Abstract
Seizures are common in patients with brain tumors, and epilepsy can significantly impact patient quality of life. Therefore, a thorough understanding of rates and predictors of seizures, and the likelihood of seizure freedom after resection, is critical in the treatment of brain tumors. Among all tumor types, seizures are most common with glioneuronal tumors (70–80%), particularly in patients with frontotemporal or insular lesions. Seizures are also common in individuals with glioma, with the highest rates of epilepsy (60–75%) observed in patients with low-grade gliomas located in superficial cortical or insular regions. Approximately 20–50% of patients with meningioma and 20–35% of those with brain metastases also suffer from seizures. After tumor resection, approximately 60–90% are rendered seizure-free, with most favorable seizure outcomes seen in individuals with glioneuronal tumors. Gross total resection, earlier surgical therapy, and a lack of generalized seizures are common predictors of a favorable seizure outcome. With regard to anticonvulsant medication selection, evidence-based guidelines for the treatment of focal epilepsy should be followed, and individual patient factors should also be considered, including patient age, sex, organ dysfunction, comorbidity, or cotherapy. As concomitant chemotherapy commonly forms an essential part of glioma treatment, enzyme-inducing anticonvulsants should be avoided when possible. Seizure freedom is the ultimate goal in the treatment of brain tumor patients with epilepsy, given the adverse effects of seizures on quality of life.
INCIDENCE OF EPILEPSY IN BRAIN TUMOR PATIENTS
Epilepsy in glioneuronal tumors and gliomas
Epilepsy can result from various types of brain tumors, but is most common in patients with low grade intrinsic lesions (Table 16.1). Across all brain tumors, glioneuronal tumors, including gangliogliomas and dysembryo-plastic neuroepithelial tumors (DNETs), are most likely to have seizure as the presenting symptom (Moreno et al., 2001; O'Brien et al., 2007; Giulioni et al., 2009; Chang et al., 2010). About three-quarters of individuals with ganglioglioma present with seizures, three-quarters of those have a temporal-lobe lesion, and nearly half of ganglioglioma patients progress to drug-resistant epilepsy (Aronica et al., 2001; Luyken et al., 2003; Southwell et al., 2012). Approximately half of patients with a DNET and epilepsy also have cortical dysplasia associated with the tumor (Chang et al., 2010).
Table 16.1.
Incidence and risk factors of epilepsy across brain tumor types
| Tumor type | Approximate incidence of seizures | Risk factor for seizures | References |
|---|---|---|---|
| Glioneuronal tumors | 70–80% | Frontotemporal, insular | Aronica et al. (2001); Luyken et al. (2003); Southwell et al. (2012) |
| Low-grade gliomas | 60–75% | Frontotemporal, insular, superficial | Chang et al. (2008a); Pignatti et al. (2002); Recht and Glantz (2008); Lee et al. (2010); You et al. (2012); Iuchi et al. (2015) |
| High-grade gliomas | 25–60% | WHO grade III, temporal lobe, superficial | Sheth (2002); van Breemen et al. (2007); Jacoby et al. (2008); Chaichana et al. (2009b); Sizoo et al. (2010) |
| Meningiomas | 20–50% | Peritumoral edema | Yao (1994); Chow et al. (1995); Lieu and Howng (2000); Oberndorfer et al. (2002) |
| Metastases | 20–35% | Melanoma, lung cancer | Oberndorfer et al. (2002); Lynam et al. (2007); Avila (2013) |
WHO, World Health Organization.
Among patients with glioma, the highest rates of epilepsy are observed in patients with low-grade gliomas (World Health Organization (WHO) grade I–II), while in high-grade glioma patients, seizures are more common with anaplastic astrocytomas (WHO grade III) than with glioblastoma multiforme (GBM: WHO grade IV) (Kim et al., 2004; Lee et al., 2010; Englot et al., 2011). In GBM, about 40–45% of patients present with epilepsy, often as secondary generalized seizure, while 15–20% develop seizures later on (Moots et al., 1995; Kerkhof et al., 2013). Smaller tumors and those growing less quickly are associated with higher rates of seizures than large, rapidly growing lesions (Moots et al., 1995; Glantz et al., 2000; Herman, 2002; Pasquier et al., 2002; Rosati et al., 2009; Chaichana et al., 2009b; Lee et al., 2010). Although the reasons for this trend are not known, possible explanations include the predilection of high-grade gliomas for brain white matter, the possibility that rapid growth might preclude epileptogenesis development, and the prospect that some patients with malignant lesions do not survive long enough to develop epilepsy (Pace et al., 2003; Rosati et al., 2009; Lee et al., 2010).
Epilepsy in meningiomas and brain metastases
While epilepsy is most common with intrinsic, intra-axial brain tumors, more than one-quarter of patients with brain metastases or extra-axial meningiomas also suffer from seizures at some point in their disease course (Table 16.1). In one study of 222 patients with meningiomas, seizures were the presenting symptoms in 26% of cases, were more frequently seen with convexity-based lesion than with tumors in other regions, and were common with lesions associated with marked peritumoral edema (Lieu and Howng, 2000). Seizures are less common in patients with brain metastases, and incidence varies by primary tumor pathology. In one large retrospective series including 470 patients with brain metastases, 24% of patients had experienced tumor-related seizures (Oberndorfer et al., 2002). While seizures occurred in only 16% of individuals with breast cancer and 21% of those with gastrointestinal metastases, seizure incidence was 29% in lung cancer patients and 67% in those with melanoma, perhaps given the propensity for intracranial hemorrhage in this tumor type.
The influence of tumor location
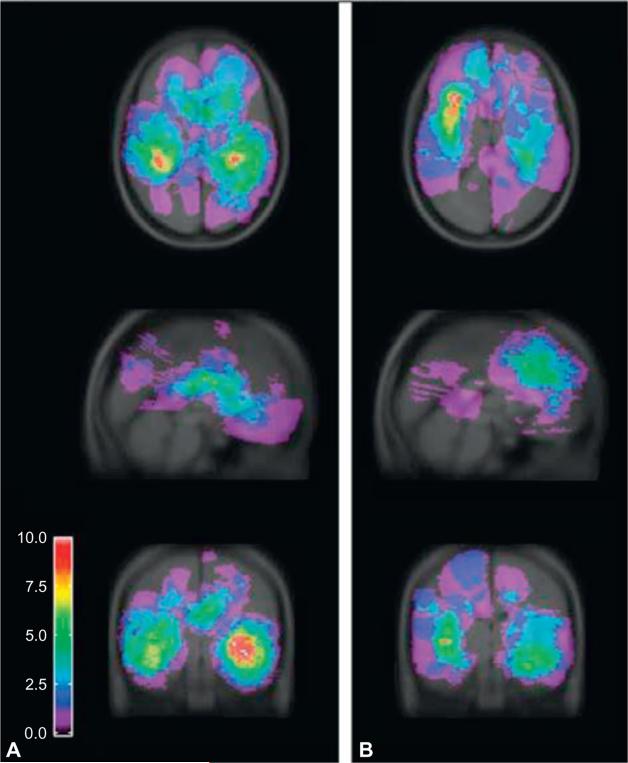
In addition to tumor type, tumor location influences the incidence of epilepsy. For instance, prior groups have noted that tumors located in superficial cortical areas are most likely to be associated with seizures (Penfield et al., 1940; Fried et al., 1994; Liigant et al., 2001; Lynam et al., 2007; Lee et al., 2010), as are lesions centered in the temporal lobe, frontal lobe, or insula (Lund, 1952; Liigant et al., 2001; Zaatreh et al., 2002, 2003; Lynam et al., 2007; Lee et al., 2010). In one prior study, Lee and others (2010) analyzed tumor location in 124 glioma patients with seizures, using a summed statistic image to map aggregate tumor location, as depicted in Figure 16.1. The authors noted that gliomas most likely to be associated with seizures were located in the temporal lobe, followed by the frontal lobe. It is likely that the inherent epileptogenicity of structures in the mesial temporal lobe contributes to seizure generation in this region (Delgado-Escueta et al., 1986; Engel et al., 2009). Furthermore, Spencer and colleagues have described that dual pathology – including gliosis, hippocampal sclerosis, and cortical dysgenesis – may further drive epilepto-genesis in tumor-related temporal-lobe epilepsy (Fish and Spencer, 1995; Spencer and Huh, 2008). Some groups have reported a lower incidence of seizures in de novo glioblastomas than those having progressed from known lower-grade gliomas (Moots et al., 1995; Rosati et al., 2009), and others have found that seizures may precede radiologic evidence of malignant tumor transformation (Rossi et al., 2010). Finally, epilepsy is reported to be more common in patients with multifocal disease than in those with a solitary tumor (Moots et al., 1995).
Fig. 16.1.
Summed statistic image demonstrating aggregate location of 124 tumors. At each voxel, the number of patients presenting with tumors is calculated. Maps are generated from the sum of the binary tumor masks for high-grade (A) and low-grade (B) gliomas. (Reproduced with permission from Lee et al., 2010, © American Medical Association. All rights reserved.)
Biologic factors
A number of molecular biologic factors have been recognized in the epileptogenesis of brain tumors. Mutation of the isocitrate dehydrogenase 1 (IDH1) enzyme, which takes part in the Krebs cycle, causes conversion of isocitrate into 2-hydroxyglutarate (Sanson et al., 2009). This product resembles structurally glutamate and may activate N-methyl-d-aspartate (NMDA) and amino-methylisoxazolepropionic acid (AMPA) receptors with ensuing epileptogenesis. In low-grade gliomas, the presence of IDH1 mutations shows a strong association with seizures as initial clinical symptom, frontal-lobe tumor location, and longer survival (Stockhammer et al., 2012). In glioblastomas, the excitatory neurotransmitter glutamate shows increased extracellular and intrasynaptic concentrations due to changes in transporter systems of the glial membrane. These abnormalities correlate with higher seizure frequency (Yuen et al., 2012; Rosati et al., 2013). Disturbances in chloride balance may play a role as well, secondary to changes in chloride co-transporters, suggesting accompanying changes in GABA metabolism and chloride transport (Huberfeld et al., 2007; Pallud et al., 2013). Besides, glutamergic stimulation of NMDA and AMPA receptors may activate intracellular mTOR, AKT, and MAPK signaling pathways, contributing both to cell growth and to epileptogenesis (Wee et al., 2014).
SEIZURES AS A PROGNOSTIC INDICATOR IN GLIOMAS
In patients with gliomas and other brain tumors, epilepsy dramatically impacts upon patients’ quality of life, causes neurocognitive deterioration, and significant morbidity may result from seizures themselves or medication side-effects (Sheth, 2002; Klein et al., 2003; Zaatreh et al., 2003; Taphoorn and Klein, 2004; Yang et al., 2010). Interestingly, however, the presence of seizures in patients with glioma may positively prognosticate overall survival. Lote and colleagues (1998) performed a retrospective study of 649 patients with glioblastoma or anaplastic glioma, and 379 individuals with low-grade glioma. High-grade glioma patients with seizures experienced significantly longer overall survival compared to those without seizures across both univariate and multivariate analyses, but this trend was not observed in individuals with low-grade glioma. However, several other groups have also found a positive association between seizure history and overall survival in low-grade glioma patients (Bartolomei et al., 1997; Stupp et al., 2003; Blumcke et al., 2004; Krzyszkowski et al., 2004; Danfors et al., 2009).
The factors underlying the survival benefit associated with seizures in glioma are not well understood, and it is possible that the onset of seizures simply leads to earlier diagnosis and thus treatment of these lesions. However, some have noted that peripherally located or slower-growing tumors that are more likely to be associated with epilepsy are also easier to treat, accounting for the association (Danfors et al., 2009). Others have proposed that gliomas causing seizures may represent a unique histopathologic tumor subtype (Bartolomei et al., 1997; Blumcke et al., 2004). Furthermore, while seizures at the time of glioma diagnosis are associated with a survival advantage, seizure recurrence after treatment portends a poor prognosis, possibly due to the association between tumor regrowth and recurrent epilepsy (Chang et al., 2008a; Danfors et al., 2009). In low-grade gliomas, relapse of seizures after a period of 6 months or more of seizure freedom is an indicator of tumor progression in about 50% of patients (Chang et al., 2008b; You et al., 2012). In GBM, recurrence or a worsening of seizures following first-line antitumor therapy heralds progression in about two-thirds of patients (Wick et al., 2005; Chang et al., 2008b; Chaichana et al., 2009a).
Thus, while early seizures in gliomas may represent a favorable prognostic indicator with respect to survival alone, late seizures are more likely to indicate progressive disease and have significant adverse effects on patient quality of life overall.
SURGICALTHERAPY
While oncologic control is typically the primary focus in the surgical treatment of brain tumors, achieving seizure freedom is also a critical goal in patients with intractable epilepsy to improve quality of life (Klein et al., 2003; Taphoorn, 2003; Villanueva et al., 2008; Duffau, 2009). This is especially true in individuals with low-grade tumors, who may survive many years or decades. Several studies have investigated seizure outcomes and predictors in surgery for glioneuronal tumors, gliomas, and meningiomas, as summarized in Table 16.2. Although seizures occur with other tumor types, including brain metastases, seizure outcomes rates and predictors have been less well studied with these lesions, and will not be discussed here.
Table 16.2.
Seizure outcomes in surgery for brain tumors associated with preoperative epilepsy
| Tumor type | Approximate seizure freedom rates | Seizure freedom predictors | References |
|---|---|---|---|
| Glioneuronal tumors | 70–90% | Gross total resection, early surgery, absence of generalized seizures | Giulioni et al. (2005); Park et al. (2008); Chang et al. (2010); Englot et al. (2012a); Southwell et al. (2012) |
| Low-grade gliomas | 65–80% | Gross total resection, early surgery, localized EEG, less severe epilepsy | Luyken et al. (2003); Zaatreh et al. (2003); Benifla et al. (2006); Chang et al. (2008a); Englot et al. (2011) |
| Meningiomas | 60–80% | Less peritumoral edema | Chow et al. (1995); Lieu and Howng (2000); Chaichana et al. (2013); Fang et al. (2013); Zheng et al. (2013) |
EEG, electroencephalogram.
Glioneuronal tumor surgery
Prior surgical studies of glioneuronal tumors causing epilepsy report postoperative seizure freedom rates between 45% and 100%, with most centers achieving this outcome in 70–90% of patients (Aronica et al., 2001; Giulioni et al., 2005, 2006, 2009; Park et al., 2008; Chang et al., 2010; Yang et al., 2011). In a retrospective series of 66 patients with ganglioglioma treated at the University of California, San Francisco (UCSF), 49 of whom presented with epilepsy, long-term seizure outcomes were evaluated after tumor removal (Southwell et al., 2012). Five years after surgery, 85% of individuals with a previous seizure history were seizure-free (Engel class I outcome). Seizure freedom was observed in 96% of patients with gross total lesionectomy, but in only 54% of those with subtotal resection. While tumor progression was noted in 38% of cases involving subtotal resection, this occurred in only 8% of patients who received gross total resection.
Seizure outcomes were also evaluated in 50 patients with DNET-related epilepsy treated at this institution, 87% of whom reached an Engel class I outcome postoperatively (Chang et al., 2010). As with gangliogliomas, seizure freedom was significantly more common with gross total resection, which was achieved in about 80% of surgeries, and seizure freedom remained resilient at a median follow-up of more than 5 years (Chang et al., 2010). The critical importance of extent of resection in glioneuronal tumor-related epilepsy surgery was also supported by a systematic review of 39 reports, including 910 patients (Englot et al., 2012b). In this study, seizure freedom was approximately 30% more likely with gross total resection than after subtotal excision, and was predicted by early surgical therapy and an absence of generalized seizures. These results suggest that excellent seizure and oncologic control can be achieved in glioneuronal tumor surgery, particularly with gross total resection and early surgical intervention.
Glioma surgery
Although the oncologic prognosis is less favorable in patients with low-grade (WHO grade II) glioma compared to those with glioneuronal tumors, survival may nonetheless exceed 10 years with aggressive management of these lesions, and seizure control is a well-known predictor of quality of life (Klein et al., 2003; Taphoorn, 2003; Villanueva et al., 2008; Duffau, 2009). In a series of 332 patients with low-grade glioma who received surgery at UCSF, 80% presented with seizures, and drug resistance was present in one-half of these (Chang et al., 2008a). Postoperative seizure freedom was seen in 67% of patients with epilepsy before surgery, with another 17% experiencing rare seizures, and favorable outcome was predicted by greater extent of tumor resection. Recurrent seizures were seen with tumor recurrence, further elucidating the association between tumor burden and seizures (Chang et al., 2008a). In a systematic review of the literature that examined 773 patients with low-grade gliomas and epilepsy across 20 surgical series, approximately 70% of individuals became seizure-free after surgery (Englot et al., 2011). Predictors of a favorable seizure outcome included greater extent of resection, a shorter duration of epilepsy, and better control of seizures with antiepileptic drugs (AEDs). Therefore, similar to glioneuronal tumors, gross total resection and early surgery are associated with improved seizure outcomes in epilepsy caused by low-grade gliomas, with other positive predictors described in the literature, including localized electroencephalogram and less severe seizure profile (Luyken et al., 2003; Zaatreh et al., 2003; Benifla et al., 2006).
Compared to low-grade gliomas, significantly fewer studies have examined the effects of surgery on seizures in high-grade (WHO grade III–IV) gliomas, as surgical treatment is nearly exclusively focused on limiting progression and improving survival (Englot et al., 2012b; Bruna et al., 2013). In one large surgical series of 648 patients with glioblastoma, 24% presented with seizures, particularly in the setting of a grade III lesion or tumor location in the temporal lobe or superficial cortex (Chaichana et al., 2009b). One year after surgical resection, 77% of individuals with preoperative seizures achieved postoperative seizure freedom, including the effects of chemoradiation with temozolomide and anti-convulsant therapy. Seizure outcomes were more favorable among patients with improved overall functional status and better preoperative seizure control, and tumor regrowth was associated with seizure recurrence. Despite the poor oncologic prognosis associated with high-grade gliomas, treatment of seizures represents a worthwhile consideration, as uncontrolled epilepsy negatively impacts quality of life in these patients (Moots et al., 1995; Glantz et al., 2000; Herman, 2002; Pasquier et al., 2002; Chaichana et al., 2009b).
Meningioma surgery
Epilepsy is less common in meningioma patients than those with intra-axial intrinsic brain tumors, and seizure outcomes with excision of these extra-axial lesions have been less well studied. Although the surgical goal in meningioma resection is typically to relieve mass effect and/or improve a focal neurologic deficit, seizure freedom is also a critical goal in treating patients with meningioma-associated epilepsy. In one patient series, Chaichana and colleagues (2013) examined 84 individuals with seizures who underwent supratentorial meningioma resection. After 48 months of postoperative follow-up, 83% of patients who had uncontrolled epilepsy preoperatively were free of seizures, and worse seizure outcomes were seen in patients with parasagittal or sphenoid wing tumors versus those in other locations. However, a clear relationship between tumor location and seizure outcome has not been clearly established in other studies.
Chow and others (1995) performed a retrospective study of 323 patients undergoing meningioma surgery: 98 (30%) had epilepsy. Seizure freedom was seen in 67% of individuals postoperatively, and this outcome was more common in patients with less peritumoral edema. Another series reported by Lieu and Howng (2000) also supports the relationship between increased brain edema and persistent seizures after meningioma resection. Also, a common observation across several investigations is that late seizure recurrence often occurs with tumor regrowth (Chow et al., 1995; Lieu and Howng, 2000; Chaichana et al., 2013). This finding again highlights the association between cytoreduction and seizure burden in brain tumor patients. Finally, the relationship between brain invasion and epileptogenicity has not been well investigated in meningiomas, and it is not known whether aggressive resection is associated with improved seizure outcomes in this patient subset, but this will be an interesting topic for further study.
Tumor surgery versus epilepsy surgery
As there are two major surgical goals for patients with tumor-related epilepsy – tumor control and seizure control – several authors have discussed whether the surgical approach in these cases should more closely resemble typical brain tumor operations versus epilepsy surgery Giulioni et al., 2009; Englot et al., 2012c; Tandon and Esquenazi, 2013). This consideration is particularly relevant with tumors in the temporal lobe, which are more prone to cause seizures than those in other locations, likely given the epileptogenicity of mesial temporal structures (White et al., 1948; Fried et al., 1994; Chang et al., 2008a; Englot et al., 2012c). While it is clear that seizure control in tumor surgery is far better after gross total resection than subtotal lesionectomy, dual pathology may also drive ictogenesis in tumoral temporal-lobe epilepsy (Fish and Spencer, 1995; Spencer and Huh, 2008). Therefore, gliosis, cortical dysgenesis, and hippo-campal sclerosis may permit continued seizures even with gross total resection of the primary lesion (White et al., 1948; Fried et al., 1994; Fish and Spencer, 1995). Some authors have thus advocated for more extensive resection in temporal-lobe cases of tumoral epilepsy, arguing that the inclusion of amygdalohippocampectomy and anterior temporal corticectomy produces improved seizure control over gross total lesionectomy alone (Jooma et al., 1995; Bilginer et al., 2009; Giulioni et al., 2009; Ogiwara et al., 2010).
In one retrospective investigation, Giulioni and others (2009) analyzed seizure outcomes in 28 patients who underwent tailored surgery for glioneuronal tumors causing temporal-lobe epilepsy. One-half of the patients underwent lesionectomy alone, while the others received lesion resection along with customized amygdalohippocampectomy and anterior temporal corticectomy. Seizure control was dramatically improved in individuals who received extended resection (93% seizure-free) compared to lesionectomy alone (43% seizure-free), although the sample size of this study was small. Extent of resection in low-grade temporal-lobe tumor resections was further examined in a meta-analysis including 1181 patients across 41 studies (Englot et al., 2012c). While subtotal lesionectomy alone led to seizure freedom in only 43% of patients, 79% of individuals achieved this outcome with gross total lesionectomy alone, and 87% were seizure-free with lesionectomy in addition to hippocampectomy and/or anterior temporal corticectomy (Englot et al., 2012c). The benefit of extended resection over gross total lesionectomy alone was larger in patients with mesial temporal tumors than those with a lateral temporal lesion. It is therefore possible that a more aggressive tailored resection may lead to better seizure outcomes in tumor-associated temporal-lobe epilepsy surgery, but further study is needed, and prospective data would be useful.
Electrocorticography (ECoG) in tumor surgery
Another important consideration in tumoral epilepsy surgery is the utilization of ECoG for mapping the epileptogenic zone. The majority of ECoG recordings of tumoral epilepsy are performed intraoperatively interic-tally (Sugano et al., 2007; Duffau, 2013), although some groups have also advocated for extraoperative ictal ECoG with subdural grid and strip electrodes in tumor-related epilepsy surgery (Sweet et al., 2013). While extraoperative ictal recordings are critical in many cases of nonlesional focal epilepsy to localize the epileptogenic zone de novo, the site of seizure onset in tumor-associated epilepsy is presumed a priori to be in the peritumoral region. Nevertheless, interictal spike mapping with intraoperative ECoG can be useful in tumoral epilepsy surgery to delineate the extent of the irritative zone through, and guide tailored resection of perilesional tissue as needed. Furthermore, intraoperative ECoG may be useful to monitor stimulation-related after-discharges in cases requiring mapping of functional cortex with direct cortical stimulation.
Several groups have reported favorable seizure outcomes using ECoG-guided tailored resection (Mikuni et al., 2006; Sugano et al., 2007; Wray et al., 2012; Duffau, 2013). However, systematic reviews of low-grade glioma and glioneuronal tumor surgery did not reveal statistical differences in seizure outcomes between resections performed with or without the use of intraoperative ECoG (Englot et al., 2011, 2012a). While this finding may suggest that the use of intraoperative ECoG is not singularly related to seizure outcome in brain tumor surgery, it is based only on retrospective associations, and conclusions may be significantly limited by patient selection bias. Specifically, intraoperative recordings may be utilized more commonly in more challenging surgical cases, such as lesions involving eloquent regions in which gross total resection is more difficult, or in cases associated with more severe epilepsy. Prospective investigations with comparable preoperative patient characteristics and correcting for extent of resection will be needed to further explore the benefit of intraoperative ECoG in tumor-related epilepsy.
Surgical considerations in pediatric patients
Although surgical techniques and treatment goals for tumor-related epilepsy are similar between adults and children, there are additional considerations in pediatric cases. While all patients are at risk for neurologic or cognitive decline with persistent uncontrolled seizures, the cumulative effects of epilepsy are a particular concern in children given ongoing neurodevelopment. Various investigators have shown that seizure control in children is associated with improved cognitive and intellectual ability (Liu et al., 2007; Souza-Oliveira et al., 2012), better memory function (Liang et al., 2012), fewer behavioral problems (Mikati et al., 2010), diminished medication use and side-effects (Keene et al., 1998a; Mikati et al., 2008), and an overall improvement in quality of life (Larysz et al., 2007; Liang et al., 2012) compared to patients with continued seizures. Family surveys have also demonstrated higher levels of education, independence, employment status, and overall satisfaction associated with seizure freedom in pediatric patients (Keene et al., 1998a, b). Also, shorter epilepsy duration has been associated with increased likelihood of postoperative seizure freedom in pediatric epilepsy surgery (Wu et al., 2010; Liava et al., 2012; Englot et al., 2013). These data suggest that early operative intervention may lead to improved neurocognitive and seizure outcomes, and thus should be strongly considered in pediatric tumoral epilepsy, even in cases with a presumed benign slow-growing lesion, if seizures are medically refractory. Finally, although the spectrum of brain tumor pathology differs between adults and children, the relationship between gross total resection and seizure freedom in pediatric tumor-related epilepsy has been clearly established in both patient populations (Giulioni et al., 2005; Englot et al., 2013; Brahimaj et al., 2014).
Conclusions regarding surgical therapy
Across all tumor pathologies and patient populations, the most consistent finding in surgical studies of brain tumor-related epilepsy is that gross total resection is associated with dramatically improved seizure outcomes compared to subtotal resection. A frequently observed relationship between tumor regrowth and seizure recurrence further exemplifies the importance of cytoreduction. Thus, gross total resection should be pursued whenever possible and safe, to maximize the likelihood of both oncologic and seizure control. Early operative intervention is also recommended in cases of medically refractory epilepsy, or when indicated due to oncologic concerns. One may also consider the use of intraoperative ECoG to guide tailored resection, and the inclusion of amygadalohippocampectomy in cases with suspected dual pathology, but prospective data clearly demonstrating superior efficacy with these approaches are not yet available.
Radiotherapy
Radiation therapy contributes to a better seizure control in low- and high-grade gliomas. Retrospective studies in low-grade gliomas indicate a 50% reduction in seizure frequency of 56–77% and a seizure freedom of 38–80% (Scerrati et al., 1994; Warnke et al., 1997; Shankar and Rajshekhar, 2003; Ruda et al., 2013). In a series of combined low- and high-grade gliomas, 77% of patients showed 50% seizure reduction and 38% seizure freedom at 12 months following radiation therapy, although patients could not discontinue AEDs (Ruda et al., 2013).
A randomized European Organisation for Research and Treatment of Cancer phase III trial on external radiotherapy in low-grade glioma to a cumulative dose of 65 Gy showed that 75% of patients became seizure-free following early and 59% following late application of radiation therapy (van den Bent et al., 2005).
Chemotherapy
The efficacy of chemotherapy with alkylating agents with temozolomide or procarbazine, lomustine, vincristine (PCV) chemotherapy for WHO grade II and III gliomas as either initial treatment or following surgery and radiotherapy is well established. These agents also help to improve seizure control (Pace et al., 2003; Kaloshi et al., 2007; Sherman et al., 2011; Ruda et al., 2012; Koekkoek et al., 2015). Administration of temozolomide results in 50% seizure reduction or more in 18–58% of patients with low-grade gliomas and in seizure freedom in 13–50% (Pace et al., 2003; Kaloshi et al., 2007; Sherman et al., 2011; Blonski et al., 2012; Koekkoek et al., 2015). Administration of PCV leads to seizure freedom in 13–60% of patients (Soffietti et al., 1998; Frenay et al., 2005; Lebrun et al., 2007).
ANTIEPILEPTIC DRUG THERAPY
Epilepsy in patients with brain tumors (EBT) is typically a focal or partial type, and for that reason its symptomatic treatment is based on the approved anticonvulsant drugs for that indication. A recent meta-analysis on the many available anticonvulsants approved for focal epilepsy in adults has been published under the aegis of the International League against Epilepsy (Glauser et al., 2013). A major factor in deciding on which AED to choose among the many approved agents are individual patient factors, defined by age, sex, organ dysfunction, comorbidity, and co-therapy. As a rule, one tries to avoid in EBT the use of the strong CYP3A4 coenzyme inducers carbamazepine, phenytoin, and phenobarbital because of risks of compromising concurrent chemo-therapy (Glantz et al., 2000; Soffietti et al., 2010). Besides, it seems preferable to avoid AEDs on which there are hardly any data available in brain tumors. For that reason, the monotherapy drugs of first choice are the evidence-based agents levetiracetam and valproic acid for focal epilepsy and for which use in EBT many data are available (Hildebrand et al., 2005; Oberndorfer et al., 2005; Lim et al., 2009; van Breemen et al., 2009; Merrell et al., 2010b; Weller et al., 2011; Kerkhof et al., 2013; Vecht et al., 2014).
Anticonvulsant monotherapy
Levetiracetam belongs together with carbamazepine, phenytoin, and zonisamide to the class 1A efficacy AEDs for focal types of epilepsy in adults (Glauser et al., 2013). About 25% of patients taking levetiracetam improve in cognitive functioning, both in general epilepsy as in EBT (Helmstaedter and Witt, 2008; de Groot et al., 2013). Another advantage is its absence of drug interactions with other agents. However, approximately 5% of patients develop irritability, aggression, or psychosis, upon which withdrawal of levetiracetam is usually indicated.
Valproic acid is the only class 1B approved anticonvulsant for focal epilepsy. Valproic acid is a broad-spectrum, well-tolerated AED that is widely applied in brain tumors, although it may cause increased appetite and trembling hands as side-effects. A major concern of the use of valproic acid is a dose-dependent risk of thrombopenia by direct toxic effects on bone marrow precursor cells, which may compromise concurrent chemotherapy (Bourg et al., 2001; Simo et al., 2012). Nevertheless, on multivariate analysis, this was the only concurrent factor determining thrombocytopenia (Simo et al., 2012). Another retrospective study showed similar hematologic toxicity irrespective of taking valproic acid, levetiracetam, or no AED (Tinchon et al., 2015). Similarly, in neurosurgical patients, postoperative bleeding or need of transfusions did not differ between patients taking valproic acid or other anticonvulsants (Ward et al., 1996; Anderson et al., 1997). Valproic acid is the only strong enzyme-inhibiting AED and may increase organ exposure to phenobarbital by CYP2C9 inhibition, and to lamotrigine by inhibition of the UGT1A4 enzyme. Protein displacement by valproic acid may lead to lower concentrations of concurrent phenytoin. In oncology, there are few known harmful drug interactions of valproic acid with chemotherapeutic agents (Bénit and Vecht, 2015).
Anticonvulsant polytherapy
In case the first monotherapy anticonvulsant gives insufficient seizure control, one can switch to the next monotherapy round, though there is a recent trend towards applying polytherapy as the following step by administration of an add-on AED (French and Faught, 2009; Brodie and Sills, 2011). Meta-analysis on pharmacoresistant epilepsy has indicated that levetiracetam is remarkably effective as add-on drug compared to other AEDs, suggesting synergistic qualities of levetiracetam in combined use with other AEDs (Otoul et al., 2005). As add-on agents, both levetiracetam and valproic acid are well tolerated (Otoul et al., 2005; Bodalia et al., 2013).
In EBT, we have adopted the policy of applying either levetiracetam or valproic acid as anticonvulsant of choice, and if necessary to combine both agents as next step. In a retrospective analysis, we observed seizure freedom in 77.7% of patients on single levetiracetam, 69.5% on single valproic acid and in 60.3% on their combination in remaining patients if either one was not effective (Kerkhof et al., 2013).
If either levetiracetam or valproic acid or its combination is insufficiently effective, one can choose lacosamide as add-on agent based on its activity and tolerability in EBT, lamotrigine for its good tolerability and indications of its synergistic activity with valproic acid, or zonisamide, considering its recent designation as class A agent for the partial epilepsies (Brodie and Yuen, 1997; French and Faught, 2009; Glauser et al., 2013; Saria et al., 2013).
For the application of anticonvulsant therapy in daily clinical practice of EBT, see Tables 16.3 and 16.4.
Table 16.3.
Characteristics of antiepileptic drugs (AEDs)
| AED | Usual dosage (mg/day) | Plasma therapeutic range (mg/L) | Common/important side-effects | Main mechanism of action | Oral bioavailability (%) | Metabolism and excretion | T1/2 (h) | Protein binding (%) |
|---|---|---|---|---|---|---|---|---|
| CBZ | 400–1600 | 4–12 | Leukopenia, hepatotoxicity,
hyponatremia, SJS/TEN |
Na+-channel blocker | 75–85 | Hepatic epoxidation, conjugation |
5–26 | 75 |
| CZP | 0.5–40 | 0.02–0.08 | Sedation, cognitive
effects, drowsiness |
GABA-receptor agonist | 90 | Hepatic reduction and acetylation |
20–60 | 85 |
| CLB | 5–40 | 0.3–3.0 | Sedation, cognitive
effects, drowsiness |
GABA-receptor agonist | 85 | Hepatic
demethylation, hydroxylation |
18–40 | 85 |
| LCM | 200–400 | 10–20 | Dizziness, headache, nausea, diplopia, blurred vision, cognitive dysfunction, skin reactions |
Slow Na+-channel blocker | >95 | Hepatic demethylation, unchanged renal excretion (40%) |
13 | <15 |
| LTG | 200–600 | 5–15 | Rash, SJS/TEN, DRESS,
headache, ataxia |
Na+-channel blocker | >95 | Hepatic glucuronidation, renal excretion (10%) |
12–60 | 55 |
| LEV | 1000–3000 | 5–30 | Somnolence, asthenia,
irritabililty, psychosis |
Binding to synaptic vesicle protein 2 (SV2A) |
>95 | Partially hydrolyzed in blood, renal excretion (65%) |
5–11 | None |
| OXC | 900–2400 | 10–35 | Somnolence, headache, diplopia, SJS, bone marrow suppression, hyponatremia |
Na+-channel blocker | >95 | Hydroxylation, glucuronidation |
8–15 | 38 |
| PB | 30–180 | 15–40 | Rash, hepatotoxicity,
impaired cognition, ataxia, mood change, SJS/TEN |
GABA-receptor agonist, glutamate antagonist, Na+-Ca+-blocker |
80–100 | Hepatic
oxidation, hydroxylation, conjugation |
46–136 | 45–60 |
| PHT | 150–400 | 10–20 | Blood dyscrasia, hepatitis, SJS,
gum hyperplasia, lupus-like reactions, hirsutism |
Na+-channel blocker | 95 95 |
Hepatic
oxidation, hydroxylation, conjugation |
24–72 | 85–95 |
| PGB | 150–600 | 2–8 | Somnolence, dizziness, ataxia | Ca+-channel blocker | 90 | No metabolism, renal excretion |
5–7 | None |
| TPM | 100–500 | 2–20 | Impaired cognition,
hepatotoxicity, weight loss, renal calculi |
Na+-channel
blocker, GABA-receptor agonist, NMDA receptor blocker |
80–95% | No metabolism, mainly renal excretion |
20–30 | 80 |
| VPA | 500–2500 | 50–100 | Hepatotoxicity, thrombo-
and neutropenia, tremor, weight gain, hair loss, ovarian cystic syndrome |
GABA-receptor agonist, Na+-channel
blocker, Glutaminergic inhibitor |
>95 | Hepatic glucuronidation, oxidation, conjugation |
8–15 | 85–95 |
| ZON | 200–600 | 20–30 | Somnolence, ataxia, dizziness, renal calculi |
Na+- and
Ca+-channel blocker |
>95 | Hepatic acetylation, glucuronidation (20%), renal excretion (30%) |
50–70 | 40–50 |
DRESS, drug reactions with eosinophilia and systemic symptoms; Na+, sodium; NMDA, N-methyl-D-aspartate; Ca+, calcium; K+, potassium; CBZ, carbamazepine; CLB, clobazam; CZP, clonazepam; GABA, gamma-aminobutyric acid; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PB, phenobarbitone; PGB, pregabalin; PHT, phenytoin; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; TPM, topiramate; VPA, valproic acid; ZON, zonisamide.
Table 16.4.
Dose regimens of symptomatic management of seizures in brain tumor-related epilepsy
| Low-grade glioma (and other primary brain tumors or brain metastasis, except glioblastoma multiforme) |
| First-line antiepileptic drug |
| Levetiracetam (LEV) |
| Medication schedule: 2 × 500 mg/day (first week 2 × 250 mg/day) |
| If necessary, up to 2 × 750–1500 mg/day |
| Therapeutic plasma range of LEV: 5–25 mg/L |
| Be alert to irritability or ill temper |
| If seizures continue: add valproic acid (VPA) |
| Dose: 20–25 mg/kg/day, can be initiated instantly |
| For example, 2 × 500 mg/day; if necessary up to 2 × 1000 mg/day |
| Therapeutic plasma range of VPA: 50–100 mg/L |
| Glioblastoma |
| First-line AED (preferably during first-line chemotherapy, including temozolomide): |
| Valproic acid (VPA) |
| Medication schedule |
| Dose: 20–25 mg/kg/day, can be initiated instantly |
| For example, 2 × 500 mg/day; if necessary up to 2 × 1000 mg/day |
| Therapeutic plasma range of VPA: 50–100 mg/L |
| Be alert to thrombopenia, particularly in association with chemotherapy |
| If seizures continue, add LEV 2 × 500 mg/day |
| If necessary, up to 2 × 750–1000 mg/day LEV (therapeutic plasma range 5–25 mg/L) |
| For both low-grade glioma and GBM, if LEV/VPA is insufficient: |
| Add lacosamide, lamotrigine, or zonisamide (in order to replace VPA or LEV) |
| To add lacosamide |
| Start 2 × 50 mg/day for 1 week, subsequently 2 × 100 mg/day |
| If necessary, increase weekly by 2 × 50 mg/day, up to 2 × 200 mg/day |
| If necessary, start at 200 mg/day in a single loading dose, and continue after 12 hours on a maintenance of 2 × 100 mg/day |
| Therapeutic plasma range 10–20 mg/L |
| To add lamotrigine |
| With VPA co-therapy: start 25 mg every other day for 2 weeks, subsequently 1 × 25 mg/day for 2 weeks, whereafter increase weekly by 25–50 mg/day, until 2 × 50–100/day |
| Without VPA co-therapy: start LTG 1 × 25 mg for 2 weeks, subsequently 50 mg/day for 2 weeks, whereafter increase the dose (bi-)weekly by 50–100 mg/day, until 2 × 100 mg/day is achieved; if necessary up to 500 mg/day |
| Therapeutic plasma range 5–15 mg/L |
| To add zonisamide |
| Initiate 2 × 25 mg/day; after 1 week 2 × 50 mg/day, whereafter if necessary, increase the dose weekly by 100 mg/day up to 2 × 150–250 mg/day |
| Therapeutic plasma range 10–40 mg/L |
When satisfactory seizure control is achieved with lacosamide, lamotrigine, or zonisamide in combination with LEV/VPA, consider tapering VPA or LEV after 1 or 2 months. With lamotrigine, it is better to maintain the use of VPA.
Prophylactic AED use
A final question is whether to initiate AED prophylaxis in the brain tumor patient without a history of seizure. Some investigators have advocated for prophylaxis in these individuals, citing successful seizure prevention, despite the risk of medication side-effects (North et al., 1983; Franceschetti et al., 1990; Forsyth et al., 2003). A randomized controlled trial of valproic acid prophylaxis in brain tumor patients suggested that patients receiving the active drug actually had a nonsignificantly increased rate of seizures compared to individuals receiving placebo (Glantz et al., 1996). Thereafter, the American Academy of Neurology advised against long-term AED prophylaxis in newly diagnosed brain tumor patients (Glantz et al., 2000), and meta-analysis of the literature provided further evidence against the prophylactic use of antiseizure medications (Sirven et al., 2004).
A notable exception is that prophylactic AEDs may be considered for 1 week after surgical resection, given the increased incidence of immediate postoperative seizures (Matthew et al., 1980; Kvam et al., 1983; Lee et al., 1989; Kuijlen et al., 1996; Glantz et al., 2000; Telfeian et al., 2001), although the evidence behind this practice remains inconclusive (De Santis et al., 2002). In a study of 121 patients undergoing glioma surgery, despite consistent perioperative AED prophylaxis, 9.1% of patients experienced a seizure within the first postoperative week, and problems with drug tolerability were not uncommon (Iuchi et al., 2015). In another observational series on meningioma surgery without seizure history, there was one single postoperative seizure in the group of 51 patients on AED prophylaxis and no seizures in 129 patients who did receive prophylaxis, suggesting little difference in seizure risk (Sughrue et al., 2011). Therefore, while AED prophylaxis remains commonly prescribed in patients with brain tumors (Siomin et al., 2005; Riva et al., 2006; Rosati et al., 2009; Lwu et al., 2010), the majority of evidence and clinical guidelines advise against this practice, and the application of perioperative prophylaxis will require further investigation.
Adverse effects of AEDs
Side-effects and toxicity related to AEDs are critical considerations in brain tumor patients, particularly given the importance of quality of life. AEDs may be associated with substantial adverse effects (Cascino, 2008; Cramer et al., 2010), such as cognitive deficits (Sisodiya et al., 2002; Wagner et al., 2003; Taphoorn and Klein, 2004), and some have suggested that first-generation medications may result in more side-effects in glioma patients than in other patients with epilepsy (Glantz et al., 2000; Batchelor and Byrne, 2006; Merrell et al., 2010a). In one large European survey of patients with epilepsy, Baker et al. (1997) observed that 31% of individuals changed AEDs at least once in the preceding year because of side-effects, and 44% were concerned about possible side-effects related to these medications.
Other investigators have demonstrated that adverse effects with AEDs have the single greatest effect on quality of life in patients with controlled seizures (Auriel et al., 2009), and that individuals would prefer to pay more for AEDs with better side-effect profiles (Lloyd et al., 2005).
Carbamazepine
Cognitive side-effects include mental decline, ataxia, and headache, and have been reported in 3.7% in EBT (Moots et al., 1995; Wick et al., 2005). Skin rash is seen in 7.4–25% of oncology patients, and hypersensitive skin reactions occur more frequently at the time of radiotherapy (Delattre et al., 1988; Micali et al., 1999). Carbamazepine-induced Stevens–Johnson syndrome occurs in almost all patients carrying the human leukocyte antigen (HLA) 1 allele B*1502 with a prevalence of 8.6% of the population at large (Amstutz et al., 2014). Presence of the HLA-A*3101 allele increases the risk of developing carbamazepine-induced hypersensitivity from 5% to 26% (McCormack et al., 2011).
Lacosamide
Lacosamide undergoes moderate hepatic metabolism through 2C19, 40% is excreted unchanged in urine, and it does not invoke drug interactions. Common side-effects are dizziness, nausea, diplopia, or blurred vision (Chung et al., 2010; Flores et al., 2012). Possibly diplopia, dizziness, or drowsiness develops more easily as a pharmacodynamic effect in combination with carbamazepine, phenytoin, or lamotrigine, as these are all sodium channel blockers (Novy et al., 2011). Withdrawal of lacosamide due to cognitive side-effects has been observed in 7.1–15.7% of cases with EBT, and in 27.8% with general epilepsy (Maschio et al., 2011a; Saria et al., 2013; Sawh et al., 2013).
Lamotrigine
Lamotrigine belongs to the AEDS with a good tolerability profile, although it may cause dizziness, ataxia, and diplopia. Skin rash is seen in 7.2% of patients, usually within 6 weeks of initiation of lamotrigine. High initial doses of lamotrigine or rapid escalation increase the risks of rash (Guberman et al., 1999). In combination with valproic acid, its metabolism is reduced via the UGT1A4 enzyme with a maximum of 50% inhibition at doses as low as 250–500 mg/day of valproic acid (Kanner and Frey, 2000; Gidal et al., 2003).
Levetiracetam
Overall side-effects of levetiracetam in EBT are seen in 5–10% of patients, often of psychiatric nature, including psychosis, depression, and aggressive behavior (Merrell et al., 2010b; Rosati et al., 2010; Maschio et al., 2011b; Rossetti et al., 2014). On the other hand, levetiracetam shows improvement in cognitive functioning in both EBT and general epilepsy (Helmstaedter and Witt, 2008; de Groot et al., 2013). Thrombopenia and dermatologic complications are rare.
Oxcarbazepine
Withdrawal due to side-effects has been observed in up to 24% of cases with EBT (Maschio et al., 2012a). During perioperative AED prophylaxis, 4% of patients developed skin rash, and 16% major skin reactions at the time of cranial radiotherapy (Mauro et al., 2007; Maschio et al., 2010).
Phenobarbital
Withdrawal due to overall side-effects of phenobarbital has been reported in 5% of cases with EBT, mainly due to cognitive dysfunction (Moots et al., 1995). In general epilepsy, the most common side-effect is sedation in 27%, usually disappearing in 1 or 2 weeks (Wang et al., 2006).
Phenytoin
In EBT, the withdrawal rate is 29.0–34.2% (Moots et al., 1995; Wick et al., 2005). In a prospective study, switching from phenytoin to levetiracetam monotherapy during the perioperative period, ataxia was seen in more than half of the phenytoin group, though not in patients on levetiracetam. Other side-effects of phenytoin were lack of energy (27%) and sleepiness (20%) (Lim et al., 2009).
Pregabalin
In two monotherapy studies in EBT, pregabalin needed to be withdrawn in 11% and 22% respectively because of peripheral edema, erectile dysfunction, depression or nausea, and 8% because of dizziness and irritability (Kerrigan and Grant, 2011; Maschio et al., 2012b). Hematotoxicity was only seen with concurrent temozolomide, though probably not related to pregabalin (Rossetti et al., 2014).
Topiramate
Although topiramate is a broad-spectrum AED with a high seizure control, it shows a high percentage of adverse effects (Otoul et al., 2005; Bodalia et al., 2013). Side-effects have been observed in 15% with EBT, including cognitive disturbances and weight loss (Maschio et al., 2008).
Valproic acid
In EBT, the withdrawal rate is 20.6%, although cognitive side-effects are uncommon (Wick et al., 2005). Hemato-logic abnormalities vary between 13.7% and 28.2% and include coagulation disorders, particularly diminished platelet aggregation and thrombocytopenia in children and the elderly. Thrombocytopenia occurs in approximately 17.7% of patients, usually asymptomatic and particularly seen with supratherapeutic drug levels (Nasreddine and Beydoun, 2008). The mechanism probably depends on dose-dependent suppression of platelet production in the bone marrow. Retrospective studies on perioperative complications in adults have not shown differences in postoperative bleeding and need for transfusions compared to patients not receiving valproic acid (Ward et al., 1996; Anderson et al., 1997; Gerstner et al., 2006).
Zonisamide
Until now, there has been hardly any experience with zonisamide in EBT. In one add-on study on 6 patients, 2 patients discontinued treatment (Maschio et al., 2009). Most common side-effects are ataxia, somnolence, agitation, irritability, and anorexia. Skin rash occurs in 1% (Baulac et al., 2012). In general epilepsy, withdrawal due to side-effects was reported as 12.3–23.4% (Chung et al., 2007; Costa et al., 2011).
Drug interactions between AEDs and chemotherapy
The potential for interaction with chemotherapy is another important consideration of the choice of a particular AED in tumor patients. CYP3A4 enzyme-inducing medications such as phenytoin, oxcarbazepine, and carbamazepine may augment the clearance of agents metabolized by the P450 system. In oncology these include several chemotherapeutic agents (cyclophosphamide, irinotecan, paclitaxel, and teniposide), a number of tyrosine kinase inhibitors (crizotinib, dasatinib, imatinib, and lapatinib) and corticosteroids such as dexamethasone that are often prescribed to reduce brain edema (Michelucci, 2006; Drappatz et al., 2007; Brown et al., 2008; Pursche et al., 2008; Raymond et al., 2008; Galanis et al., 2009; Merrell et al., 2010a).
There may be less concern with valproic acid, despite its action as enzyme inhibitor of UGT1A4 and 2C9, or with noninducing agents such as levetiracetam, lamotrigine, or lacosamide (Pursche et al., 2008).
Valproic acid as histone-deacetylase inhibitor
Of note, not all interactions between AEDs and chemo-therapeutic drugs are deleterious. Particularly, the use of valproic acid based on its action as histone-deacetylase inhibitor may provide synergistic antitumor effects with concurrent chemotherapy or radiation. By stimulating histone acetylation, abnormal balances in histone acetylation/deacetylation and of methylation of DNA promoter regions will be restored, inducing apoptosis/autophagy at the cellular level and becoming clinically apparent by sensitizing radiation/chemotherapy effects (Van Nifterik et al., 2012). Valproate is now experimentally tested in hematologic and solid tumors, including GBM, showing synergy with the alkylating agent temozolomide, expressed by longer survival times in several retrospective or post hoc analyses (Weller et al., 2011; Kerkhof et al., 2013). A recent prospective phase study in GBM showed a progression-free survival of 10 months and an overall survival of 29 months with valproic acid combined with temozolomide chemoradiation (Krauze et al., 2014).
As valproic acid represents an evidence-based choice for focal epilepsy in adults together with a large number of additional data in EBT, it is our preference to choose valproic acid during first-line chemoradiation with temozolomide (Vecht et al., 2014).
Others have observed that levetiracetam may reduce expression of DNA repair protein MGMT in vitro, and thus sensitize glioma cells to temozolomide (Bobustuc et al., 2010). Also, patients treated with enzyme-inducing AEDs paradoxically survived longer than those who did not undergo treatment (Jaeckle et al., 2009). However, it is unclear whether this observation was related to effect on the tumor or to a confounding variable.
It is important that epilepsy providers are aware of these potential interactions between AEDs and chemotherapeutic agents or tyrosine kinase inhibitors, to optimize coordination of neuro-oncologic and anti-epileptic treatment in brain tumor patients. For that purpose, we advocate therapeutic drug monitoring of plasma levels to keep dose regimens of anticonvulsants within therapeutic ranges and to avoid insufficient dosing or risks on accumulation of toxicity by AEDS.
Conclusions regarding symptomatic therapy with AEDs
EBT belongs to the type of focal epilepsies, and for that reason symptomatic therapy in EBT is determined by the evidence-based anticonvulsants for that indication. The second factor determining the choice among the approved agents are individual patient factors, usually represented by age, sex, organ dysfunction, comorbidity, or co-therapy. As concomitant chemotherapy commonly forms an essential part of glioma treatment, consensus exists that the prescribing of enzyme-inducing anticonvulsants in EBT is less appropriate. This makes levetiracetam and valproic acid the anticonvulsants of choice with both evidence-based classification for focal epilepsy in adults with a lot of additional data on their use in EBT.
If either one is ineffective for seizure control, they can well be combined as polytherapy and may show a synergistic activity. If this combination is ineffective, they can be replaced by lacosamide, lamotrigine, or zonisamide as add-on agents, although polytherapy with more than two anticonvulsant drugs has not been shown to provide additional efficacy. In case of anticonvulsant polytherapy or with concurrent chemotherapeutic treatment, drug monitoring of plasma levels helps to keep dose regimens of anticonvulsants within therapeutic ranges and avoids insufficient dosing or risks of toxicity.
References
- Amstutz U, Shear NH, Rieder MJ, et al. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55:496–506. doi: 10.1111/epi.12564. [DOI] [PubMed] [Google Scholar]
- Anderson GD, Lin YX, Berge C, et al. Absence of bleeding complications in patients undergoing cortical surgery while receiving valproate treatment. J Neurosurg. 1997;87:252–256. doi: 10.3171/jns.1997.87.2.0252. [DOI] [PubMed] [Google Scholar]
- Aronica E, Leenstra S, van Veelen CW, et al. Glioneuronal tumors and medically intractable epilepsy: a clinical study with long-term follow-up of seizure outcome after surgery. Epilepsy Res. 2001;43:179–191. doi: 10.1016/s0920-1211(00)00208-4. [DOI] [PubMed] [Google Scholar]
- Auriel E, Landov H, Blatt I, et al. Quality of life in seizure-free patients with epilepsy on monotherapy. Epilepsy Behav. 2009;14:130–133. doi: 10.1016/j.yebeh.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Avila E. Tumor Associated Epilepsy. In: Lichtor T, editor. Clinical Management and Evolving Novel Therapeutic Strategies for Patients with Brain Tumors. InTech; New York, NY: 2013. [Google Scholar]
- Baker GA, Jacoby A, Buck D, et al. Quality of life of people with epilepsy: a European study. Epilepsia. 1997;38:353–362. doi: 10.1111/j.1528-1157.1997.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Bartolomei JC, Christopher S, Vives K, et al. Low-grade gliomas of chronic epilepsy: a distinct clinical and pathological entity. J Neurooncol. 1997;34:79–84. doi: 10.1023/a:1005711321343. [DOI] [PubMed] [Google Scholar]
- Batchelor TT, Byrne TN. Supportive care of brain tumor patients. Hematol Oncol Clin North Am. 2006;20:1337–1361. doi: 10.1016/j.hoc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Baulac M, Brodie MJ, Patten A, et al. Efficacy and tolerability of zonisamide versus controlled-release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2012;11:579–588. doi: 10.1016/S1474-4422(12)70105-9. [DOI] [PubMed] [Google Scholar]
- Benifla M, Otsubo H, Ochi A, et al. Temporal lobe surgery for intractable epilepsy in children: an analysis of outcomes in 126 children. Neurosurgery. 2006;59:1203–1213. doi: 10.1227/01.NEU.0000245615.32226.83. discussion 1213–1214. [DOI] [PubMed] [Google Scholar]
- Bénit CP, Vecht CJ. Seizures and cancer: drug interactions of anticonvulsants with chemotherapeutic agents, tyrosine kinase inhibitors and glucocorticoids. Neuro Oncol Pract. 2015 doi: 10.1093/nop/npv038. http://dx.doi.org/10.1093/nop/npv038. First published online: October 11, 2015. [DOI] [PMC free article] [PubMed]
- Bilginer B, Yalnizoglu D, Soylemezoglu F, et al. Surgery for epilepsy in children with dysembryoplastic neuroepithelial tumor: clinical spectrum, seizure outcome, neuroradiology, and pathology. Childs Nerv Syst. 2009;25:485–491. doi: 10.1007/s00381-008-0762-x. [DOI] [PubMed] [Google Scholar]
- Blonski M, Taillandier L, Herbet G, et al. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J Neurooncol. 2012;106:353–366. doi: 10.1007/s11060-011-0670-x. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Luyken C, Urbach H, et al. An isomorphic subtype of long-term epilepsy-associated astrocytomas associated with benign prognosis. Acta Neuropathol. 2004;107:381–388. doi: 10.1007/s00401-004-0833-3. [DOI] [PubMed] [Google Scholar]
- Bobustuc GC, Baker CH, Limaye A, et al. Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide. Neuro Oncol. 2010;12:917–927. doi: 10.1093/neuonc/noq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodalia PN, Grosso AM, Sofat R, et al. Comparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: systematic review and network meta-analysis reveals the need for long term comparator trials. Br J Clin Pharmacol. 2013;76:649–667. doi: 10.1111/bcp.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourg V, Lebrun C, Chichmanian RM, et al. Nitrosourea-cisplatin-based chemotherapy associated with valproate: Increase of haematologic toxicity. Ann Oncol. 2001;12:217–219. doi: 10.1023/a:1008331708395. [DOI] [PubMed] [Google Scholar]
- Brahimaj B, Greiner HM, Leach JL, et al. The surgical management of pediatric brain tumors causing epilepsy: consideration of the epileptogenic zone. Childs Nerv Syst. 2014;30:1383–1391. doi: 10.1007/s00381-014-2427-2. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Sills GJ. Combining antiepileptic drugs-Rational polytherapy? Seizure-European Journal of Epilepsy. 2011;20:369–375. doi: 10.1016/j.seizure.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Yuen AWC. Lamotrigine substitution study: evidence for synergism with sodium valproate? Epilepsy Res. 1997;26:423–432. doi: 10.1016/s0920-1211(96)01007-8. [DOI] [PubMed] [Google Scholar]
- Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruna J, Miro J, Velasco R. Epilepsy in glioblastoma patients: basic mechanisms and current problems in treatment. Expert Rev Clin Pharmacol. 2013;6:333–344. doi: 10.1586/ecp.13.12. [DOI] [PubMed] [Google Scholar]
- Cascino GD. When drugs and surgery don't work. Epilepsia. 2008;49(Suppl 9):79–84. doi: 10.1111/j.1528-1167.2008.01930.x. [DOI] [PubMed] [Google Scholar]
- Chaichana KL, Parker SL, Olivi A, et al. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas Clinical article. J Neurosurg. 2009a;111:282–292. doi: 10.3171/2009.2.JNS081132. [DOI] [PubMed] [Google Scholar]
- Chaichana KL, Parker SL, Olivi A, et al. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009b;111:282–292. doi: 10.3171/2009.2.JNS081132. [DOI] [PubMed] [Google Scholar]
- Chaichana KL, Pendleton C, Zaidi H, et al. Seizure control for patients undergoing meningioma surgery. World Neurosurg. 2013;79:515–524. doi: 10.1016/j.wneu.2012.02.051. [DOI] [PubMed] [Google Scholar]
- Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008a;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008b;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- Chang EF, Christie C, Sullivan JE, et al. Seizure control outcomes after resection of dysembryoplastic neuroepithelial tumor in 50 patients. J Neurosurg Pediatr. 2010;5:123–130. doi: 10.3171/2009.8.PEDS09368. [DOI] [PubMed] [Google Scholar]
- Chow SY, Hsi MS, Tang LM, et al. Epilepsy and intracranial meningiomas. Zhonghua Yi Xue Za Zhi. 1995;55:151–155. [PubMed] [Google Scholar]
- Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure-European Journal of Epilepsy. 2007;16:296–304. doi: 10.1016/j.seizure.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Chung S, Ben-Menachem E, Sperling MR, et al. Examining the Clinical Utility of Lacosamide Pooled Analyses of Three Phase II/III Clinical Trials. CNS Drugs. 2010;24:1041–1054. doi: 10.2165/11586830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Costa J, Fareleira F, Ascencao R, et al. Clinical comparability of the new antiepileptic drugs in refractory partial epilepsy: A systematic review and meta-analysis. Epilepsia. 2011;52:1280–1291. doi: 10.1111/j.1528-1167.2011.03047.x. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Mintzer S, Wheless J, et al. Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother. 2010;10:885–891. doi: 10.1586/ern.10.71. [DOI] [PubMed] [Google Scholar]
- Danfors T, Ribom D, Berntsson SG, et al. Epileptic seizures and survival in early disease of grade 2 gliomas. Eur J Neurol. 2009;16:823–831. doi: 10.1111/j.1468-1331.2009.02599.x. [DOI] [PubMed] [Google Scholar]
- de Groot M, Douw L, Sizoo EM, et al. Levetiracetam improves verbal memory in high-grade glioma patients. Neuro Oncol. 2013;15:216–223. doi: 10.1093/neuonc/nos288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis A, Villani R, Sinisi M, et al. Add-on phenytoin fails to prevent early seizures after surgery for supratentorial brain tumors: a randomized controlled study. Epilepsia. 2002;43:175–182. doi: 10.1046/j.1528-1157.2002.24801.x. [DOI] [PubMed] [Google Scholar]
- Delattre JY, Safai B, Posner JB. Erythema multiforme and Stevens-Johnson syndrome in patients receiving cranial irradiation and phenytoin. Neurology. 1988;38:194–198. doi: 10.1212/wnl.38.2.194. [DOI] [PubMed] [Google Scholar]
- Delgado-Escueta AV, Ward AA, Jr, et al. New wave of research in the epilepsies. Adv Neurol. 1986;44:3–55. [PubMed] [Google Scholar]
- Drappatz J, Schiff D, Kesari S, et al. Medical management of brain tumor patients. Neurol Clin. 2007;25(1035-1071):ix. doi: 10.1016/j.ncl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Duffau H. Surgery of low-grade gliomas: towards a ‘functional neurooncology’. Curr Opin Oncol. 2009;21:543–549. doi: 10.1097/CCO.0b013e3283305996. [DOI] [PubMed] [Google Scholar]
- Duffau H. Brain mapping in tumors: intraoperative or extraoperative? Epilepsia. 2013;54(Suppl 9):79–83. doi: 10.1111/epi.12449. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, et al. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Berger MS, Barbaro NM, et al. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. J Neurosurg. 2011;115:240–244. doi: 10.3171/2011.3.JNS1153. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Berger MS, Barbaro NM, et al. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012a;53:51–57. doi: 10.1111/j.1528-1167.2011.03269.x. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Han SJ, Berger MS, et al. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012b;70:921–928. doi: 10.1227/NEU.0b013e31823c3a30. discussion 928. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Berger MS, Chang EF, et al. Characteristics and treatment of seizures in patients with high-grade glioma: a review. Neurosurg Clin N Am. 2012c;23:227–235. vii–viii. doi: 10.1016/j.nec.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Breshears JD, Sun PP, et al. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12:126–133. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- Fang S, Zhan Y, Xie YF, et al. Predictive value of electrocorticography for postoperative epilepsy in patients with supratentorial meningioma. J Clin Neurosci. 2013;20:112–116. doi: 10.1016/j.jocn.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Fish DR, Spencer SS. Clinical correlations: MRI and EEG. Magn Reson Imaging. 1995;13:1113–1117. doi: 10.1016/0730-725x(95)02020-t. [DOI] [PubMed] [Google Scholar]
- Flores L, Kemp S, Colbeck K, et al. Clinical experience with oral lacosamide as adjunctive therapy in adult patients with uncontrolled epilepsy: a multicentre study in epilepsy clinics in the United Kingdom (UK). Seizure-European Journal of Epilepsy. 2012;21:512–517. doi: 10.1016/j.seizure.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Forsyth PA, Weaver S, Fulton D, et al. Prophylactic anticonvulsants in patients with brain tumour. Can J Neurol Sci. 2003;30:106–112. doi: 10.1017/s0317167100053361. [DOI] [PubMed] [Google Scholar]
- Franceschetti S, Binelli S, Casazza M, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochir (Wien) 1990;103:47–51. doi: 10.1007/BF01420191. [DOI] [PubMed] [Google Scholar]
- Frenay MP, Fontaine D, Vandenbos F, et al. First-line nitrosourea-based chemotherapy in symptomatic nonresectable supratentorial pure low-grade astrocytomas. Eur J Neurol. 2005;12:685–690. doi: 10.1111/j.1468-1331.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50:63–68. doi: 10.1111/j.1528-1167.2009.02238.x. [DOI] [PubMed] [Google Scholar]
- Fried I, Kim JH, Spencer DD. Limbic and neocortical gliomas associated with intractable seizures: a distinct clinicopathological group. Neurosurgery. 1994;34:815–823. doi: 10.1227/00006123-199405000-00005. discussion 823–814. [DOI] [PubMed] [Google Scholar]
- Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27:2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner T, Teich M, Bell N, et al. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2006;47:1136–1143. doi: 10.1111/j.1528-1167.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- Gidal BE, Sheth R, Parnell J, et al. Evaluation of VPA dose and concentration effects on lamotrigine pharmacokinetics: implications for conversion to lamotrigine monotherapy. Epilepsy Res. 2003;57:85–93. doi: 10.1016/j.eplepsyres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Giulioni M, Galassi E, Zucchelli M, et al. Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurg. 2005;102:288–293. doi: 10.3171/ped.2005.102.3.0288. [DOI] [PubMed] [Google Scholar]
- Giulioni M, Gardella E, Rubboli G, et al. Lesionectomy in epileptogenic gangliogliomas: seizure outcome and surgical results. J Clin Neurosci. 2006;13:529–535. doi: 10.1016/j.jocn.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Giulioni M, Rubboli G, Marucci G, et al. Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: lesionectomy compared with tailored resection. J Neurosurg. 2009;111:1275–1282. doi: 10.3171/2009.3.JNS081350. [DOI] [PubMed] [Google Scholar]
- Glantz MJ, Cole BF, Friedberg MH, et al. A randomized, blinded, placebo-controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology. 1996;46:985–991. doi: 10.1212/wnl.46.4.985. [DOI] [PubMed] [Google Scholar]
- Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54:551–563. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- Guberman AH, Besag FMC, Brodie MJ, et al. Lamotrigine-associated rash: risk benefit considerations in adults and children. Epilepsia. 1999;40:985–991. doi: 10.1111/j.1528-1157.1999.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Witt J-A. The effects of levetiracetam on cognition: a non-interventional surveillance study. Epilepsy and Behavior. 2008;13:642–649. doi: 10.1016/j.yebeh.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Hildebrand J, Lecaille C, Perennes J, et al. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65:212–215. doi: 10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi T, Hasegawa Y, Kawasaki K, et al. Epilepsy in patients with gliomas: incidence and control of seizures. J Clin Neurosci. 2015;22:87–91. doi: 10.1016/j.jocn.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Jacoby A, Wang W, Vu TD, et al. Meanings of epilepsy in its sociocultural context and implications for stigma: findings from ethnographic studies in local communities in China and Vietnam. Epilepsy Behav. 2008;12:286–297. doi: 10.1016/j.yebeh.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Jaeckle KA, Ballman K, Furth A, et al. Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology. 2009;73:1207–1213. doi: 10.1212/WNL.0b013e3181bbfeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jooma R, Yeh HS, Privitera MD, et al. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg. 1995;83:231–236. doi: 10.3171/jns.1995.83.2.0231. [DOI] [PubMed] [Google Scholar]
- Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- Kanner AM, Frey M. Adding valproate to lamotrigine: a study of their pharmacokinetic interaction. Neurology. 2000;55:588–591. doi: 10.1212/wnl.55.4.588. [DOI] [PubMed] [Google Scholar]
- Keene DL, Loy-English I, Ventureyra EC. Long-term socioeconomic outcome following surgical intervention in the treatment of refractory epilepsy in childhood and adolescence. Childs Nerv Syst. 1998a;14:362–365. doi: 10.1007/s003810050245. [DOI] [PubMed] [Google Scholar]
- Keene DL, Loy-English I, Ventureyra EC. Patient satisfaction with surgical treatment of refractory epilepsy done in childhood and early adolescence. Childs Nerv Syst. 1998b;14:30–32. doi: 10.1007/s003810050170. [DOI] [PubMed] [Google Scholar]
- Kerkhof M, Dielemans JCM, van Breemen MS, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013;15:961–967. doi: 10.1093/neuonc/not057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan S, Grant R. Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database Syst Rev. 2011;8:CD008586. doi: 10.1002/14651858.CD008586.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OJ, Yong Ahn J, Chung YS, et al. Significance of chronic epilepsy in glial tumors and correlation with surgical strategies. J Clin Neurosci. 2004;11:702–705. doi: 10.1016/j.jocn.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54:514–520. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- Koekkoek JAF, Dirven L, Heimans JJ, et al. Seizure reduction in a low-grade glioma: more than a beneficial side effect of temozolomide. J Neurol Neurosurg Psychiatry. 2015;86:366–373. doi: 10.1136/jnnp-2014-308136. [DOI] [PubMed] [Google Scholar]
- Krauze AV, Myrehaug SD, Chang MG, et al. A phase II study of concurrent radiation therapy, temozolomide and the histone deacetylase inhibitor valproic acid for patients with glioblastoma multiforme. Neuro Oncol. 2014;16(suppl 5):v15–v16. doi: 10.1016/j.ijrobp.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyszkowski T, Czepko R, Betlej M. Prognostic value of epileptic seizures in patients with cerebral gliomas. Ann Acad Med Stetin. 2004;50:35–40. [PubMed] [Google Scholar]
- Kuijlen JM, Teernstra OP, Kessels AG, et al. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: a meta-analysis. Seizure. 1996;5:291–298. doi: 10.1016/s1059-1311(96)80023-9. [DOI] [PubMed] [Google Scholar]
- Kvam DA, Loftus CM, Copeland B, et al. Seizures during the immediate postoperative period. Neurosurgery. 1983;12:14–17. doi: 10.1227/00006123-198301000-00003. [DOI] [PubMed] [Google Scholar]
- Larysz D, Larysz P, Mandera M. Evaluation of quality of life and clinical status of children operated on for intractable epilepsy. Childs Nerv Syst. 2007;23:91–97. doi: 10.1007/s00381-006-0200-x. [DOI] [PubMed] [Google Scholar]
- Lebrun C, Fontaine D, Bourg V, et al. Treatment of newly diagnosed symptomatic pure low-grade oligodendrogliomas with PCV chemotherapy. Eur J Neurol. 2007;14:391–398. doi: 10.1111/j.1468-1331.2007.01675.x. [DOI] [PubMed] [Google Scholar]
- Lee ST, Lui TN, Chang CN, et al. Prophylactic anticonvulsants for prevention of immediate and early postcraniotomy seizures. Surg Neurol. 1989;31:361–364. doi: 10.1016/0090-3019(89)90067-0. [DOI] [PubMed] [Google Scholar]
- Lee JW, Wen PY, Hurwitz S, et al. Morphological characteristics of brain tumors causing seizures. Arch Neurol. 2010;67:336–342. doi: 10.1001/archneurol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Wang S, Zhang J, et al. Long-term outcomes of epilepsy surgery in school-aged children with partial epilepsy. Pediatr Neurol. 2012;47:284–290. doi: 10.1016/j.pediatrneurol.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Liava A, Francione S, Tassi L, et al. Individually tailored extratemporal epilepsy surgery in children: anatomo-electro-clinical features and outcome predictors in a population of 53 cases. Epilepsy Behav. 2012;25:68–80. doi: 10.1016/j.yebeh.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Lieu AS, Howng SL. Intracranial meningiomas and epilepsy: incidence, prognosis and influencing factors. Epilepsy Res. 2000;38:45–52. doi: 10.1016/s0920-1211(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Liigant A, Haldre S, Oun A, et al. Seizure disorders in patients with brain tumors. Eur Neurol. 2001;45:46–51. doi: 10.1159/000052089. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tarapore P, Chang E, et al. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma-related seizure control following craniotomy: a randomized phase II pilot study. J Neurooncol. 2009;93:349–354. doi: 10.1007/s11060-008-9781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, An N, Yang H, et al. Pediatric intractable epilepsy syndromes: reason for early surgical intervention. Brain Dev. 2007;29:69–78. doi: 10.1016/j.braindev.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Lloyd A, McIntosh E, Price M. The importance of drug adverse effects compared with seizure control for people with epilepsy: a discrete choice experiment. Pharmacoeconomics. 2005;23:1167–1181. doi: 10.2165/00019053-200523110-00008. [DOI] [PubMed] [Google Scholar]
- Lote K, Stenwig AE, Skullerud K, et al. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34:98–102. doi: 10.1016/s0959-8049(97)00374-2. [DOI] [PubMed] [Google Scholar]
- Lund M. Epilepsy in association with intracranial tumor. Acta Psychiatr Neurol Scand Suppl. 1952;81:1–149. [PubMed] [Google Scholar]
- Luyken C, Blumcke I, Fimmers R, et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44:822–830. doi: 10.1046/j.1528-1157.2003.56102.x. [DOI] [PubMed] [Google Scholar]
- Lwu S, Hamilton MG, Forsyth PA, et al. Use of peri-operative anti-epileptic drugs in patients with newly diagnosed high grade malignant glioma: a single center experience. J Neurooncol. 2010;96:403–408. doi: 10.1007/s11060-009-9977-2. [DOI] [PubMed] [Google Scholar]
- Lynam LM, Lyons MK, Drazkowski JF, et al. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. 2007;109:634–638. doi: 10.1016/j.clineuro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Zarabla A, et al. Outcome and tolerability of topiramate in brain tumor associated epilepsy. J Neurooncol. 2008;86:61–70. doi: 10.1007/s11060-007-9430-3. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Saveriano F, et al. Efficacy and tolerability of zonisamide as add-on in brain tumor-related epilepsy: preliminary report. Acta Neurol Scand. 2009;120:210–212. doi: 10.1111/j.1600-0404.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Vidiri A, et al. Rash in four patients with brain tumor-related epilepsy in monotherapy with oxcarbazepine, during radiotherapy. J Neurol. 2010;257:1939–1940. doi: 10.1007/s00415-010-5637-x. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Mingoia M, et al. Lacosamide as add-on in brain tumor-related epilepsy: preliminary report on efficacy and tolerability. J Neurol. 2011a;258:2100–2104. doi: 10.1007/s00415-011-6132-8. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Sperati F, et al. Levetiracetam monotherapy in patients with brain tumor-related epilepsy: seizure control, safety, and quality of life. J Neurooncol. 2011b;104:205–214. doi: 10.1007/s11060-010-0460-x. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Sperati F, et al. Oxcarbazepine monotherapy in patients with brain tumor-related epilepsy: open-label pilot study for assessing the efficacy, tolerability and impact on quality of life. J Neurooncol. 2012a;106:651–656. doi: 10.1007/s11060-011-0689-z. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Sperati F, et al. Effect of pregabalin add-on treatment on seizure control, quality of life, and anxiety in patients with brain tumour-related epilepsy: a pilot study. Epileptic Disord. 2012b;14:388–397. doi: 10.1684/epd.2012.0542. [DOI] [PubMed] [Google Scholar]
- Matthew E, Sherwin AL, Welner SA, et al. Seizures following intracranial surgery: incidence in the first postoperative week. Can J Neurol Sci. 1980;7:285–290. doi: 10.1017/s0317167100022757. [DOI] [PubMed] [Google Scholar]
- Mauro AM, Bomprezzi C, Morresi S, et al. Prevention of early postoperative seizures in patients with primary brain tumors: preliminary experience with oxcarbazepine. J Neurooncol. 2007;81:279–285. doi: 10.1007/s11060-006-9229-7. [DOI] [PubMed] [Google Scholar]
- McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–1143. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell RT, Anderson SK, Meyer FB, et al. Seizures in patients with glioma treated with phenytoin and levetiracetam. J Neurosurg. 2010a;113:1176–1181. doi: 10.3171/2010.5.JNS091367. [DOI] [PubMed] [Google Scholar]
- Merrell RT, Anderson SK, Meyer FB, et al. Seizures in patients with glioma treated with phenytoin and levetiracetam. J Neurosurg. 2010b;113:1176–1181. doi: 10.3171/2010.5.JNS091367. [DOI] [PubMed] [Google Scholar]
- Micali G, Linthicum K, Han N, et al. Increased risk of erythema multiforme major with combination anticonvulsant and radiation therapies. Pharmacotherapy. 1999;19:223–227. doi: 10.1592/phco.19.3.223.30917. [DOI] [PubMed] [Google Scholar]
- Michelucci R. Optimizing therapy of seizures in neurosurgery. Neurology. 2006;67:S14–S18. doi: 10.1212/wnl.67.12_suppl_4.s14. [DOI] [PubMed] [Google Scholar]
- Mikati MA, Rahi AC, Shamseddine A, et al. Marked benefits in physical activity and well-being, but not in functioning domains, 2 years after successful epilepsy surgery in children. Epilepsy Behav. 2008;12:145–149. doi: 10.1016/j.yebeh.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Mikati MA, Ataya N, Ferzli J, et al. Quality of life after surgery for intractable partial epilepsy in children: a cohort study with controls. Epilepsy Res. 2010;90:207–213. doi: 10.1016/j.eplepsyres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Mikuni N, Ikeda A, Takahashi JA, et al. A step-by-step resection guided by electrocorticography for nonmalignant brain tumors associated with long-term intractable epilepsy. Epilepsy Behav. 2006;8:560–564. doi: 10.1016/j.yebeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Moots PL, Maciunas RJ, Eisert DR, et al. The course of seizure disorders in patients with malignant gliomas. Arch Neurol. 1995;52:717–724. doi: 10.1001/archneur.1995.00540310091021. [DOI] [PubMed] [Google Scholar]
- Moreno A, de Felipe J, Garcia Sola R, et al. Neuronal and mixed neuronal glial tumors associated to epilepsy. A heterogeneous and related group of tumours. Histol Histopathol. 2001;16:613–622. doi: 10.14670/HH-16.613. [DOI] [PubMed] [Google Scholar]
- Nasreddine W, Beydoun A. Valproate-induced thrombocytopenia: a prospective monotherapy study. Epilepsia. 2008;49:438–445. doi: 10.1111/j.1528-1167.2007.01429.x. [DOI] [PubMed] [Google Scholar]
- North JB, Penhall RK, Hanieh A, et al. Phenytoin and postoperative epilepsy. A double-blind study. J Neurosurg. 1983;58:672–677. doi: 10.3171/jns.1983.58.5.0672. [DOI] [PubMed] [Google Scholar]
- Novy J, Patsalos PN, Sander JW, et al. Lacosamide neurotoxicity associated with concomitant use of sodium channel-blocking antiepileptic drugs: a pharmacodynamic interaction? Epilepsy and Behavior. 2011;20:20–23. doi: 10.1016/j.yebeh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Oberndorfer S, Schmal T, Lahrmann H, et al. The frequency of seizures in patients with primary brain tumors or cerebral metastases. An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Kaiser Franz Josef Hospital, Vienna. Wien Klin Wochenschr. 2002;114:911–916. [PubMed] [Google Scholar]
- Oberndorfer S, Piribauer M, Marosi C, et al. P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J Neurooncol. 2005;72:255–260. doi: 10.1007/s11060-004-2338-2. [DOI] [PubMed] [Google Scholar]
- O'Brien DF, Farrell M, Delanty N, et al. The Children's Cancer and Leukaemia Group guidelines for the diagnosis and management of dysembryoplastic neuroepithelial tumours. Br J Neurosurg. 2007;21:539–549. doi: 10.1080/02688690701594817. [DOI] [PubMed] [Google Scholar]
- Ogiwara H, Nordli DR, DiPatri AJ, et al. Pediatric epileptogenic gangliogliomas: seizure outcome and surgical results. J Neurosurg Pediatr. 2010;5:271–276. doi: 10.3171/2009.10.PEDS09372. [DOI] [PubMed] [Google Scholar]
- Otoul C, Arrigo C, van Rijckevorsel K, et al. Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy. Clin Neuropharmacol. 2005;28:72–78. doi: 10.1097/01.wnf.0000159956.87511.67. [DOI] [PubMed] [Google Scholar]
- Pace A, Vidiri A, Galie E, et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14:1722–1726. doi: 10.1093/annonc/mdg502. [DOI] [PubMed] [Google Scholar]
- Pallud J, Capelle L, Huberfeld G. Tumoral epileptogenicity: how does it happen? Epilepsia. 2013;54(suppl 9):30–34. doi: 10.1111/epi.12440. [DOI] [PubMed] [Google Scholar]
- Park YS, Kim DS, Shim KW, et al. Factors contributing to resectability and seizure outcomes in 44 patients with ganglioglioma. Clin Neurol Neurosurg. 2008;110:667–673. doi: 10.1016/j.clineuro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Pasquier B, Peoc HM, Fabre-Bocquentin B, et al. Surgical pathology of drug-resistant partial epilepsy. A 10-year-experience with a series of 327 consecutive resections. Epileptic Disord. 2002;4:99–119. [PubMed] [Google Scholar]
- Penfield W, Erickson T, Tarlov I. Relation of intracranial tumors and symptomatic epilepsy. Arch Neurol Psychiatry. 1940;44:300–315. [Google Scholar]
- Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- Pursche S, Schleyer E, von Bonin M, et al. Influence of enzyme-inducing antiepileptic drugs on trough level of imatinib in glioblastoma patients. Curr Clin Pharmacol. 2008;3:198–203. doi: 10.2174/157488408785747656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond E, Brandes AA, Dittrich C, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26:4659–4665. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht L, Glantz M. Neoplastic diseases. In: Engel J Jr, Pedley T, editors. Epilepsy: A Comprehensive Textbook. Lippincott, Williams, and Wilkins; New York: 2008. pp. 2637–2642. [Google Scholar]
- Riva M, Salmaggi A, Marchioni E, et al. Tumour-associated epilepsy: clinical impact and the role of referring centres in a cohort of glioblastoma patients. A multicentre study from the Lombardia Neurooncology Group. Neurol Sci. 2006;27:345–351. doi: 10.1007/s10072-006-0708-6. [DOI] [PubMed] [Google Scholar]
- Rosati A, Tomassini A, Pollo B, et al. Epilepsy in cerebral glioma: timing of appearance and histological correlations. J Neurooncol. 2009;93:395–400. doi: 10.1007/s11060-009-9796-5. [DOI] [PubMed] [Google Scholar]
- Rosati A, Buttolo L, Stefini R, et al. Efficacy and safety of levetiracetam in patients with glioma: a clinical prospective study. Arch Neurol. 2010;67:343–346. doi: 10.1001/archneurol.2009.335. [DOI] [PubMed] [Google Scholar]
- Rosati A, Poliani PL, Todeschini A, et al. Glutamine synthetase expression as a valuable marker of epilepsy and longer survival in newly diagnosed glioblastoma multiforme. Neuro Oncol. 2013;15:618–625. doi: 10.1093/neuonc/nos338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti AO, Jeckelmann S, Novy J, et al. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A phase II randomized study. Neuro Oncol. 2014;16:584–588. doi: 10.1093/neuonc/not170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Figus A, Corraine S. Early presentation of de novo high grade glioma with epileptic seizures: electroclinical and neuroimaging findings. Seizure. 2010;19:470–474. doi: 10.1016/j.seizure.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Ruda R, Bello L, Duffau H, et al. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14:55–64. doi: 10.1093/neuonc/nos199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda R, Magliola U, Bertero L, et al. Seizure control following radiotherapy in patients with diffuse gliomas: a retrospective study. Neuro Oncol. 2013;15:1739–1749. doi: 10.1093/neuonc/not109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- Saria MG, Corle C, Hu J, et al. Retrospective analysis of the tolerability and activity of lacosamide in patients with brain tumors. J Neurosurg. 2013;118:1183–1187. doi: 10.3171/2013.1.JNS12397. [DOI] [PubMed] [Google Scholar]
- Sawh SC, Newman JJ, Deshpande S, et al. Lacosamide adjunctive therapy for partial-onset seizures: a meta-analysis. PeerJ. 2013;1:e114–e114. doi: 10.7717/peerj.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerrati M, Montemaggi P, Iacoangeli M, et al. Interstitial brachytherapy for low-grade cerebral gliomas – analysis of results in a series of 36 cases. Acta Neurochir. 1994;131:97–105. doi: 10.1007/BF01401459. [DOI] [PubMed] [Google Scholar]
- Shankar A, Rajshekhar V. Radiological and clinical outcome following stereotactic biopsy and radiotherapy for low-grade insular astrocytomas. Neurol India. 2003;51:503–506. [PubMed] [Google Scholar]
- Sherman JH, Moldovan K, Yeoh HK, et al. Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg. 2011;114:1617–1621. doi: 10.3171/2010.12.JNS101602. [DOI] [PubMed] [Google Scholar]
- Sheth RD. Adolescent issues in epilepsy. J Child Neurol. 2002;17(Suppl 2):2S23–22S27. doi: 10.1177/08830738020170020801. [DOI] [PubMed] [Google Scholar]
- Simo M, Velasco R, Graus F, et al. Impact of antiepileptic drugs on thrombocytopenia in glioblastoma patients treated with standard chemoradiotherapy. J Neurooncol. 2012;108:451–458. doi: 10.1007/s11060-012-0836-1. [DOI] [PubMed] [Google Scholar]
- Siomin V, Angelov L, Li L, et al. Results of a survey of neurosurgical practice patterns regarding the prophylactic use of anti-epilepsy drugs in patients with brain tumors. J Neurooncol. 2005;74:211–215. doi: 10.1007/s11060-004-6912-4. [DOI] [PubMed] [Google Scholar]
- Sirven JI, Wingerchuk DM, Drazkowski JF, et al. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004;79:1489–1494. doi: 10.4065/79.12.1489. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM, Lin WR, Harding BN, et al. Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain. 2002;125:22–31. doi: 10.1093/brain/awf002. [DOI] [PubMed] [Google Scholar]
- Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12:1162–1166. doi: 10.1093/neuonc/nop045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffietti R, Ruda R, Bradac GB, et al. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43:1066–1073. doi: 10.1097/00006123-199811000-00035. [DOI] [PubMed] [Google Scholar]
- Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNSEANO* Task Force. Eur J Neurol. 2010;17:1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Garcia PA, Berger MS, et al. Long-term seizure control outcomes after resection of gangliogliomas. Neurosurgery. 2012;70:1406–1413. doi: 10.1227/NEU.0b013e3182500a4c. discussion 1413–1414. [DOI] [PubMed] [Google Scholar]
- Souza-Oliveira C, Escorsi-Rosset S, Terra VC, et al. Impact of pediatric epilepsy surgery on intellectual efficiency. Rev Neurol. 2012;54:214–220. [PubMed] [Google Scholar]
- Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- Stockhammer F, Misch M, Helms HJ, et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. 2012;21:194–197. doi: 10.1016/j.seizure.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Stupp R, Janzer RC, Hegi ME, et al. Prognostic factors for low-grade gliomas. Semin Oncol. 2003;30:23–28. doi: 10.1053/j.seminoncol.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Sugano H, Shimizu H, Sunaga S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure. 2007;16:120–127. doi: 10.1016/j.seizure.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Sughrue ME, Rutkowski MJ, Chang EF, et al. Postoperative seizures following the resection of convexity meningiomas: are prophylactic anticonvulsants indicated? Clinical article. J Neurosurg. 2011;114:705–709. doi: 10.3171/2010.5.JNS091972. [DOI] [PubMed] [Google Scholar]
- Sweet JA, Hdeib AM, Sloan A, et al. Depths and grids in brain tumors: implantation strategies, techniques, and complications. Epilepsia. 2013;54(Suppl 9):66–71. doi: 10.1111/epi.12447. [DOI] [PubMed] [Google Scholar]
- Tandon N, Esquenazi Y. Resection strategies in tumoral epilepsy: is a lesionectomy enough? Epilepsia. 2013;54(Suppl 9):72–78. doi: 10.1111/epi.12448. [DOI] [PubMed] [Google Scholar]
- Taphoorn MJ. Neurocognitive sequelae in the treatment of low-grade gliomas. Semin Oncol. 2003;30:45–48. doi: 10.1053/j.seminoncol.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- Telfeian AE, Philips MF, Crino PB, et al. Postoperative epilepsy in patients undergoing craniotomy for glioblastoma multiforme. J Exp Clin Cancer Res. 2001;20:5–10. [PubMed] [Google Scholar]
- Tinchon A, Oberndorfer S, Marosi C, et al. Haematological toxicity of Valproic acid compared to Levetiracetam in patients with glioblastoma multiforme undergoing concomitant radio-chemotherapy: a retrospective cohort study. J Neurol. 2015;262:179–186. doi: 10.1007/s00415-014-7552-z. [DOI] [PubMed] [Google Scholar]
- van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- van Breemen MSM, Rijsman RM, Taphoorn MJB, et al. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256:1519–1526. doi: 10.1007/s00415-009-5156-9. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- Van Nifterik KA, Van den Berg J, Slotman BJ, et al. Valproic acid sensitizes human glioma cells for temozolomide and g-radiation. J Neurooncol. 2012;107(1):61–67. doi: 10.1007/s11060-011-0725-z. [DOI] [PubMed] [Google Scholar]
- Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. 2014;19:751–759. doi: 10.1634/theoncologist.2014-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva V, Codina M, Elices E. Management of epilepsy in oncological patients. Neurologist. 2008;14:S44–S54. doi: 10.1097/01.nrl.0000340791.53413.f4. [DOI] [PubMed] [Google Scholar]
- Wagner GL, Wilms EB, Van Donselaar CA, et al. Levetiracetam: preliminary experience in patients with primary brain tumours. Seizure. 2003;12:585–586. doi: 10.1016/s1059-1311(03)00096-7. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Wu JZ, Ma GY, et al. Efficacy assessment of phenobarbital in epilepsy: a large community-based intervention trial in rural China. Lancet Neurol. 2006;5:46–52. doi: 10.1016/S1474-4422(05)70254-4. [DOI] [PubMed] [Google Scholar]
- Ward MM, Barbaro NM, Laxer KD, et al. Preoperative valproate administration does not increase blood loss during temporal lobectomy. Epilepsia. 1996;37:98–101. doi: 10.1111/j.1528-1157.1996.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Warnke PC, Berlis A, Weyerbrock A, et al. Significant reduction of seizure incidence and increase of benzodiazepine receptor density after interstitial radiosurgery in low-grade gliomas.. Advances in Stereotactic and Functional Neurosurgery 12: Proceedings of the 12th Meeting of the European Society for Stereotactic and Functional Neurosurgery; Milan. 1996; 1997. pp. 90–92. [DOI] [PubMed] [Google Scholar]
- Wee S, Niklasson M, Marinescu VD, et al. Selective calcium sensitivity in immature glioma cancer stem cells. PLoS One. 2014;22(9):e115698. doi: 10.1371/journal.pone.0115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M, Gorlia T, Cairncross JG, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77:1156–1164. doi: 10.1212/WNL.0b013e31822f02e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JC, Liu CT, Mixter WJ. Focal epilepsy; a statistical study of its causes and the results of surgical treatment; epilepsy secondary to intracranial tumors. N Engl J Med. 1948;238:891–899. doi: 10.1056/NEJM194806242382601. [DOI] [PubMed] [Google Scholar]
- Wick W, Menn O, Meisner C, et al. Pharmacotherapy of epileptic seizures in glioma patients: who, when, why and how long? Onkologie. 2005;28:391. doi: 10.1159/000086375. [DOI] [PubMed] [Google Scholar]
- Wray CD, McDaniel SS, Saneto RP, et al. Is postresective intraoperative electrocorticography predictive of seizure outcomes in children? J Neurosurg Pediatr. 2012;9:546–551. doi: 10.3171/2012.1.PEDS11441. [DOI] [PubMed] [Google Scholar]
- Wu JY, Salamon N, Kirsch HE, et al. Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology. 2010;74:392–398. doi: 10.1212/WNL.0b013e3181ce5d9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Morland TB, Schmits K, et al. A prospective study of loss of consciousness in epilepsy using virtual reality driving simulation and other video games. Epilepsy Behav. 2010;18:238–246. doi: 10.1016/j.yebeh.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I, Chang EF, Han SJ, et al. Early surgical intervention in adult patients with ganglioglioma is associated with improved clinical seizure outcomes. J Clin Neurosci. 2011;18:29–33. doi: 10.1016/j.jocn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Yao YT. Clinicopathologic analysis of 615 cases of meningioma with special reference to recurrence. J Formos Med Assoc. 1994;93:145–152. [PubMed] [Google Scholar]
- You G, Sha ZY, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro Oncol. 2012;14:230–241. doi: 10.1093/neuonc/nor205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TI, Morokoff AP, Bjorksten A, et al. Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology. 2012;79:883–889. doi: 10.1212/WNL.0b013e318266fa89. [DOI] [PubMed] [Google Scholar]
- Zaatreh MM, Spencer DD, Thompson JL, et al. Frontal lobe tumoral epilepsy: clinical, neurophysiologic features and predictors of surgical outcome. Epilepsia. 2002;43:727–733. doi: 10.1046/j.1528-1157.2002.39501.x. [DOI] [PubMed] [Google Scholar]
- Zaatreh MM, Firlik KS, Spencer DD, et al. Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology. 2003;61:636–641. doi: 10.1212/01.wnl.0000079374.78589.1b. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Chen P, Fu W, et al. Early and late postoperative seizure outcome in 97 patients with supratentorial meningioma and preoperative seizures: a retrospective study. J Neurooncol. 2013;114:101–109. doi: 10.1007/s11060-013-1156-9. [DOI] [PubMed] [Google Scholar]