Abstract
While there is general agreement on the necessity to measure glomerular filtration rate (GFR) in many clinical situations, there is less agreement on the best method to achieve this purpose. As the gold standard method for GFR determination, urinary (or renal) clearance of inulin, fades into the background due to inconvenience and high cost, a diversity of filtration markers and protocols compete to replace it. In this review, we suggest that iohexol, a non-ionic contrast agent, is most suited to replace inulin as the marker of choice for GFR determination. Iohexol comes very close to fulfilling all requirements for an ideal GFR marker in terms of low extra-renal excretion, low protein binding and in being neither secreted nor reabsorbed by the kidney. In addition, iohexol is virtually non-toxic and carries a low cost. As iohexol is stable in plasma, administration and sample analysis can be separated in both space and time, allowing access to GFR determination across different settings. An external proficiency programme operated by Equalis AB, Sweden, exists for iohexol, facilitating interlaboratory comparison of results. Plasma clearance measurement is the protocol of choice as it combines a reliable GFR determination with convenience for the patient. Single-sample protocols dominate, but multiple-sample protocols may be more accurate in specific situations. In low GFRs one or more late samples should be included to improve accuracy. In patients with large oedema or ascites, urinary clearance protocols should be employed. In conclusion, plasma clearance of iohexol may well be the best candidate for a common GFR determination method.
Keywords: glomerular filtration rate, iohexol
Introduction
Since Homer W. Smith published in 1951 his famous textbook ‘The Kidney: Structure and Function in Health and Disease’, glomerular filtration rate (GFR) has been considered as one of the best physiologic measures of kidney function [1]. The historical gold standard for GFR measurement, which is urinary clearance of inulin, is however difficult to perform in practice. Indeed, these pioneers of nephrology performed repeated urine collection every 10 or 15 min with a urinary catheter, a constant infusion of inulin and arterial samples [1, 2]. Several alternatives such as isotopic (125I-iothalamate, 51Cr-EDTA or 99Tc-DTPA) or non-isotopic (iohexol or iothalamate) ‘cold’ markers have thus been proposed to measure GFR [3]. Importantly, the term ‘alternative’ also implies that another methodology than urinary clearance can be used, i.e. plasma clearance [4–6].
Estimated GFR by equations based on creatinine and/or cystatin are less accurate than measured GFR. Although equations for estimating GFR are useful, especially outside nephrology, the precision of the equations can be relatively poor in specific clinical situations (see Part 2 of the article) [7–9]. Also, both creatinine and cystatin C are influenced by non-GFR factors [10–12]. This has been demonstrated in several and diverse clinical populations, i.e. diabetes mellitus, chronic kidney disease (CKD), renal and non-renal transplantation, patients with cirrhosis, heart failure and also in healthy subjects [13–18]. Thus, in many clinical contexts a measured GFR is required.
In Part 1 of this review, we will focus on iohexol plasma clearance, the most popular method used to measure GFR in Europe. We will review general properties, safety aspects, analytical considerations, agreement with other procedures and current available procedures of iohexol plasma clearance. In Part 2, we focus on the role of iohexol plasma clearance both in clinical practice and research.
Iohexol: general properties and safety
Iohexol is a non-ionic contrast medium, a principle developed by the Swedish radiologist Torsten Almén [19, 20]. It is mainly used for computed tomography (CT), catheter-based angiography and interventions. The first description of iohexol in humans was published in 1980 [21]. Increasing doses of iohexol (125–500 mg I/kg) were injected into 20 healthy subjects. In this seminal study, it was shown that the substance was safe and fully excreted by the kidneys. In 10 subjects, it was additionally shown that iohexol did not affect GFR measured by 51Cr-EDTA. The authors mentioned that urinary clearance of iohexol was higher than urinary clearance of 51Cr-EDTA, but no additional details were given [21]. In 1983, data from this first study were re-analysed [22]. Iohexol total clearance was identical to 51Cr-EDTA, whereas urinary clearance of iohexol was slightly higher. Finally, they showed that iohexol was distributed within the extracellular volume [22]. This iohexol distribution pattern applies also to patients with CKD and obese subjects [22–26]. Importantly for a GFR marker, other studies demonstrated that iohexol had no effect on the GFR level per se [21, 27].
The molecular weight of iohexol is 821 Da [22, 28]. The proportion of iohexol bound to protein appears to be very low. The first study described a binding to protein of only 1.5% [29], which was also confirmed by others [26, 30, 31], although one recent publication challenged these findings [32]. Regarding its molecular weight and the absence of binding to proteins, there is little doubt that this marker is freely filtrated through the glomerulus.
The question of the extra-renal clearance of a marker is important. Briefly, this question can be studied by two different methodologies: either measuring the plasma clearance in anephric patients (or in patients with very low residual function) or measuring plasma and urinary clearances, the difference between both being the extra-renal clearance. Extra-renal clearance of iohexol is low with both methodologies. Studying anephric patients, extra-renal clearance of iohexol was between 2 and 3 mL/min/1.73 m2 [24, 33–35]. Studying the difference between plasma and urinary clearances in healthy subjects, the extra-renal clearance of iohexol was between 0 and 6 mL/min/1.73 m2 [22, 26, 30, 36] or 5% [25]. These results must be compared with extra-renal clearance of other markers such as iothalamate and 51Cr-EDTA, which has been found to be between 4 and 10 mL/min/1.73 m2 for iothalamate [26, 37–39] and around 2–4 mL/min/1.73 m2 for 51Cr-EDTA [40, 41].
To be considered a reference marker for measuring GFR, there should not be any tubular secretion or absorption. Iohexol differs from iothalamate in being non-ionic, and active secretion or absorption of iohexol has to our knowledge not been demonstrated. In contrast, a concentration gradient dependent tubular transport of iothalamate and other ionic contrast media is well documented in several species, including human [42, 43].
The safety of iohexol has been extensively studied [44, 45] and is confirmed by the large number of iohexol measurements performed in countries, e.g. Sweden (in Skåne county) (∼1500 GFR measurements/year) [46, 47] and Italy (25 000 iohexol clearances since 1995 at the Clinical Research Center of Mario Negri Institute in Bergamo) (F.G., personal data). No severe adverse event, and particularly no anaphylactic reaction, occurred. This safety profile is, at least in part, explained by the current low dose of iohexol injected (5 or 10 mL versus 80–180 mL for CT scan and 130–300 mL for coronary interventions) and by the exclusion of patients with known contrast medium reactions. Even in patients with minimal renal function, iohexol doses of 10 mL (300 mg I/mL) have not been shown to be nephrotoxic by measuring serum creatinine but also urinary N-acetyl-beta-d-glucosaminidase and α1-microglobulin, at 1 and 4 days after injection [48]. Altogether, the co-authors of this review article have performed around 10 000 iohexol GFR measurements over the last 2 years. Anaphylactic shock never occurred.
Iohexol: analytical and other metrics considerations
Analytical considerations
Iohexol can be measured using different methods. High performance liquid chromatography with ultraviolet detection (HPLC-UV), X-ray fluorescence (XRF) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) are certainly the most validated methods. There are other assays, e.g. capillary electrophoresis, but such methods have been poorly validated, and nowadays are seldom used [49, 50]. HPLC-UV is clearly the most commonly used method in Europe and was historically the first method described [21] to quantify iohexol [30]. Measurement of iohexol by HPLC-UV is sensitive, specific and reproducible, enabling the use of minute doses of iohexol, as low plasma concentrations can be accurately quantified [26, 30, 45, 51–59]. The high sensitivity also allowed the development of finger-prick samples (capillary sampling). However, this capillary technique needs to be developed further, as the precision of this method cannot yet be considered sufficient [60–65]. Measurement of iohexol by XRF has been well described [45, 66–72] but requires specific instrumentation. Few studies have compared these two methods for GFR measurement purposes [69], yet these data indicate that the performance of XRF is inferior to HPLC-UV due to its lower sensitivity [69, 73]. Measurement of iohexol by LC-MS/MS has been more recently described by several authors [32, 74–79]. LC-MS/MS is theoretically a more sensitive and specific method but is more complex and costly compared with HPLC-UV.
A fundamental question is whether differences in iohexol measurement methodology will affect GFR results, as has been shown for other markers like inulin or iothalamate [80, 81]. Recent data suggest a high concordance between iohexol concentrations and plasma clearances measured by HPLC-UV or LC-MS/MS in 102 CKD and healthy subjects, but a systematic correction factor of 10% must be applied [82]. Other authors performed an inter-laboratory comparison of iohexol measurement. In 20 paediatric patients, they compared iohexol concentrations (range 15–700 µg/mL) measured by LC-MS/MS in the USA with those determined on the same samples by HPLC-UV by a European reference laboratory in Italy. The concordance between the two methods was very high both for iohexol concentrations and GFR results (difference of <10% between the two laboratories, except for one patient) [83]. Further research is still required regarding the effect of the measurement method (HPLC or LC-MS/MS) on iohexol (or iothalamate) clearance results.
Two very important analytical points should be highlighted when discussing GFR measurement. Firstly, iohexol is perfectly stable at room temperature, −20°C and −80°C [32, 84]. Iohexol clearance investigations can therefore be performed in virtually all medical settings. Samples can be measured at a tertiary care hospital and then shipped to a central laboratory. No shipment in dry ice is needed. The high stability of iohexol is a major advantage in comparison with isotopic methods that can be performed only in nuclear medicine units and for which samples are difficult to store.
Secondly, an external quality assurance programme does exist, provided by Equalis AB, Uppsala, Sweden. Two serum or plasma samples with added iohexol are distributed to participants four times per year. Additional data (patient gender, height and weight for body surface calculation, administered dose of iohexol and timing of samples) are provided to enable programme participants to calculate iohexol clearance using a one- or two-sample calculation (or both). This proficiency programme includes both HPLC-UV (the majority) and LC-MS/MS measurements. Each laboratory can compare its own results to that of other participating laboratories. In 2015, 35 laboratories participated in this programme. Agreement between overall means and spiked concentrations is high. Between-laboratory reproducibility for each survey is ∼5% [coefficient of variation (CV)]. Analysis of variance calculated from repeated distribution of the same pools over a 3-year period yields a within-laboratory reproducibility of 5%, a between-laboratory reproducibility of 3–4% and a total variation of 5–7%. The different methods of iohexol analysis perform equally well. To the best of our knowledge, such an external quality programme does not exist for other GFR markers wherefore less knowledge is available about their inter-laboratory variation [56].
Intra-individual variation
The biological variation of any physiological quantity should be considered in the interpretation of biological results. The biological CV, represents the ‘normal’ variation of a parameter that can be observed in the same individual. GFR is influenced by several physiological parameters such as diet, activity or circadian variations [71, 85–87]. In the case of measured GFR, intra-individual variation includes both biological variation and the errors due to the measurement of GFR itself. Variation is thus dependent on both the markers and measurements protocols [88]. Ideally, the GFR measurement should be performed using a standardized procedure, e.g. measurement in a fasting state, in the same environment and at the same time of day, because all these parameters are prone to increase intra-individual variation. Intra-individual variation can also differ according to the population tested. For example, intra-individual variation may be higher in CKD patients than in a healthy population [89], even if this is not found by all authors [6]. Literature gives an intra-individual variation of measured GFR (independently of the marker considered) of 4.2 to 10% [6, 23, 30, 36, 71–73, 86, 88–99]. This means that in the same given individual, GFR must increase or decrease by at least 5–15% (depending on the intra-individual CV of the method used) to be considered clinically relevant. CV of 10% is frequently considered and should be kept in mind when analysing studies comparing different GFR measurement methods, or estimated versus measured GFR. In such studies, accuracy within 10 or 30% is frequently used. Accuracy within 10% is the percentage of results with one method that are within ±10% of the results of another method, and can, with respect to intra-individual variation of GFR, be considered as an excellent agreement between the methods.
In Table 1, we summarize estimates of intra-individual variation published by co-authors of the current article when GFR is measured with iohexol.
Table 1.
Examples of GFR variability with different iohexol procedures
| Author Reference |
Sample | Protocol | Population | GFR variability (CV) |
|---|---|---|---|---|
| Krutzen [30] | 9 | PC: samples at 120 and 240 min + BM correction | Healthy | 11.4% |
| Delanaye [73] | 12 | PC: samples at 120 and 240 min + BM correction | Healthy | 4.5% |
| Eriksen [99] | 88 | PC: single-sample + Jacobsson correction | General population | 4.2% |
| Gaspari [6] | 24 | PC: samples at 120, 150, 180, 210 and 240 if eGFR >40 mL/minand at 120, 180, 240, 300, 450 and 600 min if eGFR <40 mL/min + BM correction |
Healthy and CKD | 5.6% |
eGFR, estimated glomerular filtration rate; CV, coefficient of variation; CKD, chronic kidney disease; PC, plasma clearance; BM, Brochner-Mortensen [116].
Iohexol: studies comparing iohexol with inulin clearance
To the best of our knowledge, only two studies have compared urinary clearances of iohexol and inulin [100, 101] (Table 2). Both showed excellent correlation between the two methods, but the statistical tools used in the original publications were limited and re-analysed data demonstrated less impressive concordance [3]. On the other hand, several studies have compared iohexol plasma clearances with urinary inulin clearance [52, 102–106]. In general, these studies showed a good correlation between the methods. Results are less accurate in the study published by Erley et al., but the patients included were hospitalized in an intensive care unit [104]. The value of plasma clearance (whichever marker) in this setting is probably questionable because the extracellular volume, and consequently the volume of distribution of the marker, is quite variable from patient to patient and within the same patient at different points due to changes in fluid infusions and in biological fluid loss or sequestration in different compartments [104, 107]. Another study did not describe the slope-intercept method used [102]. The studies published by Gaspari et al. in adults [52] and Berg et al. in children [105] are more relevant from a methodological point of view (see Table 2 for details). They showed low bias and relatively good precision between the two methods. Both studies included a large number of samples including a late sample (600 and 1440 min), the last point being of importance as it will be described further in the text [52, 105].
Table 2.
Studies comparing iohexol and inulin clearance
| Reference | Sample size | Population | GFR range (mL/min/1.73 m2) | Methodology | Statistics | Results | Comments |
|---|---|---|---|---|---|---|---|
| Lewis [102] | 29 | Heart transplanted (n = 10) Kidney transplanted (n = 11) Living kidney donor (n = 10) |
10–117 | In UC Io (XRF) PC: samples at 180 and 240 min |
Correlation Regression Ratio |
0.86 Io = 0.85In + 8.79 1.09 ± 0.06 |
|
| Brown [100] | 30 | Post-surgery | 10–125 | In UC Io (XRF) UC (three collections of 30 min) PC: samples at 180 and 240 min + BM correction |
Correlation Regression Ratio |
UC 0.986 Io = 0.998In − 2.31 PC 0.983 Io = 0.947In + 4.92 1.102 ± 0.286 |
|
| Lindblad [103] | 46 | Children | 25–150 | In UC (n = 54) PC (n = 20) Io (XRF) PC: samples at 60, 120 and 180 min |
Correlation | 0.766 |
|
| Gaspari [52] | 41 | CKD | 6–160 | In UC Iohexol (HPLC) PC: samples at 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, 300, 450 and 600 min AUC samples at 120 and 600 min + BM correction |
Correlation Regression BA In − Io |
AUC 0.97 Io = 0.994In + 2.339 1.02 ± 7 BM 0.982 Io = 0.994In + 1.809 |
|
| Erley [104] | 31 | Intensive care unit | 10–130 | In UC Io (XRF) PC: samples at 150, 195, 240 and 360 min (360 if estimated GFR below <30 mL/min) |
Correlation Regression BA Io − In |
0.98 Io = 0.971In + 7.65 =8.67 ± 7.21 |
|
| Sterner [101] | 20 | Healthy | 106–129 | In UC Io (HPLC) UC Infusion, plasma samples at 270, 330, 390 and 420 min and urinary collection between 300 and 360 and 360 and 420 min (bladder scan) |
Wilcoxon | In 117 mL/min Io 113 mL/min |
|
| Berg [105] | 60 | CKD children | 5–180 | In UC Io (HPLC) PC: samples at 180, 200, 220, 240, 420 and 1440 min (420 if estimated GFR between 20 and 50 mL/min and 420 and 1440 if estimated GFR <20 mL/min) + BM correction |
Correlation Regression BA (PC − In) Accuracy within 30% |
Inulin 0.921 PC = 0.9In + 9.72 2.65 ± 16.26 83.3% Iohexol PC 0.659.6 |
|
GFR, glomerular filtration rate; AUC, area under the curve; BA, Bland and Altman (bias ± standard deviation). BA calculated means that BA results have recalculated from data available in the article. BM, Brochner-Mortensen; CKD, chronic kidney disease; HPLC, high performance liquid chromatography; In, inulin; Io, iohexol; NA, not available; PC, plasma clearance; UC, urinary clearance; XRF, X-ray fluorescence. All GFR results are in mL/min or in mL/min/1.73 m2.
In a recent review article [3], authors pooled data from seven available studies comparing iohexol (both plasma or urinary clearance) with GFR measured by inulin and calculated median bias and accuracy within 30 and 10%. For iohexol urinary clearance studies, the mean (± standard deviation) GFR, measured by inulin, was 81 ± 38 mL/min/1.73 m2. Median bias (corresponding to the median difference between iohexol and inulin) was −7% [95% confidence interval (CI): −10 to 0]. Pooled accuracy within 30% was 100% (CI not reported) and accuracy within 10% was 53% (95% CI: 41–70). For plasma iohexol clearance studies, the mean GFR measured by inulin was 66 ± 40 mL/min/1.73 m2. Median bias for iohexol was +3% (95% CI: 0–6). Pooled accuracy within 30% was 86% (95% CI: 81–91); accuracy within 10% was 50% (95% CI: 43–58). We agree with the conclusions of the authors of this analysis: urinary clearance of iohexol is sufficiently accurate to reflect inulin clearance but the evidence is limited (47 measurements in 47 participants); plasma clearance of iohexol is also sufficiently accurate with moderately strong evidence (172 measurements in 172 participants) [3].
Iohexol: studies comparing iohexol with other reference markers
We will focus on studies with the most appropriate methodology, i.e. studies with low injected doses (usually no more than 5 or 10 cm3 of iohexol 300 or 240 mg I/mL) but also with the same procedure used for any markers (Table 3). Indeed, as will be discussed in the next paragraph, different procedures (urinary versus plasma clearance or different sampling times) potentially lead to differences in GFR results, which makes it impossible to know whether these differences are due to the marker itself or to the methodology. Finally, we emphasized results from studies that included appropriate statistical tools, such as Bland and Altman or accuracy analyses.
Table 3.
Studies comparing iohexol clearance with other GFR markers (except inulin)
| Reference | Sample size | Population | GFR range (mL/min/1.73 m2) | Methodology | Statistics | Results | Comments |
|---|---|---|---|---|---|---|---|
| Olsson [22] | 10 | Healthy | NA | Cr UC Collection between 120 and 240 and between 240 and 360 min Io (HPLC) UC idem |
UC 120–240 min Io: 112 mL/min Cr: 95 mL/min 240–360 min Io: 109 mL/min Cr: 97 mL/min |
|
|
| Krutzen [30] | 42 | Healthy and CKD | 24–207 | Cr PC: 120 and 240 min + BM correction Io (HPLC) idem |
Correlation Regression |
0.983 Io = 0.99Cr − 1.92 |
|
| O'Reilly [84] | 54 (100 cm3 Io) 33 (50 cm3 Io) |
NA | 30–130 | Cr PC: samples at 60, 120, 180 and 240 min Io (XRF) PC: samples at 180 and 240 min |
Correlation | 100 cm3: 0.9 50 cm3: 0.85 |
|
| Bäck [108] | 18 | Healthy | NA | It (HPLC) PC: samples at 160, 180, 200, 220, 240 and 260 min Io (HPLC) idem |
Mean GFR by It 40% higher than mean GFR by Io |
|
|
| Bäck [26] | 7 | Healthy women | 100–140 | It (HPLC) PC: samples at 2, 5, then every 5 min for 90 min then 120, 180, 200 and 220 min AUC Io (HPLC) idem |
Io: 121 mL/min It: 144 mL/min |
||
| O'Reilly [66] | 33 measurements in 12 subjects | NA | 20–100 | Dt PC: samples at 90, 135, 180 and 240 min Io (XRF) PC: samples at 180 and 240 min |
Correlation | 0.95 |
|
| Lewis [102] | 29 | Heart transplanted (n = 10) Kidney transplanted (n = 11) Living kidney donor (n = 10) |
10–117 | Dt UC: 3 collections of 20 min Io (XRF) PC: samples at 180 and 240 min |
Correlation Regression Ratio |
0.89 Io = 0.89Dt + 6.5 1.08 ± 0.06 |
|
| Effersöe [68] | 15 | Patients for urography | 22–110 | Cr and Dt PC: samples at 0, 10, 20, 30, 120, 180, 240 and 300 min AUC Io (XRF) idem |
Regression Correlation BA Cr − Io Dt − Io |
Io = 0.97Dt − 11 0.96 Io = 1.01Cr + 8 0.95 −10.8 ± 7.9 −9.4 ± 6.9 |
|
| Eriksson [109] | 98 | Diabetics | 30–150 | Cr PC: samples at 220 min + Jacobsson correction [110] Io (HPLC) idem |
Regression Correlation |
Cr = 1Io − 0.8 0.965 |
|
| Stake [111] | 11 | Children with severe CKD | 8–30 | Dt PC: samples at 5, 15, 120, 150, 180 and 210 min + Rootwelt correction [112] Io (XRF) PC: 6 samples between 180 and 1440 min + BM correction |
BA Dt − Io | Io 6 samples: 13 ± 2 mL/min Dt: 17 ± 2 mL/min |
|
| Lundqvist [113] | 31 | Plegic patients | 70–130 | Cr PC: samples at 180, 210, 240 and 270 min + BM correction Io (XRF) idem |
BA Cr − Io | Day 1: +2.1 ± 10.2 Day 2: +0.9 ± 5.9 |
|
| Nossen [24] | 8 | Severe CKD | 5–9 | It UC: 11 collections between 0 and 7200 min PC: samples at 30, 60, 120, 180, 360, 540, 1440, 1920, 2880, 4320 and 7200 min AUC Or BM correction Io (XRF) idem |
Mean | UC It 6 ± 2 Io 6 ± 2 PC: AUC It 6 ± 2 Io 8 ± 1 PC: BM Io 8 ± 1 |
|
| Rydström [92] | 122 | Healthy and CKD | 4–139 | Cr PC: samples at 180, 200, 220, 240 and 1440 min (1440 min if creatinine > 2 mg/dL) + BM correction Io (XRF) idem |
Correlation Regression |
0.986 Io = 0.971Cr − 1.368 |
|
| Lundqvist [114] | 77 | Patients for urography | 25–125 | Cr PC: samples at 180, 210, 240, 270 min + BM correction Io (XRF) PC: samples at 180 and 240 or 270 min + BM correction |
Correlation Regression BA (Io − Cr) |
0.918 Io = 0.892Cr + 6.278 2 ± 9.2 |
|
| Brandstrom [69] | 49 | CKD patients GFR >40 mL/min |
40–125 | Cr PC: samples at 150, 195 and 240 min + BM correction Io (HPLC and XRF) idem |
Regression Correlation BA Cr − Io |
XRF Io = 1.03Cr − 1.79 0.97 0.58 ± 4.95 HPLC Io = 1.05Cr − 4.43 0.96 −0.16 ± 6.17 |
|
| Pucci [72] | 32 | Diabetics | 13–151 | Cr PC: samples at 5, 10, 15, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 360, 420 and 1440 (360 and 420 if creatinine >2 mg/dL, 1440 if creatinine >5 mg/dL AUC Io (HPLC) idem |
Regression Correlation BA Cr − Io |
Io = 0.978Cr + 2.45 0.995 −0.6 ± 3.6 |
|
| Houlihan [70] | 21 | Diabetics | 50–145 | Dt PC: samples at 120, 165 and 210 min + BM correction Io (XRF) PC: samples at 120, 150, 180, 210 and 240 min + BM correction |
Regression Correlation BA Io − Dt |
Io = 0.9938Dt + 4.916 0.97 4.3 ± 7.7 |
|
| Pucci [93] | 41 | Diabetics | 29–150 | Cr PC: samples at 5, 10, 15, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 360, 420 and 1440 (360 and 420 if creatinine >2 mg/dL, 1440 if creatinine >5 mg/dL AUC Io (HPLC) idem |
Regression Correlation BA Cr − Io |
Type 1 Io = 0.978Cr + 0.132 0.999 Type 2 0.987 Io = 0.078Cr + 2.352 −0.42 ± 3.69 |
|
| Bird [115] | 56 19 |
Patients (diabetes, cancer) Healthy, studied two times (fasting or not) |
15–140 | Cr PC: samples at 20, 40, 60, 120, 180 and 240 min AUC Io (XRF) idem |
BA Cr − Io | 4 ± 7.9 | |
| Slack [58] | 10 | Cirrhotic | 45–100 | Cr PC: samples at 120, 180 and 240 min +BM correction Io (HPLC) idem |
BA Cr − Io | −1.3 ± 8.6 | |
| Seegmiller [32] | 150 | Healthy and CKD | 5–150 | It U: one collection over 45 or 60 min after 60 min + bladder scan Io (mass spectrometry) |
Passing Bablok BA relative % Io − It |
Io = 0.85It + 0.44 −15% ± 10% |
|
| Delanaye [82] | 102 | Healthy and CKD | 15–130 | It PC: samples at 120, 180, 240 and 300 min + BM correction Io (HPLC and mass spectrometry) idem |
HPLC Passing Bablok BA relative % Io − It Accuracy within 15% Mass spectrometry Passing Bablok BA relative % Io − It Accuracy within 15% |
Io = 0.88It + 7 −2% ± 13% 80% Io = 0.88It + 1 −11% ± 9% 74% |
GFR, glomerular filtration rate; AUC, area under the curve; BA, Bland and Altman (bias ± standard deviation). BA calculated means that BA results have recalculated from data available in the article. BM, Brochner-Mortensen; CKD, chronic kidney disease; Cr, 51Cr-EDTA; Dt, 99Tc-DTPA; HPLC, high performance liquid chromatography; Io, iohexol; It, iothalamate; NA, not available; PC, plasma clearance; UC, urinary clearance; XRF, X-ray fluorescence. All GFR results are in mL/min or in mL/min/1.73 m2.
51Cr-EDTA clearance is one of the most accurate methods to measure GFR [3, 116, 117]. It is thus not surprising that several authors have studied the concordance between both 51Cr-EDTA clearance and iohexol clearance [22, 30, 58, 68, 69, 72, 84, 92, 93, 109, 113–115]. All relevant studies compared plasma clearances of both markers and have found excellent agreement [30, 69, 72, 92, 93, 109, 115]. Among these studies, five authors provided Bland-Altman analyses, displaying little bias (between 0 and 4 mL/min) and excellent precision (95% of the results between 8 and 16 mL/min/1.73 m2) [58, 69, 72, 93, 115]. Based on these results, one can conclude that plasma clearance of iohexol is concordant with plasma clearance of 51Cr-EDTA.
Only a few studies compared 99Tc-DTPA with iohexol and the majority have questionable methodologies [66, 68, 70, 102, 111]. A study in 21 diabetic patients showed good correlation, acceptable bias (4 mL/min) and precision for plasma clearance of iohexol and 99Tc-DTPA [70].
Studies comparing iohexol and iothalamate clearances are of importance as iothalamate, an ionic contrast medium, is the most frequently used GFR marker in the USA and the only ‘cold’ alternative method to iohexol for measuring GFR. At least five studies have compared the two markers [24, 26, 32, 82, 108]. Two analyses, performed in healthy people, showed a constant bias in plasma clearances, with iothalamate clearances being systematically higher than iohexol clearances [26, 108]. A third one included patients with severe CKD but results were unreliable because statistical analyses were not adequate [24]. In a recent study, the authors compared urinary clearances of iohexol and iothalamate in 150 kidney transplant recipients. Contrary to the majority of other studies, iohexol was measured by LC-MS/MS. The authors found iothalamate urinary clearance to be systematically higher (+15%, constant bias thorough the GFR range) than iohexol urinary clearance [32]. Finally, another recent study compared plasma clearance of iohexol and iothalamate in 102 patients with a wide range of GFR. The authors found a good concordance between iothalamate and iohexol plasma clearance measured by HPLC, whereas iothalamate systematically overestimated (by 10%) iohexol results measured by mass spectrometry [82].
Generally, these results head towards the same direction: GFR measured with iothalamate yields higher results than iohexol. According to Seegmiller et al., there are different theoretical explanations for this discrepancy: iohexol tubular reabsorption, iothalamate tubular secretion or iohexol binding protein [32]. However, among these hypotheses, only the tubular secretion of iothalamate is well described in the literature [42, 43]. Our interpretation is that iohexol is a better marker of GFR than iothalamate because tubular secretion of iothalamate leads to GFR overestimation.
Plasma clearance: between physiology, safety and pragmatism
A variety of methodologies or procedures can be used to measure GFR with iohexol; most of these are not iohexol-specific and can be applied to other markers like 51Cr-EDTA or iothalamate. Different procedures have different degrees of complexity and precision and can therefore lead to slightly different results.
Urinary versus plasma clearance
Iohexol is almost always injected as a bolus either for urinary or plasma clearance determinations, whereas constant infusion of iohexol [30, 36, 101] for urinary clearance measurement has seldom been used (Supplementary data, Table S1). Several publications have compared iohexol urinary and plasma clearances [28, 33, 36, 48, 77, 100, 101, 118]. Among these studies, the results published by Stolz et al. should be highlighted because 342 subjects were included and adequate statistical tools were applied [77]. Most studies find plasma clearances to be higher than urinary clearances. Two important points must be emphasized.
First, the timing of the last plasma sample in multiple-sample methods is fundamental: concordance between urinary and plasma clearance improves if the last iohexol measurement was performed late. This is particularly true in the very low GFR ranges [77, 118, 119]. Therefore, in all CKD patients (i.e. GFR <45 mL/min), it should be recommended to extend plasma sampling until 5, 6 or even 8 h after injection. Gaspari et al. studied GFR in a cohort of patients with CKD (GFR <40 mL/min) and plasma samples taken hourly up to 8 h. GFR was calculated by using all available samples or by using only those collected up to 5 h after iohexol injection. The authors showed that GFR was overestimated on average by 7% in comparison with GFR measured up to 8 h (F.G., personal data). Thus, timing of the last sample is crucial. In more advanced CKD (stage 5), an additional sample after 24 h has been recommended by some groups [48, 77]. This late sample is however not always easy to implement in clinical practice. This approach has been questioned by others arguing for the circadian variation of GFR [85, 87, 120]. Moreover, a rather long interval between the two last samples could potentially make the mathematical weight of the 24 h sample exaggerated in the final GFR calculation.
Second, urinary clearance is the only option in specific clinical situations where marker distribution takes several days, i.e. in patients with severe oedema or ascites.
Both of these two last requirements apply to all plasma clearance procedures and to all GFR markers [4, 107, 121–125].
We recommend a pragmatic approach when choosing the GFR measurement method: while urinary clearance may be the most physiologic and accurate method to measure the filtering capacity of the kidney, it is time consuming and prone to errors in the measurement of urinary flows. Plasma clearance methods represent the best compromise between physiology and feasibility, both in clinical routine and research [for example: REIN (Ramipril Efficacy In Nephropathy), REIN-2, DEMAND (Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular Risk in Diabetes) and ALADIN (A Long-Acting Somatostatin on Disease Progression in Nephropathy due to Autosomal Dominant Polycystic Kidney Disease) studies] [126–129]. Monitoring plasma clearance is far less cumbersome and costly, especially in older adults or diabetic patients with bladder dysfunction and young children for whom urine collection remains challenging [130, 131]. Once again, these limitations are common in all GFR markers, including the gold standard inulin. Bladder catheterization has been used in small studies but this approach is obviously not feasible in clinical trials or clinical practice [132].
There may be one potential limitation of urinary clearance that applies specifically to iohexol, as some authors have actually hypothesized that measurement of iohexol in urine could be problematic (matrix effect). This matrix effect remains however purely speculative and other authors do not have any problem in quantification of iohexol in urine (F.G., personal data). This point is thus the subject of debate and would need further studies [118].
Plasma clearance: ok but which one?
Because of its physical characteristics (viscosity and density), iohexol is particularly suitable for plasma clearance measurement. After a bolus injection, plasma iohexol concentration will decrease constantly according to two different exponential curves (see Figure 1). The first rapid curve (fast component) corresponds to the volume of distribution, i.e. the extracellular volume [22–26]. The second curve (slow component) corresponds to the clearance of the marker by the kidney. There are several different methodologies to calculate GFR from the disappearance curves. The most precise method is to measure iohexol in both components, and to calculate the area under the curve which eventually allows GFR calculation [which corresponds to the dose of iohexol injected divided by the area under the curve (AUC)]. This method, named AUC in the current article, implies sampling of several plasma measurements at different time points. Iohexol should be sampled at very short intervals at the beginning (at 5, 10, 20, 30, 45, 60, 90 and 120 min after injection [52]), and thereafter every hour. This methodology is complex and costly, especially with necessary repeated samples during the first 2 h. For this reason, authors have proposed a mathematical correction for the first fast exponential curve. This method is frequently named as the slope-intercept method. Different mathematical corrections have been proposed [131, 133, 134, 135]. The impact of these different corrections on GFR results is probably relatively limited. The correction proposed by Brochner-Mortensen (BM) [116], initially developed for 51Cr-EDTA, is the most used in Europe:
Fig. 1.
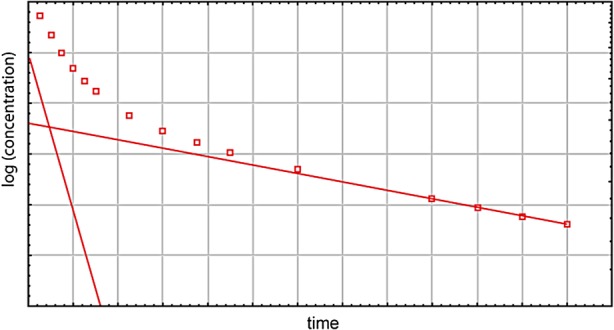
Elimination of iohexol from plasma after a single injection. Following a single injection, iohexol concentration in plasma falls as a result of both distribution and elimination. In a semi-logarithmic plot these phases can be illustrated by two lines, the slopes of which are proportional to the half-life of each phase.
BM correction= 0.990778 × C2 − 0.001218 × C22 (where C2 is the GFR calculated on the second curve only) [116]. The correction proposed by Ng et al. both for adults and children [131] has still been poorly externally validated but has been developed from a state of the art methodology and deserves further study [118, 131].
The most important limitation of the mathematical correction of the first curve area is the trend to underestimate high GFR levels (which could lead to underdiagnosis of renal hyperfiltration) [136, 137].
Different protocols have been proposed to calculate the area under the second curve (the curve that impacts the GFR result). The number and, even more important, the timing of samples are fundamental. A simple procedure would be to measure iohexol at two or three different time points. Restricting the GFR measurement to two points might theoretically expose to a greater risk of errors (if the first sample is drawn too early or the last sample not late enough). However, most studies with this protocol show that this easy-to-use procedure gives accurate and concordant results with urinary clearances or multiple-samples plasma clearances [52, 135]. When using simplified protocols, it is recommended to perform the first sample not earlier than 2 h after injection. The choice of the timing for the last sample depends on expected GFR levels [48, 52, 130]. For example, in patients with normal GFR values, the last measurement can be sampled 4 h after the injection because later samples would lead to the risk of complete clearance of iohexol. In CKD patients, the precision of the GFR calculation will be higher if iohexol is sampled later, i.e. after 5, 6 or 8 h or even after 24 h in pre-dialysis patients. Supplementary data, Table S2 summarizes studies on the agreement between different plasma clearance procedures.
Eventually, an even more simplified procedure with only one sample of iohexol can be used to calculate GFR. Different mathematical procedures have been proposed for the calculation of this single-sample method [92, 110, 114, 138–148]. The equation proposed by Jacobsson is certainly the most popular in Europe [110], and, for some investigators, the most precise method [113, 114].
GFR = (1/(t/V + 0.0016)) × ln (Dose/(V × C1) (in mL/min))
Vmale: 166 × W + 2490
Vfemale : 95 × W + 6170 [110]
Ct is the sample concentration (µg/mL) at time t (minute), V is the apparent volume of distribution (mL) and W is the weight (kg).
Determination of iohexol by the single-sample method obviously has great practical advantages. Again, the timing of the single-sample is important as it impacts the precision of the GFR calculation. As previously described, the best results are obtained when the timing of the sample is adapted to the expected GFR (based on the subjects estimated GFR), which is however sometimes difficult in very specific patients like hyperfiltrating subjects. An optimal sampling time to minimize the uncertainty in the estimate of the volume of distribution can be calculated from a formula derived by Jacobsson [110]. The sample should be late if GFR is low (300–360 min if GFR between 30 and 60 mL/min and 600 or 1440 min if GFR is below 30 mL/min) and early (180 min) if GFR is normal or high [105, 115, 149, 150]. Since weight is part of the equation, the Jacobsson correction might be questionable in subjects with extreme weights. Indeed, the estimation of extracellular volume is one predominant source of error with the single-sample method. Also, the single-sample method requires a very high precision of iohexol determination in plasma, which is why some authors have suggested that HPLC-UV be favoured over XRF with this procedure [69, 150]. In all plasma procedures, potential random analytical, blood drawing or timing errors can sometimes occur, but if there is any error, potentially outlier points can be easily identified, observing the declining curve built from multiple-samples results. Outlier points can thus be discarded (or measurement be repeated). This sort of internal control does not exist with the single-sample method. In Supplementary data, Table S3, we have summarized the available data for single-sample iohexol plasma clearance [24, 33, 69, 92, 98–101, 103, 105, 109, 114, 115, 149–154]. In general, the method demonstrated good agreement with either urinary inulin or plasma 51Cr-EDTA clearance. No study has appropriately compared the performance of single- and multiple-sample methods with inulin urinary clearance as the gold standard reference. Bird et al. found the performance of the single-sample to be at least as good as that of the multiple-sample method for normal range GFR when compared to multiple-sample 51Cr-EDTA clearance [115]. Several authors have compared concordance between single-sample and multiple-sample procedures with iohexol. Strictly speaking, such studies do not allow the performance of the two procedures to be tested, as the methods are not independent of each other, but the results of concordance between the two procedures are still of interest. Among these studies, the publication of Gaspari et al. is certainly the most relevant, including 686 patients. These authors found a slight overestimation of 3.2 mL/min/1.73 m2 of the single-sample method for GFR over 100 mL/min/1.73 m2 when comparing it with the multiple-sample method, but after logarithmic transformation the difference was the same as when using the optimal sampling time for GFR between 40 and 49 mL/min/1.73 m2 [150]. However, if bias is acceptable, concordance is not always perfect as 75% of results are concordant, concordance being defined as results within 5%. Another definition of concordance (within 10%) would lead to still more acceptable concordance, especially, once again, if the sample timing is adapted to the expected GFR.
Single-sample plasma clearance could also be used to measure residual renal function in haemodialysis patients (iohexol is injected at the end of one dialysis session and a plasma sample is captured at the start of the next treatment) [35].
Once again, all these considerations about timing and number of samples are not iohexol-specific, but also apply to other GFR markers, such as 51Cr-EDTA or iothalamate [117, 142, 143, 155–167].
A pragmatic proposal
The choice of the procedure (urinary or plasma clearance, timing and number of plasma samples) will depend on human and financial resources and specific centre expertise/experience. The choice of the procedure may depend on the reason (or the context) of why GFR is being measured. The single-sample plasma clearance technique is probably the more cost-effective procedure for population studies and renal function monitoring in large cohorts of patients. If feasible, the multiple-sample plasma clearance method is probably the most effective approach to monitor intra-patient GFR changes over time in the context of clinical trials and every-day clinical practice in individual patients. In Table 4, we make some suggestions for the choice of procedures according to the context of GFR measurement. This table represents a consensus based on the opinion of the authors of the current article.
Table 4.
Available procedures to perform iohexol clearance
| Methodology | Indication in clinical practice | Indication in clinical research | Bibliographic examples where the procedure is described into details |
|---|---|---|---|
| Urinary clearance | Increased extracellular volume (oedema, ascites, intensive care units, etc.) | Basic (physiologic) studies Specific populations (cirrhotic, intensive care, nephrotic syndrome, oedema, etc.) |
[36, 77, 125, 170] |
| Plasma clearance | |||
| Multiple samples (first or fast, second or slow exponential curves and calculation of area under the curve) | High GFR values (‘hyperfiltrating’) subjects | Development of equations to estimate GFR Studies in hyperfiltrating patients |
[52, 93, 171] |
| Multiple samples only for second and slow component (2 h after injection, 4 samples over 5 or 6 h, 1 sample/h) + BM correction | High precision determination (see text) | Development of equations to estimate GFR Clinical research with GFR as main endpoint |
[126, 172] |
| Idem + late sample (8 h or 24 h) | Pre-dialysis subjects | Research in pre-dialysis subjects | [52, 77] |
| Simplified two or three sample method (2 samples: first at 2 or 3 h and second at 4 or 5 h) + BM correction | CKD or healthy population | Development of equations to estimate GFR Clinical research with GFR as a secondary endpoint |
[69, 116] |
| Simplified single-sample method + Jacobsson correction [110] |
CKD or healthy population | Development of equations to estimate GFR Clinical research with GFR as a secondary endpoint Epidemiological research |
[14, 173] |
Suggestions (expert opinion-based) according to the clinical or experimental context.
GFR, glomerular filtration rate; CKD, chronic kidney disease; BM, Brochner-Mortensen correction [116].
Any of these methods, including the simplest procedure, can be considered as a good GFR measurement and may be considered a better reflection of ‘true’ GFR than any equation based on serum creatinine and/or cystatin C, as these biomarkers are influenced by non-GFR determinants.
The fact: we need standardization for measuring GFR
The fact that numerous different markers exist to measure GFR and that, for each marker, different protocols have been described, explains at least in part, why measured GFR has been questioned [174, 175]. Standardization is a necessary step in promoting GFR measurement. However, standardization is not easy and can be lengthy. For example, it took years to standardize the measurement of a simple biological parameter like serum creatinine [176]. For measured GFR, we need standardization at three different levels. First, the measurement of the markers in plasma must be standardized. As already mentioned, this is actually not the case for markers like inulin and iothalamate. Today, measurement of iohexol by HPLC-UV is the most popular and probably the best balance between sensitivity-specificity on one side and accessibility-cost on the other. In the analytical context, the presence of a proficiency programme (external quality control) and the stability of iohexol are two key advantages for this marker. Second, we need standardization regarding the marker used. We do not question the accuracy of isotopic methods to measure GFR, but, by nature, these methods are difficult to implement wherefore they remain limited to nuclear medicine units. Iothalamate clearance overestimates real GFR because of tubular secretion. Another major issue with iothalamate is its lack of availability, notably in Europe. Inulin is expensive and can only be used with urinary protocols. Iohexol plasma clearance seems to be the best choice considering availability, cost, safety and feasibility. Third, the procedure to measure GFR should be standardized and we have made some pragmatic propositions earlier in the text (Table 4).
Conclusions
‘Measuring GFR is cumbersome and costly’ is a sentence that has been written by numerous authors, including some of us, to justify the use of estimated GFR. It would be counterproductive to assert that measuring GFR is as simple as measuring creatinine or cystatin C. However, measured GFR is a reference method. Therefore, it should be compared to other reference methods in medicine. Troponin is a key biological parameter for cardiologists and we can compare it to serum creatinine for nephrologists. The sensitivity of troponin to detect myocardial infarction but also to predict mortality has however not questioned the relevance of coronary angiography as the reference method. The comparison might be continued, notably regarding the terms ‘cumbersome’ and ‘costly’. As we described earlier, measuring GFR by iohexol plasma clearance can be simplified, while keeping a high degree of precision. Iohexol is available worldwide and provided under the commercial name Omnipaque® or Accupaque® (240 or 300 mg I/mL) in Europe. Different bottles of iohexol exist and the price of one bottle of 20 cm3 (to be used for two or four patients, if 5 or 10 cm3 are injected) is around 10 Euros in Belgium. Including the price of the HPLC measurement, and depending on the number of samples, the global procedure will cost between 100 and 200 Euros per patient. This cost is comparable to the cost of other procedures in medicine like any CT scan. The safety of iohexol protocols is also remarkably superior to that of other reference methods in medicine such as colonoscopy or coronary angiography. According to the characteristics of iohexol, we think that iohexol plasma clearance is clearly the best alternative to have the same, standardized method for GFR measurement that would be easy to implement worldwide.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1.Smith HW. The Kidney: Structure and Function in Health and Disease. New York: Oxford University Press Inc, 1951 [Google Scholar]
- 2.Shannon JA, Smith HW. The excretion of inulin, xylose, and urea by normal and phorizinized man. J Clin Invest 1935; 14: 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soveri I, Berg UB, Björk J et al. Measuring GFR: a systematic review. Am J Kidney Dis 2014; 64: 411–424 [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Bills JE, Yigazu PM et al. Assessment of iothalamate plasma clearance: duration of study affects quality of GFR. Clin J Am Soc Nephrol 2009; 4: 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brochner-Mortensen J, Christoffersen J. Single injection (51Cr)EDTA plasma clearance determination in children using capillary blood samples. Scand J Clin Lab Invest 1977; 37: 631–633 [DOI] [PubMed] [Google Scholar]
- 6.Gaspari F, Perico N, Matalone M et al. Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol 1998; 9: 310–313 [DOI] [PubMed] [Google Scholar]
- 7.Delanaye P, Pottel H, Botev R. Con: Should we abandon the use of the MDRD equation in favour of the CKD-EPI equation? Nephrol Dial Transplant 2013; 28: 1396–1403 [DOI] [PubMed] [Google Scholar]
- 8.Delanaye P, Mariat C. The applicability of eGFR equations to different populations. Nat Rev Nephrol 2013; 9: 513–522 [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009; 20: 2305–2313 [DOI] [PubMed] [Google Scholar]
- 10.Schei J, Stefansson VTN, Mathisen UD et al. Residual associations of inflammatory markers with eGFR after accounting for measured GFR in a community-based cohort without CKD. Clin J Am Soc Nephrol 2016; 11: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melsom T, Fuskevåg OM, Mathisen UD et al. Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol 2015; 41: 7–15 [DOI] [PubMed] [Google Scholar]
- 12.Stevens LA, Schmid CH, Greene T et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009; 75: 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaspari F, Ruggenenti P, Porrini E et al. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 2013; 84: 164–173 [DOI] [PubMed] [Google Scholar]
- 14.Eriksen BO, Mathisen UD, Melsom T et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 2010; 78: 1305–1311 [DOI] [PubMed] [Google Scholar]
- 15.Delanaye P, Nellessen E, Grosch S et al. Creatinine-based formulae for the estimation of glomerular filtration rate in heart transplant recipients. Clin Transplant 2006; 20: 596–603 [DOI] [PubMed] [Google Scholar]
- 16.Masson I, Flamant M, Maillard N et al. MDRD versus CKD-EPI equation to estimate glomerular filtration rate in kidney transplant recipients. Transplantation 2013; 95: 1211–1217 [DOI] [PubMed] [Google Scholar]
- 17.Luis-Lima S, Marrero-Miranda D, González-Rinne A et al. Estimated glomerular filtration rate in renal transplantation: the nephrologist in the mist. Transplantation 2015; 99: 2625–2633 [DOI] [PubMed] [Google Scholar]
- 18.Beben T, Rifkin DE. GFR estimating equations and liver disease. Adv Chronic Kidney Dis 2015; 22: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almén T. Experimental investigations with iohexol and their clinical relevance. Acta Radiol Suppl 1983; 366: 9–19 [PubMed] [Google Scholar]
- 20.Almén T. Development of nonionic contrast media. Invest Radiol 1985; 20: S2–S9 [DOI] [PubMed] [Google Scholar]
- 21.Aakhus T, Sommerfelt SC, Stormorken H et al. Tolerance and excretion of iohexol after intravenous injection in healthy volunteers. Preliminary report. Acta Radiol Suppl 1980; 362: 131–134 [PubMed] [Google Scholar]
- 22.Olsson B, Aulie A, Sveen K et al. Human pharmacokinetics of iohexol. A new nonionic contrast medium. Invest Radiol 1983; 18: 177–182 [DOI] [PubMed] [Google Scholar]
- 23.Friedman AN, Strother M, Quinney SK et al. Measuring the glomerular filtration rate in obese individuals without overt kidney disease. Nephron Clin Pract 2010; 116: c224–c234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nossen JO, Jakobsen JA, Kjaersgaard P et al. Elimination of the non-ionic X-ray contrast media iodixanol and iohexol in patients with severely impaired renal function. Scand J Clin Lab Invest 1995; 55: 341–350 [DOI] [PubMed] [Google Scholar]
- 25.Edelson J, Shaw D, Palace G. Pharmacokinetics of iohexol, a new nonionic radiocontrast agent, in humans. J Pharm Sci 1984; 73: 993–995 [DOI] [PubMed] [Google Scholar]
- 26.Bäck SE, Krutzen E, Nilsson-Ehle P. Contrast media as markers for glomerular filtration: a pharmacokinetic comparison of four agents. Scand J Clin Lab Invest 1988; 48: 247–253 [DOI] [PubMed] [Google Scholar]
- 27.Olofsson P, Krutzen E, Nilsson-Ehle P. Iohexol clearance for assessment of glomerular filtration rate in diabetic pregnancy. Eur J Obstet Gynecol Reprod Biol 1996; 64: 63–67 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz GJ, Furth S, Cole SR et al. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 2006; 69: 2070–2077 [DOI] [PubMed] [Google Scholar]
- 29.Mutzel W, Siefert HM, Speck U. Biochemical-pharmacologic properties of iohexol. Acta Radiol Suppl 1980; 362: 111–115 [PubMed] [Google Scholar]
- 30.Krutzen E, Bäck SE, Nilsson-Ehle I et al. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med 1984; 104: 955–961 [PubMed] [Google Scholar]
- 31.Skinnemoen K. Physicochemical properties and degree of protein binding of iopentol. Acta Radiol Suppl 1987; 370: 33–36 [PubMed] [Google Scholar]
- 32.Seegmiller JC, Burns BE, Schinstock CA et al. Discordance between iothalamate and iohexol urinary clearances. Am J Kidney Dis 2016; 67: 49–55 [DOI] [PubMed] [Google Scholar]
- 33.Frennby B, Sterner G, Almen T et al. Determination of low glomerular filtration rate using iohexol clearance. Invest Radiol 1994; 29 (Suppl 2): S234–S235 [DOI] [PubMed] [Google Scholar]
- 34.Swan SK, Halstenson CE, Kasiske BL et al. Determination of residual renal function with iohexol clearance in hemodialysis patients. Kidney Int 1996; 49: 232–235 [DOI] [PubMed] [Google Scholar]
- 35.Sterner G, Frennby B, Månsson S et al. Assessing residual renal function and efficiency of hemodialysis—an application for urographic contrast media. Nephron 2000; 85: 324–333 [DOI] [PubMed] [Google Scholar]
- 36.Arvidsson A, Hedman A. Plasma and renal clearance of iohexol—a study on the reproducibility of a method for the glomerular filtration rate. Scand J Clin Lab Invest 1990; 50: 757–761 [DOI] [PubMed] [Google Scholar]
- 37.Cangiano JL, Genuth SM, Renerts L et al. Simplified measurement of glomerular filtration rate. Invest Urol 1971; 9: 34–38 [PubMed] [Google Scholar]
- 38.Evans JR, Cutler RE, Forland SC. Pharmacokinetics of iothalamate in endstage renal disease. J Clin Pharmacol 1988; 28: 826–830 [DOI] [PubMed] [Google Scholar]
- 39.Dowling TC, Frye RF, Fraley DS et al. Comparison of iothalamate clearance methods for measuring GFR. Pharmacotherapy 1999; 19: 943–950 [DOI] [PubMed] [Google Scholar]
- 40.Jagenburg R, Attman PO, Aurell M et al. Determination of glomerular filtration rate in advanced renal insufficiency. Scand J Urol Nephrol 1978; 12: 133–137 [DOI] [PubMed] [Google Scholar]
- 41.Brochner-Mortensen J, Rodbro P. Comparison between total and renal plasma clearance of [51Cr] EDTA. Scand J Clin Lab Invest 1976; 36: 247–249 [PubMed] [Google Scholar]
- 42.Odlind B, Hallgren R, Sohtell M et al. Is 125I iothalamate an ideal marker for glomerular filtration? Kidney Int 1985; 27: 9–16 [DOI] [PubMed] [Google Scholar]
- 43.Zurth C. Mechanism of renal excretion of various X-ray contrast materials in Rabbits. Invest Radiol 1983; 19: 110–115. [DOI] [PubMed] [Google Scholar]
- 44.Heron CW, Underwood SR, Dawson P. Electrocardiographic changes during intravenous urography: a study with sodium iothalamate and iohexol. Clin Radiol 1984; 35: 137–141 [DOI] [PubMed] [Google Scholar]
- 45.Aurell M. Accurate and feasible measurements of GFR—is the iohexol clearance the answer? Nephrol Dial Transplant 1994; 9: 1222–1224 [PubMed] [Google Scholar]
- 46.Nilsson-Ehle P, Grubb A. New markers for the determination of GFR: iohexol clearance and cystatin C serum concentration. Kidney Int Suppl 1994; 47: S17–S19 [PubMed] [Google Scholar]
- 47.Nilsson-Ehle P. Iohexol clearance for the determination of glomerular filtration rate: 15 years’ experience in clinical practice. eJIFCC 2002; 13: 1–5. [PMC free article] [PubMed] [Google Scholar]
- 48.Frennby B, Sterner G, Almen T et al. The use of iohexol clearance to determine GFR in patients with severe chronic renal failure—a comparison between different clearance techniques. Clin Nephrol 1995; 43: 35–46 [PubMed] [Google Scholar]
- 49.Shihabi ZK, Constantinescu MS. Iohexol in serum determined by capillary electrophoresis. Clin Chem 1992; 38: 2117–2120 [PubMed] [Google Scholar]
- 50.Rocco MV, Buckalew VM Jr, Moore LC et al. Capillary electrophoresis for the determination of glomerular filtration rate using nonradioactive iohexol. Am J Kidney Dis 1996; 28: 173–177 [DOI] [PubMed] [Google Scholar]
- 51.Cavalier E, Rozet E, Dubois N et al. Performance of iohexol determination in serum and urine by HPLC: validation, risk and uncertainty assessment. Clin Chim Acta 2008; 396: 80–85 [DOI] [PubMed] [Google Scholar]
- 52.Gaspari F, Perico N, Ruggenenti P et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 1995; 6: 257–263 [DOI] [PubMed] [Google Scholar]
- 53.Soman RS, Zahir H, Akhlaghi F. Development and validation of an HPLC-UV method for determination of iohexol in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 816: 339–343 [DOI] [PubMed] [Google Scholar]
- 54.Farthing D, Sica DA, Fakhry I et al. Simple HPLC-UV method for determination of iohexol, iothalamate, p-aminohippuric acid and n-acetyl-p-aminohippuric acid in human plasma and urine with ERPF, GFR and ERPF/GFR ratio determination using colorimetric analysis. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 826: 267–272 [DOI] [PubMed] [Google Scholar]
- 55.Castagnet S, Blasco H, Vourc'h P et al. Routine determination of GFR in renal transplant recipients by HPLC quantification of plasma iohexol concentrations and comparison with estimated GFR. J Clin Lab Anal 2012; 26: 376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luis-Lima S, Gaspari F, Porrini E et al. Measurement of glomerular filtration rate: internal and external validations of the iohexol plasma clearance technique by HPLC. Clin Chim Acta 2014; 430: 84–85 [DOI] [PubMed] [Google Scholar]
- 57.Portal AJ, McPhail MJ, Bruce M et al. Neutrophil gelatinase—associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transpl 2010; 16: 1257–1266 [DOI] [PubMed] [Google Scholar]
- 58.Slack A, Tredger M, Brown N et al. Application of an isocratic methanol-based HPLC method for the determination of iohexol concentrations and glomerular filtration rate in patients with cirrhosis. Ann Clin Biochem 2014; 51: 80–88 [DOI] [PubMed] [Google Scholar]
- 59.Bäck SE, Masson P, Nilsson-Ehle P. A simple chemical method for the quantification of the contrast agent iohexol, applicable to glomerular filtration rate measurements. Scand J Clin Lab Invest 1988; 48: 825–829 [DOI] [PubMed] [Google Scholar]
- 60.Krutzen E, Bäck SE, Nilsson-Ehle P. Determination of glomerular filtration rate using iohexol clearance and capillary sampling. Scand J Clin Lab Invest 1990; 50: 279–283 [DOI] [PubMed] [Google Scholar]
- 61.Niculescu-Duvaz I, D'Mello L, Maan Z et al. Development of an outpatient finger-prick glomerular filtration rate procedure suitable for epidemiological studies. Kidney Int 2006; 69: 1272–1275 [DOI] [PubMed] [Google Scholar]
- 62.Mafham MM, Niculescu-Duvaz I, Barron J et al. A practical method of measuring glomerular filtration rate by iohexol clearance using dried capillary blood spots. Nephron Clin Pract 2007; 106: c104–c112 [DOI] [PubMed] [Google Scholar]
- 63.Maahs DM, Bushman L, Kerr B et al. A practical method to measure GFR in people with type 1 diabetes. J Diabetes Complications 2014; 28: 667–673 [DOI] [PubMed] [Google Scholar]
- 64.Salvador CL, Tøndel C, Mørkrid L et al. Glomerular filtration rate measured by iohexol clearance: a comparison of venous samples and capillary blood spots. Scand J Clin Lab Invest 2015; 75: 710–716 [DOI] [PubMed] [Google Scholar]
- 65.Hingorani S, Pao E, Schoch G et al. Estimating GFR in adult patients with hematopoietic cell transplant: comparison of estimating equations with an iohexol reference standard. Clin J Am Soc Nephrol 2015; 10: 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Reilly PH, Jones DA, Farah NB. Measurement of the plasma clearance of urographic contrast media for the determination of glomerular filtration rate. J Urol 1988; 139: 9–11 [DOI] [PubMed] [Google Scholar]
- 67.Bäck SE, Nilsson-Ehle P. Re: Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol 1993; 149: 378. [DOI] [PubMed] [Google Scholar]
- 68.Effersöe H, Rosenkilde P, Groth S et al. Measurement of renal function with iohexol. A comparison of iohexol, 99mTc-DTPA, and 51Cr-EDTA clearance. Invest Radiol 1990; 25: 778–782 [DOI] [PubMed] [Google Scholar]
- 69.Brandstrom E, Grzegorczyk A, Jacobsson L et al. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant 1998; 13: 1176–1182 [DOI] [PubMed] [Google Scholar]
- 70.Houlihan C, Jenkins M, Osicka T et al. A comparison of the plasma disappearance of iohexol and 99mTc-DTPA for the measurement of glomerular filtration rate (GFR) in diabetes. Aust N Z J Med 1999; 29: 693–700 [DOI] [PubMed] [Google Scholar]
- 71.Bird NJ, Peters C, Michell AR et al. Reproducibilities and responses to food intake of GFR measured with chromium-51-EDTA and iohexol simultaneously and independently in normal subjects. Nephrol Dial Transplant 2008; 23: 1902–1909 [DOI] [PubMed] [Google Scholar]
- 72.Pucci L, Bandinelli S, Penno G et al. Iohexol plasma clearance in determining glomerular filtration rate in diabetic patients. Ren Fail 1998; 20: 277–284 [DOI] [PubMed] [Google Scholar]
- 73.Delanaye P, Cavalier E, Froissart M et al. Reproducibility of GFR measured by chromium-51-EDTA and iohexol. Nephrol Dial Transplant 2008; 23: 4077–4078 [DOI] [PubMed] [Google Scholar]
- 74.Lee SY, Chun MR, Kim DJ et al. Determination of iohexol clearance by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). J Chromatogr B Analyt Technol Biomed Life Sci 2006; 839: 124–129 [DOI] [PubMed] [Google Scholar]
- 75.Annesley TM, Clayton LT. Ultraperformance liquid chromatography-tandem mass spectrometry assay for iohexol in human serum. Clin Chem 2009; 55: 1196–1202 [DOI] [PubMed] [Google Scholar]
- 76.Denis MC, Venne K, Lesiege D et al. Development and evaluation of a liquid chromatography-mass spectrometry assay and its application for the assessment of renal function. J Chromatogr A 2008; 1189: 410–416 [DOI] [PubMed] [Google Scholar]
- 77.Stolz A, Hoizey G, Toupance O et al. Evaluation of sample bias for measuring plasma iohexol clearance in kidney transplantation. Transplantation 2010; 89: 440–445 [DOI] [PubMed] [Google Scholar]
- 78.Braselton WE, Stuart KJ, Kruger JM. Measurement of serum iohexol by determination of iodine with inductively coupled plasma-atomic emission spectroscopy. Clin Chem 1997; 43: 1429–1435 [PubMed] [Google Scholar]
- 79.Vicente FB, Vespa G, Miller A et al. Quantification of iohexol in serum by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS). Methods Mol Biol 2016; 1383: 185–193 [DOI] [PubMed] [Google Scholar]
- 80.Delanaye P, Thibaudin L, Souvignet M et al. Comparison of acid and enzymatic methods for inulin dosage: analytical performances and impact on glomerular filtration rate evaluation. Clin Chim Acta 2012; 413: 556–560 [DOI] [PubMed] [Google Scholar]
- 81.Seegmiller JC, Burns BE, Fauq AH et al. Iothalamate quantification by tandem mass spectrometry to measure glomerular filtration rate. Clin Chem 2010; 56: 568–574 [DOI] [PubMed] [Google Scholar]
- 82.Delanaye P, Jouret F, Le Goff C et al. Concordance between iothalamate and iohexol plasma clearance. Am J Kidney Dis 2016; Feb 2. pii: S0272-6386(16)00021-4 2016; 68:329–330 [DOI] [PubMed] [Google Scholar]
- 83.Vicente FB, Vespa GK, Carrara F et al. Determination of iohexol in human serum by a semi-automated liquid chromatography tandem mass spectrometry method. Clin Biochem 2015; 48: 679–685 [DOI] [PubMed] [Google Scholar]
- 84.O'Reilly PH, Brooman PJ, Martin PJ et al. Accuracy and reproducibility of a new contrast clearance method for the determination of glomerular filtration rate. Br Med J (Clin Res Ed) 1986; 293: 234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Acker BA, Koomen GC, Koopman MG et al. Discrepancy between circadian rhythms of inulin and creatinine clearance. J Lab Clin Med 1992; 120: 400–410 [PubMed] [Google Scholar]
- 86.Wilkinson J, Fleming JS, Waller DG. Effect of food and activity on the reproducibility of isotopic GFR estimation. Nucl Med Commun 1990; 11: 697–700 [DOI] [PubMed] [Google Scholar]
- 87.Koopman MG, Koomen GC, Krediet RT et al. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 1989; 77: 105–111 [DOI] [PubMed] [Google Scholar]
- 88.Perrone RD, Steinman TI, Beck GJ et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease Study. Am J Kidney Dis 1990; 16: 224–235 [DOI] [PubMed] [Google Scholar]
- 89.Brochner-Mortensen J, Rodbro P. Selection of routine method for determination of glomerular filtration rate in adult patients. Scand J Clin Lab Invest 1976; 36: 35–43 [DOI] [PubMed] [Google Scholar]
- 90.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 1950; 29: 496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brauner L, Westling H. On the necessity of strict bed rest during determination of 51Cr-EDTA clearance. Clin Physiol Funct Imaging 1981; 1: 175–180 [Google Scholar]
- 92.Rydström M, Tengstrom B, Cederquist I et al. Measurement of glomerular filtration rate by single-injection, single-sample techniques, using 51Cr-EDTA or iohexol. Scand J Urol Nephrol 1995; 29: 135–139 [DOI] [PubMed] [Google Scholar]
- 93.Pucci L, Bandinelli S, Pilo M et al. Iohexol as a marker of glomerular filtration rate in patients with diabetes: comparison of multiple and simplified sampling protocols. Diabet Med 2001; 18: 116–120 [DOI] [PubMed] [Google Scholar]
- 94.Agarwal R. Ambulatory GFR measurement with cold iothalamate in adults with chronic kidney disease. Am J Kidney Dis 2003; 41: 752–759 [DOI] [PubMed] [Google Scholar]
- 95.Mariat C, Alamartine E, Barthelemy JC et al. Assessing renal graft function in clinical trials: can tests predicting glomerular filtration rate substitute for a reference method? Kidney Int 2004; 65: 289–297 [DOI] [PubMed] [Google Scholar]
- 96.Froissart M, Rossert J, Jacquot C et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 2005; 16: 763–773 [DOI] [PubMed] [Google Scholar]
- 97.Ibrahim HN, Foley R, Tan L et al. Long-term consequences of kidney donation. N Engl J Med 2009; 360: 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.James TJ, Lewis AV, Tan GD et al. Validity of simplified protocols to estimate glomerular filtration rate using iohexol clearance. Ann Clin Biochem 2007; 44: 369–376 [DOI] [PubMed] [Google Scholar]
- 99.Eriksen BO, Stefansson VT, Jenssen TG et al. Elevated blood pressure is not associated with accelerated glomerular filtration rate decline in the general non-diabetic middle-aged population. Kidney Int 2016; 90: 404–410 [DOI] [PubMed] [Google Scholar]
- 100.Brown SC, O'Reilly PH. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol 1991; 146: 675–679 [DOI] [PubMed] [Google Scholar]
- 101.Sterner G, Frennby B, Mansson S et al. Determining ‘true’ glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol 2008; 42: 278–285 [DOI] [PubMed] [Google Scholar]
- 102.Lewis R, Kerr N, Van Buren C et al. Comparative evaluation of urographic contrast media, inulin, and 99mTc-DTPA clearance methods for determination of glomerular filtration rate in clinical transplantation. Transplantation 1989; 48: 790–796 [DOI] [PubMed] [Google Scholar]
- 103.Lindblad HG, Berg UB. Comparative evaluation of iohexol and inulin clearance for glomerular filtration rate determinations. Acta Paediatr 1994; 83: 418–422 [DOI] [PubMed] [Google Scholar]
- 104.Erley CM, Bader BD, Berger ED et al. Plasma clearance of iodine contrast media as a measure of glomerular filtration rate in critically ill patients. Crit Care Med 2001; 29: 1544–1550 [DOI] [PubMed] [Google Scholar]
- 105.Berg UB, Bäck R, Celsi G et al. Comparison of plasma clearance of iohexol and urinary clearance of inulin for measurement of GFR in children. Am J Kidney Dis 2011; 57: 55–61 [DOI] [PubMed] [Google Scholar]
- 106.Brown SC, O'Reilly PH. The estimate of glomerular filtration rate during urography. Acceptability of a nonionic contrast medium as a marker of renal function. Invest Radiol 1992; 27: 774–778 [DOI] [PubMed] [Google Scholar]
- 107.Skluzacek PA, Szewc RG, Nolan CR III et al. Prediction of GFR in liver transplant candidates. Am J Kidney Dis 2003; 42: 1169–1176 [DOI] [PubMed] [Google Scholar]
- 108.Bäck SE, Krutzen E, Nilsson-Ehle P. Contrast media and glomerular filtration: dose dependence of clearance for three agents. J Pharm Sci 1988; 77: 765–767 [DOI] [PubMed] [Google Scholar]
- 109.Eriksson CG, Kallner A. Glomerular filtration rate: a comparison between Cr-EDTA clearance and a single sample technique with a non-ionic contrast agent. Clin Biochem 1991; 24: 261–264 [DOI] [PubMed] [Google Scholar]
- 110.Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 1983; 3: 297–305 [DOI] [PubMed] [Google Scholar]
- 111.Stake G, Monn E, Rootwelt K et al. The clearance of iohexol as a measure of the glomerular filtration rate in children with chronic renal failure. Scand J Clin Lab Invest 1991; 51: 729–734 [DOI] [PubMed] [Google Scholar]
- 112.Rootwelt K, Falch D, Sjokvist R. Determination of glomerular filtration rate (GFR) by analysis of capillary blood after single shot injection of 99mTc-DTPA. A comparison with simultaneous 125I-iothalamate GFR estimation showing equal GFR but difference in distribution volume. Eur J Nucl Med 1980; 5: 97–102 [DOI] [PubMed] [Google Scholar]
- 113.Lundqvist S, Hietala SO, Berglund C et al. Simultaneous urography and determination of glomerular filtration rate. A comparison of total plasma clearances of iohexol and 51Cr-EDTA in plegic patients. Acta Radiol 1994; 35: 391–395 [PubMed] [Google Scholar]
- 114.Lundqvist S, Hietala SO, Groth S et al. Evaluation of single sample clearance calculations in 902 patients. A comparison of multiple and single sample techniques. Acta Radiol 1997; 38: 68–72 [DOI] [PubMed] [Google Scholar]
- 115.Bird NJ, Peters C, Michell AR et al. Comparison of GFR measurements assessed from single versus multiple samples. Am J Kidney Dis 2009; 54: 278–288 [DOI] [PubMed] [Google Scholar]
- 116.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 1972; 30: 271–274 [DOI] [PubMed] [Google Scholar]
- 117.Medeiros FS, Sapienza MT, Prado ES et al. Validation of plasma clearance of 51Cr-EDTA in adult renal transplant recipients: comparison with inulin renal clearance. Transpl Int 2009; 22: 323–331 [DOI] [PubMed] [Google Scholar]
- 118.Shafi T, Levey AS, Inker LA et al. Plasma iohexol clearance for assessing residual kidney function in dialysis patients. Am J Kidney Dis 2015; 66: 728–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frennby B, Sterner G. Contrast media as markers of GFR. Eur Radiol 2002; 12: 475–484 [DOI] [PubMed] [Google Scholar]
- 120.Sirota JH, Baldwin DS, Villareal H. Diurnal variations of renal function in man. J Clin Invest 1950; 29: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brochner-Mortensen J, Rodbro P. Optimum time of blood sampling for determination of glomerular filtration rate by single-injection [51Cr]EDTA plasma clearance. Scand J Clin Lab Invest 1976; 36: 795–800 [DOI] [PubMed] [Google Scholar]
- 122.Gaspari F, Perico N, Remuzzi G. Application of newer clearance techniques for the determination of glomerular filtration rate. Curr Opin Nephrol Hypertens 1998; 7: 675–680 [DOI] [PubMed] [Google Scholar]
- 123.Sambataro M, Thomaseth K, Pacini G et al. Plasma clearance rate of 51Cr-EDTA provides a precise and convenient technique for measurement of glomerular filtration rate in diabetic humans. J Am Soc Nephrol 1996; 7: 118–127 [DOI] [PubMed] [Google Scholar]
- 124.Maahs DM, Jalal D, McFann K et al. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol 2011; 6: 1952–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Henriksen UL, Henriksen JH. The clearance concept with special reference to determination of glomerular filtration rate in patients with fluid retention. Clin Physiol Funct Imaging 2015; 35: 7–16 [DOI] [PubMed] [Google Scholar]
- 126.Caroli A, Perico N, Perna A et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet 2013; 382: 1485–1495 [DOI] [PubMed] [Google Scholar]
- 127.Ruggenenti P, Perna A, Loriga G et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365: 939–946 [DOI] [PubMed] [Google Scholar]
- 128.Ruggenenti P, Perna A, Gherardi G et al. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 1998; 352: 1252–1256 [DOI] [PubMed] [Google Scholar]
- 129.Ruggenenti P, Lauria G, Iliev IP et al. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension 2011; 58: 776–783 [DOI] [PubMed] [Google Scholar]
- 130.Ebert N, Loesment A, Martus P et al. Iohexol plasma clearance measurement in older adults with chronic kidney disease-sampling time matters. Nephrol Dial Transplant 2015; 30: 1307–1314 [DOI] [PubMed] [Google Scholar]
- 131.Ng DK, Schwartz GJ, Jacobson LP et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int 2011; 80: 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rahn KH, Heidenreich S, Brückner D. How to assess glomerular function and damage in humans. J Hypertens 1999; 17: 309–317 [DOI] [PubMed] [Google Scholar]
- 133.Chantler C, Garnett ES, Parsons V et al. Glomerular filtration rate measurement in man by the single injection methods using 51Cr-EDTA. Clin Sci 1969; 37: 169–180 [PubMed] [Google Scholar]
- 134.Jodal L, Brochner-Mortensen J. Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part I: Analytically correct relationship between total and one-pool clearance. Scand J Clin Lab Invest 2009; 69: 305–313 [DOI] [PubMed] [Google Scholar]
- 135.Schwartz GJ, Abraham AG, Furth SL et al. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int 2010; 77: 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Sadeleer C, Piepsz A, Ham HR. How good is the slope on the second exponential for estimating 51Cr-EDTA renal clearance? A Monte Carlo simulation. Nucl Med Commun 2000; 21: 455–458 [DOI] [PubMed] [Google Scholar]
- 137.Fleming JS. An improved equation for correcting slope-intercept measurements of glomerular filtration rate for the single exponential approximation. Nucl Med Commun 2007; 28: 315–320 [DOI] [PubMed] [Google Scholar]
- 138.Tauxe WN, Maher FT, Taylor WF. Effective renal plasma flow: estimation from theoretical volumes of distribution of intravenously injected 131-I orthoiodohippurate. Mayo Clin Proc 1971; 46: 524–531 [PubMed] [Google Scholar]
- 139.Groth S, Aasted M. 51Cr-EDTA clearance determined by one plasma sample. Clin Physiol 1981; 1: 417–425 [DOI] [PubMed] [Google Scholar]
- 140.Piepsz A, Ham R, De Sadeleer C. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun 2005; 26: 175–176 [DOI] [PubMed] [Google Scholar]
- 141.Fjeldborg P, Brochner-Mortensen J. Determination of 51Cr-EDTA clearance in infants by a single capillary blood sample. Scand J Clin Lab Invest 1986; 46: 335–340 [DOI] [PubMed] [Google Scholar]
- 142.Rehling M, Rabol A. Measurement of glomerular filtration rate in adults: accuracy of five single-sample plasma clearance methods. Clin Physiol 1989; 9: 171–182 [DOI] [PubMed] [Google Scholar]
- 143.Kamper AL, Nielsen SL. 51Cr-EDTA plasma clearance in severe renal failure determined by one plasma sample. Scand J Clin Lab Invest 1989; 49: 555–559 [DOI] [PubMed] [Google Scholar]
- 144.Groth S, Aasted M. Determination of 51Cr-EDTA clearance between 15 and 40 ml/min/1.73 m2 by a single plasma sample. Scand J Clin Lab Invest 1989; 49: 711–717 [DOI] [PubMed] [Google Scholar]
- 145.Martensson J, Groth S, Rehling M et al. Chromium-51-EDTA clearance in adults with a single-plasma sample. J Nucl Med 1998; 39: 2131–2137 [PubMed] [Google Scholar]
- 146.Russell CD, Bischoff PG, Kontzen FN et al. Measurement of glomerular filtration rate: single injection plasma clearance method without urine collection. J Nucl Med 1985; 26: 1243–1247 [PubMed] [Google Scholar]
- 147.Watson WS. A simple method of estimating glomerular filtration rate. Eur J Nucl Med 1992; 19: 827. [DOI] [PubMed] [Google Scholar]
- 148.Gref M, Karp K. GFR determination in adults with a single-sample iohexol plasma clearance method based on the mean sojourn time. Nephrol Dial Transplant 2007; 22: 3166–3173 [DOI] [PubMed] [Google Scholar]
- 149.Sterner G, Frennby B, Hultberg B et al. Iohexol clearance for GFR-determination in renal failure—single or multiple plasma sampling? Nephrol Dial Transplant 1996; 11: 521–525 [PubMed] [Google Scholar]
- 150.Gaspari F, Guerini E, Perico N et al. Glomerular filtration rate determined from a single plasma sample after intravenous iohexol injection: is it reliable? J Am Soc Nephrol 1996; 7: 2689–2693 [DOI] [PubMed] [Google Scholar]
- 151.Thomsen HS, Hvid-Jacobsen K. Estimation of glomerular filtration rate from low-dose injection of iohexol and a single blood sample. Invest Radiol 1991; 26: 332–336 [DOI] [PubMed] [Google Scholar]
- 152.Stake G, Monn E, Rootwelt K et al. A single plasma sample method for estimation of the glomerular filtration rate in infants and children using iohexol, II: establishment of the optimal plasma sampling time and a comparison with the 99Tcm-DTPA method. Scand J Clin Lab Invest 1991; 51: 343–348 [DOI] [PubMed] [Google Scholar]
- 153.Rocco MV, Buckalew VM Jr, Moore LC et al. Measurement of glomerular filtration rate using nonradioactive iohexol: comparison of two one-compartment models. Am J Nephrol 1996; 16: 138–143 [DOI] [PubMed] [Google Scholar]
- 154.Chowdhury TA, Dyer PH, Bartlett WA et al. Glomerular filtration rate determination in diabetic patients using iohexol clearance—comparison of single and multiple plasma sampling methods. Clin Chim Acta 1998; 277: 153–158 [DOI] [PubMed] [Google Scholar]
- 155.Tepe PG, Tauxe WN, Bagchi A et al. Comparison of measurement of glomerular filtration rate by single sample, plasma disappearance slope/intercept and other methods. Eur J Nucl Med 1987; 13: 28–31 [DOI] [PubMed] [Google Scholar]
- 156.Aydin F, Gungor F, Cengiz AK et al. Comparison of glomerular filtration rate measurements with the two plasma sample and single plasma sample, gamma camera Gates, creatinine clearance, and prediction equation methods in potential kidney donors with normal renal function. Nucl Med Commun 2008; 29: 157–165 [DOI] [PubMed] [Google Scholar]
- 157.Itoh K, Tsushima S, Tsukamoto E et al. Reappraisal of single-sample and gamma camera methods for determination of the glomerular filtration rate with 99mTc-DTPA. Ann Nucl Med 2000; 14: 143–150 [DOI] [PubMed] [Google Scholar]
- 158.Fawdry RM, Gruenewald SM, Collins LT et al. Comparative assessment of techniques for estimation of glomerular filtration rate with 99mTc-DTPA. Eur J Nucl Med 1985; 11: 7–12 [DOI] [PubMed] [Google Scholar]
- 159.Kjaergaard KD, Jensen JD, Jespersen B et al. Reliability of (5)(1)Cr-EDTA plasma and urinary clearance as a measure of residual renal function in dialysis patients. Scand J Clin Lab Invest 2011; 71: 663–669 [DOI] [PubMed] [Google Scholar]
- 160.Mulligan JS, Blue PW, Hasbargen JA. Methods for measuring GFR with technetium-99m-DTPA: an analysis of several common methods. J Nucl Med 1990; 31: 1211–1219 [PubMed] [Google Scholar]
- 161.Galli G, Rufini V, Vellante C et al. Estimation of glomerular filtration rate with 99Tc(m)-DTPA: a comparative assessment of simplified methods. Nucl Med Commun 1997; 18: 634–641 [DOI] [PubMed] [Google Scholar]
- 162.Fleming JS, Zivanovic MA, Blake GM et al. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun 2004; 25: 759–769 [DOI] [PubMed] [Google Scholar]
- 163.Picciotto G, Cacace G, Cesana P et al. Estimation of chromium-51 ethylene diamine tetra-acetic acid plasma clearance: a comparative assessment of simplified techniques. Eur J Nucl Med 1992; 19: 30–35 [DOI] [PubMed] [Google Scholar]
- 164.Galli G, Rufini V, Meduri G. A simplified determination of glomerular filtration rate with 99Tcm-DTPA. Nucl Med Commun 1994; 15: 831–835 [DOI] [PubMed] [Google Scholar]
- 165.Groth S, Aasted M, Vestergaard B. Screening of kidney function by plasma creatinine and single-sample 51Cr-EDTA clearance determination—a comparison. Scand J Clin Lab Invest 1989; 49: 707–710 [DOI] [PubMed] [Google Scholar]
- 166.Hansen HP, Rossing P, Mathiesen ER et al. Assessment of glomerular filtration rate in diabetic nephropathy using the plasma clearance of 51Cr-EDTA. Scand J Clin Lab Invest 1998; 58: 405–413 [DOI] [PubMed] [Google Scholar]
- 167.Zuo L, Ying C, Wang M et al. Prediction of two-sample (99m)Tc-diethylene triamine pentaacetic acid plasma clearance from single-sample method. Ann Nucl Med 2005; 19: 399–405 [DOI] [PubMed] [Google Scholar]
- 168.De Sadeleer C, Piepsz A, Ham HR. Checking the consistency of the two blood samples slope-intercept method for estimating GFR using the single blood sample formula in children. Nucl Med Commun 2007; 28: 49–54 [DOI] [PubMed] [Google Scholar]
- 169.Waller DG, Keast CM, Fleming JS et al. Measurement of glomerular filtration rate with technetium-99m DTPA: comparison of plasma clearance techniques. J Nucl Med 1987; 28: 372–377 [PubMed] [Google Scholar]
- 170.Delanaye P, Cavalier E, Morel J et al. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol 2014; 15: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schaeffner ES, Ebert N, Delanaye P et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012; 157: 471–481 [DOI] [PubMed] [Google Scholar]
- 172.Ruggenenti P, Porrini EL, Gaspari F et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012; 35: 2061–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Björk J, Grubb A, Sterner G et al. Performance of GFR estimating equations stratified by measured or estimated GFR: implications for interpretation. Am J Kidney Dis 2015; 66: 1107–1108 [DOI] [PubMed] [Google Scholar]
- 174.Hsu CY, Propert K, Xie D et al. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol 2011; 22: 1931–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kwong Y-T, Stevens LA, Selvin E et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis 2010; 56: 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Piéroni L, Delanaye P, Boutten A et al. A multicentric evaluation of IDMS-traceable creatinine enzymatic assays. Clin Chim Acta 2011; 412: 2070–2075 [DOI] [PubMed] [Google Scholar]
- 177.Brochner-Mortensen J. Routine methods and their reliability for assessment of glomerular filtration rate in adults, with special reference to total [51Cr]EDTA plasma clearance. Dan Med Bull 1978; 25: 181–202 [PubMed] [Google Scholar]
- 178.Groth S, Aasted M. 51Cr-EDTA clearance determined by one plasma sample in children. Clin Physiol 1984; 4: 75–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


