Abstract
Purpose of review
To describe the recent advances made in imaging of the right heart, including deformation imaging, tissue and flow characterization by resonance imaging (MRI), and molecular imaging.
Recent findings
Recent developments have been made in the field of deformation imaging of the right heart, which may improve risk stratification of patients with heart failure and pulmonary hypertension. In addition, more attention has been given to load adaptability metrics of the right heart; these simplified indices however still face challenges from a conceptual point of view. The emergence of novel MRI sequences, such as native T1 mapping, allows better detection and quantification of myocardial fibrosis and could allow better prediction of post-surgical recovery of the right heart. Other advances in MRI include four-dimensional flow imaging, which may be particularly useful in congenital heart disease or for the detection of early stages of pulmonary vascular disease.
Summary
This review will place the recent developments in right heart imaging in the context of clinical care and research.
Keywords: coupling, heart failure, imaging, pulmonary hypertension, right heart
Introduction
In the past three decades, significant progress has been made in the imaging of the right heart. One of the most important changes has been the greater awareness given to right heart function in both clinical and research settings (1). In this review, we will highlight recent innovations in the field of right heart imaging, including myocardial deformation imaging, 3D and 4D flow magnetic resonance imaging (MRI), as well as molecular imaging. These recent developments will be placed in the context of how right heart imaging can improve the management of heart failure (HF) and pulmonary hypertension (PH). The value of right heart imaging in the setting of congenital heart disease or cardiac surgery is beyond the scope of the current review and has recently been reviewed (2,3).
Right and left heart, is there a real distinction?
While the separation between the right and left heart has clear anatomical and embryological basis, it does not reflect the complexity of the structural and functional relationships and interactions between the heart and the circulation. Anatomically, the right (RV) and left (LV) ventricles are strongly connected through the septum and myofiber architecture (4). Because of the functional interactions between the ventricles, interpretation of RV performance should always be made in the context of LV function and vice versa. In the absence of complex congenital heart disease, the effective stroke volume of both ventricles is equal on average. Consequently, RV dysfunction in patients with predominantly “left heart failure” could mainly reflect low stroke volume and not necessarily intrinsic myocardial involvement of the right heart. In addition to systolic interactions, diastolic interactions, neurohormonal factors and interventricular dyssynchrony blur the lines between the right and left heart (5–7).
The right heart, as part of the cardiopulmonary unit
In addition to interventricular interactions, a comprehensive understanding of RV function should also consider the cardiopulmonary unit (1). As shown in Figure 1, this includes a better assessment of ventriculoarterial coupling (matching between RV contractility and afterload), ventilation perfusion matching, as well as atrioventricular coupling.
Figure 1. Simplified representation of the “unfolded circulation”: right atrium (RA), right ventricle (RV), lungs, left atrium (LA), left ventricle (LV) and systemic organs.
Concepts related to coupling and matching between different physiological and anatomical entities are represented. V/Q: ventilation/perfusion. (Reproduced with permission from Laboratory of Surgical Research of the Marie Lannelongue Hospital and Springer International Publishing Switzerland).
Right heart reference values, have we made any progress?
Recent efforts have been made to develop reference values for RV structure and function assessment. In 2010, Rudski et al. published an American/European consensus document on the evaluation of the right heart, later incorporated in the 2015 American Society of Echocardiography and European Association of Cardiovascular Imaging recommendations on chamber quantification (8,9). Table 1 summarizes the different proposed thresholds for enlargement or dysfunction (8–16); a recent large community based study also proposed population derived values (17). Some controversies still exist on which threshold to use for RV longitudinal strain (RVLS) and tricuspid annular plane systolic excursion (TAPSE). For example, some authors use slightly higher thresholds than those suggested in the guidelines, such as −25% for RVLS compared to the −20% recommended, or 18 mm used for TAPSE instead of 17mm (9,12). Another area of uncertainty comes from the absence of well-established gradation for RV enlargement or dysfunction, in contrast to well-defined left heart metrics, realizing that some of the gradation will be in part arbitrary. RV ejection fraction < 35% has often been used as a criterion for moderate RV systolic dysfunction, which corresponds to an RV fractional area change (RVFAC) of approximately 25% (18). Additionally to the normative values, several right heart metrics thresholds have been proposed for risk stratification. Tables 2 and 3 summarize the main prognostic right heart metrics in HF and PH (19–31,10,32,33,12).
Table 1.
Selection of the most relevant right heart imaging metrics.
| Metrics | Reference values* | Clinical relevance and comments |
|---|---|---|
| Dimensional indices | ||
| RV volumes | Sex dependent | Increased RV volumes and decreased LV end- diastolic volume are predictors of poor survival in PH |
| RV mass | Indexed on BSA 21±4 g/m2 |
Hypertrophy is predictor of poor survival in PAH but better survival in patients with Eisenmenger Reflects adaptive response but has to be interpreted with regards to load |
| RA enlargement | >11.0 cm2/m2 | > 15.4 cm2/m2 is associated with poor survival in PAH |
| Systolic functional indices | ||
| RVEF | > 50 % | < 35 % by MRI or SPECT often used as threshold to discriminate outcome associated with poor survival in HFrEF and PH < 45 % by 3D–echocardiography usually reflects abnormal RV systolic function, though laboratories may choose to refer to age- and gender-specific values |
| RVFAC | >35 % | < 25 % denotes moderate to severe dysfunction Predictor of poor survival in PH and HF |
| TAPSE | > 17 mm | < 14 mm is predictor of poor survival in PH Load-dependent Limited value after cardiac surgery |
| S’ peak velocity | > 12 cm/s | < 8 cm/s is considered abnormal Load dependent |
| Deformation indices | ||
| Global longitudinal strain |
< −20 % | Severe often if > −15 % by speckle tracking Predictor of survival in PH (12) |
| Systolodiastolic functional index | ||
| RVMPI- pulsed tissue |
< 0.55 | > 0.88 predict poor survival in idiopathic PAH Possible pseudo-normalized in case of severe RV dysfunction |
| Diastolic metric | ||
| IVRT (TDI) corrected | < 65 ms |
> 75 ms (non corrected) has been associated with RV dysfunction Requires indexation by heart rate (IVRT divided by square root of RR interval) |
| Pulmonary flow | ||
| Pulmonary acceleration time |
> 93 ms | Useful to screen for PH (> 105 ms), particularly in case of severe tricuspid regurgitation No evidence of prognosis value, time dependent (adjustment to heart rate in theory) |
| Ventricular interdependency | ||
| Eccentricity index (EI) | No normative value, usually < 1 |
Diastolic EI predictor of poor survival in PH (no consensus on the threshold) End-systole EI reflects pressure-overload while end- diastole EI reflects volume-overload |
| Myocardial fibrosis | ||
| Delayed contrast – enhancement (gadolinium) |
Absent | Presence reflects localized fibrosis Strongly correlates with increased RV mass, volumes and pulmonary pressures |
| Native T1 mapping | T1 times: RV > LV | Able to detect diffuse fibrosis Correlates with RV-PA coupling, RV performance and pulmonary pressures in a piglet model |
| Nuclear imaging | ||
| 18F- fluorodeoxyglucose PET |
No uptake by the RV | RV/LV uptake ratio increased in PH |
| 201-thallium or 99mTc SPECT |
Uptake LV > RV | Relative increase in RV perfusion compared to LV with stress may indicate multivessel coronary artery disease (15,16) |
BSA: body surface area; HFrEF: left heart failure with reduced ejection fraction; IVRT: isovolumic relaxation time; LV: left ventricle; PA: pulmonary artery; PAH: pulmonary arterial hypertension; PET: positron emission tomography; PH: pulmonary hypertension; RA: right atrium; RV: right ventricle; RVEF: RV ejection fraction; RVFAC: RV fractional area change; RVMPI: RV myocardial performance index; SPECT: single photon emission computed tomography; TAPSE: tricuspid annular plane systolic excursion; TDI: tissue Doppler imaging.
Table 2.
Selected studies associating right heart metrics with survival in left heart failure.
| Study | Population | Number of patients |
Outcomes | Afterload metrics* |
Right ventricular metrics* |
Comments |
|---|---|---|---|---|---|---|
| Heart failure with reduced ejection fraction (HFrEF) | ||||||
| Ghio et al. 2000 (19) |
Chronic HF LVEF <35% |
140 | Death or urgent transplant during FU (2 years) |
- | TAPSE ≤ 14mm |
|
| Meyer et al. 2010 (20) |
Chronic HF LVEF ≤35% |
2008 | Death during FU (2 years) |
- | RVEF < 20% | Large nuclear study |
| Dupont et al. 2012 (21) |
Chronic and acute HF LVEF =19±9% |
724 | Death or transplant during FU (3.2 years) |
Capacitance |
Visual right ventricular systolic dysfunction Cardiac Index |
First study to demonstrate prognostic value of capacitance in HFrEF |
| Gulati et al. 2013 (22) |
Non-ischemic dilated cardiomyopathy |
250 | Death or transplant during FU (6.8 years) |
- | RVEF ≤ 45% | MRI study |
| Cameli et al. 2013 (23) |
Advanced HF referred for heart transplant |
98 | Composite endpoint (death, transplant, hospitalization, mechanical assistance) during FU (1.5 years) |
- | Free-wall RV longitudinal strain RV global longitudinal strain RVFAC |
First study to report the prognostic value of RV strain in end- stage HF |
| Aronson et al. 2013 (24) |
Acute HF HFrEF or HFpEF |
326 | Death during FU (1 year) |
- | RVFAC <35% in patients with PH |
Rare study on acute failure, irrespective of LVEF |
| Iacoviello et al. 2016 (26) |
Chronic HF LVEF <45% |
332 | Death during FU (3 years) |
- | Free-wall RV longitudinal strain RV global longitudinal strain |
First study to report the prognostic value of RV strain in stable outpatients |
| Heart failure with preserved ejection fraction (HFpEF) | ||||||
| Al- Naamani et al. 2015 (25) |
LVEF >50% with PH |
73 | Death during FU (3.6 years) |
Capacitance |
- | First study to demonstrate prognostic value of capacitance in HFpEF, more studies with multivariate models are needed |
In multivariate analysis. FU: follow-up (median or mean is displayed); HF: heart failure; LVEF: left ventricular ejection fraction; PH: pulmonary hypertension; RVEF: right ventricular ejection fraction.
Table 3.
Selected studies associating right heart metrics with survival in pulmonary hypertension.
| Study | Population | Number of patients |
Outcomes | Afterload metrics* |
Right ventricular metrics* |
Comments |
|---|---|---|---|---|---|---|
| NIH registry | ||||||
| D’Alonzo et al. 1991 (27) |
PAH | 194 | Death or heart- lung transplant during FU (2.8 years) |
MPAP Mean right atrial pressure |
Cardiac Index | Does not reflect the current survival rate Only applicable to naïve-treatment patients |
| Pulmonary Hypertension Connection registry | ||||||
| Thenappan et al. 2010 (28) |
PAH | 578 | Death during FU (3.9 years) |
MPAP Mean right atrial pressure |
Cardiac Index | Does not reflect the recent treatments available |
| French National registry | ||||||
| Humbert et al. 2010 (29) | Idiopathic, familial, drug and toxins- associated PAH |
354 | Death at 3 years | - | Cardiac Index | |
| REVEAL Registry | ||||||
| Benza et al. 2010 (30) |
Idiopathic, familial, CHD and CTD- Associated PAH |
2,716 | Death at 1 year | Pulmonary Vascular Resistance (> 32 Wood Units) |
Pericardial effusion Mean right atrial pressure (>20mmHg) |
|
| UK registry | ||||||
| Lee et al. 2012 (31) |
Idiopathic, familial, CHD and CTD associated PAH |
182 | Death at 1 and 2 years | - | Mean right atrial pressure | |
| Right Heart score | ||||||
| Haddad et al. 2015 (10) |
Idiopathic, familial, drug and toxins associated PAH |
95+87 | Death or lung transplant at 5 years |
- | RV dysfunction Severe RA enlargement Systemic blood pressure <110mmHg |
Simple Echocardiographic score in PAH |
| Others | ||||||
| Mahapatra et al. 2006 (32) |
Idiopathic PAH |
104 | Death at 4 years | Capacitance | - | |
| Haddad et al. 2011 (33) |
PAH admitted for acute right heart failure |
119 | Death or lung transplant at 90 days |
- | TR severity per grade |
Rare study on acutely deteriorated patients with PH |
| Fine et al. 2013 (12) |
Group 1, 3 and 4 PH vs. no PH |
406 + 169 | Death at 18 months |
None retained | Peak RV longitudinal strain Pericardial effusion Log (NT- proBNP) |
Large study on prognostic value of RV strain in PAH |
In multivariate analysis. CHD: congenital heart disease; CTD: connective tissue disease; MPAP: mean pulmonary arterial hypertension; PAH: pulmonary arterial hypertension; RV: right ventricle; TR: tricuspid regurgitation. For other abbreviations, see Table 2.
Pitfalls of right heart imaging
Several pitfalls in the assessment of the right heart have been described. First, as the annular motion frequently decreases after cardiac surgery following pericardial opening, it should be kept in mind that annular indices (such as TAPSE or S’ velocity) do not consequently reflect RV systolic function (34,35). This represents the most common cause of misdiagnosis of RV dysfunction post-operatively. A second important pitfall is to consider the different RV indices as equivalent; for example TAPSE is a less sensitive marker of ventricular dysfunction than RVFAC or RVLS. Third, when analyzing RV size on the apical 4-chamber view, careful attention should be given to ensure that the imaging plane reflects the major axis of the right ventricle, (i.e. neither off-axis nor foreshortened). These two considerations are especially important for accurate 2D quantification of RV size. A corollary pitfall is to draw conclusions on RV dimensions based on relative RV to LV size, as this leads to underestimation of RV size in patients with dilated LV. Forth, ventricular dysfunction should not be equated with impaired RV contractility. Evidence has been made of the potential recovery of the RV even if severely impaired in the setting of abnormal afterload, as illustrated by the remodeling after lung transplant in patients with pulmonary arterial hypertension (PAH) (36). Fifth, presence of a severe tricuspid regurgitation should systematically be assessed, as it exposes to overestimation of RV function based on volumetric metrics (such as ejection fraction, RVFAC), TAPSE or RVLS. Lastly, presence of RV artifacts using nuclear imaging, such as positron emission tomography (PET), can be caused attenuation or cardiac and respiratory motion.
Latest developments in echocardiography
Right ventricular deformation imaging
Myocardial deformation imaging has gathered a lot of attention in recent years leading to several thousand publications (37). Myocardial deformation encompasses different concepts including 1) strain, usually expressed as longitudinal, circumferential or radial strain, 2) strain rate, which represents the deformation over time and 3) velocity based parameters. As summarized in a statement paper by Voigt et al., strain may refer to either natural strain or Lagrangian strain (37). One of the landmark studies in the field is Dumesnil et al.’s that outlines the principles of axial and transverse shortening of the LV (38). Both reflect deformation of the myocardial wall, but while natural strain is expressed relative to the length at a previous time, Lagrangian strain is expressed relative to the initial length as follow: (end-systolic length – end-diastolic length)/end-diastolic length and is usually assessed using speckle tracking or by manual tracing (Figure 2) (39,40). Both concepts are related to each other mathematically but are not equivalent. Moreover, studying strain adds value to other volumetric metrics especially in cases of non-dilated ventricles (40). We recently showed that in “left heart failure”, LVEF and LV strain are more collinearly related to each other in patients with reduced ejection fraction (EF) compared to higher EF.
Figure 2. Myocardial deformation and velocity imaging of the right heart.
A: Superposed RV speckle tracking tracing with numbers representing segmental peak strain. Example from Philips tracking (developed for LV and applied to RV); specific RV tracking has also been developed by other vendors. B: Strain-time curve of the different signals. ApL indicates apex lateral; ApS, apex septum; BIS, basal interventricular septum; BL, basal lateral; GLS, global longitudinal strain; MIS, mid interventricular septum; and ML, mid lateral. (Adapted from Vonk Noordegraaf et al. Circulation 2015) (1).
Primarily developed in the LV, several studies have explored the value of longitudinal shortening of the RV-free wall in patients with advanced HF referred for heart transplant (23) and outpatients with HF (26). The software used for speckle tracking have mainly been developed for the LV. Tracking of the RV may be more challenging and is often more reliable in the basal and mid portion. Recently, Ryo et al. have developed a software evaluating both axial and surface RV strain using 3D methodology (41). Finally, it should be highlighted that right heart strain derived by MRI often focus on the circumferential strain, while strain derived by echocardiography focus on the longitudinal strain.
Three-Dimensional imaging of the right heart
3D–echocardiography (3DE) opens up the possibility of evaluating RV volumes, by overcoming the limitations of conventional 2D-echocardiography RV views with regard to orientation and reference points. A meta-analysis has indeed shown the good correlation between MRI and 3DE for RV volumes and ejection fraction assessment in patients and healthy subjects, with 3DE slightly underestimating volumes as compared to MRI (42). So far, only one multicenter study provides age-, body size-, and sex-specific reference values of 3DE derived RV volumes and EF in 507 healthy volunteers (43). Overall, women have smaller indexed RV volumes and higher EF compared to men, while older age is associated with smaller RV volumes (a decrement of 5 mL per decade for end-diastolic volume and 3 mL per decade for end-systolic volume) and higher EF (an increment of 1% per decade) (43). Lastly, a recent quantitative 3DE study have explored morphological subsets of RV adaption and remodeling in 92 patients with PH, and linked them to clinical outcomes (41). 3D RV end-systolic volume had indeed significantly better predictive values than end-diastolic volume or global strain to predict the combined endpoint of hospitalization, death, or lung transplantation.
Right ventricular - pulmonary arterial coupling
The measure of RV function routinely used in clinical practice reflects overall function and not contractility (44). The concept of ventriculoarterial coupling has been developed to describe matching between RV contractility and afterload; a ventricle that can increase its contractility in response to the increase in afterload usually stays well compensated. In PAH for example, RV contractility is increased but insufficient to match the increase in load, thus RV dysfunction ensues (1,45). This is an important distinction, as RV contractility is not decreased in PAH, as illustrated by the RV recovery post- lung transplantation in those patients.
As a related concept, there has been a recent interest in focusing on markers of load adaptability of the RV. This could help addressing two questions. The first is whether RV function is disproportionally reduced considering the ventricular wall stress or load. The second is on how to best combine right heart function and load metrics into a simple index, in order to more accurately assess RV function and PH. In the present review, we will highlight two examples. The first index proposed by Guazzi et al. is a simplified index of RV length-force relationship defined by TAPSE/systolic PAP ratio. A value < 0.36 mm/mmHg was associated with an increased cardiovascular mortality (Hazard Ratio of 10.4, [5.4–19.8], p<0.001) in 293 patients with heart failure with reduced (HFrEF) or preserved EF (HFpEF) (46). However, the applicability of this ratio in patients with PAH, who have wider range of pulmonary pressures, has not been validated yet. In addition, simple ratios may not address the question of dis/proportionality of function, as the relationships between function and afterload follows a nonlinear and often inverse fit (47,48). Figure 3 schematically represents the curvilinear fit of the relationships between RV function or end-systolic dimension, and afterload metrics (such as pressure, resistance, capacitance or estimation of the RV wall stress). The second load adaptation index proposed by Dandel et al. is defined as: (delta pressure between the RV and the RA) / [EDV/LED], estimated by [VTITR × LED] / AED, with EDV being end-diastolic volume, LED the RV length in end-diastole, AED the RV area in end-diastole and VTITR the velocity time integration of the TR signal (49). The prognostic value of this index was primarily demonstrated for the assessment of RV function recovery in patients with end-stage left HF on left ventricular assist devices (22). It was also validated in 79 patients with PAH awaiting lung transplantation, and shown to be associated with the risk of RV failure and transplant-free survival at 1 and 3 years (50). However, while this index provides complementary information about proportionality of ventricular adaptation, it does not replace remodeling or function parameters.
Figure 3. Relationships between right ventricular function (A) or end-systolic size (B) and ventricular afterload.
Based on the literature, this figure schematically represents the curvilinear fit (usually logarithmic fit) of the relationships between RV function or end-systolic dimension, and afterload (such as pressure, resistance, capacitance or estimation of the RV wall stress). Estimation of the wall stress is more challenging, but better reflects the force opposing ventricular function. The shape of the fit would be inversed if capacitance is used as afterload. Two examples are depicted on this figure (patient 1 and patient 2). Despite similar moderate right ventricular function, patients 1 and 2 differ in terms of RV adaptation. Patient 1 has a disproportional dysfunction as the function is worse than what would be expected for the mild increase in afterload compared to patient 2.
Innovations in Magnetic Resonance Imaging
Beyond volumetric and functional analysis, MRI also allows for tissue characterization, pulmonary stiffness assessment, and accurate quantification of blood flow. Table S1 compares the advantages and limitations of MRI and others imaging modalities.
Myocardial tissue characterization
Two novelties in myocardium characterization by MRI need to be mentioned. The first one is the non-inclusion of RV myocardial fatty infiltration in the recent revised Task Force diagnostic criteria for Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D) (Table 4) (51). In fact, although fatty infiltration had been considered as indicative of ARVC/D for years, recent evidence has questioned its specificity, showing the high rate of physiological fatty infiltration (without concomittant fibrosis) in healthy controls (52). The presence of regional RV akinesia or dyskinesia remains an important diagnostic criterion of ARVC/D (51). A specific MRI pattern, described as a focal “crinkling” of the RV outflow tract and subtricuspid regions (accordion sign), has been reported as a promising sign for early diagnosis of ARVC/D as only found in mutation carrier (53). The accordion sign is an example of regional RV wall motion abnormalities, similar to the regional contraction abnormalities first described by McConnell et al. two decades ago (54) in patients with acute pulmonary embolism, and recently revisited using echocardiographic strain (55).
Table 4.
2010 Revised Task Force imaging criteria for diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia.
| Major criteria | Minor criteria | |
|---|---|---|
| 2D–echocardiography | Regional RV akinesia, dyskinesia, or aneurysm and 1 of the following (end-diastole): - PLAX RVOT ≥32mm (corrected for body size PLAX/BSA ≥19mm/m2) - PSAX RVOT ≥36mm (corrected for body size PSAX/BSA ≥21mm/m2) - or fractional area change ≤33%. |
Regional RV akinesia, dyskinesia, or aneurysm and 1 of the following (end-diastole): - PLAX RVOT ≥29 to <32mm (corrected for body size PLAX/BSA ≥16 to <19mm/m2) - PSAX RVOT ≥32 to <36mm (corrected for body size PSAX/BSA ≥18 to <21mm/m2) - or fractional area change >33 to ≤40%. |
|
Magnetic Resonance Imaging |
Regional RV akinesia or dyskinesia* or dyssynchronous RV contraction And 1 or the following: - Ratio of RV end-diastolic volume to BSA ≥110mL/m2 (male) or ≥100mL/m2 (female) - or RV ejection fraction ≤40%. |
Regional RV akinesia or dyskinesia* or dyssynchronous RV contraction And 1 or the following: - Ratio of RV end-diastolic volume to BSA ≥100 to <110mL/m2 (male) or ≥90 to <100mL/m2 (female) - or RV ejection fraction >40% to ≤45%. |
| RV angiography | Regional RV akinesia, dyskinesia or aneurysm |
N/A |
The second novelty is the assessment of myocardial fibrosis by late gadolinium enhancement (LGE) or more recently T1 mapping. LGE identifies myocardial fibrosis, which has diagnostic (56) and prognostic (57) values. One of the main potential pitfalls of LGE imaging is that it may fail to adequately characterize diffuse interstitial myocardial fibrosis due to reliance on relative signal intensity changes and nulling of normal appearing myocardium (58). Quantitative assessment of the myocardial longitudinal relaxation time constant (T1) has in parallel emerged as a promising technique to assess for diffuse myocardial changes. T1 maps can be produced of non-contrast (native) myocardial T1 values (providing information on both the myocyte and the interstitium) or after gadolinium-based contrast administration (enabling quantification of the extracellular space) (59). In healthy controls, RV non-contrast T1 values have been shown to be higher than LV values, which may be explained by the higher collagen content of the RV myocardium (60,61). In the setting of RV dysfunction and PH, RV and hinge point non-contrast T1 and extra-cellular volume fraction values are elevated. Post-contrast T1 values are reduced (62,63) and may correlate with pulmonary hemodynamics, RV-PA coupling and RV function (64). Finally, several advanced techniques have been proposed to improve T1 quantification of thin walled structures such as the RV, including imaging in systole and higher resolution sequences (60,61,63,65).
Pulmonary arterial stiffness
Several parameters have been developed to provide information on local, regional or global stiffness: pulse pressure, elasticity, distensibility, compliance, capacitance, and stiffness index beta (66), as detailed in Supplementary Table S2. Among them, capacitance (invasively estimated as the ratio of SV divided by pulse pressure) has been associated to RV dysfunction, remodeling and mortality, independently from the level of resistance, in a wide spectrum of diseases (idiopathic and scleroderma-associated PAH, HFrEF and HFpEF) (21,25,32,47,67,68). Pulmonary arterial elasticity is measured as (maximal PA area – minimum area) / minimum area, using phase-contrast MRI, on the transverse perpendicular plane. It may be valuable for the detection of exercise induced pulmonary hypertension or earlier stages of pulmonary vascular disease (69).
Quantification of blood flow
Three-dimensional time-resolved (4D) flow MRI is an evolving imaging technique that yields both a vector of blood velocity and the magnitude signal intensity over an imaging volume, for each temporal phase of the cardiac cycle. 4D flow MRI allows the evaluation of blood flow including valvular regurgitation (Figure 4, left panel), quantification of biventricular volumes, function and mass, and visualization of intracardiac and extracardiac structures (70,71). A recent study demonstrated that RV volume, function, and mass can be quantified with 4D flow MRI with precision and inter-observer agreement comparable to that of cine Steady-State Free Precession (SSFP) (72). Whole heart 4D flow MRI also enables detection and visualization of both normal and abnormal right heart flow patterns (73). In patients with PH, 4D flow MRI often demonstrates a vortex pulmonary artery flow pattern (as shown in Figure 4, right panel (74)); the relative period of existence of the vortex significantly correlates to the mean pulmonary artery pressure (74,75). Peak systolic velocity, peak flow, stroke volume, and wall shear stress by 4D flow MRI are significantly lower in patients with PAH compared with healthy subjects (76,77). The prognostic value of 4D flow MRI still need to be proven; however it could offer in the future a noninvasive alternative to catheterization for flow assessment and may help early detection of RV dysfunction.
Figure 4. Four-dimensional flow MR imaging of a patient with pulmonary valvular disease (left); patients with pulmonary hypertension compared to a healthy control (right panel). Left panel.
Flow pattern of a patient with both pulmonary regurgitation (A and B, acquired during diastole) and pulmonary stenosis (D and C, acquired during systole). PA indicates main pulmonary artery; PV, pulmonary valve; and RV, right ventricle. Right panel: Typical flow patterns in the RV outflow tract at different cardiac phases for a patient with manifest PH (A, D, and G), a patient with latent PH (B, E, and H), and a normal subject (C, F, and I). At maximum outflow (A through C), flow profiles were distributed homogenously across the cross sections of the main pulmonary artery in manifest PH (A), latent PH (B), and normal (C). In later systole (D through F), a vortex was formed in manifest PH (D). No such vortex could be found in latent PH (E) or normal (F). After pulmonary valve closure (G through I), the vortex in patients with PH persisted for some time. In all cases, continuous diastolic blood flow upward along the anterior wall of the main pulmonary artery could be observed. Although this phenomenon disappeared quickly in controls (I), it was observed significantly longer in latent PH (H) and manifest PH (G). (Right panel adapted from Reiter et al. Circ Cardiovasc Imaging, 2008 (74)).
What is new in Computed Tomography imaging?
In patients with PH, computed tomography (CT) angiography is widely used to rule out chronic thromboembolic pulmonary hypertension and underlying lung disease, as well as to characterize precise anatomy in the setting of congenital heart defects (78). A recent study evaluated the utility of routinely performed non-ECG chest CT to screen for PH. Spruitj et al. showed that both PA dilation (ratio relative to the ascending aorta diameter ≥1) and RV enlargement (ratio relative to the LV diameter ≥1.2), measured on the axial view, were incremental for the detection of PH in 51 patients with advanced pre-capillary PH versus 25 non-PH controls (79). The application of this screening detection in a large population still remains to be done.
Molecular imaging, a deeper view into the biology of the right heart
Multiple molecular changes occur in a failing RV exposed to an increased afterload. Four of them represent potential targets for imaging. The first target derives from the RV metabolic shift from lipolysis towards glycolysis, which has been linked to worse ventricular function and poor survival (80). The increased uptake by the cardiomyocytes of the alternative source of energy (glucose) can be easily quantified using PET 18F-2-deoxy-2-fluoro-D-Glucose (18F-FDG). An increase in the RV to LV ratio tracer uptake has been reported in patients with PAH (81). It remains, however, unclear whether this increased ratio is explained by an increased RV glucose uptake (82) or a decreased LV uptake (83). Moreover, preclinical studies have suggested that this metabolic shift may be transient during progression of RV failure (84), which compromises the relevance of RV 18F-FDG uptake as a routine biomarker in PAH. The second target is the myocardial oxygen consumption, which can be imaged using 15O-labeled tracer or 11C-acetate tracers. An increased resting oxygen consumption by the RV, and hence a reduced efficiency has been shown in patients with PAH (85). Neurohormonal dysregulation is the third target. There has been growing evidence of the importance of upregulated sympathetic nervous system and renin-angiotensin-aldosterone system in the pathophysiology of RHF in PAH (86). Finally, angiogenesis and apoptosis are additional promising targets for detection of maladaptive RV in PH (87). While these processes have been imaged in LV diseases (88) and PH animal models, their clinical application in PAH is still pending.
New insights in right heart hemodynamics
Although the review focuses on right heart imaging, hemodynamics remains one of the most important biomarker that helps guide management in patients with PH and right-sided disease. In addition to right atrial and pulmonary pressures, three important parameters or ratio may be of clinical utility: 1) RV diastolic pressure waveforms, 2) relative PH (defined as the mean pulmonary to mean arterial pressure ratio, MPAP/MAP), 3) indices of load adaptability.
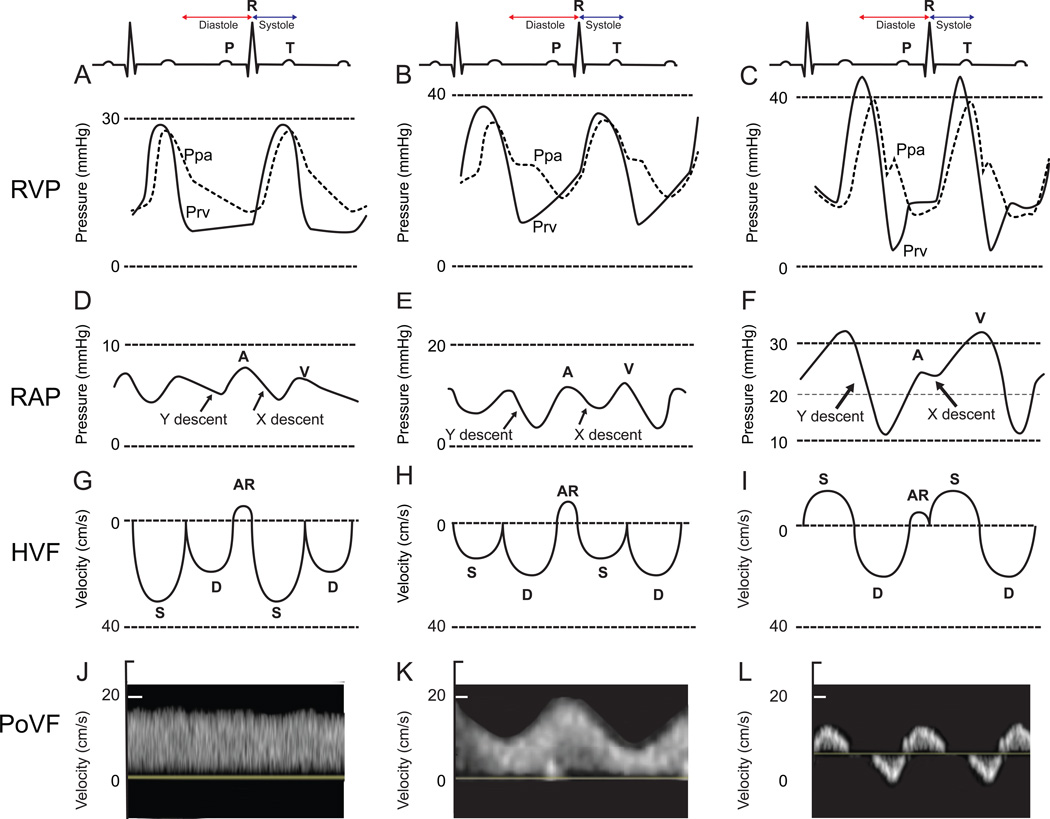
Displaying the right ventricular waveform provides insights for monitoring and management of patients. Using a pulmonary arterial catheter and transducing the RV pacing port (Paceport, Edwards Lifescience, Irvine, California), continuous RV and PA pressure waveforms can be obtained (89). As RV dysfunction occurs, the shape of the diastolic waveform progressively changes, from horizontal to an oblique aspect, followed by the appearance of a square-root sign suggesting progressive loss in RV diastolic compliance (Figure 5). These modifications observed on the RV pressure waveform can also be diagnosed using careful central venous pressure waveform analysis and Doppler hepatic and portal venous flow interrogation (90). The second metric is the relative estimation of MPAP. In case of RV failure, pulmonary pressure (MPAP) can be underestimated as it can be reduced proportionally to the decrease in systemic pressures, for example following anesthesia induction. The value of the MPAP/MAP ratio has previously been shown as the best hemodynamic predictor of post-op circulatory failure (91). The ratio is additionally associated with long term in aortic valve survival (92) and correlates with ventricular septal curvature (4). Finally, there has been a growing interest in proposing invasive load adaptability indices such as the RV functional index (RFI) measured as the systolic pressure divided by cardiac index (69). The RFI can be used to evaluate the extent to which elevated PA pressure is associated with preserved RV function. Elevated RFI may result from increasing PA pressure or decreasing cardiac index, both indicative of RV failure. Increased RFI has been associated to poor survival in 53 patients admitted to the intensive care unit with severe PH (69) and in 1439 patients undergoing cardiac surgery (91).
Figure 5. Right ventricular pressure (RVP), right atrial pressure (RAP), hepatic venous flow (HVF) and portal venous flow (PoVF) in normal patients (A,D,G,J) and typical patterns commonly observed in patients with mild (B,E,H,K) and severe (C,F,I,L) right ventricular dysfunction.
AR, atrial reversal HVF velocity; D, diastolic HVF Doppler velocity; Ppa, pulmonary artery pressure; Prv, right ventricular pressure, S, systolic HVF velocity. (Adapted from Haddad et al. Curr Opin Anaesthesiol, 2016 (3)).
Conclusions
The recent improvements in right heart imaging bring perspective into the physiology of the right heart, help early detection and prognostic stratification, and slowly bring the field in the new era of imaging biomarker guided management. Table 5 summarizes the six challenging unmet needs in the field of imaging of the right heart that are expected to be resolved in the coming ten years.
Table 5.
A look into the future of imaging the right heart.
| Unmet needs and future directions | |
|---|---|
| Echocardiography | - Validation of simple diagnostic and prognostic scores - Identifying useful load-adaptability indices - Compare the value of deformation imaging strain compared to end-systolic dimension for early detection of dysfunction and risk stratification |
|
Magnetic Resonance Imaging |
- Early detection of myocardial fibrosis and adverse remodeling using native T1 mapping sequences |
|
Computed Tomography |
- Validation of quantitative assessment of right ventricular morphology in routine chest computed tomography |
|
Positron Emission Tomography |
- Use of metabolic phenotype to tailor clinical care and management in patients with right heart failure |
Supplementary Material
Key points.
Although having anatomic and embryological basis, the separation between the right and the left ventricles is in part physiologically artificial.
Myocardial deformation imaging has gathered strong interest in imaging of the right heart; future studies will need to assess its value compared to end-systolic metrics.
Load-adaptability metrics can help answer the question of “proportionality” of ventricular adaptation to pulmonary hypertension.
Right ventricular (RV) myocardial fibrosis can be detected using Magnetic Resonance Imaging (MRI) late gadolinium enhancement and T1 mapping; 4D flow MRI is a promising tool for blood flow quantification.
Molecular imaging, such as positron emission tomography, provides information on the RV metabolism.
Acknowledgments
We would like to warmly thank Prof. Dominik Fleischmann (from the Division of Radiology, at Stanford University, USA) for his assistance with the review and mentorship, and Prof. Marlene Rabinovitch (from the Vera Moulton Wall Center and the Cardiovascular Institute, Stanford School of Medicine, Stanford University, USA) for her mentorship. We would also like to thank Dr. Marcus Chen (from the NHLBI - NIH, USA) for his permission to reproduce Table S1, as well as Prof. Olaf Mercier and Dr. David Boulate (from the Marie Lannelongue Hospitals, Le Plessis Robinson, France) for their authorization to reproduce Figure 1.
Financial support and sponsorship
This work was supported by the Stanford Cardiovascular Institute, the Vera Moulton Wall Center of Pulmonary Hypertension (Stanford University, Stanford, CA) and a NIH/NHLBI grant 5R01HL07418609. Dr. Myriam Amsallem received a Young Investigator Seed Grant from the Vera Moulton Wall Center of Pulmonary Hypertension at Stanford University.
Footnotes
Conflict of interest
None.
References
- 1.Vonk Noordegraaf A, Haddad F, Bogaard HJ, Hassoun PM. Noninvasive imaging in the assessment of the cardiopulmonary vascular unit. Circulation. 2015;131:899–913. doi: 10.1161/CIRCULATIONAHA.114.006972. [DOI] [PubMed] [Google Scholar]
- 2. D’Alto M, Dimopoulos K, Budts W, et al. Multimodality imaging in congenital heart disease-related pulmonary arterial hypertension. Heart Br Card Soc. 2016 doi: 10.1136/heartjnl-2015-308903. **Review on right heart imaging in congenital heart disease.
- 3. Haddad F, Elmi-Sarabi M, Fadel E, et al. Pearls and pitfalls in managing right heart failure in cardiac surgery. Curr Opin Anaesthesiol. 2016;29:68–79. doi: 10.1097/ACO.0000000000000284. *Review on right heart imaging after cardiac surgery.
- 4.Haddad F, Guihaire J, Skhiri M, et al. Septal curvature is marker of hemodynamic, anatomical, and electromechanical ventricular interdependence in patients with pulmonary arterial hypertension. Echocardiogr. 2014;31(6):699–707. doi: 10.1111/echo.12468. [DOI] [PubMed] [Google Scholar]
- 5.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–1724. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- 6.Belenkie I, Smith ER, Tyberg JV. Ventricular interaction: from bench to bedside. Ann Med. 2001;33:236–241. doi: 10.3109/07853890108998751. [DOI] [PubMed] [Google Scholar]
- 7.Gan CT-J, Lankhaar J-W, Marcus JT, et al. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2006;290:1528–1533. doi: 10.1152/ajpheart.01031.2005. [DOI] [PubMed] [Google Scholar]
- 8.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–788. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. **Latest American and European guidelines on right heart chambers quantification.
- 10.Haddad F, Spruijt OA, Denault AY, et al. Right Heart Score for Predicting Outcome in Idiopathic, Familial, or Drug- and Toxin-Associated Pulmonary Arterial Hypertension. JACC Cardiovasc Imaging. 2015;8:627–638. doi: 10.1016/j.jcmg.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghio S, Klersy C, Magrini G, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140:272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 12.Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6:711–721. doi: 10.1161/CIRCIMAGING.113.000640. [DOI] [PubMed] [Google Scholar]
- 13.Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–847. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 14.Zimbarra Cabrita I, Ruísanchez C, Grapsa J, et al. Validation of the isovolumetric relaxation time for the estimation of pulmonary systolic arterial blood pressure in chronic pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2013;14:51–55. doi: 10.1093/ehjci/jes093. [DOI] [PubMed] [Google Scholar]
- 15.Williams KA, Schneider CM. Increased stress right ventricular activity on dual isotope perfusion SPECT: a sign of multivessel and/or left main coronary artery disease. J Am Coll Cardiol. 1999;34:420–427. doi: 10.1016/s0735-1097(99)00193-x. [DOI] [PubMed] [Google Scholar]
- 16.Mannting F, Zabrodina YV, Dass C. Significance of increased right ventricular uptake on 99mTc-sestamibi SPECT in patients with coronary artery disease. J Nucl Med. 1999;40:889–894. [PubMed] [Google Scholar]
- 17.Kawut SM, Lima JAC, Barr RG, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiran H, Zamanian RT, McConnell MV, et al. Relationship between echocardiographic and magnetic resonance derived measures of right ventricular size and function in patients with pulmonary hypertension. J Am Soc Echocardiogr. 2014;27:405–412. doi: 10.1016/j.echo.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghio S, Recusani F, Klersy C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–842. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 20.Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupont M, Mullens W, Skouri HN, et al. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail. 2012;5:778–785. doi: 10.1161/CIRCHEARTFAILURE.112.968511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulati A, Ismail TF, Jabbour A, et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation. 2013;128:1623–1633. doi: 10.1161/CIRCULATIONAHA.113.002518. [DOI] [PubMed] [Google Scholar]
- 23.Cameli M, Righini FM, Lisi M, et al. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol. 2013;112:1778–1784. doi: 10.1016/j.amjcard.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Aronson D, Darawsha W, Atamna A, et al. Pulmonary hypertension, right ventricular function, and clinical outcome in acute decompensated heart failure. J Card Fail. 2013;19:665–671. doi: 10.1016/j.cardfail.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Al-Naamani N, Preston IR, Paulus JK, et al. Pulmonary Arterial Capacitance Is an Important Predictor of Mortality in Heart Failure With a Preserved Ejection Fraction. JACC Heart Fail. 2015;3:467–474. doi: 10.1016/j.jchf.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacoviello M, Citarelli G, Antoncecchi V, et al. Right Ventricular Longitudinal Strain Measures Independently Predict Chronic Heart Failure Mortality. Echocardiogr. 2016 doi: 10.1111/echo.13199. in press. [DOI] [PubMed] [Google Scholar]
- 27.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 28.Thenappan T, Shah SJ, Rich S, et al. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 30.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 31.Lee W-TN, Ling Y, Sheares KK, et al. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J. 2012;40:604–611. doi: 10.1183/09031936.00196611. [DOI] [PubMed] [Google Scholar]
- 32.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 33.Haddad F, Peterson T, Fuh E, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail. 2011;4:692–699. doi: 10.1161/CIRCHEARTFAILURE.110.949933. [DOI] [PubMed] [Google Scholar]
- 34.Wranne B, Pinto FJ, Hammarström E, et al. Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J. 1991;66:435–442. doi: 10.1136/hrt.66.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamborini G, Muratori M, Brusoni D, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- 36.Kusunose K, Tsutsui RS, Bhatt K, et al. Prognostic value of RV function before and after lung transplantation. JACC Cardiovasc Imaging. 2014;7:1084–1094. doi: 10.1016/j.jcmg.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Voigt J-U, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 38.Dumesnil JG, Shoucri RM, Laurenceau JL, et al. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation. 1979;59:1024–1034. doi: 10.1161/01.cir.59.5.1024. [DOI] [PubMed] [Google Scholar]
- 39.Dandel M, Lehmkuhl H, Knosalla C, et al. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev. 2009;5:133–148. doi: 10.2174/157340309788166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi Y, Ariyama M, Kobayashi Y, et al. Comparison of left ventricular manual versus automated derived longitudinal strain: implications for clinical practice and research. Int J Cardiovasc Imaging. 2016;32:429–437. doi: 10.1007/s10554-015-0804-x. [DOI] [PubMed] [Google Scholar]
- 41.Ryo K, Goda A, Onishi T, et al. Characterization of right ventricular remodeling in pulmonary hypertension associated with patient outcomes by 3-dimensional wall motion tracking echocardiography. Circ Cardiovasc Imaging. 2015:8. doi: 10.1161/CIRCIMAGING.114.003176. [DOI] [PubMed] [Google Scholar]
- 42.Shimada YJ, Shiota M, Siegel RJ, et al. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr. 2010;23:943–953. doi: 10.1016/j.echo.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Maffessanti F, Muraru D, Esposito R, et al. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging. 2013;6:700–710. doi: 10.1161/CIRCIMAGING.113.000706. [DOI] [PubMed] [Google Scholar]
- 44.Guihaire J, Haddad F, Boulate D, et al. Non-invasive indices of right ventricular function are markers of ventricular-arterial coupling rather than ventricular contractility: insights from a porcine model of chronic pressure overload. Eur Heart J Cardiovasc Imaging. 2013;14:1140–1149. doi: 10.1093/ehjci/jet092. [DOI] [PubMed] [Google Scholar]
- 45.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:22–33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 46.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:1373–1381. doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 47.Stevens GR, Garcia-Alvarez A, Sahni S, et al. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5:378–387. doi: 10.1016/j.jcmg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Bellofiore A, Chesler NC. Methods for measuring right ventricular function and hemodynamic coupling with the pulmonary vasculature. Ann Biomed Eng. 2013;41:1384–1398. doi: 10.1007/s10439-013-0752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dandel M, Potapov E, Krabatsch T, et al. Load dependency of right ventricular performance is a major factor to be considered in decision making before ventricular assist device implantation. Circulation. 2013;128:14–23. doi: 10.1161/CIRCULATIONAHA.112.000335. [DOI] [PubMed] [Google Scholar]
- 50.Dandel M, Knosalla C, Kemper D, et al. Assessment of right ventricular adaptability to loading conditions can improve the timing of listing to transplantation in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2015;34:319–328. doi: 10.1016/j.healun.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bomma C, Rutberg J, Tandri H, et al. Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2004;15:300–306. doi: 10.1046/j.1540-8167.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 53.Dalal D, Tandri H, Judge DP, et al. Morphologic variants of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy a genetics-magnetic resonance imaging correlation study. J Am Coll Cardiol. 2009;53:1289–1299. doi: 10.1016/j.jacc.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 54.McConnell MV, Solomon SD, Rayan ME, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 55.Tuzovic M, Adigopula S, Amsallem M, et al. Regional right ventricular dysfunction in acute pulmonary embolism: relationship with clot burden and biomarker profile. Int J Cardiovasc Imaging. 2015:389–398. doi: 10.1007/s10554-015-0780-1. [DOI] [PubMed] [Google Scholar]
- 56.Swift AJ, Rajaram S, Capener D, et al. LGE patterns in pulmonary hypertension do not impact overall mortality. JACC Cardiovasc Imaging. 2014;7:1209–1217. doi: 10.1016/j.jcmg.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Freed BH, Gomberg-Maitland M, Chandra S, et al. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012;14:11. doi: 10.1186/1532-429X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azevedo CF, Nigri M, Higuchi ML, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 59.Baksi AJ, Pennell DJ. T1 mapping in heart failure: from technique to prognosis, toward altering outcome. Circ Cardiovasc Imaging. 2013;6:861–863. doi: 10.1161/CIRCIMAGING.113.001178. [DOI] [PubMed] [Google Scholar]
- 60.Kawel-Boehm N, Dellas Buser T, Greiser A, et al. In-vivo assessment of normal T1 values of the right-ventricular myocardium by cardiac MRI. Int J Cardiovasc Imaging. 2014;30:323–328. doi: 10.1007/s10554-013-0326-3. [DOI] [PubMed] [Google Scholar]
- 61.Mehta BB, Auger DA, Gonzalez JA, et al. Detection of elevated right ventricular extracellular volume in pulmonary hypertension using Accelerated and Navigator-Gated Look-Locker Imaging for Cardiac T1 Estimation (ANGIE) cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:110. doi: 10.1186/s12968-015-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spruijt OA, Vissers L, Bogaard H-J, et al. Increased native T1-values at the interventricular insertion regions in precapillary pulmonary hypertension. Int J Cardiovasc Imaging. 2016;32:451–459. doi: 10.1007/s10554-015-0787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bilchick K, Mehta BB, Workman V, et al. Right Ventricular Extracellular Volume Fraction by Magnetic Resonance T1 Mapping in Pulmonary Hypertension and Heart Failure. Circulation. 2015;132:A14921–A14921. [Google Scholar]
- 64. García-Álvarez A, García-Lunar I, Pereda D, et al. Association of myocardial T1-mapping CMR with hemodynamics and RV performance in pulmonary hypertension. JACC Cardiovasc Imaging. 2015;8:76–82. doi: 10.1016/j.jcmg.2014.08.012. *This study shows the association between myocardial T1-mapping signal and pulmonary pressures and RV performance in pulmonary hypertension.
- 65.Kawel N, Nacif M, Zavodni A, et al. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012;14:27. doi: 10.1186/1532-429X-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanz J, Kariisa M, Dellegrottaglie S, et al. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:286–295. doi: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:252–260. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pellegrini P, Rossi A, Pasotti M, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–1070. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 69.Saydain G, Awan A, Manickam P, et al. Pulmonary Hypertension an Independent Risk Factor for Death in Intensive Care Unit: Correlation of Hemodynamic Factors with Mortality. Clin Med Insights Circ Respir Pulm Med. 2015;9:27–33. doi: 10.4137/CCRPM.S22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsiao A, Lustig M, Alley MT, et al. Rapid pediatric cardiac assessment of flow and ventricular volume with compressed sensing parallel imaging volumetric cine phase-contrast MRI. Am J Roentgenol. 2012;198:250–259. doi: 10.2214/AJR.11.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frydrychowicz A, Wieben O, Niespodzany E, et al. Quantification of thoracic blood flow using volumetric magnetic resonance imaging with radial velocity encoding: in vivo validation. Invest Radiol. 2013;48:819–825. doi: 10.1097/RLI.0b013e31829a4f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanneman K, Kino A, Cheng JY, et al. Assessment of the precision and reproducibility of ventricular volume, function, and mass measurements with ferumoxytol-enhanced 4D flow MRI. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25180. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.François CJ, Srinivasan S, Schiebler ML, et al. 4D cardiovascular magnetic resonance velocity mapping of alterations of right heart flow patterns and main pulmonary artery hemodynamics in tetralogy of Fallot. J Cardiovasc Magn Reson. 2012;14:16. doi: 10.1186/1532-429X-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reiter G, Reiter U, Kovacs G, et al. Magnetic resonance-derived 3-dimensional blood flow patterns in the main pulmonary artery as a marker of pulmonary hypertension and a measure of elevated mean pulmonary arterial pressure. Circ Cardiovasc Imaging. 2008;1:23–30. doi: 10.1161/CIRCIMAGING.108.780247. [DOI] [PubMed] [Google Scholar]
- 75.Reiter U, Reiter G, Kovacs G, et al. Evaluation of elevated mean pulmonary arterial pressure based on magnetic resonance 4D velocity mapping: comparison of visualization techniques. PloS One. 2013;8:e82212. doi: 10.1371/journal.pone.0082212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barker AJ, Roldán-Alzate A, Entezari P, et al. Four-dimensional flow assessment of pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension: results from two institutions. Magn Reson Med. 2015;73:1904–1913. doi: 10.1002/mrm.25326. *This study demonstrates the feasibility of assessing pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension using 4D fow MRI.
- 77.Truong U, Fonseca B, Dunning J, et al. Wall shear stress measured by phase contrast cardiovascular magnetic resonance in children and adolescents with pulmonary arterial hypertension. J Cardiovasc Magn Reson. 2013;15:81. doi: 10.1186/1532-429X-15-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. **These are the latest European guidelines on Pulmonary Hypertension.
- 79.Spruijt OA, Bogaard H-J, Heijmans MW, et al. Predicting pulmonary hypertension with standard computed tomography pulmonary angiography. Int J Cardiovasc Imaging. 2015;31:871–879. doi: 10.1007/s10554-015-0618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagaya N, Goto Y, Satoh T, et al. Impaired regional fatty acid uptake and systolic dysfunction in hypertrophied right ventricle. J Nucl Med. 1998;39:1676–1680. [PubMed] [Google Scholar]
- 81.Bokhari S, Raina A, Rosenweig EB, et al. PET imaging may provide a novel biomarker and understanding of right ventricular dysfunction in patients with idiopathic pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2011;4:641–647. doi: 10.1161/CIRCIMAGING.110.963207. [DOI] [PubMed] [Google Scholar]
- 82.Lundgrin EL, Park MM, Sharp J, et al. Fasting 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc. 2013;10:1–9. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kluge R, Barthel H, Pankau H, et al. Different mechanisms for changes in glucose uptake of the right and left ventricular myocardium in pulmonary hypertension. J Nucl Med. 2005;46:25–31. [PubMed] [Google Scholar]
- 84.Sutendra G, Dromparis P, Paulin R, et al. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med. 2013;91:1315–1327. doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 85.Wong YY, Ruiter G, Lubberink M, et al. Right ventricular failure in idiopathic pulmonary arterial hypertension is associated with inefficient myocardial oxygen utilization. Circ Heart Fail. 2011;4:700–706. doi: 10.1161/CIRCHEARTFAILURE.111.962381. [DOI] [PubMed] [Google Scholar]
- 86.de Man FS, Handoko ML, Guignabert C, et al. Neurohormonal axis in patients with pulmonary arterial hypertension: friend or foe? Am J Respir Crit Care Med. 2013;187:14–19. doi: 10.1164/rccm.201209-1663PP. [DOI] [PubMed] [Google Scholar]
- 87.Paffett ML, Hesterman J, Candelaria G, et al. Longitudinal in vivo SPECT/CT imaging reveals morphological changes and cardiopulmonary apoptosis in a rodent model of pulmonary arterial hypertension. PloS One. 2012;7:e40910. doi: 10.1371/journal.pone.0040910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amsallem M, Saito T, Tada Y, et al. Magnetic Resonance Imaging and Positron Emission Tomography Approaches to Imaging Vascular and Cardiac Inflammation. Circulation Journal. 2016 doi: 10.1253/circj.CJ-16-0224. in press. [DOI] [PubMed] [Google Scholar]
- 89.Denault A, Lamarche Y, Rochon A, et al. Innovative approaches in the perioperative care of the cardiac surgical patient in the operating room and intensive care unit. Can J Cardiol. 2014;30:459–477. doi: 10.1016/j.cjca.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 90.Laflamme M, Perrault LP, Carrier M, et al. Preliminary experience with combined inhaled milrinone and prostacyclin in cardiac surgical patients with pulmonary hypertension. J Cardiothorac Vasc Anesth. 2015;29:38–45. doi: 10.1053/j.jvca.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 91.Robitaille A, Denault AY, Couture P, et al. Importance of relative pulmonary hypertension in cardiac surgery: the mean systemic-to-pulmonary artery pressure ratio. J Cardiothorac Vasc Anesth. 2006;20:331–339. doi: 10.1053/j.jvca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Bianco JC, Qizilbash B, Carrier M, et al. Is patient-prosthesis mismatch a perioperative predictor of long-term mortality after aortic valve replacement? J Cardiothorac Vasc Anesth. 2013;27:647–653. doi: 10.1053/j.jvca.2013.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.