Abstract
Objective
Neuromyelitis optica and its spectrum disorders (NMOSD) are inflammatory demyelinating diseases (IDD) with a specific biomarker, aquaporin-4-IgG. Prior NMO/NMOSD epidemiological studies are limited by lack of aquaporin-4-IgG seroprevalence assessment, absence of population-based USA studies and under-representation of blacks. To overcome these limitations, we sought to compare NMO/NMOSD seroepidemiology across two ethnically divergent populations.
Methods
We performed a population-based comparative study of the incidence (2003–2011) and prevalence (on December 31, 2011) of NMO/NMOSD and aquaporin-4-IgG seroincidence and seroprevalence (sera collected in 80–84% of IDD) among patients with IDD diagnosis in Olmsted County, USA (82% white [Caucasian]) and Martinique (90% black [Afro-Caribbean]). Aquaporin-4-IgG was measured by M1-isoform-fluorescent-activated-cell-sorting assays.
Results
The age and sex adjusted incidence (7.3 vs 0.7/1,000,000 person-years [p<0.01]) and prevalence (10 vs 3.9/100,000[p=0.01]) in Martinique exceeded that in Olmsted County. The AQP4-IgG age and sex-adjusted seroincidence (6.5 vs 0.7/1,000,000 person-years [p<0.01]) and seroprevalence (7.9 vs 3.3/100,000[p=0.04]) were also higher in Martinique than Olmsted County. The ethnicity-specific prevalence was similar in Martinique and Olmsted County: 11.5 and 13/100,000 in blacks, and 6.1 and 4.0/100,000 in whites, respectively. NMO/NMOSD represented a higher proportion of IDD in Martinique than Olmsted County (16% vs 1.4%; p<0.01). The onset age (median, 35–37 years) and female:male distribution (8–9:1) were similar across both populations; 60% of prevalent cases were either blind in one eye, dependent on a gait aid or both.
Interpretation
This study reports the highest prevalence of NMO/NMOSD in any population (10/100,000 in Martinique), estimates it affects 16,000–17,000 in the USA (higher than previous predictions) and demonstrates it disproportionately affects blacks.
Keywords: seroprevalence, seroincidence, Devic’s disease, neuromyelitis optica spectrum disorder, ethnicity, race, transverse myelitis
Neuromyelitis Optica (NMO) is an autoimmune water channelopathy that predominantly affects astrocytes in the central nervous system (resulting in secondary demyelination) preferentially attacking optic nerve, spinal cord and circumventricular organs (e.g., area postrema).1 It is recognized as an inflammatory demyelinating disease (IDD). After aquaporin-4-IgG (AQP4-IgG) was discovered in 2004 as the first serum biomarker of any IDD, it became accepted that the NMO entity is distinct from multiple sclerosis (MS).2 AQP4-IgG, detected by clinically validated M1-isoform-fluorescent-activated-cell-sorting (M1-FACS) assays,3 is both sensitive (>80%) and highly specific (>99%) as a serum biomarker of NMO and included in 2006 NMO diagnostic criteria (which require both optic neuritis and transverse myelitis).4 AQP4-IgG specificity allowed recognition of a broader clinical spectrum of NMO with similar natural history but not meeting these criteria (e.g., recurrent AQP4-IgG-seropositive optic neuritis without transverse myelitis); these syndromes are known as NMO spectrum disorders (NMOSD).5, 6 Attacks are generally more severe and recovery less complete than in other IDD (e.g., MS);7 a single attack can render a patient permanently blind or paraplegic. Thus, knowledge of NMO/NMOSD epidemiology is important for allocation of resources and health care delivery. NMO/NMOSD prevalence rates ranging from 0.72 to 4.4/100,000 are reported in Europe.8, 9 Prior studies did not assess seroprevalence of AQP4-IgG among an unselected cohort of IDD potentially resulting in prevalence underestimation. For example, an NMO patient diagnosed prior to 2004 (when AQP4-IgG was discovered) may have been diagnosed with MS and excluded from prior NMO/NMOSD prevalence studies if they resided in a care facility without subsequent neurological follow up. In addition, population-based studies of NMO/NMOSD prevalence in the USA have not been undertaken and epidemiological studies in Africans, who are suspected to be more predisposed to NMO/NMOSD than whites, are few.10–12 To overcome pre-existing epidemiological limitations, we compared the population-based seroprevalence and seroincidence of AQP4-IgG autoimmunity and NMO/NMOSD among patients with IDD in two ethnically divergent populations (Olmsted County, USA and Martinique, French West Indies).
Methods
Study design and participants
For this population-based comparative study of the incidence and prevalence of NMO/NMOSD and AQP4-IgG seroincidence and seroprevalence among patients with central nervous system (CNS) IDD diagnosis in Olmsted County, USA and Martinique, we included patients of both sexes and all ages, including children, institutionalized individuals and ethnic minorities. The study was approved by Institutional Review Boards of the Mayo Clinic, the Olmsted Medical Center and Martinique. All patients who gave a blood sample provided written consent for blood testing. Included patients who refused to give a blood sample consented to review of their medical record for research purposes.
Olmsted County
The Olmsted County region of Minnesota is situated in the mid-west USA (44°N, 92.4°W). Its population is 145,979 and Caucasian ethnicity predominates (Table 1). The Olmsted County population demographics are summarized in Table 1. Using the Rochester Epidemiology Project medical records linkage system, a database that includes all medical practitioners in Olmsted county,13 we identified all patients with IDD (MS, NMOSD, optic neuritis, transverse myelitis, clinically isolated syndrome, acute disseminated encephalomyelitis or other CNS IDD) by searching the medical records from January 1 1985 to December 31 2011 for all diagnostic codes potentially relevant to these diseases. We cross referenced this list to a prior Olmsted County population-based MS study.14 Each potential subject was invited to participate by giving a single blood sample (collected between 2007 and 2015). We also included consenting patients whose blood samples had previously been archived in the Mayo Neuroimmunology Laboratory, avoiding the need for repeated blood draw. In our experience AQP4-IgG detection is stable in serum retested after many years of frozen storage and prior studies have used this method to calculate sensitivity and specificity or AQP4-IgG for NMO diagnosis.3
Table 1.
Comparison of the Olmsted County and Martinique 2011 populations
| Olmsted County | Martinique | |
|---|---|---|
| N (%) | N (%) | |
| Total population | 145,979 | 392,291 |
| Age | ||
| 0–17 | 36,877 (25.3%) | 92,522 (23.6%) |
| 18–39 | 42,924 (29.4%) | 96,689 (24.7%) |
| 40–64 | 47,827 (32.7%) | 140,975 (35.9%) |
| 65+ | 18,351 (12.6%) | 62,105 (15.8%) |
| Sex | ||
| Male | 71,331 (48.9%) | 180,656 (46.1%) |
| Female | 74,648 (51.1%) | 211,635 (53.9%) |
| Race | ||
| Black | 7,299 (5.0 %) | 353,062 (90.0%) |
| White | 119,703 (82.0%) | 19,615 (5.0%) |
| Other | 18,977 (13.0%) | 19,614 (5.0%) |
Martinique
Martinique is an island in the eastern Caribbean Sea (14°3N, 61°W). It has a population (December, 31, 2011) of approximately 392,291 and Afro-Caribbean ethnicity predominates. The Martinique population demographics are summarized in Table 1. The IDD population registry maintained in Martinique since 1992 from multiple sources ensures capture of all cases: hospital-based and clinic-based neurology, ophthalmology and rehabilitation services; departmental health insurance data and MS patient associations. All blood samples collected as part of this registry were sent to Mayo Clinic Rochester for quantitative assay of AQP4-IgG.
Data Collection
From the medical record we abstracted information informing race/ethnicity, age, sex, period of follow-up, co-existing autoimmune manifestations and clinical data. For stratified analysis race was divided into white (Caucasians [excluding Hispanic and Asians]) and black (those of African descent [Afro-Caribbean or African-American]) subgroups. Disability at last follow-up was assessed using the Expanded Disability Status Scale Score15 and visual acuity scores (blindness defined as acuity <20/200).
Definition of Disease
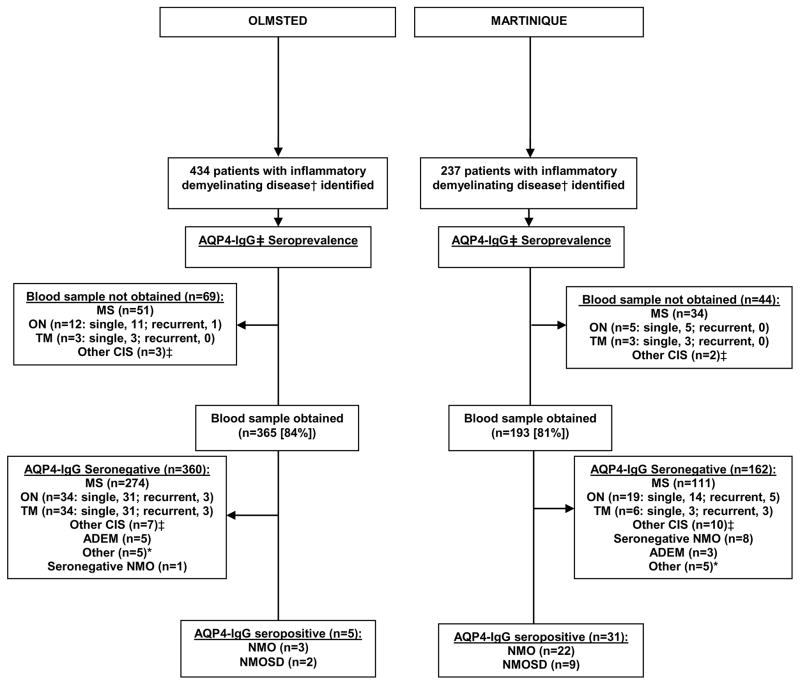
All diagnoses were assigned from retrospective medical record review by a neurologist (E.P.F, P.C.) into one of the following categories: MS, NMO/NMOSD (requiring AQP4-IgG seropositivity), optic neuritis, transverse myelitis, clinically isolated syndrome and acute disseminated myelitis or other IDD (e.g., tumefactive MS). NMO was defined by Wingerchuk 2006 criteria.4 For this study, NMOSD was defined as AQP4-IgG seropositivity with one or more of the following syndromes (but not meeting 2006 NMO criteria): single or recurrent transverse myelitis; single or recurrent optic neuritis; brainstem demyelinating attack; or cerebral demyelinating attack. Other IDD were defined by their respective diagnostic criteria (when available).16–18 In Figure 1, other clinically isolated syndrome (CIS) refers to patients with a single cerebral, cerebellar or brainstem demyelinating attack or a single demyelinating attack with multifocal CNS involvement.
Figure 1.
Flowchart of seroincidence and seroprevalence of NMOSD
† MS, NMOSD, ON, TM, CIS, ADEM or other inflammatory demyelinating disorders
*Olmsted County: Encephalomyelitis with meningeal involvement, 2; combined autoimmune retinopathy and optic neuropathy, 1; inflammatory leukoencephalopathy not otherwise specified, 1; and tumefactive demyelination, 1.
*Martinique: suspected NMOSD but AQP4-IgG seronegative, 2; Baló’s concentric sclerosis, 2; tumefactive demyelination, 1;
‡other clinically isolated syndrome (CIS) refers to patients with single episodes of cerebral, cerebellar or brainstem demyelinating attacks alone or a single demyelinating episode with multifocal CNS involvement not meeting criteria for the other diagnoses listed.
Abbreviations: ADEM, acute disseminated encephalomyelitis; AQP4-IgG, aquaporin-4-IgG; CIS, clinically isolated syndrome; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders, including NMO; ON, optic neuritis; TM, transverse myelitis.
Aquaporin-4-IgG Quantitative Assay
AQP4-IgG testing was performed in the Mayo Clinic Neuroimmunology Laboratory, by technicians blinded to diagnosis, using a clinically-validated live transfected cell-based assay (fluorescence-activated-cell-sorting AQP4-M1-isoform [M1-FACS]) which is 75–83% sensitive and 99–100% specific for NMO diagnosis.3
Prevalence and Incidence Calculation and Statistical Methodology
The prevalence calculated for the date of December 31, 2011 was the number of patients diagnosed with NMO/NMOSD per 100,000 persons. We determined the incidence from January 1, 2003 to December 31, 2011 (calculated from date of symptom onset). The exact date of symptom onset was available for all cases through retrospective medical record review. Our search and medical record review was undertaken until December 31, 2012 to assess for incident cases that had a delayed presentation. The incidence rate was the number of patients with NMO/NMOSD divided by the total number of person years at risk and was reported per 1,000,000 person-years. Age-standardized or age- and sex-standardized estimates were obtained by direct standardization to the USA 2010 total population using four age groups (0–18, 19–39, 40–64, 65+). Confidence intervals and p-values for rates assume the cases follow a Poisson distribution. Wilcoxon rank sum test or Fisher’s exact test were used for comparisons as appropriate. 95% confidence intervals and p values were reported; p values <0.05 were considered significant. Analyses were performed using SAS statistical software (SAS Institute Inc., Cary, NC).
Results
The overall population demographics of Olmsted County (USA) and Martinique are summarized in Table 1. Figure 1 shows the IDD numbers in each population, and distribution of diagnoses for the prevalence/seroprevalence cases. The clinical data of NMO/NMOSD patients from the two populations are shown in Table 2. Blood samples were received from 365/434 (84%) prevalent and 104/130 (80%) incident IDD cases within Olmsted County and from 193/237 (81%) prevalent and 111/122 (91%) incident IDD patients in Martinique. In Olmsted County 69 prevalent cases did not give a blood sample (33 refused; blood from 22 consenters was not received within the study’s timeline; 10 were unable to be contacted; 4 had died). In Martinique 44 prevalent cases did not give a blood sample (19 unable to be contacted; 13 refused; 12 precluded by severe disability/inability to travel). Review of medical records (including MRIs) revealed NMO/NMOSD diagnosis was not suspected in any case for whom serum was unavailable. A personal history of an additional autoimmune disorder was noted in five patients in Martinique (Lupus, 2; Sjögren syndrome, 2; and Type 1 diabetes, 1) and two patients in Olmsted County (Myasthenia gravis, 1; premature ovarian failure, 1).
Table 2.
Comparison of the characteristics of NMO/NMOSD patients across populations
| NMO/NMOSD, No. (%)a | |||
|---|---|---|---|
| Olmsted (n=6) | Martinique (n=39) | pb | |
| NMO/NMOSD as a proportion of inflammatory demyelinating disease in population | 6/434 (1.4%) | 39/237 (16%) | <0.01 |
| Demographics | |||
| Median onset age (range) | 37 (10–55) | 35 (14–82) | 0.93 |
| Female sex | 5 (83%) | 35 (90%) | 0.53 |
| Race | |||
| ➢White | 5 (83%) | 1 (3%) | <0.01f |
| ➢Black | 1 (17%) | 38 (97%) | <0.01f |
| Clinical Data | |||
| Median years of follow-up (range) | 12.5 (3–22) | 7 (1–37) | 0.25 |
| Personal History of autoimmunityc | 2 (33%) | 5 (13%) | 0.23 |
| Diagnosis | |||
| NMO | 4 (67%) | 30 (77%) | 0.62 |
| NMOSDd | 2 (33%) | 9 (23%) | 0.62 |
| Serology | |||
| AQP4-IgG seropositive | 5 (83%) | 31 (79%) | 1.0 |
| Clinic Coursee | |||
| Median annualized relapse rate (range) | 0.80 (0.25–1.8) | 0.67 (0.16–2.13) | 0.73 |
Except where specified
p from Fisher’s exact test for categorical variables and from Wilcoxon rank-sum test for continuous variables
Olmsted County: Myasthenia gravis, 1; premature ovarian failure, 1;
Martinique: Lupus, 2; Sjögren syndrome, 2; Type 1 diabetes, 1;
Olmsted County: AQP4-IgG seropositive recurrent longitudinally extensive transverse myelitis alone, 2;
Martinique: AQP4-IgG seropositive recurrent longitudinally extensive transverse myelitis alone, 4; AQP4-IgG seropositive recurrent optic neuritis, 3; AQP4-IgG seropositive single longitudinally extensive transverse myelitis with a cerebral attack, 1; AQP4-IgG seropositive single episode optic neuritis, 1;
The median (range) of expanded disability status scale score (EDSS) of prevalent cases was 6 (range, 4–8) in Olmsted County and 4 (range, 1–8.5) in Martinique (p=0.04); The number of prevalent NMO/NMOSD patients blind in at least one eye was 4 (67%) in Olmsted County and 14 of 36 (39%) of Martinique (p=0.37). These numbers are limited by differential follow up and small numbers in Olmsted County.
statistically significant
Abbreviations: AQP4-IgG, aquaporin-4-IgG; EDSS, expanded disability status scale score; NMO, neuromyelitis optica; NMOSD, neuromyelitis optica spectrum disorder.
Incidence and Prevalence
The overall age and sex adjusted prevalence of NMO/NMOSD on December 31, 2011 was greater in Martinique (10/100,000) than in Olmsted County (3.9/100,000), p=0.01 (Table 3). Similarly, the age and sex adjusted incidence rate of NMO/NMOSD from 2003–2011 was higher in Martinique (7.3/1,000,000) than in Olmsted County (0.7/1,000,000), p<0.001 (Table 3). The remaining prevalence/seroprevalence and incidence/seroincidence results, including stratification by sex and ethnicity are listed in Table 3. NMO/NMOSD symptom onset occurred before age 18 in one Olmsted County patient (17% of cases) and one Martinique patient (3% of cases). The ratio of MS to NMO/NMOSD was lower in Martinique (3.5:1) than Olmsted County (54:1) (Figure 1). Three incidence cases in Martinique had died at last follow-up, while the single incidence case in Olmsted County was alive at last follow-up. For the expected cases in the USA population (318,857,056) based on age-standardized prevalence rates for NMO/NMOSD, the estimates and 95% confidence intervals (CI) were: White, 9,900 (95% CI: 1,200–18,600); Black, 5,400 (95% CI: 0–16,100); and Total, 16,600 (3,000–30,000 [this latter figure assumes that the rate for non-whites and non-blacks such as Asian or Hispanic ethnicity is equal to the rates for whites]).
Table 3.
Prevalence (on December 31st 2011) and Incidence (2003–2011) of NMO/NMOSDSD and AQP4-IgG in Olmsted County and Martinique
| Olmsted County | Martinique | |||||
|---|---|---|---|---|---|---|
| Prevalent cases (2011) | Prevalencea/100,000 (95%CI)b | Prevalent cases (2011) | Prevalencea/100,000 (95% CI)b | pb | ||
| NMO/NMOSD | Overall | 6 | 3.9 (0.8, 7.1) | 39 | 10.0 (6.8, 13.2) | 0.01e |
| White | 5 | 4.0 (0.5, 7.5) | 1 | 6.1 (0.0, 18.1) | 0.47 | |
| Black | 1 | 13.0 (0.0, 38.4) | 38 | 11.5 (7.8, 15.2) | 0.59 | |
| Female | 5 | 6.5 (0.8, 12.1) | 35 | 17.4 (11.6, 23.3) | 0.02e | |
| Male | 1 | 1.4 (0.0, 4.1) | 4 | 2.5 (0.0, 4.9) | 1.0 | |
| AQP4-IgG seropositive | Overall | 5 | 3.3 (0.4, 6.2) | 31 | 7.9 (5.0, 12.6) | 0.04 |
| White | 4 | 3.2 (0.1, 6.4) | 0 | 0.0 - |
<0.001e | |
| Black | 1 | 13.0 (0.0, 38.4) | 31 | 9.3 (6.0, 12.6) | 0.51 | |
| Female | 4 | 5.2 (0.1, 10.3) | 29 | 14.3 (9.0, 19.5) | 0.02e | |
| Male | 1 | 1.4 (0.0, 4.1) | 2 | 1.2 (0.0, 2.9) | 0.58 | |
| Olmsted County | Martinique | |||||
| Incident casesc (2003–2011) | Incidencea/1,000,000 person years (95%CI)b | Incident casesd (2003–2011) | Incidencea/1,000,000 person years (95% CI)b | Pb | ||
| NMO/NMOSD | Overall | 1 | 0.7 (0.0, 2.1) | 27 | 7.3 (4.5, 10.1) | <0.001e |
| White | 1 | 0.9 (0.0, 2.6) | 0 | 0.0 - |
<0.001e | |
| Black | 0 | 0.0 - |
27 | 8.5 (5.3, 11.8) | <0.001e | |
| Female | 1 | 1.4 (0.0, 4.3) | 25 | 13.1 (7.9, 18.3) | 0.002e | |
| Male | 0 | 0.0 - |
2 | 1.2 (0.0, 3.0) | 0.64 | |
| AQP4-IgG seropositive | Overall | 1 | 0.7 (0.0, 2.1) | 24 | 6.5 (3.9, 9.2) | 0.002e |
| White | 1 | 0.9 (0.0, 2.7) | 0 | 0.0 - |
<0.001e | |
| Black | 0 | 0.0 - |
24 | 7.6 (4.5, 10.7) | <0.001e | |
| Female | 1 | 1.4 (0.0, 4.3) | 22 | 11.6 (6.7, 16.5) | 0.005e | |
| Male | 0 | 0.0 - |
2 | 1.2 (0.0, 3.0) | 0.64 | |
Rates are standardized to the age distribution of the US 2010 population when stratified by sex and race. Overall rates are standardized to the age and sex distribution of the US 2010 population; in subsequent follow-up after 12/31/2011 two prevalent NMO/NMOSD cases from Olmsted county died 16 and 26 years after diagnosis but no prevealent cases from Martinique were known to have died.
Confidence intervals and p-values assume the cases follow a Poisson distribution by Fischer’s exact test
Distribution of incident cases in Olmsted County: 2006 (n=1); 0 deaths of incident cases.
Distribution of incident cases in Martinique: 2003 (n=3); 2004 (n=3); 2005 (n=5); 2006 (n=2); 2007 (n=2); 2008 (n=2); 2009 (n=2); 2010 (n=3); 2011 (n=5); 3 deaths of incident cases (median of 4 years from symptom onset; range, 1–5).
Statistically significant
Abbreviations: CI, confidence interval; NMOSD, neuromyelitis optica spectrum disorders, including NMO, unified by AQP4-IgG seropositivity.
Discussion
The incidence and prevalence rates we documented for NMO/NMOSD disorders in Martinique (7.3/1,000,000 person-years and 10/100,000 persons), a population predominantly African in origin, are the highest reported thus far for any population,10 exceeding those of Olmsted County (population predominantly Caucasian) by a factor of approximately 2.5. Stratification by ethnicity showed similar prevalence rates in both populations (Table 3) confirming the findings of higher prevalence in blacks from prior USA tertiary referral cohorts11 and contradicting reports suggesting no ethnic differences in NMO/NMOSD prevalence.19 NMO/NMOSD morbidity was high in Olmsted County (Table 2, legend). Disability comparisons to Martinique are limited by the small sample size (n=6) in Olmsted County, the differential follow up and 3 incident cases that died in Martinique compared to no incident deaths in Olmsted County (Table 3, legend). Nonetheless, the high morbidity in Olmsted County may suggest prior reports of high rates of impairment in NMO/NMOSD in USA clinical cohorts are accurate and not due to referral bias.7 This high disease burden is noteworthy given the predisposition for those of African descent, an ethnic group in the USA with lower access to and quality of health care.20 By extrapolating from the ethnicity-specific prevalence data in Olmsted County we estimate NMO/NMOSD affects 16,000–17,000 persons in the USA, higher than previous estimates.11 The lack of NMO/NMOSD cases among Hispanics or Asians in Olmsted County probably reflects the low numbers and proportions of each ethnicity in this population (6,277 [4.3%] and 7883 [5.4%] respectively). These small numbers combined with the four fold lower proportion of Hispanics in Olmsted County than the overall USA population (4.3% Vs 17.4%) and wide confidence intervals may affect our estimate of the number of persons with NMO/NMOSD in the USA and thus these numbers should be interpreted with caution. The Olmsted County prevalence (3.9/100,000) is very similar to a Danish Caucasian population (4.4/100,000) and higher than the United Kingdom (0.72–1.96/100,000), India (2.6/100,000) and Japan (0.9/100,000).8, 9, 19, 21, 22 The Danish study evaluated AQP4-IgG among incident (but not prevalent) cases, better approximating our seroprevalence study.9 The onset age (median 35–37 years) and female:male distribution (8–9:1) were similar across both populations and comparable to prior studies.10, 11
NMO/NMOSD as a proportion of IDD was over 10 times higher in Martinique (16%) than Olmsted County (1.4%) likely due to the combined effect of the higher proportion of blacks and lower prevalence of prototypic MS in tropical regions.23 Therefore, distance from the equator and population ethnicity distribution helps determine the baseline risk of NMO/NMOSD among IDD patients and is important to consider as misdiagnosis of NMO/NMOSD as MS occurs in approximately 30%11 and MS-directed therapies may worsen NMO/NMOSD.24
The NMO/NMOSD prevalence:incidence ratio (an indicator of disease duration) was lower in Martinique (13:1) than Olmsted County (51:1) and could suggest Martinique patients die faster. However, the Martinique ratio is similar to the Danish incidence/prevalence study.9 The lower incidence in Olmsted County could be due to inherent variability with low numbers. For example, reanalysis of Olmsted County data showed that from 1994–2002 three prevalent cases would have been incident with a prevalence:incidence ratio of 14 or less, comparable to Martinique and prior studies. Alternatively, earlier cases in the larger population of Martinique whose registry began later than the search of Olmsted residents may have been underrepresented, resulting in a selection bias towards more recently identified cases. The uniform distribution of incidence in Martinique and use of symptom onset date (rather than diagnosis date) for incidence calculation ensured early incident cases were not actually prevalent cases and that differences in time to diagnosis across populations would not impact incidence rate.
By obtaining blood samples in >80% of IDD cases from each population, this seroprevalence study improved on prior methodologies,10 which likely underestimated NMO/NMOSD. This improved methodology, inclusion of AQP4-IgG seropositive NMOSD and our suspicion that mortality may have declined over time in association with improved disease recognition and treatment all may have contributed to the almost tripling of Martinique’s NMO prevalence rate from 3.6/100,000 in 1998 prior to AQP4-IgG discovery12 to 10/100,000 in this study. AQP4-IgG was assessed using M1 FACS cell-based assay that has higher sensitivity (83%) and specificity (100%) than older generation non-cell-based assays.3 Other laboratories have found similar excellent sensitivity (74%) and specificity (100%) with M23 cell-based assays25 and taking note of the AQP4-IgG assay type in future sero-epidemiological studies of NMO/NMOSD will be important as it will affect the direct comparisons of results. The differences between M1 and M23 cell-based AQP4-IgG assays are much less than the disparity between the cell-based and older generation AQP4-IgG assays (such as ELISA) in which false positives are up to 5 fold higher.26 Therefore, future seroprevalence studies using non-cell based AQP4-IgG assay techniques may be more likely to include false positive results and thus should be interpreted with caution.
The methods of identifying patients differed between populations, a medical record linkage system used in Olmsted County and a population registry used in Martinique, could impact comparison across the populations. However, the populations chosen are excellent for determining incidence/prevalence; both are well-defined and cover 94–100% of the population in each region ensuring comparisons across these two populations are valid. By capture-recapture technique the ability to diagnose inflammatory demyelinating diseases in Martinique was estimated at 94%.27 The Rochester Epidemiology Project medical records linkage system captures persons living in Olmsted County as they receive their care from local health care providers. The coverage of the population is virtually complete (100% compared to US Census estimates), but there is slight overcounting among young adults, and slight undercounting among men between 30–59 years of age.28 We undercount individuals that do not seek health care, and will only capture conditions that come to medical attention. Acute, relatively mild conditions will be undercounted in this population, because persons with these conditions will not seek treatment for symptoms that can be easily managed at home. However, we do not expect significant undercounting among our NMO/NMOSD patients because of the seriousness of this disease.
Our study has some limitations. Serum was not obtained in 16–20% potentially allowing some AQP4-IgG seropositive NMO/NMOSD cases be missed. However, medical record review of these cases showed none suspicious for NMO/NMOSD. The high risk of false positives, inherent in low probability situations,26 was minimized by using updated AQP4-IgG M1-FACS assays with specificity approaching 100%;3 medical record review suggested that all seropositive cases had compatible clinical presentations. Phenotypic spread may occur when undertaking a seroprevalence study using a serum biomarker such as AQP4-IgG but our study only evaluated AQP4-IgG in those with IDD, all AQP4-IgG seropositive cases had compatible clinical presentations confirmed by medical record review and the AQP4-IgG assay used has a specificity of 99–100% ensuring that this was not a major issue in this study. On the contrary, under-representation of patients with episodes of intractable nausea and vomiting without other neurological symptoms of NMOSD not recognized as having a brainstem IDD (these patients may initially present to gastroenterologists)29 was possible as sera were only evaluated in patients with confirmed IDD. Seronegative NMO patients meeting 2006 criteria4 were included in this study and seronegativity may be explained by immunosuppression treatment prior to testing,30 current assay detection limits, or a different diagnosis such as myelin-oligodendrocyte-glycoprotein-autoantibody associated IDD.31 Our study excluded seronegative NMOSD, which are defined in recent updated 2015 NMO/NMOSD diagnostic criteria,5 because of their lower diagnostic certainty and potential to mimic other disorders.32 Two prevalent cases in Martinique, but none in Olmsted County, met criteria for seronegative NMOSD5 suggesting their inclusion would have only slightly altered our results.
Acknowledgments
We would like to thank the Guthy-Jackson Charitable Foundation and National Institutes of Health (NS065829) for contributing to the funding for this study. This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. We would also like to acknowledge Delana Weis for her assistance as a research coordinator for this study. We thank John E. Schmeling for his expert assistance in managing sample collection processing and testing.
GLOSSARY
- AQP4-IgG
aquaporin-4-IgG
- IDD
inflammatory demyelinating disease
- M1-FACS
fluorescence-activated-cell-sorting M1-isoform of AQP4
- MS
multiple sclerosis
- NMOSD
neuromyelitis optica spectrum disorder
Footnotes
Author Contributions:
Conception and design of the study: E.P.F., P.C., J.S.S. and S.J.P.
Acquisition and analysis of data: E.P.F., P.C., B.G.W., J.S.S., D.J.J., M.M., V.A.L., C.F.L., A.McK., D.M.W., J.M., J.A.S., J.P.F., M.M., N.K., A.B.R. and S.J.P.
Drafting the manuscript or figures: E.P.F., P.C. and S.J.P.
Potential Conflicts of Interests
B.G.W., V.A.L., C.F.L., and S.J.P. receive royalties for technology license related to a test for aquaporin-4 autoantibodies for diagnosis of neuromyelitis optica and its spectrum disorders. V.A.L. and S.J.P., are named inventors on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO/AQP4-IgG as a cancer marker. E.P.F., P.C., J.S.S., D.J.J., M.Maj., A.McK., D.M.W., J.M., J.A.S., J.P.F., M.Mat., N.K., A.B.R., have no conflicts to report.
References
- 1.Flanagan EP, Weinshenker BG. Neuromyelitis optica spectrum disorders. Curr Neurol Neurosci Rep. 2014 Sep;14(9):483. doi: 10.1007/s11910-014-0483-3. [DOI] [PubMed] [Google Scholar]
- 2.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004 Dec 11–17;364(9451):2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 3.Fryer JP, Lennon VA, Pittock SJ, et al. AQP4 autoantibody assay performance in clinical laboratory service. Neurol Neuroimmunol Neuroinflamm. 2014 Jun;1(1):e11. doi: 10.1212/NXI.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006 May 23;66(10):1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015 Jun 19; doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007 Sep;6(9):805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999 Sep 22;53(5):1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 8.Jacob A, Panicker J, Lythgoe D, et al. The epidemiology of neuromyelitis optica amongst adults in the Merseyside county of United Kingdom. J Neurol. 2013 Aug;260(8):2134–7. doi: 10.1007/s00415-013-6926-y. [DOI] [PubMed] [Google Scholar]
- 9.Asgari N, Lillevang ST, Skejoe HP, Falah M, Stenager E, Kyvik KO. A population-based study of neuromyelitis optica in Caucasians. Neurology. 2011 May 3;76(18):1589–95. doi: 10.1212/WNL.0b013e3182190f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandit L, Asgari N, Apiwattanakul M, et al. Demographic and clinical features of neuromyelitis optica: A review. Mult Scler. 2015 Apr 28; doi: 10.1177/1352458515572406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012 Sep;69(9):1176–80. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 12.Cabre P, Heinzlef O, Merle H, et al. MS and neuromyelitis optica in Martinique (French West Indies) Neurology. 2001 Feb 27;56(4):507–14. doi: 10.1212/wnl.56.4.507. [DOI] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012 Dec;41(6):1614–24. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology. 2003 Nov 25;61(10):1373–7. doi: 10.1212/01.wnl.0000094316.90240.eb. [DOI] [PubMed] [Google Scholar]
- 15.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983 Nov;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 16.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002 Aug 27;59(4):499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenembaum S, Chitnis T, Ness J, Hahn JS. Acute disseminated encephalomyelitis. Neurology. 2007 Apr 17;68(16 Suppl 2):S23–36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 19.Cossburn M, Tackley G, Baker K, et al. The prevalence of neuromyelitis optica in South East Wales. Eur J Neurol. 2012 Apr;19(4):655–9. doi: 10.1111/j.1468-1331.2011.03529.x. [DOI] [PubMed] [Google Scholar]
- 20.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. Jama. 2000 May 17;283(19):2579–84. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 21.Pandit L, Kundapur R. Prevalence and patterns of demyelinating central nervous system disorders in urban Mangalore, South India. Mult Scler. 2014 Oct;20(12):1651–3. doi: 10.1177/1352458514521503. [DOI] [PubMed] [Google Scholar]
- 22.Houzen H, Niino M, Hirotani M, et al. Increased prevalence, incidence, and female predominance of multiple sclerosis in northern Japan. J Neurol Sci. 2012 Dec 15;323(1–2):117–22. doi: 10.1016/j.jns.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci. 2001 Apr;22(2):117–39. doi: 10.1007/s100720170011. [DOI] [PubMed] [Google Scholar]
- 24.Palace J, Leite MI, Nairne A, Vincent A. Interferon Beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol. 2010 Aug;67(8):1016–7. doi: 10.1001/archneurol.2010.188. [DOI] [PubMed] [Google Scholar]
- 25.Marignier R, Bernard-Valnet R, Giraudon P, et al. Aquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entity. Neurology. 2013 Jun 11;80(24):2194–200. doi: 10.1212/WNL.0b013e318296e917. [DOI] [PubMed] [Google Scholar]
- 26.Pittock SJ, Lennon VA, Bakshi N, et al. Seroprevalence of aquaporin-4-IgG in a northern California population representative cohort of multiple sclerosis. JAMA Neurol. 2014 Nov;71(11):1433–6. doi: 10.1001/jamaneurol.2014.1581. [DOI] [PubMed] [Google Scholar]
- 27.Cabre P, Signate A, Olindo S, et al. Role of return migration in the emergence of multiple sclerosis in the French West Indies. Brain. 2005 Dec;128(Pt 12):2899–910. doi: 10.1093/brain/awh624. [DOI] [PubMed] [Google Scholar]
- 28.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011 May 1;173(9):1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iorio R, Lucchinetti CF, Lennon VA, et al. Intractable nausea and vomiting from autoantibodies against a brain water channel. Clin Gastroenterol Hepatol. 2013 Mar;11(3):240–5. doi: 10.1016/j.cgh.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao Y, Fryer JP, Lennon VA, et al. Aquaporin 4 IgG serostatus and outcome in recurrent longitudinally extensive transverse myelitis. JAMA Neurol. 2014 Jan;71(1):48–54. doi: 10.1001/jamaneurol.2013.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012 Sep 18;79(12):1273–7. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan EP, Kaufmann TJ, Krecke KN, et al. Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann Neurol. 2015 Dec 17; doi: 10.1002/ana.24582. [DOI] [PubMed] [Google Scholar]