Abstract
The purpose of medicines is to improve patients' lives. Stakeholders involved in the development and lifecycle management of medicines agree that more effective patient involvement is needed to ensure that patient needs and priorities are identified and met. Despite the increasing number and scope of patient involvement initiatives, there is no accepted master framework for systematic patient involvement in industry-led medicines research and development, regulatory review, or market access decisions. Patient engagement is very productive in some indications, but inconsistent and fragmentary on a broader level. This often results in inefficient drug development, increasing evidence requirements, lack of patient-centered outcomes that address unmet medical needs and facilitate adherence, and consequently, lack of required therapeutic options and high costs to society and involved parties. Improved patient involvement can drive the development of innovative medicines that deliver more relevant and impactful patient outcomes and make medicine development faster, more efficient, and more productive. It can lead to better prioritization of early research; improved resource allocation; improved trial protocol designs that better reflect patient needs; and, by addressing potential barriers to patient participation, enhanced recruitment and retention. It may also improve trial conduct and lead to more focused, economically viable clinical trials. At launch and beyond, systematic patient involvement can also improve the ongoing benefit-risk assessment, ensure that public funds prioritize medicines of value to patients, and further the development of the medicine. Progress toward a universal framework for patient involvement requires a joint, precompetitive, and international approach by all stakeholders, working in true partnership to consolidate outputs from existing initiatives, identify gaps, and develop a comprehensive framework. It is essential that all stakeholders participate to drive adoption and implementation of the framework and to ensure that patients and their needs are embedded at the heart of medicines development and lifecycle management.
Keywords: patient involvement, medicines development
Introduction: Problem Statement
Drug development times are around 10 to 15 years1,2 and costs to bring a single new therapy to market are substantial.1–3 From the industry perspective, not putting the unmet medical needs of patients first, early in the development process, can lead to wrong priorities, wrong decisions on research design, and potentially costly late-stage failure. The complexity of clinical trials may lead to long and difficult experiences for patients4,5 and recruitment into clinical trials is ever more competitive and increasingly problematic.6 Many trials fail to achieve recruitment targets because they may be too restrictive in terms of exclusion/inclusion criteria, may impose an unfeasibly heavy burden of visits and tests on the participant, or may lack essential elements such as crossover or adaptive design, causing patients either not to enroll or to abandon a trial. Clinical or contract research organizations tasked with operational aspects of clinical trials are generally isolated from patients and patients’ needs. Furthermore, trials may include comparator or placebo groups or outcome measures that may not adequately reflect patient priorities.7–9
In every industry, product development begins with a clear understanding of the needs of the end user and aims to provide solutions that meet that need: the same should be true for medicines development. Although the purpose of medicines is to improve patients’ lives and to provide more effective health care, current patient involvement during medicines development and lifecycle management is fragmentary at best, and mostly confined to post-launch or late-stage clinical development. Without a clearly defined, timely, and methodological process, patient involvement will continue to be inconsistent and suboptimal.
Patients and biopharmaceutical companies should forge working collaborations that secure structured and integrated patient involvement at all phases of the medicines lifecycle. For this to happen in the real world, the value and benefits of patient involvement—and conversely, the consequences of failing to involve patients—need to be clear. This clarity, alongside evidence of the positive impact of patient involvement, will be a powerful driver for improvement.
Hypothesis: Routine Involvement of Patients During the Development and Lifecycle of Medicines Will Lead to Better Outcomes
Patients and their representatives can give valuable insights over the entire medicines development pathway—from preclinical laboratory-based studies to launch, and beyond launch to ultimate withdrawal from the market—for as long as that medicine is available to patients. Examples are in research scoping, study designs, recruitment, safety monitoring, understanding, and dissemination of research results (including lay summaries for nonexperts) and in describing their experiences with the use of medicines in settings outside of clinical trials.
Medicines are developed to improve patients’ lives and patients know best what makes a meaningful difference to them. Patients have a role to play alongside all other stakeholders in determining intended outcomes and priorities, acceptable uncertainty, as well as benefit/risk and value of a medicine. Their recommendations and conclusions may be different from those of regulators, payers, academic researchers, other health care professionals (HCPs), and industry,10 making it even more important that these opinions are well understood by all those making decisions.
Improved patient involvement will inspire and drive the development of innovative medicines that deliver more relevant and impactful patient outcomes. Trials and protocols will be designed to better reflect patient requirements and conducted with greater consideration of patient circumstances, allowing more patients to participate and potentially benefit from these therapies while they are still being evaluated. It also means that medicines entering the market are better able to address the actual health needs of patients for whom there may be inadequate or no specific treatments available.
Improved patient involvement has the potential to make medicine development faster, more efficient, and more productive. It can facilitate improved coordination of the process, prevent duplication of effort and inefficient resource use, and inform the wider health policy decision-making process. Although only few studies have attempted to measure the impact of patient involvement, alongside anecdotal reports, there is evidence in the literature to support these claims.11–13
Serving patients requires a deep understanding of their medical condition, especially in terms of the challenges they face in everyday living, their goals, disease symptoms and side effects of therapies, and unmet needs in terms of therapy and quality of life. These insights can be gained only through direct and constructive interactions with patients. Once the needs are clearly understood, all stakeholders—including industry, regulators, patients, patient associations and advocacy groups, purchasers of medicines (including pharmacies and hospitals), HCPs including academic and community-based researchers, physicians and nurses, politicians and legal advisors, health technology assessment (HTA) agencies, and topic-related think-tanks—can work together to develop practical and implementable solutions and achieve more meaningful outcomes.
A Common Understanding Starts With a Common Language
Selection of the term patient involvement rather than patient empowerment or patient engagement is deliberate and intentionally captures the central role that patients should play in medicines development and lifecycle management. Involvement reflects the need for patients to be active participants—valued and valuable partners—whose input, advice, and guidance is sought and implemented throughout the process. Today, a lot of different terms are used, and often the same terms are used while being differently defined or intended. This adds to and maintains the confusion. Thus, a clear definition of what is meant by patient and involvement, along with identification of key stakeholders, is critical to achieve a common understanding. First, the definition of patient needs to be wide in order to capture all relevant populations who can provide valuable insights through different lenses (Box 1). It should also be recognized that, as well as having keen insight and a different perspective, caregivers may sometimes be trying to lead 2 lives—their own and that dedicated to the patient. Second, involvement should not stop with consultation but should proactively embed patients and patient needs at the heart of the development and lifecycle of medicines. Patients’ views and opinions should be clearly sought and valued as an integral and essential part of the process, with the development of strategies and practical tools that facilitate genuine patient involvement.
Box 1. Definition of patient
Those having or at risk of having the medical condition(s) whether or not they currently receive medicines or vaccines to prevent or treat a disease—the traditional definition of a patient.
The family and those caring for those with the medical condition(s)—all of these people are in fact living with the disease.
There is already a substantial and growing number of organizations and initiatives aiming to improve patient and public involvement. This is evidence of the increasing recognition of patient involvement as a shared priority, and many valuable contributions toward this common goal are being made. However, there is as yet no consistent approach or methodology. A master framework that identifies specific stages in the development and lifecycle of medicines for patient involvement, clearly defines the scale of this involvement, and is agreed on by all stakeholders is essential and currently lacking. Present initiatives are engaging patients in discrete sections of the medicines development pathway, and many of the elements and enablers for successful patient involvement already exist. The need now is to develop a master framework that unites these sections and closes gaps in the pathway, providing much-needed guidance for productive and consistent patient involvement.
Enablers and Examples of Patient Involvement
Health Literacy
Health literacy is defined as the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions, and it can affect people of all ages, races, incomes, and education.14,15 It is universally accepted that health literacy is a critical enabler to engage and involve patients in their health care and the health of those who they care for. There are many initiatives under way that are enabled by health literacy concepts—which would better engage patients in medicines development—including provision of patient-focused materials at each stage of the medicines lifecycle. Examples include improvement of informed consent, return of results, and patient information guides or leaflets (Box 2).
Box 2. Examples of patient-focused materials
Informed Consent: The Clinical Trials Transformation Initiative’s (CTTI’s) Informed Consent Project aims to create and pilot a more effective process, including appropriate materials, for ensuring research participants’ understanding of critical informed consent elements, taking into account variability among research settings and participants (http://www.ctti-clinicaltrials.org/what-we-do/study-start-up/informed-consent).
Return of Results: The Multi-Regional Clinical Trials Center (MRCT) at Harvard University Return of Results Initiative aims to develop standards and best practices in returning clinical trial results to study participants. The aim is to create a guidance document, including templates, and to address perceived barriers to widespread implementation (http://mrct.globalhealth.harvard.edu/return-results).
Patient Information Guides and Leaflets: Patients are often presented with an overwhelming amount of information that is distributed in an uncoordinated and inconsistent manner. In response to this the FDA, the Engelberg Center for Health Care Reform, and other stakeholders have been developing a single, standardized Patient Medication Information (PMI) document which is now at the implementation stage (http://www.brookings.edu/events/2014/07/01-patient-medication-information-prescription-phrma-fda).
Expertise and Skills of Patient Advocacy Groups— Access to Information for Every Patient
The Internet and digital media resources enable patients to access almost unlimited information, to exchange experiences, and to form opinions. As a result, the individual knowledge of patients about their disease, related treatment options, and ongoing research has grown exponentially.16,17 Portals such as www.patientslikeme.com allow an exchange of disease and treatment experiences among patients and even offer tracking opportunities of the individual’s health parameters. Facebook, Internet forums, and other social media platforms are used by individuals and patient organizations to distribute information instantly.18 Global networks of patient organizations have formed to collaborate with HCPs and, in some cases, industry and to provide up-to-date information to the patient community, independent of borders and languages. Industry also provides response to specific requests from patients through their medical information departments. The informed, empowered patient is becoming the norm.
EUPATI—Enabling and Educating Patients to Give Meaningful Input Into Drug Development
Meaningful patient input into drug development and evaluation requires not just information but specific knowledge—all stakeholders are asked to contribute toward this goal. A good example is the European Patients’ Academy on Therapeutic Innovation (EUPATI), a collaborative public-private partnership project of 30 organizations that is funded by the Innovative Medicines Initiative. It was formed to increase the number and capabilities of patients and related organizations to advise on drug development. The first “class” of 50 patients will “graduate” from the EUPATI Patient Experts Training Course in November 2015. The EUPATI will also develop an Internet-based toolbox for patient advocates and a public Internet library covering all aspects of preclinical development, clinical trials, regulatory affairs, pharmacovigilance, benefit-risk assessment, and HTA in lay language.19
Clinical Trials Transformation Initiative— Enhancing Patient Involvement in Clinical Trials
The Clinical Trials Transformation Initiative (CTTI) was established by Duke University and the FDA as a public-private partnership in 2007 and brings together more than 70 organizations including academic research organizations, patient groups, industry, government, institutional review boards, and investigators. Its aim is to improve the clinical trials enterprise through identifying and promoting practices that will increase the quality and efficiency of clinical trials and, consequently, enable reliable and timely access to evidence-based prevention and treatment options. The CTTI’s Patient Leadership Council (PLC), launched in January 2013, brought together 15 patient thought leaders representing a variety of organizations engaged in clinical trials across diverse indications. The PLC initiated the CTTI’s Patient Groups and Clinical Trials project, which aims to formulate recommendations and tools that establish and support best practices for effective engagement between research sponsors and patient groups around clinical trials. The PLC also focused on delivery of presentations and events highlighting innovative programs and approaches by patient groups to overcome barriers in clinical trials. Following the success of partnership programs, PLC members have been integrated into the CTTI’s Steering Committee (as of January 2015) and representatives of the patient community now have leadership responsibilities and representation equal to all other CTTI stakeholders. A key learning from the PLC has been that the patient community must be equal partners in every aspect of the clinical trial enterprise in order to improve the quality and efficiency of clinical trials.
PCORI—Facilitating Informed Health Decision Making
Patients have unique perspectives that can change and improve health care research by potentially enhancing relevance of outcomes to actual health decisions, driving more rapid uptake of research into practice, and improving the likelihood that patients will achieve the health outcomes they desire.20 The Patient-Centered Outcomes Research Institute (PCORI) is a nonprofit, nongovernmental organization that aims to improve the quality and relevance of evidence available to help patients, caregivers, clinicians, employers, insurers, and policy makers make informed health decisions. The organization funds comparative clinical effectiveness research and supports work that will improve the methods used to conduct such studies.
Patients are increasingly well-organized and patient organizations offer many of the skills and capabilities needed for successful drug development both on a disease-specific level and for overarching topics. Their expertise and influence will lead to more significant patient involvement in the future on health policy, quality of care, the research agenda, and reimbursement decisions. The impact that patient organizations can have is well illustrated by advocacy groups focusing on a specific disease (Box 3), on a series of linked or similar diseases, or on universal health policy applicable to all diseases (Box 4). While CFF and PDF are disease specific, there has also been an emergence of competent and passionate patient organizations that cover whole ranges of conditions (Box 4).
Box 3. Examples of disease-specific knowledge and influence of patient organizations21,22
An early example of powerful patient involvement in gaining access to much needed therapies is HIV. In the 1980s HIV-infected patient advocacy groups caused a re-assessment of how much evidence is needed to gain access to potentially life-saving therapies; their tolerance for uncertainty and risk also led to a complete change of the licensing approach of promising medicines for people with HIV [21].
Cystic Fibrosis Foundation (CFF) expertise and influence spans the entire life cycle of drug development and commercialization. Among others, they offer drug discovery and development collaboration capabilities, as well as a network for clinical research and care. In 2012, fundraising revenue amounted to US$ 134 million while royalties amounted to US$ 156 million. In addition, CFF’s US$ 75 million investment into Vertex’ Kalydeco contributed to its approval in 2012 [22].
The Parkinson’s Disease Foundation (PDF) Parkinson’s Advocates in Research (PAIR) program which aims to drive development of better treatments at a faster pace by ensuring that people with Parkinson’s and care partners are primary partners in research alongside scientists, industry and government. The cornerstone of the PAIR program is a national network of more than 200 Research Advocates who complete a Learning Institute, during which they are trained by leading experts from the field about the science of Parkinson’s and the development of new treatments. Research Advocates serve as FDA patient advisors, are members of IRBs and Data Safety Monitoring Boards, advise investigators on study design and protocol and educate their peers about the importance of study participation.
Box 4. Examples of overarching patient organizations and involvement
The European Patients Forum (EPF), the European Organisation for Rare Diseases (EURORDIS) and the European AIDS Treatment Group (EATG) have been active in promoting a patient-centred philosophy and agenda within EU institutions. In the US, the National Organization for Rare Disorders (NORD) is also driving greater patient involvement.
The US National Health Council (NHC) brings together all segments of the health community to provide a united voice for the more than 133 million people with chronic diseases and disabilities and their family caregivers (NHC).
Both in Europe and the US, multistakeholder patient advocacy organizations have accumulated a huge amount of knowledge, and their expertise and influence at the systems level will lead to further evolution.
Patient & Public Involvement in Research
The principle of patient and public involvement has been embraced by many academic and governmental stakeholders with the intent to develop treatments that better meet people’s needs. Educated patient input into research planning, clinical study design, conduct, interpretation, and dissemination is expected to lead to outcomes more relevant to patients and to higher health impact to the broader population of patients. Patient and public involvement has been implemented in Europe, the United States, Canada, and Australia. For example, in the UK, the National Institute for Health Research (NIHR) is part of the government’s strategy, “Best research for best health.” The NIHR wants patients and the public to be involved in all stages of research and, together with its partners—the UK Clinical Research Collaboration and Involve—has put structures in place to achieve and facilitate this.23 A US organization that is aiming at a multistakeholder approach to change the system is FasterCures.24 Their goal is “to save lives by speeding up and improving the medical research system.” They realize that meaningful patient involvement with all stakeholders is key to achieving this ambition.
Regulators Inviting Patient Input
In both the US and Europe, a range of schemes to facilitate patient involvement in the regulatory process has been established. In the US, the Prescription Drug User Fee Act (PDUFA) aims to expedite the drug approval process and enhance patient involvement in drug development. The FDA’s Patient Focused Drug Development initiative is a commitment under the current PDUFA V to obtain patients’ input on specific disease areas as well as their conditions, impact on daily life, and available therapies. Examples of diseases explored so far include hemophilia, lung cancer, and HIV, and at least 20 public meetings will be held, each focused on a specific disease area.25 The FDA has recently requested input from stakeholders on strategies to obtain the views of patients during the medical product development process and ways to consider patients’ perspectives during regulatory discussions.26
Assessment of a product’s benefits and risks involves analysis of the severity of the condition alongside available treatment options and is a critical aspect of the FDA’s decision making as it establishes the context in which the regulatory decision is made. Based on the belief that a more systematic and comprehensive approach to obtaining the patient perspective on benefits and risk would improve the drug development and review process, the FDA has developed a structured framework for benefit-risk assessment in regulatory decision making for human drug and biologic products. PDUFA V also includes a commitment to implement this framework in the new drug approval process and a 5-year plan has been produced that describes the FDA’s approach for its further development and implementation.27 The plan will be refined and updated throughout PDUFA V, which runs until 2017, incorporating stakeholder feedback.
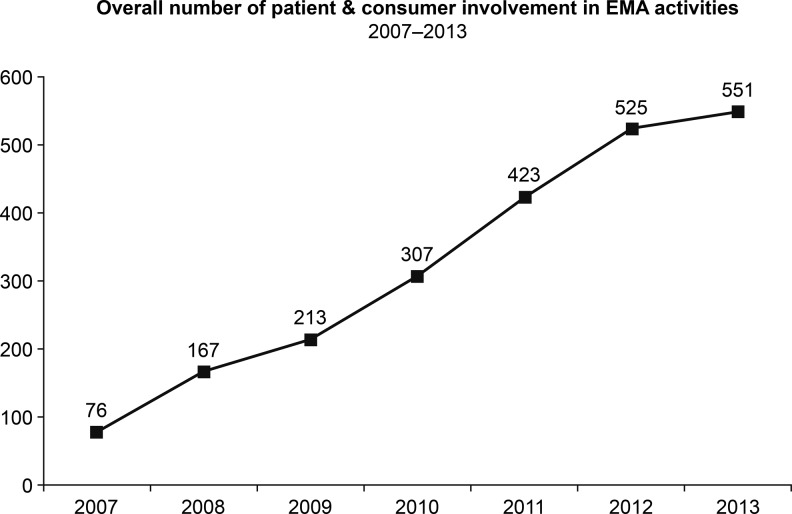
In Europe, the EMA also has multiple efforts ongoing to enhance patient involvement, including in many of its committees. In addition, the EMA’s Patients’ and Consumers’ Working Party representatives are involved in many EU-wide initiatives including the European Network of Paediatric Research, the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance, and the Pharmacoepidemiological Research on Outcomes of Therapeutics consortium.28 As a consequence of these efforts, the EMA’s interaction with patient and consumer organizations has shown substantial growth in recent years (Figure 1).
Figure 1.
Growth of EMA interactions with patients and consumer organizations between 2007 and 2013. Reproduced with permission from European Medicines Agency.29
The EMA has also developed and published terms of reference for the involvement of patients in benefit-risk discussion and evaluation within its scientific committees, its working parties, and scientific advisory groups.30 The guidance aims to ensure that involvement is consistent and efficient and provides advice on when patient involvement may be valuable, defines expectations from patient involvement in benefit/risk evaluation, and advises on appropriate processes for patient engagement and consultation. In September 2014, the EMA launched a pilot project to involve patients in the assessment of the benefits and risks of medicines in its Committee for Medicinal Products for Human Use (CHMP). Patients have been invited to present their views on medicines for which there is an unmet medical need and where the CHMP has concerns. Patients may also be invited to give their views in cases where the CHMP is considering whether to recommend the withdrawal, suspension, or revocation of a marketing authorization, or a restriction of indication of an authorized medicine.31
HTA Bodies and Payer Organizations
Health technology assessment agencies in several countries have also focused on improving patient involvement and are asking patients to engage at the time of reimbursement decisions for payer decision making. Current examples are listed (Table 1), although wide variation is seen between agencies on how patient engagement is conducted and how much impact it has on decisions. In addition, the overarching organization, Health Technology Assessment International, which has members from 59 countries, has an Interest Sub-Group for Patient and Citizen Involvement in HTA (PCISG). The PCISG aims to promote and develop methodologies to incorporate patients’ perspectives in HTAs, facilitate sharing of best practice in patient and citizen involvement in the HTA process, and provide support for countries with limited experience of patient and citizen engagement in HTA.32
Table 1.
Countries engaging with patients during reimbursement decisions for payer decision making.
| Australia | Pharmaceutical Benefits Advisory Committee |
| Canada | Canadian Agency for Drugs and Technologies in Health |
| England and Wales | National Institute for Health and Care Excellence |
| France | French National Authority for Health |
| Germany | Institute for Quality and Efficiency in Healthcare as well as Joint Federal Committee |
| New Zealand | Pharmaceutical Management Agency |
| Scotland | Scottish Medicines Consortium |
| Sweden | Dental and Pharmaceutical Benefits Agency |
| The Netherlands | National Health Care Institute (formerly College voor zorgverzekeringen, Health Care Insurance Board) |
| United States | Patient-Centered Outcomes Research Institute |
Legislation
The FDA’s Safety and Innovation Act, which reauthorized the PDUFA, incorporates legislation that aims to increase patient participation in medical product regulation. Section 1137 aims to gain patient views during the medical product development process and regulatory discussions, while section 907 evaluates the inclusion of demographic subgroups in clinical trials.33,34 The FDA has also developed guidance for industry on the collection of race and ethnicity data in clinical trials.35 An FDA report reviewing the collection, analysis, and availability of demographic subgroup data for FDA-approved medical products concluded that current statutes, regulations, and policies provide a solid framework for product sponsors in their applications on the inclusion and analysis of demographic subgroups and that generally sponsors incorporate demographic profiles and subset analyses in their applications.36 EMA Paediatric Regulation,37 which came into force in January 2007, has established patient representation at the Paediatric Committee. In addition, EMA legislation on pharmacovigilance, which came into effect in July 2012, saw the establishment of PRAC and a legal requirement for the engagement of patients and HCPs in the regulatory process, including direct consumer reporting of suspected adverse drug events.38
Potential Barriers to Patient Involvement
A number of reports have highlighted key issues in the involvement of patients in the health care process, many of which have also been identified in the setting of patient involvement in medicines development. Examples of these perceived risks and barriers are given (Table 2).31,39–43 Ongoing and planned patient involvement initiatives likely will identify additional barriers and seek solutions to overcome them.
Table 2.
Perceived risks and barriers to patient involvement.
| Education and training |
|
| Communication |
|
| Perceptions and cultural barriers |
|
| Evidence |
|
| Structure, support, and resources |
|
| Legal and regulatory |
|
The above examples of initiatives that aim to secure patient input demonstrate the substantial headway that is already being made. However, the focus is too often on the expected medical outcome, but a patient’s aspiration is the motivation to take the journey to get to the desired medical outcomes. From the patient perspective, the quality care trifecta includes not only the medical outcome but also the journey to reach that outcome and the individual’s personal aspirations—all 3 must be in balance (Box 5). By engaging with patients to capture and incorporate their wants and needs into the lifecycle of medicines, industry will be more effective in developing and providing treatments that help people on their journey to better health.
Box 5. Examples illustrating the need to balance medical outcome with the medical journey and individual aspirations
A father with diabetes and heart disease wants to follow his doctor’s orders to reach his desired medical outcome to be in better health, but his journey to reach that outcome is difficult to manage. He is a bus driver and the prescribed medications make him drowsy. This man’s aspiration is to make sure he can work to provide a better life for his children. It is only by prioritising this aspiration and altering the journey by finding a treatment that will allow him to keep working, that the patient can achieve the desired medical outcome.
A single mother with breast cancer who has a child diagnosed with autism and a parent with early signs of dementia struggles to keep her family together. Working part-time, she is emotionally underwater. Her life aspiration is unclear. She lives in a rural community eight hours away from an academic medical centre and relies on the local community health clinic and pharmacy chain for her care. Her journey to better health may involve social services to help her financially and to provide care for her parent. It is by identifying quality measures that matter to each unique patient that medical outcomes become achievable.
The Path to a Master Framework for Integrated and Systematic Patient Involvement
The ultimate goal is to ensure that medicines deliver more relevant and impactful patient outcomes by addressing unmet patient needs, and medicine development is faster, more efficient, and more productive through systematic patient involvement. This can be accomplished only through open dialogue on a peer-to-peer basis with patient representatives and when a rational, structured process for integrated patient involvement is developed and accepted by all stakeholders. Systemwide progress to achieve consistent patient involvement will require stakeholders to work together on a noncompetitive basis. The framework to deliver improved patient involvement will benefit all partners, thus fostering cooperation rather than competition. Development of the framework through equal noncompetitive contribution is essential to ensure that the framework is valid and accepted by all. Collaboration should be across borders and regardless of affiliation in order to establish uniform standards that promote full and meaningful patient involvement during the entire lifecycle of medicines. Many groups have considered opportunities for patient involvement during the development and registration of medicines, and a good example from the US National Health Council is shown in Figures 2 and 3.
Figure 2.
Patient engagement in the R&D process. Reproduced with permission from the National Health Council.44
Figure 3.
Patient engagement in regulatory decision making. Reproduced with permission from the National Health Council.44
This work provides a sound basis for further refinement; a rational and synergistic approach would be to integrate existing successful initiatives to drive development of a master framework for patient engagement that covers the entire medicines pathway. Key steps toward this end would be to:
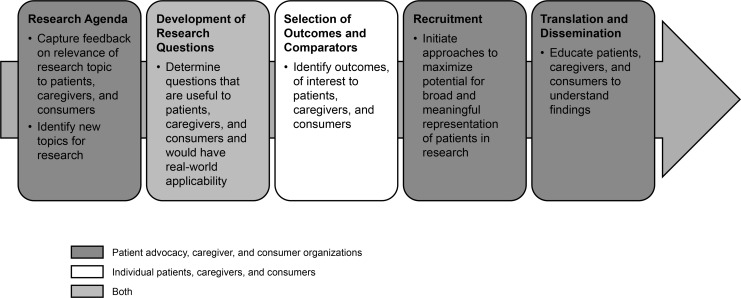
Map the medicine lifecycle and agree on essential and optimal time points for patient engagement (a good example from the CTTI is given in Figure 4)
Define goals of patient involvement at each time point/period, specific activities and required outcomes, as well as resource and capability needs
Outline methods of engagement as well as current regulations regarding interactions of patients with industry, academic, regulatory, and community groups, and identify potential challenges
Map existing stakeholder initiatives to identify gaps, avoid duplication, and improve synergies
Develop, disseminate, and drive implementation of the master framework
Figure 4.
Patient roles in the clinical trials continuum. Adapted from Parkinson’s Disease Foundation materials and developed by the Clinical Trials Transformation Initiative. Reproduced with permission from the Parkinson’s Disease Foundation.
A Call to Action
Patients and society need more effective, needs-based, and targeted development of medicines and, once developed and proven to show added value, rapid access to therapies that meet their medical needs. Most stakeholders agree that more effective patient involvement is essential in order to better prioritize and drive rational, strategic medicines development and lifecycle management.45 Despite currently fragmentary approaches, the plethora of schemes demonstrate widespread acceptance of the value of constructive collaboration. Development and validation of a master framework for systematic patient involvement in industry-led medicines research and development is the crucial next step to create better medicines and better health.
There are fundamental success criteria that will need to be met in order to successfully develop and establish a master framework. Framework development should be driven by a multinational partnership with balanced representation of stakeholders working together in line with agreed on principles to ensure openness, inclusiveness, transparency, and credibility. The framework must be supported and endorsed by patient organizations across diverse areas of illness, health, and policy; the FDA, EMA, and other regulators; HTA bodies and payers globally; medical and other relevant professional organizations; and a critical mass of biopharmaceutical companies. We call all stakeholders in the medicine development chain to collaborate, actively share outputs from existing initiatives, and develop specific projects that will remove the current barriers, build professional capacity on patient involvement in industry and patient advocates through education and training, and fill existing gaps in order to make continuous patient involvement a reality. We are currently actively working toward forming an open network to develop such a framework and would urge stakeholders to contribute to and support its development and adoption. A collaborative inclusive approach and widespread implementation of the framework will help to ensure that patients and their needs are embedded at the heart of medicines development.
Acknowledgments
The authors thank Diana Hughes (affiliated with Pfizer Inc, New York, at the time of manuscript preparation) for her valuable input in the planning of this article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The following authors are employees of GlaxoSmithKline (J.A., M.M.); UCB Biopharma (L.D.); MSD (Europe) Inc (A.J.); Merck & Co, Inc (J.R.); Pfizer Inc (R.F.S.); and Novartis Pharma AG (G.T.). The opinions expressed in this article are those of the authors and do not necessarily reflect the views of their employers or organizations.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Pharmaceutical Research and Manufacturers of America. Biopharmaceutical research industry profile. www.phrma.org/sites/default/files/pdf/PhRMA%20Profile%202013.pdf. Published July 2013. Accessed September 2014.
- 2. European Federation of Pharmaceutical Industries and Associations. Pricing of medicines. www.efpia.eu/topics/industry-economy/pricing-of-medicines. Published 2014. Accessed December 2014.
- 3. Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–214. [DOI] [PubMed] [Google Scholar]
- 4. Luce BR, Kramer JM, Goodman SN, et al. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009;151:206–209. [DOI] [PubMed] [Google Scholar]
- 5. Djulbegovic B, Hozo I, Ioannidis JP. Improving the drug development process: more not less randomized trials. JAMA. 2014;311:355–356. [DOI] [PubMed] [Google Scholar]
- 6. Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open. 2012;2:e000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petit-Zeman S. Why patients and clinicians should set priorities for cardiac researchers. Br J Card Nurs. 2012;7:95–97. [Google Scholar]
- 8. Stewart R, Oliver S. A Systematic Map of Studies of Patients’ and Clinicians’ Research Priorities. Oxford, UK: James Lind Alliance; 2008. [Google Scholar]
- 9. Mann H, Djulbegovic B. Comparator bias: why comparisons must address genuine uncertainties. In: JLL Bulletin 2012: Commentaries on the History of Treatment Evaluation. www.jameslindlibrary.org. Accessed September 2014.
- 10. Franson TR, Peay H. Benefit-risk assessments in rare disorders: the case for therapeutic development in Duchenne muscular dystrophy as the prototype for new approaches. www.phrma.org/catalyst/understanding-patient-perspective-critical-in-benefit-risk-assessment. Accessed September 2014.
- 11. Barber R, Beresford P, Boote J, Cooper C, Faulkner A. Evaluating the impact of public involvement on research: a prospective case study. Int J Consum Stud. 2011;35:609–615. [Google Scholar]
- 12. Staley K. Exploring Impact: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh, UK: INVOLVE; 2009. [Google Scholar]
- 13. van Thiel G, Stolk P. Priority medicines for Europe and the world. A public health approach to innovation. Update 2013. Update on 2004 background paper, BP 8.5 patient and citizen involvement. www.who.int/medicines/areas/priority…/BP8_5Stakeholder.pdf. Accessed October 2014.
- 14. Department of Health and Human Services. Quick guide to health literacy fact sheet. Health literacy basics. http://health.gov/communication/literacy/quickguide/factsbasic.htm. Accessed December 2014.
- 15. Institute of Medicine. Roundtable on health literacy. http://www.iom.edu/Activities/PublicHealth/HealthLiteracy.aspx. Accessed December 2014.
- 16. Hale TM, Pathipati AS, Zan S, Jethwani K. Representation of health conditions on Facebook: content analysis and evaluation of user engagement. J Med Internet Res. 2014;16:e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greene JA, Choudhry NK, Kilabuk E, et al. Online social networking by patients with diabetes: a qualitative evaluation of communication with Facebook. J Gen Intern Med. 2011;26:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rozenblum R, Bates DW. Patient-centred healthcare, social media and the Internet: the perfect storm? BMJ Qual Saf. 2013;22:183–186. [DOI] [PubMed] [Google Scholar]
- 19. European Patients’ Academy on Therapeutic Innovation. www.patientsacademy.eu. Accessed September 2014.
- 20. Frank L, Basch E, Selby JV; Patient-Centered Outcomes Research Institute. The PCORI perspective on patient-centered outcomes research. JAMA. 2014;312:1513–1514. [DOI] [PubMed] [Google Scholar]
- 21. Manganiello M, Anderson M. Back to basics: HIV/AIDS advocacy as a model for catalyzing change. forces4quality.org/node/6968. Accessed September 2014.
- 22. Cystic Fibrosis Foundation. Annual report 2012. www.cff.org. Accessed September 2014.
- 23. Thornton H. Patient and public involvement in clinical trials. BMJ. 2008;336:903–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FasterCures. www.fastercures.org/. Accessed September 2014.
- 25. Prescription Drug User Fee Act, 21 USC, 1992.
- 26. Food and Drug Administration activities for patient participation in medical product discussions; establishment of a docket; request for comments. Fed Regist. 2014;79(213):65410–65411. [Google Scholar]
- 27. Food and Drug Administration. Enhancing benefit-risk assessment in regulatory decision-making. http://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm326192.htm. Accessed December 2014.
- 28. Bere N. Overview of EMA’s interaction with patients and consumers organisations (2013). www.ema.europa.eu. Accessed September 2014.
- 29. European Medicines Agency. Annual report on European Medicines Agency’s interaction with patients, consumers, healthcare professionals and their organisations (2013). www.ema.europa.eu. Published 2015.
- 30. European Medicines Agency. Incorporating patients’ views during evaluation of benefit-risk by the EMA Scientific Committees. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2014/09/WC500173508.pdf. Accessed December 2014.
- 31. European Medicines Agency. Patients to discuss benefit-risk evaluation of medicines with the Committee for Medicinal Products for Human Use (press release, September 26, 2014). http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/09/news_detail_002172.jsp&mid=WC0b01ac058004d5c1. Accessed December 2014.
- 32. Health Technology Assessment International. http://www.htai.org/about-htai/what-is-htai.html. Accessed December 2014.
- 33. Food and Drug Administration Safety and Innovation Act 2012. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/default.htm. Accessed September 2014.
- 34. Public Law 112-144 (July 9, 2012). http://www.gpo.gov/fdsys/pkg/PLAW-112publ144/pdf/PLAW-112publ144.pdf. Accessed December 2014.
- 35. Food and Drug Administration. Guidance for industry. Collection of race and ethnicity data in clinical trials. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126396.pdf. Published 2005. Accessed December 2014.
- 36. Food and Drug Administration. Collection, analysis, and availability of demographic subgroup data for FDA-approved medical products (August 2013). http://www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM365544.pdf. Published 2013. Accessed December 2014.
- 37. Regulation (EC) No. 1901/2006 of the European Parliament and of the Council (December 12, 2006). http://ec.europa.eu/health/files/eudralex/vol-/reg_2006_1901/reg_2006_1901_en.pdf. Accessed December 2014.
- 38. European Medicines Agency. Pharmacovigilance legislation. www.ema.europa.eu/ema/. Published 2012. Accessed September 2014.
- 39. Eurobarometer Qualitative Study. Patient involvement—aggregate report 2012. ec.europa.eu/…/eurobaro_patient_involvement_2012_en.pdf. Accessed October 2014.
- 40. Health policy brief: patient engagement. Health Affairs. www.healthaffairs.org/healthpolicybriefs/. Published February 14, 2013. Accessed December 2014.
- 41. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. European Patients’ Forum manifesto for the 2014 European elections. Patient involvement = healthier Europe. Background briefing no. 4 www.eu-patient.eu/…/EPFManifesto_backgroundpaper4_NOV13.pdf. Published November 2013. Accessed October 2014.
- 43. National Health Council comments on Strategy for American Innovation request for information. www.nationalhealthcouncil.org/. Published September 23, 2014. Accessed October 2014.
- 44. National Working Group on Evidence-Based Health Care. The role of the patient/consumer in establishing a dynamic clinical research continuum: models of patient/consumer inclusion. http://www.evidencebasedhealthcare.org/images/download/W.G.PatientConsumerBklt2%288-6%29.pdf. Published August 2008.
- 45. Hirsch BR, Schulman KA. The economics of new drugs: can we afford to make progress in a common disease? Am Soc Clin Oncol Educ Book. 2013. doi:10.1200/EdBook_AM.2013.33.e126.43. [DOI] [PubMed] [Google Scholar]