Abstract
Here we discuss three RNA therapeutic technologies exploiting various oligonucleotides that bind RNA by base-pairing in a sequence-specific manner yet have different mechanisms of action and effects. RNA interference and antisense oligonucleotides downregulate gene expression by enzyme-dependent degradation of targeted mRNA. Steric blocking oligonucleotides block access of cellular machinery to pre-mRNA and mRNA without degrading the RNA. Through this mechanism, blocking oligonucleotides can redirect alternative splicing, repair defective RNA, restore protein production or also downregulate gene expression. Moreover, they can be extensively chemically modified, resulting in more drug-like properties. The ability of RNA blocking oligonucleotides to restore gene function makes them suited for treatment of genetic disorders. Positive results from clinical trials for the treatment of Duchenne muscular dystrophy show that this technology is close to realizing its clinical potential.
INTRODUCTION
The majority of currently marketed drugs are small molecules that target proteins such as enzymes and receptors, which represent a relatively small subset of total cellular proteins. In contrast, oligonucleotides are macromolecules that target pre-mRNA and mRNA, the carriers of genetic information before it is translated into proteins. Because mRNAs code for all cellular proteins, oligonucleotides targeting mRNA could prove to be effective for targets and diseases that are not treatable by current drugs. For example, genetic diseases — where the defect in the gene can be best repaired by manipulating DNA or RNA rather than a protein — such as Duchenne muscular dystrophy (DMD) and spinal muscular atrophy (SMA) are such diseases discussed below. This review covers approaches that exploit oligonucleotides: RNA intereference (RNAi), antisense oligonucleotides (ASO), and steric-blocking oligonucleotides. The three approaches involve the binding of complementary oligonucleotides to target RNA through base pairing, and therefore all three are, in essence, operating by an antisense mechanism. However, they differ substantially in their downstream mechanisms of action and the functional outcomes that they produce.
RNAi and ASOs — which will be discussed only briefly as they have been extensively reviewed previously 1, 2 — modulate gene expression by inducing enzymatic degradation of targeted mRNA and removal of the disease-causing gene product, such as an oncogene or a pro-inflammatory cytokine. Since cellular enzymes need to recognize these antisense compounds, they can only be chemically modified to a limited degree, which limits our ability to enhance their pharmacological qualities. Antisense compounds that modulate RNA function by blocking access of cellular machinery to the RNA, and so do not lead to degradation of the target RNA will be the main focus of the Review. This different mode of action leads to outcomes such as repair of a defective RNA or generation of a novel that cannot be achieved by the use of RNAi or ASO. Furthermore, because RNA-blocking oligonucleotides do not need to exploit cellular enzymes for their activity, they can be subjected to more extensive chemical modifications that improve their drug-like qualities.
RNAi, ASOs and blocking oligonucleotides have poor intracellular uptake, which is a major impediment to their use as therapeutics. This is the main reason why fomivirsen (3), an antiviral drug, is the only approved antisense drug. Recent advances in chemistries that improve the intracellular delivery of antisense oligonucleotides, as well as differences that characterize the three technologies, are highlighted in this review. Aptamers, which are more complex than oligonucleotides RNA-based drugs and interact directly with proteins rather than complementary RNA are not covered in this article (4).
Oligonucleotides that induce degradation of target mRNA
RNA interference
RNA interference (RNAi) was first demonstrated in a nematode (5) (Caenorhabditis elegans), where delivery of exogenous long, double-stranded RNA (dsRNA) effectively silenced the expression of a gene (encoding for a myofilament protein) by inducing degradation of a homologous host mRNA. The mechanism that mediated gene silencing involved degradation of dsRNA into short interfering RNAs (siRNA), double–stranded RNA fragments that are 21–22 nucleotides long and interact with a multi-protein RNA-Induced Silencing Complex (RISC). Within the RISC, the siRNA is unwound, the sense strand is discarded, and the antisense or guide strand binds mRNA. When siRNA is fully complementary to its target, an endonuclease, AGO2, a component of RISC, cleaves the mRNA 10 and 11 nucleotides downstream from the 5′-end of the antisense strand. (6) (Fig. 1A)
Figure 1. Mechanisms of oligonucleotide-induced downregulation of gene expression.
A. SiRNA. Synthetic double-stranded short interfering RNA (siRNA) is complexed with components of RNA interference (RNAi) pathway, dicer, AGO2 and other proteins, forming RNA-interference-silencing-complex, RISC. RISC binds to a target mRNA via the unwound guide strand of siRNA, allowing AGO 2 to degrade the RNA. RISC-bound siRNA can also bind with mismatches to unintended mRNAs, leading to significant off-target effects (see main text).
B. Antisense gapmer oligonucleotides. These usually have a PS backbone with flanks additionally modified with 2′MOE or 2′OMe residues (Red in figure. flank modifications increase resistance of the ASO to degradation and enhance binding to target mRNA. The unmodified “gap” in a gapmer/mRNA duplex is recognized by RNase H, a ribonuclease that degrades duplexed mRNA.
C. Translation suppressing oligomers (TSO). PMO and their derivatives or oligonucleotides fully substituted with 2′MOE or 2′OMe residues are not recognized by RISC or RNase H and do not lead to RNA degradation. Nevertheless, they lead to downregulation of gene expression by steric blockade of ribosome access to mRNA and suppression of protein translation.
D. External Guide Sequence (EGS) and RNase P. A PPMO is designed to hybridize to targeted bacterial mRNA and form stem-loop structures such that the resulting duplex resembles tRNA. In bacteria, a tRNA processing ribozyme RNase P recognizes this structure and cleaves mRNA.
Although RNAi could not initially be detected in mammalian cells, later studies showed that these cells lacked the ability to cleave dsRNA into siRNA. The discovery that synthetic siRNA can enter RISC and degrade targeted mRNA when delivered to cultured human cells (7) as evidenced by rapid increase in the amount of research related to siRNA. A query for siRNA in PubMed brings 234 citations in 2002, the first full year after the siRNA discovery, 33,009 in the 2002–2010 period, and 7241 in 2010. Notably, a first successful in vivo study was carried out as early as 2003, using naked siRNA to knock-down FAS mRNA in a mouse model of fulminant hepatitis. ((8)) The growth in the amount of RNAi research also resulted in the founding of companies — such as Alnylam and SIRNA — focused on the development of siRNA as a promising therapeutic platform.
Systemically delivered, unmodified siRNA is rapidly degraded by nucleases circulating in the blood; its half-life in plasma is minutes, and its uptake into target organs and cells is generally poor, in spite of some success in liver delivery (8). Chemical modifications to promote metabolic stability and improve target cell penetration have been introduced to overcome such problems. However, it became apparent that the interaction of siRNAs with the cellular RISC machinery presents a challenge for their use as therapeutics, because only limited chemical modifications can be introduced into the siRNA for it to remain functional within RISC. In the antisense strand, phosphorothioate internucleotide linkages at the 3′-end, and 2′-O-methyl (2′-OMe) nucleotide substitutions (Fig. 2) in one or two internal nucleotides are tolerated and improve the resistance of the siRNA to nucleases. The sense strand can be modified more heavily (that is, more internal nucleotides can tolerate 2′-OMe nucleotide substitutions) without substantially reducing efficacy (reviewed in 9).
Figure 2.
Oligonucleotide Chemistries. Top. All oligonucleotides are negatively charged. Phosphorothioate (PS) backbone and 2′MOE and 2′OMe substituents increase resistance to degradation and promote protein binding. In addition, LNA modification markedly increases binding of the oligonucleotide to the target mRNA. Bottom. In Phosphorodiamidate morpholino oligomers (PMO) ribose (RNA) or deoxyribose (DNA) are replaced with morpholine rings and the phosphorothioate or phosphodiester (RNA) groups are replaced with uncharged phosphorodiamidate groups, resulting in a compound that is neutral and very resistant to degradation. Positively charged piperazine residues in PMOplus or positively charged, arginine rich peptides in PPMO dramatically improve intracellular uptake of the oligomers.
Because of the difficulty in achieving intracellular siRNA delivery, they were administered locally in the majority of initial clinical trials. These included intravitreal injection for treatment of macular degeneration (ClinicalTrials.gov, NCT00363714), intranasal delivery for respiratory syncytial virus (RSV) (ClinicalTrials.gov, NCT00658086), and direct injections in skin lesions for Pachyonychia congenita, a rare genetic skin disorder (NCT00716014). It was noted that human intranasal delivery results only in minimal systemic distribution of the drug (10), indicating that the bioavailability of unmodified siRNAs in the absence of delivery agents in humans is poor.
In two clinical trials that are currently underway the problems associated with systemic siRNA delivery were tackled by combining siRNA with delivery-enhancing agents. In one trial, a cocktail of two siRNAs, ALN-VSP02, was formulated with lipid particles and targeted to kinesin spindle protein and VEGF mRNAs, which are essential for tumor proliferation and tumor-supporting angiogenesis, respectively. (http://phx.corporate-ir.net/phoenix.zhtml?c=148005&p=irol-newsArticle2&ID=1570823&highlight=; NCT00882180). In the second trial, siRNA was formulated in a cyclodextrin-adamantene polyethylene glycol particle that included a targeting component, human transferrin protein (CALAA-01), which targeted the siRNA to ribonucleotide reductase mRNA (NCT00689065). These delivery-enhancing moieties should improve cellular uptake of siRNA; if successful, these clinical studies will be of substantial interest.
A series of lipid-based, liver-directed siRNA carrier particles was recently tested in a mouse model of hemophilia and in healthy cynomolgus monkeys. Systemic delivery of the formulated siRNAs reduced the levels of target mRNAs, factor VII and GAPDH, respectively, at remarkably low doses of 0.01 mg/kg in mice and 0.1 mg/kg in monkeys (11). A single high dose of the preparation was well tolerated in both species. Since the effects of the siRNAs were examined only in the liver, it is not known if they were taken up by other tissues. Nevertheless, these results represent dramatic improvements in siRNA delivery to specific target tissues, in this case the liver.
Another problem associated with RNAi technology — which stems from mechanism of RNAi — is off-target effects of siRNAs. Short dsRNAs, termed micro RNAs (miRNAs), are produced in mammalian cells and as a class they control the efficiency of the translation of a large number of mRNAs. Synthetic siRNAs may interfere with this process, thus mimicking the effects of miRNAs. Specifically, siRNAs can enter RISC and bind with certain base-pairs mismatched to untargeted mRNAs, so acting like endogenous miRNAs and leading to off-target gene silencing (12). In addition, siRNAs can activate an innate immune response via activation of Toll-like receptors, which leads to undesirable side effects such as induction of pro-inflammatory cytokines or interferon-α (IFNα) (6, 12). Taken together, these developments indicate that systemic delivery of chemically modified siRNA is not very effective and that more work will be needed to achieve sufficient therapeutic activity and specificity in the absence of delivery agents.
It has now been over a decade since the discoveries of RNAi and siRNAs, but in spite of some promising clinical trial results, industry remain cautious about the potential of RNAi-based drugs For example, although Merck acquired SIRNA for US$1.1 billion, Merck was quoted in 2009 as remaining sceptical about the development of siRNA -based drugs (13) while Roche, Novartis and Pfizer (14; http://www.genomeweb.com/rnai/pfizer-shut-down-oligo-therapeutics-unit-part-restructuring; http://www.proactiveinvestors.com/companies/news/19679/will-rnai-therapeutics-ever-succeed-roche-pfizer-abbott-merck-novartis-trim-commitments-19679.html decided to dramatically reduce or eliminate their RNAi research programs, which raised the question “is RNAi dead?” in a 2011 editorial (15). This is very reminiscent of a previous question “does antisense exist?” raised in a commentary in 1995 (16). The history of antisense oligonucleotides (ASOs, see Box 1), which seem now well on their way to becoming drugs, suggests that RNAi may come back as a therapeutically viable technology. As a possible harbinger of further progress, an upcoming clinical trial will test chemically modified siRNA for the treatment of diabetic macular edema (ClinicalTrials.gov, NCT01445899).
Box 1. The Ups and Downs of Antisense Oligonucleotides.
In 1978 Zamecnik and Stephenson found that a 13-nucleotide long oligodeoxynucleotide complementary to a target sequence in Rous sarcoma virus (RSV) RNA inhibited viral replication and protein translation in vitro (17; 96). The field of antisense oligonucleotides (ASO) was born. Remarkably, in this pioneering work, the authors already introduced chemical modifications at the oligonucleotide 3′ and 5′ ends to reduce its degradation by cellular nucleases, which improved its activity, and they demonstrated that the same ASO used against another avian virus was less effective, presumably because the three mismatches in the target sequence weakened its binding. Thus, two key themes—improvements in ASO efficacy via chemical modifications and the need for specificity controls—were established at the very birth of the field.
It took almost 10 years before the next major advancement in the field. The most consequential was the introduction of phosphorothioate (PS) internucleotide linkages (97) (Fig. 2, top) followed by the addition of 2′-O-modified nucleotides at the 5′ and 3′ ends, which protected the ASOs from degradation by nucleases (98). These modifications dramatically increased the stability of ASOs in cell culture and in vivo, whilst still allowing RNase H cleavage of RNA in PS-ASO/RNA duplexes (reviewed in 18). A large number of PS-ASOs drug candidates were progressed through various stages of drug development but only one, fomivirsen, (Vitravene) was registered in 1998 as a treatment for CMV-induced retinitis in immunocompromised patients with AIDS. The drug, a 21mer, was delivered through intraocular injection. This choice of indication and delivery contributed to the success of the drug. Intravitreal distribution of the drug dramatically limited the necessary dose (330 μg/0.05 mL) and eliminated systemic exposure of the patient to the drug, avoiding any potential side effects. Thus, 20 years after Zamecnik’s discovery, the first antisense drug reached the market (3).
Although off-target effects have not been a serious issue for PS-ASOs, this backbone imparts a significant, hybridization-independent toxicity profile that varies with different sequences. The effects include increased coagulation time, pro-inflammatory effects and activation of the complement pathway. At higher concentrations, PS-ASOs lead to renal tubule changes and thrombocytopenia (99). In addition, PS-ASOs that contain certain sequences induce a strong immunostimulatory response through interactions with Toll-like receptors (100 [or bind directly to proteins, leading to unexpected, spurious effects. These results led to an article entitiled: “Does antisense exist?” (16). Some companies continued development of PS-ASOs until very recently.
Oblimersen, developed which was developed by Genta as a potential anticancer drug is one such example. Oblimersen targets the mRNA of BCL2, an anti-apoptotic gene over-expressed in numerous cancers. It is noteworthy how difficult the attempts to bring oblimersen to market have been. Since 1999, this drug has been tested in 45 clinical trials (http://clinicaltrials.gov/ct2/results?term=oblimersen). Unfortunately, recent trials for treatment of advanced melanoma and myeloma, which enrolled almost 1000 patients, demonstrated either very modest effects (for the treatment of melanoma) or negative (treatment of myeloma) effects of the drug (101,102). These efforts were certainly costly. In April 2002 Genta and Aventis (now Sanofi) entered into a development agreement for oblimersen that was valued at the time at $480 million (http://www.forbes.com/2002/04/29/0429genta.html). Today, the stock of Genta is no longer listed on a regular NASDAQ stock exchange board and after additional trials that missed the expected primary targets the oblimirsen program was terminated (103).
Antisense oligonucleotides
Remarkable progress in oligonucleotide drug development has been made since the first application of a short fragment of unmodified DNA in cell culture as an antisense oligonucleotide (ASO) by Paul Zamecnik in 1978 (17). Numerous chemical modifications that improve the drug-like properties of DNA have been introduced (see Box 1), an antisense drug has been marketed, and successful clinical trials are progressing, as described below. Currently, a typical ASO drug candidate is about 20 nucleotides long and has a phosphorothioate (PS) linkage (Fig. 2, top) between the nucleosides that form the backbone. In addition, five nucleotides at each flank are further modified (Fig. 2, top) to protect the ASO from exonucleases, increasing its stability in vivo. This design leaves a central 10-nucleotide phosphorothioate gap that allows cleavage of targeted mRNA by RNase H [glossary] (18) (Fig. 1B). The modified flanks also improve the binding of ASO to mRNA and reduce the side effects that are associated with the presence of phosphorothioate residues (Box 1). In contrast to siRNA, which tolerates only limited modifications to remain RISC-compatible, more extensive chemical modifications in ‘gapmers’ (that is, ASO containing a phosphorothioate gap do not abrogate RNase H activity. One such modification, 2′MOE (2′-O-(2′-methoxyethyl)-ribonucleoside) (Fig. 2, top), is present in two ASOs that have recently been successful in clinical trials: mipomersen (also known as ISIS 301012) and custirsen (also known as OGX-111) (19, 20).
Mipomersen is a 2′MOE PS ASO gapmer, which targets mRNA for liver-expressed apolipoprotein B-100 (apoB-100), a protein involved in the production of low-density lipoprotein cholesterol (LDL-C or ‘bad’ cholesterol). Mipomersen reduces the levels of apoB-100 protein that is secreted from liver cells into the bloodstream. In a recent trial, mipomersen lowered LDL-C in patients with homozygous familial hypercholesterolemia who had very high (up to 300 mg/dL) LDL-C levels by ~25% compared with a ~3% decrease for placebo. Twenty-six out of thirty-four treated patients incurred injection-site reactions, and four had a significant increase in alanine aminotransferase, possibly an indication of liver damage (19). These signals need to be carefully monitored, especially in a chronic disease.
Custirsen (also know as OGX-011) is also a PS-ASO gapmer with 2′-MOE flanks that targets the mRNA of clusterin, an anti-apoptotic chaperone protein that is upregulated in cancer cells. Significant declines in serum clusterin levels were seen in the Phase 2 trial of custirsen in patients with advanced metastatic prostate cancer, who were treated with a combination of custirsen and docetaxel or docetaxel alone, with 41 patients in each arm (20). OGX-011 was well tolerated, although fever was seen in some patients. The most encouraging outcome was the median overall survival in patients treated with custirsen, which was ~24 months compared with ~17 months for patients treated with docetaxel alone. Differences in other measures were not statistically significant. Nevertheless, on the strength of these results, two Phase 3 trials were initiated (http://ir.oncogenex.com/releasedetail.cfm?ReleaseID=512563).
The same compound was not effective in a Phase II study of women with metastatic breast cancer (21) suggesting that the efficacy of the drug may be cancer-specific. If custirsen and mipomersen are approved in the near future, 20 years will have elapsed from the discovery of the first antisense oligonucleotide to the approval of first generation antisense drug, phosphorothioate fomivirsen (Vitravene), and another 10 years to the approval of the new, second-generation, gapmer-type antisense drugs. This timescale is similar to that for development of monoclonal antibodies, another class of macromolecular drugs (see also Box 1 and Outlook section).
Oligonucleotides that block target pre-mRNA and mRNA
In parallel to the development of gapmer ASOs, oligonucleotides were developed that do not induce RNase H cleavage of mRNA but act by blocking target RNA without inducing its degradation. Early examples included oligonucleoside-methylphosphonates (22) and phosphorodiamidate morpholino oligomers, PMOs (23) (Fig. 2, bottom). The outcomes of this approach depend on what nucleotide sequence elements in mRNA or pre-mRNA are targeted. They can include modulation of splicing when pre-mRNA is targeted blockade of mRNA translation or RNA folding, and external guide sequence (EGS)-directed mRNA degradation by a tRNA-processing ribozyme, RNase P. They can also be used to block toxic RNAs, which would otherwise sequester protein factors at their expanded triplet repeats (Figs. 1 and 3). These applications are highlighted in more detail below.
Figure 3.
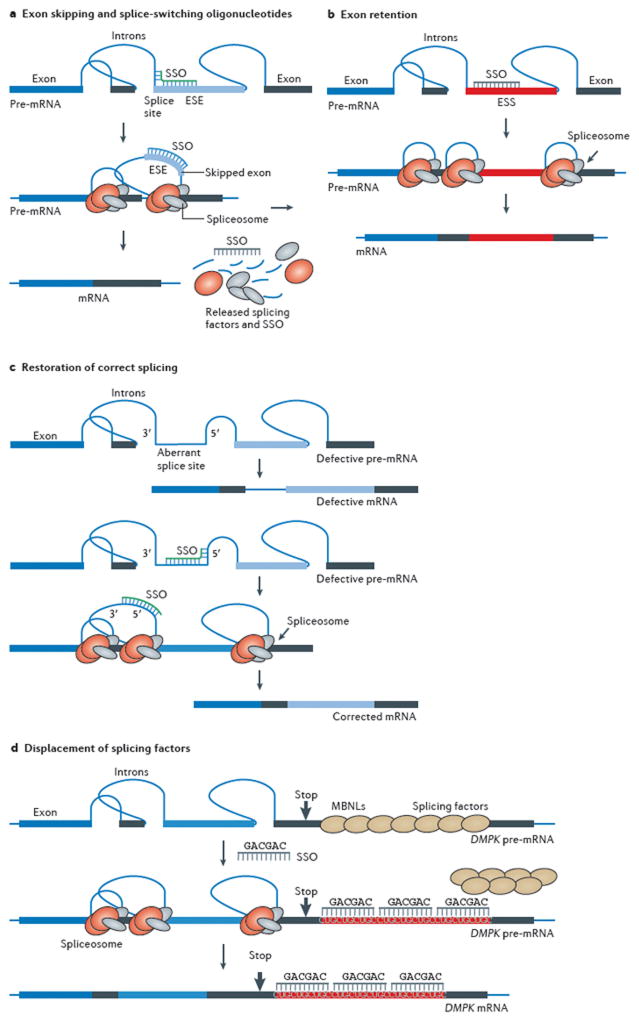
Mechanisms of oligonucleotide induced modulation of gene expression. A. Exon skipping and splice switching oligonucleotides (SSO). A chemically modified, RNA blocking oligonucleotide targeted to a splice site or an exon-internal exon splicing enhancer (ESE) in pre-mRNA prevents proper assembly of the spliceosome on the exon and redirects splicing to another pathway, effecting skipping of the targeted exon. Such alternatively spliced mRNA may code for a novel protein with favorable properties, may restore translation, if exon skipping restores a reading frame, as is the case in DMD, or may change the balance of alternative splice variants These outcomes cannot be accomplished by ASO or siRNA, which degrade target mRNA. B. Exon retention by SSO. Some exons are poorly spliced into mRNA because they contain exon splicing silencer (ESS) elements. An SSO designed to block an ESS interferes with this element’s role in splicing and promotes exon inclusion, as has been demonstrated in the case of SMA, a genetic disorder. C. Restoration of correct splicing and RNA repair by SSO. Top. An intron mutation may create and/or activate aberrant splice sites, which leads to inclusion of an intronic fragment into the spliced mRNA, in essence creating a pseudo exon and interfering with the translational reading frame. Bottom. An SSO targeted to the aberrant splicing elements restores correct splicing and allows translation of the correct, fully functional protein. D. Displacement of splicing factors from triplet repeats. An extended triplet repeat CUGCUGCUG… in DMPK pre-mRNA attracts a splicing factor, muscleblind (MBNL) and titrates it out from the nucleoplasm. As a result, DMPK and several other pre-mRNAs are not properly processed, preventing translation of a number of proteins and causing myotonic dystrophy, a neuromuscular disorder. A modified, steric-blocking oligonucleotide displaces MBNL, allowing it to participate in splicing of appropriate mRNAs and restoring function the affected muscle.
Splice-Switching Oligonucleotides (SSO)
Oligonucleotide-induced modulation of splicing leads to several outcomes in cell culture and in vivo that have potential therapeutic value. These outcomes are not achievable with siRNA or classic gapmer ASOs, which only downregulate gene expression. Oligonucleotides that modulate pre-mRNA splicing (SSO, splice switching oligonucleotides) can repair defective RNA and restore the production of essential proteins; they can generate novel proteins with desirable properties and they can regulate the presence of disease related splice-variant proteins. The latter outcome is achieved by modulation of alternative splicing of pre-mRNA. Since over 95% of all human genes produce splice-variant proteins by alternative splicing, modulation of alternative splicing may be applicable to a multitude of diseases. (24)
To modulate pre-mRNA splicing, oligonucleotides must block RNA sequences that are essential for splicing and prevent the interaction of splicing factors—RNA-binding proteins, small nuclear RNAs (snRNAs), and other components of the spliceosome—with the pre-mRNA. The chemistries that have been shown to work in animal models include PNAs, alternating LNA/deoxynucleotide oligonucleotides, fully modified (non-gapmer) 2′-substituted oligonucleotides, and PMO-based oligomers (26, 27, 28 see also 25 for review) (Fig. 2A). The latter two SSO chemistries have been used in clinical trials that tested splicing modulation as a treatment for Duchenne muscular dystrophy (DMD) (Fig. 3A.). (29–32)
Duchenne muscular dystrophy
DMD is a severe genetic disorder that affects 1 in 3500 newborn males. It causes muscle wasting, leading to loss of mobility by the age of 10–12 years, and death in the mid 20s due to failure of respiratory and cardiac functions. The genetic defects that underlie DMD are mostly DNA deletions within the gene that codes for dystrophin, a protein that connects intracellular actin filaments to the sarcolemma membrane and as such is essential for maintaining the latter’s integrity. In its absence, muscle fibres disintegrate at a rate that outpaces muscle regeneration mechanisms. In a majority of cases of DMD the deletions disrupt the translational reading frame, abrogating expression of dystrophin protein. In contrast, in a milder disease, Becker muscular dystrophy (BMD) the deletions mostly preserve the reading frame, allowing the production of truncated but partially functional dystrophin. Remarkably, even though the mutant dystrophin produced in BMD may have large internal deletions, and is produced at levels below normal, its activity is largely sufficient to retain, muscle function that in some individuals is close to normal. This observation indicates that a drug that could partially restore the function of dystrophin protein could be of clinical value for the treatment of DMD (33 reviewed in 34.).
With 2.4 million base pairs, the dystrophin gene is the largest gene in humans. It comprises 79 exons, 34 of which code for full codons (termed here as “in-frame” exons). Deletion of an in-frame exon(s) does not disrupt the overall reading frame and allows translation of dystrophin that retains the correct amino acid sequences at the termini of the molecule but is missing its internal portion. Such deletions usually are found in patients with BMD. In the remaining 45 exons the last, first or both terminal codons are split between the two adjacent exons. Most of these exons are “out-of-frame”, and, in contrast to most in-frame exons, their deletion disrupts translation of dystrophin protein and so causes DMD. The same is true for a deletion that starts at an in-frame exon but ends in an out-of-frame exon (33). It follows that SSO-induced skipping of the adjacent out-of frame exon should restore the reading frame and convert severe DMD to milder BMD (35) (Fig. 3A). This approach would be applicable to about 85% of DMD patients, as some mutations cannot be corrected by exon skipping. For example, about 17% of patients from the population that responds to exon skipping, with deletions of out-of-frame exons 50 or 52 would benefit from skipping of exon 51 and restoration of the reading frame. SSO-induced skipping of four more exons, (exons 44, 45, 50, and 53) could benefit about 27% of patients from this population (36).
PMO and 2′OMe PS
The proof of concept of exon skipping in DMD was demonstrated over 10 years ago (37) by treatment of muscle cells from mdx mouse, a mouse with muscular dystrophy caused by nonsense mutation in exon 23 of DMD gene (38), with 2′-OMe PS SSO in. Following this finding, similar results were obtained in muscle cells from patients with DMD and after intramuscular injections of 2′-OMe PS SSO in the mdx mouse 39, 40, 41
2′OMe PS SSOs and PMO SSOs were subsequently tested by systemic administration in mdx mouse and dog models of DMD 26, 42, 43, 44). In mdx mouse, PMO SSOs were more effective than 2′-OMe PS SSOs in restoring the reading frame of dystrophin mRNA (42,43). A single intravenous dose of PMO SSO at 80 mg/kg induced dystrophin expression in about 25% muscle fibres in the quadriceps, which was the muscle that responded best to treatment. Seven weekly injections increased the percentage of muscle fibres that expressed dystrophin to over 80%. A three-dose treatment improved the function of tibialis anterior muscle to nearly 80% of normal, even though the level of dystrophin in this muscle was only 20% of normal. This is an important result, suggesting that below-normal dystrophin levels may still be clinically beneficial.
PMO SSOs were also effective in a dog model of DMD (44). The importance of this study was the demonstration of the efficacy of exon skipping in a large animal, with clear improvement in the mobility of the dog. Whereas an untreated dog had difficulty finishing a 15-metre run, a dog treated with 5 weekly injections at 120 mg/kg ran quickly and completed the course without difficulty. This performance was achieved when the level of dystrophin in the muscles of the treated animals was on average 26% of normal, confirming in a more challenging animal model that complete restoration of dystrophin is not essential for clinically relevant effects. In both the dog and mouse models of DMD 2′OMe PS SSO was less effective. For example, a single intravenous injection of the compound at ~80 mg/kg resulted in less than 10% of dystrophin positive fibers in the mdx mouse quadriceps (32). Nevertheless, both 2′OMe SSOs and PMO SSOs entered into clinical trials as a treatment for DMD. These trials were recently extensively reviewed 45) and will be discussed here only briefly.
In trials that tested the safety of the drug given by single, intramuscular injections, 2′-OMePS SSO (PRO-051) (29) and PMO SSO (AVI-4658) (30) were delivered into tibialis anterior and extensor digitorum brevis (EDB) muscles, respectively. Both drugs caused accurate skipping of exon 51, PRO051 at 0.8 mg, and AVI-4658 at two doses, 0.9 mg and 0.09 mg. Importantly, with both drugs, dystrophin protein correctly localized to the muscle fibre membranes, irrespective of the size of the exon deletions in individual patients, which varied from five exons, 45–50, to a single exon, 50. This indicated that in every subject the rescued dystrophin was functional, inasmuch as it reconstituted a dystroglycan complex, normally located in the sarcolemma of healthy muscle. AVI-4658 produced a statistically significant increase in dystrophin-positive fibers of up to 79% of normal and above the background of the control sample from a saline-treated contralateral EDB muscle. In PRO-051 trial, up to 97% of dystrophin positive fibers as compared to normal muscle were detected. However, that study lacked a negative control, which made quantitative analysis of newly generated dystrophin questionable.
PRO-051 and AVI-4658, given systemically, were also evaluated in clinical trials (31, 32). PRO-051 was initially delivered weekly by subcutaneous injection for 5 weeks at 0.5–6.0 mg/kg doses. In a continuation study, all patients were treated 6–15 months later with 6.0 mg/kg weekly dose and the results were reported after 12 weeks. In support of the proof of principle for exon skipping therapy, muscle biopsies collected after the last injection showed the presence of dystrophin-positive fibres in most patients. However, because background control, pre-treatment biopsies were collected for only a few subjects the validity of that assessment is again debatable. The improvement in the clinical status of the patients was measured by a 6-minute walk test (6MWT) after the additional 12-week treatment. A slight, but not statistically significant increase in the covered distance was noted (31).
Systemic delivery of AVI-4658 was by weekly intravenous infusion at doses ranging from 0.5–20.0 mg/kg for 12 weeks; pre- and post-treatment biopsies were collected from every patient. Seven patients responded well to treatment; their mean dystrophin fluorescence intensity increased from 8·9% to 16·4% of normal control. One patient treated at 20 mg/kg exhibited 55% of dystrophin-positive fibers over negative control. Overall there was significant dose dependent increase in new dystrophin protein in the muscle biopsies. Furthermore, there was a dose-dependent, statistically significant decrease in inflammatory infiltrate in the biopsy material, suggesting that restoration of dystrophin even at low levels ameliorates certain aspects of the disease. Similar to 2′OMe PS SSO, PMO SSO did not achieve statistically significant distance increase in the 6MWT pre- vs. post treatment (32).
The above two clinical studies also show differences between the PRO-051 and AVI-4658 chemistries in terms of tolerability. AVI-4658 was well tolerated and there were no drug-related adverse effects at any dose (32). In contrast, all patients treated in a continuation study with PRO-051 for 12 weeks at 6 mg/kg exhibited proteinuria, a possible sign of drug-related kidney damage 31).
The safety of AVI-4658 was further confirmed in animal studies. At doses up to 320 mg/kg in monkeys and up to 960 mg/kg in mice, there were no observable toxic effects (46). In mdx mice, there was no PMO SSO-induced toxicity after a single injection even at 3 g/kg of body weight, (47). A study of the pharmacokinetics and pharmacodynamics of a 2′OMe PS SSO that induced skipping of exon 23 in mdx mice demonstrated high accumulation of the compound in kidney and liver; however, an analysis of potential toxic effects in these organs was not reported (48).
Peptide conjugated PMO
PMO SSOs did not generate appreciable dystrophin in the heart of dystrophic dogs (44) and, even in high doses, only low levels in the hearts of mdx mice (47). Considering that patients with DMD usually die of heart or respiratory failure, this is an important issue. The synthesis of improved, second generation, peptide-conjugated PMOs (PPMOs) (Fig. 2, bottom) was a significant advance. In mdx mice, a PPMO (AVI-5225) that included a positively charged, arginine-rich peptide was superior to PMO and other chemistries in generating dystrophin (49). At 12.5 mg/kg it skipped exon 23 in mdx mice and restored dystrophin protein in skeletal muscles to close to normal levels, which remained unchanged for up to 11 weeks. The PPMO treatment led to a long-term reduction of cardiac hypertrophy (50) and also prevented heart failure in mdx mice challenged with the β-adrenergic receptor antagonist, dobutamine, whereas 60% of untreated mice died (51).
Even more convincing evidence for the high efficacy of PPMO-induced exon skipping was obtained in utrophin/dystrophin double-knockout (dKO) mouse (utr−/mdx−) with very severe muscular dystrophy. Lacking utrophin, a muscle protein that partially compensates for the lack of dystrophin in mdx mice, dKO mice are largely immobile and have an average lifespan of 8.2 weeks, compared to about 2 years for mdx mice. Treatment with a PPMO targeted to exon 23 given at 25 mg/kg/wk for 6 weeks, beginning at 10 days of age, dramatically increased the animals’ mobility, food-seeking behavior and the turgor of the tail to close to normal values (52). Variants of arginine-rich PPMOs, that are particularly effective in restoring dystrophin in mdx mouse heart muscle have been recently reported (53).
It should be noted that in a preliminary study in monkeys 12 weekly doses of a PPMO targeted to DMD exon 50 resulted in kidney toxicity, which was not observed at a four-week treatment. (http://investorrelations.avibio.com/phoenix.zhtml?c=64231&p=irol-newsArticle&ID=1406001&highlight=) Since PMOs are very stable in serum, intracellularly (54) and in vivo (Eckhoff and Kole, unpublished data) it seems possible that less frequent dosing may reduce kidney accumulation of the compound and alleviate this problem.
Other indications for SSOs
Therapeutic application of SSOs is most advanced in DMD but, as described in more detail in this section, these compounds are also being studied as potential treatments for two other genetic diseases, spinal muscular atrophy (SMA) and β-thalassemia, and for rheumatoid arthritis, a major inflammatory condition. Although in each condition a desired outcome is production of a functional protein, a different mechanism of splicing modulation is exploited to achieve this goal. In SMA, the SSOs blocks either exonic or intronic splicing silencers (ESS or ISS) and promote inclusion of an otherwise skipped exon (Fig. 3B). In thalassemia, an aberrant splice site is blocked, and splicing is redirected to the correct pathway (Fig. 3C). In treatment for rheumatoid arthritis, a functional TNF-α receptor is converted by exon skipping into a soluble decoy receptor that inhibits TNF-α activity. This flexibility in the possible approaches indicates that SSO-induced modulation of splicing has the potential to be clinically beneficial in many diseases.
Spinal Muscular Atrophy
SMA, a genetic neuromuscular disease with an incidence of approximately one in 6,000 live births, is characterized by loss of lower motor neurons in the spinal cord, and results in progressive paralysis and muscle atrophy. There is no treatment for this disorder, which in its severest form leads to early infant death. The disease is caused by mutations in the gene survival of motor neuron 1 (SMN1), which codes for SMN, a protein that is essential for preservation of motor-neuron integrity (55). Humans also carry SMN2, a very closely related variant of the SMN1 gene, but SMN2 produces SMN protein only in low amounts, insufficient to fully compensate for the loss of SMN1-derived protein. The low activity of the SMN2 gene is caused by a translationally silent mutation that weakens recognition of exon 7 by the splicing machinery and its inclusion into SMN2 mRNA during splicing (56). A systematic screen of 2′-MOE PS SSOs targeted to SMN2 pre-mRNA exon 7 identified a compound that blocked an ESS located in exon 7 and increased inclusion of the exon and restoration of SMN protein in cell culture (57) (Fig. 3B). Highly effective SSOs targeting ISS in the introns flanking SMN2 exon 7 were also identified (58, 59). In a mouse model of SMA, 4 weeks of systemic SSO administration, increased inclusion of SMN2 exon 7 by approximately 5-fold in liver, 3-fold in kidney and 2-fold in quadriceps of treated mice (51). Because oligonucleotides do not seem to cross the blood-brain barrier (27) it was not surprising that there was no effect in the spinal cord, the key therapeutic target tissue. The experiments provided an important, in vivo proof of principle for treatment of the disease and demonstrated that SSOs can be used for exon skipping and also for exon retention.
To bypass [MF: Author wishes to use the word ‘bypass’] the blood-brain barrier, 2′-OMe PS SSO (60) or 2′-O-MOE PS SSO (61,62) were delivered by direct intracerebroventricular injections into embryos or newborn pups of mice with SMA. This produced striking results: SSOs were distributed throughout the CNS, including the spinal cord, led to improvement in the righting response of the mice, and delayed the onset of tail and ear necrosis. In the next step toward clinical trials, intrathecal and intracerebroventricular infusions of 2′OMOE PS SSO delivered the compound to the spinal cord in monkeys. This tissue contained >8 μg/g of the oligonucleotide, the level anticipated to have therapeutic effects based on the results in the mouse models (62). If the SSO passes the FDA-mandated safety tests in animals, clinical tolerability and efficacy trials in humans will follow. Since there is little evidence that large, charged oligonucleotides can traverse a mature blood-brain barrier one could assume that the use of SSOs in patients with SMA will require intrathecal delivery. However, an unexpected finding that systemic delivery of 2′-O-MOE PS SSO gave a much more striking phenotypic rescue than ICV delivery in a mouse model of severe SMA (63) suggests otherwise.
SSO ICV administration efficiently restored SMN expression in the CNS, including in motor neurons, but only increased mean survival from 10 to 17 days, consistent with earlier results in another mouse model (62). In contrast, subcutaneous SSO administration increased the mean survival to 250 days, with some mice surviving longer than 1 year, even though only limited splicing correction was observed in the CNS. Combined ICV and systemic administration was slightly better than systemic administration alone. These data suggest that, at least in this mouse model, SMA is not solely a defect of motor neurons. Low levels of circulating IGF-1— which is synthesised in the liver — observed in this animal were attributed to reduced mRNA levels of the IGF-interacting acid-labile subunit (IGFALS). Systemic ASO treatment restored IGFALS mRNA and IGF1 protein to normal levels. Given that IGF1 has neurotrophic activity these results illustrate how pleiotropic peripheral defects could contribute to motor-neuron disease in SMA.
It remains to be seen to what extent these recent observations in mice with severe SMA are clinically relevant. This in turn will determine whether systemic SSO administration—alone or in combination with ICV administration—should be tested in clinical trials. Such approach will require additional safety and tolerability studies in non-human primates mandated by regulatory authorities before such trials could be conducted. Nevertheless, a treatment for this devastating disease appears to be on the horizon.
β-thalassemia
β-thalassemia is caused by mutations in the β-globin gene that decreases the production of β-globin, a subunit of haemoglobin, resulting in insufficient levels of hemoglobin to carry oxygen throughout the body 64. That decrease also results in an excess of α-globin subunit, which contributes to the damage to red blood cells. Blood transfusions are the main current treatment, but in developing countries — where the disease is prevalent— these carry the risk of blood-borne infections. Most common mutations induce aberrant splicing of β-globin pre-mRNA, which abrogates correct translation of the β-globin protein 64 One such mutation at nucleotide 654 of intron 2 (IVS2-654)of β-globin pre-mRNA creates a new 5′ splice site and concomitantly activates a pre-existing, cryptic 3′ splice site, leading to retention of a pseudo-exon in the spliced mRNA (Fig. 3C). That correct splicing can be restored by SSOs targeted to β-globin pre-mRNA was demonstrated in vitro in 1993 (65) but obtaining results in vivo proved difficult to achieve. Several chemistries (e.g., 2′OMe PS, PNA and PMO) were ineffective in the delivery of the SSO to erythroid progenitor cells in a transgenic mouse model of IVS2-654 thalassemia (66). The only effective SSO variant was a PPMO. A three-week, i.v. treatment course with the PPMO restored the levels of correct human β-globin mRNA six-fold over control. The mRNA was properly translated in circulating red blood cells and their morphology was improved 67).
An elegant follow-up to this work was treatment of IVS2-654 mice with siRNA, which lowered production of α-globin subunits, combined with a vector expressing antisense RNA that modulated splicing of IVS2-654 β-globin pre-mRNA and increased the level of β-globin. This combination lead to a detectable increase in hemoglobin production in treated IVS2-654 mice (68). Clearly, replacing transfusions with an oligonucleotide that restores hemoglobin production would be a substantial advance for individuals with β-thalassemia.
β-thalassemia is one the most common genetic disorders worldwide, particularly in south east Asia (69) and IVS2-654 is the most common mutation in gene encoding β-globin in China (68). However, because of the economic status of the affected countries there is apparent little commercial interest in advancing these new treatment options. Given the current high cost of oligonucleotides and/or gene therapy, it remains to be seen if China and other Asian countries will accept treatments that, if developed, would currently be very expensive.
Alternative splicing
While the above examples highlight the application of SSOs to repair of defective mRNA for the possible treatment of genetic disorders, these compounds may also be used to manipulate alternative splicing of genes that are not defective or mutated. Since most genes use alternative splicing to express multiple isoforms of mRNA and consequently proteins, this approach could have wide applicability. In early studies, manipulation of spliced was tested in vitro in cancer cell lines by switching the splicing pattern of Bcl-x pre-mRNA from anti-apoptotic Bcl-xL to pre-apoptotic Bcl-xS splice variants (70, 71). Oligonucleotide-induced skipping of exon 7 in pre-mRNA of TNF-α receptor 2 (TNFR2) illustrates testing of this principle in vivo in mouse models of inflammatory disease.
Exon 7 in TNFR2 pre-mRNA codes for a trans-membrane domain in the membrane-bound receptor protein. Removal of this domain has a dual effect: it generates a soluble, secreted form of the receptor (Δ7TNFR2), which acts as a decoy receptor, binding TNF-α and removing it from the bloodstream, and it reduces the level of functional, membrane-bound TNFR2. TNFR2 mediates the activity of tumour necrosis factor-α (TNF-α), a cytokine induced in rheumatoid arthritis, psoriasis and other inflammatory diseases. In mice, injections of exon 7–targeted oligonucleotides with locked nucleic acid backbone (LNA)(Fig. 2, top) at 25 mg/kg induced high levels of Δ7TNFR2, which persisted in the circulation for 30 days. This treatment prevented TNF-α-induced acute liver inflammation, and delayed the onset and reduced the severity of collagen-induced arthritis (72). LNA SSO could potentially be injected less frequently than the currently marketed drug etanercept, and because patients would express their own Δ7TNFR2, the potential for an immune response would be reduced. Another recent approach was to reduce TNF-α directly by an oligonucleotide that intercalates into the DNA of TNF-α gene inhibiting its expression. (73)
RNA-blocking oligonucleotides for other indications
Myotonic Dystrophy
Several neuromuscular diseases, including Huntington’s disease, spinocerebellar ataxia, and myotonic dystrophy, are caused by expansion of triplet repeats, most frequently, CTG and CAG. In healthy individuals the repeats are 5 to 35 triplets in length but this number may increase in their descendents and cause disease when the expansion reaches 80 to >2500 repeats. While in Huntington’s disease and ataxias, defective proteins are produced that cause disease, in myotonic dystrophy the CTG repeat is in the 3′-untranslated region (3′UTR) of the DMPK gene and in principle the correct DMPK protein could still be produced. However, the repeat generates a defective, expanded CUG repeat containing RNA (CUGexp mRNA), which is not correctly spliced and accumulates in the cell nucleus in distinct foci, preventing export of DMPK mRNA to the cytoplasm [74] More importantly, the repeat in the accumulated CUGexp mRNA tightly binds and traps a splicing factor, muscleblind-like 1 (MBNL1) protein, which is then unavailable for splicing of pre-mRNA from several other genes, including the muscle-specific chloride channel (CLCN1). Lack of this channel results in muscle hyperexcitability, causing myotonia (75).
In a mouse model of myotonia, a 25-mer PMO-based compound complementary to the CUG repeat displaced bound MBNL-1 from the repeat and dispersed RNA foci in the fibres of an injected muscle (Fig. 3D). Moreover, this treatment restored correct splicing of CLCN1 and other affected genes. Correction of CLCN1 mRNA rescued production of the chloride channel protein and restored its transmembrane chloride ion conductance. As a result, myotonia of the treated muscle was markedly reduced (76). Similar results were obtained by intramuscular injections of 21-mer 2′-OMe PS oligonucleotides (77). Interestingly, the oligonucleotide treatment may not only halt the course of the disease but also prevent its onset. Direct injection of CAG-repeat-targeted LNA oligonucleotide into muscle of the myotonic mouse suppressed further expansion of the repeat. This suggests that early oligonucleotide treatment may stabilize the repeats at subpathogenic lengths. (78)
Targeting splicing, as described in the above sections, shows that the splicing machinery, in spite, or perhaps because of its complexity and plasticity, provides an amenable and remarkably broad platform for manipulation by oligonucleotides. The achievable effects include repair of splicing defects and restoration of protein function in SMA and β-thalassemia or in the case of DMD, repair of mRNA in which deletions affect protein translation. Those approaches led to generation of partially functional dystrophin proteins. The possibility that a novel, clinically useful protein can be generated by manipulation of alternative splicing is exemplified by Δ7TNFR2, which showed anti-inflammatory effect in mice. Finally, simple blocking of the binding of a splicing factor to an extended triplet repeat has pleiotropic effects because it restores the correct splicing and expression of several genes, ameliorating myotonic dystrophy, a disorder whose etiology only became understood in the last few years.
Antibacterials and antivirals
In addition to inducing production of functional proteins, RNA blocking oligonucleotides can also be used to down-regulate the production of undesirable proteins by several mechanisms. These include redirection of splicing that prevents protein production, blocking of protein translation (Fig. 1C), and generation of a so-called external guide sequence (EGS), which guides the tRNA-processing ribozyme, RNase P, to cleave EGS-targeted mRNA (Fig. 1D). Several reports showed the effectiveness of these approaches as antibacterial and antiviral treatments, as we now highlight below.
Oligonucleotides are rarely tested as antibacterials, because the bacterial cell wall presents a formidable obstacle to entry of the oligonucleotide into the cell. Surprisingly, very short, 11-mer, PMOs administered to E. coli-infected mice downregulated the expression of the acyl carrier protein (AcP), which is essential for E. coli lipid biosynthesis [79]. Further work demonstrated that oligomers with the same sequence but either conjugated to a cell penetrating peptide (PPMO) or containing positively charged subunits within the backbone (PMOplus) (Fig. 2, bottom) were even more effective. All mice treated with either chemistry survived, whereas all control mice died 12 h post-infection. On a molar basis, PPMO was more effective than ampicillin, a small-molecule antibiotic (80, 81).
PPMOs were also used to inhibit bacterial growth and expression of specific genes in cultures of E. coli (Gram-negative) and B. subtilis (Gram-positive) bacteria. A unique feature of this work (82) is that PPMOs acted as EGSs, which are short RNAs designed bind to targeted mRNA and form a complex with a three-dimensional structure that somewhat resembles tRNA (Fig. 1D). This structure is recognized in bacterial and eukaryotic cells by a tRNA-processing ribozyme, RNase P, which cleaves the EGS-bound mRNA at the first base-pair of the duplex formed by the EGS and the target mRNA (see 83 for review). Surprisingly, when mRNA was hybridized to an EGS with a PMO backbone that was chemically very different from that of RNA (Fig. 2), the complex was still recognized and the mRNA cleaved by RNase P. Indeed, treatment of chloramphenicol-resistant E. coli with a mixture of two PPMO EGSs targeted to chloramphenicol-resistance gene mRNA reduced bacterial survival by 99.9% in the presence of the antibiotic compared to chloramphenicol-only treated bacteria (84). This efficacy suggests that the PPMO EGS approach may be effective against infections by antibiotic-resistant strains of bacteria.
A recent paper summarized the effects of PPMOs conjugated to a different basic peptide (84). This peptide, derived from a protein from human T cells and with no biological activity on its own, was a remarkable facilitator of transport of a covalently attached PMO into bacteria. The new conjugate is 10- to 100-fold more effective in killing a wide variety of bacteria — including E. coli, S. aureus and Klebsiella —than the previously tested compounds. The new conjugate is also effective in reducing survival of drug-resistant bacteria. If the PMO conjugates with sequences targeted to eukaryotic mRNAs and designed to be recognised as an EGS by eukaryotic RNase P are delivered to eukaryotic cells, the new agent might also be effective against eukaryotic gene targets such as viral RNAs (85)
The effective treatment of monkeys infected with Ebola or Marburg viruses by PMO-based compounds provides a striking example of therapy by RNA-blocking morpholino oligomers that inhibit viral mRNA translation (86). Ebola and Marburg are deadly hemorrhagic viruses, which in an outbreak kill the majority of infected individuals. The only countermeasure is quarantine and prevention of the spread of the virus.
The results indicate that treatment of virus-infected (~1000-fold the minimum lethal dose) macac monkeys with PMOplus drug candidates for Ebola Zaire (AVI-6002) and Marburg (AVI-6003) resulted in 60% and 100% survival, respectively, at 15 days post-infection. This level of survival has not been observed previously; untreated monkeys on average survive no more than 10–11 days (86 and Patrick Iversen, personal communication). At 15 days post infection, both viruses were undetectable in the blood of treated animals. Furthermore, surviving monkeys were resistant to infection when re-challenged with the virus 60 days after initial treatment, suggesting that the animals were able to mount an effective immune response once the infection progress was slowed down (Patrick Iversen, personal communication. The first safety clinical trials, (ClinicalTrials.gov NCT01353027 and NCT01353040) for the AVI-6002 and AVI-6003 have been initiated (http://www.avibio.com/our-programs/infectious-diseases/hemorrhagic-viruses/).
AVI-6002 and AVI-6003 each target two independent genes that are essential for viral survival. This same double-target approach was used against several other viruses that included clinically important RSV, HSV1 (87,88), the picornavirus family (89) and influenza viruses (90–92). Oligomers were targeted to the AUG sites or the highly conserved terminal sequence of appropriate genes. This double-target design is anticipated to minimize the emergence of resistant mutant viruses (86).
The increasing prevalence of bacterial and viral outbreaks and drug-resistant strains of these organisms indicate that there is a need for novel anti-bacterial and anti-viral drugs. Oligonucleotides may be a rapid, albeit for now expensive, answer to this problem.
Outlook
The only two approved nucleic-acid-based drugs, fomivirsen (3), an antisense oligonucleotide, and pegaptanib, an RNA aptamer (4) which is not discussed in this review, have reached the market and both are delivered locally by intravitreal injection. It could be argued that oligonucleotide-based drugs cannot be effective systemically because these large polar molecules do not follow the so-called Lipinski rule 93). This empirical rule states that among other characteristics effective drugs invariably have molecular weights below 500 Daltons and are fairly soluble in both polar and non-polar solvents. Although oligonucleotides clearly do not meet these criteria, in our opinion, which is supported by the results reviewed above, the field is on the verge of success. Novel chemistries that enhance oligonucleotide cellular uptake are especially promising. Likewise, the potential of RNA therapeutics to reach heretofore undruggable targets and affect untreatable diseases certainly warrants the efforts to turn these compounds into effective drugs.
A comparison to monoclonal antibodies (mAbs) is instructive. These molecules are as far from Lipinski rule as are oligonucleotides and yet they have now reached a worldwide market of over 40 billion dollars and are used against serious chronic diseases such as cancer, multiple sclerosis and rheumatoid arthritis. Antigen specific mAbs were first produced in 1975 (94), but with the exception of a single mAb approved by FDA in 1986 the majority reached the market about 10 years later (95). We believe that oligonucleotide and RNA therapeutics have the potential to follow a similar or shorter timeline and reach a similar level of success.
Table 1.
| Steric blocking oligonucleotides | Antisense oligonucleotides | siRNA | External Guide Sequence | ||
|---|---|---|---|---|---|
| Technology | Splice switching oligonucleotide (SSO) | Translation suppressing oligonucleotide (TSO) | Antisense oligonucleotide (ASO) (for examples, hybrid ASO and gapmer ASO | Short interfering RNA (siRNA) | External guide sequence (EGS)*. |
| Target | re-mRNA | mRNA | mRNA | mRNA | mRNA |
| Mechanism of action | blocks access of splicing machinery to splicing elements in targeted pre-mRNA, redirecting the process to a different pathway. | blocks access of the translation machinery, in particular ribosomes, to the translation initiation codon, in targeted mRNA. | induces degradation of targeted mRNA by RNase H, reducing translation of undesirable protein. | siRNA induces degradation of targeted mRNA by components of RISC, reducing translation of undesirable protein. | binds to targeted mRNA, forming a structure that is recognized by RNase P, which cleaves mRNA, preventing translation of undesirable protein |
| Outcome | Redirection of splicing to prevent generation of undesirable splice variant proteins Generation of desired mRNA splice variants and production of desired proteins. | Inhibition of translation of undesirable protein | Degradation of mRNA, preventing translation of undesirable protein | Degradation of mRNA, preventing translation of undesirable protein | Degradation of mRNA, preventing translation of undesirable protein |
| Compatible chemistries | Chemically modified oligonucleotides that are highly resistant to degradation by cellular enzymes and do not support activity of RNase H or RISC. E.g. PMO, LNA, 2′OMe or 2′MOE fully substituted oligonucleotides | Chemically modified oligonucleotides that are highly resistant to degradation by cellular enzymes and do not support activity of RNase H or RISC. E.g. PMO, LNA, 2′OMe or 2′MOE fully substituted oligonucleotides | Oligonucleotides with chemically modified flanks that are resistant to degradation by cellular enzyme and do not support RNase H activity, and a central core that allows RNase H degradation of target mRNA. Eg. Oligonucleotides with 2′OMe, 2′MOE, LNA flanks and a deoxynucleotide phosphorothio ate core | Short, 21–23 nucleotide long, double stranded RNA, with limited chemical modifications that allow siRNA to degrade targeted mRNA via RISC. Eg. 1–2 2′OMe nucleotides at the ends of the RNA and a single 2′OMe nucleotide close to the centre of the strand | Short RNAs and oligonucleot ides that form tRNA-like structures with targeted mRNA and allow cleavage of mRNA by RNase P. E.g. Short, ≤20 nucleotides long RNA or PMO |
| Advantages/applications | High specificity; restoration of defective proteins in rare disease | High specificity; effective inhibition of translation in viruses and drug-resistant bacteria | High specificity; effective degradation of mRNA in liver diseases | High specificity; very effective in degrading mRNA in cell culture. Effective in local delivery in lung, eye | High specificity; particularly effective inhibiting growth of drug-resistant bacteria |
| Disadvantages | Poor intracellular uptake Chemistry-dependent toxicities and plasma stability |
Poor intracellular uptake Chemistry dependent toxicities |
Poor intracellular uptake Chemistry dependent toxicities |
Poor intracellular uptake Chemistry dependent toxicities Relatively poor stability in plasma Inherent off-target effects can be managed by careful design |
Poor intracellular uptake Chemistry dependent toxicities |
Short RNA-forming structured complexes with targeted mRNA that are recognized by a tRNA processing enzyme, RNase P
Box 2. Pre-mRNA splicing as a therapeutic target.
The discovery of RNA splicing (104, 105) was followed by the identification of an intronic mutation in the human β-globin gene, which corrupted splicing of β-globin pre-mRNA, even though the natural splice sites were not mutated and remained potentially functional (106, 107). That defect prevented proper translation of β-globin protein, causing β-thalassemia, a genetic blood disorder. The single point mutation in a β-globin intron 1 created an additional, aberrant 3′ splice site, which redirected the spliceosome away from the natural 3′ splice site. This aberrant splicing pathway, which occurred even though the correct 3′ splice was still present in the pre-mRNA, suggested that splice-site selection occurs by competition between splice sites for the components of the spliceosome assembling on the pre-mRNA. If this hypothesis was correct it seemed likely that blocking the aberrant 3′ splice site with an oligonucleotide might redirect the spliceosome back to the natural splice site, restoring proper splicing and translation of β-globin protein, with obvious potential for clinical application. This ability to repair the RNA and resurrect the missing protein, leading to amelioration of disease, is the key distinguishing feature of oligonucleotide-induced modulation of pre-mRNA splicing. It cannot be achieved by siRNA or classical antisense gapmer oligonucleotides, which lead only to degradation of the targeted RNA (25). The discovery of alternative splicing showed that this mechanism of competition between splice sites is not limited to just mutant genes, but that it also controls expression of the vast majority of native, properly functioning genes (108). Thus, the splice switching oligonucleotides (SSO) form a basis for a platform technology with a potential to treat many diseases.
The repair of defective β-globin pre-mRNA, which restored correct splicing of β-globin mRNA, was first demonstrated in splicing extracts from HeLa cells (65). Incidentally, the notion that this approach may be of clinical relevance was considered farfetched at the time. A reviewer of our paper opined that the experiments are “molecular gymnastics that will never amount to anything”. Yet splice-switching oligonucleotides (SSO) delivered systemically have proven effective in vivo in animal models of thalassemia (67, 68), spinal muscular atrophy (SMA) (61–63) and Duchenne muscular dystrophy (26), three devastating genetic disorders that affect children. More importantly, the application of SSO as drugs for treatment of DMD has already been tested in intramuscular clinical trials (29, 30) and in recently completed systemic clinical trials (31,32).
Glossary terms
- RNA intereference (RNAi)
A form of post-transcriptional gene silencing in which expression or transfection of double stranded RNA induces degradation by nucleases of the homologous endogenous transcripts, resulting in reduction or loss of gene activity
- Short interfering RNAs (siRNA)
synthetic, short, 21–22 nucleotide long double-stranded RNAs with chemical modifications designed to increase their stability and cellular uptake. One strand of siRNA hybridizes to target mRNA and allows mRNA degradation
- RNA-Induced Silencing Complex (RISC)
a multi-protein complex, which when combined with siRNA effects mRNA degradation. A key component of RISC is an endonuclease, AGO2, which cleaves the targeted mRNA within the siRNA-mRNA duplex.
- External Guide Sequence (EGS)
a short RNA designed to bind to targeted mRNA and form a structure that is recognized by a tRNA processing ribozyme, RNase P. RNase P cleaves the mRNA, downeregulating the function of a targeted gene.
- Antisense oligonucleotide (ASO)
defined here as oligonucleotides that bind to complementary mRNA by base pairing and induce cleavage of target mRNA by RNase H, an enzyme that degrades RNA in RNA-DNA duplex
- Splice-switching oligonucleotide (SSO)
Chemically modified oligonucleotides that block sequences in pre-mRNA that are involved in pre-mRNA splicing and redirect mRNA splicing pathways. SSO-pre-mRNA duplex is not recognized by RNase H or RISC and the pre-mRNA is not cleaved.
- Translation suppressing oligonucleotide (TSO)
modified oligonucleotides that block mRNA sequences near the initiation of translation codon, AUG, interfere with the binding of ribosomes to mRNA and inhibit translation of undesirable proteins. TSO-mRNA duplex is not recognized by RNase H or RISC and the mRNA is not cleaved.
- Cryptic splice site
a splice site that is not functional under normal conditions but becomes activated if a mutation modifies its sequence to resemble a functional splice site or an adjacent normal splice site is inactivated by a mutation.
- Exon and intron splicing enhancer and silencer sequences
sequences present in pre-mRNA that contribute to modulation of splicing. They can enhance or inhibit splicing, that is, inclusion of a given exon into mature mRNA, and can be located either within the exons or introns.
- Translational reading frame
Arrangement of mRNA nucleotides into triplets (codons) that when read by the ribosome are translated into one amino acid per codon. The reading frame usually starts with a translation initiation codon, AUG. For example AUG UUU ACA GCA. A deletion of a nucleotide, for example U, changes the reading frame to AUG UUA CAG CA, preventing translation of a desired protein. [Mariam - the example might be problematic if we cannot use bold]
- Alternative splicing
Splicing of pre-mRNA such that a pre-mRNA is spliced to yield more than one kind of mRNA, that is, different splice variants, frequently including or excluding an exon.
References
- 1.Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nature Rev Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodchild J. Therapeutic oligonucleotides. Methods Mol Biol. 2011;764:1–15. doi: 10.1007/978-1-61779-188-8_1. [DOI] [PubMed] [Google Scholar]
- 3.Crooke ST. Vitravene — another piece in the mosaic. Antisense Nucleic Acid Drug Dev. 1998;8:vii–viii. doi: 10.1089/oli.1.1998.8.vii. [DOI] [PubMed] [Google Scholar]
- 4.Moshfeghi AA, Puliafito CA. Pegaptanib sodium for the treatment of neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2005;14:671–682. doi: 10.1517/13543784.14.5.671. [DOI] [PubMed] [Google Scholar]
- 5.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 6.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nature Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 8.Song E, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nature Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 9.Watts JK, Deleavey GF, Damha MJ. Chemically modified siRNA: tools and applications. Drug Discov Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 10.DeVincenzo J, et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN - RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Semple SC, et al. Rational design of cationic lipids for siRNA delivery. Nature Biotech. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 12.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nature Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 13.Bonetta L. RNA-based therapeutics: ready for delivery? Cell. 2009;136:581–584. doi: 10.1016/j.cell.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Couzin-Frankel J. Drug research. Roche exits RNAi field, cuts 4800 jobs. Science. 2010;330:1163. doi: 10.1126/science.330.6008.1163. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM. Is RNAi dead? Mol Ther. 2011;19:1001–1002. doi: 10.1038/mt.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein CA. Does antisense exist? Nature Med. 1995;1:1119–1121. doi: 10.1038/nm1195-1119. [DOI] [PubMed] [Google Scholar]
- 17.Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci USA. 1978;75:280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima W, Wu H, Crooke ST. In: Antisense Drug Technology: Principles, Strategies, and Applications. 2. Crooke ST, editor. CRC Press; Boca Raton, Florida: 2007. pp. 47–74. [Google Scholar]
- 19.Raal FJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 20.Chi KN, et al. Randomized Phase II study of docetaxel and prednisone with or without OGX -011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:4247–4254. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]
- 21.Chia S, et al. Phase II trial of OGX - 011 in combination with docetaxel in metastatic breast cancer. Clin Cancer Res. 2009;15:708–713. doi: 10.1158/1078-0432.CCR-08-1159. [DOI] [PubMed] [Google Scholar]
- 22.Smith CC, Aurelian L, Reddy MP, Miller PS, Ts’o PO. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci USA. 1986;83:2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stirchak EP, Summerton JE, Weller DD. Uncharged stereoregular nucleic acid analogs: 2. Morpholino nucleoside oligomers with carbamate internucleoside linkages. Nucleic Acids Res. 1989;17:6129–6141. doi: 10.1093/nar/17.15.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nature Rev Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sazani P, Graziewicz M, Kole R. In: Antisense Drug Technology: Principles, Strategies, and Applications. 2. Crooke ST, editor. CRC Press; Boca Raton: 2007. pp. 89–114. [Google Scholar]
- 26.Lu QL, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sazani P, et al. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nature Biotech. 2002;20:1228–1233. doi: 10.1038/nbt759. [DOI] [PubMed] [Google Scholar]
- 28.Dillman J, et al. Efficient and persistent splice switching by systemically delivered LNA oligonucleotides in mice. Mol Ther. 2006;14:471–475. doi: 10.1016/j.ymthe.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 29.van Deutekom JC, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 30.Kinali M, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof –of - concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goemans NM, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 32.Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, Phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Ommen GJ, van Deutekom J, Aaartsma-Rus A. The therapeutic potential of antisense-mediated exon skipping. Curr Opin Mol Ther. 2008;10:140–149. [PubMed] [Google Scholar]
- 34.Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2:731–740. doi: 10.1016/s1474-4422(03)00585-4. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo M, et al. Exon skipping during splicing of dystrophin mRNA precursor due to an intraexon deletion in the dystrophin gene of Duchenne muscular dystrophy kobe. J Clin Invest. 1991;87:2127–2131. doi: 10.1172/JCI115244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aartsma-Rus A, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat. 2009;30:293–299. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- 37.Wilton SD, et al. Specific removal of the nonsense mutation from the mdx dystrophin mRNA using antisense oligonucleotides. Neuromuscul Disord. 1999;9:330–338. doi: 10.1016/s0960-8966(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 38.Bulfield G, Siller W, Wight P, Moore K. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu QL, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nature Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- 40.Takeshima Y, et al. Oligonucleotides against a splicing enhancer sequence led to dystrophin production in muscle cells from a Duchenne muscular dystrophy patient. Brain Dev. 2001;23:788–790. doi: 10.1016/s0387-7604(01)00326-6. [DOI] [PubMed] [Google Scholar]
- 41.van Deutekom JC, et al. Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum Mol Genet. 2001;10:1547–1554. doi: 10.1093/hmg/10.15.1547. [DOI] [PubMed] [Google Scholar]
- 42.Heemskerk HA, et al. In vivo comparison of 2′-O-methyl phosphorothioate and morpholino antisense oligonucleotides for Duchenne muscular dystrophy exon skipping. J Gene Med. 2009;11:257–266. doi: 10.1002/jgm.1288. [DOI] [PubMed] [Google Scholar]
- 43.Alter J, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nature Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- 44.Yokota T. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muntoni F, Wood MJ. Targeting RNA to treat neuromuscular disease. Nature Rev Drug Discov. 2011;10:621–637. doi: 10.1038/nrd3459. [DOI] [PubMed] [Google Scholar]
- 46.Sazani P, Weller DL, Shrewsbury SB. Safety pharmacology and genotoxicity evaluation of AVI-4658. Int J Toxicol. 2010;29:143–156. doi: 10.1177/1091581809359206. [DOI] [PubMed] [Google Scholar]
- 47.Wu B, et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 2010;17:132–140. doi: 10.1038/gt.2009.120. [DOI] [PubMed] [Google Scholar]
- 48.Heemskerk H, et al. Preclinical PK and PD studies on 2′-O-methyl-phosphorothioate RNA antisense oligonucleotides in the mdx mouse model. Mol Ther. 2010;18:1210–1217. doi: 10.1038/mt.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jearawiriyapaisarn N, et al. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jearawiriyapaisarn N, Moulton HM, Sazani P, Kole R, Willis MS. Long-term improvement in mdx cardiomyopathy after therapy with peptide-conjugated morpholino oligomers. Cardiovasc Res. 2010;85:444–453. doi: 10.1093/cvr/cvp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu B, et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goyenvalle A, et al. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol Ther. 2010;18:198–205. doi: 10.1038/mt.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin H, et al. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol Ther. 2011;19:1295–1303. doi: 10.1038/mt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Youngblood DS, Hatlevig SA, Hassinger JN, Iversen PL, Moulton HM. Stability of cell-penetrating peptide–morpholino oligomer conjugates in human serum and in cells. Bioconjug Chem. 2007;18:50–60. doi: 10.1021/bc060138s. [DOI] [PubMed] [Google Scholar]
- 55.Lefebvre S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 56.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hua Y, Vickers TA, Baker BF, Bennett CF, Krainer AR. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007;5:e73. doi: 10.1371/journal.pbio.0050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh NK, Singh NN, Androphy EJ, Singh RN. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol Cell Biol. 2006;26:1333–1346. doi: 10.1128/MCB.26.4.1333-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hua Y, Vickers TA, Okunola HL, Bennett CF, Krainer AR. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet. 2008;82:834–848. doi: 10.1016/j.ajhg.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams JH, et al. Oligonucleotide-mediated survival of motor neuron protein expression in CNS improves phenotype in a mouse model of spinal muscular atrophy. J Neurosci. 2009;29:7633–7638. doi: 10.1523/JNEUROSCI.0950-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hua Y, et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010;24:1634–1644. doi: 10.1101/gad.1941310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Passini MA, et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med. 2011;3:72ra18. doi: 10.1126/scitranslmed.3001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hua Y, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gibbons R, Higgs DR, Olivieri NF, Wood WG. In: The Thalassaemia Syndromes. 4. Weatherall DJ, Clegg JB, editors. Ch 7. Blackwell Science; Oxford: 2001. pp. 287–356. [Google Scholar]
- 65.Dominski Z, Kole R. Restoration of correct splicing in thalassemic pre-mRNA by antisense oligonucleotides. Proc Natl Acad Sci USA. 1993;90:8673–8677. doi: 10.1073/pnas.90.18.8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis J, et al. A common human β globin splicing mutation modeled in mice. Blood. 1998;91:2152–2156. [PubMed] [Google Scholar]
- 67.Svasti S, et al. RNA repair restores hemoglobin expression in IVS2-654 thalassemic mice. Proc Natl Acad Sci USA. 2009;106:1205–1210. doi: 10.1073/pnas.0812436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie SY, et al. Correction of β654-thalassaemia mice using direct intravenous injection of siRNA and antisense RNA vectors. Int J Hematol. 2011;93:301–310. doi: 10.1007/s12185-010-0727-1. [DOI] [PubMed] [Google Scholar]
- 69.Cao A, Galanello R. β-thalassemia. Genet Med. 2010;12:61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 70.Taylor JK, Zhang QQ, Wyatt JR, Dean NM. Induction of endogenous Bcl-xS through the control of Bcl-x pre-mRNA splicing by antisense oligonucleotides. Nature Biotech. 1999;17:1097–1100. doi: 10.1038/15079. [DOI] [PubMed] [Google Scholar]
- 71.Mercatante DR, Bortner CD, Cidlowski JA, Kole R. Modification of alternative splicing of Bcl-x pre-mRNA in prostate and breast cancer cells. analysis of apoptosis and cell death. J Biol Chem. 2001;276:16411–16417. doi: 10.1074/jbc.M009256200. [DOI] [PubMed] [Google Scholar]
- 72.Graziewicz MA, et al. An endogenous TNF-α antagonist induced by splice-switching oligonucleotides reduces inflammation in hepatitis and arthritis mouse models. Mol Ther. 2008;16:1316–1322. doi: 10.1038/mt.2008.85. [DOI] [PubMed] [Google Scholar]
- 73.Paquet J, et al. Alternative for anti-TNF antibodies for arthritis treatment. Mol Ther. 2011;19:1887–1895. doi: 10.1038/mt.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller JW, et al. Recruitment of human muscleblind proteins to (CUG)n expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee JE, Cooper TA. Pathogenic mechanisms of myotonic dystrophy. Biochem Soc Trans. 2009;37:1281–1286. doi: 10.1042/BST0371281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wheeler TM, et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science. 2009;325:336–339. doi: 10.1126/science.1173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mulders SA, et al. Triplet-repeat oligonucleotide mediated reversal of RNA toxicity in myotonic dystrophy. Proc Natl Acad Sci USA. 2009;106:13915–13920. doi: 10.1073/pnas.0905780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakamori M, Gourdon G, Thornton CA. Stabilization of expanded (CTG)•(CAG) repeats by antisense oligonucleotides. Mol Ther. 2011;19:2222–2227. doi: 10.1038/mt.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geller BL, Deere J, Tilley L, Iversen PL. Antisense phosphorodiamidate morpholino oligomer inhibits viability of Escherichia coli in pure culture and in mouse peritonitis. J Antimicrob Chemother. 2005;55:983–988. doi: 10.1093/jac/dki129. [DOI] [PubMed] [Google Scholar]
- 80.Mellbye BL, Puckett SE, Tilley LD, Iversen PL, Geller BL. Variations in amino acid composition of antisense peptide-phosphorodiamidate morpholino oligomer affect potency against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother. 2009;53:525–530. doi: 10.1128/AAC.00917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mellbye BL, et al. Cationic phosphorodiamidate morpholino oligomers efficiently prevent growth of Escherichia coli in vitro and in vivo. J Antimicrob Chemother. 2010;65:98–106. doi: 10.1093/jac/dkp392. [DOI] [PubMed] [Google Scholar]
- 82.Shen N, et al. Inactivation of expression of several genes in a variety of bacterial species by EGS technology. Proc Natl Acad Sci USA. 2009;106:8163–8168. doi: 10.1073/pnas.0903491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lundblad EW, Altman S. Inhibition of gene expression by RNase P. Nature Biotech. 2010;27:212–221. doi: 10.1016/j.nbt.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Wesolowski D, et al. Basicpeptide–morpholino oligomer conjugate that is very effective in killing bacteria by gene-specific and nonspecific modes. Proc Natl Acad Sci USA. 2011;108:16582–16587. doi: 10.1073/pnas.1112561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang X, et al. Engineered external guide sequences effectively block viral gene expression and replication in cultured cells. J Biol Chem. 2011;286:322–330. doi: 10.1074/jbc.M110.158857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warren TK, et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nature Med. 2010;16:991–994. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- 87.Lai SH, et al. Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice. Mol Ther. 2008;16:1120–1128. doi: 10.1038/mt.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eide K, et al. Reduction of herpes simplex virus type-2 replication in cell cultures and in rodent models with peptide-conjugated morpholino oligomers. Antivir Ther. 2010;15:1141–1149. doi: 10.3851/IMP1694. [DOI] [PubMed] [Google Scholar]
- 89.Stone JK, et al. A morpholino oligomer targeting highly conserved internal ribosome entry site sequence is able to inhibit multiple species of picornavirus. Antimicrob Agents Chemother. 2008;52:1970–1981. doi: 10.1128/AAC.00011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gabriel G, Nordmann A, Stein DA, Iversen PL, Klenk HD. Morpholino oligomers targeting the PB1 and NP genes enhance the survival of mice infected with highly pathogenic influenza A H7N7 virus. J Gen Virol. 2008;89:939–948. doi: 10.1099/vir.0.83449-0. [DOI] [PubMed] [Google Scholar]
- 91.Lupfer C, et al. Inhibition of influenza A H3N8 virus infections in mice by morpholino oligomers. Arch Virol. 2008;153:929–937. doi: 10.1007/s00705-008-0067-0. [DOI] [PubMed] [Google Scholar]
- 92.Paessler S, et al. Inhibition of alphavirus infection in cell culture and in mice with antisense morpholino oligomers. Virology. 2008;376:357–370. doi: 10.1016/j.virol.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 94.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 95.Waldmann TA. Immunotherapy: past, present and future. Nature Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 96.Stephenson ML, Zamecnik PC. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci USA. 1978;75:285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsukura M, et al. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci USA. 1987;84:7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agrawal S, Mayrand SH, Zamecnik PC, Pederson T. Site-specific excision from RNA by RNase H and mixed-phosphate-backbone oligodeoxynucleotides. Proc Natl Acad Sci USA. 1990;87:1401–1405. doi: 10.1073/pnas.87.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geary RS, Yu RZ, Levin AA. In: Antisense Drug Technologies: Principles, Strategies, and Applications. 2. Crooke ST, editor. CRC Press; Boca Raton, Florida: 2007. pp. 183–217. [Google Scholar]
- 100.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 101.Agarwala SS, et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951) Eur J Cancer. 2009;45:1807–1814. doi: 10.1016/j.ejca.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 102.Chanan-Khan AA, et al. Phase III randomised study of dexamethasone with or without oblimersen sodium for patients with advanced multiple myeloma. Leuk Lymphoma. 2009;50:559–565. doi: 10.1080/10428190902748971. [DOI] [PubMed] [Google Scholar]
- 103.Morrison T. Genta plunges on failed Phase III survival analysis for Genasense. BioWorld website [online] 2011 [Google Scholar]
- 104.Berget SM, Moore C, Sharp PA. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci USA. 1977;74:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chow LT, Gelinas RE, Broker TR, Roberts RJ. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- 106.Spritz RA, et al. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci USA. 1981;78:2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Busslinger M, Moschonas N, Flavell RA. β+ thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981;27:289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- 108.Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta. 2009;1792:14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]