Abstract
Background
The most abundant and potent carcinogenic tobacco-specific nitrosamines in tobacco and tobacco smoke is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). In vivo, NNK is rapidly metabolized to both the (R)- and (S)-enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), which possesses similar carcinogenic properties as NNK. The major detoxification pathway for both NNAL enantiomers is glucuronidation by UDP-glucuronosyltransferase (UGT) enzymes including UGT2B10 and UGT2B17.
Methods
NNAL-N-Gluc, (R)-NNAL-O-Gluc, (S)-NNAL-O-Gluc and free NNAL were simultaneously and directly quantified in the urine of smokers by LC-MS analysis. Genotypes were determined by Taqman-assay using genomic DNA.
Results
The average percentage of total urinary NNAL that existed as NNAL-N-Gluc, (R)-NNAL-O-Gluc, (S)-NNAL-O-Gluc, and free-NNAL in 180 active smokers was 23.2%, 21.7%, 26.9% and 28.2%, respectively. The functional knock-out polymorphism in the UGT2B10 gene at codon 67 (Asp>Tyr) was significantly (P<0.0001) associated with a 93% decrease in creatinine-adjusted NNAL-N-Gluc. The polymorphic whole-gene deletion of the UGT2B17 gene was associated with significant (P=0.0048) decreases in the levels of creatinine-adjusted (R)-NNAL-O-Gluc, with a 32% decrease in the levels of urinary (R)-NNAL-O-Gluc/(S)-NNAL-O-Gluc among subjects with the UGT2B17 (*2/*2) genotype as compared to subjects with the UGT2B17 (*1/*1) genotype.
Conclusions
These results suggest that functional polymorphisms in UGT2B10 and UGT2B17 are associated with a reduced detoxification capacity against NNAL and may therefore affect individual cancer risk upon exposure to tobacco.
Impact
This is the first report to clearly demonstrate strong genotype-phenotype associations between both the UGT2B10 codon 67 Asp<Tyr genotype and urinary NNAL-N-Gluc levels and between the UGT2B17 copy number variant and urinary (R)-NNAL-O-Gluc levels in smokers.
Keywords: Carcinogen-detoxifying enzymes, Tobacco, Carcinogen metabolism, NNAL, UGT
Introduction
Cigarette smoking and smokeless-tobacco exposure are the dominant risk factors for many epithelial cancers including lung, oral, pancreatic and bladder cancers (1), with 85-90% of lung cancer cases directly attributed to tobacco exposure and the relative risk for the development of lung cancer for tobacco smokers is ~20 times higher than the risk for non-smokers (2). Tobacco-specific nitrosamines (TSNA) are produced by nitrosation of tobacco alkaloids, including nicotine, during the tobacco curing and fermentation process and are typically present in substantial amounts in unburned tobacco and in tobacco smoke. The most abundant and potent carcinogenic TSNAs are 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N’-nitrosonornicotine (NNN) (3-5) [e.g., cigarettes: NNK, 19-10,745 ng/cigarette; NNN, 20-58,000 ng/cigarette (6, 7)]. In mainstream cigarette smoke, the total level of NNN plus NNK, is 2-10 times that of the carcinogenic polycyclic aromatic hydrocarbon, benzo(a)pyrene (4). Tissue-specific carcinogenicity is observed in rodent tissues for NNN and NNK (5, 8-11), with NNK much more potent than NNN in inducing lung adenocarcinomas in rodents (12). NNK is metabolized into intermediates which bind to human lung DNA (13), transform human epithelium in vitro (14), and induce non-tumorigenic human bronchial epithelial cells to become neoplastically transformed in nude mice after subcutaneous transplantation (15). The lifetime dose of 1.6 mg NNK/kg body wt estimated for the average 2 pack-a-day U.S. smoker (4) is similar to the cumulative dose of 1.8 mg NNK/kg body wt which induced lung tumors in rats (8). Therefore, NNK is strongly implicated as a human lung carcinogen.
Urinary NNK metabolites have been assessed as effective biomarkers of tobacco exposure and cancer risk (7). In vivo, NNK is rapidly metabolized to both the (R)- and (S)-enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), which possess similar carcinogenic properties as NNK (16). It has been found that (S)-NNAL is stereoselectively retained and has a higher tumorigenicity as compared to (R)-NNAL in rat lung (17, 18), and studies in smokeless tobacco users indicate that NNAL may also be stereo-selectively retained in humans (19).
The major detoxification pathway for both NNAL enantiomers is glucuronide conjugation on the nitrogen within the pyridine ring (NNAL-N-Gluc) or on the hydroxyl group on the NNAL side-chain (NNAL-O-Gluc). UGT2B10 was shown to exhibit high N-Gluc activity against nicotine as well as several tobacco-specific nitrosamines including NNAL (20, 21). A functional knock-out polymorphism in the UGT2B10 gene at codon 67 (Asp>Tyr) (rs61750900) was associated with a decrease of ~95% in NNAL-N-Gluc formation in human liver microsomes (HLM) (22) and large decreases of both nicotine-N-Gluc and cotinine-N-Gluc formation in the urine of smokers (23). No studies have as yet been performed specifically examining the role of the UGT2B10 codon 67 polymorphism on urinary NNAL-N-Gluc levels in vivo in smokers.
Three hepatic UGTs, 2B17, 2B7 and 1A9, have been shown to be active in NNAL-O-Gluc formation (24, 25). Studies of NNAL glucuronidation in vitro have shown that UGTs 2B7 and 2B17 exhibit preferential glucuronidation activity for (S)- and (R)-NNAL, respectively, while UGT1A9 is relatively non-stereospecific (26). Common functional variants are known to exist for UGTs 2B7 and 2B17. A polymorphic whole-gene deletion of the UGT2B17 gene has been shown to be associated with significant decreases in the formation of total NNAL-O-Gluc and (R)-NNAL-O-Gluc in HLM (24, 26) and with increased risk for lung adenocarcinoma in women (27). While a missense polymorphism at codon 268 (His>Tyr) of the UGT2B7 gene was associated with a 1.4-fold decrease in total NNAL-O-Gluc formation activity in HLM (28), no studies have yet been performed examining these polymorphisms in vivo in smokers.
The goal of the present study was to assess the levels of NNAL and its glucuronides in the urine of smokers and to determine whether the levels of NNAL-N-Gluc and individual NNAL-O-Gluc diastereomers are associated with functional polymorphisms in the UGT2B10, UGT2B17 and UGT2B7 genes. Using a sensitive ultra-performance liquid chromatrography-mass spectrometry (UPLC-MS) method developed for the direct assessment of NNAL, NNAL-N-Gluc and each of the NNAL-O-Gluc diastereomers, functional polymorphisms in UGT2B10 and UGT2B17 were shown to be associated with the levels of NNAL-N-Gluc and (R)-NNAL-O-Gluc, respectively, in the urine of smokers.
Materials and Methods
Chemicals and materials
NNAL and NNAL-N-Gluc standards were purchased from Toronto Research Chemicals (Toronto, Canada). UDP-glucuronic acid (UDPGA) was obtained from Sigma-Aldrich (St. Louis, MO). UPLC-MS grade solvents including methanol, formic acid, ammonium-formate and ammonium-bicarbonate were purchased from Fisher Scientific. MilliQ ultra-pure water was used for all analysis. D4-NNAL was kindly provided by Shantu Amin (Penn State University). D4-NNAL-N-Gluc and D4-NNAL-O-Gluc were biochemically synthesized by incubating D4-NNAL with UDPGA and bovine liver microsomes, prepared as previously described (21, 26), for 2 h at 37°C, and then purified by high-performance liquid chromatrography (HPLC).
Subjects and specimens
Spot urine specimens and matching oral buccal cell or blood (10 cc) samples were collected from Caucasian current and former smokers who were a subgroup of healthy control subjects in two large case-control studies: 110 self-reported current smokers (smoked at least 100 cigarettes lifetime and had smoked at least one cigarette in the year prior to interview) and 29 former smokers (smoked at least 100 cigarettes lifetime but had not smoked for at least 1 year prior to interview) were from a lung cancer case-control study conducted at the H. Lee Moffitt Cancer Center in Tampa, FL (27), and 87 self-reported current smoker subjects were from a colorectal cancer case-control study conducted at Penn State University College of Medicine in Hershey, PA (29-32). Oral buccal cell or blood genomic DNA was extracted as previously described (27, 29-32) and urine and DNA specimens were stored at −80°C prior to analysis.
The HLM used for these studies were prepared from normal human liver tissue as described previously (33). Liver microsomes were prepared through differential centrifugation and stored as −80°C.
Genotyping analysis
The UGT2B10 codon 67 Asp>Tyr polymorphism (rs61750900) was determined by real-time PCR using a custom design TaqMan SNP genotyping Assay (w1506724762000, Life Technologies). Because a custom design assay was used, real-time results were confirmed by PCR-RFLP analysis in three subjects of each of the three UGT2B10 genotypes [Asp/Asp (*1/*1), Asp/Tyr (*1/*2), Tyr/Tyr (*2/*2)] using the HinFI restriction enzyme, as described previously (20). UGT2B17 copy number variant (CNV) genotypes were determined by real-time PCR using a CNV genotyping assay (Hs03185327_c, Life Technologies) using RNase P as a control (Cat # 4403326, Life Technologies). The UGT2B7 codon 268 His>Tyr polymorphism (SNP ID: hCV32449742; rs7439366, rs7438284) was determined by real-time PCR using a pre-designed TaqMan SNP genotyping Assay (c32449742_20, Life Technologies). All real-time PCR was performed in the Washington State University-Spokane Genomics Core Facility using a Bio-Rad CFX384 real-time PCR machine.
Synthesis and purification of (S)-, (R)- and D4-NNAL glucuronide standards
D4-NNAL-N-Gluc and D4-NNAL-O-Gluc were synthesized by using Bovine liver microsomes (5 mg protein/ml reaction) in glucuronidation reaction containing D4-NNAL and UDPGA and then collected and purified by HPLC as previously described (26). (S)-NNAL-O-Gluc and (R)-NNAL-O-Gluc standards were synthesized by human liver microsomes (5 mg protein/ml reaction) in glucuronidation reaction containing racemic NNAL (26) and UDPGA, and were collected and purified by UPLC method described below with the mass spectrometer disconnected.
UPLC-MS analysis
A 10-μl aliquot of each urine specimen was spiked with 5 μl of an internal standard mixture that included D4-NNAL, D4-NNAL-N-Gluc and D4-NNAL-O-Gluc (0.1 ppm each). After the addition of 10 μl ammonium-formate (0.5 M), the mixture was vortexed to mix thoroughly. All precipitate was removed by centrifugation at 16,000 g for 10 min at 4°C. The supernatant was transferred into 350 μl conical glass sample vials for UPLC-MS analysis.
Urine samples prepared as above were analyzed using an Acquity LC-MS system (Waters Corporation) consisting of an Acquity UPLC pump and an autosampler fitted with an Acquity HSS T3 (100 × 2.1 mm, 1.8 μm) UPLC column and a Xevo G2-S Qtof mass spectrometer located within the Washington State University-Spokane Mass Spectrometry Core Facility. LC peak separation was performed at a 25°C column temperature and the sample injection volume was 5 μl with a flow rate of 0.3 mL/min using the following conditions: 13 min with 100% buffer A (5 mM ammonium-formate and 0.01% formic acid in water), followed by a linear gradient for 8 min to 85% buffer A:15% buffer B (100% methanol), and a subsequent linear gradient for 1 min to 100% buffer B. Flow continued for 4 min in 100% buffer B before re-equilibrium in 100% buffer A for 2 min. Ammonium-bicarbonate at 0.5 M was infused at 10 microL/min with post column elution flow.
The Waters Xevo G2-S Qtof mass spectrometer was operated in positive electrospray ionization MS/MS sensitive mode, with capillary voltage at 0.6 kV. Nitrogen was used for both cone and desolvation gases at 50 L/h and 800 L/h, respectively. Ultra-pure argon was used as the collision gas with a flow rate of 0.1 L/h for collision-induced dissociation. The source temperature was 120°C, de solvation gas temperature was 500°C. The dwell time for each ion was 0.1 sec. The cone voltage was 30 V and the collision energies were 15, 20 and 22 volts for NNAL, NNAL-N-Gluc and NNAL-O-Gluc, respectively. The MS/MS transition (m/z) traces for quantification for NNAL-N-Gluc, NNAL-O-Gluc and NNAL are 386.2>180.1 (IS: 390.2>184.2), 386.2>162.1 (IS: 390.2>166.1) and 210.1>180.1 (IS: 214.2 >184.2), respectively.
Urinary creatinine levels were determined after dilution of a small urine specimen 1000-fold in water, with 10 μL of the diluted sample mixed with 10 μL of 0.1 ppm D3-creatinine for LC-MS analysis using the same LC-MS system described above. LC separation was performed at a 40°C column temperature and the sample injection volume was 2 μl with a flow rate of 0.4 mL/min using the following conditions: 1 min with 100% buffer A (5 mM ammonium-acetate in water), followed by a linear gradient for 2 min to 100% buffer B (100% acetonitrile), and continued for 1 min in 100% buffer B before re-equilibration in 100% buffer A for 1 min. Creatinine was detected in positive MS mode with the cone voltage at 20 V. The MS (m/z) traces for quantification for creatinine is 114.06 (IS: 117.08), with a linear range of detection from 2 to 20,000 ppm.
Quantification
Standard curves were constructed by plotting the ratio of analyte (NNAL, (R)-NNAL-O-Gluc, (S)-NNAL-O-Gluc, NNAL-N-Gluc or creatinine) peak area vs the peak area of the corresponding internal standard, and comparing ratio that to a standard curve of eleven analyte concentrations ranging from 0.01 to 10 ppb for NNAL and NNAL metabolites and 2 to 20,000 ppm for creatinine. (R)-NNAL-O-Gluc and (S)-NNAL-O-Gluc were quantified independently as described in the Results (see below). Urinary free NNAL, NNAL-N-Gluc, (S)-NNAL-O-Gluc, (R)-NNAL-O-Gluc and creatinine concentrations were calculated using Waters’ TargetLynx software.
Statistical analysis
All statistical analysis was performed using Prism version 6.01 (GraphPad Software, San Diego, CA). Nonparametric Spearman correlation analysis was used to measure the relationship between creatinine-adjusted NNAL-N-Gluc, (R)-NNAL-O-Gluc or (S)-NNAL-O-Gluc and nicotine-Gluc and 3′-hydroxy (OH)-cotinine-Gluc, which were analyzed in a subset of the same specimens in previous studies (23, 34). Where indicated (when data were not normally distributed), a square root transformation was applied before analysis. The Student’s t-test and the ANOVA linear regression trend test were used to compare the levels of urinary, (i) NNAL-N-Gluc in smokers stratified by UGT2B10 codon 67 Asp>Tyr genotypes, (ii) (R)-NNAL-O-Gluc stratified by UGT2B17 copy number variant genotypes, and (iii) (S)-NNAL-O-Gluc stratified by UGT2B7 copy number variant genotypes.
Results
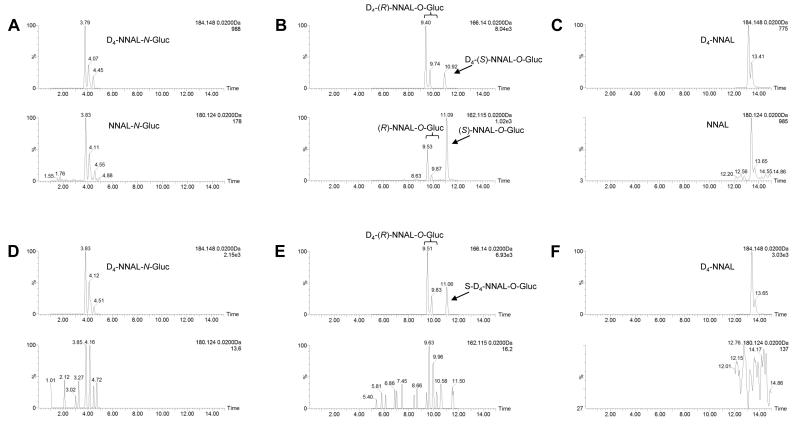
Urinary unconjugated NNAL and individual NNAL glucuronides were separated by UPLC-MS as described in the Materials and Methods. Peaks corresponding to D4-NNAL-N-Gluc, D4-NNAL-O-Gluc and D4-NNAL internal standards were observed at 3.79 – 4.45, 9.53 – 10.92, and 13.16 – 13.43 min, respectively (Figure 1, panels A, B, and C). Corresponding NNAL peaks from the urine of smokers matched well with the internal standards, with retention times of 3.83-4.55 min, 9.53 – 11.10 min, and 13.4 – 13.65 min for NNAL-N-Gluc, NNAL-O-Gluc, and free NNAL, respectively. No NNAL or NNAL glucuronide peaks were observed in the urine specimens from former-smokers (Figure 1, panels D, E, and F).
Figure 1. LC-MS analysis of NNAL and its metabolites in smoker’s urine vs former smoker’s urine.
Urine samples were treated with ammonium formate to normalize the pH and then spiked with the D4 labeled internal standard (IS) and analyzed as described in the Materials and Methods. Urine from one representative smoker showing clear peaks for NNAL-N-Gluc (panel A), (R)-NNAL-O-Gluc and (S)-NNAL-O-Gluc (panel B), and free NNAL (panel C) and one former smoker that shows no distinguishable peaks for NNAL-N-Gluc (panel D), (R)-NNAL-O-Gluc or (S)-NNAL-O-Gluc (panel E), or NNAL (panel F). Internal standards [D4-NNAL-N-Gluc, D4-(R)-NNAL-O-Gluc, D4-(S)-NNAL-O-Gluc, and D4-NNAL] are shown in the corresponding top panels.
NNAL exists as two interchangeable E and Z rotamers of two enantiomers, (R) and (S). To characterize the glucuronide peaks formed from (S)- versus (R)-NNAL using the UPLC-MS methodology described in this study, (S)-NNAL and (R)-NNAL were each purified to >99% (26), and were independently incubated with HLM and UDPGA as previously described (21). As observed in Figure 2, HLM-generated (S)-NNAL-N-Gluc E/Z rotamers were observed at retention times of 3.99 and 4.37 min (panel A), while the E/Z rotamers of (R)-NNAL-N-Gluc were observed at 3.75 and 4.16 min (panel C). HLM-generated (S)-NNAL-O-Gluc peaks (E and Z) were observed at a retention time of 11.16 min (Figure 2, panel B). (R)-NNAL-O-Gluc E/Z rotamer peaks were better separated and observed at 9.53 and 9.86 min; a minor (S)-NNAL-O-Gluc peak was also observed at 11.12 min due to a small amount of (S)-NNAL in the purified (R)-NNAL substrate (Figure 2, panel D).
Figure 2. Human liver microsome incubations with pure NNAL enantiomers.
(S)- and (R)-NNAL were separately incubated with human liver microsomes (5 mg protein/ml reaction) as described in the Materials and Methods. Peaks corresponding to (S)-NNAL-N-Gluc (panel A), (S)-NNAL-O-Gluc (panel B), (R)-NNAL-N-Gluc (panel C), and (R)-NNAL-O-Gluc (panel D) are shown.
NNAL and NNAL glucuronides were analyzed in urine specimens from 197 self-reported current smokers as well as 29 former-smokers. All subjects were of European American decent with an age range of 23 to 80 (mean age = 57 y). Fifty-five percent of the current-smokers and 59% of the former-smokers were male. Although subjects were recruited from two separate studies, the average age (56 versus 57 years) and sex distribution (42% versus 49% female) were similar between studies. The minimum levels of detection of free NNAL, NNAL-N-Gluc, (S)-NNAL-O-Gluc, and (R)-NNAL-O-Gluc were 0.1 nM, 0.1 nM, 0.025 nM and 0.05 nM, respectively. The method reproducibility was evaluated by running 5 randomly-chosen urine specimens in triplicate, performed on three consecutive days. As shown in Table 1, there was good reproducibility for both NNAL-O-Gluc diastereomers and free NNAL. Similar to that observed using other methodologies (35), NNAL-N-Gluc determinations were slightly more variable than other metabolites in the present study. The assay accuracy was studied by spiking D4-labeled free NNAL, NNAL-N-Gluc, (S)-NNAL-O-Gluc, and (R)-NNAL-O-Gluc standards into a smoker’s urine sample at concentrations of 2.5, 1.25 and 0.625 ppb. The lowest rate of recovery was for NNAL-N-Gluc at 83.5% at the lowest concentration tested (0.625 ppb), with the recovery >90% for all other metabolites at all concentrations examined (see Table 2). Linear increases were observed for free NNAL, NNAL-N-Gluc, (S)-NNAL-O-Gluc, and (R)-NNAL-O-Gluc from 0.04 to 10 ppb (results not shown).
Table 1.
Reproducibility of method.a
| specimen | NNAL-N-Gluc | (R)-NNAL-O-Gluc | (S)-NNAL-O-Gluc | Free NNAL |
|---|---|---|---|---|
| 1 | 0.61 ± 0.11 | 0.47 ± 0.08 | 0.57 ± 0.05 | 0.64 ± 0.02 |
| 2 | 0.50 ± 0.01 | 0.45 ± 0.09 | 0.30 ± 0.01 | 0.16 ± 0.03 |
| 3 | below DLb | 0.11 ± 0.02 | 0.15 ± 0.01 | 0.27 ± 0.04 |
| 4 | 0.43 ± 0.12 | 0.80 ± 0.07 | 1.29 ± 0.05 | 1.57 ± 0.25 |
| 5 | 0.48 ± 0.06 | 0.90 ± 0.11 | 0.81 ± 0.03 | 0.52 ± 0.07 |
All values are in nM concentrations, shown as mean ± SD of 3 LC-MS runs per sample analyzed on three consecutive days.
Values were below the detection limit (DL).
Table 2.
Recovery of urinary NNAL metabolites.
| NNAL-N-Gluc (n=3) | R-NNAL-O-Gluc (n=3) | S-NNAL-O-Gluc (n=3) | NNAL (n=3) | |||||
|---|---|---|---|---|---|---|---|---|
| ppb added | Detected | Recovery | Detected | Recovery | Detected | Recovery | Detected | Recovery |
| 2.5 | 2.67 | 101% | 3.12 | 113% | 2.95 | 107% | 2.64 | 96.3% |
| 1.25 | 1.39 | 100% | 1.71 | 114% | 1.38 | 89.8% | 1.47 | 98.8% |
| 0.625 | 0.66 | 83.5% | 1.02 | 118% | 0.93 | 107% | 0.81 | 92.5% |
| 0 | 0.14 | - | 0.29 | - | 0.26 | - | 0.24 | - |
No detectable levels of any of the four NNAL metabolites were found in any of the 29 former smoker’s urine specimens tested (results not shown). Of the 197 urine specimens from subjects determined to be ‘current smokers’ based on interview criteria, several exhibited free NNAL or NNAL metabolite levels that were below the level of detection. To assess the role of UGT genotypes on NNAL metabolite levels in the urine of the 197 subjects determined to be ‘current smokers’ based on interview criteria, only those urine specimens considered to be from ‘active smokers’, previously defined as those subjects with total urinary NNAL (NNAL-N-Gluc + (R)-NNAL-O-Gluc + (S)-NNAL-O-Gluc + free NNAL) levels of >0.23 nM (36), were considered for further analysis. This resulted in the exclusion of 17 urine specimens; of these, no detectable levels of any of the four NNK metabolites examined in this study (free NNAL or any of the three NNAL-Gluc metabolites) were observed in 10 specimens. (S)-NNAL-O-Gluc was detected in 178 subjects, ranging from 0.04 - 5.04 nM; (R)-NNAL-O-Gluc was detected in 171 subjects with a range of 0.052 nM - 4.16 nM, NNAL-N-Gluc was detected in 161 subjects with a range of 0.10 nM - 4.76 nM, and free NNAL was detected in 167 subjects with a range of 0.11 nM - 2.18 nM. These large ranges in urinary NNAL and NNAL-Gluc levels are suggestive of large inter-individual differences in NNAL metabolism and excretion.
The average level of urinary creatinine-adjusted free NNAL, (R)-NNAL-O-Gluc, (S)-NNAL-O-Gluc and NNAL-N-Gluc in the 180 ‘active smokers’ was 0.78, 0.59, 0.72 and 0.62 pmol/mg creatinine, respectively (Table 3). The average percentages of NNAL-N-Gluc, (R)-NNAL-O-Gluc, (S)-NNAL-O-Gluc and NNAL in total urinary NNAL excreted among the 180 subjects were 23.2%, 21.7%, 26.9% and 28.2%, respectively. The total creatinine-adjusted NNAL levels were comparable between urine specimens from subjects recruited from the two different studies (2.52 versus 2.84 pmol/mg creatinine). The average level of urinary creatinine-adjusted free NNAL, (R)-NNAL-O-Gluc, (S)-NNAL-O-Gluc and NNAL-N-Gluc, was, in pmol/mg creatinine, 0.63 versus 0.89, 0.54 versus 0.62, 0.67 versus 0.77 and 0.68 versus 0.57, respectively.
Table 3.
Creatinine-adjusted NNK metabolite levels (pmol/mg creatinine) in the urine of smokers (n=180).a
| NNAL-N-Gluc | (R)-NNAL-O-Gluc | (S)-NNAL-O-Gluc | Total NNAL-Gluc | Free NNAL | Total NNAL | |
|---|---|---|---|---|---|---|
| Mean | 0.62 ± 0.49 | 0.59 ± 0.44 | 0.72 ± 0.50 | 1.93 ± 1.23 | 0.78 ± 0.60 | 2.71 ± 1.61 |
| Range | 0.06 - 3.23b | 0.10 - 2.78c | 0.06 - 2.96d | 0.14 - 6.31 | 0.11- 3.29e | 0.26 - 9.45 |
All concentrations listed are in pmol/mg creatinine (mean ± SD) for 180 metabolite-informative subjects.
n=161; 19 subjects were below the detection limit for NNAL-N-Gluc;
n=171; 9 subjects were below the detection limit for (R)-NNAL-O-Gluc;
n=178; 2 subjects were below the detection limit for (S)-NNAL-O-Gluc;
n=167; 13 subjects were below the detection limit for free NNAL.
UGT2B10 has been shown to be the primary hepatic enzyme involved in the formation of NNAL-N-Gluc (21). UGT2B10 was previously shown to be the major enzyme involved in the glucuronidation of nicotine and the UGT2B10 codon 67 SNP was correlated with decreased levels of nicotine-N-Gluc in the urine of smokers (37). In an analysis of a subset of the smoker’s urine specimens (n=103) for whom nicotine-N-Gluc and 3′-OH-cotinine-Gluc were measured in previous studies (34, 37) and for whom NNAL metabolites were measured in the present study, a significant association was observed between the creatinine-adjusted levels of urinary NNAL-N-Gluc and nicotine-N-Gluc (r2=0.46, P<0.0001; Table 4), which is consistent with UGT2B10 being active in the N-Gluc formation of both nicotine and NNAL in vivo. Consistent with UGT2B10 not being active in the in vitro formation of 3′-OH-cotinine-Gluc and with UGT2B10 codon 67 genotypes exhibiting no correlation with 3′-OH-cotinine-Gluc levels in smoker’s urine in previous studies (34), a much weaker association was observed between the creatinine-adjusted levels of urinary NNAL-N-Gluc and 3′-OH-cotinine-Gluc in the present study (r2=0.12, P =0.0005; Table 3).
Table 4.
Spearman Correlation coefficients between creatinine-adjusted NNAL glucuronides (pmol/mg creatinine) and creatinine-adjusted nicotine-Gluc or 3′-OH-cotinine-Gluc (pmol/mg creatinine) in 103 smoker’s urinea.
| NNAL-N-Gluc | (R)-NNAL-O-Gluc | (S)-NNAL-O-Gluc | |
|---|---|---|---|
| nicotine-Gluc | 0.46 (4.1E-15) | 0.075 (5.0E-3) | 0.12 (3.9E-4) |
| 3′-OH-cotinine-Gluc | 0.12 (4.5E-4) | 0.24 (1.2E-7) | 0.35 (3.6E-11) |
Values represent r2, with P-values in parenthesis.
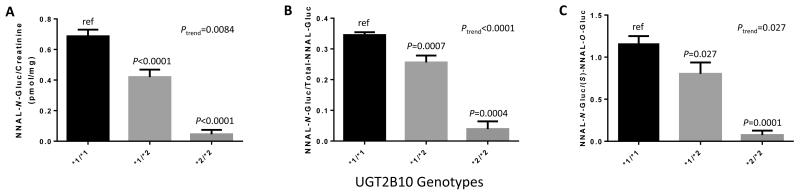
An Asp>Tyr SNP at codon 67 of the UGT2B10 gene has been previously shown to be associated with a loss of enzyme function and decreased hepatic glucuronidation activity (22). Informative genotyping data were obtained for the UGT2B10 codon 67 polymorphism for 174 of the 180 urinary NNAL metabolite-informative specimens. The allelic prevalence of the Tyr-encoding UGT2B10*2 allele was 11.5% in the population examined in this study, with 4 subjects exhibiting the UGT2B10 (*2/*2) genotype, and the allelic distribution of this polymorphism was within Hardy-Weinberg equilibrium (P=0.205). As shown in Figure 3, there was a strong correlation between UGT2B10 genotype and creatinine-adjusted NNAL-N-Gluc levels in the 174 smoker’s urine specimens analyzed in the present study (panel A). Significant decreases of 93% (P<0.0001) and 39% (P<0.0001) were also observed for creatinine-adjusted NNAL-N-Gluc among subjects with the UGT2B10 (*2/*2) and (*1/*2) genotypes, respectively, as compared to subjects with the UGT2B10 (*1/*1) genotype (Figure 3, panel A); a similarly significant pattern was observed after normalizing by cigarettes/day or after incorporating cigarettes per day in a linear regression model (results not shown). A highly significant (P=0.0084) trend was observed between decreasing creatinine-adjusted NNAL-N-Gluc levels and increasing number of the UGT2B10*2 allele. Similar significant data were observed when comparing UGT2B10 genotypes vs NNAL-N-Gluc/total-NNAL-Gluc (Figure 3, panel B) or NNAL-N-Gluc/(S)-NNAL-O-Gluc (Figure 3, panel C).
Figure 3. Association between the UGT2B10 codon 67 polymorphism and urinary NNAL-N-Gluc levels in smokers.
Subjects were stratified by UGT2B10 codon 67 Asp>Tyr genotype, with the UGT2B10*1 allele corresponding to the wild-type UGT2B1067Asp and the UGT2B10*2 allele corresponding to the UGT2B1067Tyr variant. A, UGT2B10 genotypes versus creatinine-adjusted NNAL-N-Gluc (pmol/mg creatinine); B, UGT2B10 genotypes versus the ratio of NNAL-N-Gluc/total-NNAL-Gluc; and C, UGT2B10 genotypes versus the ratio of NNAL-N-Gluc/(S)-NNAL-O-Gluc. All values are expressed as the mean ± SEM for 174 genotype-informative subjects. For comparative analysis between genotypes, the Students t-test was performed on squared-root transformed data for panel A only; non-transformed data were used for panels B and C.
UGTs 2B7 and 2B17 have been shown to be among the primary hepatic enzymes involved in the formation of NNAL-O-Gluc (24, 34), with recent studies demonstrating that UGT2B17 is more active against (R)-NNAL than (S)-NNAL while UGT2B7 is more active against (S)-NNAL (26). These enzymes were also shown to be active in the formation of 3′-OH-cotinine-Gluc but not nicotine-Gluc (20, 23, 34). Consistent with the in vitro activity observed for UGT2B17 against (R)-NNAL, a stronger correlation was observed between the levels of creatinine-adjusted urinary (R)-NNAL-O-Gluc and 3′-OH-cotinine-Gluc (r2=0.24, P<0.0001; Table 4) than that observed between creatinine-adjusted urinary (R)-NNAL-O-Gluc and nicotine-N-Gluc (r2=0.075, P=0.005; Table 4). Consistent with the in vitro activity observed for UGT2B7 against (S)-NNAL, a stronger correlation was also observed between the levels of creatinine-adjusted urinary (S)-NNAL-O-Gluc and 3′-OH-cotinine-Gluc (r2=0.35, P<0.0001; Table 4) than that observed between creatinine-adjusted urinary (S)-NNAL-O-Gluc and nicotine-Gluc (r2=0.12, P=0.0004; Table 4).
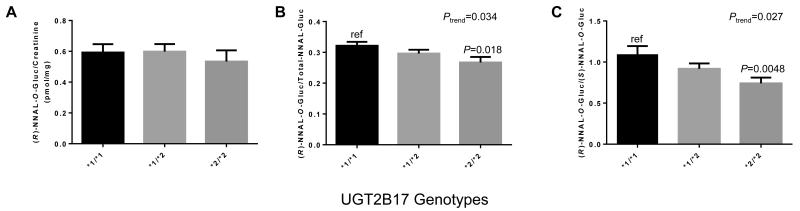
The UGT2B17 whole-gene deletion polymorphism has been previously shown to be associated with decreased 3′-OH-cotinine-Gluc formation in HLM (24), with urinary 3′-OH-cotinine-Gluc levels in smokers (38) and with decreased NNAL-O-Gluc formation in HLM (24). Informative genotyping data were obtained for the UGT2B17 copy number variant for all 180 urinary NNAL metabolite-informative specimens. The gene deletion UGT2B17*2 allelic prevalence of 37.5% was observed in this population with 25 subjects exhibiting the UGT2B17 (*2/*2) genotype. The allele distribution was within Hardy-Weinberg equilibrium (P=0.82). As shown in Figure 4, there were significant correlations between UGT2B17 genotype and (R)-NNAL-O-Gluc/total NNAL-Gluc (panel B; P=0.018) and (R)-NNAL-O-Gluc/(S)-NNAL-O-Gluc (panel C; P=0.0048) in the 180 smoker’s urine specimens, with a 32% decrease in the levels of urinary (R)-NNAL-O-Gluc/(S)-NNAL-O-Gluc among subjects with the UGT2B17 (*2/*2) genotype as compared to subjects with the UGT2B17 (*1/*1) genotype (Figure 4, panel C). Significant trends towards decreasing levels of urinary (R)-NNAL-O-Gluc/total NNAL-Gluc (Ptrend=0.034) as well as (R)-NNAL-O-Gluc/(S)-NNAL-O-Gluc (Ptrend=0.027) was observed in subjects with increasing numbers of the UGT2B17*2 allele (panels B and C, respectively).
Figure 4. Association between the UGT2B17 deletion polymorphism and urinary (R)-NNAL-O-Gluc levels in smokers.
Subjects were stratified by UGT2B17 copy number variant (CNV) genotypes, with the UGT2B17*1 allele corresponding to the wild-type single-gene copy number and the UGT2B17*2 allele corresponding to the UGT2B17 gene deletion variant. A, UGT2B17 CNV genotypes versus creatinine-adjusted (R)-NNAL-O-Gluc (pmol/mg creatinine); B, UGT2B17 CNV genotypes versus the ratio of (R)-NNAL-O-Gluc/total-NNAL-Gluc; and C, UGT2B17 CNV genotypes versus the ratio of (R)-NNAL-O-Gluc/(S)-NNAL-O-Gluc. All values are expressed as the mean ± SEM for 180 genotype-informative subjects.
Of the 168 subjects for whom informative UGT2B7 codon 268 (His>Tyr) data were obtained, 25% (n=42) exhibited the homozygous wild-type UGT2B7 His/His (*1/*1) genotype, 46% (n=78) exhibited the heterozygous wild-type UGT2B7 (*1/*2) genotype, and 29% (n=48) exhibited the homozygous UGT2B7 (*2/*2) genotype, a distribution that was consistent with Hardy-Weinberg equilibrium (P=0.36). No significant association was found between the UGT2B7 codon 268 genotype and urinary (R)- or (S)-NNAL-O-Gluc levels, whether examined as urinary concentrations or as a ratio with total NNAL-Gluc (data not shown).
Discussion
The present study is the first to directly examine the role of functional variants in UGT2B7, UGT2B10 and UGT2B17 in NNK metabolism in smokers. Previous studies have shown that while both UGT2B10 and UGT1A4 exhibit NNAL-N-Gluc formation activity, UGT2B10 exhibited a 6-fold lower Km for NNAL than UGT1A4 (22) and the functional polymorphism at codon 67 of the UGT2B10 gene resulted in an 11-fold decrease in NNAL-N-Gluc formation in HLM (22). Results from the present study are highly consistent with these in vitro data, with smokers homozygous for the UGT2B10*2 variant exhibiting a 23-fold decrease in the levels of urinary NNAL-N-Gluc in comparison to smokers exhibiting the wild-type UGT2B10 (*1/*1) genotype. Therefore, it is estimated that UGT2B10 is responsible for more than 90% of NNAL-N-Gluc formation in vivo, indicating that UGT1A4 may be responsible for less than 10% of the elimination of NNAL by NNAL-N-Gluc formation in smokers with the wild-type UGT2B10 (*1/*1) genotype.
The data from the present study are also consistent with UGT2B10 playing an important role in the hepatic N-glucuronidation and excretion of other tobacco constituents. A strong association was observed between the levels of urinary NNAL-N-Gluc and urinary nicotine-Gluc but not the O-glucuronidated metabolite of 3′-OH-cotinine in smokers in the present study, a pattern consistent with the N-glucuronidating capacity of UGT2B10 previously described in in vitro studies (20, 37, 39).
The UGT2B17 copy number variant was associated with the urinary levels of (R)-NNAL-O-Gluc but not (S)-NNAL-O-Gluc in smokers in the present study, a pattern consistent with recent in vitro studies demonstrating that UGT2B17 prefers (R)-NNAL over (S)-NNAL as a substrate (26). These data are also consistent with previous in vitro studies demonstrating that the UGT2B17 deletion was associated with decreases in total NNAL-O-Gluc formation (24) and, more recently, with (R)-NNAL-O-Gluc formation (26) in HLM. The fact that the UGT2B17 deletion was associated with a maximum decrease of 27% in the urinary ratio of (R)-NNAL-O-Gluc/(S)-NNAL-O-Gluc suggests that other UGTs, presumably UGTs 2B7 and 1A9, may also play important roles in the excretion of (R)-NNAL in vivo. This is consistent with the fact that these enzymes are all hepatically well-expressed (40). It is also interesting that despite the relatively low percentage of urinary (R)-NNAL-O-Gluc associated with the UGT2B17 deletion in smokers, a significant correlation was observed between the UGT2B17 deletion and increased risk for lung adenocarcinoma in women (27). This is likely due to the fact that UGT2B17 is relatively well-expressed in lung, a pattern not observed for other NNAL-glucuronidating enzymes including UGTs 2B7, 1A9 or 2B10 (41, 42). To better examine this, studies are currently underway attempting to examine the relationship between the UGT2B17 deletion genotype and NNAL glucuronidating activities in human lung microsomes.
A limitation of the present study is the fact that all of the urine specimens examined in this study were from Caucasian subjects. It would be interesting to expand to incorporate subjects from other racial or ethnic groups. For instance, a functional splice site SNP in UGT2B10 (rs116294140) is relatively prevalent in African Americans but was not incorporated into the present analysis due to its low prevalence (<1%) in Caucasians (43). Another limitation is that dietary information was not collected from the individual subjects examined in this study. Therefore, the potential influence of other factors including specific dietary exposures on NNAL metabolism could not be performed in this study.
The average urinary levels of NNAL and its metabolites in smokers were below 1 nM in the present study. This is more than 10,000-fold lower than the levels of urinary nicotine and its major metabolite, cotinine (44, 45). It is therefore a technical challenge to directly quantify NNAL and its metabolites in the urine of tobacco users. Previous studies from the Hecht lab (University of Minnesota) have examined urinary NNAL and its glucuronides by measuring, (i) free NNAL, (ii) free NNAL + NNAL-N-Gluc, and (iii) total-NNAL, with the levels of both NNAL-N-Gluc and total NNAL-O-Gluc mathematically-derived. This methodology incorporates several sample processing steps and corresponding LC-MS runs using up to 180 μL of urine specimen and a nano-LC-MS instrument (35). This method is sensitive, reaching levels as low as 0.10 nM for the quantification of urinary free NNAL (35, 46). Limitations of this procedure include the fact that NNAL-N-Gluc and NNAL-O-Gluc are not directly measured, and that the (R)- and (S)-NNAL-O-Gluc diasteriomers are not measured individually. A LC-MS method for the simultaneous quantification of intact NNAL-O-Gluc and NNAL-N-Gluc (as well as free NNAL) was recently described by Yao et al (47). This method is highly sensitive, reaching detection levels as low as 0.007, 0.039 and 0.055 nM for free NNAL, NNAL-N-Gluc and NNAL-O-Gluc, respectively, and incorporates a purification step using dichloromethane and a further extraction step by solid-phase extraction which concentrates each urine sample 100-fold. The (R)- and (S)-NNAL-O-Gluc diastereomers are not measured individually within this methodology.
The UPLC-MS method developed in the present study is the first to simultaneously and directly quantify the urinary levels of free NNAL, NNAL-N-Gluc, and the individual (R)-NNAL-O-Gluc and (S)-NNAL-O-Gluc diastereomers in tobacco users. It requires only 10 μL of urine sample, no sample incubation or extraction steps, and a single UPLC-MS analysis. The average percentage of NNAL-N-Gluc, NNAL-O-Gluc [(R)-NNAL-O-Gluc + (S)-NNAL-O-Gluc] and free-NNAL in the urine samples from 158 smokers in the present study were 23%, 50% and 27%, respectively, which is highly consistent with that observed in smokers in previous studies [22%, 48% and 31%, respectively (35)]. These data suggest that the values obtained using the methodology described in the present study are highly accurate. While the lower limit of detection for urinary free NNAL in the current study is similar to those of Hecht’s studies (35), it is approximately 14-fold higher than the detection limits observed for NNAL using the Yao et al methodology (47). However, the limits of detection observed in the present study for NNAL-N-Gluc (0.10 nM) and each of the NNAL-O-Gluc diastereomers [0.025 nM for (S)-NNAL-O-Gluc and 0.050 nM for (R)-NNAL-O-Gluc] are similar to that observed for NNAL-N-Gluc (0.039 nM) and total NNAL-O-Gluc (0.055 nM) in the Yao et al methodology (47). In the present study, the lower limits of detection observed for urinary NNAL-N-Gluc, (R)-NNAL-O-Gluc, and (S)-NNAL-O-Gluc are similar to or lower than that observed for free NNAL (0.1 nM). Together, these data suggest that the methodology described in the present study is quite sensitive for the direct detection of these glucuronidated NNAL metabolites.
It has been found that (S)-NNAL is stereoselectively retained in rat lung and has a higher tumorigenicity than (R)-NNAL, and (R)-NNAL exhibits a higher rate of glucuronidation in rats (17, 18, 48) and the A/J mouse (49, 50). Data from previous studies suggest that (S)-NNAL may also be stereoselectively retained in humans (19). However, the levels of urinary (S)-NNAL-O-Gluc were higher than the levels of urinary (R)-NNAL-O-Gluc in smokers (19), tobacco chewers (19) and in the patas monkey (51), a pattern consistent with the results from the present study where the mean levels of urinary (S)-NNAL-O-Gluc was approximately 25% higher than the mean levels of urinary (R)-NNAL-O-Gluc in smokers. These data could reflect higher levels of (S)-NNAL formed from NNK during NNK metabolism after ingestion/inhalation, or be due to a preferential glucuronidation of (S)-NNAL, in primates vs. rodents. Further studies will be required to examine this in full.
As indicated in Table 2, there were individual values for NNAL metabolites that were below the detection limit; these raw values were used as true values for this study. To confirm the UGT genotype:urinary NNAL metabolite phenotype associations observed in this study, we also applied another strategy in dealing with values that are below the limit of detection by arbitrarily setting any non-detectable value equal to one-half of the minimum level of detection for that metabolite (0.05 nM for NNAL-N-Gluc, 0.025 nM for (R)-NNAL-O-Gluc, 0.0125 nM for (S)-NNAL-O-Gluc, and 0.05 nM for free NNAL). Virtually identical statistically significant results were observed for all associations using this alternate strategy (results not shown).
In summary, NNAL-N-Gluc, (R)-NNAL-O-Gluc, (S)-NNAL-O-Gluc and free NNAL in smoker’s urine were simultaneously and directly determined using a simple UPLC-MS analysis to demonstrate, for the first time, strong genotype-phenotype associations between both the UGT2B10 codon 67 Asp<Tyr genotype and urinary NNAL-N-Gluc levels and between the UGT2B17 copy number variant and urinary (R)-NNAL-O-Gluc levels in smokers. These results suggest that functional polymorphisms in UGT2B10 and UGT2B17 are associated with reduced detoxification capacity against NNAL and therefore may affect individual cancer risk upon tobacco exposure.
Acknowledgements
This work was supported in part by the National Institutes of Health, National Institutes of Environmental Health Sciences [Grant R01-ES025460, to P Lazarus] and the Health Sciences and Services Authority of Spokane, WA [Grant WSU002292, to P Lazarus]. We thank the China Scholarship Council for financial support [Scholarship to Shaman Luo]. We also thank the Mass Spectrometry Core facility at Washington State University Spokane for their help with LC-MS.
Footnotes
The authors declare that there are no conflicts of interest.
References
- 1.Hecht SS. Tobacco smoke carcinogens and lung cancer. Journal of the National Cancer Institute. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 2.Shopland DR, Eyre HJ, Pechacek TF. Smoking-attributable cancer mortality in 1991: is lung cancer now the leading cause of death among smokers in the United States? J Natl Cancer Inst. 1991;83:1142–8. doi: 10.1093/jnci/83.16.1142. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann D, Adams JD, Lisk D, Fisenne I, Brunnemann KD. Toxic and carcinogenic agents in dry and moist snuff. J Natl Cancer Inst. 1987;79:1281–6. [PubMed] [Google Scholar]
- 4.Hoffmann D, Hecht SS. Springer Handbook of Experimental Carcinogenesis and Mutagenesis. Springer Publications; New York: 1989. Advances in tobacco carcinogenesis; pp. 63–102. [Google Scholar]
- 5.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical research in toxicology. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 6.Akopyan G, Bonavida B. Understanding tobacco smoke carcinogen NNK and lung tumorigenesis. Int J Oncol. 2006;29:745–52. [PubMed] [Google Scholar]
- 7.Yuan JM, Butler LM, Stepanov I, Hecht SS. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 2014;74:401–11. doi: 10.1158/0008-5472.CAN-13-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann D, Rivenson A, Amin S, Hecht SS. Dose-response study of the carcinogenicity of tobacco-specific N-nitrosamines in F344 rats. J Cancer Res Clin Oncol. 1984;108:81–6. doi: 10.1007/BF00390978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann D, Brunnemann KD, Prokopczyk B, Djordjevic MV. Tobacco-specific N-nitrosamines and Areca-derived N-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. Journal of toxicology and environmental health. 1994;41:1–52. doi: 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- 10.Belinsky SA, Devereux TR, White CM, Foley JF, Maronpot RR, Anderson MW. Role of Clara cells and type II cells in the development of pulmonary tumors in rats and mice following exposure to a tobacco-specific nitrosamine. Exp Lung Res. 1991;17:263–78. doi: 10.3109/01902149109064417. [DOI] [PubMed] [Google Scholar]
- 11.Oreffo VI, Lin HW, Padmanabhan R, Witschi H. K-ras and p53 point mutations in 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced hamster lung tumors. Carcinogenesis. 1993;14:451–5. doi: 10.1093/carcin/14.3.451. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS, Chen CB, Ohmori T, Hoffmann D. Comparative carcinogenicity in F344 rats of the tobacco-specific nitrosamines, N'-nitrosonornicotine and 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1980;40:298–302. [PubMed] [Google Scholar]
- 13.Foiles PG, Akerkar SA, Carmella SG, Kagan M, Stoner GD, Resau JH, et al. Mass spectrometric analysis of tobacco-specific nitrosamine-DNA adducts in smokers and nonsmokers. Chem Res Toxicol. 1991;4:364–8. doi: 10.1021/tx00021a017. [DOI] [PubMed] [Google Scholar]
- 14.Parsa I, Foye CA, Cleary CM, Hoffmann D. Differences in Metabolism and Biological Effects of NNK in Human Target Cells. Vol. 23. Cold Spring Harbor, NY: 1986. pp. 233–44. Banbury Report No. 23. [Google Scholar]
- 15.Klein-Szanto AJ, Iizasa T, Momiki S, Garcia-Palazzo I, Caamano J, Metcalf R, et al. A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1992;89:6693–7. doi: 10.1073/pnas.89.15.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmella SG, Akerkar S, Hecht SS. Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers' urine. Cancer research. 1993;53:721–4. [PubMed] [Google Scholar]
- 17.Lao Y, Yu N, Kassie F, Villalta PW, Hecht SS. Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2007;20:235–45. doi: 10.1021/tx060207r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman CL, Wu Z, Upadhyaya P, Hecht SS. Stereoselective metabolism and tissue retention in rats of the individual enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), metabolites of the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 2004;25:1237–42. doi: 10.1093/carcin/bgh120. [DOI] [PubMed] [Google Scholar]
- 19.Hecht SS, Carmella SG, Ye M, Le KA, Jensen JA, Zimmerman CL, et al. Quantitation of metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone after cessation of smokeless tobacco use. Cancer Res. 2002;62:129–34. [PubMed] [Google Scholar]
- 20.Chen G, Blevins-Primeau AS, Dellinger RW, Muscat JE, Lazarus P. Glucuronidation of nicotine and cotinine by UGT2B10: loss of function by the UGT2B10 Codon 67 (Asp>Tyr) polymorphism. Cancer research. 2007;67:9024–9. doi: 10.1158/0008-5472.CAN-07-2245. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Dellinger RW, Sun D, Spratt TE, Lazarus P. Glucuronidation of tobacco-specific nitrosamines by UGT2B10. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:824–30. doi: 10.1124/dmd.107.019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Dellinger RW, Gallagher CJ, Sun D, Lazarus P. Identification of a prevalent functional missense polymorphism in the UGT2B10 gene and its association with UGT2B10 inactivation against tobacco-specific nitrosamines. Pharmacogenetics and genomics. 2008;18:181–91. doi: 10.1097/FPC.0b013e3282f4dbdd. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Giambrone NE, Dluzen DF, Muscat JE, Berg A, Gallagher CJ, et al. Glucuronidation Genotypes and Nicotine Metabolic Phenotypes: Importance of Functional UGT2B10 and UGT2B17 Polymorphisms. Cancer Research. 2010;70:7543–52. doi: 10.1158/0008-5472.CAN-09-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarus P, Zheng Y, Aaron Runkle E, Muscat JE, Wiener D. Genotype-phenotype correlation between the polymorphic UGT2B17 gene deletion and NNAL glucuronidation activities in human liver microsomes. Pharmacogenetics and genomics. 2005;15:769–78. doi: 10.1097/01.fpc.0000175596.52443.ef. [DOI] [PubMed] [Google Scholar]
- 25.Ren Q, Murphy SE, Zheng Z, Lazarus P. O-Glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) by human UDP-glucuronosyltransferases 2B7 and 1A9. Drug metabolism and disposition: the biological fate of chemicals. 2000;28:1352–60. [PubMed] [Google Scholar]
- 26.Kozlovich S, Chen G, Lazarus P. Stereospecific Metabolism of the Tobacco-Specific Nitrosamine, NNAL. Chem Res Toxicol. 2015 doi: 10.1021/acs.chemrestox.5b00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher CJ, Muscat JE, Hicks AN, Zheng Y, Dyer AM, Chase GA, et al. The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:823–8. doi: 10.1158/1055-9965.EPI-06-0823. [DOI] [PubMed] [Google Scholar]
- 28.Wiener D, Fang JL, Dossett N, Lazarus P. Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer research. 2004;64:1190–6. doi: 10.1158/0008-5472.can-03-3219. [DOI] [PubMed] [Google Scholar]
- 29.Ashmore JH, Lesko SM, Miller PE, Cross AJ, Muscat JE, Zhu J, et al. Association of dietary and supplemental iron and colorectal cancer in a population-based study. European Journal of Cancer Prevention. 2013 doi: 10.1097/CEJ.0b013e32836056f8. [DOI] [PubMed] [Google Scholar]
- 30.Ashmore JH, Lesko SM, Muscat JE, Gallagher CJ, Berg AS, Miller PE, et al. Association of dietary and supplemental folate intake and polymorphisms in three FOCM pathway genes with colorectal cancer in a population-based case-control study. Genes, chromosomes & cancer. 2013;52:945–53. doi: 10.1002/gcc.22089. [DOI] [PubMed] [Google Scholar]
- 31.Miller PE, Lazarus P, Lesko SM, Cross AJ, Sinha R, Laio J, et al. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr Cancer. 2013;65:202–26. doi: 10.1080/01635581.2013.756534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller PE, Lazarus P, Lesko SM, Muscat JE, Harper G, Cross AJ, et al. Diet index-based and empirically derived dietary patterns are associated with colorectal cancer risk. J Nutr. 2010;140:1267–73. doi: 10.3945/jn.110.121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiener D, Doerge DR, Fang JL, Upadhyaya P, Lazarus P. Characterization of N-glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human liver: importance of UDP-glucuronosyltransferase 1A4. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:72–9. doi: 10.1124/dmd.32.1.72. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Giambrone NE, Lazarus P. Glucuronidation of trans-3'-hydroxycotinine by UGT2B17 and UGT2B10. Pharmacogenetics and genomics. 2012;22:183–90. doi: 10.1097/FPC.0b013e32834ff3a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chemical research in toxicology. 2013;26:1209–17. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011;13:202–8. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Giambrone NE, Jr., Dluzen DF, Muscat JE, Berg A, Gallagher CJ, et al. Glucuronidation genotypes and nicotine metabolic phenotypes: importance of functional UGT2B10 and UGT2B17 polymorphisms. Cancer research. 2010;70:7543–52. doi: 10.1158/0008-5472.CAN-09-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmella SG, Chen M, Zarth A, Hecht SS. High throughput liquid chromatography-tandem mass spectrometry assay for mercapturic acids of acrolein and crotonaldehyde in cigarette smokers' urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;935:36–40. doi: 10.1016/j.jchromb.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassenaar CA, Conti DV, Das S, Chen P, Cook EH, Ratain MJ, et al. UGT1A and UGT2B genetic variation alters nicotine and nitrosamine glucuronidation in european and african american smokers. Cancer Epidemiol Biomarkers Prev. 2015;24:94–104. doi: 10.1158/1055-9965.EPI-14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallon JK, Neubert H, Hyland R, Goosen TC, Smith PC. Targeted quantitative proteomics for the analysis of 14 UGT1As and -2Bs in human liver using NanoUPLC-MS/MS with selected reaction monitoring. J Proteome Res. 2013;12:4402–13. doi: 10.1021/pr4004213. [DOI] [PubMed] [Google Scholar]
- 41.Jones NR, Lazarus P. UGT2B gene expression analysis in multiple tobacco carcinogen-targeted tissues. Drug Metab Dispos. 2014;42:529–36. doi: 10.1124/dmd.113.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallagher CJ, Balliet RM, Sun D, Chen G, Lazarus P. Sex differences in UDP-glucuronosyltransferase 2B17 expression and activity. Drug metabolism and disposition: the biological fate of chemicals. 2010;38:2204–9. doi: 10.1124/dmd.110.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35:2526–33. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- 45.Radwan G, Hecht SS, Carmella SG, Loffredo CA. Tobacco-specific nitrosamine exposures in smokers and nonsmokers exposed to cigarette or waterpipe tobacco smoke. Nicotine Tob Res. 2013;15:130–8. doi: 10.1093/ntr/nts099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SL, Carmella SG, Ming X, Vielguth E, Stram DO, Le Marchand L, et al. Variation in levels of the lung carcinogen NNAL and its glucuronides in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:561–9. doi: 10.1158/1055-9965.EPI-14-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao L, Zheng S, Guan Y, Yang J, Liu B, Wang W, et al. Development of a rapid method for the simultaneous separation and determination of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its N- and O-glucuronides in human urine by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2013;788:61–7. doi: 10.1016/j.aca.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Wang M, Villalta PW, Lindgren BR, Upadhyaya P, Lao Y, et al. Analysis of pyridyloxobutyl and pyridylhydroxybutyl DNA adducts in extrahepatic tissues of F344 rats treated chronically with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2009;22:926–36. doi: 10.1021/tx900015d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hecht SSS, T. E, Trushin N. Corrigendum: Absolute configuration of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol formed metabolically from4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 2000;21:850. [PubMed] [Google Scholar]
- 50.Upadhyaya P, Kenney PM, Hochalter JB, Wang M, Hecht SS. Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers and metabolites in the A/J mouse. Carcinogenesis. 1999;20:1577–82. doi: 10.1093/carcin/20.8.1577. [DOI] [PubMed] [Google Scholar]
- 51.Hecht SS, Trushin N, Reid-Quinn CA, Burak ES, Jones AB, Southers JL, et al. Metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the patas monkey: pharmacokinetics and characterization of glucuronide metabolites. Carcinogenesis. 1993;14:229–36. doi: 10.1093/carcin/14.2.229. [DOI] [PubMed] [Google Scholar]