Reliable information regarding the current prevalence of peripheral T-cell lymphoma (PTCL) entities is missing. Herein we report on the frequency of PTCL entities in France between 2010 and 2013. Using Lymphopath, a national lymphoma network established by the French National Cancer Agency, which covers about 70 % of all lymphomas currently diagnosed in France, we found that PTCL comprised 6.5 % of non-cutaneous lymphomas with angioimmunoblastic T-cell lymphoma (AITL) being the most frequent (739 cases; 36 %), followed by peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) (550 cases, 27%). These data were verified in an independent data set from a transnational research consortium active in three European countries. In comparison to epidemiologic data reported previously, we show that AITL is by far the most common PTCL subtype. In light of the results of recent molecular findings highlighting the heterogeneity of T-cell lymphomas and the advent of targeted therapies, these data have important implications for both basic and clinical research.
Peripheral T-cell lymphomas (PTCLs) represent diverse and complex diseases, estimated to represent an overall 10–15% of all lymphomas worldwide, with the highest incidence rates occurring in Asia.1,2 The relative prevalence of PTCL entities delineated according to the criteria of the REAL (1994)/WHO (2001) classification systems, was evaluated in multiple institutions in the late 1990s and early 2000s, based on retrospective cohorts of patients. In those series, PTCL-NOS (a “by default” diagnosis for cases not fulfilling the criteria for other more specific entities) was the most frequent entity, followed by anaplastic large cell lymphoma (ALCL) and AITL, while extranodal entities, in general, accounted for a small proportion of the cases.1,3,4
This worldwide epidemiology was most recently addressed by the International PTCL study, which reviewed more than 1,300 patients diagnosed with PTCL between 1990 and 2002 in North America, Europe and Asia.5 In this cohort, PTCL-NOS was the most common diagnosis (25,9%), followed by AITL (18,5%), representing 30.4% and 21.7% of non-cutaneous PTCL, respectively (Figure 1).5 This study also confirmed geographic variations in the distribution of PTCL entities, notably demonstrating that the highest frequencies of AITL and enteropathy-associated T-cell lymphoma (EATL) are in Europe.
Figure 1.
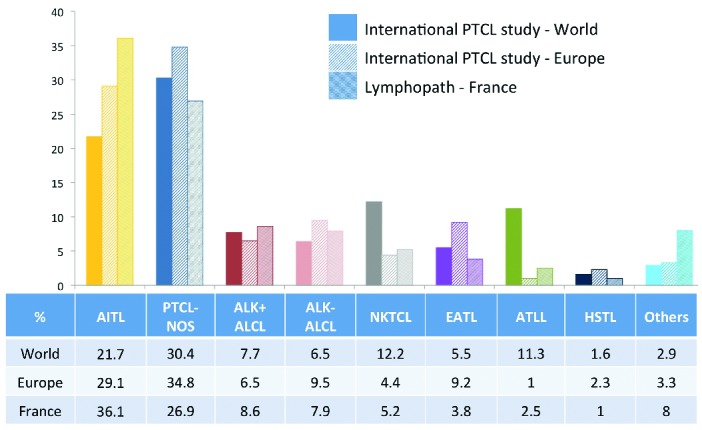
Relative frequency of non-cutaneous PTCL entities according to the International peripheral T-cell lymphoma (PTCL) project (worldwide (n=1314) and European (n=450) statistics)5 and in the Lymphopath registry (France) (n=2046). ALCL: anaplastic large cell lymphoma; ALK: anaplastic lymphoma kinase; ATLL: adult T-cell leukemia/lymphoma; EATL: enteropathy-associated T-cell lymphoma; HSTL: hepatosplenic T-cell lymphoma; NK/T: extranodal NK/T-cell lymphoma; PTCL-NOS: peripheral T-cell lymphoma, not otherwise specified. For the International T-cell lymphoma project, the percentage of the different non-cutaneous PTCL entities have been adjusted, after exclusion of cases not confirmed to be T-cell lymphomas (for the international statistics) and exclusion of categories corresponding to cutaneous lymphomas and “other disorders”.
Here, we provide recent data obtained from a large prospective survey in France. The prevalent analysis of PTCL was derived from data collected through Lymphopath, a national network of 33 expert reference centers for hematopathology which was established by the French National Cancer Agency in 2010. Non-expert pathologists are encouraged to refer every newly diagnosed lymphoma for review to a Lymphopath center. Diagnoses provided by experts, following slide review and additional ancillary techniques performed in the reference center, are entered into a central database. Of the 31,401 non-cutaneous lymphomas reviewed in Lymphopath over four years (2010–2013), there were 2,046 cases of PTCLs (6.5%) which comprised: 739 AITL (36.1%), 550 PTCL-NOS (26.9%), 176 ALK+ ALCL (8.6%), 162 ALK− ALCL (7.9%), 107 extranodal NK/T-cell lymphomas, nasal-type (5.2%), 77 EATL (3.8%), 52 adult T-cell leukemia/lymphoma (2.5%) and 20 hepatosplenic T-cell lymphomas (1.0%). The remaining 163 cases (8%) included 74 cases of NK/T-cell leukemias (15 aggressive NK-cell leukemias, 14 T-prolymphocytic leukemias and 45 large granular lymphocyte leukemias), and 89 other, or unclassifiable cases. Thus, in Lymphopath, AITL represents the most frequent non-cutaneous PTCL entity (36.1%) followed by PTCL-NOS (26.9%), which is distinctly less recurrent.
In comparison to the European data of the International PTCL study (Figure 1), the current findings differ principally with respect to the relative prevalence of AITL and PTCL-NOS.5 The reason for this discrepancy is unclear. The international study comprised a total of 450 European cases collected from eight submitting centers (two centers from Spain and Italy, and one center each from France, Germany, UK, and Norway), whereas the current analysis encompasses a much higher number of cases, estimated to be comprised of more than 70% of all new lymphoma diagnoses in France. Yet, despite the coverage not being exhaustive, it thus far represents the most recent and largest prospectively collected series validated by expert hematopathologists, with unrestricted access to ancillary diagnostic tools.
Although a selection bias inherent to the design of the international study (retrospective collection, limited number of submitting centers, university medical centers only) cannot be excluded, the differences observed may in fact reflect geographical differences within Europe. An overrepresentation of EATL in Nordic countries is well recognized, but this largely rare disease has only minimal impact on global statistics. Interestingly, according to the data collected by the national Swedish Lymphoma Registry over a 10-year period (2000–2009), the 755 PTCL (non-cutaneous and non-leukemic) registered in Sweden comprised 34% PTCL-NOS, 29% ALCL, only 14% AITL, and 9% EATL, a distribution also markedly different from both the published European statistics and from our findings, thus further suggesting geographic variations.6
Another potentially conflicting difference is that the international study cohort comprises cases diagnosed between 1990 and 2002 and reviewed with reference to the 2001 WHO classification, while the Lymphopath dataset is derived from a more recent observation period (2010–2013). In the interim, it was discovered that AITL is associated to CD10 expression and derived from T follicular helper (TFH) cells. These perceptions were incorporated into the description of AITL in the 2008 WHO classification,2 and since then CD10 and novel markers associated to TFH differentiation have been validated for diagnostic use and increasingly incorporated into routine practice.7 Thus, consideration must be given to whether the higher prevalence of AITL observed recently might be linked in some way to the availability of novel ancillary tools to support the diagnosis.
Confronted with the lack of a similar systematic database antedating Lymphopath in France, we used another non-overlapping set of PTCL patients to compare the distribution of PTCL entities over an earlier time period. This set of patients was collected through Tenomic, a transnational research consortium on T-cell lymphomas involving several LYSA (Lymphoma Study Association) centers in France, Belgium and Switzerland. The Tenomic database (approved by the ethical committee “CPP Ile-de-France IX 08-009”) is a retrospective and prospective collection of PTCL samples with available frozen tissue, and corresponding clinical annotations (Online Supplementary Methods), including a subset of patients enrolled in GELA/LYSA studies. All Tenomic cases are reviewed by at least two expert hematopathologists belonging to the consortium, and classified according to the 2008 WHO criteria. The Tenomic database comprised 623 non-anaplastic PTCL cases diagnosed between 1999 and 2009, reviewed after 2008. Remarkably, within the limits of this retrospective collection, there was an even higher ratio of AITL (n=293) to PTCL-NOS (n=171) in the Tenomic dataset (1.7:1) than in that of Lymphopath (1.35:1). According to the detailed records available for 236 AITL cases (Online Supplementary Methods and Online Supplementary Table S1), the characteristic morphological features of AITL (polymorphic cellular infiltrate (100%), arborizing vessels (98%) blast cells (94%), clear cells (69%) and sinus sign (72%)), were present in most cases. In addition, an expansion of follicular dendritic cells (FDCs), regarded as a hallmark of AITL,7 was present in 93% of the cases, and EBV-positive blasts were evidenced in 77%. The percentages of cases expressing CD10 (83%) and the TFH-associated molecules PD1 (78%), CXCL13 (76%) and BCL6 (62%) in the neoplastic cells were comparative to those previously reported.7 In addition, among the 246 patients who had available follow-up data, the frequencies of B symptoms (67%), advanced stage disease (98%), IPI score>2 (94%), hypergammaglobulinemia (64%) and positive Coombs’ test (41%) (Table 1), were overall similar to those recorded in other series,8–11 providing evidence that the pathological AITL diagnoses were concordant with the clinical and biological features of that entity. The five-year overall survival (OS) for the entire group, whose treatment characteristics are detailed in the Online Supplementary Table S2, was 34% (IC 95% [27%–40%]) (Online Supplementary Figure S1), which is also in agreement with other series.8–11
Table 1.
Clinical characteristics of 246 AITL patients with follow-up data from the Tenomic collection with comparison with other published series.

Interestingly, 43 of the 293 Tenomic AITL cases (14.6%) had initially been diagnosed as PTCL-NOS, and reclassified as AITL cases by expert review. The pathological and clinical features (shown in Online Supplementary Tables S3 and S4) of these reclassified cases did not differ from those of the entire cohort, suggesting that the apparent “increase” in AITL frequency may at least partly reflect the underrecognition or underdiagnosis of this entity in previous years.
This report further confirms the poor long-term outcome for AITL patients, and in view of the high prevalence of this disease there are essential, but as yet unfulfilled, needs for new therapeutic options. Importantly, the response to novel agents in AITL patients may be distinctively different from that seen in other PTCL patients.12
In that respect, the recent findings of recurrent mutations in genes coding for enzymes controlling DNA methylation (TET2, IDH2 and DNMT3),13,14 and in RHOA (coding for a small GTPase),15 offer rationale for the use of demethylating agents and/or specific inhibitors in AITL patients.16 As a whole, the data presented herein are highly relevant at a time when there is a shift towards the development of individualized therapies in PTCL, and should be taken into consideration for the design of future clinical studies.
Acknowledgments
The authors thank Karen Leroy from the Tumor tissue Bank (Plateforme de Ressources Biologiques, GH Henri Mondor, AP-HP, Créteil, France), the LYSA platform for their expert assistance with histological techniques and statistical analyses and reporting, Marc Maynadier, Mylène Dandoit (CHU Dijon), Nadia Lansalot-Amara (CHU Toulouse), Magali Marie-Sainte (GH Mondor, Créteil), Séverine Lepuil (CHU Pessac) for the Lymphopath database.
Footnotes
Appendix: list of Lymphopath expert pathologists
G. Delsol, L. Lamant, P. Brousset, C. Laurent, CHU Purpan de Toulouse; P. Gaulard, C. Copie-Bergman, J. Moroch, N. Ortonne, Hôpital Henri Mondor, Créteil; J. Briere, V. Meignin, Hôpital Saint-Louis, Paris; T. Molina, N. Brousse, D. Canioni, S. Fraitag, Hôpital Necker, Paris; B. Fabiani, JF Flejou, Hôpital Saint Antoine, Paris; F. Charlotte, E. Labouyrie, Hôpital La Pitié Salpêtrière, Paris; A.Martin, Hôpital Avicenne, Bobigny; S. Prevot, C. Guettier, M. Raphaél, Hôpital A. Béclère, Clamart/Kremlin Bicêtre; J. Bosq, P. Dartigues, Institut Gustave Roussy, Villejuif; V. Costes, T. Rousset, CHU de Montpellier; A. de Mascarel, M. Parrens, B. Vergier, CHU de Bordeaux; I. Soubeyran, Institut Bergonié, Bordeaux; F. Berger, A. Traverse-Glehen, B. Balme, CHU de Lyon Sud; Dr C Chassagne-Clément, Dr A Decouvelaere, Dr A Fouchardière, Centre Léon Bérard, Lyon; B. Fabre, CHU de Grenoble; M. Peoc’h, CHU de Saint Etienne; A. Pilon, P. Dechelotte, F. Franck, CHU de Clermont-Ferrand; L. Xerri, B. Chataille, Institut Paoli Calmettes, Marseille; I. Peyrotte, JF Michiels, P. Hofman, O. Vire, CHU de Nice; A. Moreau, C. Bossard, CHU de Nantes; M-C. Rousselet, A. Croué, CHU d’Angers; P. Tas, CHU de Rennes; F. Arbion, A. de Muret, CHU de Tours; I. Quintin-Roue, CHU de Brest; MC Copin, B. Bouchindhomme, C. Delattre, CHU de Lille; H. Sevestre, CHU d’Amiens; J-M. Picquenot, A. François, P. Couville, Centre Henri Becquerel, Rouen; M. Galateau-Salle, CHU de Caen; L. Martin, T. Petrella, CHU de Dijon, S. Degano-Valmary, CHU Besançon; F. Plénat, CHU de Nancy; M. Patey, CHU de Reims; S. Thiebault, CH de Mulhouse; M. Delage, B. Petit, CHU Limoges. Participants to the Tenomic consortium: A. Martin, Hôpital Avicenne, Bobigny, France; I. Soubeyran, P. Soubeyran, Institut Bergonié, Bordeaux, France; P. Dechelotte, A. Pilon, O. Tournilhac, Hôtel-Dieu, Clermont Ferrand, France; P. Gaulard, C Copie-Bergman, MH Delfau, A Plonquet, F Le Bras, J Dupuis, C. Haïoun, Hôpital H Mondor, Créteil, France; T. Petrella, L. Martin, JN Bastié, O Casasnovas CHU, Dijon, France; B. Fabre, R. Gressin, D. Leroux, MC Jacob CHU, Grenoble, France; L. de Leval, B. Bisig, A. Cairoli, CHUV, Lausanne, Suisse; C. Bonnet, CHU Sart-Tilman, Liège, Belgique; M.C. Copin, B. Bouchindhomme, F. Morschhauser, CHU, Lille, France; B. Petit, A. Jaccard, Hôpital Dupuytren, Limoges, France; F. Berger, B. Coiffier, CHU Sud, Lyon, France;T. Rousset, P. Quittet, G. Cartron, Hôpital Gui de Chauliac-St Eloi, Montpellier, France; S. Thiebault, B. Drenou, Hôpital E. Muller, Mulhouse, France; K. Montagne, C. Bastien, S. Bologna, CHU de Brabois, Nancy, France; C. Bossard, S. Le Gouill, Hôtel-Dieu, Nantes, France; J. Brière, D. Sibon, C. Gisselbrecht, Hôpital St Louis, Paris, France; B. Fabiani, A Aline-Fardin, P. Coppo, Hôpital Saint-Antoine, Paris, France; F. Charlotte, J. Gabarre, Hôpital Pitié-Salpétrière, Paris, France; T. Molina, J. Bruneau, D. Canioni, V. Verkarre, E Macintyre, V. Asnafi, O. Hermine, R. Delarue, JP Jaïs, Hôpital Necker, Paris, France; M. Parrens, JP Merlio, K. Bouabdallah, Hôpital Haut Lévêque, Bordeaux, France; S. Maugendre-Caulet, P. Tas, F. Llamas-Gutierrez T. Lamy, CHU Pontchaillou, Rennes, France; JM Picquenot, F. Jardin, C. Bastard, Centre H Becquerel, Rouen, France; M. Peoch’, J. Cornillon, CHU, Saint Etienne, France; L. Lamant, G. Laurent, L. Ysebaert, Hôpital Purpan, Toulouse, France; J. Bosq, P. Dartigues, V. Ribrag, Institut G Roussy, Villejuif, France; M. Patey, A. Delmer, Hôpital R. Debré, Reims, France; JF Emile, K. Jondeau, Hôpital Ambroise Paré, Boulogne, France; MC Rousselet, M Hunault, CHU, Angers, France; C. Badoual, Hôpital européen Georges Pompidou, Paris; C. Legendre, S. Castaigne, AL Taksin, CH Versailles, Le Chesnay, France; J. Vadrot, B Joly, A. Devidas, CH Sud francilien, Corbeil, France; G. Damaj, Service d’Hématologie, CHU Caen, France; P Dessen, G Meurice, Institut G Roussy, Villejuif, France; M Delorenzi, E Missiaglia, MP Dobay, Swiss Institut of Bioinformatics, Lausanne, Suisse; F Radvanyi, E Chapeaublanc, Institut Curie, Paris, France; S Spicuglia, CIML, Marseille, France; J Soulier, Hôpital St Louis, Paris, France; C Thibault, IGBMC, Illkirsch, France; V. Fataccioli, project coordinator, GH Henri-Mondor Albert-Chenevier, APHP, Créteil, France
Funding: this study was supported in part by grants from the Institut National du Cancer (INCa), a Programme Hospitalier de Recherche Clinique (PHRC 2007 – Code Projet: AOM 07236), the Fondation pour la Recherche Médicale (DEQ 2010/0318253), the Association pour la Recherche Thérapeutique, Génétique et Immunologique dans les Lymphomes (ARTGIL) and the Plan Cancer Research Programme (Belgium, LdL). The Lymphopath network is granted by the Institut National du Cancer (INCa)
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9(7):717–20. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2008. [Google Scholar]
- 3.Melnyk A, Rodriguez A, Pugh W, et al. Evaluation of the Revised European-American Lymphoma classificaiton confirms the clinical relevance of immunophenotype in 560 cases of aggressive non-Hodgkin’s lymphoma. Blood. 1997;89: 4514–4520. [PubMed] [Google Scholar]
- 4.Lopez-Guillermo A, Cid J, Salar A, et al. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol. 1998;9(8):849–855. [DOI] [PubMed] [Google Scholar]
- 5.Vose J, Armitage J, Weisenburger D. International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 6.Ellin F, Landstrom J, Jerkeman M, et al. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124(10):1570–1577. [DOI] [PubMed] [Google Scholar]
- 7.Attygalle AD, Cabecadas J, Gaulard P, et al. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward - report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014;64(2):171–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mourad N, Mounier N, Briere J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111(9):4463–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86(5):282–292. [DOI] [PubMed] [Google Scholar]
- 10.Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013; 31(2):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multi-center cooperative study in Japan. Blood. 2012;119(12):2837–2843. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014;123(17):2636–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemonnier F, Couronne L, Parrens M, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120(7):1466–1469. [DOI] [PubMed] [Google Scholar]
- 14.Cairns RA, Iqbal J, Lemonnier F, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119(8):1901–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakata-Yanagimoto M, Enami T, Yoshida K, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46(2):171–175. [DOI] [PubMed] [Google Scholar]
- 16.Cheminant M, Bruneau J, Kosmider O, et al. Efficacy of 5-Azacytidine in a TET2 mutated angioimmunoblastic T cell lymphoma. Br J Haematol. 2015;168(6):913–916. [DOI] [PubMed] [Google Scholar]


