Abstract
Objectives
Isolated Acute Vestibular Syndrome (iAVS) presentations to the Emergency Department (ED) pose management challenges given concerns for posterior circulation strokes. False negative brain imaging may erroneously reassure clinicians, while HINTS-plus examination outperforms imaging to screen for strokes in iAVS. We studied the feasibility of implementing HINTS-plus testing in the ED, aiming to reduce neuroimaging in patients with iAVS.
Methods
We launched an institutional Quality Improvement initiative, using DMAIC methodology. The outcome measures (proportion of iAVS subjects that had HINTS-plus examinations and underwent neuroimaging by CT/MRI) were compared before and after the established intervention. The intervention consisted of formal training for neurologists and emergency physicians on how to perform, document, and interpret HINTS-plus and implementation of novel iAVS management algorithm. Neuroimaging was not recommended if HINTS-plus suggested peripheral vestibular etiology. If a central process was suspected, brain MRI/MR angiogram was performed. Head CT was reserved only for thrombolytic time-window cases.
Results
In the first 2 months post-implementation, HINTS-plus testing performance by neurologists increased from 0% to 80% (p = 0.007), and by ED providers from 0% to 9.09% (p = 0.367). Head CT scans were reduced from 18.5% to 6. 25%. Brain MRI use was reduced from 51.8% to 31.2%. 60% of the iAVS subjects were discharged from the ED; none were readmitted or had another ED presentation in the ensuing 30 days.
Conclusions
Implementation of HINTS-plus evaluation in the ED is valuable and feasible for neurologists, but challenging for emergency physicians. Future studies should determine the ‘dose-response’ curve of educational interventions.
Keywords: Stroke, Vertigo, Ocular motor, Quality Improvement, Emergency Medicine
Introduction
Assessment of acute dizziness and vertigo is a major clinical challenge1,2. Acute vestibular syndrome (AVS), consisting of the following complaints -vertigo, dizziness, imbalance, gait instability, oscillopsia, nausea/vomiting, plus/minus sudden onset hearing loss, is responsible for approximately 400,000 to 800,000 US Emergency Department (ED) visits annually3. It can be caused by acute peripheral or central vestibulopathies. Posterior circulation strokes account for 3%–5% of ED vertigo and dizziness presentations4 and 35% of them are initially missed5. This may be due to use of outdated diagnostic paradigms, incomplete neurological examination, and low sensitivity of brain imaging, as currently employed2. Brain CT scans have low sensitivity (approximately 16%) and often miss acute infarction in the posterior fossa3. Although better than CT, diffusion weighted imaging (DWI) MRI performed within 24 hours from symptom onset still misses about 20% of acute posterior fossa infarctions4.
A bedside ocular motor examination named HINTS (Head-Impulse, Nystagmus, and Test-of-Skew) was reported to accurately identify stroke as a cause of AVS3. A positive HINTS-plus test, defined as bilaterally normal horizontal head impulse test (lack of a corrective saccade on head impulse testing), central-appearing nystagmus (direction changing in eccentric gaze), skew deviation, new unilateral hearing loss, or any combination of these, is reported to suggest central pathology and have 99.9 % sensitivity and 97% specificity in detecting posterior circulation infarcts6. A benign HINTS-plus examination (unilaterally abnormal head impulse test with a corrective saccade, plus direction-fixed horizontal nystagmus opposite the abnormal impulse, plus absent skew deviation, plus normal hearing) “rules out” stroke better than a negative DWI MRI in the first 48 hours after symptom onset3,6 and denotes a peripheral vestibular etiology.
Neuroimaging in these patients unnecessarily increases cost, as head CT adds little to the diagnostic impression and exposes patients to needless radiation2,7. A negative CT or DWI MRI may falsely reassure clinicians that they have ruled out acute posterior circulation strokes4. Although HINTS outperforms imaging (as well as vascular risk stratification scores such as ABCD2) to screen for stroke in AVS6, the HINTS examination is unfamiliar to many emergency physicians and neurologists.
To address known challenges in diagnosing AVS patients2, we launched an institutional Quality Improvement (QI) initiative, using the DMAIC methodology (Define, Measure, Analyze, Improve, Control)8.
Methods
Define
We recognized the following problems: (1) detailed neurological examination and HINTS-plus testing are not performed by emergency physicians or neurologists evaluating patients with AVS; (2) neuroimaging is overused in patients who present to the ED with isolated AVS. Based on these observations, we sought interventions with the following aims: (1) educate emergency physicians and neurologists about HINTS testing with a goal of ≥50% of neurology consultation notes and ≥30% of the ED notes will have HINTS documentation; (2) decrease unnecessary brain imaging in patients with isolated AVS with a goal to reduce CT use in isolated AVS patients by 50% and brain MRI by 20% within 2 months, from the baseline imaging rates.
Measure
Our primary outcome measures were the proportion of patients with isolated AVS that had proper ocular motor examinations and underwent neuroimaging by CT or MRI. The process measures were the performance of ocular motor exam and hearing testing, the correct documentation and the accurate interpretation of HINTS-plus testing.
Analyze
We identified the following reasons why brain imaging may be excessively performed in AVS: incomplete neurological examinations, lack of neurologic consultation in the ED, deficiency of HINTS-plus testing, lacking knowledge about the interpretation and significance of HINTS-plus findings, the lack of awareness about false reassurance provided by negative brain imaging.
Improve
The QI initiative stakeholders included neurology and stroke trainees, neurology attending physicians and ED physicians. All participants attended formal teaching sessions (hands-on, lectures and online tutorials) on how to perform, document and interpret the HINTS-plus testing. A template was created to standardize documentation in the electronic medical record (EMR). We taught physicians to document horizontal head impulse tests findings as having a corrective saccade absent (i.e., normal) or present (i.e., abnormal) and its direction. Team members were trained how to check for nystagmus in all gaze directions and how to document the identified pattern; of note, in the nystagmus template phrase, the participants could choose amongst direction changing eccentric gaze, pure vertical, pure torsional, horizontal unidirectional, and other pattern, and were encouraged to describe the findings. Participants were taught how to check for skew deviation with the cover-uncover test and asked to document the presence or absence of a vertical deviation and note which was the hypertropic or hyperphoric eye. The test for hearing loss was asked to be performed by finger rub.
The target population was patients presenting to ED with isolated AVS. ED physicians were encouraged to consult neurology/stroke team for detailed examinations. All providers were encouraged to perform HINTS-plus testing and document the findings using the template. Teams were asked to follow a standard management algorithm. If HINTS-plus testing showed central findings (bilaterally normal horizontal head impulse test, central-appearing nystagmus, skew deviation, new unilateral hearing loss, or any combination of these), brain MRI and head and neck MR angiogram were recommended. Head CT was advised only for cases presented within the time-window for thrombolytic therapy. If HINTS-plus testing demonstrated a peripheral pattern (unilateral corrective saccade, plus direction-fixed horizontal nystagmus opposite the abnormal corrective saccade, plus absent skew deviation, plus normal hearing), the recommendation was to defer brain imaging and pursue peripheral vestibulopathy management. If HINTS-plus testing was inconclusive (exhibited any other pattern different than central or peripheral), clinical judgment for therapeutic decision and neuroimaging was recommended.
Every isolated AVS patient seen by neurology or stroke team was added to a shared patients list created in the EMR. The principal investigator reviewed the list three times weekly to provide stakeholders accurate and timely feedback.
Medical records from isolated AVS cases seen two months prior to QI implementation and the months during the implementation were reviewed and analyzed. The outcome measures were compared before and after the established intervention using the software SAS V 9.4, Cary, NC.
Results
Baseline data were collected in 27 isolated AVS cases that presented to the ED in the two months prior to the QI initiative. Among these, 8 neurology consultations were obtained. None of these cases had HINTS-plus evaluation. More than half of patients underwent neuroimaging (head CT 18.5 %; brain MRI 51.8%).
Within 2 months after QI initiation, there were 84 ED presentations with vertigo or dizziness without associated medical conditions; 16 were adjudicated to be isolated AVS by chart review, 5 of which underwent neurology consultation (Figure A). Fisher’s exact test was used to compare the two proportions. The effect of our interventions in the first 2 months post implementation is shown in Table 1. The complete HINTS-plus testing performance by neurologists increased from 0% to 80% (p = 0.007), and by ED providers from 0% to 9.09% (p = 0.367). 87.5 % of the isolated AVS patients (n=14), and 60% of the isolated AVS patients that followed the proposed protocol (n=3) were discharged from the ED; none were readmitted or had another presentation to the ED in the ensuing 30 days.
Figure.
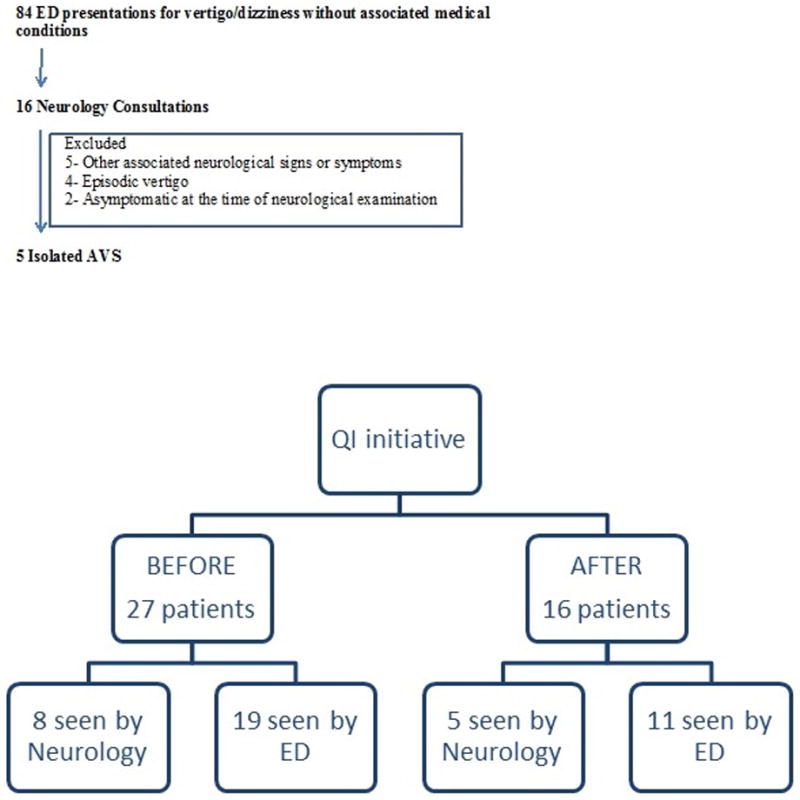
Diagrams illustrating the number of patients screened for isolated AVS and HINTS protocol by the Neurology Team (Figure A) and the number of patients with isolated AVS that were evaluated before and after the QI initiative was implemented (Figure B).
Table 1.
Pre- versus post-intervention comparison of outcomes
| Parameter | Pre-Intervention | Post-Intervention | Fisher’s Exact p-value |
|---|---|---|---|
| Ocular motor testing proportion by neurologists | 0% (n=0/8) | 80% (n=4/5) | 0.007 |
| Ocular motor testing proportion by ED providers | 0% (n=0/19) | 9.1% (n=1/11) | 0.367 |
| Head impulse testing proportion by neurologists | 12.5% (n=1/8) | 60% (n=3/5) | 0.217 |
| Head impulse testing proportion by ED providers | 5.2 % (n=1/19) | 9 % (n=1/11) | 1.0 |
| Nystagmus testing proportion by neurologists | 100% (n=8/8) | 100% (n=5/5) | 1.0 |
| Nystagmus testing proportion by ED providers | 31.5% (n=6/19) | 91% (n=10/11) | 0.002 |
| Skew deviation testing proportion by neurologists | 0% (n=0/8) | 60% (n=3/5) | 0.035 |
| Skew deviation testing proportion by ED providers | 0 % (n=0/19) | 9% (n=1/11) | 0.367 |
| Head CT imaging | 18.5% (n=5/27) | 6.3% (n=1/16) | 0.386 |
| Brain MR imaging | 51.8% (n=14/27) | 31.2% (n=5/16) | 0.221 |
Control
We continued to collect data beyond the initial phase to demonstrate sustained improvement. The results were communicated to neurology and ED teams in a timely fashion. Our interventions have been adopted as routine neurological care in evaluation of patients with isolated AVS. The impact on the total cost of care is pending determination, but overall rates of imaging for AVS patients declined during the study period.
While monitoring the performance of ocular motor examination, we reviewed charts and, as needed, provided feedback to clinicians. If HINTS documentation or interpretation was deemed inappropriate or questionable to any team member, an astute second look was performed by the principal investigator for clarification. Judicious and constructive opinion was provided to the stakeholders based on the preliminary analysis of the data. Further educational videos and suitable feedback were provided to enhance their comfort and attainment. For emergency physicians, nystagmus identification was found to be the easiest, while head impulse and skew deviation interpretation was not as readily adopted. Given their expressed lack of confidence with these HINTS-plus testing elements, it was not surprising that the number of neurology consultations in the ED increased from 14.8% to 31.25%.
An initial hurdle was adjudication of what isolated AVS represents and when HINTS-plus interpretation can be applied as per the protocol. Neurologists were consulted for 19% of vertigo and dizziness ED presentations, but only 31% of those were isolated AVS cases (Figure B). Challenging cases were apparent AVS patients that became asymptomatic by the time of neurology evaluation (secondary to vestibular suppressants or resolved spontaneously) and had a central HINTS pattern. We also noted that the HINTS-based protocol that we recommended to clinicians was inappropriately used in a few non-isolated AVS cases (i.e., with episodic vertigo or non-isolated AVS associated with additional neurological or ENT symptoms).
Discussion
We found that a multifaceted intervention including teaching sessions and continuous feedback to providers was able to improve consulting neurologist but not emergency physician documentation of eye movement examinations in AVS. We observed a trend towards decreased imaging use by both CT and MRI in these patients. Our study suggests that implementation of HINTS-plus evaluation to differentiate central from peripheral disorders in patients with AVS in the ED is probably feasible for neurologists but may prove challenging for emergency physicians, absent more extensive implementation interventions than the ones we used here. We were unable to replicate a recent study suggesting that emergency physicians can readily learn to use eye movement assessments with relatively limited training9, as our impact on emergency physician documentation was minimal.
Our study is limited by its small sample, incomplete case ascertainment, lack of eye movement recordings to validate clinician findings, and use of a single performance site. Not all patients presenting to the ED with vertigo were evaluated by neurologists. From those evaluated by neurologists, some had associated neurological symptoms or signs (ataxic gait, dysmetria, severe new-onset headache, etc), so the proposed protocol could not be applied appropriately. It is unknown whether our experience would generalize well to other centers.
Future studies should seek to determine the ‘dose-response’ curve of educational interventions for emergency physicians, perhaps assisted by new technologies. For example, quantitative portable video-oculography (VOG) could make testing more objective and reduce training needs10. A phase II, randomized controlled trial comparing VOG-guided care to current standard care to assess accuracy of diagnoses and initial management decisions for ED patients with a chief symptom of vertigo or dizziness is currently underway (AVERT: Acute Video-oculography for Vertigo in Emergency Rooms for Rapid Triage; ClinicalTrials.gov Identifier: NCT02483429).
In conclusion, while we await the results of ongoing studies, neurologists may prove a more receptive audience than ED physicians for education and QI initiatives designed to improve HINTS testing.
Acknowledgments
Dr. David E. Newman-Toker reports NIH grant support and research equipment loaned by GN Otometrics and Interacoustics. Dr. Newman-Toker’s effort was partly supported by a grant from the National Institutes of Health via the National Institute on Deafness and Other Communication Disorders (1 U01 DC013778-01A1). The funding agency was not involved in the writing, approval, or decision to publish this manuscript.
Dr. Shlee S. Song served on the scientific advisory board for Boehringer-Ingelheim and Portola Pharmaceuticals and is funded by NIH, California Community Foundation, and GenenTech grants for MR Witness (P50 NS051343), RHAPSODY (U01 NS088312), SUCCEED, and PRISMS stroke clinical trials.
Footnotes
Disclosures:
Drs. Oana M. Dumitrascu, Sam Torbati and Mourad Tighiouart have no discolsures.
References
- 1.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. 2011;183(9):E571–592. doi: 10.1503/cmaj.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerber KA, Newman-Toker DE. Misdiagnosing Dizzy Patients: Common Pitfalls in Clinical Practice. Neurol Clin. 2015;33(3):565–575, viii. doi: 10.1016/j.ncl.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40(11):3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman-Toker DE. Missed stroke in acute vertigo and dizziness: It is time for action, not debate. Annals of neurology. 2016;79(1):27–31. doi: 10.1002/ana.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37(10):2484–2487. doi: 10.1161/01.STR.0000240329.48263.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2013;20(10):986–996. doi: 10.1111/acem.12223. [DOI] [PubMed] [Google Scholar]
- 7.Saber Tehrani AS, Coughlan D, Hsieh YH, et al. Rising annual costs of dizziness presentations to U.S. emergency departments. Acad Emerg Med. 2013;20(7):689–696. doi: 10.1111/acem.12168. [DOI] [PubMed] [Google Scholar]
- 8.Kassardjian CD, Williamson ML, van Buskirk DJ, Ernste FC, Hunderfund AN. Residency Training: Quality improvement projects in neurology residency and fellowship: applying DMAIC methodology. Neurology. 2015;85(2):e7–e10. doi: 10.1212/WNL.0000000000001732. [DOI] [PubMed] [Google Scholar]
- 9.Vanni S, Nazerian P, Casati C, et al. Can emergency physicians accurately and reliably assess acute vertigo in the emergency department? Emerg Med Australas. 2015;27(2):126–131. doi: 10.1111/1742-6723.12372. [DOI] [PubMed] [Google Scholar]
- 10.Newman-Toker DE, Curthoys IS, Halmagyi GM. Diagnosing Stroke in Acute Vertigo: The HINTS Family of Eye Movement Tests and the Future of the “Eye ECG”. Semin Neurol. 2015;35(5):506–521. doi: 10.1055/s-0035-1564298. [DOI] [PMC free article] [PubMed] [Google Scholar]


