Abstract
Malformations of cortical development (MCDs) compose a diverse range of disorders that are common causes of neurodevelopmental delay and epilepsy. With improved imaging and genetic methodologies, the underlying molecular and pathobiological characteristics of several MCDs have been recently elucidated. In this Review, we discuss genetic and molecular alterations that disrupt normal cortical development, with emphasis on recent discoveries, and provide detailed radiological features of the most common and important MCDs.
INTRODUCTION
The term malformation of cortical development was first introduced in 1996 to describe a group of disorders that result from disturbances of the normal developmental processes of the human cerebral cortex and cause a wide range of developmental disorders of the cortex that are common causes of neurodevelopmental delay and epilepsy. 1 As a means of elucidating these disorders, a classification scheme was developed, based on the earliest stage at which the neurodevelopment process is first disrupted, and has been subsequently updated. 2-3
In this review, we discuss fundamental concepts underlying each component of the classification scheme3-4, discuss recently elucidated genetic mutations and disruptions of molecular pathways and provide detailed imaging features of the most common and important MCDs. The malformations are discussed based upon the developmental process that is affected by the presumed causative mutation, with defects in earliest processes discussed earliest and those proposed to affect the latest processes discussed last.
GROUP I. MALFORMATIONS SECONDARY TO ABNORMAL CELL PROLIFERATION OR APOPTOSIS
This group can be further subdivided into malformations resulting from reduced proliferation or elevated apoptosis, elevated proliferation or reduced apoptosis, or abnormal proliferation, which cause primary microcephalies, megalencephalies, and dysplastic malformations (focal cortical dysplasias and non-neoplastic gangliogliomas), respectively. Converging molecular and genetic evidence indicates that many malformations associated with abnormal neuronal proliferation, including megalencephalies and focal cortical dysplasias, result from mutations affecting the mammalian target of rapamycin (mTOR) pathway, which serves as a central regulator of growth and homeostasis. Given the shared pathobiology, this review will discuss and classify several neuronal proliferation abnormalities as mTOR pathway related malformations.
Microcephalies
Present at birth, primary microcephaly (also known as true microcephaly or microcephaly vera) is defined by the clinical finding of a head circumference more than 3 standard deviations below the age- and sex-related population mean5 (Figure 1 and Table). The majority of genes associated with primary microcephaly affect pathways involved with neurogenesis and cell replication; the protein products of many genes function at multiple steps of progenitor division and neuronal migration. These include cell cycle progression and checkpoint regulation (MCPH1, CENPJ, CDK5RAP2), 6 mitotic spindle formation (WDR62, NDE1), 7 and centrosome duplication and maturation (NDE1, CDK5RAP2). 6,8 Importantly, mutations of genes encoding proteins involved with formation of microtubules (TUBA1A, TUBB2B, TUBB3, TUBG1) 9-11 and microtubule-associated proteins (MAPs, including LIS1, DCX, DYNC1H, KIF5C, NDE1) 11-13, which play a crucial role in cellular cytoskeleton development and centrosome formation, are also associated with microcephaly (Figure 1 and Table). Although WDR62 14 and ASPM 15 mutations may also be associated with cortical malformations, most genetic microcephalies manifest as subtle alterations in gyral patterning, cortical surface area and size of the corpus callosum. 15-16
Figure 1.
Axial T1 (A), T2 (B) and sagittal T2 (C) MRI images illustrating the morphologic characteristics of microcephaly associated with a TUBA1A mutation. Impaired tubulin functions explain the microcephaly (mitosis/cell proliferation), the lissencephalic cerebral cortex (neuron migration), extremely small cerebellum and pons (neuron proliferation, cell migration, axon navigation), and absence of the corpus callosum with interhemispheric cyst (absent corpus callosum allows extension of the third ventricle into the interhemispheric fissure).
Table.
Genetic and imaging findings associated with MCDs
| MCD Type | Group | Associated Genes | Associated Pathways and etiology | Imaging Findings |
|---|---|---|---|---|
| Microcephaly | Group I |
MCPH1, CENPJ,
CDK5RAP2, WDR62, NDE1, NDE1, ASPM, CDK5RAP2, TUBA1A, TUBB2B, TUBB3, TUBG1, LIS1, DCX, DYNC1H, KIF5C, NDE1 |
Neurogenesis and cell replication, tubulin and microtubule- associated proteins (MAP) |
Small head size, small cerebellum and pons and lissencephaly (with tubulin and MAP-associated genes) |
|
Megalencephaly
spectrum |
Group I |
AKT3, PIK3CA,
and PIK3R2 |
mTOR | Focal (localized), hemispheric or diffuse cortical enlargement, cerebellum and deep gray nuclei also enlarged, gray/white boundary blurring |
| FCD type IIa | Group I |
MTOR,
DEPDC5,and PIK3CA |
mTOR | Gray/white matter blurring with apparent cortical thickness |
| FCD type IIb | Group I |
MTOR, DEPDC5,
NPRL3 |
mTOR | Cortical/sulcal T2 hyperintensity may extend to ventricular surface (transmantle sign) |
| Tubulinopathies | Group II |
TUBA1A,
TUBB2B, TUBB3, TUBG1, LIS1, DCX, DYNC1H, KIF5C, NDE1 |
Microtubule structure and function |
Microcephaly, lissencephaly, fused basal ganglia (BG), cortical dysgyria, callosal abnormalities, asymmetric brainstem and small cerebellar vermis |
| Variant lissencephalies | Group II |
ARX, DCX, RELN
and VLDR |
Reelin |
ARX -Lissencephaly, callosal abnormalities, dysmorphic BG, hydrancephaly Reelin – lissencephaly in anterior-posterior gradient, cortical thicknening, small cerebellum and vermis |
|
Gray matter
heterotopia |
Group II |
FLNA and ARFGEF2 |
Neuroependyma/neuroepitheliium | Normal gray matter in abnormal locations |
|
Cobblestone
malformations |
Group II |
GPR56, LAMB1,
LAMB2, LAMC3 and SRD5A3 |
Dystroglycanopathies affecting pial limiting membrane |
Lissencephaly/pachygyria or polymicrogyria (PMG), possible cerebellar involvement |
| Polymicrogyria (PMG) | Group III | 1p36.3 and 22q11.2 mutations, mTOR genes |
Etiology can be from prenatal ischemic, teratogenic or infectious brain injury |
Perisylvian bilateral PMG (most common), associated with schizencephaly |
A separate group of microcephalies, commonly called postmigrational microcephalies, 3 are characterized by normal or slightly diminished head circumference at birth (up to 2 standard deviations below the mean), with severe microcephaly developing during the first two postnatal years. Examples include the congenital variant of Rett syndrome, caused by FOXG1 mutations and associated with early frontal lobe degeneration and thinning of the callosal genu, 17-19 pontocerebellar hypoplasia associated with CASK mutations, 20-21 Angelman syndrome (with UBE3A mutations), 22 and some pontocerebellar hypoplasias associated with mutations of genes coding for proteins involved in protein synthesis (e.g., TSEN54, TSEN2, TSEN34, RATS2). 3, 23
It is important to emphasize that the current classification scheme for microcephalies 3 relies predominantly on clinical features and inheritance. Although tremendous progress has been made over the past decade on elucidating the molecular basis of many microcephalies, the processes of brain growth and development are so complex that our current level of knowledge is insufficient for the development of a comprehensive, or even useful, genetic or pathway-based classification.
Overgrowth Disorders
mTOR pathway related malformations
Recent studies have shown that many macrocephalic malformations results from mutations of genes that encode components of the mammalian target of rapamycin (mTOR) pathway, an intracellular signaling pathway that plays an important role in regulating cellular growth and homeostasis. 24-25 Although mTOR pathway functions are still incompletely understood, it is now well established that MTOR is a protein kinase that links with other proteins to form two major complexes termed mTORC1 (a complex of 7 proteins) and mTORC2 (a complex of 8 proteins), and that these complexes coordinate many anabolic and catabolic processes in response to growth factors and nutrients. 24 The mTORC1 branch is the better characterized of the two complexes; it integrates input from at least five major intra- and extracellular chemicals (amino acids – particularly leucine and arginine, neurotransmitters, glucose, growth factors and guidance molecules), unfavorable extracellular conditions (stress, hypoxia), and stimulates ribosome biogenesis, transcription, translation, cell growth/proliferation/differentiation and autophagy. The mTOR pathway can be activated or inhibited by a number of upstream signaling mechanisms, in response to environmental or metabolic demands, or by activation of other pathways (such as RAS/MAPK pathway of growth factor mediated cell proliferation, which stimulates PI3K activity). 24-25 Within the central nervous system, activation of PIK3CA or AKT3 or inhibition of TSC leads to hyperactivation of the mTOR pathway, resulting in ribosome biogenesis and elevated messenger RNA translation and, eventually, increased proliferation with production of the dysmorphic neurons seen with type II focal cortical dysplasias and dysplastic megalencephalies. 26-30
Recent work has implicated several mTOR pathway-associated genetic mutations in the pathogenesis of MCD secondary to abnormal cell proliferation. Using next generation sequencing approaches, including whole exome and targeted deep sequencing, mutations in AKT3, PIK3CA, and PIK3R2 have been identified in dysplastic megalencephaly (DMEG, formerly known as hemimegalencephaly), megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome, megalencephaly-capillary malformation syndrome, congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE) syndrome and focal cortical dysplasias). 26-30 Moreover, recent work suggests that PIK3R2 mutations may be among the most common causes of nonsyndromic polymicrogyria. 30 It is important to note that the majority of these mTOR-associated MCD mutations are not inherited (non-germline). Occurring ‘de novo’, these mutations are most often somatic (present only in the affected individual and not family members), and can be mosaic (present only in a subset of neurons). 31 Absent in peripheral leukocytes, somatic mutations in mTOR-associated AKT3 of patients with DMEG were only detected in brain tissue surgically resected for refractory epilepsy, indicating that abnormalities of a small number of neurons can cause cortex-wide dysfunction. 26 Similarly, although germline mutations of TSC1 or TSC2 are present in patients with tuberous sclerosis (TSC), ‘second hit’ somatic mutations have been shown in both non-CNS and CNS TSC-related lesions (including subependymal giant cell astrocytomas and the commonly found cortical tubers), suggesting that additional somatic mutations may be required for localized regions of dysgenesis even in the presence of germline mutations.
Mutations within the mTOR pathway genes lead to the characteristic morphologic MCD abnormalities visualized by structural MRI. With DMEG, although only 8-35% of cells (neurons and glia) carry the disease causing mutation, 28,31 the morphologic abnormalities vary considerably; the abnormality most commonly affects less than half of a hemisphere (Figure 2a), sometimes referred to as localized megalencephaly, 32 but may involve an entire cerebral hemisphere (Figure 2b,c) or, rarely, the cerebellum (Figure 2d). In addition to the variation in extent and location of hemispheric involvement, the gyral pattern varies considerably from patient to patient ranging from varying appearances of polymicrogyria to pachygyria and, in some patients, regions of lissencephaly. Other findings may include loss of gray/white matter differentiation, blurring of the cortico-medullary junction, enlargement of deep gray matter structures (Figure 2b), and asymmetrical enlargement of the lateral ventricle with the larger ventricle usually on the more dysplastic side (Figure 2b and Table). Possibly as a result of mosaicism, the authors’ experience in reviewing 51 MRIs for this review suggests that each DMEG seems to have a different morphologic appearance: differences in degree of enlargement, topologic location, cortical thickness, gyral pattern, ventricular size and in both extent and characteristics of white matter involvement.
Figure 2.
Axial and coronal T1 and T2 MRI images illustrating wide range of dysplastic megalencephaly resulting from the mosaic nature of the disorder. Image A shows gyral enlargement and blurring of cortical-white matter junction (arrows) affecting the left temporal lobe (localized megalencephaly). Image B shows right hemisphere dysgenesis with dilation of the right lateral ventricle, anterior pachygyria (small white arrows), posterior lissencephaly (large white arrows), very dysmorphic basal ganglia (in center of hemisphere) and regions of abnormal white matter (black arrows). Image C shows pachygyric frontal cortex in the affected right hemisphere (large white arrows) with multiple areas of white matter hyperintensity (small white arrows), impaired myelination (relatively hyperintense white matter), and epsilateral dysmorphic ventriculomegaly. Image D shows enlarged, dysmorphic right cerebellar hemisphere (cerebrum of this patient not shown).
Focal cortical dysplasias (FCDs) are a related group of disorders with more localized (compared to DMEG) dysplastic neurons that cause epilepsy. 33 Although they are classified together, histology, genetic analyses, topography of the lesions and imaging characteristics suggest that FCD types Ia, Ib, IIa and IIb are likely different entities. FCD Ia and Ib can be histologically distinguished by the distribution of cortical neurons, with Ia having abnormalities of vertical lamination and Ib having disturbed horizontal lamination. In the authors’ experience, some cases of FCD Ib have a histological appearance resembling laminar necrosis and an imaging appearance of chronic infarction, 33 raising the possibility that these may be late, epileptogenic stages of fetal cortical infarcts. FCD 1a may be extensive, involving frontal, parietal and temporal lobes and, on imaging, appears to be associated with diminished or delayed myelination of underlying white matter. No genetic mutations have been consistently associated with FCD Ia or Ib. Due to their rarity and the lack of good analyses to date, FCD type I will not be further discussed, except to mention that it seems to be quite different than DMEG and FCD II spectrum disorders, and that it is likely that, as our understanding of these disorders improves, FCD Ib (and possibly FCD Ia) will likely be classified elsewhere in the MCD classification system.
Somatic mutations of several mTOR pathway genes (MTOR, DEPDC5, NPRL3) are associated with focal cortical dysplasia type IIb (FCD IIb) 31,34; in this disorder, a localized cluster of dysplastic cells is present, often extending radially inward, with histology similar to the dysmorphic cells in DMEG but the cluster is smaller and, usually, more localized. 31 Germline mutations of the same genes may cause generalized epilepsy with a normal-appearing brain or may cause a variety of malformations ranging from DMEG to localized pachygyria. 29, 31,34 Similar to TSC 35, it has been suggested that the focal lesions in type II FCD are the result of a second, mosaic mutation superimposed upon the germline mutation. 34
FCD IIa is also associated with medically refractory epilepsy 33 and with mutations of genes that encode components of the mTOR pathway. 36 Imaging of patients with FCD type IIa (characterized by localized dyslamination associated with dysmorphic and slightly large neurons) most commonly demonstrates blurring of the gray/white matter boundary with apparent cortical thickening (Figure 3a), whereas FCD type IIb has a more variable imaging appearance, varying from that of a T2 hyperintense cortical tuber to a T2/FLAIR hyperintensity at the bottom of the sulcus and extending a variable length into the subcortical white matter 37 to an abnormal ‘funnel’ shaped signal extending from the dysplastic cortex to the superolateral margin of the lateral ventricle (transmantle sign) 38 (Figure 3b and Table). Tissue from some of these FCD Type II lesions have been found to contain mosaic mutations of MTOR and DEPDC5, while both localized and non-localized familial epilepsy has been associated with germline mutations of these genes and PIK3CA. 29 Currently, it appears that both a germline mutation and a somatic mutation of components of the PI3K-AKT3-TSC2-mTOR-S6K1 pathway may be necessary for developing malformations in this group, but the puzzle is still being solved.
Figure 3.
Focal cortical dysplasia (FCD) type IIa and IIb. With FCD type IIa (A and B), the most common appearance is blurring of the gray/white matter boundary (white arrows), seen here in the the left superior frontal gyrus. In FCD type IIb (C and D), abnormal ‘funnel’ shaped T2 hyperintensity (the transmantle sign, arrows in C) is seen within the left parietal lobe extending radially to the left lateral ventricle. Note that the “sign” often is only partly seen in (D); this is because it is oriented obliquely with respect to the axial plane and is, therefore, only partially seen on axial images.
GROUP II. MALFORMATIONS SECONDARY TO ABNORMAL CELL MIGRATION
Heterotopia (due to incomplete neuronal migration), ‘cobblestone’ malformations (due to overmigration of neurons through defects in the glial limiting membrane) and lissencephalies are classified as MCDs resulting primarily from abnormal neuronal migration. Recent evidence suggests that lissencephaly, heterotopia and some polymicrogyria-like cortical malformations may result from mutations of genes that encode tubulin or microtubule-associated-protein (MAP) genes, which are critical for the migration of neurons. In light of these shared pathobiological mechanisms, we discuss cortical malformations secondary to abnormal function of microtubules during the processes of neuronal migration and axonal navigation/pathfinding (commonly called tubulinopathies) and consider heterotopia, variant lissencephaly and cobblestone malformations in separate sections.
Tubulinopathies
Microtubules (MTs) are polymers composed of alternating heterodimers of α- and β-tubulin; they are integral components of the cellular cytoskeleton. Due to their involvement in mitosis/centrosome formation, organization of intracellular structure, axonal pathfinding, and protein transport, MTs play a crucial role in normal brain development and function (for additional details see references 4 and 12). MTs formation is initiated by bonding of a heterodimer composed of α-tubulin (encoded by TUBA genes) and β-tubulin (encoded by TUBB genes) to a complex composed of γ-tubulin and proteins in centrosome walls 39-40; many additional heterodimers then polymerize to form the microtubules by following actin and myosin scaffolding and reacting to surrounding chemical signals that initiate further polymerization or depolymerization. 40
Disorders of MT formation/function result in multiple brain abnormalities including microcephaly (impaired mitosis); lissencephaly, pachygyria, band heterotopia and other types of cortical dysgenesis (impaired neuronal migration); anomalies of white matter tracts and cranial nerves (impaired axonal pathfinding); and malformations of the midbrain and hindbrain (impairment of both neuronal migration and axonal pathfinding). 4 Highly associated with heterozygous missense mutations of tubulin and MAP genes, these malformations have a range of characteristic features on both physical examination and on imaging studies (best seen on structural MRI). Patients with tubulin mutations (TUBA1A, TUBB2B, TUBB3, and TUBG) typically have a) microcephaly (impaired mitosis) (see Figure 1), b) varying degrees of cerebral cortical dysgenesis due to both undermigration (impaired neuronal migration along radial glia) and overmigration into the subarachnoid space secondary to defects in attachment of the radial glia to the glial/pial limiting membrane similar to cobblestone malformations 41, c) absent or dysmorphic corpus callosum and other white matter pathways/cranial nerves (impaired axonal navigation, including cranial neuropathies) 42, d) basal ganglia abnormalities (included fused striatum due to impaired formation of the anterior limb of the internal capsule), e) cortical ‘dysgyria’ (sometimes mistakenly called polymicrogyria; probably impaired neuronal migration and axonal navigation), and f) asymmetric brainstem and small cerebellar vermis (probably a combined defect of neuronal migration and axonal navigation) 11-13 (Table and Figure 4). Patients with mutations in microtubule-associated proteins (MAPs) (LIS1, DCX, KIF5C, KIF2A and DYNC1H1) usually demonstrate similar morphologic abnormalities – pachygyria, small cerebellar vermis, callosal dysgenesis 11,12 -- but may have a milder impairment of brain growth.13
Figure 4.
Axial and sagittal T1 and T2 MRI images of a patient with a severe TUBA1A mutation showing several aspects of ‘tubulinopathies’ including a lissencephalic cortex, absent anterior limb internal capsule resulting in ‘fusion’ of the basal ganglia, dysmorphic corpus callosum, and small cerebellum/ pons.
Variant lissencephalies
During normal brain development, the cerebral cortex begins as two layers of cells; these will eventually become the marginal zone (layer 1) and the subplate (transient layer 7). Subsequent neurons migrate beyond the subplate to form layers 6 to 2. Neurons in layer 7, known as the subplate, synapse with axons from thalamic neurons and other cortical neurons until the cortical areas that are the final destinations of these axons mature, at which time they migrate to their final synaptic connections. 43-44 Lissencephaly (smooth brain) is a malformation that occurs when too few neurons migrate all the way to the cortex; it is characterized by a thick cerebral cortex that has no sulci (complete lissencephaly) or abnormally broad gyri that are separated by shallow sulci (incomplete lissencephaly or pachygyria). A related malformation, subcortical band heterotopia (also known as double cortex), develops when only a subset of neurons has the deleterious mutation. The initial descriptions of band heterotopia were of mutations of DCX involving a single X chromosome in women 45 but it was rapidly discovered that there were many cases without DCX mutations 46 and it is now recognized that mutations of many tubulin and MAP genes can result in similar phenotypes. 11-13 Band heterotopia are easily recognized on MRIs due to the smooth, sometimes undulating band of subcortical gray matter, composed of incompletely migrated neurons. The band can have variable thickness, and may or may not parallel the overlying cortex. Histologically, lissencephalies can be identified by the number of affected cortical layers and include two, three and four layered forms. Characterized by a normal molecular zone containing Cajal-Retzius cells (layer I), a relatively thick pyramidal cell zone (layer II), a cell-sparse zone (layer III), and a deep cellular layer of heterotopic neurons (layer IV), the four-layered subtype is the most common form of lissencephaly. 47
Although the histologic aspects are interesting, lissencephalies are better categorized on a genetic basis. Classic lissencephalies (including two and four-layered forms) have been shown to result mainly from microtubule-associated dysfunction, mostly from mutations of tubulin and MAP genes (see above). In contrast, ‘variant’ lissencephalies result mainly from mutations in the Aristaless-related homeobox (ARX) gene and the Reelin (RELN and VLDR) signaling pathways. 48-49 Structural imaging can distinguish the phenotypic manifestations of ARX and Reelin pathway abnormalities. 49-50
Due to its integral role in normal brain development, including cell migration, axonal guidance, GABAergic neurogenesis, and transcription regulation, ARX mutations can result in a wide range of brain abnormalities due to reduction of GABAergic neurons in the basal ganglia and cerebral cortex. 51-53 The cortical malformation associated with ARX mutations has been described as a three-layered cortex with diminished number of GABAergic neurons and scarcity of myelinated axons in the white matter. 48 As seen with structural MRI, mutations of ARX result in a small lissencephalic brain with agenesis of the corpus callosum, small dysmorphic basal ganglia, defects in the thalamocortical tracts and massive hydrocephalus that results in herniation of ventricular ependyma through choroidal fissures, often mislabeled as hydranencephaly. 54 Genitalia are often ambiguous. 50
Like ARX, Reelin has multiple functions in normal brain development. During the late stage of neuronal migration, Reelin (RELN) binds APOER2 and VLDLR, resulting in conformational change in integrin α5β1, which in turn binds to fibronectin on Cajal-Retzius cells in the molecular layer (layer 1) allowing neurons to successfully pass into the submolecular layer and form a six-layered cerebral cortex. 55 Within the cerebellum, Purkinje cells establish proper orientation for radial migration to the cortex only after binding of Reelin to APOER2 and VLDLR. 56 Reelin also interacts with the mTOR pathway to stimulate protein translation for dendrite development and with the MEK-Erk pathway in the hippocampus to produce chemical transcripts for learning and memory in the hippocampus. 57 On MRI, Reelin pathway disorders result in a gradient of disorders ranging from a simplified gyral pattern with slight cortical thickening to frank lissencephaly with substantial thickening, an anterior to posterior gradient of gyration, and a variably small, smooth cerebellum with disproportionate vermian involvement (Table). 4
Gray matter heterotopia
Neuronal accumulations in abnormal locations, commonly called heterotopia, vary widely in location and size; phenotypes vary from periventricular nodular heterotopia (PNH) which are thought to result from loss of radial glial cell attachment to disrupted neuroependyma 58, to subcortical heterotopia (many types, causes not established) 59 to subpial heterotopia, which may be part of a continuum with cobblestone cortex, and likely result from loss of radial glial attachment to a disrupted glial limitans. 60 Disruption of the neuroepithelium (neuroependyma), likely due to membrane ‘gaps’ and impaired adhesion of neural progenitors and radial glial cells to the neuroependyma, leads to impaired neuronal migration from the ventricular zone. 58 Classic PNH is associated with mutations in FLNA, and ARFGEF2, which have been hypothesized to be associated with vesicle trafficking and neuroependymal repair during neuron proliferation and intracellular transport. 58
The majority of patients with heterotopia have epilepsy with seizures localizing to the heterotopic nodules 61, the overlying cortex 62 or both. Classic PNH occur in or near the ventricular walls (Table); they may occur in isolation or associated with other CNS malformations. Several recent studies have noted that posterior heterotopia (localized in the temporal and occipital horns and trigones) are associated with other malformations of the posterior brain, including cerebellar dysgenesis, corpus callosum abnormalities, malrotated hippocampi and temporal lobe cortical dysgenesis (for additional details see reference 4). Patients with the X-linked form of PNH (such as mutations in the FLNA gene) most often demonstrate bilateral contiguous periventricular heterotopia that spare the temporal horns but may be associated with mild cerebellar vermian hypoplasia. More complex forms of heterotopia include subcortical heterotopia, a heterogeneous group of conditions characterized by a continuous neuronal conglomerate that extends from the ependyma to the cortex, the causes of which remain poorly understood. 3-4, 59 “Band heterotopia” (double cortex) is considered a subset of lissencephaly (see variant lissencephaly section above).
Cobblestone malformations
Previously known as type II lissencephaly 63 cobblestone malformations (also called cobblestone lissencephaly) result from impaired cerebral and cerebellar basement membrane (glial limitans) formation, most often due to defects in linkage with radial glia. The first cobblestone malformations described were due to defective binding of laminins in the glial limitans to alpha-dystroglycan on the basal end of radial glia with resultant defects in the pial limiting membrane and aberrant terminal neuron migration. 64 Mutations in genes within glycosylation pathways, including LAMB1, LAMB2, LAMC3 and SRD5A3, have also been associated with cobblestone malformations. 65-66 Although not directly involving a glycosylation defect, mutations of GPR56 and Col3A1 have also been shown to be associated with murine cobblestone malformations and cobblestone-like human cortical dysgenesis and likely cause disruption of glial limitans integrity in the forebrain and the rostral cerebellum. 60,67-68 We have seen a patient with a compound heterozygous COL18A1 mutation that resembled polymicrogyria in infancy but looked like a GPR56 mutation with broad gyri and thickened cortex later in childhood (Figure 5). ‘Dystroglycanopathies’ often affect other organ systems, but often result in congenital muscular dystrophies (elevated serum creatine kinase levels, hypotonia, cardiac abnormalities) and ocular anomalies (buphthalmos, congenital glaucoma). 69
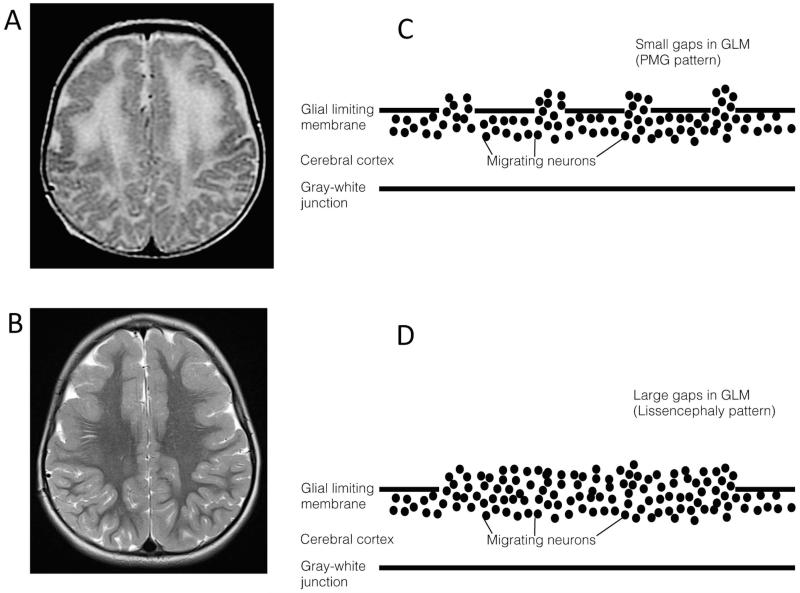
Figure 5.
Cobblestone malformations. Axial MRI T2 images from a patient with a compound heterozygous COL18A mutation at 6 months (A) demonstrating a polymicrogyria-like pattern (likely secondary to relatively small gaps in the glial limitans and decreased myelination at this stage) and at age 8 (B) showing a pachygyric and lissencephalic pattern. As illustrated in the right panels, a polymicrogyric (PMG) pattern may result from migration of relatively few neurons through small gaps in the glial limiting membrane (C) whereas a lissencephalic pattern may occur due to greater number of neurons migrating through larger gaps in the glial limiting membrane (D).
The integrity of the glial limiting membrane GLM and the GLM-radial glia linkage influence the clinical phenotype of cobblestone malformations. Similarly, the MRI phenotype varies depending on the size of the gaps within the PLM and the amount of neuronal tissue that migrates through the gaps in the GLM. 3 Brains with small gaps with minimal neuronal overmigration look normal or nearly normal on MRI and have small surface bumps on nearly normal appearing cortex on gross pathology. 69 Gaps of an intermediate size look like polymicrogyria on MRI and on gross pathology, 70 while large gaps have a large neuronal overmigration that results in a smooth appearing cortical surface and may lead to initial diagnoses of lissencephaly (Table) (Figure 5c,d). 4,69
GROUP III. MALFORMATIONS SECONDARY TO ABNORMAL POST-MIGRATIONAL DEVELOPMENT
Polymicrogyria
Polymicrogyria (PMG) is a common phenotype characterized histologically by findings of overfolding and abnormal lamination of the cortex and grossly by an excessive number of small convolutions.71,72 Presentation varies with the location and the extent of the PMG, but most patients have developmental delay or behavioral problems in childhood and ~70% develop seizures during the first 5 years of life. 71 Despite the large number of patients affected, PMG pathogenesis is poorly understood: it very likely multifactorial; its presentation and outcome vary because of its causal heterogeneity; and not all disorders with multiple small gyri are true polymicrogyria, even though they have multiple small gyri and may, therefore, be given that diagnosis71.
PMG can result from prenatal ischemic, teratogenic or infectious brain injury. 71-75 Although there are numerous examples in the literature of “genetic” PMG, a closer look at many of these suggests that the causation may be uncertain. For example, cobblestone malformations (one form of PMG, discussed above) appear as multiple small gyri when imaged before myelination because overmigration of neurons through medium-sized defects in the glia limitans looks like polymicrogyria on MR imaging (Figure 5a); follow-up imaging after myelination often shows a very different appearance (Figure 5b). Others have diagnosed PMG based upon slight focal irregularity of the cortical-white matter junction, even though the images show an abnormally thick cortex with a smooth cortical surface and a nearly completely smooth gray-white junction of most of the malformation 76; in such cases, one should suspect a cobblestone malformation with variably sized gaps in the glia limitans, particularly if an ocular anomaly, muscular weakness or myelination delay is present.
Schizencephaly is much less common than polymicrogyria, and is associated with young maternal age, maternal alcohol use and lack of prenatal care. 73 Patients with schizencephaly (Figure 6) also typically present with delayed motor or cognitive development or with seizures. Imaging shows gray matter extending from the cortex to the ventricle, nearly always with CSF (sometimes containing blood vessels) within the cleft. 74,75 In nearly half of the patients, an irregular, polymicrogyria-like gray matter lines the ‘clefts’ of schizencephaly; PMG is found outside the cleft in the ipsilateral hemisphere in two-thirds of affected patients, and the cortex surrounding the schizencephalic cleft is nearly always polymicrogyria. 74 Approximately one third of patients with unilateral schizencephaly have polymicrogyria in the same location of the contralateral hemisphere. 74 About one-half of schizencephalies are bilateral, again with PMG commonly present in the cleft or the cortex surrounding it. 74,75 As the clefts are often found in vascular distributions (parasagittal or perisylvian being among the most common) and the two malformations so commonly occur together, many have proposed that PMG and schizencephaly can result from similar events of differing severity. 74
Figure 6.
Schizencephalies, with closed and open lips. Image (A) shows bilateral, symmetrically located closed lip schizencephalies (black arrows). Note that the right sided lesion is completely closed but the left sided one has separated walls near the entrance to the ventricle. Image (B) shows an open-lip schizencephaly extending upward in the right hemisphere and two separate closed lip schizencephalies (arrows, one horizontal and one vertical, connecting with 3 sulci) in the left hemisphere. Note that the gray matter linking the clefts has an appearance of polymicrogyria in some places.
As for genetic causes, deletions in 1p36.3 and 22q11.2 have been identified in patients with PMG 77, and PMG may occur in microcephaly syndromes including WDR62 mutations and macrocephaly syndromes associated with mutations of mTOR pathway genes such as PIK3R2 and PI4KA (see reference 77 for discussion); however, most of the causal genes and chemical biology underlying these loci have not been identified. PMG is nearly always seen in patients with Aicardi syndrome, but no causative gene has been identified and, thus, it is not yet certain whether the PMG is acquired or caused by mosaic mutation(s).
On imaging, PMG can be seen involving practically any part of the cerebrum, ranging from small areas localized to the frontal or posterior sylvian cortex to diffuse PMG affecting the entire cerebrum. The most common location, by far, is the perisylvian cortex, particularly the posterior perisylvian region; it is often bilateral (Table). In comparison, the parasagittal and, particularly, the medial cortices are less frequently involved. The imaging appearance varies considerably, from crowded microgyri that are not resolved by MRI and, therefore, appear as thick, irregular cortex, to small areas of small, almost feathery gyri (Figure 7). Large, thick-appearing gyri in a setting of PMG are virtually always composed of multiple very small gyri that can only be resolved if the image is acquired with high resolution and the reader magnifies the image to see the irregularities in the gyral wall (in Figure 7, the deep appearing sulci, in the suprasylvian cortices, are not the microgyri; the microgyri are seen within the cortex, as shown by very small arrows in 7B and 7D). In one study, all perisylvian affected cases demonstrated a tapering of PMG severity as the malformation extended away from the perisylvian region. 71 Although the white matter may be comparatively normal, signal abnormalities within the white matter in PMG may suggest an infectious (cytomegalovirus) etiology or other, PMG-like, syndromes such as cobblestone malformations (see section above). Although polymicrogyria is almost inevitably seen in patients with schizencephaly, the presence of schizencephaly is rather uncommon in patients with polymicrogyria. In one series of 328 patients with PMG, 71 only two had schizencephaly; in both cases, the lesions were perisylvian and directly contralateral to each other.
Figure 7.
Different appearances of polymicrogyria. A,D. Patient 1 has three small, fine microgyri (very small arrows in right frontal lobe in D), small microgyri (medium arrows in left frontal lobe), and larger, coarser and deeper PMG (large arrows left posterior sylvian cortex). B,E. Patient 2 has extensive fonto-temporo-parietal PMG with cortex appearing thick at first glance, but tiny true microgyria can be seen in a few areas (very small arrows in left frontal and parietal lobes in B).
C,F. Patient 3 has thick cortex with nearly smooth inner surface in right temporal lobe (large black arrows in C and F). Note the marked discrepancy in white matter volume of the temporal lobes, which is much diminished on the left; this is also manifested by the difference in cerebral peduncles illustrated by central, different-sized black arrows in C).
CONCLUSIONS
Malformations of cortical development result from a diverse molecular disruption of normal brain development and manifest as a wide array of anatomical and functional phenotypes. Continued focus on delineating the molecular pathways that are disturbed in these disorders may facilitate early therapeutic interventions and will lead to improved understanding of normal brain development.
ACKNOWLEDGEMENT
This work was supported by the RSNA Resident/Fellow Scholar Award (Desikan) and NIH grants U24DA041123 (Dale) and R01NS035129 (Walsh).
Footnotes
AUTHOR CONTRIBUTIONS
RSD and AJB contributed to conception and design of the study, and drafting a significant portion of the manuscript or figures.
POTENTIAL CONFLICTS OF INTEREST
We report no potential conflicts of interest.
REFERENCES
- 1.Barkovich AJ, Kuzniecky RI, Dobyns WB, et al. A classification scheme for malformations of cortical development. Neuropediatrics. 1996;27:59–63. doi: 10.1055/s-2007-973750. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Kuzniecky RI, Jackson GD, et al. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–87. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- 3.Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–69. doi: 10.1093/brain/aws019. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Dobyns WB, Guerrini R. Malformations of cortical development and epilepsy. Cold Spring Harb Perspect Med. 2015;5:a022392. doi: 10.1101/cshperspect.a022392. doi: 10.1101/cshperspect.a022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmore EC, Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol. 2013 Jul;2(4):461–78. doi: 10.1002/wdev.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornton GK, Woods CG. Primary microcephaly: do all roads lead to Rome? Trends Genet. 2009 Nov;25(11):501–10. doi: 10.1016/j.tig.2009.09.011. doi: 10.1016/j.tig.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu TW, Mochida GH, Tischfield DJ, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet. 2010 Nov;42(11):1015–20. doi: 10.1038/ng.683. doi: 10.1038/ng.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkuraya FS, Cai X, Emery C, et al. Human mutations in NDE1 cause extreme microcephaly with lissencephaly. Am J Hum Genet. 2011 May 13;88(5):536–47. doi: 10.1016/j.ajhg.2011.04.003. doi: 10.1016/j.ajhg.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirier K, Saillour Y, Bahi-Buisson N, et al. Mutations in the neuronal ß-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum Mol Genet. 2010 Nov 15;19(22):4462–73. doi: 10.1093/hmg/ddq377. doi: 10.1093/hmg/ddq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breuss M, Heng JI, Poirier K, Tian G, et al. Mutations in the β-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep. 2012 Dec 27;2(6):1554–62. doi: 10.1016/j.celrep.2012.11.017. doi: 10.1016/j.celrep.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirier K, Lebrun N, Broix L, Tian G, et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet. 2013 Jun;45(6):639–47. doi: 10.1038/ng.2613. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahi-Buisson N, Poirier K, Fourniol F, et al. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014 Jun;137:1676–700. doi: 10.1093/brain/awu082. Pt 6. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- 13.Mutch CA, Poduri A, Sahin M, et al. Disorders of Microtubule Function in Neurons: Imaging Correlates. AJNR Am J Neuroradiol. 2016 Mar;37(3):528–35. doi: 10.3174/ajnr.A4552. doi: 10.3174/ajnr.A4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu TW, Mochida GH, Tischfield DJ, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet. 2010 Nov;42(11):1015–20. doi: 10.1038/ng.683. doi: 10.1038/ng.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passemard S, Titomanlio L, Elmaleh M, et al. Expanding the clinical and neuroradiologic phenotype of primary microcephaly due to ASPM mutations. Neurology. 2009 Sep 22;73(12):962–9. doi: 10.1212/WNL.0b013e3181b8799a. doi: 10.1212/WNL.0b013e3181b8799a. [DOI] [PubMed] [Google Scholar]
- 16.Rimol LM, Agartz I, Djurovic S, et al. Sex-dependent association of common variants of microcephaly genes with brain structure. Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):384–8. doi: 10.1073/pnas.0908454107. doi: 10.1073/pnas.0908454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahi-Buisson N, Nectoux J, Girard B, et al. Revisiting the phenotype associated with FOXG1 mutations: two novel cases of congenital Rett variant. Neurogenetics. 2010 May;11(2):241–9. doi: 10.1007/s10048-009-0220-2. doi: 10.1007/s10048-009-0220-2. [DOI] [PubMed] [Google Scholar]
- 18.Le Guen T, Bahi-Buisson N, Nectoux J, et al. A FOXG1 mutation in a boy with congenital variant of Rett syndrome. Neurogenetics. 2011 Feb;12(1):1–8. doi: 10.1007/s10048-010-0255-4. doi: 10.1007/s10048-010-0255-4. [DOI] [PubMed] [Google Scholar]
- 19.Kortüm F, Das S, Flindt M, et al. The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J Med Genet. 2011 Jun;48(6):396–406. doi: 10.1136/jmg.2010.087528. doi: 10.1136/jmg.2010.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Najm J, Horn D, Wimplinger I, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008 Sep;40(9):1065–7. doi: 10.1038/ng.194. doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- 21.Takanashi J, Arai H, Nabatame S, et al. Neuroradiologic features of CASK mutations. AJNR Am J Neuroradiol. 2010 Oct;31(9):1619–22. doi: 10.3174/ajnr.A2173. doi: 10.3174/ajnr.A2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997 Jan;15(1):70–3. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 23.Namavar Y, Barth PG, Kasher PR, et al. Clinical, neuroradiological and genetic findings in pontocerebellar hypoplasia. Brain. 2011 Jan;134:143–56. doi: 10.1093/brain/awq287. Pt 1. doi: 10.1093/brain/awq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012 Apr 13;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laplante M, Sabatini DM. mTOR Signaling. Cold Spring Harb Perspect Biol. 2012 Feb 1;4(2):pii. doi: 10.1101/cshperspect.a011593. a011593. doi: 10.1101/cshperspect.a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poduri A, Evrony GD, Cai X, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012 Apr 12;74(1):41–8. doi: 10.1016/j.neuron.2012.03.010. doi: 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivière JB, Mirzaa GM, O'Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012 Jun 24;44(8):934–40. doi: 10.1038/ng.2331. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet. 2012 Jun 24;44(8):941–5. doi: 10.1038/ng.2329. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Gama AM, Geng Y, Couto JA, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol. 2015 Apr;77(4):720–5. doi: 10.1002/ana.24357. doi: 10.1002/ana.24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirzaa GM, Campbell CD, Solovieff N, et al. Association of MTOR Mutations With Developmental Brain Disorders, Including Megalencephaly, Focal Cortical Dysplasia, and Pigmentary Mosaicism. JAMA Neurol. 2016 Jul 1;73(7):836–45. doi: 10.1001/jamaneurol.2016.0363. doi: 10.1001/jamaneurol.2016.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poduri A, Evrony GD, Cai X, et al. Somatic mutation, genomic variation, and neurological disease. Science. 2013 Jul 5;341(6141):1237758. doi: 10.1126/science.1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahashi M, Sato N, Yagishita A, et al. Clinical and imaging characteristics of localized megalencephaly: a retrospective comparison of diffuse hemimegalencephaly and multilobar cortical dysplasia. Neuroradiology. 2009 Dec;51(12):821–30. doi: 10.1007/s00234-009-0579-7. doi: 10.1007/s00234-009-0579-7. [DOI] [PubMed] [Google Scholar]
- 33.Blümcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011 Jan;52(1):158–74. doi: 10.1111/j.1528-1167.2010.02777.x. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim JC, Scerri T, Fanjul-Fernández M, et al. Familial cortical dysplasia caused by mutation in the mammalian target of rapamycin regulator NPRL3. Ann Neurol. 2016 Jan;79(1):132–7. doi: 10.1002/ana.24502. doi: 10.1002/ana.24502. [DOI] [PubMed] [Google Scholar]
- 35.Tsai V, Parker WE, Orlova KA, et al. Fetal brain mTOR signaling activation in tuberous sclerosis complex. Cereb Cortex. 2014 Feb;24(2):315–27. doi: 10.1093/cercor/bhs310. doi: 10.1093/cercor/bhs310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen LA, Mirzaa GM, Ishak GE, et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015 Jun;138:1613–28. doi: 10.1093/brain/awv045. Pt 6. doi: 10.1093/brain/awv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey AS, Mandelstam SA, Maixner WJ, et al. The surgically remediable syndrome of epilepsy associated with bottom-of-sulcus dysplasia. Neurology. 2015 May 19;84(20):2021–8. doi: 10.1212/WNL.0000000000001591. doi: 10.1212/WNL.0000000000001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barkovich AJ, Kuzniecky RI, Bollen AW, et al. Focal transmantle dysplasia: a specific malformation of cortical development. Neurology. 1997 Oct;49(4):1148–52. doi: 10.1212/wnl.49.4.1148. [DOI] [PubMed] [Google Scholar]
- 39.Kollman JM, Merdes A, Mourey L, et al. Microtubule nucleation by γ-tubulin complexes. Nat Rev Mol Cell Biol. 2011 Oct 12;12(11):709–21. doi: 10.1038/nrm3209. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuijpers M, Hoogenraad CC. Centrosomes, microtubules and neuronal development. Mol Cell Neurosci. 2011 Dec;48(4):349–58. doi: 10.1016/j.mcn.2011.05.004. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Fallet-Bianco C, Laquerrière A, Poirier K, et al. Mutations in tubulin genes are frequent causes of various foetal malformations of cortical development including microlissencephaly. Acta Neuropathol Commun. 2014 Jul 25;2:69. doi: 10.1186/2051-5960-2-69. doi: 10.1186/2051-5960-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chew S, Balasubramanian R, Chan WM, et al. A novel syndrome caused by the E410K amino acid substitution in the neuronal β-tubulin isotype 3. Brain. 2013 Feb;136:522–35. doi: 10.1093/brain/aws345. Pt 2. doi: 10.1093/brain/aws345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 1993 Mar;117(3):1031–47. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- 44.Ayoub AE, Kostovic I. New horizons for the subplate zone and its pioneering neurons. Cereb Cortex. 2009 Aug;19(8):1705–7. doi: 10.1093/cercor/bhp025. doi: 10.1093/cercor/bhp025. [DOI] [PubMed] [Google Scholar]
- 45.Gleeson JG, Allen KM, Fox JW, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998 Jan 9;92(1):63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 46.Gleeson JG, Luo RF, Grant PE, et al. Genetic and neuroradiological heterogeneity of double cortex syndrome. Ann Neurol. 2000 Feb;47(2):265–9. [PubMed] [Google Scholar]
- 47.Forman MS, Squier W, Dobyns WB, et al. Genotypically defined lissencephalies show distinct pathologies. J Neuropathol Exp Neurol. 2005 Oct;64(10):847–57. doi: 10.1097/01.jnen.0000182978.56612.41. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura K, Yanazawa M, Sugiyama N, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002 Nov;32(3):359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- 49.Hong SE, Shugart YY, Huang DT, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000 Sep;26(1):93–6. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- 50.Bonneau D, Toutain A, Laquerrière A, et al. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): clinical, magnetic resonance imaging, and neuropathological findings. Ann Neurol. 2002 Mar;51(3):340–9. doi: 10.1002/ana.10119. [DOI] [PubMed] [Google Scholar]
- 51.Okazaki S, Ohsawa M, Kuki I, et al. Aristaless-related homeobox gene disruption leads to abnormal distribution of GABAergic interneurons in human neocortex: evidence based on a case of X-linked lissencephaly with abnormal genitalia (XLAG) Acta Neuropathol. 2008 Oct;116(4):453–62. doi: 10.1007/s00401-008-0382-2. doi: 10.1007/s00401-008-0382-2. [DOI] [PubMed] [Google Scholar]
- 52.Colasante G, Collombat P, Raimondi V, et al. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008 Oct 15;28(42):10674–86. doi: 10.1523/JNEUROSCI.1283-08.2008. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colasante G, Simonet JC, Calogero R, et al. ARX regulates cortical intermediate progenitor cell expansion and upper layer neuron formation through repression of Cdkn1c. Cereb Cortex. 2015 Feb;25(2):322–35. doi: 10.1093/cercor/bht222. doi: 10.1093/cercor/bht222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato M, Das S, Petras K, et al. Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum Mutat. 2004 Feb;23(2):147–59. doi: 10.1002/humu.10310. [DOI] [PubMed] [Google Scholar]
- 55.Sekine K, Kubo K, Nakajima K. How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex? Neurosci Res. 2014 Sep;86:50–8. doi: 10.1016/j.neures.2014.06.004. doi: 10.1016/j.neures.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Leto K, Arancillo M, Becker EB, et al. Consensus Paper: Cerebellar Development. Cerebellum. 2015 Oct 6; doi: 10.1007/s12311-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ventruti A, Kazdoba TM, Niu S, et al. Reelin deficiency causes specific defects in the molecular composition of the synapses in the adult brain. Neuroscience. 2011 Aug 25;189:32–42. doi: 10.1016/j.neuroscience.2011.05.050. doi: 10.1016/j.neuroscience.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 58.Ferland RJ, Batiz LF, Neal J, et al. Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia. Hum Mol Genet. 2009 Feb 1;18(3):497–516. doi: 10.1093/hmg/ddn377. doi: 10.1093/hmg/ddn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barkovich AJ, Kuzniecky RI. Gray matter heterotopia. Neurology. 2000 Dec 12;55(11):1603–8. doi: 10.1212/wnl.55.11.1603. [DOI] [PubMed] [Google Scholar]
- 60.Bahi-Buisson N, Poirier K, Boddaert N, et al. GPR56-related bilateral frontoparietal polymicrogyria: further evidence for an overlap with the cobblestone complex. Brain. 2010 Nov;133(11):3194–209. doi: 10.1093/brain/awq259. doi: 10.1093/brain/awq259. [DOI] [PubMed] [Google Scholar]
- 61.Scherer C, Schuele S, Minotti L, et al. Intrinsic epileptogenicity of an isolated periventricular nodular heterotopia. Neurology. 2005 Aug 9;65(3):495–6. doi: 10.1212/01.wnl.0000172350.25380.c7. [DOI] [PubMed] [Google Scholar]
- 62.Tassi L, Colombo N, Cossu M, et al. Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain. 2005 Feb;128:321–37. doi: 10.1093/brain/awh357. Pt 2. [DOI] [PubMed] [Google Scholar]
- 63.Dobyns WB, Kirkpatrick JB, Hittner HM, et al. Syndromes with lissencephaly. II: Walker-Warburg and cerebro-oculo-muscular syndromes and a new syndrome with type II lissencephaly. doi: 10.1002/ajmg.1320220118. [DOI] [PubMed] [Google Scholar]
- 64.Myshrall TD, Moore SA, Ostendorf AP, et al. Dystroglycan on radial glia end feet is required for pial basement membrane integrity and columnar organization of the developing cerebral cortex. J Neuropathol Exp Neurol. 2012 Dec;71(12):1047–63. doi: 10.1097/NEN.0b013e318274a128. doi: 10.1097/NEN.0b013e318274a128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radmanesh F, Caglayan AO, Silhavy JL, et al. Mutations in LAMB1 cause cobblestone brain malformation without muscular or ocular abnormalities. Am J Hum Genet. 2013 Mar 7;92(3):468–74. doi: 10.1016/j.ajhg.2013.02.005. doi: 10.1016/j.ajhg.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radner S, Banos C, Bachay G, et al. β2 and γ3 laminins are critical cortical basement membrane components: ablation of Lamb2 and Lamc3 genes disrupts cortical lamination and produces dysplasia. Dev Neurobiol. 2013 Mar;73(3):209–29. doi: 10.1002/dneu.22057. doi: 10.1002/dneu.22057. [DOI] [PubMed] [Google Scholar]
- 67.Luo R, Jeong SJ, Jin Z, et al. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12925–30. doi: 10.1073/pnas.1104821108. doi: 10.1073/pnas.1104821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo R, Jin Z, Deng Y, Strokes N, et al. Disease-associated mutations prevent GPR56-collagen III interaction. PLoS One. 2012;7(1):e29818. doi: 10.1371/journal.pone.0029818. doi: 10.1371/journal.pone.0029818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Devisme L, Bouchet C, Gonzalès M, et al. Cobblestone lissencephaly: neuropathological subtypes and correlations with genes of dystroglycanopathies. Brain. 2012 Feb;135:469–82. doi: 10.1093/brain/awr357. Pt 2. doi: 10.1093/brain/awr357. [DOI] [PubMed] [Google Scholar]
- 70.Meilleur KG, Zukosky K, Medne L, et al. Clinical, pathologic, and mutational spectrum of dystroglycanopathy caused by LARGE mutations. J Neuropathol Exp Neurol. 2014 May;73(5):425–41. doi: 10.1097/NEN.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leventer RJ, Jansen A, Pilz DT, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010 May 133;:1415–27. doi: 10.1093/brain/awq078. Pt 5. doi: 10.1093/brain/awq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jansen AC, Robitaille Y, Honavar M, Mullatti N, Leventer RJ, Andermann E, Andermann F, Squier W. The histopathology of polymicrogyria: a series of 71 brain autopsy studies. Developmental Medicine and Child Neurology. 2016 Jan;58:39–48. doi: 10.1111/dmcn.12840. doi:10.1111/dmcn.12840. [DOI] [PubMed] [Google Scholar]
- 73.Dies KA, Bodell A, Hisama FM, et al. Schizencephaly: Association With Young Maternal Age, Alcohol Use, and Lack of Prenatal Care. J Child Neurol. 2013;28:198–203. doi: 10.1177/0883073812467850. doi: 10.1177/0883073812467850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayashi N, Tsutsumi Y, Barkovich AJ. Morphological features and associated anomalies of schizencephaly in the clinical population: detailed analysis of MR images. Neuroradiology. 2002 May;44(5):418–27. doi: 10.1007/s00234-001-0719-1. [DOI] [PubMed] [Google Scholar]
- 75.Guerrini R, Dobyns WB. Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 2014 Jul;13(7):710–26. doi: 10.1016/S1474-4422(14)70040-7. doi: 10.1016/S1474-4422(14)70040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barak T, Kwan KY, Louvi A, et al. Recessive LAMC3 mutations cause malformations of occipital cortical development. Nat Genet. 2011 Jun;43(6):590–4. doi: 10.1038/ng.836. doi: 10.1038/ng.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stutterd CA, Leventer RJ. Polymicrogyria: a common and heterogeneous malformation of cortical development. Am J Med Genet C Semin Med Genet. 2014 Jun;166C(2):227–39. doi: 10.1002/ajmg.c.31399. doi: 10.1002/ajmg.c.31399. [DOI] [PubMed] [Google Scholar]