Abstract
Hypoxia is a major hallmark of the tumor microenvironment that is strictly associated with rapid cancer progression and induction of metastasis. Hypoxia inhibits disulfide bond formation and impairs protein folding in the Endoplasmic Reticulum (ER). The stress in the ER induces the activation of Unfolded Protein Response (UPR) pathways via the induction of protein kinase RNA-like endoplasmic reticulum kinase (PERK). As a result, the level of phosphorylated Eukaryotic Initiation Factor 2 alpha (eIF2α) is markedly elevated, resulting in the promotion of a pro-adaptive signaling pathway by the inhibition of global protein synthesis and selective translation of Activating Transcription Factor 4 (ATF4). On the contrary, during conditions of prolonged ER stress, pro-adaptive responses fail and apoptotic cell death ensues. Interestingly, similar to the activity of the mitochondria, the ER may also directly activate the apoptotic pathway through ER stress-mediated leakage of calcium into the cytoplasm that leads to the activation of death effectors. Apoptotic cell death also ensues by ATF4-CHOP- mediated induction of several pro-apoptotic genes and suppression of the synthesis of anti-apoptotic Bcl-2 proteins. Advancing molecular insight into the transition of tumor cells from adaptation to apoptosis under hypoxia-induced ER stress may provide answers on how to overcome the limitations of current anti-tumor therapies. Targeting components of the UPR pathways may provide more effective elimination of tumor cells and as a result, contribute to the development of more promising anti-tumor therapeutic agents.
Keywords: Tumor, PERK, eIF2α, Unfolded Protein Response, ER stress, CHOP, apoptosis
1. INTRODUCTION
A tumor is an abnormal mass of tissue composed of cells with continual unregulated proliferation, which can occur in any part of the body. There are two types of tumors that vary significantly – benign and malignant. The differences between them are very important in cancer pathology due to the fact that not all tumors are cancerous [1]. A benign tumor grows and remains confined to its original location, not invading the surrounding tissue or spreading to other sites. On the contrary, a malignant tumor has the ability to occupy neighbouring healthy tissues and may spread from its primary area to other parts of the body via the vascular and lymphatic system in a process commonly termed metastasis [1, 2]. Metastasis is a major clinical problem during tumor therapy [3]. It is a highly complex process and is further complicated by the uncertainty of when it may occur after diagnosis of the primary tumor; varying from months to years or not at all in some patients. [4]. The majority of cancers grow as solid tumors [2] and can be classified into three general groups such as carcinomas, sarcomas, and leukemias or lymphomas according to the type of tissue they arise from [1].
Cancer constitutes a primary health problem and has a major impact on society. Tens of millions of people have been diagnosed with cancer worldwide and more than half of them finally die from it [5]. More than a million cases are diagnosed with cancer and more than 500,000 die of cancer each year in the United States [1]. The newest data confirm that cancer constitutes the second cause of human mortality, after cardiovascular diseases, in highly developed countries [6], and affects half of men and one third of women during their lifetimes [2]. Current estimates suggest that cancers of the breast, prostate, lung, and colon account for more than half of all cancer cases in highly developed countries [1].
Due to the significant improvement in both medical treatment and prevention of cardiovascular diseases, cancer may become the main cause of death worldwide [5].
Cancer comprises more than 200 different human disease entities [7] and results from perturbations of cellular responses at the molecular level that leads to significant alterations of normal properties in healthy cells. Cancer is closely connected with abnormal proliferation of cells that evade apoptosis and have continual unregulated growth, presenting in almost any tissue of the body. Each cancer has its own unique pattern of behaviour based on the location of the cancer, mutations of the cells, and an individual's molecular makeup. Due to the fact that there are more than a hundred types of cancers and the different factors that make them unique, elimination of cancer through anti-tumor drugs is still inadequate [1, 2].
A range of stressful cellular conditions accompanied by tumor development may trigger disruptions of Endoplasmic Reticulum (ER) homeostasis and lead to ER stress, further activating Unfolded Protein Response (UPR) branches. This results in subsequent phosphorylation of Eukaryotic Initiation Factor 2 alpha (eIF2α) by activated protein kinase RNA-like endoplasmic reticulum kinase (PERK), and rapid downregulation of global protein synthesis and preferential translation of genes. These products may contribute to adaptations of the tumor cell to hypoxic conditions. In addition, long-term activation of the UPR axis may evoke a paradoxical response via the initiation of apoptotic cell death. Several lines of evidence suggest a pivotal role of PERK/eIF2α/ATF4/CHOP in tumor progression. Knowledge in this area may contribute to the development of modern treatment for the elimination of cancer [8-10].
2. HYPOXIA INDUCES ENDOPLASMIC RETICULUM STRESS AND THE UNFOLDED PROTEIN RESPONSE
The ER is a multifunctional organelle, that plays a central role in protein folding, biosynthesis of phospholipids, and maintaining calcium homeostasis [11]. Sufficient oxidation within the ER environment is required for the formation of disulfide bonds, which stabilize the conformation of mature proteins [12, 13]. The ER has a quality control system to eliminate unfolded or misfolded proteins from the secretory pathway and exports only the properly folded proteins to their final destinations [14]. Disrupted ER homeostasis engages a range of stress-response signaling pathways collectively termed Unfolded Protein Response [15]. Numerous pathological conditions and chemical compounds, such as thapsigargin and tunicamycin, are implicated in disruption of ER functions and thereby can lead to the activation of ER stress conditions within cells [16, 17]. Stress stimuli primarily include: decreased oxygen levels, viral infections, deprivation of nutrients (especially a glucose deficiency caused by perturbation in protein glycosylation), fast cell proliferation, changes in redox homeostasis, increased level of protein synthesis, and decreased levels of calcium ions in the ER lumen [18]. These disruptions rapidly lead to the aggregation of unfolded and misfolded proteins in the lumen of the ER, further activating the UPR signaling branches as a cellular adaptive program to cope with stress conditions, recover the homeostatic state, and resume cell division [9, 19, 20]. However, prolonged activation of the UPR, in which the initial aim was to support cell survival, may convert to the pro-apoptotic signaling network [21-23].
There is abundant evidence that this dychotomic ER stress-mediated response pathway is strictly associated with the pathogenesis of many human disease entities including cancer, neurodegenerative diseases, type 2 diabetes, renal disease, and atherosclerosis [24]. Currently, the main target of numerous studies is to develop a method to pharmacologically switch the UPR signal form pro-survival to pro-apoptotic in tumor cells and trigger their death [16].
Hypoxia refers to areas of low oxygen tension. It is a physiologically important dynamic hallmark, which is present in all solid tumors. Moreover, hypoxia plays a central role in tumor invasion, metastatic cascade, and resistance to current treatment modalities [25, 26]. Tumor hypoxia results from an insufficient supply of oxygen [27]. Normal cells have oxygen levels ranging from 3.1% to 8.7%. The oxygen concentration of tumor cells may vary from 0.01-3.9% O2, which in comparison to normal cells is significantly lower [28]. The uncontrolled growth of tumor cells forming an enlarging mass causes the distance between existing blood vessels to increase and creates areas of acute and chronic hypoxia [27]. Insufficient supply of nutrients stimulates tumor cells to form new vascular networks through the process of angiogenesis [29, 30]. The majority of tumors synthesize growth factors such as Basic Fibroblast Growth Factor (bFGF), Transforming Growth Factor α (TGFα), and Vascular Endothelial Growth Factor (VEGF) that possess the ability to enhance angiogenesis [30]. Newly-formed blood vessels significantly differ from vascular networks in normal, healthy tissue [31] due to the immature structure in comparison to their normal counterparts [32]. Tumors have significant interruptions in blood flow [33] and have poorly oxygenated areas spread within the mass [34, 35]. Generally, hypoxia constitutes a prominent physiologic barrier to cell survival, but paradoxically, tumor cells can adapt to survive in the extremely low concentrations of oxygen. As a result, tumor cells under hypoxic stress become more resistant to chemotherapy since drug diffusion to the hypoxic areas is limited [35-37]. In addition, poor oxygenation causes resistance of tumor cells to anti-tumor therapy because the presence of oxygen is essential for effective DNA damage during radiotherapy and for activation of the significant cytotoxicity of anti-tumor therapeutic agents [37, 38]. For instance, Brizel et al. showed that individuals with head and neck cancers with hypoxic tumors had a decreased survival time in comparison with patients with better-oxygenated tumors [39]. Moreover, Gatenby et al. also reported that head and neck cancers with hypoxic conditions are more resistant to radiation [40]. These studies emphasize that regions of low oxygen availability have a significant effect on clinical outcomes, leading to decreased effectiveness of anti-cancer therapy.
3. THE IMPACT OF HYPOXIA-INDUCIBLE FACTOR 1 ON TUMOR CELL SURVIVAL
Characteristics of fast-growing tumor cells include slower formation of new blood vessels compared to the rate of tumor growth, pathological architecture of blood vessels, as well as blood flow disturbances [32]. These features are closely connected with initiation of various pathological mechanisms of microenvironmental stress. To survive unfavourable conditions, tumor cells must adapt to low concentrations of oxygen through the induction of stress response signaling pathways including activation of the hypoxia-inducible factor 1 (HIF-1) [41, 42], which is known as a key mediator of oxygen homeostasis. HIF-1 plays a fundamental role in regulation of the expression of numerous genes that exert control over a wide range of intracellular processes that promote angiogenesis, anaerobic glycolysis, and erythropoiesis [43, 44]. Generally, HIF-1 is composed of two subunits such as HIF-1α and HIF-1β [45], but only one of them - HIF-1β is constitutively expressed in cells. Activity of the HIF-1α subunit is regulated by numerous post-transcriptional modifications such as hydroxylation, acetylation, and phosphorylation [46]. Under normal aerobic conditions hydroxylation and acetylation of the oxygen-dependent domain of HIF-1α targets it for degradation by the ubiquitin-proteasome pathway [47], which makes it experimentally undetectable. Adversely, in response to hypoxic conditions HIF-1α becomes stable and accumulates in the nucleus where it forms a heterodimer with the HIF-1β subunit. After dimerization, the HIF-1 complex is activated and may control the expression of various hypoxia-regulated genes [45, 48, 49] including Vascular Endothelial Growth Factor (VEGF) and erythropoietin (EPO), which regulate angiogenesis and erythropoiesis, respectively. Furthermore, HIF-1 coordinates regulation of the expression of other genes essential for cell survival and proliferation [46]. These include various glycolytic genes involving glucose transporters and glycolytic enzymes such as Glucose Transporter 1 (GLUT1) and Phosphoglycerate Kinase (PGK), which support uptake of glucose and alter the utilization of energy sources from aerobic to glycolytic metabolism [48]. Overexpression of HIF-1 has been reported in many types of cancers as one of the cellular responses to hypoxic stress conditions that results in adaptation of the cancer cells to oxygen deprivation [47].
HIF-1 transcription factor plays an important role in the response of tumor cells to hypoxic conditions and is essential for regulating the expression of genes involved in homeostatic processes [50]. However, its activation alone is insufficient to repair all the damage that occurs in the cell under limited oxygen concentrations. Under more severe hypoxic conditions, cells elicit other adaptive strategies. These immediate reactions result in the attenuation of global protein synthesis, which is closely connected with the promotion of tumor cell survival by reducing energy consumption [19].
4. THE ROLE OF ENDOPLASMIC RETICULUM IN PROMOTING CYTOPROTECTIVE VERSUS APOPTOTIC RESPONSES DURING TUMORIGENESIS
Due to the excessive proliferation of malignant tumor cells and their insufficient vascularisation, a wide range of pathological conditions, including hypoxia, may trigger disruption of ER homeostasis through the accumulation of unfolded and misfolded proteins within its lumen. Interestingly, under hypoxic conditions, energy demand for protein synthesis significantly decreases in comparison to the normoxic cells. The significant ATP reduction that is due to oxygen and energy deprivation is strictly affiliated with a rapid decrease in the rate of protein translation that is crucial for the adaptation of tumor cells to survive in the adverse conditions [32]. As a response to extreme hypoxic conditions, stress response signaling pathways, including the UPR, are activated [42, 51]. The UPR is a specific cellular process that, depending on the severity of stress conditions and the exposure time of cells to unfavourable factors, may lead to different outcomes such as cell adaptation to stress or cell death by apoptosis [52]. Thus, the UPR signaling network is divided into two different pathways - cytoprotective and apoptotic [53]. Even though tumor cells grow in size and spread from their initial area of development to other parts of the body, the UPR can still play a crucial role in maintaining the ER homeostasis. This kind of UPR response is tightly associated with its pro-adaptive role, since the main aim of its activation is overturning the capacity of the ER to proper protein folding and reduction of protein synthesis. Inversely, if the adaptive response is insufficient, cells undergo apoptosis [42]. Altogether, the key role of the UPR in cancer pathogenesis is to protect tumor cells from the apoptotic process [9]. Activation of the UPR, as a response to the accumulation of unfolded and misfolded proteins in the ER lumen causes increased synthesis of molecular chaperone proteins to enhance protein refolding, elimination of the misfolded proteins, as well as inhibition of protein translation [51].
The UPR is also divided into three branches that may be triggered by specific ER transmembrane proteins such as: PERK, Inositol Regulating Enzyme 1 (IRE1), and Activating Transcription Factor 6 (ATF6) [54]. The ER lumen is crowded with special chaperones like immunoglobulin heavy chain-binding protein (BiP), also termed glucose-regulated protein 78 (GRP78), that belong to the heat-shock family of proteins [55]. Under normal conditions all of these stress sensors are held in the inactive state by forming a complex with ER chaperone BiP/GRP78. After aggregation of misfolded and unfolded proteins within the ER lumen, BiP/ GRP78 are released from the lumenal domains of ER receptors [56]. That dissociation immediately leads to the rapid activation of all three ER stress receptors. Finally, three arms of the UPR become active and pro-survival, or if exposed to prolonged ER stress pro-apoptotic, transmitting signals to the cell cytosol and nucleus [42].
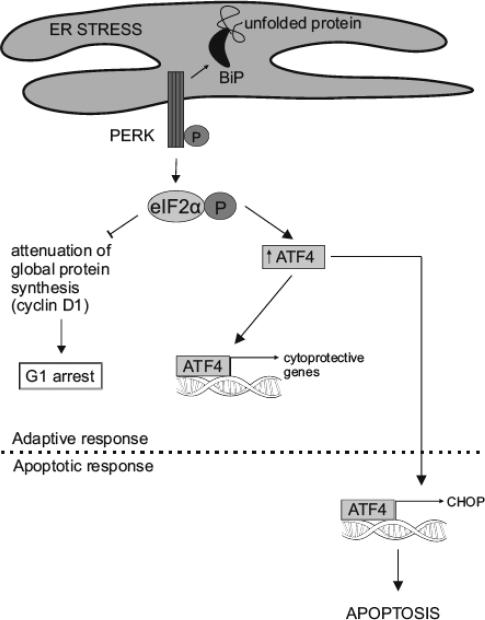
There is abundant evidence that activation of UPR signaling pathways and subsequent eIF2α phosphorylation play a pivotal role in solid tumor growth, invasion, and angiogenesis. It has been reported in a range of different cancer models that tumor cells in the primary tumor upregulate UPR networks, whereas healthy tissue surrounding a growing tumor do not [9, 57]. Therefore, tumor hypoxia is correlated with a significant increase in eIF2α phosphorylation [58]. This results in the attenuation of global protein translation within tumor cells as well as induction of translation of only selective mRNAs, including activating transcription factor 4 (ATF4) [59]. Generally, ATF4 is a transcription factor that regulates a wide range of genes, which play a crucial role in cell adaptation to stress conditions, but paradoxically, during long-term ER stress, ATF4 may also stimulate genes of CCAAT-enhancer-binding protein homologous protein (CHOP), which is responsible for initiation of the apoptotic cascade [60] (Fig. 1).
Fig. (1).
PERK-dependent Unfolded Protein Response. Under normal physiological conditions, ER transmembrane receptor PERK is present in an inactive state, since it is associated with BiP chaperones. As a response to the accumulation of unfolded or misfolded proteins within ER lumen, under ER stress, BiP dissociates from the lumenal domain of PERK. This leads to oligomerization and trans-autophosphorylation of PERK, which becomes an active kinase with the ability to phosphorylate α subunits of the eIF2. The result is suppression of global protein translation, which causes the cell cycle to arrest in the G1 phase as well as the induction of preferential translation of ATF4, which upregulates expression of genes responsible for restoring cell homeostasis. Under prolonged ER stress, when pro-adaptive UPR fails, PERK may also trigger pro-apoptotic signals through activation of downstream CHOP, which promotes apoptosis.
5. ACTIVATION OF THE PERK/EIF2A/ATF4 AXIS OF THE UPR UNDER STRESS CONDITIONS
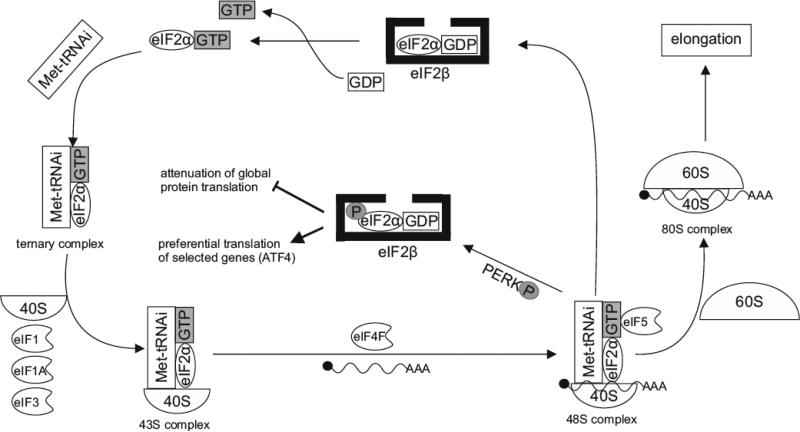
Translation initiation is a highly complex process in eukaryotic cells. Interestingly, it is strictly dependent on eIF2α, which is composed of three general subunits: α, β and γ [61]. The heterotrimeric protein forms a ternary complex with guanosine triphosphate (GTP) and initiator-methionyl-tRNA (Met)-tRNAi. Subsequently, this complex combines with the smaller ribosomal subunit 40S bound to eukaryotic initiation factors such as: eIF1, eIF1A and eIF3, resulting in the formation of a preinitiation complex 43S [62, 63]. The preinitiation complex 43S combines with mRNA and heterotetramer eukaryotic initiation factor 4F (eIF4F) consisting of the subunits: eIF4E, eIF4A, eIF4B, eIF4G, eIF4F. eIF4F facilitates binding of mRNA to the preinitiation factor 43S [64], which migrates downstream of mRNA for the AUG initiation codon. Following the proper codon-anticodon pairing, the 48S preinitiation complex is created. That process requires consumption of energy, thus the GTP associated with eIF2α is hydrolyzed to guanosine diphosphate (GDP) by GTPase-activating protein eIF5. Hydrolysis of GTP releases the eIF2-GDP complex and all of the eukaryotic initiation factors from the ribosome [65]. The next event is the assembly of a complete 80S initiation complex, ready to start the elongation process through binding of the larger 60S ribosomal subunit to the pre-initiation complex [66] (Fig. 2).
Fig. (2).
Mechanisms of initiation of translation. eIF2α-GTP complex binds to (Met)-tRNAi that forms a ternary complex, which associates with 40S ribosomal subunit and combines with eIF1, eIF1A and eIF3 resulting in the creation of pre-initiation complex 43S. This combines with both mRNA and heterotetramer eIF4F. Afterwards, the anticodon of (Met)-tRNAi pairs with the AUG codon of mRNA, which forms the 48S pre-initiation complex. That process requires consumption of energy, since GTP is hydrolysed to GDP. Subsequently, complex eIF2α-GDP and other initiator factors are released. After association of the 60S subunit of the ribosome with the 48S pre-initiation complex, the complete 80S initiation complex is created. Formation of a new ternary complex requires replacement of GDP to GTP by eIF2β. The process is inhibited by phosphorylation of eIF2α by PERK under stress conditions of the ER, which results in attenuation of global protein translation and triggers preferential translation of selected genes such as ATF4.
Generation of a new initiation complex strictly depends on the conversion of GDP, bound to eIF2α, to GTP. This exchange is catalyzed by guanine nucleotide exchange factor eIF2β [67]. eIF2α phosphorylation of Ser51 results in repression of global protein synthesis and preferential translation of selected genes. It can be inferred that phosphorylation of eIF2α is a master regulator of cell adaptation to ER stress conditions. It is also a significant checkpoint under which not only global protein translation, but also cell proliferation are blocked [68]. Furthermore, phosphorylated eIF2α has a negative influence on eIF2β functioning and acts as its major inhibitor [69].
There is a large body of evidence that numerous stress conditions that are strictly associated with the environment of the tumor may lead to rapid activation of the PERK/eIF2α/ATF4 branches of the UPR [70]. PERK is a type of I transmembrane ER receptor with a serine/threonine cytoplasmic domain [71]. Molecular chaperones, such as BiP/GRP78, play a vital role in maintaining the lumenal domain of PERK with kinase activity in its inactive state [72].
As a consequence of ER stress conditions BiP/GRP78 proteins dissociate from PERK, which results in homo-oligomerization and autophosphorylation of stress-sensing PERK domains [73].
Assembly of a new ternary complex requires involvement of protein eIF2β, since complex eIF2α-GDP is an inactive form of eIF2α. Whereas eIF2β creates the complex with eIF2α-GDP, the replacement of GDP by GTP ensues [74]. The process is inhibited when activated PERK phosphorylates its main substrate - eIF2α at Ser51. As a result, formation of a new ternary complex is abrogated, and global protein synthesis does not ensue [75, 76].
Moreover, cell survival branches of the UPR network are also closely connected with a major tumor phenomenon, referred to as tumor dormancy [77]. Collectively, exposure of cells to stress conditions and activation of UPR trigger PERK-dependent eIF2α phosphorylation which results in repression of protein translation, involving cyclin D1 – a prominent cell cycle regulator, which plays a key role in controlling G1/S transition [78]. Downregulation of cyclin D1 causes the cell cycle to arrest and to give some time for cellular recovery from hypoxic conditions. It can be concluded that the pro-adaptive UPR response promotes cellular dormancy by cell cycle inhibition in G1 phase [79].
6. MECHANISMS OF ENDOPLASMATIC RETICULUM STRESS-INDUCED APOPTOSIS
Apoptosis is a key mechanism for maintaining tissue homeostasis by selective elimination of cells that are damaged, mutated, or represent a threat to the whole organism, such as pre-cancerous cells [80, 81]. The major molecular components of the apoptotic machinery are represented by the family of cysteine proteases termed caspases, which are essential for the regulation of apoptosis signaling [82]. Caspases are initially translated as inactive procaspases that are activated by numerous cell stresses, including oxygen depletion. They are divided into two different classes based on their position in the apoptotic signaling pathway. The first are the initiator caspases, which include caspases 8, 9 and 12. The second class is comprised of effector caspases and the crucial members of this class are caspases 3, 6, and 7. Generally, the initiator caspases play a pivotal role in the activation of the caspase cascade that rapidly leads to apoptosis [83, 84].
The proteins of the Bcl-2 family are well-characterized regulators of apoptotic cell death [85]. They are localized in the mitochondrial membrane and play a fundamental role in regulation of apoptotic cell death, since they are responsible for the activation of caspases [86, 87]. The Bcl-2 family of genes are comprised of 20 members, including both death and survival genes [88], and their products are divided into two functional classes such as anti-apoptotic and pro-apoptotic. Most of the anti-apoptotic proteins have all four Bcl-2 homology (BH) domains. On the contrary, the pro-apoptotic Bcl-2 family of proteins is subdivided into two subclasses. Some pro-apoptotic Bcl-2 proteins (Bax, Bak, Bok) contain BH1, BH2, and BH3 domains, but other pro-apoptotic Bcl-2 proteins (Bid, Bim, Bad, Bmf, Bik, Blk, Noxa, Puma, Hrk) have only one conservative domain, namely BH3 (Table 1) [81, 89, 90]. The Bcl-2 family of proteins is strictly associated with the regulation of mitochondrial outer membrane (MOM) integrity. Under stress conditions anti-apoptotic Bcl-2 proteins are functionally suppressed. Adversely, pro-apoptotic Bcl-2 proteins, such as Bax and Bak, trigger increased permeability of the MOM. This creates proteolipid pores, which allow the release of pro-apoptotic mitochondrial proteins into the cell cytoplasm [91]. Disturbances in ER homeostasis also contribute to the activation of the latter group of pro-apoptotic proteins such as Bid, Bad, Bim, Noxa, and Puma that belong to the BH3 domain-only members of the Bcl-2 family of proteins. These proteins are essential for induction of cell apoptosis, due to the fact that they may bind to the members of anti-apoptotic Bcl-2 proteins, which cause inhibition of their biological activity [81].
Table 1.
The functional and structural classification of the Bcl-2 family members of proteins.
| Bcl-2 family of proteins | |||
|---|---|---|---|
| Function | Pro-apoptotic | Anti-apoptotic | |
| Name of a protein class | Bax-like proteins | BH3 domain-only proteins | Bcl-2-like proteins |
| Presence of Bcl-2 homology (BH) domain/motif | BH1-BH3 domains | BH3 domain | BH1-BH4 domains |
| Members of a Bcl-2 family of proteins | Bax, Bak, Bok | Bid, Bim, Bad, Bmf, Bik, Blk, Noxa, Puma, Hrk | Bcl-2, Bcl-XL, Bcl-w, Mcl-1, A1, Bcl-B |
The mitochondrial outer membrane permeability is critical in apoptosis, such that if cytochrome c is released into the cytoplasm a large caspase-activating complex, termed apoptosome, is created. That multi-protein complex, excluding cytochrome c, contains Apaf-1 and procaspase 9, which exists in the cell cytosol in an inactive state. After cellular interaction with cytochrome c, procaspase-9 undergoes a conformational change and becomes active. As a result, caspase 9 may subsequently facilitate activation of caspase 3 – the major effector caspase that is primarily responsible for cell destruction [92, 93].
ER and mitochondria are connected not only structurally, but also functionally. Bcl-2 anti-apoptotic proteins are located both in the MOM and ER nuclear envelope [94, 95]. Moreover, the pro-apoptotic Bax-like proteins, including BAX and BAK, are also localized in the ER membrane. Calcium ion leakage into the cytosol may induce caspase-dependent apoptotic cell death. The group of BH3 domain- only proteins is also engaged in ER-induced apoptosis. They may bind to the anti-apoptotic Bcl-2 family of proteins that are localized in the ER membrane and inhibit their activity. Furthermore, BH-3 domain-only proteins also possess the ability to rapidly activate pro-apoptotic proteins such as Bax and Bak that subsequently triggers leakage of calcium ions, activation of caspase signaling cascade and, as a result, apoptotic cell death [87, 96].
Studies show that prolonged ER stress may render tumor cells more vulnerable to apoptotic cell death [15]. In situations where ER mechanisms that are responsible for the proper folding of proteins fail, the apoptotic branch is activated. Remarkably, this suggests that during prolonged ER stress, when adaptive responses are insufficient to sustain ER homeostasis, PERK-dependent signaling pathways may elicit pro-apoptotic signals and, as a result, rapid cell death ensues [97]. Due to the fact that ER stress-induced apoptosis is closely associated with the pathophysiology of several diseases, including cancer, current research has focused on how long-term ER stress can activate apoptosis [13]. Little is known about the mechanisms that are involved in ER-induced apoptosis. However, it is clear that calcium ions, which are released form the ER lumen under ER stress, play a central role in activation of caspases strictly connected with apoptotic cell death [32, 87].
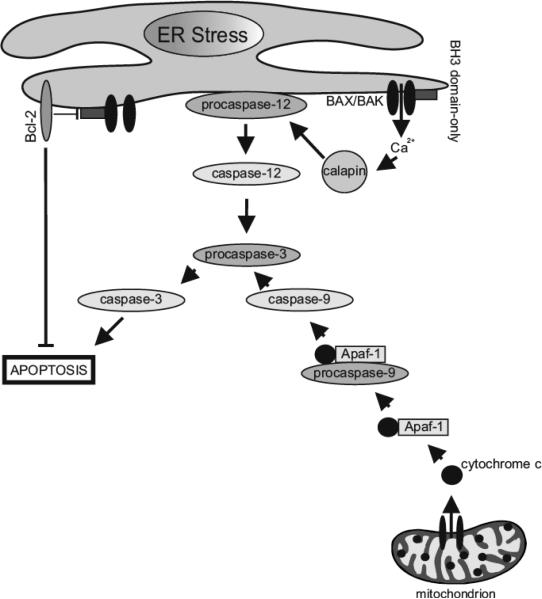
Numerous studies were undertaken to explain the mechanism that is associated with activation of caspases under ER stress conditions. It was a great mystery, but in the year 2000 Nakagawa and Yuan described caspase-12, which is localized at the cytosolic face of the ER [98]. Nakagawa et al. reported that mice are resistant to ER- induced apoptosis when they are deficient in caspase-12, but their cells still undergo apoptosis in response to other death signals. It is worthy to note that caspase-12, like all caspases, is synthetized as an inactive protein. Interestingly, it becomes active only in response to stress conditions of the ER, including perturbations in calcium homeostasis and aggregation of unfolded proteins, but not by other apoptotic signals [99]. Caspase-12 may be activated by calcium-activated protease calpain as a result of calcium ion leakage from the lumen of the ER [98]. Activated caspase-12 triggers downstream of apoptotic pathways by cleaving and activating caspase 9, and subsequently caspase 3. Essentially, caspase-12 possesses the ability to activate caspase-9 without the involvement of cytochrome c. It can be concluded that activation specific for ER caspase-12 leads to the caspase cascade in response to ER stress conditions that, as a consequence, trigger cell death through ER-induced apoptosis [100] (Fig. 3).
Fig. (3).
ER stress-mediated apoptosis. ER and mitochondria may directly initiate signaling pathways for the activation of the caspase cascade that results in apoptotic cell death. Members of all classes of the Bcl-2 family of proteins localize not only to the outer mitochondrial membrane, but also to the ER membrane. The anti-apoptotic Bcl-2 members of family proteins may inhibit activation of pro-apoptotic Bcl-2 proteins such as Bax/Bak. Under ER stress conditions active BH3 domain-only proteins trigger the oligomerization of pro-apoptotic proteins like Bax and Bak, creation of Bax/Bak pores within mitochondrial outer membrane, and subsequently its permeabilization. As a consequence, cytochrome c is released into the cell cytosol where it binds to Apaf-1 and procaspase-9 and forms an apoptosome complex. This triggers the activation of the caspase cascade, which finally may elicit mitochondria-mediated apoptosis. On the other hand, prolonged ER stress can trigger caspase-12-mediated apoptosis. Caspase-12, associated with the cytoplasmic face of the ER membrane, is activated under chronic ER stress in response to calcium leakage from the ER lumen. This leads to rapid activation of the calcium-calpain-caspase-12-caspase-3 cascade and, as a result, ER stress induced apoptotic cell death ensues.
7. PROLONGED ENDOPLASMATIC RETICULUM STRESS TRIGGERS ACTIVATION OF CHOP-MEDIATED APOPTOSIS PATHWAY
A wide range of stress stimuli and perturbations in cellular homeostasis may cause aggregation of misfolded and unfolded proteins within the ER lumen. Under ER stress the UPR branches are rapidly activated [43]. The duration, as well as severity of ER stress are the major signals that allow the determination of when the signaling pathway switches from pro-survival to pro-apoptotic [42]. The main aim of activated UPR branches is to rebalance the ER homeostasis, but under severe and prolonged stress conditions the pro-apoptotic arm of UPR is activated and cells undergo apoptosis [101].
Activation of PERK and subsequent phosphorylation of eIF2α results in global attenuation of new protein synthesis to restore cell homeostasis. However, activated PERK and elevated levels of phosphorylated eIF2α also lead to increased translation of selected mRNAs. This includes ATF4, a transcription factor that promotes expression of a wide range of genes implicated in the enhancement of cell recovery and adaptation to stress conditions [16, 102]. ATF4 stimulates expression of numerous genes involved in cell metabolism, nutrient uptake, and antioxidation. On the other hand, under prolonged ER stress conditions, ATF4 promotes expression of transcription factors such as C/EBP homologous protein (CHOP), also termed growth arrest and DNA damage inducible gene (GADD135) [103, 104]. CHOP is a transcription factor that may promote cell demise in numerous ways. It has been repeatedly suggested that CHOP gene expression is strictly involved in cell death by apoptosis, but research has not confirmed existence of any relationship between induction of CHOP and apoptosis. The McCullough et al. study shows that increased expression of CHOP leads to downregulation of the expression of the anti-apoptotic protein Bcl-2 genes, upregulation of pro-apoptotic BH3 domain-only proteins genes such as BIM, and disruption of redox homeostasis, which rapidly evokes apoptosis [105]. The mechanisms that may explain how apoptotic cell death is induced by CHOP still remain unclear but, there is ample evidence that it is implicated in numerous human disease entities, including diabetes, neurodegenerative diseases, ischemic diseases, and tumor development [106].
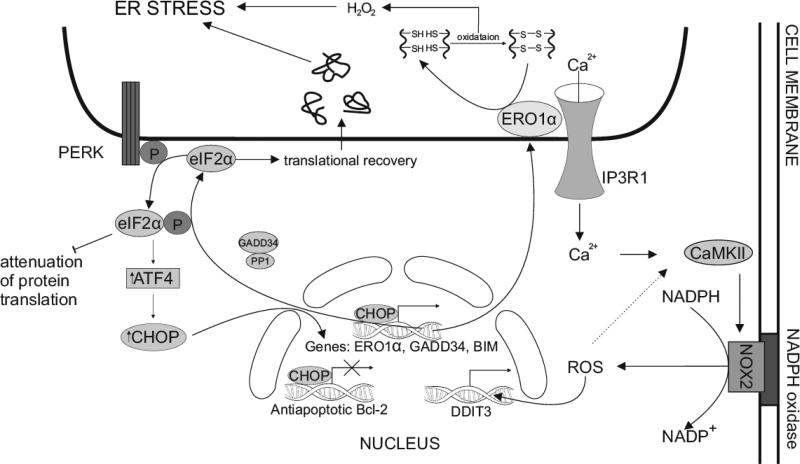
Additionally, CHOP is involved in another pro-apoptotic mechanism, since it directly transactivates the growth arrest and DNA damage-inducible protein GADD34, which promotes sufficient dephosphorylation of the α subunit of eIF2 and leads to protein translation recovery in stressed cells. Thereby, in CHOP −/− stressed cells with impaired expression of GADD34 proteins, the load within the ER lumen is significantly decreased [107]. This negative feedback loop possesses a broad role in restoration of protein synthesis when eIF2α is phosphorylated upon stress conditions. Downstream of PERK, ATF4 and CHOP directly activate GADD34 that associates with protein phosphatase 1 (PP1) to dephosphorylate eIF2α. In conclusion, CHOP directly leads to translational recovery, increases ER nascent protein loads, and thus promotes ER stress and apoptotic cell death [19, 108]. It is worthy to note that CHOP promotes expression of ER oxidoreductin 1α (ERO1α) genes. In physiological conditions, these products, which are enzymes strictly associated with the ER membrane, play a pivotal role in the formation of disulfide bonds in newly translated proteins. Moreover, ERO1α requires molecular oxygen to oxidize PDI, which catalyses the formation of disulfide bonds. Therefore, it could be assumed, that under prolonged ER stress conditions ERO1α leads to the production of H2O2 and promotes a hyperoxidizing environment that causes apoptosis [109, 110].
Interestingly, high concentrations of reactive oxygen species (ROS) in the ER lumen may subsequently activate the ER calcium release channel inositol-1,4,5-trisphosphate receptor 1 (IP3R1), which causes calcium leakage from the ER lumen into the cell cytoplasm. Finally, the calcium-sensing kinase termed calcium/calmodulin-dependent protein kinase II (CaMKII) is activated and may induce various apoptotic signaling pathways. One of the consequences of the activation of the CHOP–ERO1α–IP3R1–CaMKII pathway is induction of cell membrane bound nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) subunit 2 (NOX2), which results in the generation of ROS [24].
Essentially, ROS induced by NADPH oxidase may create a positive feedback loop axis, since they markedly enhance activation of CaMKII. As a result, generated ROS can markedly amplify expression of DDIT3 genes that encode CHOP, signifying that high levels of ROS in cell cytoplasm as well as enhanced expression of CHOP promote apoptotic cell death [42].
The Han et al. investigation confirms the role of CHOP as a transcription factor, which in conjunction with ATF4 enhances expression of various genes encoding proteins that cause an elevated level of protein synthesis. Their outcomes emphasize a dual role of eIF2α, in which the unphosphorylated form promotes cell survival and the phosphorylated form causes switching of the signal to markedly enhance apoptotic cell death. Only ATF4 and CHOP are selectively translated when eIF2α is phosphorylated. The signal for apoptosis is generated as a result of generation of ROS when the rate of protein translation increases before restoration of proteostasis [111] (Fig. 4).
Fig. (4).
Mechanisms of CHOP-induced apoptosis under prolonged ER stress. Sustained PERK-mediated phosphorylation of eIF2α and increased expression of ATH4 under prolonged stress conditions of the ER lead to CHOP-induced apoptotic cell death through various signaling pathways. CHOP transcriptionally attenuates Bcl-2 anti-apoptotic family of proteins and adversely activates the pro-apoptotic BH3- domain only proteins, such as BIM. Moreover, CHOP directly activates the expression of GADD34 genes, in which the products create a complex with PP1 resulting in dephosphorylation of eIF2α and inhibition of PERK-mediated translational attenuation. CHOP also markedly enhances expression of ERO1α genes. ERO1α, oxidoreductase ER enzyme, generates ER-localized H2O2, thus causing a hyperoxidized environment in the ER lumen. ERO1α also binds to the ER calcium channels IP3R1 and promotes release of calcium ions into the cell cytosol. As a consequence, calcium-sensing enzyme CaMKII is activated, which, in turn, triggers induction of NADPH oxidase subunit 2 (NOX2) and subsequent generation ROS, that through a positive feedback loop causes activation of CaMKII, thereby promoting expression of genes DDIT3 that encode transcription factor CHOP.
Based on the newest data, cell death is also triggered by CHOP-mediated apoptosis, which is closely correlated with suppression of cell cycle regulator protein 21 (p21/WAF1) [112]. Generally, the pivotal role of p21 is its inhibition of the cell cycle in the G1 phase following the interaction with cyclin-dependent kinase (Cdk) [113]. The expression of p21 is dependent on tumor suppressor p53. Under stress conditions the tumor protein 53 (p53) up-regulates p21 that results in repression of the cell cycle at the G1 phase [114]. Recent data report that there is a continuous interplay between CHOP – a fundamental factor that trigger apoptosis, and p21 – an inhibitor of cell cycle progression. The possible crosstalk between p21 and CHOP may explain the transition from the pro-adaptive to the pro-apoptotic state of UPR under non-physiological conditions. Mihailidou et al. emphasizes a dual activity of p21 that is tightly connected with strong pro-apoptotic activity and, on the other hand, pro-adaptive suppression of cell cycle in the G1 phase. CHOP is therefore a regulator of p21 expression. During low and moderate stress conditions the process is stimulated, but during times of acute and chronic stress the process is supressed, leading to apoptotic cell death. This evidence indicates that in tumor diseases, the shift of UPR from pro-survival to the proapoptotic pathway may be affiliated with crosstalk between the PERK/eIF2α/ATF4/CHOP pathway and protein p21 [115, 116].
CONCLUSION
Areas of tissue hypoxia are the main hallmark of the microenvironment of a solid tumor, further leading to rapid cancer progression. Mammalian cells have specific transmembrane receptors, which in response to stress signals cause inhibition of translation, initiation and, as a result, attenuation of protein synthesis. The low oxygen tension causes hypoxic stress within tumor cells and is closely associated with the irregular vasoconstriction and vasodilation of tumor blood vessels. In response to the depletion of oxygen levels, tumor cells grow characteristically and show increased metastatic potential. Moreover, there is ample evidence that poorly-oxygenated tumors have a poorer prognosis and become more resistant to current treatment modalities such as radiotherapy and chemotherapy compared to better-oxygenated tumors.
Tumorigenesis is a multistep process closely associated with significant alterations on the molecular level within tumor cells. Tumor hypoxia induces a global attenuation of protein synthesis due to the fact that deficiency of oxygen has been regarded as a potential process that activates ER stress and leads to initiation of the UPR pathways. This consists of a cascade of events resulting in the attenuation of protein synthesis as well as activation of protein degradation pathways. Hypoxia also triggers multiple cellular response pathways that modify gene expression at the transcriptional level. Interestingly, extreme, prolonged stress conditions of the ER may also contribute to induction of the apoptotic pathway, mainly through the promoted expression of transcription factors such as ATF4 and CHOP. Regulation of protein synthesis in response to stress conditions concerns the PERK-mediated phosphorylation of translation initiation factor eIF2, necessary for the initiation of protein synthesis. An increased level of phosphorylated eIF2α has been reported in individuals with cancer, which indicates PERK kinase activation during tumor progression.
Currently, available anti-tumor treatment is insufficient due to the fact that patients suffer from various side effects. The different modalities of treatment are all characterized by non-specific action in both normal and tumor cells. For example, it has been reported that small molecular drugs currently used in chemotherapy target healthy cells including hair follicles, bone marrow, or the digestive tract, frequently resulting in the development of secondary adverse effects such as anaemia, alopecia, nausea, and vomiting. In addition, radiotherapy based on utilizing high-energy radio waves to kill tumor cells may also damage healthy tissues and lead to similar side effects.
Due to the fact that deficiency of oxygen has been established as a significant barrier to tumor therapy, better understanding of the mechanisms that are involved in the survival of tumor cells in hypoxic stress conditions is a promising avenue in the aim of developing more effective anti-cancer treatments. Current molecular insights into the mechanisms that allow the signaling switch from the pro-adaptive into the pro-apoptotic pathways are still insufficient. However, it is highly possible that utilising inhibitors of the components of the UPR pathways as anti-neoplastic therapeutics, may be an effective way to eliminate tumor cells and, as a result, may contribute to the development of novel, targeted anti-tumor drugs.
ACKNOWLEDGEMENTS
This work was supported by grant HARMONIA no. 2013/10/M/NZ1/00280 from the Polish National Science Centre, grant PRELUDIUM no. 2015/19/N/NZ3/00055 from the Polish National Science Centre and by grant of Medical University of Lodz, Poland no. 502-03/5-108-05/502-54-170.
Biography
 D. Pytel
D. Pytel
 I. Majsterek
I. Majsterek
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Cooper GM. The cell: a molecular approach. 2nd ed. ASM Press Sinauer Associates; Washington, D.C. Sunderland, Massachusetts: 2000. [Google Scholar]
- 2.Sudhakar A. History of Cancer, Ancient and Modern Treatment Methods. J Cancer Sci Ther. 2009;1:1–4. doi: 10.4172/1948-5956.100000e2. [DOI] [PubMed] [Google Scholar]
- 3.Schirrmacher V, Schwartz-Albiez R. Cancer metastasis: molecular and cellular biology, host, immune response, and perspectives for treatment. Springer-Verlag; Berlin ; New York: 1989. [Google Scholar]
- 4.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10(Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma X, Yu H. Global burden of cancer. Yale J Biol Med. 2006;79:85–94. [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 7.Schulz WA. Molecular biology of human cancers: an advanced student's textbook. Springer; Dordrecht, The Netherlands ; Norwell, MA: 2005. [Google Scholar]
- 8.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–95. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–77. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 10.Wang WA, Groenendyk J, Michalak M. Endoplasmic reticulum stress associated responses in cancer. Biochim Biophys Acta. 2014;1843:2143–9. doi: 10.1016/j.bbamcr.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–8. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267–73. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–91. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 15.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–28. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schonthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo) 2012;2012:857516. doi: 10.6064/2012/857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bobrovnikova-Marjon E, Pytel D, Riese MJ, et al. PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate Akt activation, and promote adipocyte differentiation. Mol Cell Biol. 2012;32:2268–78. doi: 10.1128/MCB.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pytel D, Seyb K, Liu M, et al. Enzymatic Characterization of ER Stress-Dependent Kinase, PERK, and Development of a High-Throughput Assay for Identification of PERK Inhibitors. J Biomol Screen. 2014;19:1024–34. doi: 10.1177/1087057114525853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- 20.Pytel D, Majsterek I, Diehl JA. Tumor progression and the different faces of the PERK kinase. Oncogene. 2015;35:1207–15. doi: 10.1038/onc.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fribley A, Zhang K, Kaufman RJ. Regulation of apoptosis by the unfolded protein response. Methods Mol Biol. 2009;559:191–204. doi: 10.1007/978-1-60327-017-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitnis NS, Pytel D, Bobrovnikova-Marjon E, et al. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol Cell. 2012;48:353–64. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chitnis N, Pytel D, Diehl JA. UPR-inducible miRNAs contribute to stressful situations. Trends Biochem Sci. 2013;38:447–52. doi: 10.1016/j.tibs.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 26.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 27.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 28.Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–8. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 29.Lodish HF. Molecular cell biology. 4th ed. W.H. Freeman; New York: 2000. [Google Scholar]
- 30.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 31.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- 32.Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- 33.Hockel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28:36–41. [PubMed] [Google Scholar]
- 34.Koumenis C, Wouters BG. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res. 2006;4:423–36. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- 35.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 36.Teicher BA. Physiologic mechanisms of therapeutic resistance. Blood flow and hypoxia. Hematol Oncol Clin North Am. 1995;9:475–506. [PubMed] [Google Scholar]
- 37.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–68. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 38.Majsterek I, Slupianek A, Hoser G, Skorski T, Blasiak J. ABL-fusion oncoproteins activate multi-pathway of DNA repair: role in drug resistance? Biochimie. 2004;86:53–65. doi: 10.1016/j.biochi.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 40.Gatenby RA, Kessler HB, Rosenblum JS, et al. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988;14:831–8. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- 41.Mann MJ, Hendershot LM. UPR activation alters chemosensitivity of tumor cells. Cancer Biol Ther. 2006;5:736–40. doi: 10.4161/cbt.5.7.2969. [DOI] [PubMed] [Google Scholar]
- 42.Vandewynckel YP, Laukens D, Geerts A, et al. The paradox of the unfolded protein response in cancer. Anticancer Res. 2013;33:4683–94. [PubMed] [Google Scholar]
- 43.Li Y, Luo L, Thomas DY, Kang CY. Control of expression, glycosylation, and secretion of HIV-1 gp120 by homologous and heterologous signal sequences. Virology. 1994;204:266–78. doi: 10.1006/viro.1994.1531. [DOI] [PubMed] [Google Scholar]
- 44.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 46.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 47.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70:1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 48.Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol Cell Biol. 2008;28:5106–19. doi: 10.1128/MCB.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80:51–60. [PMC free article] [PubMed] [Google Scholar]
- 51.Atkins C, Liu Q, Minthorn E, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 52.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–76. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Saha S, Bhanja P, Partanen A, et al. Low intensity focused ultrasound (LOFU) modulates unfolded protein response and sensitizes prostate cancer to 17AAG. Oncoscience. 2014;1:434–45. doi: 10.18632/oncoscience.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Y, Hendershot LM. The mammalian endoplasmic reticulum as a sensor for cellular stress. Cell Stress Chaperones. 2002;7:222–9. doi: 10.1379/1466-1268(2002)007<0222:tmeraa>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwab M. Encyclopedia of cancer. 3rd ed. Springer; Heidelberg ; New York: 2011. [Google Scholar]
- 56.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 57.Mahadevan NR, Zanetti M. Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J Immunol. 2011;187:4403–9. doi: 10.4049/jimmunol.1101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283:31153–62. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blais JD, Filipenko V, Bi M, et al. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol. 2004;24:7469–82. doi: 10.1128/MCB.24.17.7469-7482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151:217–9. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- 61.Suragani RN, Ghosh S, Ehtesham NZ, Ramaiah KV. Expression and purification of the subunits of human translational initiation factor 2 (eIF2): phosphorylation of eIF2 alpha and beta. Protein Expr Purif. 2006;47:225–33. doi: 10.1016/j.pep.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Harford JB, Morris DR. mRNA metabolism & post-transcriptional gene regulation. Wiley-Liss; New York: 1997. [Google Scholar]
- 63.Simón C, Pellicer A, Reijo Pera R. Stem cells in reproductive medicine: basic science and therapeutic potential. 3rd ed. Cambridge University Press; Cambridge: 2013. [Google Scholar]
- 64.Clemens MJ. Protein phosphorylation in cell growth regulation. Harwood Academic Publishers; Australia: 1996. [Google Scholar]
- 65.Asano K, Clayton J, Shalev A, Hinnebusch AG. A multifactor complex of eukaryotic initiation factors, eIF1, eIF2, eIF3, eIF5, and initiator tRNA(Met) is an important translation initiation intermediate in vivo. Genes Dev. 2000;14:2534–46. doi: 10.1101/gad.831800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceci M, Gaviraghi C, Gorrini C, et al. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature. 2003;426:579–84. doi: 10.1038/nature02160. [DOI] [PubMed] [Google Scholar]
- 67.Hinnebusch AG. Translational regulation of yeast GCN4. A window on factors that control initiator-trna binding to the ribosome. J Biol Chem. 1997;272:21661–4. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 68.Rajesh K, Krishnamoorthy J, Kazimierczak U, et al. Phosphorylation of the translation initiation factor eIF2alpha at serine 51 determines the cell fate decisions of Akt in response to oxidative stress. Cell Death Dis. 2015;6:e1591. doi: 10.1038/cddis.2014.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clemens MJ. Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol. 2001;27:57–89. doi: 10.1007/978-3-662-09889-9_3. [DOI] [PubMed] [Google Scholar]
- 70.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–97. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 71.Cui W, Li J, Ron D, Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr D Biol Crystallogr. 2011;67:423–8. doi: 10.1107/S0907444911006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carrara M, Prischi F, Nowak PR, Kopp MC, Ali MM. Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. Elife. 2015:4. doi: 10.7554/eLife.03522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 74.Spirin AS. Ribosomes. Kluwer Academic/Plenum; New York: 1999. [Google Scholar]
- 75.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–29. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kimball SR. Eukaryotic initiation factor eIF2. Int J Biochem Cell Biol. 1999;31:25–9. doi: 10.1016/s1357-2725(98)00128-9. [DOI] [PubMed] [Google Scholar]
- 77.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97:12625–30. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 79.Raven JF, Baltzis D, Wang S, et al. PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2alpha phosphorylation. J Biol Chem. 2008;283:3097–108. doi: 10.1074/jbc.M709677200. [DOI] [PubMed] [Google Scholar]
- 80.Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 2005;24:1546–56. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813:508–20. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 82.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 83.Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–46. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. 2013;5:a008672. doi: 10.1101/cshperspect.a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 87.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–18. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 88.Renehan AG, Booth C, Potten CS. What is apoptosis, and why is it important? BMJ. 2001;322:1536–8. doi: 10.1136/bmj.322.7301.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coultas L, Pellegrini M, Visvader JE, et al. Bfk: a novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ. 2003;10:185–92. doi: 10.1038/sj.cdd.4401204. [DOI] [PubMed] [Google Scholar]
- 90.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27(Suppl 1):S2–19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elkholi R, Floros KV, Chipuk JE. The Role of BH3-Only Proteins in Tumor Cell Development, Signaling, and Treatment. Genes Cancer. 2011;2:523–37. doi: 10.1177/1947601911417177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baliga B, Kumar S. Apaf-1/cytochrome c apoptosome: an essential initiator of caspase activation or just a sideshow? Cell Death Differ. 2003;10:16–8. doi: 10.1038/sj.cdd.4401166. [DOI] [PubMed] [Google Scholar]
- 93.Cain K, Bratton SB, Cohen GM. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie. 2002;84:203–14. doi: 10.1016/s0300-9084(02)01376-7. [DOI] [PubMed] [Google Scholar]
- 94.Su J, Zhou L, Xia MH, Xu Y, Xiang XY, Sun LK. Bcl-2 family proteins are involved in the signal crosstalk between endoplasmic reticulum stress and mitochondrial dysfunction in tumor chemotherapy resistance. Biomed Res Int. 2014;2014:234370. doi: 10.1155/2014/234370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zong WX, Li C, Hatzivassiliou G, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–94. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 100.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–94. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 101.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Jin Y, Williams TA, Burtenshaw SM, Martyn AC, Lu R. Amino acid deprivation induces CREBZF/Zhangfei expression via an AARE-like element in the promoter. Biochem Biophys Res Commun. 2010;391:1352–7. doi: 10.1016/j.bbrc.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 103.Dey S, Baird TD, Zhou D, Palam LR, Spandau DF, Wek RC. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem. 2010;285:33165–74. doi: 10.1074/jbc.M110.167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 105.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2015;47:146–7. doi: 10.1093/abbs/gmu128. [DOI] [PubMed] [Google Scholar]
- 107.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–56. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 110.Simmen T, Lynes EM, Gesson K, Thomas G. Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM). Biochim Biophys Acta. 2010;1798:1465–73. doi: 10.1016/j.bbamem.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–90. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mkrtchian S. Targeting unfolded protein response in cancer and diabetes. Endocr Relat Cancer. 2015;22:C1–4. doi: 10.1530/ERC-15-0106. [DOI] [PubMed] [Google Scholar]
- 113.Harper JW, Elledge SJ, Keyomarsi K, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jeong JH, Kang SS, Park KK, Chang HW, Magae J, Chang YC. p53-independent induction of G1 arrest and p21WAF1/CIP1 expression by ascofuranone, an isoprenoid antibiotic, through downregulation of c-Myc. Mol Cancer Ther. 2010;9:2102–13. doi: 10.1158/1535-7163.MCT-09-1159. [DOI] [PubMed] [Google Scholar]
- 115.Mihailidou C, Papazian I, Papavassiliou AG, Kiaris H. CHOP-dependent regulation of p21/waf1 during ER stress. Cell Physiol Biochem. 2010;25:761–6. doi: 10.1159/000315096. [DOI] [PubMed] [Google Scholar]
- 116.Mihailidou C, Chatzistamou I, Papavassiliou AG, Kiaris H. Improvement of chemotherapeutic drug efficacy by endoplasmic reticulum stress. Endocr Relat Cancer. 2015;22:229–38. doi: 10.1530/ERC-15-0019. [DOI] [PubMed] [Google Scholar]