ABSTRACT
In the presence of alanine, AldR, which belongs to the Lrp/AsnC family of transcriptional regulators and regulates ald encoding alanine dehydrogenase in Mycobacterium smegmatis, changes its quaternary structure from a homodimer to an octamer with an open-ring conformation. Four AldR-binding sites (O2, O1, O4, and O3) with a consensus sequence of GA/T-N2-NWW/WWN-N2-A/TC were identified upstream of the M. smegmatis ald gene by means of DNase I footprinting analysis. O2, O1, and O4 are required for the induction of ald expression by alanine, while O3 is directly involved in the repression of ald expression. In addition to O3, both O1 and O4 are also necessary for full repression of ald expression in the absence of alanine, due to cooperative binding of AldR dimers to O1, O4, and O3. Binding of a molecule of the AldR octamer to the ald control region was demonstrated to require two AldR-binding sites separated by three helical turns between their centers and one additional binding site that is in phase with the two AldR-binding sites. The cooperative binding of AldR dimers to DNA requires three AldR-binding sites that are aligned with a periodicity of three helical turns. The aldR gene is negatively autoregulated independently of alanine. Comparative analysis of ald expression of M. smegmatis and Mycobacterium tuberculosis in conjunction with sequence analysis of both ald control regions led us to suggest that the expression of the ald genes in both mycobacterial species is regulated by the same mechanism.
IMPORTANCE In mycobacteria, alanine dehydrogenase (Ald) is the enzyme required both to utilize alanine as a nitrogen source and to grow under hypoxic conditions by maintaining the redox state of the NADH/NAD+ pool. Expression of the ald gene was reported to be regulated by the AldR regulator that belongs to the Lrp/AsnC (feast/famine) family, but the underlying mechanism was unknown. This study revealed the regulation mechanism of ald in Mycobacterium smegmatis and Mycobacterium tuberculosis. Furthermore, a generalized arrangement pattern of cis-acting regulatory sites for Lrp/AsnC (feast/famine) family regulators is suggested in this study.
INTRODUCTION
NAD(H)-dependent alanine dehydrogenase (Ald; EC 1.4.1.1) catalyzes the oxidative deamination of l-alanine to pyruvate and reversibly catalyzes the reductive amination of pyruvate to l-alanine. Ald is required for mycobacteria to utilize alanine as a nitrogen source via the oxidative deamination reaction (1–3). It was also proposed that Ald helps mycobacteria maintain the redox state of the NADH/NAD+ pool under respiration-inhibiting conditions, such as hypoxic conditions, by regenerating NAD+ from NADH via its reductive amination reaction (2, 4, 5). The enzyme forms a hexamer of identical subunits with the N-terminal catalytic domain and the C-terminal NAD(H)-binding domain (6, 7). Ald was shown to be one of the major antigens found in culture filtrates of Mycobacterium tuberculosis (8, 9).
Expression of the ald gene as well as the synthesis and activity of Ald were observed to be increased under oxygen-limiting conditions in M. tuberculosis and Mycobacterium smegmatis (2–4, 10–14). Other studies reported that expression of the ald gene was upregulated in Mycobacterium marinum during persistence within its host granulomas and in M. tuberculosis under nutrient starvation conditions (5, 15). Expression of the ald gene was demonstrated to be strongly induced in M. smegmatis and M. tuberculosis grown in the presence of alanine (2, 3, 14).
Expression of the ald gene in M. smegmatis is under the control of the AldR transcriptional regulator that belongs to the Lrp/AsnC (leucine-responsive regulatory protein/asparagine synthase C) family. AldR is a direct sensor of alanine and serves as both an activator of ald expression in the presence of alanine and a repressor in the absence of alanine (14). We also demonstrated that hypoxic induction of ald results from increased intracellular levels of alanine in M. smegmatis under hypoxic conditions. Inhibition of the respiratory electron transport chain under hypoxic conditions leads to a higher ratio of NADH to NAD+, by which cellular levels of alanine might increase through the reductive amination reaction by Ald. Like other members of the Lrp/AsnC family, AldR is composed of two distinct domains, the N-terminal DNA-binding domain containing a winged helix-turn-helix (HTH) motif and the C-terminal ligand-binding domain called RAM (regulation of amino acid metabolism) (14). The C-terminal domain is also known to be involved in dimerization and further higher-order oligomerization (16–21). Most members of the Lrp/AsnC family adopt a ringlike octamer structure (either open- or closed-ring structure) consisting of four dimers (16, 18–27). In many cases, binding of the cognate amino acids to Lrp/AsnC regulators leads to changes in their quaternary structure (oligomerization state) (14, 17, 21, 23, 24, 26–28). It was demonstrated previously that alanine not only alters the quaternary structure of AldR from a dimer to a higher-order oligomer (hexamer or octamer) but also leads to an increase in the binding affinity of AldR for the ald control region of M. smegmatis (14). Three putative AldR-binding sites (O1, O2, and O3) were identified upstream of the ald gene in M. smegmatis on the basis of sequence analysis and EMSAs (electrophoretic mobility shift assays) (14). However, it remains to be clarified how AldR regulates ald expression in both positive and negative ways and the role that each AldR-binding site plays in the regulation of ald expression. In this study, we identified another AldR-binding site (O4) in the ald control region by means of DNase I footprinting analysis and determined how the AldR-binding sites are implicated in the positive and negative regulation of ald, depending on the presence and absence of alanine. Based on comparative analyses of ald regulation in M. smegmatis and M. tuberculosis, we concluded that a common theme exists with regard to the regulation mechanism of ald in both mycobacteria.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. M. smegmatis strains were grown in Middlebrook 7H9 medium (Difco, Sparks, MD) supplemented with 0.2% (wt/vol) glucose as a carbon source and 0.02% (vol/vol) Tween 80 as an anticlumping agent at 37°C. For treatment of M. smegmatis cultures with alanine, M. smegmatis strains were grown to an optical density at 600 nm (OD600) of 0.5 to 0.6 on a gyratory shaker (200 rpm). Following the addition of 25 mM l-alanine to the cultures, the strains were further grown for 1 h. Escherichia coli strains were grown in Luria-Bertani (LB) medium at 37°C. Ampicillin (100 μg/ml for E. coli), hygromycin (200 μg/ml for E. coli and 50 μg/ml for M. smegmatis), and kanamycin (50 μg/ml for E. coli and 30 μg/ml for M. smegmatis) were added to the growth medium when required. The construction of the plasmids used in this study is described in the supplemental material.
DNA manipulation and electroporation.
Recombinant DNA manipulations were performed according to standard protocols or the manufacturers' instructions (29). The introduction of plasmids into M. smegmatis strains was carried out by electroporation, as described previously (30).
5′ rapid amplification of cDNA ends.
The 5′ end of mRNA containing M. tuberculosis ald was determined by means of 5′ rapid amplification of cDNA ends (RACE). Total RNA was isolated from an M. smegmatis strain containing pNBV1MTaldR grown aerobically, as described previously (31). Nine micrograms of total RNA was reverse transcribed into cDNA by using 18 pmol of the M. tuberculosis ald-specific primer MTBald_RACE_1 (5′-CTGCCGCCTTGAAATCCGCG-3′) and RT-&GO Mastermix reverse transcriptase (MPbio, Eschwege, Germany) according to the manufacturer's instructions. The resulting cDNA was then purified with a QIAquick PCR purification kit (Qiagen, Valencia, CA). 5′ RACE was performed as described previously (32). Briefly, the addition of the poly(A) tail to cDNA was performed by using terminal deoxynucleotidyl transferase (Thermo Scientific, Rockford, IL) and dATP. Primary PCR was carried out by using the poly(A)-tailed cDNA as a template and the primers oligo(dT) anchor (5′-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTV-3′, where V is A, C, or G) and MTBald_RACE_1. For secondary PCR, the PCR anchor primer (5′-GACCACGCGTATCGATGTCGAC-3′) and the nested gene-specific primer R_MTBald (5′-CAAAATCGATGAGCACCTCATGGCCAC-3′) were used. PCR products were cloned into pMD20-T, and the nucleotide sequences of the cloned PCR products were then determined by DNA sequencing.
Purification of AldR.
Purification of C-terminally His6-tagged AldR was carried out as described previously (14).
β-Galactosidase assay and determination of protein concentration.
β-Galactosidase activity was assayed spectrophotometrically as described previously (33). The protein concentration was determined by using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) and bovine serum albumin as a standard.
DNase I footprinting analysis.
DNase I footprinting was carried out by using fluorescence (TAMRA [6-carboxytetramethylrhodamine])-labeled DNA fragments and purified AldR protein. TAMRA-labeled DNA fragments of 321 bp containing the ald-aldR intergenic region were generated by PCR using the primers TAMRA_pUC19 (5′-TAMRA-GTTTTCCCAGTCACGACGTTGTA-3′) and pUC19_reverse (5′-AGGAAACAGCTATGACCATG-3′). pALDFootF and pALDFootR were used for PCR as the templates. The PCR products were purified after agarose gel electrophoresis, and DNA concentrations were then determined by using a NanoDrop 2000 spectrophotometer (Thermo). DNA binding reaction mixtures were composed of 5 pmol of labeled DNA probes, purified AldR (0.5 or 1 nmol), 20 mM Tris-HCl (pH 8.0), 0.6 mM MgCl2, 5.6 mM KCl, 0.1 mM dithiothreitol (DTT), and 11.1% (vol/vol) glycerol in a final volume of 90 μl. When necessary, AldR protein was treated with 20 mM l-alanine, and the mixture was incubated for 30 min at 25°C prior to binding reactions for 10 min at 25°C. DNase I (TaKaRa, Tokyo, Japan) was diluted in buffer containing 20 mM Tris-HCl (pH 8.0), 1 mM MgCl2, 50 mM NaCl, 1 mM DTT, and 10% (vol/vol) glycerol to a final concentration of 10 mU/μl. DNase I digestion was initiated by the addition of 10 μl of diluted DNase I to the binding reaction mixtures, conducted for 30 s at 25°C, and stopped by the addition of 200 μl of stop solution containing 40 mM EDTA in 20 mM Tris-HCl (pH 8.0). DNA was purified by phenol-chloroform-isoamyl alcohol (25:24:1) extraction and ethanol precipitation. The pellets were dissolved in loading buffer (5:1 [vol/vol] mixture of deionized formamide and 25 mM EDTA [pH 8.0] with 50 mg/ml blue dextran) and analyzed by electrophoresis on 6% (wt/vol) denaturing polyacrylamide gels with 7 M urea in 0.8× TTE (Tris-taurine-EDTA) buffer using an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA). Reference sequencing was performed by using a Thermo Sequenase dye primer manual cycle sequencing kit (USB, Cleveland, OH) with the primer TAMRA_pUC19 and the template plasmid (pALDFootF or pALDFootR).
Electrophoretic mobility shift assay.
A 108-bp DNA fragment containing the O1 and O2 sites was generated by PCR using pBSMS2659 as a template and the primers F_EMSA_O1O2 (5′-CGGTCGATGTCGTCGAGGTC-3′) and R_EMSA_O1O2 (5′-GAAAATTCGTTAGGATTGTG-3′). To obtain a 108-bp DNA fragment containing the O3 and O4 sites, PCR was carried out by using pBSMS2659 as a template and the primers F_EMSA_O3O4 (5′-GATCCTTCACTCACGGCAC-3′) and R_EMSA_O3O4 (5′-ATCGCTCCTTCAGAAGGGG-3′). DNA fragments of 243 bp containing the ald control region with the wild-type (WT) and mutated AldR-binding sites (O2, O1, O4, and O3) were amplified by PCR using the primers F_EMSA (5′-AACCATCGATAGCATGATCGCTCCTTCAGAAG-3′) and R_218bp (5′-ATTGTCTAGATATGCGTGCATCGTCGTGCAAC-3′) and pBSMS2659, pBSO2PM, pBSO1PM, pBSO4PM, pBSO3PM, and pBSO2O3PM as the templates, respectively. EMSA was performed as described previously (14).
Transmission electron microscopy (TEM).
Five microliters of AldR in the presence of l-alanine at ∼5 μg/ml was loaded onto an electron microscopy (EM) grid covered with continuous carbon film that was freshly glow discharged. After 1 min of sample adsorption, the grid was washed with droplets of deionized water and then negatively stained with 0.75% (wt/vol) uranyl formate for 1 min. Excess stain solution was blotted by using a piece of filter paper, and the grid was air dried. The sample was imaged under a minimal electron dose (20 to ∼30 electrons/Å2) at an underfocus of between 0.8 μm and 1.5 μm by using a Tecnai G2 Sprit transmission electron microscope (FEI, Hillsboro, OR) equipped with a Lab6 gun and operating at 120 kV. The images were recorded at a nominal magnification of ×67,000 by using an UltraScan 4000 charge-coupled-device (CCD) camera (Gatan, Pleasanton, CA), which corresponds to 1.6-Å/pixel sampling at the specimen level. Electron micrographs of negatively stained AldR that showed minimal astigmatism and drift were selected for image processing using EMAN2 software suite (34). From 61 raw frames, 4,151 particles were manually selected and boxed into 128- by 128-pixel boxes. The particles were corrected for contrast transfer function and then subjected to iterative alignment, classification, and averaging.
RESULTS
Structure of AldR in the presence of l-alanine.
Gel filtration chromatography with purified AldR previously demonstrated that AldR exists as a homodimer in amino-acid-free solution and that the presence of alanine converts AldR dimers into a higher-order structure with a molecular mass corresponding to a homohexamer (14). Since most regulatory proteins of the Lrp/AsnC (or feast/famine) family adopt ringlike octamer structures with either a closed- or open-ring conformation (16, 18–27) and there is no known member of this family with a quaternary structure of a homohexamer, we assumed that AldR also has a quaternary structure of a homooctamer in the presence of alanine, which was supported by the suggestion that the AldR ortholog (Rv2779c) of M. tuberculosis has a homooctameric structure (35). To determine whether AldR adopts a closed- or open-ring octamer structure in the presence of alanine, we performed TEM analysis on purified AldR. TEM images of negatively stained AldR revealed that AldR exhibited a horseshoe-shaped morphology in the presence of alanine (Fig. 1A, circles), although no clear symmetry was identifiable from the particles. Subsequent class averaging confirmed such a morphology (Fig. 1B), where the open gap of the horseshoe-shaped particle closely resembles the gap between the dimers seen in the open-ring octamer of FL11 of Pyrococcus sp. strain OT3 in the presence of arginine (Fig. 1C, arrowhead). Whereas the alanine-bound C-terminal domains of the AldR octamer were clearly observed, the N-terminal DNA-binding domains were absent in the class average (Fig. 1C, marked in red), suggesting that these domains are mobile due to a flexible hinge between the DNA-binding domain and the C-terminal domain.
FIG 1.
Transmission electron microscopy analysis of AldR in the presence of l-alanine. (A) Electron micrograph of negatively stained AldR. (B) Representative class average of AldR that exhibits an open-ring conformation. (C) Superimposition of the FL11 open-ring octamer structure (PDB accession number 2ZNY) onto the class average.
Identification of AldR-binding sites in the upstream region of ald and their roles in the regulation of ald expression.
In our previous study, three putative AldR-binding sites (O2, O1, and O3) were identified upstream of the ald gene in M. smegmatis (14). In order to define precisely the number and location of AldR-binding sites in the ald control region as well as the DNA bending sites resulting from AldR binding, DNase I footprinting analysis was performed with purified AldR and 321-bp TAMRA-labeled DNA fragments containing the ald-aldR intergenic region (Fig. 2). For both ald coding and noncoding strands, binding of AldR protected DNA from DNase I cleavage at four sites (O2, O1, O4, and O3), at positions −133 to −108, −92 to −67, −61 to −36, and −30 to −5, respectively, with respect to the transcriptional start point (TSP) (+1) (Fig. 2, gray bars). In good agreement with our previous EMSA results (14), the addition of 20 mM alanine to the reaction mixtures resulted in more clearly protected windows for both coding and noncoding strands, confirming that alanine increases the binding affinity of AldR for four AldR-binding sites. As shown in Fig. 2 and 3, the three AldR-binding sites (O2, O1, and O3) share a consensus sequence (GA-N2-WWN/NWW-N2-TC, where W is A or T) consisting of the dimeric dyad (GA-N7-TC) and two consecutive W nucleotides in the central sequence (WWN or NWW). The O4 site identified in this study shows the sequence deviating from the consensus sequence where TC is replaced with AC. O1 and O4 as well as O4 and O3 are separated from each other by 31 bp (three helical turns) between their centers, whereas the centers of O2 and O1 are separated by 41 bp (four helical turns).
FIG 2.
DNase I footprinting analysis of the ald control region bound by AldR. The DNA fragments containing the noncoding (A) and coding (B) strands labeled with TAMRA at their 5′ ends were incubated with increasing concentrations of purified AldR (0.5 and 1 nmol) in the absence (−Ala) or presence (+Ala) of 20 mM l-alanine and then subjected to DNase I footprinting reactions, as described in Materials and Methods. The amounts of AldR protein used are given below the lanes. The schematic diagrams at the top depict the TAMRA-labeled DNA probes containing the AldR-binding sites. TAMRA is indicated by the gray spheres. The regions protected by AldR and the AldR-binding consensus sequences (GA-N7-TC; O1, O2, O3, and O4) are marked by gray bars and black lines on the right, respectively. The numbered asterisks indicate the hypersensitive sites resulting from AldR binding. Lanes G, A, T, and C represent the sequence ladders.
FIG 3.
Nucleotide sequences of the ald control regions of M. smegmatis mc2155 and M. tuberculosis H37Rv. The TSP for ald of M. smegmatis was previously reported by Feng et al. (2). The position of TSP of M. tuberculosis ald was determined by 5′ RACE analysis in this study. The TSPs are indicated by +1. The −10 and −35 regions of the putative ald promoters deduced from the TSPs are enclosed in boxes. The AldR-binding sites (O1, O2, O3, and O4) are marked below their sequences, and the GA and TC regions in the consensus sequence (GA/T-N2-NWW/WWN-N2-T/AC) are shaded. Our previous study reported that O1 of M. smegmatis has an extended inverted repeat (14), which is indicated by dashed lines. The start codons of ald and aldR are highlighted in boldface type, and the arrows below or above the start codons indicate the transcriptional direction. The hypersensitive sites detected by DNase I footprinting are marked by numbered asterisks. Abbreviations: MTB, ald control region of M. tuberculosis; MSMEG, ald control region of M. smegmatis.
The presence of AldR in the reaction mixtures led to a significant increase in the intensity of two bands, at sites *2 and *3, when the labeled noncoding strands were used for footprinting analysis (Fig. 2A). These AldR-induced hypersensitive sites were observed irrespective of the presence or absence of alanine, implying that the binding of both AldR dimers and AldR octamers to the ald control region results in DNA bending or curvature between O1 and O4 as well as between O4 and O3. When the labeled coding strands were employed for footprinting analysis, the band corresponding to site *2 and another adjacent band (site *2′) were detected between O1 and O4 (Fig. 2B). As shown in Fig. 2B, the DNA band denoted by *1 arose from the coding strands only when both AldR and alanine were present in the footprinting reaction mixtures. The intensity of the DNA band corresponding to site *1 was also enhanced with the addition of both AldR and alanine to the reaction mixtures, when the labeled noncoding strands were used (Fig. 2A). This result indicates that binding of the alanine-bound AldR octamer to the ald control region brings about DNA bending or looping between O2 and O1, whereas binding of the AldR dimers does not.
Since the genetic organization of the aldR-ald loci of M. smegmatis and M. tuberculosis is well conserved and the regulation pattern of M. tuberculosis ald is known to be similar to that of M. smegmatis, e.g., upregulation of ald expression by alanine as well as under hypoxic conditions, we assumed that the expression of M. tuberculosis ald is regulated by AldR and that cis-acting regulatory elements involved in the regulation of ald expression might be conserved in the ald control region of M. tuberculosis. Prior to comparing the ald upstream region of M. tuberculosis with that of M. smegmatis, we determined the TSP of M. tuberculosis ald by means of 5′ RACE analysis that was performed with total RNA isolated from aerobically grown cells of M. smegmatis carrying the ald gene of M. tuberculosis and its control region on the multicopy vector pNBV1 in trans. cDNA fragments from 5′ RACE reactions were subcloned into pMD20-T, and 8 recombinant plasmids were used to determine the 5′ terminus of the ald transcript. DNA sequencing analysis revealed two 5′-terminal nucleotides that correspond to nucleotides A and C, 21 and 20 bp upstream of the ald start codon, respectively (Fig. 3). Since purine nucleotides are more frequently used as a TSP than pyrimidine nucleotides (36) and the shorter mRNA starting with C might be a degraded product of the longer one starting with A, the “A” nucleotide was taken as the TSP of M. tuberculosis ald. When aligning the ald upstream regions of M. smegmatis and M. tuberculosis by placing both TSPs at the same position as a reference point, we found that four AldR-binding sites and the ald promoter in the ald control region of M. tuberculosis are located at the same positions as those of M. smegmatis. Although the nucleotide sequences of the AldR-binding sites of M. smegmatis and M. tuberculosis comply relatively well with the consensus sequence for AldR-binding sites (GA-N2-WWN/NWW-N2-TC), some variations were found. As shown in Fig. 3, TC in the dimeric dyad is replaced with AC in the O4 site of M. smegmatis, and GT occurs in place of GA in the O1 site of M. tuberculosis, which led us to suggest a revised consensus sequence for the AldR-binding sites, GA/T-N2-WWN/NWW-N2-T/AC.
The aldR gene of M. smegmatis has a “GTG” start codon, while that of M. tuberculosis begins with an “ATG” start codon (Fig. 3). In order to ascertain the roles of the identified AldR-binding sites in the regulation of ald expression in M. smegmatis, we determined promoter activities of ald in M. smegmatis strains grown in the presence and absence of alanine by using several ald::lacZ transcriptional fusions (pNCO1PM, pNCO2PM, pNCO3PM, pNCO4PM, and pNCO2O3PM) that are derivatives of pALDLACZ and contain mutations within the AldR-binding sites (Fig. 4). Both the GA-to-CC mutation in the dimeric dyad and the WW-to-CC mutation in the central sequence were introduced into the corresponding AldR-binding sites to abolish AldR binding. As controls, wild-type (WT) and ΔaldR mutant strains harboring pALDLACZ were included in the experiment. In good agreement with our previous results (14), ald expression was strongly induced by alanine in the WT strain containing pALDLACZ (control WT). Expression of the ald gene was shown to be derepressed in the ΔaldR mutant with pALDLACZ (control ΔaldR mutant) grown in the absence of alanine relative to that in the control WT strain grown without the addition of alanine, and the presence of alanine did not lead to the induction of ald expression in the control ΔaldR mutant, confirming our previous suggestion that AldR acts as both an activator and a repressor depending on the presence and absence of alanine. When O2, O1, or O4 was mutated, ald expression was no longer induced by alanine, as judged by β-galactosidase activities detected in the WT strains with pNCO2PM, pNCO1PM, and pNCO4PM. In contrast, an O3 mutation led to a strong derepression of ald in the absence of alanine and an ∼1.5-fold increase in the ald expression level in the presence of alanine relative to the expression level of ald in the control WT strain grown in the presence of alanine. Despite the mutation within O3, the presence of alanine was shown to bring about a 2.4-fold induction of ald expression. Since O3 overlaps the promoter of ald (Fig. 3), it is possible that the mutation within O3 alters the intrinsic promoter strength of ald and that the increased expression level of ald in the WT strain with pNCO3PM is a consequence of changes in the ald promoter. To examine this possibility, we determined the promoter activity of ald in the ΔaldR mutant strain with either pALDLACZ or pNCO3PM (Fig. 5). The promoter activity of ald in the ΔaldR mutant with pNCO3PM was slightly decreased relative to that detected in the mutant with pALDLACZ in the presence and absence of alanine, indicating that the intrinsic promoter strength of ald is not increased by the O3 mutation. Taken together, the results shown in Fig. 4 and 5 suggest that O2, O1, and O4 are necessary for the induction of ald expression by alanine and that O3 is involved in AldR-mediated repression of ald not only in the absence of alanine but also to some extent in the presence of alanine.
FIG 4.
Effect of mutations in the AldR-binding sites (O1, O2, O3, and O4) on ald expression. The ald promoter activities were determined by using ald::lacZ transcriptional fusions containing mutations in AldR-binding sites (pNCO1PM, pNCO2PM, pNCO3PM, pNCO4PM, and pNCO2O3PM) that are derivatives of pALDLACZ. The schematic diagrams depicting the transcriptional fusions used are presented at the bottom. The dimeric dyads of the AldR-binding sites are indicated by shading and arrows. Mutations within O1, O2, O3, and O4 are indicated by asterisks. As a control, pALDLACZ was included in the experiment. M. smegmatis wild-type and ΔaldR mutant strains harboring the transcriptional fusion plasmids were grown aerobically to an OD600 of 0.5 to 0.6. Following the addition of 25 mM l-alanine to the cultures, the strains were further grown for 1 h (+Ala). As controls, the M. smegmatis strains were further grown for 1 h without the addition of l-alanine (−Ala). Cell-free crude extracts were used to measure β-galactosidase activity. All values provided are the averages of the results from three independent determinations. Error bars indicate the standard deviations.
FIG 5.
Effect of O3 mutation on ald expression in the ΔaldR mutant strain of M. smegmatis. The ald promoter activities were determined by using pNCO3PM. As a control, pALDLACZ was included in the experiment. M. smegmatis ΔaldR strains harboring the transcriptional fusion plasmids were grown in the presence (+Ala) and absence (−Ala) of l-alanine, as described in the legend to Fig. 4. All values provided are the averages of the results from three independent determinations. Error bars indicate the standard deviations.
In the absence of alanine, expression of ald was derepressed the most in the WT strain with pNCO3PM and was not derepressed at all in the WT strain with pNCO2PM relative to the expression level of ald in the WT control strain. Derepression levels of ald in the WT strains grown in the absence of alanine were in the descending order of O3, O4, and O1 mutations, indicating that the O3 site is most important for the repression of ald expression. This derepression order (O3, O4, and O1) coincides with the order of the AldR-binding sites in the ald control region. The expression level and regulation pattern of ald in the WT strain with pNCO2O3PM were shown to be similar to those in the ΔaldR control mutant, implying that mutations of both O2 and O3 might abolish the binding of AldR to the ald control region. In this respect, it is plausible that the O4 mutation also severely affects the binding of AldR to the ald control region.
Binding of the AldR octamer to the ald control region.
Given that four AldR dimers form an open ringlike octamer with their DNA-binding domains facing out, it is plausible that a molecule of the AldR octamer can simultaneously bind to several AldR-binding sites with DNA wrapping around the AldR octamer. In order to determine how many AldR-binding sites are required for efficient binding of the AldR octamer to the ald control region and to what extent mutation of each AldR-binding site affects the binding affinity of AldR for the ald control region, we performed EMSAs using purified AldR and 243-bp DNA fragments containing the WT or mutated AldR-binding sites (O1PM, O2PM, O3PM, O4PM, and O2O3PM) in the presence of alanine. As shown in Fig. 6A and B, the O2PM and O3PM DNA fragments containing mutations within O2 and O3, respectively, were retarded by AldR to a similar extent as the WT DNA fragment, as judged by the levels of free DNA. In contrast, O4 mutation significantly affected the binding of AldR octamers to the ald control region. The O1PM DNA fragments were retarded by AldR to a lesser extent than the WT, O2, and O3 fragments. Mutations of both O2 and O3 virtually abolished the binding of AldR to the O2O3PM fragments, indicating that the occurrence of only O1 and O4 in DNA fragments is not sufficient for AldR binding. We further examined whether the AldR octamer can bind to DNA fragments containing only two tandem AldR-binding sites (O1O2 and O3O4). As shown in Fig. 6C, the O1O2 and O3O4 fragments were not noticeably retarded, even when large amounts of AldR (20 and 40 pmol) were applied for EMSAs, confirming the requirement for at least three AldR-binding sites for AldR binding.
FIG 6.
EMSAs showing the binding of purified AldR to the mutated ald control regions. (A) The 243-bp DNA fragments (50 fmol, corresponding to 7.5 ng) containing the wild-type (WT) or mutated AldR-binding sites (O1PM, O2PM, O3PM, O4PM, and O2O3PM) were incubated with various concentrations of purified AldR in the presence of 20 mM l-alanine. The amounts of AldR used are given above the lanes. The arrow indicates the bands of free DNA. (B) Quantitation of the band intensity of free DNA in panel A was performed with the ImageJ densitometry program (version 1.37). The band intensity of the free DNA without AldR is set as 100, and the relative values are plotted as a function of the AldR concentration. (C) The 108-bp DNA fragments (100 fmol, corresponding to 6.7 ng), which contain either O1 and O2 (O1O2) or O3 and O4 (O3O4), were incubated with various concentrations of purified AldR in the presence of 20 mM l-alanine and subjected to native PAGE. (D) Schematic diagrams of the DNA fragments used for EMSAs.
Using pALDLACZ-derived ald::lacZ transcriptional fusions (pNCO1O2-5bp and pNCO1O2-10bp) that contain insertions of 5 bp and 10 bp of DNA between O2 and O1, respectively, we examined whether the distance between O2 and O1 and their relative phase on the DNA helix are important in the induction of ald expression (Fig. 7). The insertion of 5 bp (half a helical turn) leads to changes in both distance and relative phase between O2 and O1, while the insertion of 10 bp changes the distance between O2 and O1 without alterations in their relative phase on the DNA helix. The ald gene was almost completely repressed in the WT strain with pNCO1O2-5bp even in the presence of alanine. When 10 bp was inserted between O2 and O1, ald expression was marginally induced by alanine in comparison with the 5-bp insertion. This result indicates that both the distance between O2 and O1 and their relative phase are important in the induction of ald expression by alanine and that the relative phase might be more important than the distance between O2 and O1.
FIG 7.
Effect of insertion mutations between O1 and O2 on ald expression. The ald promoter activities were determined by using ald::lacZ transcriptional fusions (pNCO1O2-5bp and pNCO1O2-10bp) with insertions of 5 bp (GCAGC) and 10 bp (GCAGCACCAG) of DNA between O1 and O2, respectively. The transcriptional fusions are schematized. The numbers between the two adjacent AldR-binding sites indicate the distances between their centers in base pairs. As a positive control, pALDLACZ was included in the experiment. M. smegmatis wild-type strains harboring the transcriptional fusions were grown in the presence (+Ala) and absence (−Ala) of l-alanine, as described in the legend to Fig. 4. All values provided are the averages of the results from three independent determinations. Error bars indicate the standard deviations.
Regulation of the M. tuberculosis ald gene.
The conserved AldR-binding sites and the presence of the aldR gene in M. tuberculosis led us to speculate that the regulation of ald expression in M. tuberculosis might be similar to that in M. smegmatis. Due to the high homology of amino acid sequences (75% identity) between AldR proteins of M. smegmatis and M. tuberculosis (AldR-MS and AldR-MT, respectively), we assumed that AldR-MT could functionally substitute for AldR-MS. To examine this assumption, we measured the expression level of M. smegmatis ald (ald-MS) in the ΔaldR mutant of M. smegmatis with pNBV1MTaldR that contains the aldR gene of M. tuberculosis (aldR-MT) and its upstream region (Fig. 8). Expression of ald-MS was derepressed in the ΔaldR mutant of M. smegmatis with the empty vector pNBV1 grown in the absence of alanine compared to that in the WT strain of M. smegmatis with pNBV1. No induction of ald-MS by alanine was observed in the ΔaldR mutant of M. smegmatis with pNBV1. The introduction of aldR-MT and aldR-MS into the ΔaldR mutant of M. smegmatis by using pNBV1MTaldR and pNBV1aldR, respectively, resulted in the restoration of ald-MS induction by alanine. Unexpectedly, expression of ald-MT was only slightly induced by alanine in the WT strain of M. smegmatis with pNBV1. However, when aldR-MS was expressed from a multicopy plasmid, pNBV1aldR, expression of ald-MT was strongly induced in the presence of alanine and repressed in the absence of alanine. This result indicates that the expression of ald-MT is regulated in a way similar to that of ald-MS and that the regulation of ald-MT expression requires higher levels of AldR-MS than does the regulation of ald-MS expression. When aldR-MT was expressed from pNBV1MTaldR in the ΔaldR mutant of M. smegmatis, expression of ald-MT was normally regulated in response to alanine, confirming the compatibility between AldR-MT and AldR-MS in the regulation of ald expression.
FIG 8.
Expression patterns of M. tuberculosis ald in M. smegmatis and compatibility test for AldR of M. tuberculosis and M. smegmatis. WT and ΔaldR mutant strains of M. smegmatis harboring the ald::lacZ transcriptional fusion (pMV306lacZald or pMV306lacZMTald) in their chromosomes, which contains the ald control region of M. smegmatis or M. tuberculosis, respectively, were grown as described in the legend to Fig. 4. The expression plasmids pNBV1aldR and pNBV1MTaldR, which carry the genes encoding AldR of M. smegmatis and M. tuberculosis, respectively, were used for a complementation test. As controls, WT and ΔaldR mutant strains of M. smegmatis containing the empty vector pNBV1 were included in the experiment. The ald promoter activities were measured by determining the β-galactosidase activity. All values provided are the averages of the results from three independent determinations. Error bars indicate the standard deviations.
Negative autoregulation of aldR.
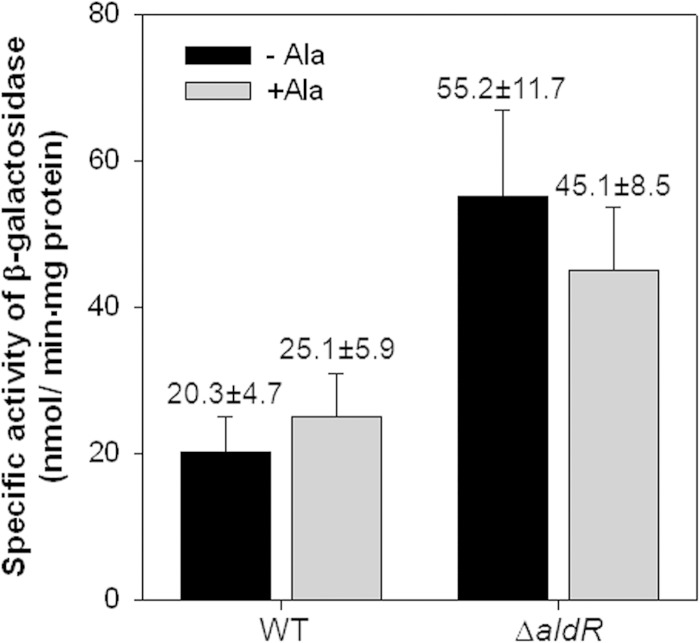
To investigate whether the expression of aldR is autoregulated in M. smegmatis, we determined aldR expression levels in the wild-type and ΔaldR mutant strains of M. smegmatis by using an aldR::lacZ transcriptional fusion, pALDRLACZ. As shown in Fig. 9, the expression of aldR was 1.8- and 2.7-fold increased in the ΔaldR mutant grown in the presence and absence of alanine, respectively, compared to that in the wild-type strain grown under the same conditions. This result clearly indicates that AldR serves as a repressor for the regulation of its own gene. Most Lrp/AsnC family regulators are known to negatively regulate the expression of their own genes independently of effector molecules, thereby keeping their cellular levels in a steady state (37–47).
FIG 9.
Expression of the aldR gene in WT and ΔaldR mutant strains of M. smegmatis. M. smegmatis WT and ΔaldR strains containing the aldR::lacZ transcriptional fusion plasmid (pALDRLACZ) were grown in the presence (+Ala) and absence (−Ala) of l-alanine, as described in the legend to Fig. 4. All values provided are the averages of the results from three independent determinations. Error bars indicate the standard deviations.
DISCUSSION
Quaternary structure of AldR in the presence of alanine.
The basic assembly and DNA-binding unit of AldR was proposed to be the homodimer, as in other members of the Lrp/AsnC family (14, 16, 18–23, 25, 26). Purified AldR exists as a homodimer in amino-acid-free solution and assembles into a higher-order structure in the presence of alanine (14). All the Lrp/AsnC family regulators whose three-dimensional structures were determined by crystallography (Lrp from E. coli, AsnC from E. coli, LrpC from Bacillus subtilis, LrpA from M. tuberculosis, Grp from Sulfolobus tokodaii, LrpA from Pyrococcus furiosus, and FL11 from Pyrococcus sp. OT3) have ringlike structures of a homooctamer (tetramer of dimers) (16, 18–27), which strongly implies that AldR also has a quaternary structure of a homooctamer in the presence of alanine. Our TEM images of purified AldR in the presence of alanine showed negatively stained particles with a horseshoe shape. The size and shape of AldR oligomers are in accord with the open quaternary structures of the arginine-bound form of FL11, E. coli Lrp cocrystallized with DNA, and the Gly102Thr mutant form of M. tuberculosis LrpA (20, 23, 26, 27), indicating that the AldR octamer likely adopts an open-ring structure. It was previously reported that FL11 dimers assemble into closed- and open-ring octamers in the presence of lysine and arginine, respectively. The molecular mass of the open-ring octamer form of FL11 was determined by means of MALS (multiangle light scattering) analysis to be 120 kDa, corresponding to the homohexameric molecular mass, while that of the closed-ring octamer was 160 kDa (26). The particle (molecular) shape is known to affect the velocity of migration through resins in gel filtration chromatography. This implies that the molecular mass of alanine-bound AldR determined by gel filtration chromatography might be underestimated due to its open-ring conformation. On the basis of previous findings together with the TEM images presented here, we suggest that the quaternary structure of AldR in the presence of alanine is an open-ring homooctamer.
Optimal binding of AldR to the ald control region requires three AldR-binding sites that have proper arrangement and phasing with one another.
The first step to reveal the mechanism of ald regulation was to identify cis-regulatory elements in the upstream region of ald. According to previously reported footprinting results, a long stretch of DNA was generally protected by Lrp/AsnC family regulators (48–61), which indicates the presence of multiple binding sites on DNA. Likewise, four AldR-binding sites (O2, O1, O4, and O3) with a consensus sequence of GA/T-N2-WWN/NWW-N2-T/AC were identified upstream of the ald gene by sequence analysis in conjunction with DNase I footprinting analysis and EMSAs. The consensus sequence of the AldR-binding sites is similar to those (GA-N2-WWW-N2-TC) of FL11 from Pyrococcus sp. OT3, Lrp from E. coli, and MdeR from Pseudomonas putida (22, 26, 62). Both the dimeric dyad (GA/T-N7-T/AC) and WW of three central nucleotides were previously demonstrated to be important for AldR binding (14).
The centers of the four sites are separated by integral numbers (3 or 4) of helical turns (O2-4ht-O1-3ht-O4-3ht-O3, where ht stands for helical turns), indicating that all the AldR-binding sites are centered on the same face of the DNA helix. In this study, several lines of evidence that indicate that a molecule of the AldR octamer simultaneously binds to three AldR-binding sites were obtained. First, EMSA analysis revealed that the binding affinity of AldR octamers for DNA fragments containing four AldR-binding sites (O2, O1, O4, and O3) was similar to that for DNA fragments with three consecutive AldR-binding sites (O2, O1, and O4 as well as O1, O4, and O3) (Fig. 6A). If the AldR octamer binds to four AldR-binding sites using its four DNA-binding domains, the binding affinity of AldR for the DNA fragments containing the four AldR-binding sites may well be higher than that for the DNA fragments with only three consecutive AldR-binding sites. Because four DNA-binding domains of the AldR octamer face out on the periphery of the ringlike structure, simultaneous binding of the AldR octamer to the four binding sites might lead to nearly complete DNA wrapping around the AldR octamer, causing intramolecular steric interference of the wrapping DNA. Second, EMSA also revealed that only two AldR-binding sites separated by three or four helical turns are not sufficient for binding of the AldR octamer, which was supported in vivo by the observation that both O2 and O3 mutations rendered the regulation pattern of ald expression similar to that in the ΔaldR mutant of M. smegmatis (Fig. 4). Third, the O3 site, which overlaps the ald promoter, should not be occupied by AldR for the induction of ald expression in the presence of alanine.
It was predicted that the two adjacent dimers of the Lrp octamer with a rotational angle of 90° optimally bind to two target DNA sites that are separated by three helical turns between their centers (20, 27). Our EMSA results showed that the strong binding of the AldR octamer to DNA requires two AldR-binding sites separated by three helical turns (31 bp) and an additional AldR-binding site that is positioned apart from one of the two AldR-binding sites by three or more helical turns between their centers (Fig. 6). This implies that two AldR-binding sites separated by three helical turns serve as anchoring sites for binding of the AldR octamer, and the additional binding site, which is in phase with the other AldR-binding sites, appears to stabilize the AldR-DNA complex. Multiple binding sites for Lrp/AsnC family regulators with at least two of them being separated by three helical turns were found in the control regions of many of their target genes (e.g., serA, micF, ompC, ilvIH, papBA, gltBDF, daa, sfa, and clp regulated by E. coli Lrp; asnC regulated by E. coli AsnC; and putA regulated by Rhodobacter capsulatus PutR) (47–50, 53, 56, 57, 60–63). This allowed us to suggest a generalized arrangement pattern of cis-acting regulatory sites for Lrp/AsnC family regulators in the corresponding control regions: two binding sites separated by three helical turns and at least one additional binding site that is in the same phase with one of the two AldR-binding sites on the DNA helix. Multiple binding sites with an appropriate arrangement are required for the regulation of the genes that are under the control of Lrp/AsnC family regulators, probably due to both the low binding affinity of the regulators for a single binding site and the presence of theoretically too many binding sites on the chromosomal DNA. For example, the probability that a random 11-mer matches the AldR-binding motif (GA/T-N2-NWW/WWN-N2-A/TC) is 3/512 (1/4 × 1/2 × 24/64 × 1/2 × 1/4) if A, T, C, and G are equally present in the chromosome.
Promoter assays using ald::lacZ transcriptional fusions with mutations within AldR-binding sites showed that three consecutive AldR-binding sites (O2, O1, and O4) are necessary for the induction of ald expression by alanine (Fig. 4). This result and the requirement for three AldR-binding sites for AldR binding strongly suggest that a molecule of the AldR octamer simultaneously binds to O2, O1, and O4 to induce ald expression in the presence of alanine. In this case, the ald promoter overlapping O3 is free and therefore accessible to RNA polymerase. The AldR octamer bound upstream of the ald promoter between positions −43 and −126 (from O2 to O4) seems to promote the binding of RNA polymerase to the promoter, probably through protein-protein interactions. Transcriptional activators are known to generally bind upstream of their target promoters between positions −30 and −80 relative to the transcriptional start points of their target genes to enhance the binding of RNA polymerase to the promoters or the formation of an open complex (64, 65).
The mutation within O3 led to the derepression of ald even in the presence of alanine, with the induction effect of alanine on ald expression being retained. This finding indicates that there is competition between the O2, O1, and O4 and the O1, O4, and O3 binding sites for binding to the AldR octamer in the presence of alanine (Fig. 10). When an AldR octamer binds to the O1, O4, and O3 sites, ald expression is repressed. In contrast, binding of the AldR octamer to the O2, O1, and O4 sites, as mentioned above, induces ald expression. Our EMSA results demonstrated that the AldR octamer binds equally well to both the O2, O1, and O4 sites and the O1, O4, and O3 sites. This indirectly corroborates our suggestion that the AldR octamer adopts an open-ring structure rather than a closed-ring structure. If the AldR octamer has a closed-ring structure, all four AldR dimers are spatially arranged at the same rotational angle of 90° with the adjacent AldR dimers. In this case, the binding affinity of the AldR octamer for the O1, O4, and O3 sites is expected to be higher than that for the O2, O1, and O4 sites, since O1 and O4 as well as O4 and O3 are separated from each other by the same distance (three helical turns), and the simultaneous binding of two adjacent dimers of the AldR octamer to O2 and O1 separated by four helical turns might be less favorable due to some tension generated by DNA looping between the two AldR dimers. It was previously suggested that two adjacent dimers of E. coli Lrp with a rotational angle of 122.5° bind to two target DNA sites separated by four helical turns without causing DNA looping (20, 27). This suggestion together with our TEM image of AldR octamers provide a structural rationale for the equally efficient binding of the AldR octamer to the O2, O1, and O4 sites and the O1, O4, and O3 sites. When the AldR octamer with an open-ring conformation binds to the O2, O1, and O4 sites, the two AldR dimers (dimers A and D) (Fig. 10) neighboring the disrupted dimer interface likely bind to O2 and O1, and one of the remaining AldR dimers likely binds to O4.
FIG 10.

Model for the regulation of ald expression by AldR. The numbers between the two adjacent AldR-binding sites indicate the distances between their central T nucleotides in base pairs. The hypersensitive sites detected by DNase I footprinting are marked by numbered asterisks. The promoter region (−35 and −10) of ald overlaps the O3 site. The AldR monomers are represented by gray ovals, and the AldR-binding sites are depicted as black cylinders. RNAP, RNA polymerase.
Under conditions of repression of ald, i.e., in the absence of or at low concentrations of alanine, AldR exists as a homodimer (14). Our previous EMSA results (14) and the footprinting results presented here demonstrated that AldR dimers have a lower binding affinity for the AldR-binding sites than do AldR octamers. As judged by the derepression levels of ald in the absence of alanine, it is evident that, among the AldR-binding sites, O3 is most important for ald repression in the absence of alanine and that O1 and O4 are also required for full repression of ald (Fig. 4). Given that the AldR dimer binds to O3 to repress ald expression in the absence of alanine by occluding the access of RNA polymerase to the ald promoter, this finding indicates the cooperative binding of AldR dimers to O1, O4, and O3 that are spaced apart from the neighboring site(s) by three helical turns between their centers. In other words, the AldR dimer bound to O4 facilitates or stabilizes the binding of AldR dimers to O3 and O1 through protein-protein interactions. Likewise, the occupation of O1 by the AldR dimer has the same effect on the binding of AldR to O4, thereby exerting an indirect effect on the binding of AldR to O3. Protein-protein interactions between the AldR dimers bound to O1, O4, and O3 induce DNA bending between O1 and O4 as well as between O4 and O3, which was manifested by the AldR-induced hypersensitive sites (sites *2 and *3) observed by DNase I footprinting analysis in the absence of alanine (Fig. 2A). Since the O2 mutation did not lead to derepression of ald in the absence of alanine, the AldR dimer bound to O2 appears not to be involved in the cooperative binding of AldR, which might be attributable to the longer interval (four helical turns) between O2 and O1. Maximal cooperative binding of LrpB dimers of Sulfolobus solfataricus was previously suggested to require three binding sites that are aligned with a periodicity of three helical turns, like the O1, O4, and O3 binding sites in the ald control region (66).
Model for positive and negative regulation of ald expression by the AldR transcription factor in M. smegmatis and M. tuberculosis.
Based on the results presented here, we propose a model explaining the regulation of ald expression in response to alanine availability (Fig. 10). In the presence of alanine, an AldR octamer with an open-ring conformation binds to either the O2, O1, and O4 sites or the O1, O4, and O3 sites. Binding of the AldR octamer to the O2, O1, and O4 sites leads to the activation of ald expression by recruiting RNA polymerase to the ald promoter, while binding of the AldR octamer to the O1, O4, and O3 sites results in the repression of ald expression. When these activation and repression effects are added up, the outcome is the induction of ald expression in the presence of alanine. In the absence of alanine, AldR dimers bind to O2, O1, O4, and O3 sites, and the occupation of O3 by the AldR dimer blocks the access of RNA polymerase to the ald promoter, thereby repressing ald expression. Binding of AldR dimers to O1, O3, and O4 sites is cooperative through protein interactions between them, which enables full repression of ald expression in the absence of alanine.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A2004243).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00453-15.
REFERENCES
- 1.Chen JM, Alexander DC, Behr MA, Liu J. 2003. Mycobacterium bovis BCG vaccines exhibit defects in alanine and serine catabolism. Infect Immun 71:708–716. doi: 10.1128/IAI.71.2.708-716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Z, Caceres NE, Sarath G, Barletta RG. 2002. Mycobacterium smegmatis l-alanine dehydrogenase (Ald) is required for proficient utilization of alanine as a sole nitrogen source and sustained anaerobic growth. J Bacteriol 184:5001–5010. doi: 10.1128/JB.184.18.5001-5010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giffin MM, Modesti L, Raab RW, Wayne LG, Sohaskey CD. 2012. ald of Mycobacterium tuberculosis encodes both the alanine dehydrogenase and the putative glycine dehydrogenase. J Bacteriol 194:1045–1054. doi: 10.1128/JB.05914-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutter B, Dick T. 1998. Increased alanine dehydrogenase activity during dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett 167:7–11. doi: 10.1111/j.1574-6968.1998.tb13200.x. [DOI] [PubMed] [Google Scholar]
- 5.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 6.Agren D, Stehr M, Berthold CL, Kapoor S, Oehlmann W, Singh M, Schneider G. 2008. Three-dimensional structures of apo- and holo-L-alanine dehydrogenase from Mycobacterium tuberculosis reveal conformational changes upon coenzyme binding. J Mol Biol 377:1161–1173. doi: 10.1016/j.jmb.2008.01.091. [DOI] [PubMed] [Google Scholar]
- 7.Tripathi SM, Ramachandran R. 2008. Crystal structures of the Mycobacterium tuberculosis secretory antigen alanine dehydrogenase (Rv2780) in apo and ternary complex forms captures “open” and “closed” enzyme conformations. Proteins 72:1089–1095. doi: 10.1002/prot.22101. [DOI] [PubMed] [Google Scholar]
- 8.Andersen AB, Andersen P, Ljungqvist L. 1992. Structure and function of a 40,000-molecular-weight protein antigen of Mycobacterium tuberculosis. Infect Immun 60:2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raynaud C, Etienne C, Peyron P, Laneelle MA, Daffe M. 1998. Extracellular enzyme activities potentially involved in the pathogenicity of Mycobacterium tuberculosis. Microbiology 144:577–587. doi: 10.1099/00221287-144-2-577. [DOI] [PubMed] [Google Scholar]
- 10.Usha V, Jayaraman R, Toro JC, Hoffner SE, Das KS. 2002. Glycine and alanine dehydrogenase activities are catalyzed by the same protein in Mycobacterium smegmatis: upregulation of both activities under microaerophilic adaptation. Can J Microbiol 48:7–13. doi: 10.1139/w01-126. [DOI] [PubMed] [Google Scholar]
- 11.Starck J, Kallenius G, Marklund BI, Andersson DI, Akerlund T. 2004. Comparative proteome analysis of Mycobacterium tuberculosis grown under aerobic and anaerobic conditions. Microbiology 150:3821–3829. doi: 10.1099/mic.0.27284-0. [DOI] [PubMed] [Google Scholar]
- 12.Rosenkrands I, Slayden RA, Crawford J, Aagaard C, Barry CE III, Andersen P. 2002. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J Bacteriol 184:3485–3491. doi: 10.1128/JB.184.13.3485-3491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voskuil MI, Visconti KC, Schoolnik GK. 2004. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis 84:218–227. doi: 10.1016/j.tube.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Jeong JA, Baek EY, Kim SW, Choi JS, Oh JI. 2013. Regulation of the ald gene encoding alanine dehydrogenase by AldR in Mycobacterium smegmatis. J Bacteriol 195:3610–3620. doi: 10.1128/JB.00482-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan K, Knaak T, Satkamp L, Humbert O, Falkow S, Ramakrishnan L. 2002. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc Natl Acad Sci U S A 99:3920–3925. doi: 10.1073/pnas.002024599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard PM, Smits SH, Sedelnikova SE, Brinkman AB, de Vos WM, van der Oost J, Rice DW, Rafferty JB. 2001. Crystal structure of the Lrp-like transcriptional regulator from the archaeon Pyrococcus furiosus. EMBO J 20:990–997. doi: 10.1093/emboj/20.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Rosner MH, Calvo JM. 2001. Leucine-regulated self-association of leucine-responsive regulatory protein (Lrp) from Escherichia coli. J Mol Biol 312:625–635. doi: 10.1006/jmbi.2001.4955. [DOI] [PubMed] [Google Scholar]
- 18.Reddy MC, Gokulan K, Jacobs WR Jr, Ioerger TR, Sacchettini JC. 2008. Crystal structure of Mycobacterium tuberculosis LrpA, a leucine-responsive global regulator associated with starvation response. Protein Sci 17:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thaw P, Sedelnikova SE, Muranova T, Wiese S, Ayora S, Alonso JC, Brinkman AB, Akerboom J, van der Oost J, Rafferty JB. 2006. Structural insight into gene transcriptional regulation and effector binding by the Lrp/AsnC family. Nucleic Acids Res 34:1439–1449. doi: 10.1093/nar/gkl009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de los Rios S, Perona JJ. 2007. Structure of the Escherichia coli leucine-responsive regulatory protein Lrp reveals a novel octameric assembly. J Mol Biol 366:1589–1602. doi: 10.1016/j.jmb.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrivastava T, Ramachandran R. 2007. Mechanistic insights from the crystal structures of a feast/famine regulatory protein from Mycobacterium tuberculosis H37Rv. Nucleic Acids Res 35:7324–7335. doi: 10.1093/nar/gkm850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koike H, Ishijima SA, Clowney L, Suzuki M. 2004. The archaeal feast/famine regulatory protein: potential roles of its assembly forms for regulating transcription. Proc Natl Acad Sci U S A 101:2840–2845. doi: 10.1073/pnas.0400109101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada M, Ishijima SA, Suzuki M. 2009. Interactions between the archaeal transcription repressor FL11 and its coregulators lysine and arginine. Proteins 74:520–525. doi: 10.1002/prot.22269. [DOI] [PubMed] [Google Scholar]
- 24.Okamura H, Yokoyama K, Koike H, Yamada M, Shimowasa A, Kabasawa M, Kawashima T, Suzuki M. 2007. A structural code for discriminating between transcription signals revealed by the feast/famine regulatory protein DM1 in complex with ligands. Structure 15:1325–1338. doi: 10.1016/j.str.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Kumarevel T, Nakano N, Ponnuraj K, Gopinath SC, Sakamoto K, Shinkai A, Kumar PK, Yokoyama S. 2008. Crystal structure of glutamine receptor protein from Sulfolobus tokodaii strain 7 in complex with its effector L-glutamine: implications of effector binding in molecular association and DNA binding. Nucleic Acids Res 36:4808–4820. doi: 10.1093/nar/gkn456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama K, Ishijima SA, Koike H, Kurihara C, Shimowasa A, Kabasawa M, Kawashima T, Suzuki M. 2007. Feast/famine regulation by transcription factor FL11 for the survival of the hyperthermophilic archaeon Pyrococcus OT3. Structure 15:1542–1554. doi: 10.1016/j.str.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Shrivastava T, Dey A, Ramachandran R. 2009. Ligand-induced structural transitions, mutational analysis, and ‘open’ quaternary structure of the M. tuberculosis feast/famine regulatory protein (Rv3291c). J Mol Biol 392:1007–1019. doi: 10.1016/j.jmb.2009.07.084. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Calvo JM. 2002. Leucine-induced dissociation of Escherichia coli Lrp hexadecamers to octamers. J Mol Biol 318:1031–1042. doi: 10.1016/S0022-2836(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Green MR. 2012. Molecular cloning: a laboratory manual, 4th ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 30.Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim MJ, Park KJ, Ko IJ, Kim YM, Oh JI. 2010. Different roles of DosS and DosT in the hypoxic adaptation of mycobacteria. J Bacteriol 192:4868–4875. doi: 10.1128/JB.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuss AM, Glaeser J, Klug G. 2009. RpoH(II) activates oxidative-stress defense systems and is controlled by RpoE in the singlet oxygen-dependent response in Rhodobacter sphaeroides. J Bacteriol 191:220–230. doi: 10.1128/JB.00925-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh JI, Kaplan S. 1999. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry 38:2688–2696. doi: 10.1021/bi9825100. [DOI] [PubMed] [Google Scholar]
- 34.Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ. 2007. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Dey A, Ramachandran R. 2014. Cloning, overexpression, purification and preliminary X-ray analysis of a feast/famine regulatory protein (Rv2779c) from Mycobacterium tuberculosis H37Rv. Acta Crystallogr F Struct Biol Commun 70:97–100. doi: 10.1107/S2053230X13033128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendoza-Vargas A, Olvera L, Olvera M, Grande R, Vega-Alvarado L, Taboada B, Jimenez-Jacinto V, Salgado H, Juarez K, Contreras-Moreira B, Huerta AM, Collado-Vides J, Morett E. 2009. Genome-wide identification of transcription start sites, promoters and transcription factor binding sites in E. coli. PLoS One 4:e7526. doi: 10.1371/journal.pone.0007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell SD, Jackson SP. 2000. Mechanism of autoregulation by an archaeal transcriptional repressor. J Biol Chem 275:31624–31629. doi: 10.1074/jbc.M005422200. [DOI] [PubMed] [Google Scholar]
- 38.Brinkman AB, Dahlke I, Tuininga JE, Lammers T, Dumay V, de Heus E, Lebbink JHG, Thomm M, de Vos WM, van der Oost J. 2000. An Lrp-like transcriptional regulator from the archaeon Pyrococcus furiosus is negatively autoregulated. J Biol Chem 275:38160–38169. doi: 10.1074/jbc.M005916200. [DOI] [PubMed] [Google Scholar]
- 39.Jafri S, Evoy S, Cho KY, Craighead HG, Winans SC. 1999. An Lrp-type transcriptional regulator from Agrobacterium tumefaciens condenses more than 100 nucleotides of DNA into globular nucleoprotein complexes. J Mol Biol 288:811–824. doi: 10.1006/jmbi.1999.2715. [DOI] [PubMed] [Google Scholar]
- 40.Cho KY, Winans SC. 1996. The putA gene of Agrobacterium tumefaciens is transcriptionally activated in response to proline by an Lrp-like protein and is not autoregulated. Mol Microbiol 22:1025–1033. doi: 10.1046/j.1365-2958.1996.01524.x. [DOI] [PubMed] [Google Scholar]
- 41.Ernsting BR, Atkinson MR, Ninfa AJ, Matthews RG. 1992. Characterization of the regulon controlled by the leucine-responsive regulatory protein in Escherichia coli. J Bacteriol 174:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calvo JM, Matthews RG. 1994. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev 58:466–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madhusudhan KT, Huang N, Sokatch JR. 1995. Characterization of BkdR-DNA binding in the expression of the bkd operon of Pseudomonas putida. J Bacteriol 177:636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolling R, Lother H. 1985. AsnC: an autogenously regulated activator of asparagine synthetase A transcription in Escherichia coli. J Bacteriol 164:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brinkman AB, Ettema TJG, de Vos WM, van der Oost J. 2003. The Lrp family of transcriptional regulators. Mol Microbiol 48:287–294. doi: 10.1046/j.1365-2958.2003.03442.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Wu J, Friedberg D, Plakto J, Calvo JM. 1994. Regulation of the Escherichia coli lrp gene. J Bacteriol 176:1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keuntje B, Masepohl B, Klipp W. 1995. Expression of the putA gene encoding proline dehydrogenase from Rhodobacter capsulatus is independent of NtrC regulation but requires an Lrp-like activator protein. J Bacteriol 177:6432–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nou X, Braaten B, Kaltenbach L, Low DA. 1995. Differential binding of Lrp to two sets of pap DNA binding sites mediated by PapI regulates Pap phase variation in Escherichia coli. EMBO J 14:5785–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiese DE II, Ernsting BR, Blumenthal RM, Matthews RG. 1997. A nucleoprotein activation complex between the leucine-responsive regulatory protein and DNA upstream of the gltBDF operon in Escherichia coli. J Mol Biol 270:152–168. doi: 10.1006/jmbi.1997.1057. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Calvo JM. 1993. Lrp, a global regulatory protein of Escherichia coli, binds co-operatively to multiple sites and activates transcription of ilvIH. J Mol Biol 229:306–318. doi: 10.1006/jmbi.1993.1036. [DOI] [PubMed] [Google Scholar]
- 51.Peeters E, Thia-Toong TL, Gigot D, Maes D, Charlier D. 2004. Ss-LrpB, a novel Lrp-like regulator of Sulfolobus solfataricus P2, binds cooperatively to three conserved targets in its own control region. Mol Microbiol 54:321–336. doi: 10.1111/j.1365-2958.2004.04274.x. [DOI] [PubMed] [Google Scholar]
- 52.Peeters E, Albers SV, Vassart A, Driessen AJ, Charlier D. 2009. Ss-LrpB, a transcriptional regulator from Sulfolobus solfataricus, regulates a gene cluster with a pyruvate ferredoxin oxidoreductase-encoding operon and permease genes. Mol Microbiol 71:972–988. doi: 10.1111/j.1365-2958.2008.06578.x. [DOI] [PubMed] [Google Scholar]
- 53.Ferrario M, Ernsting BR, Borst DW, Wiese DE II, Blumenthal RM, Matthews RG. 1995. The leucine-responsive regulatory protein of Escherichia coli negatively regulates transcription of ompC and micF and positively regulates translation of ompF. J Bacteriol 177:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gazeau M, Delort F, Fromant M, Dessen P, Blanquet S, Plateau P. 1994. Structure-function relationship of the Lrp-binding region upstream of lysU in Escherichia coli. J Mol Biol 241:378–389. doi: 10.1006/jmbi.1994.1514. [DOI] [PubMed] [Google Scholar]
- 55.Zhi J, Mathew E, Freundlich M. 1999. Lrp binds to two regions in the dadAX promoter region of Escherichia coli to repress and activate transcription directly. Mol Microbiol 32:29–40. doi: 10.1046/j.1365-2958.1999.01314.x. [DOI] [PubMed] [Google Scholar]
- 56.Marasco R, Varcamonti M, La Cara F, Ricca E, De Felice M, Sacco M. 1994. In vivo footprinting analysis of Lrp binding to the ilvIH promoter region of Escherichia coli. J Bacteriol 176:5197–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nou X, Skinner B, Braaten B, Blyn L, Hirsch D, Low D. 1993. Regulation of pyelonephritis-associated pili phase-variation in Escherichia coli: binding of the PapI and the Lrp regulatory proteins is controlled by DNA methylation. Mol Microbiol 7:545–553. doi: 10.1111/j.1365-2958.1993.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 58.Roesch PL, Blomfield IC. 1998. Leucine alters the interaction of the leucine-responsive regulatory protein (Lrp) with the fim switch to stimulate site-specific recombination in Escherichia coli. Mol Microbiol 27:751–761. doi: 10.1046/j.1365-2958.1998.00720.x. [DOI] [PubMed] [Google Scholar]
- 59.Rhee KY, Parekh BS, Hatfield GW. 1996. Leucine-responsive regulatory protein-DNA interactions in the leader region of the ilvGMEDA operon of Escherichia coli. J Biol Chem 271:26499–26507. doi: 10.1074/jbc.271.43.26499. [DOI] [PubMed] [Google Scholar]
- 60.Graveline R, Garneau P, Martin C, Mourez M, Hancock MA, Lavoie R, Harel J. 2014. Leucine-responsive regulatory protein Lrp and PapI homologues influence phase variation of CS31A fimbriae. J Bacteriol 196:2944–2953. doi: 10.1128/JB.01622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Woude MW, Low DA. 1994. Leucine-responsive regulatory protein and deoxyadenosine methylase control the phase variation and expression of the sfa and daa pili operons in Escherichia coli. Mol Microbiol 11:605–618. doi: 10.1111/j.1365-2958.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki M. 2003. The DNA-binding specificity of eubacterial and archaeal FFRPs. Proc Jpn Acad 79B:213–222. doi: 10.2183/pjab.79B.213. [DOI] [Google Scholar]
- 63.Yang L, Lin RT, Newman EB. 2002. Structure of the Lrp-regulated serA promoter of Escherichia coli K-12. Mol Microbiol 43:323–333. doi: 10.1046/j.1365-2958.2002.02744.x. [DOI] [PubMed] [Google Scholar]
- 64.Raibaud O, Schwartz M. 1984. Positive control of transcription initiation in bacteria. Annu Rev Genet 18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- 65.Collado-Vides J, Magasanik B, Gralla JD. 1991. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev 55:371–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peeters E, van Oeffelen L, Nadal M, Forterre P, Charlier D. 2013. A thermodynamic model of the cooperative interaction between the archaeal transcription factor Ss-LrpB and its tripartite operator DNA. Gene 524:330–340. doi: 10.1016/j.gene.2013.03.118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.