiencies result in LVSD. As more data are generated from PLHIV worldwide, the causes and mediators of HIV-associated LVSD will be better understood.
Epidemiology of HIV-associated HF in LMICs in the pre-ART era
Studies of HIV-associated HF in the pre-ART era in LMICs were heterogeneous, with wide variation in patient population, patient selection, and definitions of HF outcomes, yielding a wide range in prevalence estimates. Observational studies from SSA indicated an HF prevalence of approximately 50% and an incidence of any cardiac abnormality of 55% over a 7-year period (12,13,38). LVSD was most common among symptomatic patients, whereas asymptomatic PLHIV more often had HF with preserved ejection fraction (EF) (59). HIV-associated HF occurred most often among young persons with CD4+ T-cell counts of <100 cells/mm3, lower socioeconomic status, longer duration of HIV infection, higher viral load, and advanced HIV stage (14,43). In-hospital mortality rates due to HIV-associated HF reached 15% (13).
Epidemiology of HIV-associated HF in LMICs in the ART era
Studies in the ART era have shown that HF is still a relevant form of HIV-associated cardiovascular disease (Table 1)(60-70). There have been 2 large prospective observational studies from SSA in the ART era that shed light on the contemporary epidemiology of HIV-associated HF: the HoS (Heart of Soweto) and THESUS-HF (the Sub-Saharan Africa Survey of Heart Failure) studies (7,22). In addition, a number of other studies also inform the clinical characteristics and outcomes of patients with HIV-associated HF in LMICs in the ART era.
Table 1. Studies of HIV and HF in LMICs in the ART Era.
| Study, Year (Ref #) | Study Site (s) | Study Type | Sample Size (n) | HIV-Positive/Negative (n) | Key Findings |
|---|---|---|---|---|---|
| Jain et al. 2014 (60) | India | Cross-sectional descriptive study | 100 | 100/0 | Diastolic dysfunction (43%) and dilated cardiomyopathy (18%) were the most common abnormalities observed in HIV-infected patients. |
| Shaboodien et al., 2013 (39) | South Africa | Case-comparison study | 33 | 14/19 | Myocarditis was present in 44% of HIV-associated cardiomyopathy cases, 36% of heart transplant recipients, and 25% of participants with idiopathic dilated cardiomyopathy. Myocarditis was acute in 50% of HIV- and heart transplant-associated myocarditis and was chronic in all with idiopathic dilated cardiomyopathy. |
| Sliwa et al., 2013 (61) | Multiple in sub-Saharan Africa | International observational registry | 1,006 | 65/500 | Among patients with HF, HIV status predicts 180-day mortality. |
| Chillo et al., 2012 (62) | Tanzania | Cross-sectional descriptive study | 102 | 102/0 | 10% of HIV-positive patients with cardiomyopathy (systolic dysfunction), 34% with hypertensive heart disease. |
| Damasceno et al., 2012 (22) | Multiple in sub-Saharan Africa | International observational registry | 1,006 | 65/435 | HIV-associated cardiomyopathy was rarely seen (2.6% [26/1000 cases]). |
| Ige et al., 2012 (63) | Nigeria | Cross-sectional descriptive study | 300 | 150/150 | LVSD is significantly more frequent in HIV-infected children and worsens with increasing age. |
| Okoromah et al., 2012 (64) | Nigeria | Cross-sectional observational study | In HIV-infected children, the prevalence rates of both dilated cardiomyopathy and LVSD were 33.7%. | ||
| Olusegun-Joseph et al., 2012 (65) | Nigeria | Cross-sectional descriptive study | 150 | 100/50 | Echocardiographic abnormalities were significantly more common in the cases than the controls (78% vs. 16%, respectively; p < 0.001), including systolic dysfunction (30% vs. 8%, respectively; p < 0.05) and diastolic dysfunction (32% vs. 8%, respectively; p < 0.01). |
| Pepeta et al., 2012 (66) | South Africa | Retrospective | 34 | 34/0 | There was improvement in LV function in patients with HIVAC treated with antiretroviral therapy. |
| Schwartz et al., 2012 (67) | Botswana | Cross-sectional descriptive study | 179 | 106/73 | HIV infection was strongly associated with pericarditis and cardiomyopathy; 18% of HIV+ patients had hypertensive heart disease. Prevalence of HIV among patients with cardiomegaly was higher than in the general population |
| Sliwa, et al., 2012 (7) | South Africa | Prospective clinical registry | 53,28 | 518/4,810 | HIV-related cardiac disease was a minor contributor to overall disease burden (4% prevalence). Cardiomyopathy was the most common HIV-related cardiac disease. |
| Tantchou Tchoumi et al., 2011 (68) | Cameroon | Prospective observational | 462 | 7/455 | 7 of 462 patients (1.6%) of cases had HIVAC. |
| El Hattaoui et al., 2008 (42) | Morocco | Cross-sectional descriptive study | 238 | 158/80 | Cardiomyopathy was more common in HIV-infected patients with AIDS than in those with non-AIDS or HIV-uninfected patients. Cardiomyopathy was more common among HIV-infected patients with CD4 count below 100. |
| Twagirumukiza, et al., 2007 (43) | Rwanda | Prospective observational | 416 | 416/0 | Predictors of dilated cardiomyopathy were low socioeconomic status, duration of HIV infection, CD4 count, HIV viral load, advanced stage of HIV, and low plasma level of selenium. |
| Lubega et al., 2005 (69) | Uganda | Cross-sectional descriptive study | 230 | 230/0 | Heart abnormalities were common in children with symptomatic HIV disease and included sinus tachycardia (21%), LVSD (17%), and right ventricular dilation (14%). |
| Diogenes et al., 2003 (70) | Brazil | Case report | 1 | 1/0 | Perinatally-infected 1-year-old child with severe cardiomyopathy had full reversal of disease after 6 years of treatment and follow-up, with normal LV dimension and contractility. |
AIDS = acquired immune deficiency syndrome; ART = antiretroviral therapy; HF = heart failure; HIVAC= ■ ■ ■; LMICs = low- and middle-income countries; LVSD = left ventricular systolic dysfunction.
Epidemiology and Clinical Characteristics
The HoS study is the largest and most comprehensive registry of de novo presentations of any cardiac disease in SSA (71). Of 5,328 cardiac presentations between 2006 and 2008, there were 518 PLHIV (7). Most PLHIV (54%) were taking ART at the time of enrollment, were younger, and had lower blood pressure, higher heart rate, and lower body mass index than the rest of the HoS cohort. Among PLHIV, HIV-associated HF was the most common diagnosis (38%). The average LVEF of those with HIV-associated HF was 46%.
There were more women than men with HIV-associated HF in the HoS study. Overall, the women in HoS were younger (∼6 years younger than men), and men were more likely to smoke tobacco. The viral load was significantly higher (110,000 vs. 19,000 RNA copies/ml), and CD4+ T-cell counts were significantly lower (180 vs. 211 cells/mm3) in patients with HIV-associated HF than in PLHIV without HF. Valve dysfunction, right HF, and hypertension were common comorbidities, and no PLHIV with HF were reported to have coronary artery disease.
The THESUS-HF study was a multinational prospective registry of patients with acute decompensated HF (ADHF) admitted to university hospitals in 9 African countries between 2007 and 2010 (22). Endemic causes of HF (i.e., HIV, rheumatic heart disease, pericarditis, and others) were compared with emerging causes such as hypertension and ischemic heart disease. Of 1,006 patients enrolled, 50% underwent testing for HIV, and 65 were found to be infected with HIV. HIV was the cause of HF in 2.6% of all cases. Most of these patients were from Mozambique, Cameroon, and Uganda, countries that also have high rates of HIV. Patients with endemic causes of HF (including HIV) were younger by 10 to 15 years and were less often smokers, hypertensive, or diabetic. According to echocardiographic results, LV systolic and diastolic dimensions were larger in patients with endemic causes; however, there were no significant differences in LVEF.
Most, but not all (72,73), observational studies from LMICs in the ART era are generally consistent with the above findings, showing prevalence of HIV-associated HF between 1% and 5% (68,74,75). PLHIV usually have HF diagnosed in the third decade of life and are more often women. Both systolic and diastolic dysfunctions are more common (∼30% prevalence) in PLHIV than in controls without HIV (65), and rarely, diastolic dysfunction is more prevalent (42,60). Ventricular dimensions and mass are large in PLHIV with HF in SSA, similar to findings from North America (65,76). Up to one-third of African HIV-infected children have LVSD (63,64). Cardiac dysfunction in HIV-exposed infants is identifiable shortly after birth in high-income countries (77).
Antiretroviral Therapy
Pediatric studies suggest a relationship between type of ART and LVSD. In Brazil, HIV-infected children treated with AZT were more likely to develop HF than children treated with combination ART (78). Conversely, combination ART, but not AZT monotherapy, improves LV function in children with HIV-associated HF (70). In a retrospective study from South Africa, 18 HIV-infected children with HF receiving ART were compared with 16 children with HIV-associated HF cared for before national rollout of ART (66). After 38 months of follow-up, the children treated with ART had improved LV fractional shortening compared with the nontreated controls. Four of the ART-treated children had worsening cardiac function that improved upon discontinuation of AZT, stavudine, or didanosine.
Outcomes
In SSA, patients with endemic causes of HF (including HIV) have slightly longer lengths of hospital stay for ADHF than those with emerging causes (9.8 vs. 8.7 days, respectively) (22). In-hospital mortality (28% vs. 14%, respectively), 60-day mortality (13% vs. 9.0%, respectively), and 180-day mortality (21% vs. 16%, respectively) are higher in the endemic causes group. HIV status is a significant predictor of death at 180 days for patients with ADHF (61).
Treatment
There are no specific international guidelines for the treatment of HIV-associated HF (24,25). There are no controlled trials from LMICs addressing the most effective treatment strategy. As such, data from the broader international studies guides management. Restoration of immune status and viral load suppression may ameliorate cardiac dysfunction as long as medications causing cardiac dysfunction are avoided (49,50). Clinicians should be aware of the potential drug-drug interactions (e.g., with calcium channel blockers, HMG-CoA reductase inhibitors, antiarrhythmic drugs, and ART) as well as cardiac side effects of certain ART. Online tools detailing significant drug interactions for ART and HF medication are available (79).
There have been no trials to assess whether guideline-directed medical therapy for HF is also suitable for PLHIV. Nonetheless, use of angiotensin-converting enzyme inhibitors (26%), β-adrenergic blockers (29%), and spironolactone (35%) is low (7,22). The use of digoxin in HIV-associated HF may have particular relevance because of its inhibitory effects on HIV viral replication as demonstrated in vitro (80). Trials are needed to further elucidate whether this translates into clinical benefit in humans with HIV-associated HF.
Diastolic Dysfunction in HIV+ Patients
Among PLHIV with HF, LVSD is currently less common in high-income countries. Diastolic dysfunction, however, is present in up to 64% of asymptomatic PLHIV taking ART in high-income countries (81,82) and appears to be independent of traditional risk factors including age and hypertension (76). Early stage diastolic dysfunction is often the only echocardiographic abnormality found in asymptomatic PLHIV (83). Asymptomatic PLHIV have higher LV mass than HIV-uninfected subjects, and higher LV mass is inversely proportional to nadir CD4+ T-cell count (76). The mechanisms of diastolic dysfunction in PLHIV are as yet unknown but may involve direct myocardial effects of HIV (84). Data from LMICs in this field are sorely needed.
Impact of HIV Infection on Cardiac Deformation
Early detection of subclinical myocardial dysfunction, assessed by 2-dimensional strain and strain rate using speckle tracking, may maximize treatment benefits by identifying individuals who would benefit from preventive strategies (85). Asymptomatic, otherwise healthy PLHIV have lower strain and strain rates than HIV-uninfected people (86). HIV infection appears to affect mainly longitudinal systolic cardiac strain with preserved circumferential myocardial deformation (Figure 3) (87). Magnetic resonance imaging shows that these subclinical changes may relate to myocardial fibrosis and steatosis (84). Identification of early markers of myocardial dysfunction may identify PLHIV at high risk for cardiac dysfunction and thus allow early initiation of life-saving therapy. Such data are not yet available from LMICs.
Figure 3. Myocardial Deformation Tracings in HIV-Infected Individuals With Normal LVEF.

(A) Normal global longitudinal strain (GLS) pattern in asymptomatic PLHIV (average GLS = −19%). (B) Abnormal GLS pattern in asymptomatic PLHIV (average GLS = −6%). (Illustrations courtesy of Duke University Medical Center Cardiac Diagnostic Unit.) HIV = human immunodeficiency virus; LVEF = left ventricular ejection fraction; PLHIV = people living with HIV.
Other Risk Factors for HIV-associated HF in LMICs
Data are mixed regarding the prevalence of traditional cardiovascular risk factors in PLHIV in LMICs. A meta-analysis examining the association between HIV and cardiovascular risk factors in SSA showed that the burden of most factors was generally low (88). PLHIV had lower body mass indexes (standard mean differences [SMD]: 0.3 kg/m2), lower high-density lipoprotein (HDL; SMD: −0.6 mg/dl) and higher triglyceride levels (SMD 0.3 mg/dl) than their HIV-uninfected counterparts. ART use was associated with higher HDL (SMD 0.4 mg/dl), low-density lipoprotein (LDL; SMD: 0.4 mg/dl), and lower hemoglobin A1C levels (SMD: −0.3%). Smoking, however, is common in HIV-infected adults (22%) and adolescents (13% to 17%) in SSA (89).
High blood pressure is 1 of the most common causes of HF in SSA (22,23,73,75). Among PLHIV in SSA, the overall rate of hypertension is 8% to 19% (90). HIV infection is also associated with ambulatory non-dipping status, which suggests an underlying dysregulation of the cardiovascular system (91). Higher blood pressure increases mortality in HIV-infected men with well-controlled HIV disease nearly 3-fold than in those with more advanced disease (92).
Tuberculosis infection is a common antecedent among PLHIV with HF. Two-thirds of patients with moderate-to-large tuberculous pericardial effusions in Africa have HIV infection (93). Patients tend to be young and have low CD4+ T-cell counts, and development of tuberculous pericarditis is usually regarded as progression to end-stage HIV disease (94).
In contrast to high-income countries, where acute coronary syndromes are more common in PLHIV than in those infected with HIV (3,4), coronary artery disease and ischemic cardiomyopathy are rarely reported in PLHIV in LMICs (7). When coronary artery disease is identified in PLHIV, patients tend to be younger and are more commonly smokers, and coronary thrombosis predominates over coronary atherosclerosis (95).
Research Directions for PLHIV with HF
There are many opportunities for discovery in the field of HIV-associated HF in LMICs. From an epidemiological perspective, it remains unclear whether the prevalence of HF is higher among PLHIV than with HIV-uninfected patients and whether there are true sex-based differences. Most data for HIV-associated HF in LMICs emanate from a small number of countries in SSA with high HIV burden. However, there may also be important geographic differences among countries in Africa and Latin America and the Caribbean, East Asia, and the Pacific, Eastern Europe, Central Asia, South Asia, and the Middle East that have yet to be described.
Establishing diagnostic criteria for HIV-associated HF should be a high priority. Diagnostic criteria should consider the degree of immunosuppression and stage of HIV, because causes of HIV-associated HF likely vary depending on HIV clinical stage.
There is also a need to clarify the appropriate therapy for HIV-associated LVSD. It is unclear whether guideline-directed medical therapies for HF, as described by society guidelines, have similar efficacy in ART-treated PLHIV and/or whether specific therapies (e.g., digoxin) would provide additional benefit for HIV-associated HF. The tachycardia and low blood pressure that are common in HIV-associated HF suggest a potential role for medications that affect sinoatrial node rate without lowering blood pressure (96).
The best approach to integrating care for HF in HIV treatment programs needs to be systematically evaluated. Many HIV treatment programs in LMICs have benefitted from strengthening of the overall health care system. These investments should be leveraged and expanded to address other comorbid chronic diseases.
It is unknown whether identifying LV dysfunction in an asymptomatic PLHIV warrants further action. Evaluating for contractile reserve with stress echocardiography, diastolic dysfunction, and abnormal myocardial deformation may identify higher-risk patients (97,98), but it is unknown whether a strategy incorporating such techniques alters clinical decision making for patients with HIV-associated HF. Last, the long-term impact of HIV on cardiac structure and function needs to be described in the ART era in order to guide care for children, as the natural history of cardiac function in perinatally HIV-infected children is unknown.
Conclusions
In the ART era, HF remains an important contributor to the cardiovascular disease burden in PLHIV in LMICs. Although the causative factors related to HIV-associated HF have shifted over time, HIV-infected patients in LMICs are at risk of developing HF even as access to effective ART expands. Outcomes for these patients are grim, and HIV infection increases in-hospital and post-discharge HF mortality. Ischemic HF is reportedly rare but may become more prevalent as cardiovascular disease epidemiology shifts. A number of causative factors appears to be operating simultaneously. Both adults and children are affected, yet early institution of ART may ameliorate the prognosis. Further insights into the disease may be gained by leveraging efforts from the numerous cardiovascular disease registries and HIV clinical care programs in LMICs. Finally, novel imaging techniques and parameters of cardiac function may reveal early manifestations of cardiac disease in PLHIV that could pave the way for early screening, institution of therapy, and prevention. The magnitude of HIV in LMICs should prompt the scientific community and funders of research to fill the knowledge gaps and improve our ability to manage and prevent HF in PLHIV.
Central Illustration. Current Knowledge, Knowledge Gaps, and Future Research.

Directions for HIV-associated heart failure in low- and middle-income countries. HIV = human immunodeficiency virus.
Acknowledgments
Dr. Mayosi has received research grants from Pfizer and Servier Laboratories; travel support from Roche and Novartis; clinical trial support from Cadila Pharmaceuticals; research and educational grants from AstraZeneca and Novartis; and an honorarium from Sanofi. Dr. Velazquez is member of advisory committees for Novartis and Alnylam; and has received grant support from Alnylam and Pfizer.
Abbreviations and Acronyms
- ADHF
acute decompensated heart failure
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- AZT
zidovudine
- DNA
deoxyribonucleic acid
- EF
ejection fraction
- HF
heart failure
- LMICs
low- and middle-income countries
- LVSD
left ventricular systolic dysfunction
- PLHIV
people living with human immunodeficiency virus
- SSA
sub-Saharan Africa
- Tat
transactivator of transcription
Footnotes
All other authors report that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabin CA. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med. 2013;11:251. doi: 10.1186/1741-7015-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Québec's public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–53. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 6.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–43. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012;33:866–74. doi: 10.1093/eurheartj/ehr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorgis L, Cottenet J, Molins G, et al. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127:1767–74. doi: 10.1161/CIRCULATIONAHA.113.001874. [DOI] [PubMed] [Google Scholar]
- 9.Esser S, Gelbrich G, Brockmeyer N, et al. Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol. 2013;102:203–13. doi: 10.1007/s00392-012-0519-0. [DOI] [PubMed] [Google Scholar]
- 10.Joint United Nations Programme on HIV/AIDS (UNAIDS) Geneva: World Health Organization; 2013. [Accessed February 20, 2015]. Report on the Global AIDS Epidemic 2013. Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 11.Magula NP, Mayosi BM. Cardiac involvement in HIV-infected people living in Africa: a review. Cardiovasc J S Afr. 2003;14:231–7. [PubMed] [Google Scholar]
- 12.Hakim JG, Matenga JA, Siziya S. Myocardial dysfunction in human immunodeficiency virus infection: an echocardiographic study of 157 patients in hospital in Zimbabwe. Heart. 1996;76:161–5. doi: 10.1136/hrt.76.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niakara A, Drabo YJ, Kambire Y, Nebie LV, Kabore NJ, Simon F. [Cardiovascular diseases and HIV infection: study of 79 cases at the National Hospital of Ouagadougou (Burkina Faso) Bull Soc Pathol Exot. 2002;95:23–6. [PubMed] [Google Scholar]
- 14.Nzuobontane D, Blackett KN, Kuaban C. Cardiac involvement in HIV infected people in Yaounde, Cameroon. Postgrad Med J. 2002;78:678–81. doi: 10.1136/pmj.78.925.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie PF, Jacob AJ, Foreman AR, Elton RA, Brettle RP, Boon NA. Heart muscle disease related to HIV infection: prognostic implications. BMJ. 1994;309:1605–7. doi: 10.1136/bmj.309.6969.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbaro G. Pathogenesis of HIV-associated heart disease. AIDS. 2003;17(Suppl 1):S12–20. doi: 10.1097/00002030-200304001-00003. [DOI] [PubMed] [Google Scholar]
- 17.Bloomfield GS, Velazquez EJ. HIV and cardiovascular disease in sub-Saharan Africa: the Sutton Law as applied to global health. J Am Coll Cardiol. 2013;61:2395. doi: 10.1016/j.jacc.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 18.Callender T, Woodward M, Roth G, et al. Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001699. doi: 10.1371/journal.pmed.1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrosy AP, Gheorghiade M, Chioncel O, Mentz RJ, Butler J. Global perspectives in hospitalized heart failure: regional and ethnic variation in patient characteristics, management, and outcomes. Curr Heart Fail Rep. 2014;11:416–27. doi: 10.1007/s11897-014-0221-9. [DOI] [PubMed] [Google Scholar]
- 20.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloomfield GS, Barasa FA, Doll JA, Velazquez EJ. Heart Failure in Sub-Saharan Africa. Curr Cardiol Rev. 2013;9:157–73. doi: 10.2174/1573403X11309020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1,006 Africans from 9 countries. Arch Intern Med. 2012;172:1386–94. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
- 23.Stewart S, Wilkinson D, Hansen C, et al. Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation. 2008;118:2360–7. doi: 10.1161/CIRCULATIONAHA.108.786244. [DOI] [PubMed] [Google Scholar]
- 24.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 26.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005;112:3577–83. doi: 10.1161/CIRCULATIONAHA.105.542894. [DOI] [PubMed] [Google Scholar]
- 28.Bloomfield GS, Khazanie P, Morris A, et al. HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr. 2014;67(Suppl 1):S40–53. doi: 10.1097/QAI.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis W. Cardiomyopathy in AIDS: a pathophysiological perspective. Prog Cardiovasc Dis. 2000;43:151–70. doi: 10.1053/pcad.2000.9031. [DOI] [PubMed] [Google Scholar]
- 30.Duan M, Yao H, Hu G, Chen X, Lund AK, Buch S. HIV Tat induces expression of ICAM-1 in HUVECs: implications for miR-221/-222 in HIV-associated cardiomyopathy. PLoS One. 2013;8:e60170. doi: 10.1371/journal.pone.0060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabrese LH, Proffitt MR, Yen-Lieberman B, Hobbs RE, Ratliff NB. Congestive cardiomyopathy and illness related to the acquired immunodeficiency syndrome (AIDS) associated with isolation of retrovirus from myocardium. Ann Intern Med. 1987;107:691–2. doi: 10.7326/0003-4819-107-5-691. [DOI] [PubMed] [Google Scholar]
- 32.Fiala M, Popik W, Qiao JH, et al. HIV-1 induces cardiomyopathyby cardiomyocyte invasion and gp120, Tat, and cytokine apoptotic signaling. Cardiovasc Toxicol. 2004;4:97–107. doi: 10.1385/ct:4:2:097. [DOI] [PubMed] [Google Scholar]
- 33.Jacob AJ, Sutherland GR, Bird AG, et al. Myocardial dysfunction in patients infected with HIV: prevalence and risk factors. Br Heart J. 1992;68:549–53. doi: 10.1136/hrt.68.12.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Twu C, Liu NQ, Popik W, et al. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion- and death receptor-controlled pathways. Proc Natl Acad Sci U S A. 2002;99:14386–91. doi: 10.1073/pnas.212327899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopes de Campos WR, Chirwa N, London G, et al. HIV-1 subtype C unproductively infects human cardiomyocytes in vitro and induces apoptosis mitigated by an anti-Gp120 aptamer. PLoS One. 2014;9:e110930. doi: 10.1371/journal.pone.0110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecoeur H, Borgne-Sanchez A, Chaloin O, et al. HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell death & disease. 2012;3:e282. doi: 10.1038/cddis.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaboodien G, Engel ME, Syed FF, Poulton J, Badri M, Mayosi BM. The mitochondrial DNA T16189C polymorphism and HIV-associated cardiomyopathy: a genotype-phenotype association study. BMC Med Genet. 2009;10:37. doi: 10.1186/1471-2350-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longo-Mbenza B, Seghers KV, Phuati M, Bikangi FN, Mubagwa K. Heart involvement and HIV infection in African patients: determinants of survival. Int J Cardiol. 1998;64:63–73. doi: 10.1016/s0167-5273(97)00321-5. [DOI] [PubMed] [Google Scholar]
- 39.Shaboodien G, Maske C, Wainwright H, et al. Prevalence of myocarditis and cardiotropic virus infection in Africans with HIV-associated cardiomyopathy, idiopathic dilated cardiomyopathy and heart transplant recipients: a pilot study: cardiovascular topic. Cardiovasc J Afr. 2013;24:218–23. doi: 10.5830/CVJA-2013-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondy KE, Gottdiener J, Overton ET, et al. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52:378–86. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 41.Cerrato E, D'Ascenzo F, Biondi-Zoccai G, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–6. doi: 10.1093/eurheartj/ehs471. [DOI] [PubMed] [Google Scholar]
- 42.El Hattaoui M, Charei N, Boumzebra D, Aajly L, Fadouach S. Prevalence of cardiomyopathy in HIV infection: prospective study on 158 HIV patients. Med Mal Infect. 2008;38:387–91. doi: 10.1016/j.medmal.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Twagirumukiza M, Nkeramihigo E, Seminega B, Gasakure E, Boccara F, Barbaro G. Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: a multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res. 2007;5:129–37. doi: 10.2174/157016207779316288. [DOI] [PubMed] [Google Scholar]
- 44.Joy EJ, Ander EL, Young SD, et al. Dietary mineral supplies in Africa. Physiol Plant. 2014;151:208–29. doi: 10.1111/ppl.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Look MP, Rockstroh JK, Rao GS, et al. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase (GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. Eur J Clin Nutr. 1997;51:266–72. doi: 10.1038/sj.ejcn.1600401. [DOI] [PubMed] [Google Scholar]
- 46.Zazzo JF, Chalas J, Lafont A, Camus F, Chappuis P. Is nonobstructive cardiomyopathy in AIDS a selenium deficiency-related disease? J Parenter Enteral Nutr. 1988;12:537–8. doi: 10.1177/0148607188012005537. [DOI] [PubMed] [Google Scholar]
- 47.Kavanaugh-McHugh AL, Ruff A, Perlman E, Hutton N, Modlin J, Rowe S. Selenium deficiency and cardiomyopathy in acquired immunodeficiency syndrome. J Parenter Enteral Nutr. 1991;15:347–9. doi: 10.1177/0148607191015003347. [DOI] [PubMed] [Google Scholar]
- 48.Lemmer CE, Badri M, Visser M, Mayosi BM. A lower body mass index is associated with cardiomyopathy in people with HIV infection: evidence from a case comparison study. S Afr Med J. 2011;101:119–21. doi: 10.7196/samj.4348. [DOI] [PubMed] [Google Scholar]
- 49.Pugliese A, Isnardi D, Saini A, Scarabelli T, Raddino R, Torre D. Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Infect. 2000;40:282–4. doi: 10.1053/jinf.2000.0672. [DOI] [PubMed] [Google Scholar]
- 50.Bijl M, Dieleman JP, Simoons M, van der Ende ME. Low prevalence of cardiac abnormalities in an HIV-seropositive population on antiretroviral combination therapy. J Acquir Immune Defic Syndr. 2001;27:318–20. doi: 10.1097/00126334-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 51.Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr HIV Res. 2006;4:79–85. doi: 10.2174/157016206775197664. [DOI] [PubMed] [Google Scholar]
- 52.Currie PF, Boon NA. Immunopathogenesis of HIV-related heart muscle disease: current perspectives. AIDS. 2003;17(Suppl 1):S21–8. doi: 10.1097/00002030-200304001-00004. [DOI] [PubMed] [Google Scholar]
- 53.Herskowitz A, Willoughby SB, Baughman KL, Schulman SP, Bartlett JD. Cardiomyopathy associated with antiretroviral therapy in patients with HIV infection: a report of six cases. Ann Intern Med. 1992;116:311–3. doi: 10.7326/0003-4819-116-4-311. [DOI] [PubMed] [Google Scholar]
- 54.Lipshultz SE, Orav EJ, Sanders SP, Hale AR, McIntosh K, Colan SD. Cardiac structure and function in children with human immunodeficiency virus infection treated with zidovudine. N Engl J Med. 1992;327:1260–5. doi: 10.1056/NEJM199210293271802. [DOI] [PubMed] [Google Scholar]
- 55.Luo L, Ye Y, Liu Z, et al. Assessment of cardiac diastolic dysfunction in HIV-infected people without cardiovascular symptoms in China. Int J STD AIDS. 2010;21:814–8. doi: 10.1258/ijsa.2010.010168. [DOI] [PubMed] [Google Scholar]
- 56.Currie PF, Goldman JH, Caforio AL, et al. Cardiac autoimmunity in HIV related heart muscle disease. Heart. 1998;79:599–604. doi: 10.1136/hrt.79.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frustaci A, Petrosillo N, Francone M, Verardo R, Ippolito G, Chimenti C. Biopsy-proven autoimmune myocarditis in HIV-associated dilated cardiomyopathy. BMC Infect Dis. 2014;14:3855. doi: 10.1186/s12879-014-0729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herskowitz A, Wu TC, Willoughby SB, et al. Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late-stage infection with human immunodeficiency virus. J Am Coll Cardiol. 1994;24:1025–32. doi: 10.1016/0735-1097(94)90865-6. [DOI] [PubMed] [Google Scholar]
- 59.Longo-Mbenza B, Seghers LV, Vita EK, Tonduangu K, Bayekula M. Assessment of ventricular diastolic function in AIDS patients from Congo: a Doppler echocardiographic study. Heart. 1998;80:184–9. doi: 10.1136/hrt.80.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain N, Reddy DH, Verma SP, et al. Cardiac abnormalities in HIV-positive patients: results from an observational study in India. J Int Assoc Provid AIDS Care. 2014;13:40–6. doi: 10.1177/1545109712456740. [DOI] [PubMed] [Google Scholar]
- 61.Sliwa K, Davison BA, Mayosi BM, et al. Read-mission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. Eur Heart J. 2013;34:3151–9. doi: 10.1093/eurheartj/eht393. [DOI] [PubMed] [Google Scholar]
- 62.Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Afr. 2012;23:90–7. doi: 10.5830/CVJA-2011-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ige OO, Oguche S, Bode-Thomas F. Left ventricular systolic function in Nigerian children with human immunodeficiency virus infection. Congenital heart disease. 2012;7:417–22. doi: 10.1111/j.1747-0803.2012.00676.x. [DOI] [PubMed] [Google Scholar]
- 64.Okoromah CA, Ojo OO, Ogunkunle OO. Cardiovascular dysfunction in HIV-infected children in a sub-Saharan African country: comparative cross-sectional observational study. J Trop Pediatr. 2012;58:3–11. doi: 10.1093/tropej/fmr009. [DOI] [PubMed] [Google Scholar]
- 65.Olusegun-Joseph DA, Ajuluchukwu JN, Okany CC, Mbakwem AC, Oke DA, Okubadejo NU. Echocardiographic patterns in treatment-naïve HIV-positive patients in Lagos, south-west Nigeria. Cardiovasc J Afr. 2012;23:e1–6. doi: 10.5830/CVJA-2012-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pepeta L, Cilliers AM. Impact of highly active antiretroviral therapy on paediatric human immunodeficiency virus-associated left ventricular dysfunction within the Johannesburg teaching hospital complex. Cardiol Young. 2012;22:564–73. doi: 10.1017/S1047951112000078. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz T, Magdi G, Steen TW, Sjaastad I. HIV as a risk factor for cardiac disease in Botswana: a cross-sectional study. Int Health. 2012;4:30–7. doi: 10.1016/j.inhe.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Tantchou Tchoumi JC, Ambassa JC, Kingue S, et al. Occurrence, aetiology and challenges in the management of congestive heart failure in sub-Saharan Africa: experience of the Cardiac Centre in Shisong, Cameroon. Pan Afr Med J. 2011;8:11. doi: 10.4314/pamj.v8i1.71059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lubega S, Zirembuzi GW, Lwabi P. Heart disease among children with HIV/AIDS attending the paediatric infectious disease clinic at Mulago Hospital. Afr Health Sci. 2005;5:219–26. doi: 10.5555/afhs.2005.5.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diogenes MS, Carvalho AC, Succi RC. Reversible cardiomyopathy subsequent to perinatal infection with the human immunodeficiency virus. Cardiol Young. 2003;13:373–6. [PubMed] [Google Scholar]
- 71.Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371:915–22. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 72.Owusu IK, Adu-Boakye Y. Prevalence and aetiology of heart failure in patients seen at a teaching hospital in Ghana. J Cardiovasc Dis Diagn. 2013;1:1–4. [Google Scholar]
- 73.Ogah OS, Stewart S, Falase AO, et al. Contemporary profile of acute heart failure in Southern Nigeria: data from the Abeokuta Heart Failure Clinical Registry. J Am Coll Cardiol HF. 2014;2:250–9. doi: 10.1016/j.jchf.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Onwuchekwa AC, Asekomeh GE. Pattern of heart failure in a Nigerian teaching hospital. Vasc Health Risk Manag. 2009;5:745–50. doi: 10.2147/vhrm.s6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makubi A, Hage C, Lwakatare J, et al. Contemporary aetiology, clinical characteristics and prognosis of adults with heart failure observed in a tertiary hospital in Tanzania: the prospective Tanzania Heart Failure (TaHeF) study. Heart. 2014 doi: 10.1136/heartjnl-2014-305599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsue PY, Hunt PW, Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–9. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiovascular status of infants and children of women infected with HIV-1 (P(2)C(2) HIV): a cohort study. Lancet. 2002;360:368–73. doi: 10.1016/S0140-6736(02)09607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herdy GV, Pinto CA, Lopes VG, et al. Study of the cardiac alterations in HIV-infected children consequent to the antiretroviral therapy. Prospective study of 47 cases. Arq Bras Cardiol. 2003;80:311–20. doi: 10.1590/s0066-782x2003000300007. [DOI] [PubMed] [Google Scholar]
- 79.HIV-Druginteractions. [Accessed June 17, 2015]; Available at: http://www.hiv-druginteractions.org.
- 80.Wong RW, Balachandran A, Ostrowski MA, Cochrane A. Digoxin suppresses HIV-1 replication by altering viral RNA processing. PLoS Pathog. 2013;9:e1003241. doi: 10.1371/journal.ppat.1003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schuster I, Thoni GJ, Ederhy S, et al. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol. 2008;101:1213–7. doi: 10.1016/j.amjcard.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 82.Reinsch N, Neuhaus K, Esser S, et al. Prevalence of cardiac diastolic dysfunction in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2010;11:156–62. doi: 10.1310/hct1103-156. [DOI] [PubMed] [Google Scholar]
- 83.Karavidas A, Tsiachris D, Lazaros G, et al. Doppler tissue imaging unmasks right ventricular function abnormalities in HIV-infected patients. Cardiol J. 2010;17:587–93. [PubMed] [Google Scholar]
- 84.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–22. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- 85.Kraigher-Krainer E, Shah AM, Gupta DK, et al. mpaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendes L, Silva D, Miranda C, et al. Impact of HIV infection on cardiac deformation. Rev Port Cardiol. 2014;33:501–9. doi: 10.1016/j.repc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Al-Naami G, Kiblawi F, Kest H, Hamdan A, Myridakis D. Cardiac mechanics in patients with human immunodeficiency virus: a study of systolic myocardial deformation in children and young adults. Pediatr Cardiol. 2014;35:1046–51. doi: 10.1007/s00246-014-0896-4. [DOI] [PubMed] [Google Scholar]
- 88.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and metaanalysis. Int J Epidemiol. 2013;42:1754–71. doi: 10.1093/ije/dyt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desalu OO, Oluboyo PO, Olokoba AB, et al. Prevalence and determinants of tobacco smoking among HIV patients in North Eastern Nigeria. Afr J Med Med Sci. 2009;38:103–8. [PubMed] [Google Scholar]
- 90.Bloomfield GS, Hogan JW, Keter A, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in western Kenya. PLoS One. 2011;6:e22288. doi: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borkum M, Wearne N, Alfred A, Dave JA, Levitt NS, Rayner B. Ambulatory blood pressure profiles in a subset of HIV-positive patients pre and post antiretroviral therapy. Cardiovasc J Afr. 2014;25:153–7. doi: 10.5830/CVJA-2014-029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bloomfield GS, Hogan JW, Keter A, et al. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14:284. doi: 10.1186/1471-2334-14-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mayosi BM, Ntsekhe M, Bosch J, et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med. 2014;371:1121–30. doi: 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ntsekhe M, Hakim J. Impact of human immunodeficiency virus infection on cardiovascular disease in Africa. Circulation. 2005;112:3602–7. doi: 10.1161/CIRCULATIONAHA.105.549220. [DOI] [PubMed] [Google Scholar]
- 95.Becker AC, Jacobson B, Singh S, et al. The thrombotic profile of treatment-naive HIV-positive Black South Africans with acute coronary syndromes. Clin Appl Thromb Hemost. 2011;17:264–72. doi: 10.1177/1076029609358883. [DOI] [PubMed] [Google Scholar]
- 96.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 97.Wever-Pinzon O, Bangalore S, Romero J, Silva Enciso J, Chaudhry FA. Inotropic contractile reserve can risk-stratify patients with HIV cardiomyopathy: a dobutamine stress echocardiography study. J Am Coll Cardiol Img. 2011;4:1231–8. doi: 10.1016/j.jcmg.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lai H, Redheuil A, Tong W, et al. HIV infection and abnormal regional ventricular function. Int J Cardiovasc Imaging. 2009;25:809–17. doi: 10.1007/s10554-009-9493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.World Health Organization. Global Health Observatory Data Repository. [Accessed June 17, 2015];2013 Available at: http://www.who.int/gho/en/
- 100.U.S. Central Intelligence Agency World Factbook. [Accessed June 17, 2015]; Available at: https://www.cia.gov/library/publications/the-world-factbook/rankorder/2157rank.html.
- 101.Centers for Disease Control Taiwan (ROC) [Accessed June 17, 2015]; Available at: http://archive.is/www.cdc.gov.tw.


