Abstract
Objective
Structural brain imaging studies in Obsessive-Compulsive Disorder (OCD) have produced inconsistent findings. This may be partially due to limited statistical power from relatively small samples and clinical heterogeneity related to variation in disease profile and developmental stage.
Methods
To address these limitations, we conducted a meta- and mega-analysis of data from OCD sites worldwide. T1 images from 1,830 OCD patients and 1,759 controls were analyzed, using coordinated and standardized processing, to identify subcortical brain volumes that differ in OCD patients and healthy controls. We additionally examined potential modulating effects of clinical characteristics on morphological differences in OCD patients.
Results
The meta-analysis indicated that adult patients had significantly smaller hippocampal volumes (Cohen’s d=−0.13; p=5.1x10−3, % difference −2.80) and larger pallidum volumes (d=0.16; p=1.6x10−3, % difference 3.16) compared to adult controls. Both effects were stronger in medicated patients compared to controls (d=−0.29; p=2.4x10−5, % difference −4.18 and d=0.29; p=1.2x10−5, % difference 4.38, respectively). Unmedicated pediatric patients had larger thalamic volumes (d=0.38, p=2.1x10−3) compared to pediatric controls. None of these findings were mediated by sample characteristics such as mean age or field strength. Overall the mega-analysis yielded similar results.
Conclusion
Our study indicates a different pattern of subcortical abnormalities in pediatric versus adult OCD patients. The pallidum and hippocampus seem to be of importance in adult OCD, whereas the thalamus seems to be key in pediatric OCD. This highlights the potential importance of neurodevelopmental alterations in OCD, and suggests that further research on neuroplasticity in OCD may be useful.
Introduction
Obsessive-compulsive disorder (OCD) is a neurodevelopmental disorder that affects 1–3% of the population (1; 2). In more than 50% of all OCD cases, symptoms emerge during childhood or adolescence (1; 3), and in more than 40% of these cases the disorder persists into adulthood (4). OCD symptoms have been associated with structural and functional brain abnormalities in the parallel cortico-striato-thalamo-cortical circuits and other related brain networks, involving fronto-parietal, fronto-limbic and cerebellar regions (5; 6).
Several studies have shown volumetric abnormalities in different deep grey matter structures, mainly the basal ganglia (7–10). Meta-analyses have repeatedly, although not consistently, reported larger volumes in the lenticular nucleus extending to the caudate (11–14). In addition, Pujol et al. (7) showed that the relative enlargement of striatal areas in OCD patients was driven by an older age of the subject and a longer disease duration, suggesting that basal ganglia alterations progress throughout the disease course, supported by the mega-analysis from the OCD Brain Imaging Consortium (OBIC) (15). These findings led to the hypothesis that preservation of basal ganglia volume resulted from neuroplastic changes due to chronic compulsivity.
Although these findings suggest ongoing neuroplasticity, a lifespan approach has seldom been used to understand the variation in structural abnormalities in OCD (5). Studying the brain characteristics of disease during childhood may minimize the potentially confounding effects of neuroplastic changes associated with chronic symptomatology and long-term treatment. Pediatric studies have been sparse and small, leaving the extant findings inconclusive and variable. For example, some studies reported increased thalamus volume in adult (16; 17) and pediatric OCD patients (18), supported by two meta-analyses (14; 19) showing larger thalamus volumes in OCD patients when pediatric and adult data were combined. In contrast, several recent meta-analyses showed no differences in thalamus volumes while combining adult and pediatric subjects (11–13). The variation across studies may partially be explained by variations in the developmental and disease stages of the subjects included.
In view of the clinical heterogeneity of OCD, relatively small samples, differences in data acquisition, data processing protocols, and statistical analyses further contribute to the inconsistent findings. Different segmentation algorithms may give variable estimates of subcortical volumes and thus their sensitivity to regionalized group differences (20). To overcome the heterogeneity in image processing and to increase sample sizes, especially regarding pediatric data, we initiated the OCD Working-Group within the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium (21).
The ENIGMA-OCD Working-Group is an international collaboration and its current aim is to identify subcortical imaging markers that differ in OCD patients and healthy controls, both in children and in adults. Therefore, we conducted a meta- and mega-analysis on structural Magnetic Resonance Imaging (MRI) data of 1,830 OCD patients and 1,759 healthy controls. The mega-analysis ensures information preservation and enables the examination of specific effects of demographic and clinical parameters. By employing meta- and mega-analysis we sought to investigate whether the mega-analytical design has greater sensitivity to detect more subtle brain abnormalities from increased statistical power.
In this study, we investigated nine regions of interest (i.e. seven subcortical grey matter regions, lateral ventricle, and total intracranial volume) in OCD patients compared to healthy controls by performing the largest meta- and mega-analysis to date. In additional exploratory analyses, we examined potential modulating effects of demographic, clinical, and methodological characteristics on subcortical brain volume in OCD. Based on previous meta- and mega-analyses, we expected subcortical brain volumes to vary across developmental stage showing differences between pediatric and adult OCD, and disease profile and stage, including co-morbidity.
Methods
Samples
The ENIGMA-OCD Working-Group includes 35 datasets from 25 international research institutes, with neuroimaging and clinical data from OCD patients and controls, including both children and adults. We considered subjects ≥18 years as adults and subjects <18 years as children. Since previous literature suggested differential effects between pediatric and adult samples, we performed separate meta- and mega-analysis for adult and pediatric data. Demographics and clinical characteristics of the participants in each center are shown in Tables 1 and 2, respectively. In total, we analyzed data from 3,589 subjects including 1,830 OCD patients (N=335 children, N=1,495 adults) and 1,759 controls (N=287 children, N=1,472 adults). All local IRBs permitted the use of extracted measures of the completely anonymized data.
Table 1.
ENIGMA-OCD Working-Group Demographics (age in years), sex, and OCD patients-control breakdown for participating sites
| Study # | Site PI | Site, Country | Field Strength | Age Controls | Age OCD patients | % Male Controls | % Male OCD | Total N Controls | Total N OCD | Total N | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||||||
| 1 | Benedetti | Milan, ITA | 3T | 34.0 | 12.3 | 35.0 | 10.4 | 73 | 71 | 62 | 66 | 128 |
| 2 | Beucke | Berlin, GER | 1.5T | 31.9 | 9.5 | 32.4 | 9.7 | 49 | 50 | 104 | 92 | 196 |
| 3 | Cheng | Kunming, CHN I | 1.5T | 31.4 | 8.0 | 30.6 | 10.2 | 33 | 38 | 40 | 24 | 64 |
| 4 | Kunming, CHN II | 3T | 26.2 | 4.2 | 32.9 | 10.6 | 28 | 55 | 95 | 56 | 151 | |
| 5 | Denys | Amsterdam, NLD | 3T | 39.6 | 10.3 | 33.8 | 9.6 | 44 | 21 | 25 | 24 | 49 |
| 6 | van den Heuvel | Amsterdam, NLD I | 1.5T | 31.6 | 7.7 | 33.5 | 9.2 | 39 | 30 | 49 | 54 | 103 |
| 7 | Amsterdam, NLD II | 3T | 39.6 | 11.4 | 38.3 | 10.1 | 47 | 48 | 38 | 42 | 80 | |
| 8 | Hoexter | Sao Paulo, BRA I | 1.5T | 27.6 | 7.8 | 31.5 | 10.1 | 35 | 44 | 37 | 50 | 87 |
| 9 | Koch | Munchen, GER | 3T | 30.2 | 9.0 | 31.1 | 9.7 | 40 | 33 | 75 | 72 | 147 |
| 10 | Kwon | Seoul, KOR I | 1.5T | 24.0 | 3.6 | 24.8 | 5.4 | 56 | 76 | 104 | 45 | 149 |
| 11 | Seoul, KOR II | 1.5T | 24.9 | 5.3 | 28.8 | 6.8 | 64 | 56 | 45 | 34 | 79 | |
| 12 | Seoul, KOR III | 3T | 26.3 | 6.9 | 26.3 | 6.8 | 61 | 61 | 89 | 90 | 179 | |
| 13 | Mataix-Cols | Stockholm, SWE | 1.5T | 36.1 | 11.3 | 38.7 | 10.9 | 36 | 43 | 33 | 44 | 77 |
| 14 | Menchon | Barcelona, ESP | 1.5T | 33.1 | 10.2 | 34.8 | 9.2 | 45 | 50 | 66 | 117 | 183 |
| 15 | Nakamae | Kyoto, JPN I | 1.5T | 30.3 | 7.8 | 31.7 | 9.3 | 52 | 49 | 48 | 82 | 130 |
| 16 | Kyoto, JPN II | 3T | 30.0 | 7.4 | 33.3 | 9.7 | 48 | 35 | 42 | 34 | 76 | |
| 17 | Nakao | Fukuoka, JPN | 3T | 39.3 | 13.0 | 36.6 | 10.0 | 39 | 42 | 41 | 81 | 122 |
| 18 | Reddy | Bangalore, IND I | 1.5T | 27.2 | 6.4 | 27.5 | 6.3 | 74 | 59 | 46 | 44 | 90 |
| 19 | Bangalore, IND II | 3T | 26.3 | 5.0 | 29.6 | 8.0 | 62 | 52 | 156 | 208 | 364 | |
| 20 | Simpson | New York, USA | 3T | 28.3 | 8.0 | 29.6 | 8.0 | 52 | 52 | 33 | 33 | 66 |
| 21 | Spalletta | Rome, ITA | 3T | 36.5 | 10.5 | 36.7 | 11.6 | 59 | 67 | 128 | 84 | 212 |
| 22 | Stein | Cape Town, ZAF | 3T | 30.6 | 10.8 | 30.7 | 10.8 | 38 | 50 | 29 | 22 | 51 |
| 23 | Tolin | Conneticut, USA | 3T | 48.0 | 11.9 | 32.1 | 12.0 | 22 | 67 | 32 | 27 | 59 |
| 24 | Walitza | Zurich, CHE I | 3T | 32.9 | 9.2 | 31.2 | 7.7 | 28 | 47 | 18 | 17 | 35 |
| 25 | Wang | Shanghai, CHN | 3T | 26.2 | 7.5 | 29.6 | 9.3 | 54 | 57 | 37 | 53 | 90 |
| adult samples combined | 1472 | 1495 | 2967 | |||||||||
|
| ||||||||||||
| 1 | Anorld | Ontario, CAN | 3T | 12.3 | 2.2 | 12.9 | 2.4 | 54 | 58 | 13 | 40 | 53 |
| 2 | Fitzgerald | Michigan, USA | 3T | 12.9 | 2.9 | 13.9 | 2.6 | 52 | 49 | 67 | 74 | 141 |
| 3 | Gruner | Conneticut, USA | 3T | 14.2 | 2.2 | 14.3 | 2.1 | 52 | 57 | 23 | 23 | 46 |
| 4 | Hoexter | Sao Paulo, BRA II | 3T | 12.0 | 2.4 | 12.6 | 2.5 | 57 | 61 | 28 | 28 | 56 |
| 5 | Huyser | Amsterdam, NLD | 3T | 13.3 | 2.5 | 13.6 | 2.5 | 36 | 37 | 25 | 27 | 52 |
| 6 | Lazaro | Barcelona, ESP I | 1.5T | 14.6 | 2.3 | 14.6 | 2.0 | 47 | 58 | 32 | 31 | 63 |
| 7 | Barcelona, ESP II | 3T | 14.6 | 2.1 | 14.6 | 2.0 | 55 | 60 | 44 | 58 | 102 | |
| 8 | Reddy | Bangalore, IND III | 3T | 13.1 | 2.1 | 14.6 | 2.0 | 50 | 56 | 14 | 18 | 32 |
| 9 | Soreni | Ontario, CAN | 3T | 11.2 | 3.1 | 13.4 | 2.5 | 52 | 40 | 21 | 20 | 41 |
| 10 | Walitza | Zurich, CHE II | 3T | 14.6 | 1.3 | 15.7 | 1.4 | 50 | 81 | 20 | 16 | 36 |
| paediatric samples combined | 287 | 335 | 622 | |||||||||
|
| ||||||||||||
| 35 | total | 1759 | 1830 | 3589 | ||||||||
Abbreviations: Obsessive Compulsive Disorder (OCD), Principal Investigator (PI), Standard Deviation (SD)
Table 2.
ENIGMA-OCD Working-Group Clinical characteristics of OCD patients. Percentage of OCD patients using medication, disease severity (measured with the YBOCS), age of onset of OCD, percentage of patients with a comorbid anxiety disorder, and percentage of patients with a comorbid depression are listed.
| Study # | Site PI | Site, Country | % medicated OCD patients | YBOCS | Age of onset | % Comorbid lifetime anxiety | % Comorbid lifetime depression | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||||
| Adult samples | |||||||||
| 1 | Benedetti | Milan, ITA | 64 | 30.9 | 5.6 | 16.0 | 6.1 | 1.52 | 10.61 |
| 2 | Beucke | Berlin, GER | 40 | 20.1 | 7.1 | 17.2 | 7.8 | 11.96 | 18.48 |
| 3 | Cheng | Kunming, CHN I | 71 | 31.0 | 6.1 | 26.8 | 10.4 | 50.00 | 16.67 |
| 4 | Kunming, CHN II | 68 | 28.2 | 6.3 | 27.2 | 10.7 | 89.29 | 28.57 | |
| 5 | Denys | Amsterdam, NLD | 63 | 26.6 | 6.2 | 18.1 | 6.9 | 4.17 | 41.67 |
| 6 | van den Heuvel | Amsterdam, NLD I | 0 | 22.7 | 6.1 | 14.4 | 7.7 | 22.22 | 33.33 |
| 7 | Amsterdam, NLD II | 0 | 21.5 | 6.1 | 15.5 | 6.9 | 40.48 | 52.38 | |
| 8 | Hoexter | Sao Paulo, BRA I | 20 | 27.2 | 6.1 | 13.1 | 7.0 | 62.00 | 54.00 |
| 9 | Koch | Munchen, GER | 60 | 20.9 | 6.2 | 17.0 | 6.7 | – | – |
| 10 | Kwon | Seoul, KOR I | 24 | 20.2 | 6.0 | 17.4 | 5.2 | 0.00 | 0.00 |
| 11 | Seoul, KOR II | 0 | 23.9 | 6.5 | 18.9 | 6.6 | 0.00 | 2.94 | |
| 12 | Seoul, KOR III | 2 | 26.5 | 6.5 | 19.0 | 6.4 | 1.11 | 2.22 | |
| 13 | Mataix-Cols | Stockholm, SWE | 41 | 25.9 | 7.7 | 18.4 | 9.2 | 27.27 | 34.09 |
| 14 | Menchon | Barcelona, ESP | 97 | 25.5 | 5.8 | 21.4 | 8.5 | 20.51 | 18.80 |
| 15 | Nakamae | Kyoto, JPN I | 49 | 25.2 | 6.4 | 25.1 | 9.4 | 9.76 | 21.95 |
| 16 | Kyoto, JPN II | 0 | 22.4 | 6.9 | 25.2 | 9.1 | 8.82 | 20.59 | |
| 17 | Nakao | Fukuoka, JPN | 88 | 22.5 | 5.6 | 24.6 | 9.5 | – | 35.80 |
| 18 | Reddy | Bangalore, IND I | 0 | 25.8 | 7.3 | 21.7 | 7.5 | 15.91 | 18.18 |
| 19 | Bangalore, IND II | 40 | 25.8 | 6.3 | 22.0 | 7.6 | 7.69 | 15.38 | |
| 20 | Simpson | New York, USA | 0 | 25.5 | 3.7 | 15.0 | 7.0 | 21.21 | 30.30 |
| 21 | Spalletta | Rome, ITA | 88 | 23.4 | 8.9 | 18.9 | 10.9 | 9.52 | 9.52 |
| 22 | Stein | Cape Town, ZAF | 41 | 22.9 | 4.2 | 13.6 | 6.6 | 0.00 | 0.00 |
| 23 | Tolin | Conneticut, USA | 78 | 22.7 | 4.8 | – | 44.44 | 40.74 | |
| 24 | Walitza | Zurich, CHE I | 59 | 17.1 | 9.9 | 16.7 | 7.8 | 47.06 | 47.06 |
| 25 | Wang | Shanghai, CHN | 0 | 25.5 | 5.1 | 23.3 | 10.3 | 0.00 | 0.00 |
|
| |||||||||
| Paediatric samples | |||||||||
| 1 | Anorld | Ontario, CAN | 53 | 20.9 | 7.8 | 8.7 | 2.6 | 25.00 | 17.50 |
| 2 | Fitzgerald | Michigan, USA | 50 | 18.7 | 7.8 | 9.9 | 3.0 | 50.00 | 6.76 |
| 3 | Gruner | Conneticut, USA | 52 | 26.9 | 4.5 | – | 43.48 | 39.13 | |
| 4 | Hoexter | Sao Paulo, BRA II | 46 | 26.9 | 5.4 | 7.2 | 3.0 | 21.43 | 0.00 |
| 5 | Huyser | Amsterdam, NLD | 0 | 25.1 | 5.0 | 10.9 | 2.8 | 48.15 | 25.93 |
| 6 | Lazaro | Barcelona, ESP I | 55 | 22.2 | 6.0 | 12.4 | 2.2 | 16.13 | 3.23 |
| 7 | Barcelona, ESP II | 79 | 18.6 | 7.4 | 12.0 | 2.4 | 25.86 | 5.17 | |
| 8 | Reddy | Bangalore, IND III | 83 | 22.6 | 7.3 | 13.1 | 2.1 | 22.22 | 5.56 |
| 9 | Soreni | Ontario, CAN | 0 | 22.8 | 4.3 | – | – | – | |
| 10 | Walitza | Zurich, CHE II | 56 | 14.7 | 1.0 | 11.1 | 2.2 | 50.00 | 6.25 |
Abbreviations: Obsessive Compulsive Disorder (OCD), Principal Investigator (PI), Standard Deviation (SD), Yale Brown Obsessive Compulsive Scale (YBOCS)
Image acquisition and processing
Structural T1-weighted MRI brain scans were acquired and analyzed locally. Images were acquired at different field strengths (i.e., 1.5T and 3T). The acquisition parameters of each sample are listed in Supplementary Table 1. The images were analyzed using the fully automated and validated segmentation software FreeSurfer v5.3. (22) following standardized protocols to harmonize analysis and quality control processes across multiple sites (http://enigma.ini.usc.edu/protocols/imaging-protocols/). Segmentation of nine regions of interest, including seven subcortical grey matter structures, i.e., nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus, the lateral ventricle volumes (mean bilateral and right and left side separately), and total intracranial volume were visually inspected for accuracy (Supplementary Information 2).
Meta-analysis of subcortical brain volumes
We examined differences between OCD patients and controls across samples by performing a meta-analysis on the mean of the left and right hemisphere measures of each subcortical structure. The meta-analysis was based on multiple linear regression models, with the mean subcortical brain volume as the outcome measure and a binary indicator of diagnosis (0=controls, 1=patients) as the predictor of interest. All models were controlled for age, sex, and intracranial volume. Effect size estimates, adjusted for age, sex, and intracranial volume, were calculated using Cohen’s d-metric computed from the t-statistic of the diagnosis indicator variable from the regression models.
To explore the influence of sex and age on between-group subcortical volume differences, we assessed the significance of diagnosis-by-sex and diagnosis-by-age interaction effects within each sample. Further, multiple linear regression models were used to investigate the within-group effects of age at onset, disease duration, disease severity (using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and the Children’s Y-BOCS (23; 24) total severity score) as continuous variables. To further study the neurodevelopmental aspects of disease within the adult samples, we performed separate stratified meta-analyses comparing early-onset OCD patients (<18 years) to controls, and late-onset OCD patients (≥ 18 years) to controls. Stratified meta-analyses were also performed for medicated and non-medicated patients. Likewise separate stratified analyses were performed to investigate comorbid major depressive disorder (MDD), comorbid anxiety disorders, and OCD symptom dimensions (using the Y-BOCS symptom checklist; Supplementary Information 5 symptom dimension analyses).
All regression models and effect size estimates were fit at each site separately. Subsequently, a final Cohen’s d-effect size estimate was obtained using an inverse variance-weighted random-effect meta-analysis model with the R package ‘metaphor’ (version 1.9-118). The meta-analysis of disease severity, age at onset, and disease duration were exceptions. The scores on these variables were considered as continuous variables, so effect sizes are reported using Pearson’s r, a partial-correlation after removing nuisance variables (age, sex, and intracranial volume). The final meta-analyzed Pearson’s r was estimated following the same inverse variance-weighted random-effect meta-analysis models used for the other meta-analyses (Supplementary Information 3).
Moderator analyses
Meta-regressions were performed to examine the effects of moderator variables on meta-analysis effect sizes. We tested whether hypothesized moderating factors such as the mean age of each sample, field strength, percentage of patients taking antidepressants and percentage of patients taking antipsychotics influenced the effect size estimates of the OCD patients versus controls comparison of all subcortical volumes across samples included in the meta-analysis. Each moderator variable was separately included as a fixed effect predictor in a meta-regression model. We report uncorrected P-values with a significance threshold determined by Bonferroni correction for testing nine regions of interest (P=0.05/9= 5.6×10−3).
Power Analysis
Sample sizes that achieve 80% power to detect group differences given the presented effect sizes were calculated based two-sided t-tests assuming unequal variance with G*Power v3.2.1. (25). See Supplementary Information 4 for full details of the power analysis.
Mega-analysis of subcortical brain volumes
We also performed a mega-analysis by pooling all volumetric measurements. The mega-analysis of each mean ((left+right)/2) subcortical volume was performed using the following model: Brain volume= βageXage+ βsexXsex+ βintracranial volumeXintracranial volume+ βdiagnosisXdiagnosis+ βcohort1Xcohort1+….+ βcohort35Xcohort35+ ε. Similar to the meta-analysis, several covariates of interest were investigated using this regression model. Results were considered significant if they exceeded the Bonferroni corrected P-value threshold 5.6×10−3.
Results
We included data of 25 adult cohorts and 10 pediatric cohorts. The adult meta- and mega-analysis contained 1,495 OCD patients and 1,472 controls and the pediatric meta- and mega-analysis contained 335 OCD patients and 287 controls. An overview of the number of participants included per cohort is given in Table 1. Supplementary Information 5 describes which sites were included in the analyses regarding the clinical characteristics, and what was considered a sufficient amount of data.
Meta-analysis
OCD patients versus healthy controls
Adult comparison
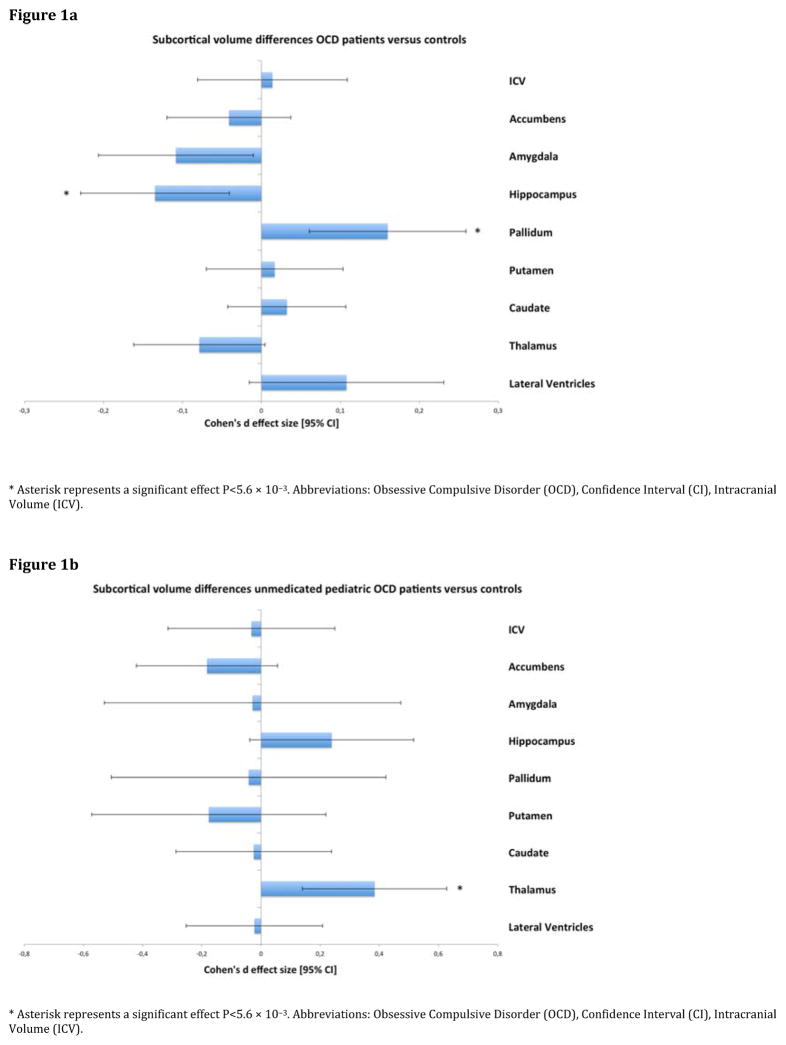
Results from the analysis comparing all adult OCD patients (N=1,495) to all adult controls (N=1,472) across nine regions of interest volumes are provided in Figure 1a and Table 3. Compared to controls, adult OCD patients showed significantly smaller hippocampal volume (Cohen’s d [95% confidence interval]: d=−0.13 [−0.23, −0.04]; P-value= 5.08×10−3, % difference −2.80) and larger pallidum volume (d=+0.16 [0.06, 0.26]; P-value= 1.60×10−3, % difference 3.16). No significant diagnosis-by-sex or diagnosis-by-age interaction effect for any of the subcortical volumes was observed.
Figure 1.
(a) Cohen’s d-effect sizes 95% CI for differences in subcortical brain volumes between adult OCD patients and healthy controls. (b) Cohen’s d-effect sizes 95% CI for differences in subcortical brain volumes between unmedicated pediatric OCD patients and pediatric healthy controls. Effect sizes were corrected for age, sex, and intracranial volume.
Table 3.
Full meta-analytic results for each mean structure for the OCD patients versus controls comparison, controlling for age, sex, scan center, and intracranial volume. Adjusted Cohen’s d is reported.
| Cohen’s d (OCD–HC) | Standard error | 95% CI | % Difference | P-value | I2 | Number of controls | Number of patients | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lateral Ventricles | 0,108 | 0,063 | −0,016 | to | 0,231 | 1,712 | 0,087 | 61,327 | 1466 | 1491 |
| Thalamus | −0,079 | 0,042 | −0,162 | to | 0,005 | −1,851 | 0,064 | 12,542 | 1387 | 1375 |
| Caudate | 0,032 | 0,038 | −0,043 | to | 0,107 | 0,844 | 0,399 | 0,003 | 1424 | 1441 |
| Putamen | 0,017 | 0,044 | −0,070 | to | 0,103 | 0,380 | 0,704 | 16,141 | 1335 | 1365 |
| Pallidum | 0,160 | 0,051 | 0,061 | to | 0,259 | 3,156 | 1,60 × 10− 3 | 32,877 | 1312 | 1336 |
| Hippocampus | −0,135 | 0,048 | −0,229 | to | −0,040 | −2,802 | 5,08 × 10− 3 | 32,692 | 1440 | 1444 |
| Amygdala | −0,108 | 0,050 | −0,206 | to | −0,010 | −2,163 | 0,031 | 37,194 | 1418 | 1452 |
| Accumbens | −0,041 | 0,040 | −0,120 | to | 0,037 | −1,025 | 0,305 | 8,384 | 1446 | 1465 |
| ICV* | 0,014 | 0,048 | −0,081 | to | 0,109 | 0,286 | 0,775 | 35,547 | 1470 | 1493 |
Abbreviations: Obsessive Compulsive Disorder (OCD), Healthy Control (HC), Intracranial Volume (ICV), Confidence Interval (CI)
Controlled for age, sex and scan center
Pediatric comparison
None of the subcortical volumes was significantly different between pediatric OCD cases (N=335) and controls (N=287) after Bonferroni correction (Supplementary Table 2).
Influence of medication on subcortical volume
Adult comparisons
Compared to controls, medicated OCD patients (N=654) showed larger lateral ventricles (d=+0.24 [0.08, 0.41]; P-value= 2.95×10−3, % difference 2.97) and a larger pallidum volume (d= +0.29 [0.16, 0.42]; P-value= 1.20×10−5, % difference 4.38) as well as a smaller hippocampal volume (d=− 0.29 [−0.43, −0.16]; P-value= 2.39×10−5, % difference −4.18). We did not detect any significant differences between unmedicated OCD patients (N=821) and healthy controls, nor between medicated OCD patients and unmedicated OCD patients. See Supplementary Table 3a–c for full meta-analytic details regarding medication influence on the adult comparisons.
Pediatric comparisons
Figure 1b and Table 4 show that the unmedicated pediatric OCD patients (N=159), compared with controls, had larger thalamic volume (d= +0.38 [0.14, 0.63]; P-value= 2.09×10−3, % difference 3.08). Further, we found smaller nucleus accumbens volume in medicated pediatric OCD patients (N=170) compared with controls (d= −0.32 [−0.54, −0.09]; P-value= 5.25×10−3, % difference −2.79). No significant differences were detected between medicated and unmedicated pediatric OCD patients (Supplementary Table 4a–b).
Table 4.
Full meta-analytic results for each mean structure for the pediatric unmedicated OCD patients versus pediatric controls comparison, controlling for age, sex, scan center, and intracranial volume. Adjusted Cohen’s d is reported
| Cohen’s d (unmedicated pediatric OCD–HC) | Standard error | 95% CI | % Difference | P-value | I2 | Number of controls | Number of patients | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lateral Ventricles | −0,022 | 0,118 | −0,253 | to | 0,209 | −0,189 | 0,850 | 0,000 | 216 | 115 |
| Thalamus | 0,384 | 0,125 | 0,139 | to | 0,628 | 3,078 | 2,09 × 10− 3 | 0,000 | 201 | 103 |
| Caudate | −0,024 | 0,134 | −0,288 | to | 0,239 | −0,182 | 0,855 | 14,641 | 198 | 109 |
| Putamen | −0,177 | 0,202 | −0,572 | to | 0,219 | −0,875 | 0,382 | 59,152 | 204 | 104 |
| Pallidum | −0,042 | 0,237 | −0,506 | to | 0,423 | −0,176 | 0,860 | 66,561 | 174 | 87 |
| Hippocampus | 0,239 | 0,141 | −0,038 | to | 0,516 | 1,688 | 0,091 | 22,715 | 210 | 107 |
| Amygdala | −0,029 | 0,256 | −0,530 | to | 0,473 | −0,112 | 0,911 | 72,254 | 188 | 89 |
| Accumbens | −0,183 | 0,122 | −0,422 | to | 0,056 | −1,500 | 0,134 | 0,004 | 203 | 111 |
| ICV* | −0,033 | 0,144 | −0,314 | to | 0,249 | −0,226 | 0,821 | 29,531 | 219 | 116 |
Abbreviations: Obsessive Compulsive Disorder (OCD), Healthy Control (HC), Intracranial Volume (ICV), Confidence Interval (CI)
Controlled for age, sex and scan center
Influence of comorbid MDD on subcortical volume in adult OCD
Adult comparisons
Supplementary Table 5a–c shows that compared to controls, OCD patients with a comorbid lifetime diagnosis of depression (N=325) had smaller hippocampal volume (d=−0.27 [−0.43, −0.12]; P-value= 6.43×10−4, % difference −3.41) and larger lateral ventricles (d= +0.29 [0.14, 0.44]; P-value= 1.16×10−4, % difference 3.85). OCD patients without a comorbid lifetime diagnosis of MDD (N=1,041) present larger pallidum volume (d=+0.19 [0.09, 0.29]; P-value= 1.56×10−4, % difference 3.78) and smaller hippocampal volume (d=−0.16 [−0.25, −0.06]; P-value= 1.04×10−3, % difference −3.28). No significant subcortical volume differences were observed between OCD patients with and without a comorbid lifetime depression.
Pediatric comparisons
Too few pediatric samples had sufficient numbers of subjects with MDD to permit analyses (Supplementary Information 5).
Influence of a comorbid anxiety disorder on subcortical volume
Adult comparisons
Compared to controls, patients without a comorbid anxiety diagnosis (N=1002) showed bigger pallidum volume (d= +0.17 [0.05, 0.28]; P-value= 4.70×10−3, % difference 2.83) and smaller hippocampal volume (d= −0.20 [−0.30, −0.10]; P-value= 1.51×10−4, % difference −3.79). We did not detect any significant differences between OCD patients with a comorbid anxiety diagnosis (N=291) and controls. The comparison between OCD patients with and without a comorbid anxiety diagnosis showed that OCD patients with a comorbid lifetime anxiety diagnosis had larger intracranial volume (d= +0.41 [0.12, 0.70]; P-value= 5.08×10−3, % difference 2.80) (Supplementary Table 6a–c).
Pediatric comparisons
Too few pediatric samples had sufficient numbers of subjects with comorbid anxiety disorders to permit analyses (Supplementary Information 5).
Influence of symptom dimensions on subcortical volume
Adult comparisons
Regression analyses within OCD patients on symptom dimensions (N=1,151) showed no association of the presence of a particular symptom dimension and volume of any of the subcortical volumes.
Pediatric comparisons
Insufficient data on the symptom dimensions was available to perform meta-analyses (Supplementary Information 5).
Influence of age of onset and disease duration on subcortical volume
Stratified analyses (Supplementary Table 7a–c) show that adult OCD patients with an early disease-onset (N=626) exhibited larger pallidum volumes (d=+0.25 [0.12, 0.38]; P-value= 2.30×10−4, % difference 3.68) and that patients with a late disease-onset (N=794) exhibited smaller hippocampal volume (d=−0.18 [−0.29, −0.08]; P-value= 7.87×10−4, % difference −3.36) than controls. No significant differences in subcortical brain volume were found when comparing early onset with late-onset adult OCD patients. In addition, we did not observe any significant association between age of onset nor disease duration - as continuous variables - and subcortical volumes in the adult (N=1420) nor pediatric (N=285) OCD group (Supplementary Table 8a–b and 9a–b).
Association of disease severity with subcortical volumes
We did not detect any significant associations, neither in adult (N=1,455) nor in pediatric (N=328) OCD patients, between disease severity and subcortical volumes (Supplementary Table 10 and 11).
Moderator analyses
Mean age of each sample and field strength did not moderate case-control differences in subcortical volumes in the adult or pediatric meta-analysis. The percentage of patients using an SSRI or antipsychotic medication of each adult sample did not moderate the subcortical volume differences (Supplementary 12 and 13).
Mega-analysis
Adult OCD
Results of the adult mega-analysis are shown in Supplementary Table 14. Overall the results of the mega-analysis yielded similar results as the meta-analysis. The case-control mega-analysis indicated a larger pallidum volume (β=0.06; P-value= 1.02×10−4) and smaller hippocampal volume (β=−0.05; P-value= 4.66×10−4). The pallidum (β=0.09; P-value= 5.50×10−7) and hippocampus (β=−0.09; P-value= 1.99×10−7) effects were more pronounced in the comparison between medicated OCD patients and controls. Early-onset patients showed larger pallidum volumes (β=0.08; P-value= 8.42×10−6) than controls. Patients with a late disease-onset (β=−0.06; P-value= 8.23×10−5) and patients with a comorbid depression (β=−0.07; P-value= 2.75×10−4) presented smaller hippocampal volumes compared to controls.
Pediatric OCD
Results of the pediatric mega-analysis are shown in Supplementary Table 15. Pediatric OCD patients, compared with controls, have a larger thalamus volume (β=0.08; P-value= 5.47×10−3). The thalamic effect was more pronounced in patients without a comorbid anxiety disorder (β=0.11; P-value= 9.60×10−4) and in patients without a comorbid depression (β=0.09; P-value= 2.16×10−3).
Discussion
This worldwide collaborative analysis identified distinct subcortical volume alterations in pediatric and adult OCD. The adult meta- and mega-analyses were consistent and results showed that, compared with controls, adult OCD patients had significantly smaller hippocampal and larger pallidum volumes. Both findings were more pronounced in the subsample of medicated OCD patients versus controls. Furthermore, the smaller hippocampal volume seemed to be driven, at least partly, by the OCD patients with comorbid depression and late disease-onset. Indeed jackknife resampling showed a robust pallidum effect and a hippocampal effect dependent on site characteristics (data not shown). The larger pallidum finding was more pronounced in the adult OCD patients with an early disease-onset. The pediatric mega-analysis showed larger thalamus in OCD based on the main group comparison, whereas the meta-analysis only showed this in unmedicated pediatric OCD patients compared with controls. The pediatric mega-analysis also suggests that larger thalamic volume in pediatric OCD patients is specific to those without comorbid anxiety or depression. The finding of a larger thalamic volume in pediatric OCD is in line with some previous research in pediatric OCD patients (18; 26). Notably, Gilbert et al. (18) suggested a normalizing effect of pharmacological treatments on thalamic volume in pediatric OCD. The current adult meta- and mega-analyses did not reveal group differences in thalamic volume, consistent with the most recent meta-analyses of OCD (11–13). The only meta-analytic findings of thalamic enlargement in OCD included pediatric patients (14; 19). These results provide evidence of a clear distinction in thalamic volume across pediatric and adult OCD, and suggest that an increased thalamic volume may be an early marker of the disease, unrelated to disease severity, and may be related to altered neurodevelopment. Indeed, patients with other neurodevelopmental disorders such as Tourette’s syndrome (27) and ADHD (28), also present a morphologically enlarged thalamus.
Most previous research (11; 13–15; 19) did not report volumetric differences in the hippocampal complex of OCD patients. The (para)hippocampal regions are specifically vulnerable to stress-related toxic changes (29). Greater volume loss in these regions may thus be related to chronic stress and the exaggerated emotional responsiveness seen in OCD (30). The hippocampal effect in OCD patients was more pronounced in medicated patients and seemed to be driven, at least partly, by the OCD patients with a comorbid major depression (31). These two findings are probably not independent, since patients with comorbidities are often the patients who receive medication. Further, Selles et al (32) showed that a comorbid depression is associated with a late-onset of the disease. This is in line with our finding that the hippocampal effect seemed to be driven by late-onset OCD patients. Other ENIGMA disease working-groups, such as those focusing on MDD (33), schizophrenia (34), and bipolar disorder (35), also observed smaller hippocampal volume in patients, which suggests that the hippocampal abnormalities in OCD are disease non-specific, and possibly related to chronic stress and comorbid depression.
Our results suggest a key role for the pallidum in adult OCD patients. Prior meta-analyses have reported greater lenticular (i.e., putamen and pallidum) volume in OCD patients (11–14). On the contrary patients with other anxiety disorders showed decreased lenticular nucleus volume (13). Since repetitive behaviors differentiate OCD from other anxiety disorders, the increased lenticular volume in OCD may reflect these unique symptoms (13). Our analyses also suggested that the early-onset adult OCD patients drive the pallidum effect. We, therefore, hypothesize that a larger pallidum in OCD patients could be the consequence of disease chronicity. Notably, the ENIGMA-schizophrenia (34) Working-Group also observed a larger pallidum in schizophrenia patients compared to controls. Future ENIGMA research will enable cross-diagnosis analyses to further investigate common and distinct neural substrates across psychiatric disease groups.
Our analyses could not replicate findings of increased putamen and caudate nucleus volumes observed in smaller meta-analyses (11–14). Note that, these studies used different segmentation techniques. One may argue that the technique might influence findings in case of adjacent structures such as the pallidum and putamen (36). Our current observations suggest that subcortical alterations in adult OCD may be limited to the pallidum and hippocampus rather than widespread.
This study constitutes the largest meta- and mega-analysis of subcortical brain volumes in OCD to date. Strengths of this study include the sample size (N=3,589) and inclusion of both adults and children. Another strength is our strategy that ensured great methodological homogeneity by standardizing brain segmentation techniques and statistical models across all participating samples, which increased the power to detect small effects. A similar strategy has been used in parallel by other ENIGMA working-groups (33–35). This method generates highly significant findings and allows us to systematically investigate the effects of clinical characteristics on brain alterations in OCD patients.
This study also had limitations. First, a recent study showed effects of workstation vendor and operating system version on brain volume and cortical thickness estimates (37). Indeed the individual sites did differ in operating system and workstation vendor. Additionally, Schoemaker et al. 2016 showed that FreeSurfer tends to overestimate subcortical volumes in children (38). However, this non-systematic error probably affects patients and controls equally. Second, although we have pooled an enormous amount of data, subjects with comorbidities and subjects categorized to each specific symptom dimension especially in the pediatric datasets were still limited. However, the key variable, i.e., the CY-BOCS score, the gold standard clinical instrument in pediatric OCD research, was present in all subjects. Third, the structure labelled as “thalamus” by FreeSurfer’s segmentation algorithm may contain both white matter and grey matter. We, therefore, cannot conclude that this thalamic enlargement involves grey matter enlargement solely. Fourth, our findings indicate medication effects. It should be noted, however, that only current medication status has been taken into consideration. It is difficult to attribute the results to direct effects of the medication itself. Furthermore, the range of medications that are generally prescribed to OCD patients is very broad. Although we have tested whether different types of medication influenced our findings, we were not able to calculate relative doses of different medication types and analyze medication effects in a more fine-grained fashion due to the retrospective nature of our study. Thus we need to interpret these findings with caution.
Despite these limitations, results of this first initiative of the ENIGMA-OCD Working-Group clearly indicate a key role of the thalamus and pallidum in the pathophysiology of pediatric and adult OCD, respectively. Our findings suggest a different pattern of subcortical abnormalities in pediatric and adult OCD patients, which is in line with the developmental nature of OCD and neuroplastic changes during the course of the disease. The current study is a first step toward identifying robust brain volume alterations in OCD patients. An important next step is to apply similar methods in order to identify robust cortical imaging markers on cortical thickness and surface area measures associated with OCD.
Supplementary Material
Acknowledgments
Acknowledgements, funding sources and conflict of interests can be found in Supplementary Information 6.
Dan J. Stein has received research grants and/or consultancy honoraria from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun in the past 3 years.
The ENIGMA-Obsessive Compulsive Disorder Working-Group gratefully acknowledges support from the NIH BD2K award U54 EB020403-02 (PI: Dr. Thompson). Supported by the Neuroscience Campus Amsterdam (NCA), IPB-grant to Dr. Schmaal & Dr. van den Heuvel; the Hartmann Muller Foundation (No. 1460 to Dr. Brem); the International Obsessive-Compulsive Disorder Foundation (IOCDF) Research Award to Dr. Gruner; the Deutsche Forschungsgemeinschaft (DFG) (KO 3744/2-1 to Dr. Koch); the Marató TV3 Foundation grants 01/2010 and 091710 to Dr. Lazaro; the Wellcome Trust and a pump priming grant from the South London and Maudsley Trust, London, UK (Project grant no. 064846) to Dr. Mataix-Cols; the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT KAKENHI No. 26461753 to Dr. Nakamae); the Government of India grants to Prof. Y.C. Janardhan Reddy (SR/S0/HS/0016/2011) and Dr. Janardhanan C. Narayanaswamy (DST INSPIRE faculty grant -IFA12-LSBM-26) of the Department of Science and Technology; the Government of India grants to Prof. Y.C. Janardhan Reddy (No.BT/PR13334/Med/30/259/2009) and Dr. Janardhanan C. Narayanaswamy (BT/06/IYBA/2012) of the Department of Biotechnology; the Wellcome-DBT India Alliance grant to Dr. Ganesan Venkatasubramanian (500236/Z/11/Z); the Carlos III Health Institute (CP10/00604, PI13/00918, PI13/01958, PI14/00413/PI040829); FEDER funds/European Regional Development Fund (ERDF), AGAUR (2014 SGR 1672 and 2014 SGR 489); a “Miguel Servet” contract (CP10/00604) from the Carlos III Health Institute to Dr. Soriano-Mas; the Italian Ministry of Health (RC10-11-12-13-14-15A to Dr. Spalletta); the Swiss National Science Foundation (No. 320030_130237 to Dr. Walitza); and the Netherlands Organization for Scientific Research (NWO VIDI 917-15-318 to Dr. van Wingen).
Further we wish to acknowledge Juliane Ball, Ph.D., Elizabeth Buimer, B.Sc., Kenji Fukui, M.D., Ph.D., Jin Narumoto, M.D., Ph.D., Seiji Nishida, M.D., Ph.D., Reto Iannaccone Ph.D., Yuki Sakai, M.D., Ph.D., Tobias U. Hauser, Ph.D., Anri Watanabe, M.D., and Kei Yamada, M.D., Ph.D.
Footnotes
Previous presentations: Preliminary results were presented at the International College of Obsessive Compulsive Spectrum Disorders (ICOCS), Amsterdam, The Netherlands, September 02, 2015.
Disclosures: All authors have no conflicts of interest related to this study.
References
- 1.Ruscio M, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe - A critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 2005;15:357–376. doi: 10.1016/j.euroneuro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Nestadt G, Samuels J, Bienvenu O, Liang K, LaBuda M, Walkup J, Grados M, Hoehn-Saric R. A Family Study of Obsessive-compulsive Disorder. Arch Gen Psychiatry. 2000;57:358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- 4.Stewart SE, Geller D, Jenike M, Pauls D, Shaw D, Mullin B, Faraone SV. Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr Scand. 2004;110:4–13. doi: 10.1111/j.1600-0447.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 5.Van den Heuvel Oa, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, Denys D, Goudraiaan aE, Veltman DJ. Brain Circuitry of Compulsivity [Internet] Eur Neuropsychopharmacol. 2015:1–18. doi: 10.1016/j.euroneuro.2015.12.005. In Press. [DOI] [PubMed] [Google Scholar]
- 6.Milad MR, Rauch SL. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert AR, Keshavan MS, Diwadkar V, Nutche J, MacMaster F, Easter PC, Buhagiar CJ, Rosenberg DR. Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: A preliminary voxel-based morphometry study. Neurosci Lett. 2008;435:45–50. doi: 10.1016/j.neulet.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szeszko PR, Christian C, MacMaster F, Lencz T, Mirza Y, Taormina SP, Easter P, Rose M, Michalopoulou G, Rosenberg DR. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: An optimized voxel-based morphometry study. Am J Psychiatry. 2008;165:1299–1307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- 10.Zarei M, Mataix-Cols D, Heyman I, Hough M, Doherty J, Burge L, Winmill L, Nijhawan S, Matthews PM, James A. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 2011;70:1083–1090. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Peng Z, Lui SSY, Cheung EFC, Jin Z, Miao GD, Jing J, Chan RCK. Brain structural abnormalities in obsessive-compulsive disorder: Converging evidence from white matter and grey matter. Asian J Psychiatr. 2012;5:290–296. doi: 10.1016/j.ajp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 13.Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 14.Rotge J-Y, Langbour N, Guehl D, Bioulac B, Jaafari N, Allard M, Aouizerate B, Burbaud P. Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2010;35:686–691. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, Stein DJ, Fouche JP, Soriano-Mas C, Sato JR, Hoexter MQ, Denys D, Nakamae T, Nishida S, Kwon JS, Jang JH, Busatto GF, Cardoner N, Cath DC, Fukui K, Jung WH, Kim SN, Miguel EC, Narumoto J, Phillips ML, Pujol J, Remijnse PL, Sakai Y, Shin NY, Yamada K, Veltman DJ, Van Den Heuvel Oa. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 16.Atmaca M, Yildirimb H, Ozdemirb H, Aydinb A, Tezcana E, Ozlera S. Volumetric MRI assessment of brain regions in patients with refractory obsessive-compulsive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 2006;30:1051–1057. doi: 10.1016/j.pnpbp.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Kursad Poyraz a. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert R, Moore GJ, Keshavan MS, Paulson La, Narula V, Mac Master FP, Stewart CM, Rosenberg DR. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Arch Gen Psychiatry. 2000;57:449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- 19.Rotge J-Y, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Meta-analysis of brain volume changes in obsessive-compulsive disorder. [Internet] Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–66. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson PM, Andreassen Oa, Arias-Vasquez A, Bearden CE, Boedhoe PS, Brouwer RM, Buckner RL, Buitelaar JK, Bulaeva KB, Cannon DM, Cohen Ra, Conrod PJ, Dale AM, Deary IJ, Dennis EL, de Reus Ma, Desrivieres S, Dima D, Donohoe G, Fisher SE, Fouche J-P, Francks C, Frangou S, Franke B, Ganjgahi H, Garavan H, Glahn DC, Grabe HJ, Guadalupe T, Gutman Ba, Hashimoto R, Hibar DP, Holland D, Hoogman M, Pol HEH, Hosten N, Jahanshad N, Kelly S, Kochunov P, Kremen WS, Lee PH, Mackey S, Martin NG, Mazoyer B, McDonald C, Medland SE, Morey Ra, Nichols TE, Paus T, Pausova Z, Schmaal L, Schumann G, Shen L, Sisodiya SM, Smit DJa, Smoller JW, Stein DJ, Stein JL, Toro R, Turner Ja, van den Heuvel M, van den Heuvel Oa, van Erp TGM, van Rooij D, Veltman DJ, Walter H, Wang Y, Wardlaw JM, Whelan CD, Wright MJ, Ye J. ENIGMA and the Individual: Predicting Factors that Affect the Brain in 35 Countries Worldwide. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.11.057. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 23.Scahill L, Riddle Ma, McSwiggin-Hardin M, Ort SI, King Ra, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive. Arch Gen Psychiatry. 1989;48:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 25.Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behav Res Methods, Instruments, Comput. 1996;28:1–11. [Google Scholar]
- 26.Rosenberg DR, Benazon NR, Gilbert A, Sullivan A, Moore GJ. Thalamic volume in pediatric obsessive-compulsive disorder patients before and after cognitive behavioral therapy [Internet] Biol Psychiatry. 48:294–300. doi: 10.1016/s0006-3223(00)00902-1. [DOI] [PubMed] [Google Scholar]
- 27.Miller MB. Enlargement of thalamic nuclei in tourette syndrome. Arch Gen Psychiatry. 2010;67:955–964. doi: 10.1001/archgenpsychiatry.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Sanchez-Pena J, Miller AM, Chakravarty MM, Klahr K, Durkin K, Greenhill LL, Peterson BS. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, Hatton SN, Bennett MR. Stress-Induced Grey Matter Loss Determined by MRI Is Primarily Due to Loss of Dendrites and Their Synapses. Mol Neurobiol. 2012:1–17. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- 30.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder. The orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoner N, Soriano-Mas C, Pujol J, Alonso P, Harrison BJ, Deus J, Hernández-Ribas R, Menchón JM, Vallejo J. Brain structural correlates of depressive comorbidity in obsessive-compulsive disorder. Neuroimage. 2007;38:413–421. doi: 10.1016/j.neuroimage.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 32.Selles RR, Storch E, Lewin AB. Variations in Symptom Prevalence and Clinical Correlates in Younger Versus Older Youth with Obsessive–Compulsive Disorder [Internet] Child Psychiatry Hum Dev. 2014;45:666–674. doi: 10.1007/s10578-014-0435-9. [DOI] [PubMed] [Google Scholar]
- 33.Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman a, Niessen WJ, Vernooij MW, Ikram Ma, Wittfeld K, Grabe HJ, Block a, Hegenscheid K, Völzke H, Hoehn D, Czisch M, Lagopoulos J, Hatton SN, Hickie IB, Goya-Maldonado R, Krämer B, Gruber O, Couvy-Duchesne B, Rentería ME, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie Na, Wright MJ, Hall GB, MacQueen GM, Frey EM, Carballedo a, van Velzen LS, van Tol MJ, van der Wee NJ, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Nickson T, McIntosh aM, Papmeyer M, Whalley HC, Sussmann JE, Godlewska BR, Cowen PJ, Fischer FH, Rose M, Penninx BWJH, Thompson PM, Hibar DP. Subcortical brain alterations in major depressive disorder. findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2015:1–7. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen Oa, Agartz I, Westlye LT, Haukvik UK, Dale aM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin a, Machielsen MWJ, Koenders L, de Haan L, Veltman DJ, Satterthwaite TD, Wolf DH, Gur RC, Gur RE, Potkin SG, Mathalon DH, Mueller Ba, Preda a, Macciardi F, Ehrlich S, Walton E, Hass J, Calhoun VD, Bockholt HJ, Sponheim SR, Shoemaker JM, van Haren NEM, Pol HEH, Ophoff Ra, Kahn RS, Roiz-Santiañez R, Crespo-Facorro B, Wang L, Alpert KI, Jönsson EG, Dimitrova R, Bois C, Whalley HC, McIntosh aM, Lawrie SM, Hashimoto R, Thompson PM, Turner Ja. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2015:1–7. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hibar D. ENIGMA Bipolar disorder working group findings from 1, 747 cases and 2, 615 controls. 2014;3:66–68. [Google Scholar]
- 36.Grimm O, Pohlack S, Cacciaglia R, Plichta M, Demirakca T, Flor H. Amygdala and hippocampal volume. A comparison between manual segmentation, Freesurfer and VBM. J Neurosci Methods. 2015;253:254–261. doi: 10.1016/j.jneumeth.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Gronenschild EHBM, Habets P, Jacobs HIL, Mengelers R, Rozendaal N, van Os J, Marcelis M. The Effects of FreeSurfer Version, Workstation Type, and Macintosh Operating System Version on Anatomical Volume and Cortical Thickness Measurements. PLoS One. 2012;7:e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoemaker D, Buss C, Head K, Sandman Ca, Davis EP, Chakravarty MM, Gauthier S, Pruessner J. Hippocampus and amygdala volumes from magnetic resonance images in children. Assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage. 2016;129:1–14. doi: 10.1016/j.neuroimage.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.