Abstract
Neurofibromatosis type 2 is an autosomal-dominant multiple neoplasia syndrome that results from mutations in the NF2 tumour suppressor gene located on chromosome 22q. It has a frequency of one in 25 000 livebirths and nearly 100% penetrance by 60 years of age. Half of patients inherit a germline mutation from an affected parent and the remainder acquire a de novo mutation for neurofibromatosis type 2. Patients develop nervous system tumours (schwannomas, meningiomas, ependymomas, astrocytomas, and neurofibromas), peripheral neuropathy, ophthalmological lesions (cataracts, epiretinal membranes, and retinal hamartomas), and cutaneous lesions (skin tumours). Optimum treatment is multidisciplinary because of the complexities associated with management of the multiple, progressive, and protean lesions associated with the disorder. We review the molecular pathogenesis, genetics, clinical findings, and management strategies for neurofibromatosis type 2.
Introduction
Neurofibromatosis type 2 is a multiple neoplasia syndrome that results from a mutation in the NF2 tumour suppressor gene on chromosome 22q12. The disorder occurs in one in 25 000 livebirths and is inherited as an autosomal dominant trait. It has wide phenotypic variability and nearly 100% penetrance by 60 years of age.1,2 Improvements in diagnosis and treatment have led to a rise in the diagnostic prevalence from one in 210 000 in 1992, to one in 100 000 people in 2005.1,2
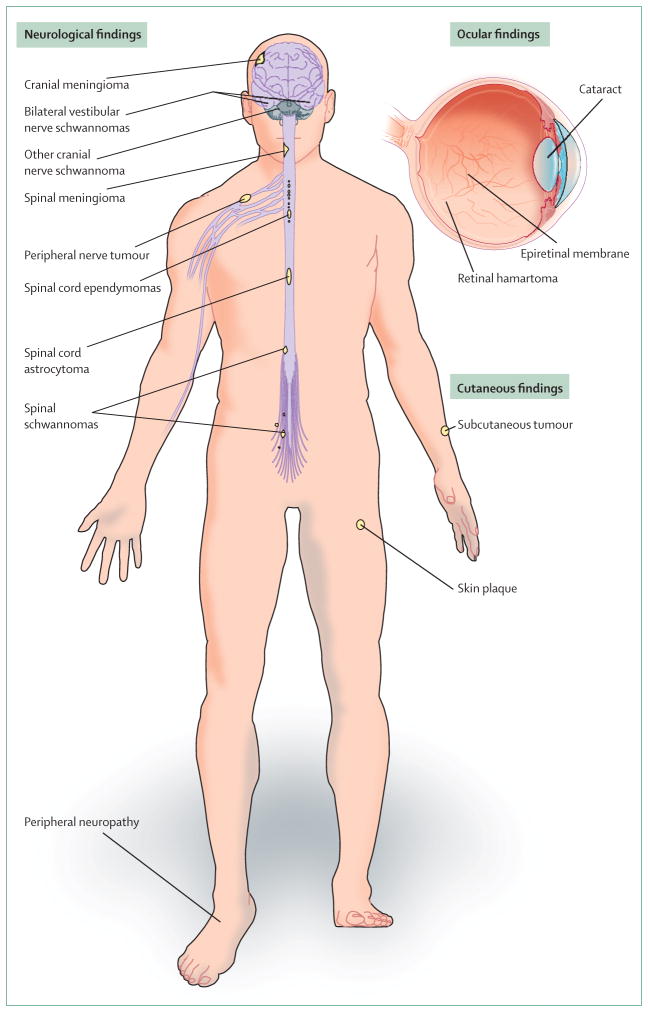
Patients are predisposed to development of lesions of the nervous system, eyes, and skin (figure 1, table 1). Bilateral vestibular nerve schwannomas are the distinctive feature of neurofibromatosis type 2, but affected people can develop schwannomas in other cranial, spinal, and peripheral nerves. Other nervous system tumours associated with the disorder include meningiomas, ependymomas, astrocytomas, and rarely, neurofibromas. Peripheral neuropathies can develop independently of compressive tumours. Ocular abnormal findings include cataracts, epiretinal membranes, and retinal hamartomas. Cutaneous tumours—usually schwannomas—are a frequent finding.
Figure 1.
Clinical manifestations of neurofibromatosis type 2
Table 1.
Frequency of lesions associated with neurofibromatosis type 2
| Frequency of association with NF2 | |
|---|---|
| Neurological lesions | |
| Bilateral vestibular schwannomas | 90–95%3–5 |
| Other cranial nerve schwannomas | 24–51%4–9 |
| Intracranial meningiomas | 45–58%3–5,10 |
| Spinal tumours | 63–90%4,5,10–13 |
| Extramedullary | 55–90% |
| Intramedullary | 18–53% |
| Peripheral neuropathy | Up to 66%4,5,14 |
|
| |
| Ophthalmological lesions | |
|
| |
| Cataracts | 60–81%3–5,15 |
| Epiretinal membranes | 12–40%15,16 |
| Retinal hamartomas | 6–22%4,5,17 |
|
| |
| Cutaneous lesions | |
|
| |
| Skin tumours | 59–68%3,4,18,19 |
| Skin plaques | 41–48% |
| Subcutaneous tumours | 43–48% |
| Intradermal tumours | rare |
NF2=neurofibromatosis type 2.
Search strategy and selection criteria.
We searched PubMed using the search term “neurofibromatosis type 2” or “NF2” in combination with the terms “nervous system”, “schwannoma”, “meningioma”, “ependymoma”, or “cataract”. We also searched the reference lists of reports identified by this search strategy and selected those we judged relevant. We have used mainly reports published between December, 1998, and December, 2008, but have included relevant landmark publications before December, 1998. Pertinent articles not identified by this search were also referenced.
The multiple, progressive, and protean features associated with neurofibromatosis type 2 present substantial management challenges. Patients should be managed in specialty centres with a skilled multidisciplinary team, consisting of a neurosurgeon, otolaryngologist, neurologist, geneticist, ophthalmologist, pathologist, radiologist, audiologist, and experienced nursing staff.20,21 Optimum management includes screening of populations at risk, early diagnosis, close surveillance, and development of treatment frameworks on the basis of the natural history of each associated feature (panels 1 and 2).
Panel 1. Patient populations at risk for neurofibromatosis type 2.
First degree relative with neurofibromatosis type 2 (affected parent, sibling, or children)
People younger than age 30 years with a unilateral vestibular schwannoma or meningiomas
People with multiple spinal tumours (schwannomas, meningiomas)
People with cutaneous schwannomas
Data from Evans and co-workers.21
Panel 2. Recommended intervals for screening children of an affected parent.
Ophthalmological examination yearly from infancy
Neurological examination yearly from infancy
Audiology with auditory brainstem evoked potentials yearly from infancy
Presymptomatic genetic testing; one test from 10 years of age*
Cranial MRI at 10–12 years of age*
Spinal MRI at 10–12 years of age* (every 2–3 years)
Other populations at risk (panel 1) should have complete screening (excluding genetic testing) at the time of risk factor identification.*Earlier than age 10 years in severely affected families and families in which early detection of disease would aid family preparation for future events related to neurofibromatosis type 2. Data from Evans and co-workers.21
Molecular biology
The NF2 tumour suppressor gene was identified in 1993. It has 17 exons that encode for a 69 kDa protein product called merlin (moesin-ezrin-radixin-like protein) or schwannomin.22,23 Consistent with Knudson’s two-hit hypothesis of tumorigenesis, tumour formation initiates when both alleles of this gene are inactivated.24 Patients either inherit a germline mutation of one affected allele from a parent or acquire a de novo mutation of an allele at the postzygotic stage of embryogenesis. Subsequently, tumours develop in susceptible target organs (ie, nervous system, eyes, and skin) from cells that lose function of the wild type (normal) NF2 allele.
Somatic inactivation of both alleles of this gene happens in sporadic schwannomas (>90%), meningiomas (50%), and ependymomas (5%).25,26 Conditional mouse models with selective inactivation of the NF2 gene in Schwann cells or leptomeningeal cells develop schwannomas and meningiomas, respectively.27,28 These findings and models support the tumour suppressor role of merlin and provide a powerful method for investigation of genetic interactions, protein function, and potential targeted treatments.
Two isoforms (I and II) of merlin exist in people.29,30 Only isoform I is able to undergo the necessary protein folding that enables tumour suppressor activity.31 Merlin is a 595 aminoacid protein encoded by exons 1–15 and 17. It is composed of three domains: a tri-lobed aminoterminal protein 4·1-ezrin–radixin–moesin (FERM) domain, an α helical domain, and a carboxyterminal domain (figure 2). Phosphorylation is a key regulator of merlin conformation and tumour suppressive activity. Phosphorylation at serine-518 by p21-activated kinases and cyclic AMP dependent-protein kinase A prevents intramolecular folding, resulting in inactivation and relocalisation of merlin (open form of merlin).31–36 Reversal of serine-518 phosphorylation is caused by myosin phosphatase-1 protein phosphatase-1δ. Dephosphorylated merlin assumes its closed and active state through intramolecular association within the FERM domain and then between the FERM and carboxyterminal domains. Upstream regulation of p21-activated kinases, cyclic AMP dependent-protein kinase A, and myosin phosphatase-1 protein phosphatase-1δ activity help determine the phosphorylation status of merlin. Merlin is a unique tumour suppressor protein because it localises to the cell membrane–cytoskeletal interface.37
Figure 2. Molecular biology of merlin.
(A) Merlin isoform I has three structural regions: a tri-lobed aminoterminal protein 4·1-ezrin-radixin-moesin (FERM) domain, an α helical domain, and a carboxyterminal domain. PKA=cAMP dependent protein kinase A. PAK=p21-activated kinases. MYPT-1-PP1 δ=myosin phosphatase-1 protein phosphatase-1. ser518=serine-518. (B) Active state of merlin. In this state, merlin mediates its tumour suppressor activity through the downstream regulation of various mitogenic intracellular signalling pathways. Most important of these activities is its effects on the phosphoinositide-3 kinase (PI3K)-signalling pathway (PI3K–Akt–MTOR) and the mitogen-activated protein kinase (MAPK) signalling pathway (Ras–Raf–MEK–ERK). EGFR=endothelial growth factor receptor. PIKE-L=phosphatidylinositol 3-kinase enhancer long form. eiF3c=eukaryotic initiation factor 3 subunit c. TRBP=transactivation responsive RNA binding proteins. RalGDS=Ral guanine-nucleotide dissociation stimulator. Rho GTPases=Rho guanosine triphosphatases. N-WASP=Neuronal Wiskott-Aldrich syndrome protein.
Abnormal or absent merlin function (as in neurofibromatosis type 2) can disrupt tumour suppression via various mechanisms, but the importance of merlin’s many protein interactions in this process has not been fully elucidated. Merlin’s tumour suppressive effects seem to be mediated indirectly through its membrane organisation of proteins (ie, CD44, epidermal growth factor receptor, layilin), cell-to-cell adhesion (ie, β catenin ε cadherin, β1 integrin, paxillin), and cytoskeletal architecture (ie, βII spectrin, F-actin, Rho guanosine triphosphatases, neuronal Wiskott-Aldrich syndrome protein), or through interaction with cytosolic proteins (ie, phosphatidylinositol 3-kinase enhancer long form, eukaryotic initiation factor 3 subunit c, transactivation responsive RNA binding proteins, and Ral guanine-nucleotide dissociation stimulator). These effects result in downstream regulation of various mitogenic signalling pathways, including, most importantly, the phosphoinositide-3 kinase (PI3K) signalling pathway and the mitogen-activated protein kinase (MAPK) signalling pathway (figure 2).38–45 These oncogenic pathways are crucial for promotion of cell growth, protein translation, and cellular proliferation.
Drugs targeting these pathways (ie, sorafenib, trastuzumab, lapatinib, LY294002, protein kinase inhibitors, and p21-activated kinase inhibitors) and tumour angiogenesis (bevacizumab) are under preclinical or early clinical investigation and are potential treatments for neurofibromatosis type 2.
Diagnosis and clinical presentation
Diagnosis is based on clinical criteria; the presence of a constitutional NF2 mutation is not part of present diagnostic criteria. The Manchester diagnostic criteria21—which are the most widely used—include patients without a family history of this disorder or bilateral vestibular schwannoma but who have multiple other related lesions (table 2).3,46,47 Because more than half of patients do not have a positive family history and might present with clinical features other than vestibular schwannoma, the Manchester criteria are more sensitive than were early diagnostic models, while maintaining a high specificity.48 Peripheral neuropathy has yet to be incorporated into clinical classification schemes for diagnosis.
Table 2.
Manchester criteria for clinical diagnosis of neurofibromatosis type 2 (NF2) according to primary finding
| Additional findings needed for diagnosis | |
|---|---|
| Bilateral vestibular schwannomas | None |
| Family history | Unilateral vestibular schwannoma or two NF2-associated lesions (meningioma, glioma, neurofibroma, schwannoma, or cataract) |
| Unilateral vestibular schwannoma | Two NF2-associated lesions associated with the disorder (meningioma, glioma, neurofibroma, schwannoma, or cataract) |
| Multiple meningiomas | Unilateral vestibular schwannoma or two other NF2-associated lesions (glioma, neurofibromas, schwannoma, or cataract) |
Data from Evans and co-workers.21
Neurofibromatosis type 2 generally presents in young adulthood (age 20–30 years) with hearing loss from a vestibular schwannoma. Hearing loss is often unilateral initially, and can be accompanied by tinnitus, dizziness, and imbalance.3,4 Although up to 30% of children might present with the same symptoms, they more frequently present with visual disturbance (cataract, hamartomas, intracranial tumour), skin tumours, mononeuropathy (facial paresis, foot drop), symptomatic spinal cord tumours, or non-vestibular intracranial tumours, than do their adult counterparts.49–51 Therefore, of special importance is that clinicians screening young children examine the skin and eyes and undertake a thorough neurological examination. Furthermore, because more than half of patients have no family history of neurofibromatosis type 2, clinicians should be aware of other populations at risk (panel 1).
Genetics and clinical phenotypes
Th neurofibromatoses consist of three distinct diseases—neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. Neurofibromatosis type 2 (formerly central neurofibromatosis or bilateral acoustic neurofibromatosis) was first described in 1822 in a deaf patient with tumours in the skull, dura mater, and brain.52 For several decades, it became inextricably intertwined with neurofibromatosis type 1 (formerly von Recklinghausen’s disease or peripheral neurofibromatosis), which is more common than is neurofibromatosis type 2.53 In 1920, heritability of neurofibromatosis type 2 was described in three generations of a family with vestibular schwannomas.54 Autosomal dominant transmission was reported in 1930 in a family with 38 affected members across five generations.55 In 1987, neurofibromatosis type 1 and type 2 were localised to different chromosomes by genetic linkage analysis, and the two disorders were formally separated.56–58
Type 1 neurofibromatosis is a genetically and phenotypically distinct autosomal-dominant tumour syndrome associated with a mutation in the NF1 tumour suppressor gene located on chromosome 17q11. It is characterised by many café-au-lait maculae, multiple cutaneous and subcutaneous neurofibromas, plexiform neurofibromas, intertriginous freckling, optic pathway gliomas, Lisch nodules, and bony dysplasia.58–60 Schwannomatosis—a third genetically distinct peripheral-nerve tumour syndrome—was then identified and linked with a mutation in the SMARCB1 tumour suppressor gene located on chromosome 22q11.61 Schwannomatosis is characterised by multiple non-intradermal schwannomas in the absence of vestibular schwannomas.62
Results of clinical studies1,4,63 assessing the range of neurofibromatosis type 2 manifestations showed that phenotypic expression and natural history were similar for family members but differed between families. Specific genotype and phenotype correlations have been identified that help to explain the interfamilial clinical heterogeneity. Generally, constitutional NF2 non-sense or frameshift mutations that create truncated proteins are associated with severe disease, whereas mis-sense mutations and inframe or large deletions are associated with mild disease.64,65 Splice-site mutations are associated with variable disease severity, although mutations located within exons 1–5 are related to more severe disease than are mutations on exons 11–15.66
These genotype–phenotype effects extend to relative risk of mortality. Neurofibromatosis type 2 patients with mis-sense mutations have a lower risk of mortality than do patients with non-sense or frameshift mutations.20 Although associations between mutation types and disease features (presence of intracranial meningiomas and cataracts, numbers of spinal tumours and peripheral nerve tumours) exist, the behaviour of specific tumours seems to vary independently of mutation type.6,11,64,67–69
Neurofibromatosis type 2 is an unusual inherited disorder because of the high frequency of somatic mosaicism in patients with de novo mutations.70–72 In patients with mosaicism, the mutation takes place after conception, resulting in two separate cell lineages. Most people with mosaicism carry the mutation in a proportion of their cells that is too small for detection in lymphocytes but can be detected in tumour tissue. The extent of clinical features suggests the time at which the mutation took place during development, and hence, patients with mosaicism tend to have mild generalised or even localised disease (eg, unilateral vestibular schwannoma with ipsilateral tumours).
Only a portion of the germ cells in a person with NF2 mosaicism are likely to carry the mutation, and thus the risk of transmission to offspring will be less than the expected one in two (50%) for an inherited mutation. However, children who inherit the mutation from a mosaic parent will probably have more severe disease than will the parent because the mutation will be present in all their somatic cells. Estimates72 suggest that 33% of de novo patients who present with bilateral vestibular schwannoma and up to 60% of such patients who present with a unilateral vestibular schwannoma have mosaicism.
Presymptomatic genetic testing is an important aspect of the management of families because early diagnosis improves clinical care. Once the mutation has been identified in an affected patient, DNA from family members at risk can be tested for the specific mutation. In a patient with the inherited form of the disease, the combined use of direct gene sequencing of all exons and multiple ligation-dependent probe amplification has a 91–95% sensitivity in the detection of a mutation.21,73 In patients with de novo neurofibromatosis type 2 (who might have mosaicism), tiered analysis with exon scans of blood samples and tumours, and combined deletion and duplication analysis by multiple ligation-dependent probe amplification will detect 80–85% of mutations.74
In the UK, presymptomatic genetic testing of children at risk of neurofibromatosis type 2 is recommended at around age 10 years—the age at which most medical centres would begin MRI screening of these children. Justification for genetic testing at this age is based on the knowledge that, in many families, MRI studies would reveal neurofibromatosis type 2 status before the age of consent.21 In the USA, presymptomatic genetic testing of children who are at risk and younger than the age of consent is tailored to the needs of individual families. Parents’ perceived benefits of knowing that a child carries the mutation are that they can initiate surveillance to detect early disease and prepare the family or patient for future events related to the disorder.75
Although risk of transmission to offspring from parents with an inherited germline mutation has an autosomal-dominant inheritance pattern (one in two risk), the risk of transmission to the children of those with a de novo mutation is substantially lower than for the autosomal-dominant type. De novo neurofibromatosis type 2 patients with a negative blood mutation analysis and bilateral vestibular schwannomas have a one in eight risk of transmission. This risk is further lessened to one in 12 when the patient has only a unilateral vestibular schwannoma. Reduction in risk is proportional to the decreased likelihood that germ cell precursors are within the population of cells harbouring the mutation in each clinical state.72
Neurological manifestations
Vestibular schwannomas
Bilateral vestibular schwannomas are the distinctive feature of neurofibromatosis type 2 and are identified in 90–95% of patients (table 1).3,4,5 Sporadic vestibular schwannomas more frequently originate within the inferior vestibular portion of cranial nerve VIII (vestibulocochlear nerve), but in this disorder this predilection is not present.76–78 Although more than 99% of vestibular schwannomas in neurofibromatosis type 2 are benign, they remain a substantial cause of morbidity because of their location.79
Hearing loss and tinnitus (often unilateral at onset) are the presenting symptoms in 60% of adults and up to 30% of children.18,21,49–51 Although results from a retrospective auditory analysis80 of newly diagnosed patients revealed that hearing in an untreated ear will probably remain stable for up to 2 years, some patients can develop rapid hearing loss that is unrelated to tumour size or growth rate. The rate of hearing loss often differs between the ears in affected individuals.80 Results of a meta-analysis81 of longitudinal studies in people with neurofibromatosis type 2 with vestibular schwannomas show that these tumours have highly variable growth rates that decrease inversely with age.
Vestibular schwannomas vividly enhance and are best seen by high resolution contrast-enhanced, T1-weighted MRI (figure 3). T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI sequences are used to accurately quantify peritumoral oedema and cysts.
Figure 3. MRI and histological features of schwannomas in neurofibromatosis type 2.
(A) Axial T1-weighted contrast-enhanced MRI of a man’s brain reveals bilateral vestibular schwannomas (arrows). Intracanlicular extension (arrowheads) of tumours into the internal auditory meatus is clearly delineated. (B) Axial T1-weighted contrast-enhanced MRI of the cervical spine reveals a dumbbell shaped spinal schwannoma (arrows), showing mass effect on adjacent spinal cord (arrowheads) in a young man. (C) Axial T1-weighted contrast-enhanced, fat-suppressed MRI of a woman’s chest shows a schwannoma (arrow) of an intercostal nerve. (D) Haematoxylin and eosin stained section of an excised vestibular schwannoma reveals a spindle-cell lesion, with cells that assume intermixed dense (Antoni A) (a) and loose (Antoni B) (b) arrangements.
At operation, neurofibromatosis type 2 associated vestibular schwannomas are tan in colour and assume a more lobular growth pattern than do sporadic schwannomas.53,82 Typically, they are also more adherent to adjacent cranial nerves and incorporate more native nerve fascicles.83 Histological investigation reveals a spindle-cell lesion with intermixed dense (Antoni A) and loose (Antoni B) cellular arrangements (figure 3). Verocay bodies, enhanced nuclear pleomorphism, and hyalinised vessels are generally seen.
No direct genotype–phenotype association for hearing loss in neurofibromatosis type 2 has been established, and tumour size and growth rate do not predict hearing status; hence, regional practice patterns and individual practitioner experience seem to most influence management of vestibular schwannomas in neurofibromatosis type 2. Complete surgical resection is curative but the timing of treatment remains controversial and the risks of surgery should be balanced against the tumour’s natural history. Early surgical management of small vestibular schwannomas (less than 3 cm in diameter) can preserve serviceable hearing and normal function of the facial nerves in 30–65% and 75–92% of patients, respectively.7,76 This treatment framework is generally reserved for patients with intact hearing in both ears. Bilateral resection can be thought about when hearing preservation is successful in the first ear. Even when hearing is lost, early removal might allow for maintenance of cochlear nerve integrity, enabling cochlear implantation for auditory restoration.
Overall, surgical results for preservation of hearing and facial nerves in neurofibromatosis type 2—even for small tumours—has generally been much poorer than is reported.7,76 This difference might be attributed to the decentralised delivery of health care and the requisite learning curve surgeons undergo before achieving results similar to those from highly specialised centres.84,85 Thus, conventional management strategies are typically conservative and recommend surgical resection of tumours 2–3 cm in size only if serviceable hearing is lost or rapid growth identified. Patients with tumours that enlarge beyond this size who maintain serviceable hearing should be advised that operative morbidity (facial paralysis, lower cranial nerve injury) rises with tumour growth. Some patients might choose to wait until brainstem compression or raised intracranial pressure necessitate removal, with the understanding that this wait-and-see approach will result in heightened operative risks, including facial paralysis, and inevitable hearing loss.
To avoid the risks of surgery without delaying treatment, researchers in several centres have examined the potential effectiveness of stereotactic radiosurgery for vestibular schwannomas in neurofibromatosis type 2. Small tumours (less than 3 cm) and those not associated with peritumoral oedema or cysts, are most likely to respond to surgery. Local control (represented by stable size) has been reported in 74–100% of vestibular schwannomas in neurofibromatosis type 2 (mean follow-up 54 months).86–89 Measurable hearing and normal function of the facial nerve were maintained in 33–57% and 92–100% of patients, respectively.86–89 Extended follow-up of patients showing continued local control and an absence of malignant disease induced by radiation is needed before stereotactic radiosurgery can be validated as a primary treatment option.79,90 Other concerns are the increased difficulty of preservation of facial nerve function at subsequent operations because of scar formation, and maintenance of cochlear nerve viability for future hearing rehabilitation procedures.
The high rate of bilateral hearing loss associated with bilateral vestibular schwannomas has resulted in the development of strategies to restore auditory function. Patients who are deaf with an anatomically and physiologically intact cochlear nerve are candidates for cochlear implantation that can provide sustained auditory improvement.91,92 When the physiological integrity of the cochlear nerve is compromised, an auditory brainstem implant might be an option for hearing rehabilitation.
Debate exists about the ideal timing for auditory brainstem implantation. Some centres place an auditory brainstem implant at first vestibular schwannoma removal with intent for future use, whereas others wait until second tumour surgery. Neurofibromatosis type 2 patients with these implants might obtain measurable assistance with environmental sound and lip reading, but only a small subset gain significant speech understanding.93,94 The restricted hearing performance achieved through stimulation of the cochlear nucleus with an auditory brainstem implant has led to the investigation of auditory midbrain implants that stimulate the inferior colliculus (proximal auditory pathway site).95
Meningiomas
Meningiomas are the second most common tumour related to neurofibromatosis type 2. Intracranial meningiomas are present in 45–58% of patients and intradural extramedullary spinal meningiomas are present in about 20% (table 1).3,4,5,10 Intracranial meningiomas are frequently multiple and they develop at a younger age than do their counterparts with sporadic cases of meningiomas.4,5,49,51 Up to 20% of children presenting with a meningioma will have neurofibromatosis type 2, thereby necessitating full clinical screening and longitudinal follow-up.51,96 Meningiomas produce clinical symptoms related to their size and anatomical location. Although cerebral convexity meningiomas become large before causing symptoms, small tumours of the optic nerve sheath, skull base, and spinal canal can cause profound symptoms as a result of their close relation with delicate structures. The presence of intracranial meningiomas is associated with a 2 · 5-fold rise in relative risk of mortality.20
Meningiomas homogeneously enhance and are best seen with contrast-enhanced T1-weighted MRI (figure 4). T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI sequences can be used to show peritumoral oedema or cysts. A region of enhancement referred to as a dural tail often extends from the central tumour mass along the dura. They are reddish tan in colour and typically well encapsulated. All major histological subtypes (meningothelial, fibroblastic, psammomatous, and transitional) can develop in neurofibromatosis type 2, although the fibroblastic variant is over represented.98,99 Meningiomas associated with the disorder frequently have heightened proliferative activity and a greater rate of atypical and anaplastic grades than do sporadic meningiomas.98,99 Most meningiomas of the cerebral hemispheres and spinal canal can be resected safely and fully. Because of their anatomical location, complete surgical resection of meningiomas originating from the optic nerve sheath and skull base might be associated a dural tail often extends from the central tumour mass along the dura. They are reddish tan in colour and typically well encapsulated. All major histological subtypes (meningiothelial, fibroblastic, psammomatous, and transitional) can develop in neurofibromatosis type 2, although the fibroblastic variant is over represented.97,98
Figure 4. MRI features of meningiomas in neurofibromatosis type 2.
(A) Axial T1-weighted contrast-enhanced MRI of a woman’s brain show several enhancing intracranial meningiomas (arrows). (B) Sagittal T1-weighted contrast-enhanced MRI of the cervical spine in a young man shows a spinal meningioma (arrows) with classic dural tail sign (green arrowheads). An enhancing intramedullary tumour (probable ependymoma) (black arrowhead) is visible in the adjacent spinal cord.
Meningiomas associated with the disorder frequently have heightened proliferative activity and a greater rate of atypical and anaplastic grades than do sporadic meningiomas.97,98
Most meningiomas of the cerebral hemispheres and spinal canal can be resected safely and fully. Because of their anatomical location, complete surgical resection of meningiomas originating from the optic nerve sheath and skull base might be associated with substantial neurological morbidity.99,100
The limited operability of these tumours in sporadic cases promoted use of adjuvant stereotactic radiosurgery for local control of residual tumour.101,102 Results of a report103 of radiotherapy for meningiomas in neurofibromatosis type 2 showed a 5-year progression-free survival rate of 86%, but the study did not have the long term follow-up needed to address concerns about radiation-induced malignant transformation and adjacent tumour development.104 Until these data are available, clinicians must balance the potential toxicities of radiation therapy with the morbidity of progressive disease and surgical intervention in management of high risk meningiomas.
Spinal cord ependymomas
Ependymomas account for more than 75% of intramedullary spinal-cord tumours associated with neurofibromatosis type 2.11,10,105,106 They are present in 18–53% of patients but cause clinical symptoms in fewer than 20% (table 1).10–13 These intramedullary tumours develop more frequently in neurofibromatosis type 2 patients with constitutional non-sense and frameshift mutations than in those with other NF2 mutations.10
Clinical features of spinal cord ependymomas result from their size and location along the spinal axis. Patients with symptomatic intramedullary spinal cord lesions typically present with recumbent back pain (56%), weakness (28%), or sensory disturbances (16%).107–111 Spinal cord ependymomas are isointense or slightly hyperintense on non-enhanced T1-weighted MRI and enhance homogeneously after contrast administration. Frequently, the presence of multiple lesions along the central canal of the spinal cord can give rise to an appearance resembling a string of pearls on contrast-enhanced MRI (figure 5). Ependymomas are hyperintense on T2-weighted MRI.10–13 A haemosiderin cap (T2-weighted sequences) might be identified at the superior and inferior poles of the tumour.112,113
Figure 5. MRI and histological features of an ependymoma in neurofibromatosis type 2.
(A) Sagittal T1-weighted MRI of the cervical spine in a man reveals a subtle swelling (arrows) of the spinal cord at C7, suggesting the presence of an intramedullary lesion. (B) Sagittal T1-weighted contrast-enhanced MRI in the same patient shows the homogeneous pattern of enhancement in a central location within the spinal cord and frequent multiplicity of ependymomas (string of pearls) (arrows). (C) Haematoxylin and eosin stained section of an excised ependymoma reveals a well delineated, moderately cellular tumour with a monomorphic nuclear morphology that is characterised by round to oval nuclei with salt and pepper speckling of the chromatin (arrows). Occasional perivascular pseudorosette (asterisk) and ependymal rosette arrangements are typical histological findings.
Spinal cord ependymomas are purple–tan lesions. Histopathology typically reveals a WHO grade II lesion displaying a well delineated, moderately cellular tumour with round–oval nuclei. Occasional perivascular pseudorosette and ependymal rosette arrangements can be seen (figure 5). Although surgery for symptomatic spinal cord ependymomas in patients with neurofibromatosis type 2 is effective and curative, serial observation is most often used in the management of asymptomatic tumours. These ependymomas frequently remain quiescent and asymptomatic for many years, obviating the need for surgery with its attendant risks. The strongest predictor of postoperative neurological function after surgery is preoperative neurological status; therefore, detailed neurological surveillance to assess for early onset of signs and symptoms might best determine the timing for resection.107,108,111 Because most tumours are WHO grade II, gross total resection often negates the need for postoperative adjunctive therapy.
Peripheral neuropathy
Most neurofibromatosis type 2 patients will develop peripheral neuropathy during their lifetime. Although many cases can be attributed to tumours within or compressing a nerve, some are not associated with tumours. Results of studies3,4,14 showed that up to 66% of patients develop signs of neural dysfunction in the absence of a compressive tumour (table 1).
Growing recognition of non-tumour-related peripheral neuropathies unrelated to tumours in neurofibromatosis type 2 came from reports114–121 in which patients with focal amyotrophy, distal symmetric sensorimotor neuropathy, or mononeuropathy multiplex have been identified. Age of onset ranged from 7 to 41 years and the duration of symptoms ranged from 3 months to 50 years. Peripheral neuropathy (ie, facial palsy, foot drop) can pre-date the appearance of tumours (ie, vestibular schwannoma, spinal tumours) and can be the sentinel finding in patients who eventually are diagnosed.51
Peripheral neuropathy unexplained by tumour after craniospinal MRI should be investigated with electrodiagnostic studies, including nerve conduction studies and needle electromyography. MR-neurography might be useful for diagnosis of previously unidentified peripheral-nerve tumours or non-neoplastic causes that result in focal neuropathy.122
Potential pathogenetic mechanisms underlying development of peripheral neuropathy in neurofibromatosis type 2 are cumulative compression by multiple tumourlets along the length of an adjacent peripheral nerve, presence of schwannosis (Schwann cell proliferation with entangled axons without frank tumour formation), effects of local toxic or metabolic determinants of pathological cells, or dysfunction of Schwann cells attributable to reduced amounts of merlin dosage from haploinsufficiency.14,123
Histopathology of peripheral nerves in patients with peripheral neuropathy has consistently revealed myelinated and unmyelinated fibre loss and abnormal Schwann cell proliferation with or without an onion bulb-like appearance.14,114–120,123 Treatment of peripheral neuropathies not related to tumours in neurofibromatosis type 2 focuses on symptom management, including medical treatment of neuropathic pain (ie, gabapentin, pregabalin) and use of general principles of preventive and palliative care.
Other neurological manifestations
Schwannomas are the most frequently identified nerve sheath tumour in this condition and can develop along the course of non-vestibular cranial nerves (III–VII, IX–XII), spinal nerves, and peripheral nerves (figure 3). Up to 51% of patients harbour non-vestibular cranial nerve schwannomas that most often arise from cranial nerves III (oculomotor), V (trigeminal), and VII (facial) (table 1).5,7–9 Although tumours of the lower cranial nerves develop less often than do those of the upper cranial nerves, they are more frequently associated with symptoms. Schwannomas do not seem to grow on cranial nerves I (olfactory) and II (optic)—probably because they are central nervous system derivatives and do not have Schwann cell investment.
Schwannomas of the spinal nerve root are frequently multiple, and they account for almost 90% of extramedullary spinal tumours.10,12 Over time, 30% of patients with these schwannomas develop symptoms necessitating excision (table 1).3,10,12 Schwannomas can form along the course of any peripheral nerve, resulting in discrete peripheral neuropathies. Nodular subcutaneous schwannomas are identified in 43–48% of patients and often cause pain and are sensitive to pressure.3,19 Neurofibromas, by contrast, are rarely identified in neurofibromatosis type 2 and are distinguished from schwannomas histologically by their loose and haphazard arrangement of Schwann cells and fibroblasts within a collagen–myxoid matrix. Symptomatic lesions of peripheral nerves are mainly treated by surgical resection.
Intramedullary astrocytomas of the spinal cord (diffuse and pilocytic), and rarely, intramedullary schwannomas have been reported in neurofibromatosis type 2.10–13,105,106,124 Diffuse astrocytomas typically are ill-defined T2-weighted hyperintense lesions that do not enhance on MRI and occupy a more eccentric location within the spinal cord than do ependymomas. Pilocytic astrocytomas and intramedullary schwannomas enhance on T1-weighted MRI and to distinguish between tumours based on imaging can be difficult. Presence of pronounced T2-weighted hyperintensity on MRI within the spinal cord surrounding the lesion is suggestive of a schwannoma.125 Resection remains the treatment for symptomatic lesions.126 Adjunctive radiotherapy can be used to treat residual diffuse astrocytomas and might improve survival in sporadic cases, but the applicability of these findings in neurofibromatosis type 2 remains uncertain.127
Benign intracranial calcified deposits (on CT) are frequently identified within the choroid plexus, cerebellum, and cerebral parenchyma.128 Post-mortem studies have revealed the presence of small dysplastic glial foci in the cerebral cortex and basal ganglia. These glial hamartias (microhamartomas) do not seem to be preneoplastic lesions.129 Meningioangiomatosis—a multifocal cortical plaque-like proliferation of meningovascular elements—might be identified at autopsy but is not associated with epilepsy in neurofibromatosis type 2.130
Ocular manifestations
Lens opacities are an important diagnostic marker. 60–81% of neurofibromatosis type 2 patients have cataracts (table 1).3–5,15 Only cataracts identified in patients younger than 50 years can be regarded as specific to the disorder; including opacities located in the posterior subcapsular or capsular, and peripheral cortical region of the lens (figure 6).131,132 Small size and peripheral location of cortical wedge opacities necessitates a careful ophthalmological examination after maximum dilation. Cataracts interfere with vision in 10–25% of patients and might need extraction.5,15,133
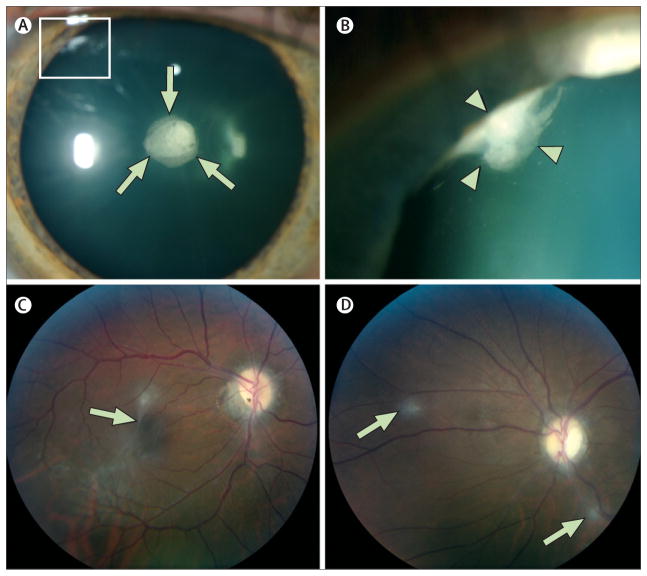
Figure 6. Ocular findings in neurofibromatosis type 2.
(A) Slit-lamp examination in a man reveals a cataract in the posterior subcapsular and capsular (arrows) region and a wedge cataract in the peripheral cortical region (box). (B) Maximum dilation delineates the wedge cataract (box in panel A) in the peripheral cortical region (arrowheads). (C) Fundoscopic examination of another man with an epiretinal membrane (arrow) and (D) retinal hamartomas (arrows).
Other ocular manifestations are epiretinal membranes and retinal hamartomas (figure 6).134,135 Epiretinal membranes are translucent, semitranslucent, or whitish grey membranes with prominent whitish edges demarcating their borders. Even in patients with severe clinical manifestations of neurofibromatosis type 2—in up to 80% of whom epiretinal membranes have been identified—these membranes are usually not the cause of loss of visual acuity loss (table 1).136 Retinal hamartomas (6–22% of patients) are slightly raised masses most frequently identified in the macula that frequently reduce visual acuity.4,5,16 On fundoscopic examination, they are characterised by enhanced pigmentation and varying amounts of thickened, grey–white retinal and epiretinal tissue.137
Cutaneous manifestations
Skin tumours are present in 59–68% of patients with this disorder and include skin plaques, subcutaneous tumours, and intradermal tumours (table 1).3,4,19 They have a raised frequency in severely affected patients and are often multiple (mean skin tumours; 7·1).3,4,19 No predilection to site has been substantiated. Most skin tumours are schwannomas, although histologically confirmed neurofibromas or mixed tumours have been occasionally identified.19,129
Skin plaques are well circumscribed, slightly raised, roughened areas that typically are less than 2 cm and display slight hyperpigmentation and hypertrichosis (figure 7).3,19,138 They are present in 41–48% of patients and might be hairless, smooth, and soft in patients under 10 years (table 1).3,17,19 Subcutaneous tumours develop along peripheral nerves and can be palpated or seen as fusiform or nodular swellings (figure 7). These tumours are present in 43–48% of patients and are often painful and sensitive to pressure (table 1).3,19 Intradermal tumours are less frequently identified than are other skin lesions in neurofibromatosis type 2 and appear as epicutaneous, well demarcated, soft lesions with violaceous colouring. Café-au-lait maculae are well defined flat, hyperpigmented areas of skin that represent a non-specific finding. They are present in 33–48% of patients with the disorder but are often singular and inconspicuous.3,4,19
Figure 7. Cutaneous findings in neurofibromatosis type 2.
(A) Careful examination of a boy reveals skin plaque (arrows) of the forehead. These lesions are well circumscribed, slightly raised, roughened areas that typically display slight hyperpigmentation, hypertrichosis, and often measure less than 2 cm in diameter. (B) Subcutaneous tumours (arrows) seen as fusiform swellings along the course of peripheral nerves on the forearms of a woman.
Disease progression and survival
Average age at symptom onset in neurofibromatosis type 2 is 20 years but diagnosis is delayed on average for 7 years.3–5,139 Although the range of disease progression is highly variable, most patients are rendered deaf and many will eventually need wheelchair assistance. Early age at symptom onset and the presence of intracranial meningiomas at diagnosis—both cardinal markers of increased disease severity—are associated with a heightened risk of early mortality.20 In 1992, although mean actuarial survival in a 150-patient cohort was 62 years, more than 40% of these patients were expected to die by age 50 years.3 Since that time, the 15-year mean actuarial survival from time of diagnosis has probably extended because of advances in treatment available at specialised centres and the recognition of mildly affected people with mosaic disease.
Conclusion
Increased understanding of the clinical manifestations of neurofibromatosis type 2 in conjunction with improved precision of genetic tests and imaging studies have improved early diagnosis of patients. Further insight into the molecular pathogenesis and natural history of lesions in this disorder along with advances in treatment and restorative modalities will lead to improved management of these patients.
Acknowledgments
We thank the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health for their support of this Seminar.
Footnotes
Contributors
ARA and RRL participated in writing, editing, and generation of all figures and tables. DMP was involved in writing and editing and developed figures 3–5, and 7. JAB was involved in writing and editing the introduction and imaging sections and assisted with figures 3–5. HJK participated in writing and editing of the introduction and vestibular schwannoma section. ETT participated in writing and editing the section on ocular findings and generated figure 6. ZZ was involved in the writing and editing of the molecular biology section and assisted in the generation of figure 2. All authors have seen and approved the final version.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Evans DGR, Huson SM, Donnai D, et al. A genetic study of type 22 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 1992;29:841–46. doi: 10.1136/jmg.29.12.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DGR, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26:93–97. doi: 10.1097/00129492-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Evans DGR, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–18. [PubMed] [Google Scholar]
- 4.Parry DM, Eldridge R, Kaiser-Kupfer MI, Bouzas EA, Pikus A, Patronas N. Neurofibromatosis 2(NF2): clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet. 1994;52:450–61. doi: 10.1002/ajmg.1320520411. [DOI] [PubMed] [Google Scholar]
- 5.Mautner VF, Lindenau M, Baser ME, et al. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996;38:880–86. doi: 10.1097/00006123-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Baser ME, Kuramoto L, Joe H, et al. Genotype-phenotype correlations for nervous system tumors in neurofibromatosis 2: a population-based study. Am J Hum Genet. 2004;75:231–39. doi: 10.1086/422700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samii M, Matthies C, Tatagiba M. Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery. 1997;40:696–706. doi: 10.1097/00006123-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Fisher LM, Doherty JK, Lev MH, Slattery WH., 3rd Distribution of nonvestibular cranial nerve schwannomas in neurofibromatosis 2. Otol Neurotol. 2007;28:1083–90. doi: 10.1097/MAO.0b013e31815a8411. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka G, Saito K, Nagatani T, Yoshida J. Age at symptom onset and long-term survival in patients with neurofibromatosis type 2. J Neurosurg. 2003;99:480–83. doi: 10.3171/jns.2003.99.3.0480. [DOI] [PubMed] [Google Scholar]
- 10.Patronas NJ, Courcoutsakis N, Bromley CM, Katzman GL, MacCollin M, Parry DM. Intramedullary and spinal canal tumors in patients with neurofibromatosis 2: MR imaging findings and correlation with genotype. Radiology. 2001;218:434–42. doi: 10.1148/radiology.218.2.r01fe40434. [DOI] [PubMed] [Google Scholar]
- 11.Dow G, Biggs N, Evans G, Gillespie J, Ramsden R, King A. Spinal tumors in neurofibromatosis type 2. Is emerging knowledge of genotype predictive of natural history? J Neurosurg Spine. 2005;2:574–79. doi: 10.3171/spi.2005.2.5.0574. [DOI] [PubMed] [Google Scholar]
- 12.Mautner VF, Tatagiba M, Lindenau M, et al. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AMJR Am J Roentgenol. 1995;165:951–57. doi: 10.2214/ajr.165.4.7676998. [DOI] [PubMed] [Google Scholar]
- 13.Rennie ATM, Side L, Kerr RSC, Anslow P, Pretorius P. Intramedullary tumours in patients with neurofibromatosis type 2: MRI features associated with a favourable prognosis. Clin Radiol. 2008;63:193–200. doi: 10.1016/j.crad.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Sperfeld AD, Hein C, Schroder JM, Ludolph AC, Hanemann CO. Occurrence and characterization of peripheral nerve involvement in neurofibromatosis type 2. Brain. 2002;125:996–1004. doi: 10.1093/brain/awf115. [DOI] [PubMed] [Google Scholar]
- 15.Bosch MM, Boltshauser E, Harpes P, Landau K. Ophthalmologic findings and long-term course in patients with neurofibromatosis type 2. Am J Ophthalmol. 2006;141:1068–77. doi: 10.1016/j.ajo.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Ragge NK, Baser ME, Klein J, et al. Ocular abnormalities in neurofibromatosis 2. Am J Ophthalmol. 1995;120:634–41. doi: 10.1016/s0002-9394(14)72210-x. [DOI] [PubMed] [Google Scholar]
- 17.Ragge NK, Baser ME, Riccardi VM, Falk RE. The ocular presentation of neurofibromatosis 2. Eye. 1997;11:12–18. doi: 10.1038/eye.1997.3. [DOI] [PubMed] [Google Scholar]
- 18.Maccollin M, Mautner VF. The diagnosis and management of neurofibromatosis 2 in childhood. Semin Pediatr Neurol. 1998;5:243–52. doi: 10.1016/s1071-9091(98)80003-x. [DOI] [PubMed] [Google Scholar]
- 19.Mautner VF, Lindenau M, Baser ME, Kluwe L, Gottschalk J. Skin abnormalities in neurofibromatosis 2. Arch Dermatol. 1997;133:1539–43. [PubMed] [Google Scholar]
- 20.Baser ME, Friedman JM, Aeschliman D, et al. Predictors of the risk of mortality in neurofibromatosis 2. Am J Hum Genet. 2002;71:715–23. doi: 10.1086/342716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans DGR, Baser ME, O’Reilly B, et al. Management of the patient and family with neurofibromatosis 2: a consensus conference statement. Br J Neurosurg. 2005;19:5–12. doi: 10.1080/02688690500081206. [DOI] [PubMed] [Google Scholar]
- 22.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–21. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 23.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 24.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–23. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumanski JP, Carlbom E, Collins VP, Nordenskjold M. Deletion mapping of a locus on human chromosome 22 involved in the oncogenesis of meningioma. Proc Natl Acad Sci USA. 1987;84:9275–79. doi: 10.1073/pnas.84.24.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seizinger BR, Rouleau G, Ozelius LJ. Common pathogenetic mechanism for three tumor types in bilateral acoustic neuro-fibromatosis. Science. 1987;236:317–19. doi: 10.1126/science.3105060. [DOI] [PubMed] [Google Scholar]
- 27.Giovannini M, Robanus-Maandag E, Niwa-Kawakita M, et al. Schwann cell hyperplasia and tumors in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes Dev. 1999;13:978–86. doi: 10.1101/gad.13.8.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalamarides M, Niwa-Kawakita M, Leblois H, et al. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev. 2002;16:1060–65. doi: 10.1101/gad.226302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianchi AB, Hara T, Ramesh V, et al. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994;6:185–92. doi: 10.1038/ng0294-185. [DOI] [PubMed] [Google Scholar]
- 30.Pykett MJ, Murphy M, Harnish PR, George DL. The neurofibromatosis 2(NF2) tumor suppressor gene encodes multiple alternatively spliced transcripts. Hum Mol Genet. 1994;3:559–64. doi: 10.1093/hmg/3.4.559. [DOI] [PubMed] [Google Scholar]
- 31.Sherman L, Xu HM, Geist RT, et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–09. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 32.Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O. Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem. 2004;279:18559–66. doi: 10.1074/jbc.M313916200. [DOI] [PubMed] [Google Scholar]
- 33.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–99. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 34.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–79. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- 35.Shaw RJ, Paez JG, Curto M, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 36.Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–54. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- 37.Scherer SS, Gutmann DH. Expression of the neurofibromatosis 2 tumor suppressor gene product, merlin, in Schwann cells. J Neurosc Res. 1996;46:595–605. doi: 10.1002/(SICI)1097-4547(19961201)46:5<595::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scoles DR, Yong WH, Qin Y, Wawrowsky K, Pulst SM. Schwannomin inhibits tumorigenesis through direct interaction with the eukaryotic initiation factor subunit c(eIF3c) Hum Mol Genet. 2006;15:1059–70. doi: 10.1093/hmg/ddl021. [DOI] [PubMed] [Google Scholar]
- 40.Ryu CH, Kim SW, Lee KH, et al. The merlin tumor suppressor interacts with Ral guanine nucleotide dissociation stimulator and inhibits its activity. Oncogene. 2005;24:5355–64. doi: 10.1038/sj.onc.1208633. [DOI] [PubMed] [Google Scholar]
- 41.Rong R, Tang X, Gutmann DH, Ye K. Neurofibromatosis 2(NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci USA. 2004;101:18200–05. doi: 10.1073/pnas.0405971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JY, Kim H, Ryu CH, et al. Merlin, a tumor suppressor, interacts with transactivation-responsive RNA-binding protein and inhibits its oncogenic activity. J Biol Chem. 2004;279:30265–73. doi: 10.1074/jbc.M312083200. [DOI] [PubMed] [Google Scholar]
- 43.Lim JY, Kim H, Jeun SS, Kang SG, Lee KJ. Merlin inhibits growth hormone-regulated Raf-ERKs pathways by binding to Grb2 protein. Biochem Biophys Res Commun. 2006;340:1151–57. doi: 10.1016/j.bbrc.2005.12.122. [DOI] [PubMed] [Google Scholar]
- 44.Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–27. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- 45.Wiederhold T, Lee MF, James M, et al. Magicin, a novel cytoskeletal protein associates with the NF2 tumor suppressor merlin and Grb2. Oncogene. 2004;23:8815–25. doi: 10.1038/sj.onc.1208110. [DOI] [PubMed] [Google Scholar]
- 46.Evans DGR, Sainio M, Baser ME. Neurofibromatosis type 2. J Med Genet. 2000;37:897–904. doi: 10.1136/jmg.37.12.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.NIH Consensus development conference: Acoustic neuroma. NIH Consen Statement. 1991;9:1–24. [PubMed] [Google Scholar]
- 48.Baser ME, Friedman JM, Wallace AJ, Ramsden RT, Joe H, Evans DGR. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology. 2002;59:1759–65. doi: 10.1212/01.wnl.0000035638.74084.f4. [DOI] [PubMed] [Google Scholar]
- 49.Nunes F, MacCollin M. Neurofibromatosis 2 in the pediatric population. J Child Neurol. 2003;18:718–24. doi: 10.1177/08830738030180101301. [DOI] [PubMed] [Google Scholar]
- 50.Ruggieri M, Iannetti P, Polizzi A, et al. Earliest clinical manifestations and natural history of neurofibromatosis type 2 (NF2) in childhood: a study of 24 patients. Neuropediatrics. 2005;36:21–34. doi: 10.1055/s-2005-837581. [DOI] [PubMed] [Google Scholar]
- 51.Evans DGR, Birch JM, Ramsden RT. Paediatric presentation of type 2 neurofibromatosis. Arch Dis Child. 1999;81:496–99. doi: 10.1136/adc.81.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wishart JH. Case of tumours in the skull, dura mater and brain. Edinburgh Med Surg J. 1822;18:393–97. [PMC free article] [PubMed] [Google Scholar]
- 53.Cushing H. Tumors of the nervus acusticus and the syndrome of the cerebellopontine angle. Philadelphia: W.B. Saunders Company; 1917. [Google Scholar]
- 54.Feiling A, Ward E. A familial form of acoustic tumour. BMJ. 1920;10:496–97. doi: 10.1136/bmj.1.3093.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardner WJ, Frazier CH. Bilateral acoustic neurofibromas: a clinical study and field survey of a family of five generations with bilateral deafness in thirty-eight - members. Arch Neurol Psychiatr. 1930;23:266–302. [Google Scholar]
- 56.Rouleau GA, Wertelecki W, Haines JL. Genetic linkage of bilateral acoustic neurofibromatosis to a DNA marker on chromosome 22. Nature. 1987;329:246–48. doi: 10.1038/329246a0. [DOI] [PubMed] [Google Scholar]
- 57.Seizinger BR, Rouleau GA, Ozelius LJ, et al. Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell. 1987;49:589–94. doi: 10.1016/0092-8674(87)90534-4. [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health Consensus Development Conference Statement on Neurofibromatosis. Neurofibromatosis Res Newsl. 1987;3:3–6. [Google Scholar]
- 59.Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–92. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 60.Wallace MR, Marchuk DA, Andersen LB, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–6. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 61.Hulsebos TJM, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80:805–10. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baser ME, Friedman JM, Evans DGR. Increasing the specificity of diagnostic criteria for schwannomatosis. Neurology. 2006;66:730–32. doi: 10.1212/01.wnl.0000201190.89751.41. [DOI] [PubMed] [Google Scholar]
- 63.Eldridge R, Parry DM, Kaiser-Kupfer MI. Neurofibromatosis 2 (NF2): clinical heterogeneity and natural history based on 39 individuals in 9 families and 16 sporadic cases. Am J Hum Genet. 1991;49(suppl):133. [Google Scholar]
- 64.Parry DM, MacCollin MM, Kaiser-Kupfer MI, et al. Germ-line mutations in the neurofibromatosis 2 gene: correlations with disease severity and retinal abnormalities. Am J Hum Genet. 1996;59:529–39. [PMC free article] [PubMed] [Google Scholar]
- 65.Evans DGR, Trueman L, Wallace A, Collins S, Strachan T. Genotype/phenotype correlations in type 2 neurofibromatosis (NF2): evidence for more severe disease associated with truncating mutations. J Med Genet. 1998;35:450–55. doi: 10.1136/jmg.35.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baser ME, Kuramoto L, Woods R, et al. The location of constitutional neurofibromatosis 2 (NF2) splice site mutations is associated with the severity of NF2. J Med Genet. 2005;42:540–46. doi: 10.1136/jmg.2004.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baser ME, Makariou EV, Parry DM. Predictors of vestibular schwannoma growth in patients with neurofibromatosis type 2. J Neurosurg. 2002;96:217–22. doi: 10.3171/jns.2002.96.2.0217. [DOI] [PubMed] [Google Scholar]
- 68.Baser ME, Kuramoto L, Joe H, et al. Genotype-phenotype correlations for cataracts in neurofibromatosis 2. J Med Genet. 2003;40:758–60. doi: 10.1136/jmg.40.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baser ME, Kluwe L, Mautner VF. Germ-line NF2 mutations and disease severity in neurofibromatosis type 2 patients with retinal abnormalities. Am J Hum Genet. 1999;64:1230–23. doi: 10.1086/302338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kluwe L, Mautner VF. Mosaicism in sporadic neurofibromatosis 2 patients. Human Mol Genet. 1998;7:2051–55. doi: 10.1093/hmg/7.13.2051. [DOI] [PubMed] [Google Scholar]
- 71.Moyhuddin A, Baser ME, Watson C, et al. Somatic mosaicism in neurofibromatosis 2: prevalence and risk of disease transmission to offspring. J Med Genet. 2003;40:459–63. doi: 10.1136/jmg.40.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans DGR, Ramsden RT, Shenton A, et al. Mosaicism in neurofibromatosis type 2: an update of risk based on uni/bilaterality of vestibular schwannoma at presentation and sensitive mutation analysis including multiple ligation-dependent probe amplification. J Med Genet. 2007;44:424–28. doi: 10.1136/jmg.2006.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evans DGR, Ramsden RT, Shenton A, et al. What are the implications in individuals with unilateral vestibular schwannoma and other neurogenic tumors? J Neurosurg. 2008;108:92–96. doi: 10.3171/JNS/2008/108/01/0092. [DOI] [PubMed] [Google Scholar]
- 74.Ahronowitz I, Xin W, Kiely R, Sims K, MacCollin M, Nunes FP. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat. 2007;28:1–12. doi: 10.1002/humu.20393. [DOI] [PubMed] [Google Scholar]
- 75.Twomey JG, Bove C, Cassidy D. Presymptomatic genetic testing in children for neurofibromatosis 2. J Pediatr Nurs. 2008;23:183–94. doi: 10.1016/j.pedn.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Brackmann DE, Fayad JN, Slattery WH, Iii, et al. Early proactive management of vestibular schwannomas in neurofibromatosis type 2. Neurosurgery. 2001;49:274–83. doi: 10.1097/00006123-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Slattery WH, Iii, Brackmann DE, Hitselberger W. Hearing preservation in neurofibromatosis type 2. Am J Otol. 1998;19:638–43. [PubMed] [Google Scholar]
- 78.Khrais T, Romano G, Sanna M. Nerve origin of vestibular schwannoma: a prospective study. J Laryngol Otol. 2008;122:128–31. doi: 10.1017/S0022215107001028. [DOI] [PubMed] [Google Scholar]
- 79.Baser ME, Evans DGR, Jackler RK, Sujansky E, Rubenstein A. Neurofibromatosis 2, radiosurgery and malignant nervous system tumours. Br J Cancer. 2000;82:998. doi: 10.1054/bjoc.1999.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masuda A, Fisher LM, Oppenheimer ML, Iqbal Z, Slattery WH. Hearing changes after diagnosis in neurofibromatosis type 2. Otol Neurotol. 2004;25:150–54. doi: 10.1097/00129492-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 81.Baser ME, Mautner VF, Parry DM, Evans DGR. Methodological issues in longitudinal studies: vestibular schwannoma growth rates in neurofibromatosis 2. J Med Genet. 2005;42:903–06. doi: 10.1136/jmg.2005.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sobel RA, Wang Y. Vestibular (acoustic) schwannomas: histologic features in neurofibromatosis 2 and in unilateral cases. J Neuropathol Exp Neurol. 1993;52:106–13. doi: 10.1097/00005072-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Jaaskelainen J, Paetau A, Pyykko I, Blomstedt G, Palva T, Troupp H. Interface between the facial nerve and large acoustic neurinomas. Immunohistochemical study of the cleavage plane in NF2 and non-NF2 cases. J Neurosurg. 1994;80:541–47. doi: 10.3171/jns.1994.80.3.0541. [DOI] [PubMed] [Google Scholar]
- 84.Buchman CA, Chen DA, Flannagan P, Wilberger JE, Maroon JC. The learning curve for acoustic tumor surgery. Laryngoscope. 1996;106:1406–11. doi: 10.1097/00005537-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 85.Charabi S, Tos M, Thomsen J, Borgesen SE. Suboccipital acoustic neuroma surgery: results of decentralized neurosurgical tumor removal in Denmark. Acta Oto-Laryngologica. 1992;112:810–15. doi: 10.3109/00016489209137478. [DOI] [PubMed] [Google Scholar]
- 86.Kida Y, Kobayashi T, Tanaka T, Mori Y. Radiosurgery for bilateral neurinomas associated with neurofibromatosis type 2. Surg Neurol. 2000;53:383–90. doi: 10.1016/s0090-3019(00)00174-9. [DOI] [PubMed] [Google Scholar]
- 87.Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60:460–68. doi: 10.1227/01.NEU.0000255340.26027.53. [DOI] [PubMed] [Google Scholar]
- 88.Roche PH, Regis J, Pellet W, et al. Neurofibromatosis type 2. Preliminary results of gamma knife radiosurgery of vestibular schwannomas [Neurofibromatose de type 2 Resultats preliminaires de la radiochirurgie gamma knife des schwannomes vestibulaires.] Neurochirurgie. 2000;46:339–54. [PubMed] [Google Scholar]
- 89.Rowe JG, Radatz MWR, Walton L, Soanes T, Rodgers J, Kemeny AA. Clinical experience with gamma knife stereotactic radiosurgery in the management of vestibular schwannomas secondary to type 2 neurofibromatosis. J Neurol Neurosurg Psychiatry. 2003;74:1288–93. doi: 10.1136/jnnp.74.9.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rowe J, Grainger A, Walton L, Radatz M, Kemeny A. Safety of radiosurgery applied to conditions with abnormal tumor suppressor genes. Neurosurgery. 2007;60:860–63. doi: 10.1227/01.NEU.0000255426.08926.95. [DOI] [PubMed] [Google Scholar]
- 91.Hoffman RA, Kohan D, Cohen NL. Cochlear implants in the management of bilateral acoustic neuromas. Am J Otol. 1992;13:525–28. [PubMed] [Google Scholar]
- 92.Neff BA, Wiet RM, Lasak JM, et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007;117:1069–72. doi: 10.1097/MLG.0b013e31804b1ae7. [DOI] [PubMed] [Google Scholar]
- 93.Otto SR, Brackmann DE, Hitselberger WE, Shannon RV, Kuchta J. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96:1063–71. doi: 10.3171/jns.2002.96.6.1063. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz MS, Otto SR, Shannon RV, Hitselberger WE, Brackmann DE. Auditory brainstem implants. Neurotherapeutics. 2008;5:128–36. doi: 10.1016/j.nurt.2007.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lim HH, Lenarz T, Joseph G, et al. Electrical stimulation of the midbrain for hearing restoration: insight into the functional organization of the human central auditory system. J Neurosci. 2007;27:13541–51. doi: 10.1523/JNEUROSCI.3123-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evans DG, Watson C, King A, Wallace AJ, Baser ME. Multiple meningiomas: differential involvement of the NF2 gene in children and adults. J Med Genet. 2005;42:45–48. doi: 10.1136/jmg.2004.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Antinheimo J, Haapasalo H, Haltia M, et al. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J Neurosurg. 1997;87:610–14. doi: 10.3171/jns.1997.87.4.0610. [DOI] [PubMed] [Google Scholar]
- 98.Perry A, Giannini C, Raghavan R, et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropath Exp Neurol. 2001;60:994–1003. doi: 10.1093/jnen/60.10.994. [DOI] [PubMed] [Google Scholar]
- 99.Larson JJ, Van Loveren HR, Balko MG, Tew JM., Jr Evidence of meningioma infiltration into cranial nerves: clinical implications for cavernous sinus meningiomas. J Neurosurg. 1995;83:596–99. doi: 10.3171/jns.1995.83.4.0596. [DOI] [PubMed] [Google Scholar]
- 100.Couldwell WT, Fukushima T, Giannotta SL, Weiss MH. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84:20–28. doi: 10.3171/jns.1996.84.1.0020. [DOI] [PubMed] [Google Scholar]
- 101.Couldwell WT, Kan P, Liu JK, Apfelbaum RI. Decompression of cavernous sinus meningioma for preservation and improvement of cranial nerve function: technical note. J Neurosurg. 2006;105:148–52. doi: 10.3171/jns.2006.105.1.148. [DOI] [PubMed] [Google Scholar]
- 102.Kondziolka D, Levy EI, Niranjan A, Flickinger JC, Lunsford LD. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44–50. doi: 10.3171/jns.1999.91.1.0044. [DOI] [PubMed] [Google Scholar]
- 103.Wentworth S, Pinn M, Bourland JD, et al. Clinical experience with radiation therapy in the management of neurofibromatosis associated central nervous system tumors. Internat J Radiat Onc Biol Phys. 2008;73:208–13. doi: 10.1016/j.ijrobp.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 104.Evans DGR, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006;43:289–94. doi: 10.1136/jmg.2005.036319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee M, Rezai AR, Freed D, Epstein FJ. Intramedullary spinal cord tumors in neurofibromatosis. Neurosurgery. 1996;38:32–37. doi: 10.1097/00006123-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 106.Egelhoff JC, Bates DJ, Ross JS, Rothner AD, Cohen BH. Spinal MR findings in neurofibromatosis types 1 and 2. AJNR Am J Neuroradiol. 1992;13:1071–77. [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrante L, Mastronardi L, Celli P, Lunardi P, Acqui M, Fortuna A. Intramedullary spinal cord ependymomas – a study of 45 cases with long-term follow-up. Acta Neurochir (Wien) 1992;119:74–79. doi: 10.1007/BF01541785. [DOI] [PubMed] [Google Scholar]
- 108.Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993;79:204–09. doi: 10.3171/jns.1993.79.2.0204. [DOI] [PubMed] [Google Scholar]
- 109.Chang UK, Choe WJ, Chung SK, Chung CK, Kim HJ. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncolog. 2002;57:133–39. doi: 10.1023/a:1015789009058. [DOI] [PubMed] [Google Scholar]
- 110.McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–32. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 111.Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H. Results of microsurgical treatment for intramedullary spinal cord ependymomas: analysis of 36 cases. Neurosurgery. 1999;44:264–69. doi: 10.1097/00006123-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 112.Fine MJ, Kricheff II, Freed D, Epstein FJ. Spinal cord ependymomas: MR imaging features. Radiology. 1995;197:655–58. doi: 10.1148/radiology.197.3.7480734. [DOI] [PubMed] [Google Scholar]
- 113.Lowe GM. Magnetic resonance imaging of intramedullary spinal cord tumors. J NeuroOncol. 2000;47:195–210. doi: 10.1023/a:1006462321234. [DOI] [PubMed] [Google Scholar]
- 114.Kilpatrick TJ, Hjorth RJ, Gonzales MF. A case of neurofibromatosis 2 presenting with a mononeuritis multiplex. J Neurol Neurosurg Psychiatry. 1992;55:391–93. doi: 10.1136/jnnp.55.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gijtenbeek JMM, Gabreels-Festen AAWM, Lammens M, Zwarts MJ, Van Engelen BGM. Mononeuropathy multiplex as the initial manifestation of neurofibromatosis type 2. Neurology. 2001;56:1766–68. doi: 10.1212/wnl.56.12.1766. [DOI] [PubMed] [Google Scholar]
- 116.Overweg-Plandsoen WC, Brouwer-Mladin R, Merel P, de Vries L, Bijlsma EK. Neurofibromatosis type 2 in an adolescent boy with polyneuropathy and a mutation in the NF2 gene. J Neurol. 1996;243:724–26. doi: 10.1007/BF00873979. [DOI] [PubMed] [Google Scholar]
- 117.Hagel C, Lindenau M, Lamszus K, Kluwe L, Stavrou D, Mautner VF. Polyneuropathy in neurofibromatosis 2: clinical findings, molecular genetics and neuropathological alterations in sural nerve biopsy specimens. Acta Neuropathol. 2002;104:179–87. doi: 10.1007/s00401-002-0535-7. [DOI] [PubMed] [Google Scholar]
- 118.Trivedi R, Byrne J, Huson SM, Donaghy M. Focal amyotrophy in neurofibromatosis 2. J Neurol Neurosurg Psychiatry. 2000;69:257–61. doi: 10.1136/jnnp.69.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iwata A, Kunimoto M, Inoue K. Schwann cell proliferation as the cause of peripheral neuropathy in neurofibromatosis-2. J Neurol Sci. 1998;156:201–04. doi: 10.1016/s0022-510x(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 120.Thomas PK, King RHM, Chiang TR, Scaravilli F, Sharma AK, Downie AW. Neurofibromatous neuropathy. Muscle Nerve. 1990;13:93–101. doi: 10.1002/mus.880130202. [DOI] [PubMed] [Google Scholar]
- 121.Kuo HC, Chu CC, Jung SM, Huang CC. An unusual clinical course of peripheral neuropathy in neurofibromatosis type 2. J Clin Neuromuscul Dis. 2004;5:195–201. doi: 10.1097/01.cnd.0000108899.38238.7a. [DOI] [PubMed] [Google Scholar]
- 122.Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643–82. doi: 10.1016/j.ncl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 123.Hanemann CO, Diebold R, Kaufmann D. Role of NF2 haploinsufficiency in NF2-associated polyneuropathy. Brain Pathol. 2007;17:371–76. doi: 10.1111/j.1750-3639.2007.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siddiqui AA, Shah AA. Complete surgical excision of intramedullary schwannoma at the craniovertebral junction in neurofibromatosis type-2. Br J Neurosurg. 2004;18:193–96. doi: 10.1080/02688690410001681109. [DOI] [PubMed] [Google Scholar]
- 125.Colosimo C, Cerase A, Denaro L, Maira G, Greco R. Magnetic resonance imaging of intramedullary spinal cord schwannomas: Report of two cases and review of the literature. J Neurosurg. 2003;99(suppl 1):114–17. doi: 10.3171/spi.2003.99.1.0114. [DOI] [PubMed] [Google Scholar]
- 126.Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg. 1992;77:355–59. doi: 10.3171/jns.1992.77.3.0355. [DOI] [PubMed] [Google Scholar]
- 127.Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM. Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg. 1995;83:590–95. doi: 10.3171/jns.1995.83.4.0590. [DOI] [PubMed] [Google Scholar]
- 128.Mayfrank L, Mohadjer M, Wullich B. Intracranial calcified deposits in neurofibromatosis type 2. A CT study of 11 cases. Neuroradiology. 1990;32:33–37. doi: 10.1007/BF00593938. [DOI] [PubMed] [Google Scholar]
- 129.Wiestler OD, Von Siebenthal K, Schmitt HP, Feiden W, Kleihues P. Distribution and immunoreactivity of cerebral micro-hamartomas in bilateral acoustic neurofibromatosis (neurofibromatosis 2) Acta Neuropathol. 1989;79:137–43. doi: 10.1007/BF00294370. [DOI] [PubMed] [Google Scholar]
- 130.Omeis I, Hillard VH, Braun A, Benzil DL, Murali R, Harter DH. Meningioangiomatosis associated with neurofifbromatosis: report of 2 cases in a single family and review of the literature. Surg Neurol. 2006;65:595–603. doi: 10.1016/j.surneu.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 131.Pearson-Webb MA, Kaiser-Kupfer MI, Eldridge R. Eye findings in bilateral acoustic (central) neurofibromatosis: association with presenile lens opacities and cataracts but absence of Lisch nodules. N Engl J Med. 1986;315:1553–54. doi: 10.1056/NEJM198612113152419. [DOI] [PubMed] [Google Scholar]
- 132.Bouzas EA, Freidlin V, Parry DM, Eldridge R, Kaiser-Kupfer MI. Lens opacities in neurofibromatosis 2: further significant correlations. Br J Ophthalmol. 1993;77:354–57. doi: 10.1136/bjo.77.6.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bouzas EA, Parry DM, Eldridge R, Kaiser-Kupfer MI. Visual impairment in patients with neurofibromatosis 2. Neurology. 1993;43:622–23. doi: 10.1212/wnl.43.3_part_1.622. [DOI] [PubMed] [Google Scholar]
- 134.Kaye LD, Rothner AD, Beauchamp GR, Meyers SM, Estes ML. Ocular findings associated with neurofibromatosis type II. Ophthalmology. 1992;99:1424–29. doi: 10.1016/s0161-6420(92)31789-0. [DOI] [PubMed] [Google Scholar]
- 135.Landau K, Dossetor FM, Hoyt WF, Muci-Mendoza R. Retinal hamartoma in neurofibromatosis 2. Arch Ophthalmol. 1990;108:328–29. doi: 10.1001/archopht.1990.01070050026011. [DOI] [PubMed] [Google Scholar]
- 136.Meyers SM, Gutman FA, Kaye LD, et al. Retinal changes associated with neurofibromatosis 2. Trans Am Ophthalmol Soc. 1995;93:245–57. doi: 10.1016/s0002-9394(14)70558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bouzas EA, Parry DM, Eldridge R, Kaiser-Kupfer MI. Familial occurrence of combined pigment epithelial and retinal hamartomas associated with neurofibromatosis 2. Retina. 1992;12:103–07. doi: 10.1097/00006982-199212020-00005. [DOI] [PubMed] [Google Scholar]
- 138.Martuza RL, Eldridge R. Neurofibromatosis 2. (Bilateral acoustic neurofibromatosis) N Engl J Med. 1988;318:684–88. doi: 10.1056/NEJM198803173181106. [DOI] [PubMed] [Google Scholar]
- 139.Kanter WR, Eldridge R, Fabricant R, Allen JC, Koerber T. Central neurofibromatosis with bilateral acoustic neuroma: genetic, clinical and biochemical distinctions from peripheral neurofibromatosis. Neurology. 1980;30:851–59. doi: 10.1212/wnl.30.8.851. [DOI] [PubMed] [Google Scholar]