Abstract
The public health, tourism, fisheries and ecosystem impacts from harmful algal blooms (HABs) have all increased over the last few decades. This has led to heightened scientific and regulatory attention, and the development of many new technologies and approaches for research and management. This in turn, is leading to significant paradigm shifts with regard to, e.g., our interpretation of the phytoplankton species concept (strain variation), the dogma of their apparent cosmopolitanism, the role of bacteria and zooplankton grazing in HABs, and our approaches to investigating the ecological and genetic basis for the production of toxins and allelochemicals. Increasingly, eutrophication and climate change are viewed and managed as multifactorial environmental stressors that will further challenge managers of coastal resources and those responsible for protecting human health. Here we review HAB science with an eye towards new concepts and approaches, emphasizing, where possible, the unexpected yet promising new directions that research has taken in this diverse field.
Keywords: red tide, shellfish toxicity, fish kills, ecogenomics, monitoring, climate change
INTRODUCTION
Virtually every coastal region of the world is affected by harmful algal blooms (HABs) commonly called “red tides” (Fig.1). Since the latter term red tide erroneously includes many blooms that discolor the water but cause no harm, and also excludes blooms of highly toxic cells that cause problems at low (and essentially invisible) cell concentrations, scientists prefer the term HAB. HABs are most common in coastal marine ecosystems, but they may also affect the open ocean, and brackish or freshwater ecosystems. Most HAB phenomena are caused by blooms of microscopic algae or phytoplankton, including certain cyanobacteria (“blue-green” algae), although the term also applies to harmful blooms of macroalgae (seaweeds). HAB events are typically associated with rapid proliferation and/or high biomass accumulation of toxic or otherwise noxious microalgae at the sea surface or in the water column, but even low cell numbers of highly toxic planktonic species or accumulation of cells on benthic substrates may cause problems.
Fig. 1.

Noctiluca scintillans “red tide” at a pristine tourism resort off the east coast of Tasmania, SE Australia, believed to represent a recent climate-driven range extension from Sydney coastal waters (Source: Erin Watson, University of Tasmania).
The HAB designation is a societal concept rather than a scientific definition – blooms are considered to fit the HAB criterion if they cause injury to human health, socio-economic interests or to components of aquatic ecosystems. Some HAB species are toxigenic, and produce blooms that cause illness and death of fish, seabirds, mammals, and other marine life, often via toxin transfer through the food web. In addition, human consumers of seafood contaminated by these toxins may also be poisoned, suffering acute toxic symptoms and even fatalities in extreme cases. Further toxic threats to human health are posed by toxic aerosols and water-borne compounds that cause respiratory and skin irritation when released from toxic cells.
Certain HAB species can directly release compounds that are, strictly speaking, not toxins (i.e., reactive oxygen species, polyunsaturated fatty acids, mucilage) but can be injurious and even lethal to finfish, especially when held captive in aquaculture operations. Non-toxic HABs cause damage to ecosystems, fisheries resources, and recreational facilities, often due to the high biomass of accumulated algae, which can create noxious scums and foam, shade other phytoplankton and seagrass beds, and cause faunal mortalities via decay and oxygen depletion.
The diversity of HAB species and their impacts presents a significant challenge to those responsible for management of coastal resources and the protection of public health. HABs are complex oceanographic phenomena that require multidisciplinary study and methodologies ranging from molecular and cell biology to large-scale field surveys, numerical modelling, and remote sensing. Our knowledge base and ability to understand and manage these phenomena have expanded greatly in the past several decades. Consequently, there are many excellent books and papers that review this progress (e.g., Hallegraeff 1993; Anderson et al. 1998; Hallegraeff et al. 2003; Granéli & Turner 2006). The purpose of this contribution is not to duplicate these efforts, but rather to highlight new discoveries, technological advances, and paradigm shifts in our view of HABs.
TRENDS
For many years, HAB research was conducted in relative isolation. Individual researchers or countries ran programs independently, with few taking a global perspective. Given the diversity of HABs and their impacts, it was difficult to perceive trends in incidence – regionally, nationally, or globally. The first efforts to provide a global perspective on HAB trends were by Anderson (1989) and Hallegraeff (1993) who argued that there was a global increase in the frequency, magnitude, and geographic extent of HAB events over the preceding two decades. There were multiple reasons proposed for this expansion, including: natural dispersal of species by currents and storms; dispersal through human activities such as ballast water discharge and shellfish translocation; improved detection of HABs and their toxins due to better chemical instrumentation and improved communication among scientists; increased aquaculture operations in coastal waters; and stimulation of HABs as a result of cultural eutrophication or perhaps even climate change.
More than 20 years later, there is a general scientific consensus that globally, the number of toxic blooms, the resulting economic losses, the types of resources affected, and the number of toxins and toxic species reported have all increased over the last few decades (Fig. 2). Arguments and disagreements arise, however, over the reasons for the expansion in particular regions, or on whether there has been any increase at all or even a decrease in other areas. It is far too easy to extrapolate from one region to another without adequate scientific justification, or to infer a trend without sufficient long-term data. With the benefit of hindsight we can highlight regional cases where expansion has occurred, and where probable mechanisms can be identified. One example of a HAB expansion caused by storms or other natural events is in the Gulf of Maine (USA), where a major tropical storm stimulated and dispersed a large, regional Alexandrium red tide throughout the region in 1972. This led to the deposition of dormant cysts in waters that had virtually no history of paralytic shellfish poisoning (PSP), but that has had annual outbreaks nearly every year thereafter (Anderson 1998). An example of ship ballast water-mediated range expansion is the introduction of the toxic dinoflagellate Gymnodinium catenatum into Tasmania in the mid-1970s (see review of molecular, plankton and cyst evidence by Bolch & de Salas (2007), from where it has since dispersed to mainland Australia.
Fig. 2.

Distribution of events where PSP toxins were detected in shellfish or fish– 1970 versus 2009. (Source: US National Office for Harmful Algal Blooms).
It is also clear that some aspects of the apparent “expansion” are due to heightened and more effective scientific and regulatory attention to the problem. In effect, we are better defining the boundaries of a problem whose scale we never fully understood or appreciated. A prominent example in this regard is associated with the amnesic shellfish poisoning (ASP) syndrome, which caused more than a hundred cases of human illness and several deaths in 1987 after consumption of mussels from Atlantic Canada (Bates et al. 1989). The causative agent of ASP was promptly identified as the neurotoxin domoic acid, produced by several species of marine diatoms. Following this discovery novel analytical methods were quickly introduced into the regulatory structure and used for scientific research. A few years later, the unusual mortality of seabirds in California led to the identification of domoic acid as the cause, with that discovery attributed directly to reports from the 1987 Canadian event (Work et al. 1993). The subsequent documentation of ASP toxins year after year along virtually much of the west coast of the US (Anderson et al. 2008) leaves no doubt that the toxin had been present in those waters for many years, but had never been properly identified. Twenty-five years after the Atlantic Canadian poisoning event, ASP is now a recognized public health threat in many countries.
A number of researchers have investigated potential linkages between HAB expansion and eutrophication (e.g., Smayda 1989; Anderson et al. 2002; Glibert et al. 2008). As discussed below, in some instances, the linkage is clear and unequivocal, whereas in others, it is subtle, or even non-existent (Anderson et al. 2008).
KEY DEVELOPMENTS IN HAB SCIENCE AND TECHNOLOGY
The significant public health, tourism, fisheries, and ecosystem impacts from HABs have led to major research programs worldwide, seeking to understand these complex phenomena. Progress has been rapid in many areas, and new approaches and technologies for research and management are now available.
Genetics and Taxonomy
Harmful algal blooms are often nearly monospecific events. Correctly assessing the precise taxonomic identity of the causative organism thus becomes crucial in deciding whether knowledge on toxinology, physiology and ecology gained from similar blooms in other parts of the world can be confidently applied to the local situation. Resolution of the species concept for harmful algae has become a profound issue of discussion at all major conferences dealing with toxic phytoplankton. Some argue that for the sake of stability of nomenclature, conservative morphological traits must remain the “gold standard” for traditional species classification, and if genetic differences cannot be linked to differences in morphology, we cannot speak of different species. Others contend that genetic differences are sufficient to separate species, even when these assignments differ from those that would be made on the basis of morphology. Increasingly nuclear or plastid DNA sequences, in combination with lipid, pigment and toxin biochemistry are now used to redefine existing morphospecies.
Phylogenetic reconstruction of relationships among HAB taxa are most often predicated upon sequence analysis of one or a few genes, typically including the ribosomal DNA (rDNA), internal transcribed spacer (ITS) or cytochrome oxidase (cox1). Ribotyping and application of conservative single gene markers such as rDNA have been valuable in resolving taxonomic and phylogenetic issues among HAB species, but revealing cryptic diversity at the intraspecific and population level requires a finer discrimination. For example, molecular sequencing of culture strains revealed that the widely reported “cosmopolitan” coastal diatom Skeletonema costatum, includes five distinct morphotaxa, at least some of which have discrete regional distributions and distinct ecophysiological characteristics (Kooistra et al. 2008). The dinoflagellate genera Gymnodinium/Gyrodinium, initially separated on the basis of girdle displacement. With support from a new molecular phylogeny, these genera were redefined on the basis of shape of the apical groove, allowing for the creation of the family Kareniaceae for the fucoxanthin containing fish-killing dinoflagellate genera Karenia (10+ spp now known.), Karlodinium (8 spp.) and Takayama (6 spp.) (Daugbjerg et al. 2000; de Salas et al. 2008). This combination of improvements in morphotaxonomy coupled with the development of molecular probes (see below) also led to the new recognition that Florida “Karenia brevis” red tides are in fact often multispecies blooms (Steidinger et al. 2008).
Looking alike does not necessarily mean genetically identical, and looking different does not mean genetically isolated. Morphospecies designations therefore sometimes can be of limited use for ecological purposes. The dinoflagellate Alexandrium tamarense is known to exist as toxic and non-toxic strains, bioluminescent and non-bioluminescent populations, and cold-water and warm-water forms. Furthermore, ribosomal RNA (rRNA) sequences of isolates of the A. tamarense species complex clustered more logically on the basis of geographic origin than morphotaxonomy (Scholin & Anderson 1994; Scholin et al. 1995; Fig.3). Morphologically indistinguishable populations thus can hide cryptic genotypes, some of which are consistently toxic whereas others (European and Tasmanian ribotypes, now termed Groups III and V; Lilly et al. 2007) are mostly nontoxic. In such cases it is critical that molecular probes used in shellfish and phytoplankton monitoring programs discriminate between ribotypes rather than simply morphospecies. On the basis of rRNA studies the appearance of the temperate Asian (Group IV) ribotype of A. tamarense in 1983 in the Mediterranean could only be explained by human-assisted introduction (Lilly et al. 2002). However, when Massaret et al. (2009) examined these same strains using hypervariable microsatellite markers this revealed relationships that were not apparent from rRNA studies on the same group. Mediterranean populations were shown to be a distinct lineage and therefore other origins must now be explored.
Fig.3.

Molecular biogeography of the dinoflagellate Alexandrium tamarense/catenella species complex based on large subunit ribosomal RNA sequences. Black arrows indicate natural dispersal, whereas clear arrows suggest human-assisted dispersal. After Scholin et al. 1995; Ruiz Sebastian et al. 2005. [Note, per correspondence between Gustaaf Hallegraeff and Fiona Martin – this figure to be redrawn to be similar to figure 2]
The same principles apply to the ciguatera-causing benthic dinoflagellate genus Gambierdiscus. Until recently only a single species of G. toxicus was recognized even though a >100-fold variation in toxicity and variations in ciguatoxin profiles have long been known. Molecular sequencing in combination with a reexamination of morphotaxonomy allowed discrimination of 12 species, of which 5 are endemic to the Atlantic (including the Caribbean/West Indies and Gulf of Mexico), 5 to the tropical Pacific, and 2 species, G. carpenteri and G. caribaeus are circumtropically distributed. The differences in Gambierdiscus species composition in the Atlantic and Pacific correlate with structural differences in the ciguatoxins reported from Atlantic and Pacific fish (Litaker et al. 2009).
Because of the apparent continuity of the world’s oceans, similar hydrological environments in different oceans tend to have morphologically similar phytoplankton assemblages (“latitudinal cosmopolitanism”; Taylor & Pollingher 1987) and many scientists have long claimed that marine protists have had ample evolutionary time to reach and inhabit all suitable environments. Molecular taxonomy is, however, increasingly rejecting the dogma of widespread cosmopolitanism of microalgae. Genetic, reproductive and morphological variation in 193 global strains of the “cosmopolitan” marine diatom Pseudo-nitzschia pungens allowed for the discrimination of 3 ITS (internal transcribed spacer) clades with different geographic distributions. Clade II was restricted to the NE Pacific, clade III originated from geographically widely separated areas (Vietnam, China and Mexico), but only clade I was recovered in all global locations in temperate coastal waters (Casteleyn et al. 2008).
Molecular genetic analysis with markers such as microsatellites and amplified fragment length polymorphism (AFLP) (Alpermann et al. 2009) have yielded novel insights into the linkage between genetic traits within populations of Alexandrium and the apparent lack of correlation with highly variable but clonally stable phenotypic characters, such as the production of toxins and allelochemicals. This has engendered new HAB concepts on the ecological and evolutionary significance of these characteristics in the context of population and bloom development. Similarly, Nagai et al. (2009) used microsatellites to elucidate reasons for the expansion of Cochlodinium polykrikoides red tides in Japan and Korea, being able to resolve large-scale genetic transfer from west to east via the Tushima Warm Current as well as frequent apparent human-assisted dispersal within the Seto Inland Sea.
Ecogenomics and Gene Expression and Function
The recent development and application of advanced technologies from the generically defined ‘omics sciences (genomics, transcriptomics, proteomics, metabolomics) coupled with bioinformatics platforms has already provided deep and often revolutionary shifts in understanding the ecology and evolution of HAB species and bloom dynamics. Although the field remains in its infancy, noteworthy contributions to HAB research have already been made in addressing the following questions: 1) what are the phylogenetic relationships among HAB taxa that account for their patterns of evolution; 2) what are the biosynthetic genes and metabolic pathways involved in biosynthesis of toxins and other allelochemicals; 3) what are the patterns of diversity at the population and species level within and among natural blooms; and 4) what are the mechanisms of gene expression and regulation within cells of HAB taxa and which govern intra- and inter-specific responses to putative competitors and/or grazers? The ecogenomic approach to HAB research provides the opportunity to quantify functions and interactions of organisms (cells to populations) at an ecosystem level – relevant to causes and consequences of HABs – by determining the relationships to ecological and evolutionary processes.
Conventional genomics is often based upon the sequencing and annotation of whole genomes, with subsequent bioinformatic focus on structure and function of key groups of genes. This approach has been successfully applied to elucidate the comparative structure and function of gene clusters for the toxin analogues of saxitoxins and microcystins of toxigenic HAB-forming cyanobacteria, such as Raphidiopsis brookii and Cylindrospermopsis raciborskii (Stucken et al. 2010) with genome sizes of <4 mB. The much larger size of the nuclear genomes of eukaryotic microalgae has severely restricted the whole-genome sequencing approach – there is only one published fully sequenced and annotated closed genome for a HAB species – that of the brown tide species Aureococcus anophagefferens (Gobler et al. 2011). This paucity of whole genomes for HAB taxa is being addressed, albeit not systematically. A draft genome (300 mB) of the toxigenic diatom Pseudo-nitzschia multiseries, which produces the neurotoxin domoic acid, has been generated (Armbrust 2009) and complete annotation is expected to yield insights into pathways of toxigenesis and iron acquisition relevant to bloom dynamics. Nevertheless, most toxigenic HAB taxa belong to the dinoflagellates, a group that has proven to be particularly intractable with respect to genomic analysis. The dinoflagellate nuclear genome is typically large, often >250 gB – in the case of Alexandrium this is more than 40 times the size of the human genome! This size poses an impediment to whole genome sequencing, even with the implementation of massively parallel high throughput (Roche 454) pyrosequencing technology. Further complexities and peculiarities of the dinoflagellate genome – permanently condensed chromatin, general lack of histones, frequent base-pair substitution, high G-C base pair ratio, compound the genome sequencing problem.
Much can be learned via comparative phylogenomic approaches, even in the absence of complete genomes for HAB taxa. For example, many dinoflagellate phycotoxins are linear or ladder-frame polyethers derived via polyketide biosynthesis, leading to the genomic search for polyketide synthase (PKSs) genes involved in these pathways (John et al. 2008).
Some of the complexities inherent in the genome of eukaryotic HAB species, and in particular the dinoflagellates, have recently been addressed via transcriptomics, or gene expression profiling. The transcriptome comprises all of the mRNA transcripts expressed within a given time frame, and therefore can provide dynamic insights into shifts in gene expression relevant to mechanisms such as toxin biosynthesis or bloom growth kinetics. Several HAB taxa have been recently subjected to analysis of expressed sequence tags (ESTs), each of which is a short sub-sequence of a transcribed cDNA sequence. Although the EST analysis is restricted by the fact that only a fraction of the transcribed genome is surveyed and only when the corresponding mRNA is expressed, this approach has been effectively used to identify gene transcripts, discover new genes and determine sequence homology for various HAB taxa. EST databases are now extensive and representative of a wide diversity of organisms, including the HAB species Alexandrium fundyense (Hackett et al. 2005), A. catenella (Uribe et al. 2008), A. ostenfeldii (Jaeckisch et al. 2008), A. minutum (Yang et al. 2010), Karenia brevis (Lidie et al. 2005), Chrysochromulina polylepis (John et al. 2010) and Prymnesium parvum (La Claire 2006), with more in the pipeline awaiting further annotation and verification.
An EST library constructed for the dinoflagellate Alexandrium minutum (Yang et al. 2010), combined with the application of an oligonucleotide microarray uncovered the presence of 192 genes that were differentially expressed between toxic and non-toxic strains of this species (Fig 4). Nevertheless, although potential candidate genes were detected for possible involvement in growth regulation and/or toxin biosynthesis, no confirmed hits for the PSP toxin biosynthetic genes found in cyanobacteria were detected. A major advance in this regard was reported by Hackett et al. (in press), who assembled comprehensive transcriptome datasets for several saxitoxin-producing dinoflagellates and a related non-toxic species. They identified 265 putative homologs of 14 cyanobacterial saxitoxin synthesis genes, including all of the genes directly involved in toxin synthesis, as described by Kellmann et al. (2008). Putative homologs of four proteins grouped closely in phylogenies with cyanobacteria and are likely the functional homologs of sxtA, sxtG and sxtB in dinoflagellates. However, the phylogenies do not support the transfer of these genes directly between toxic cyanobacteria and dinoflagellates. The saxitoxin synthesis pathway was likely assembled independently in the distantly related cyanobacteria and dinoflagellates. The independent evolution of STX production in these two ecologically distinct groups of organisms suggests that this toxin may confer a benefit to producers that we do not yet fully understand.
Fig. 4.
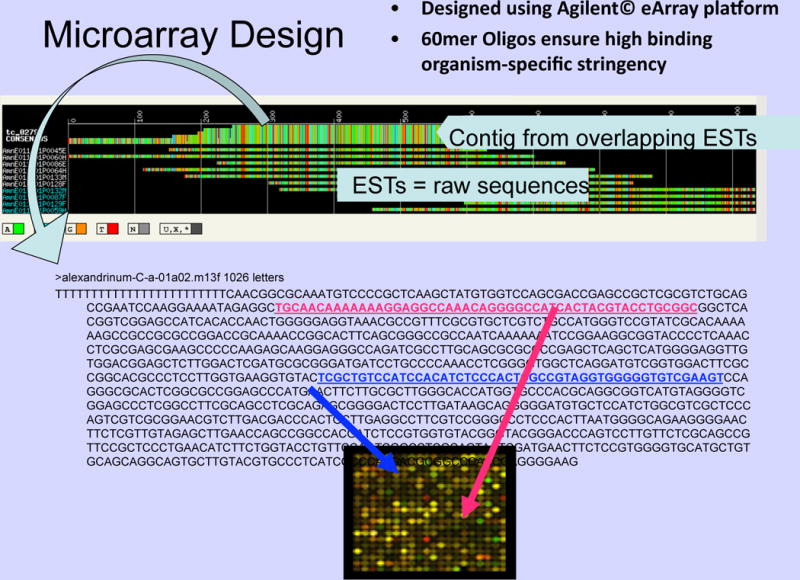
Oligonucleotide microarray for studies of gene expression in the toxigenic dinoflagellate Alexandrium minutum (Source: I. Yang, Alfred Wegener Institute for Polar and Marine Research). The microarray was derived from the transcriptomic analysis of the corresponding cDNA and sequencing of >15,000 expressed sequence tags (ESTs) (Yang et al. 2010). Gene expression patterns can be determined from automated scanning of the color pattern generated via hybridization and fluorescence labelling.
In dinoflagellate species producing polyketide toxins, including spirolides (Jaekisch et al. 2008) and brevetoxins (Monroe & van Dolah 2008), search strategies based on EST- or cDNA libraries were successful in identifying a range of polyketide synthases (PKS) from high sequence conservation in several PKS domains. Yet it remains unclear which of these genes are responsible for production of the corresponding toxins.
Sequence analysis based upon a normalized cDNA library of the fish-killing haptophyte Chrysochromulina polylepis (John et al. 2010) revealed several genes putatively related to toxin synthesis and 13 putative polyketide synthase (PKS)-related gene sequences. Semi-quantitative reverse-transcription polymerase chain reaction (RT- PCR), following expression of PKS genes over the photocycle of synchronized cultures provided the first study showing the expression of PKS genes in a toxic haptophyte.
The development and application of cDNA and oligonucleotide microrrays, a well established technology in biomedical and environmental studies, provides a quantum leap forward for HAB research. Patterns of gene expression and regulation can be screened simultaneously using thousands of gene probes spotted on the “lab on a chip”. We can now survey genes related to growth dynamics, toxigenesis, interspecific interactions, and ecophysiology, to name but a few of the processes under study. In novel study of predator-prey interactions and possible defense mechanisms, an EST-based oligonucleotide microarray for the dinoflagellate A. minutum was used to demonstrate a shift in gene expression of 14 genes in response to a copepod grazer, concomitant with an increase in dinoflagellate cell toxin content (Yang et al. 2010).
Transcriptomic analysis is, however, subject to a critical limitation, as this type of analysis only applies to expression at the transcriptional level. Many key genes may be post-translationally modified, thereby affecting the structure and function of proteins. In fact a high degree of post-translational modification of biosynthetic gene products may be a contributing factor in the failure of microarray analysis to confirm expression of toxin- specific genes in Alexandrium (Yang et al. 2010).
Analysis of the proteome, comprising the structure and function of the entire protein complement, can address the issue of post-translational modification but has not often been applied to HAB taxa. Earlier proteome analysis was dependent upon generation of a “protein fingerprint” by two-dimensional (2-D) gel electrophoresis. By this method, Chan et al. (2005) claimed to be able to resolve several groups of proteins as “taxonomic markers” discriminating toxic versus non-toxic strains of A. minutum. This approach has been complemented or superseded by electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) of whole proteins or peptide-digests followed by de novo sequencing or peptide mass fingerprinting by tandem mass spectrometry (e.g., Chan et al. 2006). Proteomics has provided valuable information on the effects and mode action of the dinoflagellate metabolite yessotoxin on a human liver cell line (Young et al. 2009), and analysis of the plastid proteome of the Florida red-tide dinoflagellate Karenia brevis detected an electron transfer protein (plastocyanin) inherited from green algae that may contribute to ecological success in iron utilization (Nosenko et al. 2006). Nevertheless, much promise remains to be fulfilled in the application of proteomics to studies of HAB ecophysiology and bloom dynamics.
The study of the profile of low-molecular weight metabolites within cells has yet to be applied to HAB research in a concerted fashion. There are no apparent major technological impediments, and the metabolomics approach has already been successfully applied to the dinoflagellate endosymbiont Symbiodinium reviewed by Gordon and Leggatt (2010). Among HAB species, metabolic fingerprinting of K. brevis extracts by mass spectrometry was not successful in identifying specific compound(s) responsible for allelopathy (Prince et al. 2008). This frontier field is likely to be more fruitful when more comprehensive spectral libraries become available and as structural- functional relationships of the metabolites are better defined.
Diversity and interaction of phycotoxins and allelochemicals
The Known Phycotoxins
Toxins produced by HAB microalgae are termed phycotoxins to reflect their algal origin. Structurally the toxins of eukaryotic microalgae can be classified into several major groups, the most prominent of which are: 1) linear and macrocyclic polyethers, e.g. okadaic acid and dinophysistoxins; 2) ladder-frame polyethers, e.g. brevetoxins and ciguatoxins; 3) macrocyclic imines, such as spirolides and gymnodimine; 4) tetrahydropurines, e.g. saxitoxin and analogues; and 5) toxic secondary amines, including domoic acid. Historically the discovery of these phycotoxins proceeded from cases of human illness of unknown etiology, often linked to consumption of contaminated seafood. This was followed by bioassay guided isolation, usually with reference to a mammalian model, such as the laboratory mouse. This led inevitably to the naming of phycotoxin-associated syndromes according to the toxin vector and characteristic symptoms elicited in humans - amnesic shellfish poisoning (ASP), ciguatera fish poisoning (CFP), diarrhetic shellfish poisoning (DSP), neurotoxic shellfish poisoning (NSP), paralytic shellfish poisoning (PSP), and azaspiracid shellfish poisoning (AZP).
Unfortunately, the focus on human symptomology has done little to advance understanding of the origin, structural and functional diversity, and ecological and evolutionary significance of these toxins. The initial breakthrough in exploring these issues coincided with the development of high-resolution analytical separation and detection technology, specifically high-performance liquid chromatography. Resolution of multiple toxin components allowed the biogeographical and taxonomic characterization of toxigenic phytoplankton according to their specific toxin profile, e.g. PSP toxins within the dinoflagellate genus Alexandrium (Cembella et al. 1987; Anderson et al. 1994). Furthermore, biotransformation processes can be studied in shellfish and finfish following direct ingestion or food web vectoral transfer from toxigenic plankton.
The next major analytical innovation was the development and implementation of liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) for phycotoxins (reviewed by Quilliam 2003), first applied to the analysis of okadaic acid and dinophysistoxins. This LC-MS/MS technology permits definitive analysis of virtually all phycotoxins (<4000 M.W.), both lipophilic and hydrophilic, typically at sub- picomolar concentrations from variety of matrices including seawater, plankton and seafood. Rapid advances in mass spectrometry have improved sensitivity by several orders of magnitude, such that it is now possible to quantitatively detect many phycotoxins directly from the water column during or subsequent to blooms at detection limit of one or a few toxic cell equivalents. Creative methods development in mass spectrometry now provides the opportunity for simultaneous broad spectrum screening for a host of ion masses from multiple structural groups of toxins in a single extraction (Quilliam 2003; Hiller et al. 2007). A major drawback of mass spectrometry, namely the requirement for laboratory operation, has now been somewhat alleviated following successful on board LC-MS/MS analysis of phycotoxins of freshly harvested plankton from the water column, providing near-real time toxin profiles during an oceanographic cruise (Krock et al. 2009).
Given this prowess for phycotoxin analysis, how has this technology altered our knowledge and concepts regarding toxigenic HABs? Since the structural elucidation of the first known phycotoxin, the tetrahydropurine neurotoxin saxitoxin and the re- discovery of domoic acid as a phycotoxin, literally hundreds of phycotoxin analogues have been found in plankton, seawater and seafood matrices, belonging to >20 structural groups. Nevertheless, it has been more than a decade since the characterization of an entirely new major group of phycotoxins, the azaspiracids (James et al. 2003), associated with acute human toxicity. It does appear that the golden age of discovery of new classes of phycotoxins causing as yet unidentified human toxin syndromes may be behind us.
This is not to denigrate the importance of discoveries of new microalgal species or populations associated with known toxins, or the reevaluation of the toxigenicity of relatively well characterized species. Based upon structural arguments and similarities to other cyclic imine toxins spirolides and gymnodimine, it has long been suspected that pinnatoxins, known previously only from shellfish, originated from toxic dinoflagellates. The recent association of a peridinoid dinoflagellate from New Zealand with production of pinnatoxins E and F has now confirmed this link (Rhodes et al. 2010).
The application of toxin analysis by high resolution mass spectrometry with screening of clonal cell isolates from field populations has yielded surprising and novel fndings on the origin of toxins in marine food webs. This combined approach served to identify and confirm the unexpected association of the marine dinoflagellate Alexandrium ostenfeldii with the occurrence of spirolides in shellfish from Atlantic Canada (Cembella et al. 2001), although these toxins had been known and characterized from shellfish several years before. Perhaps even more dramatic was the recent discovery of the culprit organism of azaspiracid poisoning (AZP) – a small previously overlooked dinoflagellate named Azadinium spinosum (Tillmann et al. 2008). This work on clonal cultures obtained by serial dilution of mixed field plankton populations from the North Sea served to overturn the prior association, also supported by LC-MS/MS, of azaspiracid toxins with the heterotrophic dinoflagellate Protoperidinium crassipes as source organism (James et al. 2003). New paradigms suggest that many components of planktonic food webs, including protists, meta-zooplankton and ichthyoplankton may act as planktonic vectors of phycotoxins.
The technology of toxin profiling has also proven to be a valuable complement to molecular methods for defining HAB population genetics and diversity, particularly because the toxin phenotype revealed in the profile is generally a rather stable characteristic, at least for dinoflagellates. Analysis of spirolide toxin profiles from natural populations and isolates of Alexandrium ostenfeldii from the Gulf of Maine revealed not only the regional diversity among populations but also the presence of five distinct toxin phenotypes among isolates (Gribble et al. 2005). This example merely illustrates the fact that biosynthesis of particular toxin analogues is subject to inter- and intraspecific variation, including at the population level and even in some cases among clones within a population. Furthermore, in general, recent studies have supported the concept that the distribution of phycotoxins is not only patchy in a biogeographical sense, but also that phycotoxins are subject to biotransformation and selective uptake and elimination kinetics within components of the food web. Investigations guided by chemical analysis of toxins in food web components are therefore crucial to determining the culprit organisms of shellfish and finfish toxicity and the development of rational monitoring programs for HAB taxa.
Given the high potency and exquisite toxicity of the known phycotoxins in humans and in mammalian models (e.g., laboratory mice and cultured cell lines), it has long been assumed that these compounds serve a defensive function in the bloom ecology and evolution of the toxigenic species. In mammalian systems, the mode of action of phycotoxins is often related to effects on ion channels in cell membranes or on enzyme inhibition. Earlier concepts therefore suggested that phycotoxins were chemical weapons in the “watery arms race” sensu Smetacek (2001), and acted as defensive compounds against predators or competitors. Consideration of allelochemical interactions in the plankton (reviewed by Cembella 2003) has revealed that chemically mediated effects of phycotoxin-producing HAB taxa are complex and often equivocal, but the evidence does not support a primary role for the known phycotoxins as defensive compounds against protistan or most metazoan competitors or predators.
Ichthyotoxicity
There are other toxic effects induced by certain HAB species, in addition to those associated with the known phycotoxins causing syndromes of human poisoning, but for which the toxic components and modes of action are generally poorly characterized. These “toxins” can be loosely categorized as those responsible for morbidity and mortality of marine fauna, e.g. ichthyotoxicity, and those that mediate allelochemical interactions in the plankton or other components of the food web. There may also be certain congruity, overlap and functional interactions with the known phycotoxins produced by various HAB species. In certain cases of apparent “ichthotoxicity” no toxic agent is in fact responsible. Massive fish kills may occur simply be the result of high algal biomass build-up and subsequent bacterial decay of the bloom generating anoxic conditions (so-called indiscriminate fish kills). In other cases mechanical damage of fish gills may be responsible (e.g. caused by the spiny diatom Chaetoceros convolutus in British Columbia; Rensel (1993)), notably when fish are held captive in aquaculture operations. Clogging of fish gills by algal mucus has sometimes been invoked (Jenkinson & Arzul 1998), but more commonly fish suffocate themselves through production of excessive gill mucus generated as a protective response to environmental irritants.
Rarely have ichthyotoxic chemical molecules been conclusively identified as the causative agent of fish kills, with the exception of brevetoxin by Karenia brevis, karlotoxin by Karlodinium veneficum, gymnocin by some strains of Karenia mikimotoi, and brevisulcatic acid by Karenia brevisulcata. Polyether toxins described as pymnesins (Prym1 and Prym2) have been isolated from the fish-killing haptophyte Prymnesium parvum, with haemolytic and cytolytic properties, but these compounds have not been definitively linked to either fish kills or allelochemical interactions (Tillmann 2003). Some research groups claimed reactive oxygen species (ROS) to be the primary cause of fish kills by e.g. Chattonella marina (Oda et al. 1992), but this has not been substantiated by fish bioassays with chemically generated superoxide or hydrogen peroxide (Marshall et al. 2003; Woo et al. 2006). Other research groups have focused on PUFA as toxic agents (Gentien et al. 2007), but with highly variable results, some of which have now been attributed to the incidence of uncharacterized lipid degradation products (Mooney et al. 2011).
Studies on ichthyotoxicity are highly dependent upon sensitive and reliable standardized bioassay systems. Research groups seldom work with identical algal culture strains, however, or with identical bioassay systems for fish toxicity, i.e. they often used different fish strains or species, juveniles or adults, and varying exposure times. Brine shrimp assays or mammalian hematocyte assays are not always good model systems for fish gills. An important recent breakthrough has been the development of a standard fish gill bioassay system, which has been adapted for use with living algal cultures and lends itself to automation in a plate reader system measuring cell viability indicator dyes (Dorantes-Aranda et al. 2011). It now is becoming clear that algal cells must rupture for significant ichthyotoxicity to occur, that some PUFAs such as EPA but not OPA nor OTA, can cause significant gill damage, and that ROS sometimes may be involved in the generation of highly toxic lipid peroxidation products (Marshall et al. 2003, Mooney et al. 2011). Hopefully, improved understanding of HAB fish killing mechanisms in future may aid in the design of effective mitigation strategies.
Allelochemicals
Smayda (1997) suggested that HAB species have evolved four major strategies to offset the ecological disadvantages of having low nutrient uptake capabilities: (1) vertical migration to reach deep nutrients; (2) mixotrophy; (3) allelochemically enhanced interspecific competition; and (4) allelochemical antipredation defense mechanisms. The latter two adaptations involve the production and release of secondary metabolites (see reviews in Cembella 2003; Legrand et al. 2003).
Recent intensive research efforts have focused on determining the chemical nature and mode of action of the allelochemicals produced by certain HAB taxa and that affect species interactions, but which are typically distinct from the known phycotoxins. The expression of “toxic” allelochemical activity by multiple clones of the dinoflagellate Alexandrium tamarense against the cryptophyte Rhodomonas salina and the predatory dinoflagellate Oxyrrhis marina including loss of mobility and cell lysis was shown to be unrelated to the PSP toxin content or composition of the Alexandrium isolate (Alpermann et al. 2010). Preliminary characterization of the lytic toxins from A. tamarense (Ma et al. 2010) indicated that these compounds are macromolecular or large aggregates (>5 kD), and further analysis indicated that they are neither proteinaceous nor primarily polysaccharide-derived. Tillmann et al. (2008) demonstrated that six species of Alexandrium produce lytic substances and other allelochemicals capable of immobilizing and lysing a variety of protist species. The exact chemical nature of the compounds involved remains to be determined, as does their ecological role, but they are distinct from the known phycotoxins and thus can cause broad-based trophodynamic effects. They may be involved in food or nutrient acquisition and feeding, as is well established for the mixotrophic haptophyte Prymnesium parvum which releases lytic compounds that immobilize or kill motile prey before ingestion (Skovgaard & Hansen 2003).
Attempts to characterize allelochemicals produced by the red tide dinoflagellate Karenia brevis, which are growth inhibitors against certain diatoms, have also not been yet successful at total structural elucidation, but at least one group comprises polar, unstable compounds of low molecular weight, unrelated to brevetoxins. The karlotoxin- producing dinoflagellate Karlodinium veneficum provides perhaps the best described current model of the mode of action of allelochemical activity associated with a rather well-defined groups of toxins. Adolf et al. (2007) showed that grazer susceptibility to membrane lysis by karlotoxins was due to the corresponding sterol composition of the potential predators - those containing predominantly desmethyl sterols are susceptible and those with mainly 4-α methyl sterols are resistant. The mode of action of karlotoxins by membrane pore formation may also account for the known ichthyotoxicity of Karlodinium spp., as well as contributing to the success of in situ blooms against competitors and predators.
Toxin production may have important implications for the maintenance and dynamics of harmful algal blooms by inhibiting grazing. Fish and zooplankton avoid dense concentrations of certain HAB species, and laboratory studies indicate that some HAB species are rejected by at least some predators or grazers either by pre-ingestive selection or after ingestion of a threshold dosage of toxic cells (Turner & Granéli 2006). A breakdown of grazing control has been implicated in the brown tides in Narragansett Bay and in Texas and removal or loss of the grazer population has been reported to precede or accompany bloom development (Montagna et al. 1993). To date, however, evidence that some HAB species are toxic to their potential grazers such as copepods, is equivocal (Turner 2006). Whereas some authors reported pre-ingestive rejection of toxic Alexandrium strains by copepods (Huntley et al. 1986), others have found that some copepods were unaffected and fed at high rates on both toxic and non-toxic Alexandrium cells (Teegarden & Cembella 1996). The extent to which any of the above biological interactions occur in natural waters, and affect HAB dynamics and toxicity is not well known, and represents an important line of inquiry which underpins our quest to understand the ecological basis for HAB toxin and allelochemical production.
Bloom dynamics
HAB events are characterized by the accumulation and occasional dominance of particular species of toxic or harmful algae, resulting from a combination of physical, chemical, and biological mechanisms. Given the diversity of HABs species and habitats in which they occur, there exist few unifying principles that explain blooms in all habitats. Several species-specific studies within specific ecosystems have been particularly successful in advancing our knowledge on the bloom dynamics of HABs. These include Karenia brevis within the Gulf of Mexico (e,g., Walsh & Kirkpatrick 2008), Alexandrium fundyense in the Gulf of Maine (Anderson et al. 2005c), and Prorocentrum donghaiense in the East China Sea (Zhou et al. 2008). Measurements of primary productivity, chlorophyll, or other bulk phytoplankton community parameters are clearly of limited value when only a single, toxic organism is of interest. This has led to many advances in cell detection, gene expression, allelopathy, grazing, and other areas that have been applied across the spectrum of HAB species.
HABs can be initiated from cells present at low concentrations, sometimes persisting in the background for months before a bloom develops (the hidden flora concept). Other HABs are delivered into a specific region via advection after developing elsewhere (e.g., Raine et al. 2010). In such cases, the population increases can be significant and alarming, but should not be attributed to in situ growth. Still other HABs are initiated from resting cysts that germinate from bottom sediments, significantly impacting many aspects of HAB phenomena (Garcés et al. 2010). Cyst or spore germination provides the inoculum for blooms, and the transformation back to the resting state can remove substantial numbers of vegetative cells from the population and act as a major factor in bloom decline. Cysts are also important for population dispersal; they permit a species to survive through adverse conditions, and since sexuality is typically required for their formation, they facilitate genetic recombination (Massaret et al. 2009). They can even be important sources of toxin to shellfish and other benthic animals.
A current example of the importance of cysts is in the Gulf of Maine, (US), where widespread blooms of Alexandrium fundyense occur, initiated from cyst accumulations in major “seedbeds” in the region (Anderson et al. 2005b). Cyst abundance has been mapped and quantified for a number of years, exhibiting considerable interannual variability. A strong relationship has been demonstrated between the abundance of cysts in surface sediments and the size of the subsequent A. fundyense bloom and extent of shellfish toxicity in that region (McGillicuddy et al., submitted ms). In other areas, the size of the inoculum of vegetative cells from cyst germination is thought to be important in bloom initiation, but does not determine the eventual bloom size – that being regulated by factors that affect cell growth and accumulation. For example, Takeuchi et al. (1995) suggest that A. catenella cysts in Tanabe Bay (Japan) sediments germinate to yield an inoculum of 10–100 cells l−1, and Anderson (1998) calculated an excystment inoculum of ~100 cells l−1 for A. fundyense blooms in Cape Cod salt ponds. In both of these areas, the eventual blooms can exceed 100,000 or even 1,000,000 cells l−1, so this requires many divisions from that initial small germination inoculum.
Once a population is established, its range and biomass are affected by physical controls such as the transport and accumulation of biomass in response to water flows (e.g., Franks and Anderson 1992), by the swimming behavior of organisms (Kamykowski 1974) and by the maintenance of suitable environmental conditions (including temperature and salinity, stratification, irradiance, and nutrient supply). These factors all interact to determine the timing, location, and ultimate biomass achieved by the bloom, as well as its impacts.
Physical processes that are likely to influence the population dynamics of HAB species are operative over a broad range of spatial and temporal scales. Large-scale circulation patterns affect the distribution of water masses and their associated HABs. Eddies from the open ocean can, for example, impinge on shelf regions, transporting HABs and nutrients to nearshore waters. This type of transport has been invoked for the delivery of the Florida red tide organism Karenia brevis to nearshore waters from an offshore zone of initiation (Walsh & Kirkpatrick 2008). Another prominent example is the wind-driven delivery of Dinophysis acuminata cells into Bantry Bay in southwest Ireland (Raine et al. 2010).
Physical processes at intermediate scales can lead to the formation of convergence zones, fronts, and upwelling. For example, a linkage has been demonstrated between tidally generated fronts and the sites of massive blooms of Karenia mikimotoi in the North Sea (Holligan 1979). The pattern generally seen is a high surface concentration of cells at the frontal convergence, sometimes visible as a red tide. Chlorophyll concentrations are generally lower and more uniform on the well-mixed side of the front. Offshore, the bloom may be harmless, but when movement of the front and its associated cells brings toxic HAB populations into contact with fish and other susceptible resources, toxicity or massive mortalities can result.
An emerging concept that also highlights the importance of small-scale physical processes in HAB development is in the formation of what are called “thin layers” (Figure 5). Off the French coast, a thin layer of dinoflagellates, including the HAB species Dinophysis cf. acuminata, is frequently observed in the proximity of the thermocline (Gentien et al. 1995). Other HAB species are also known to form thin, subsurface layers at scales as small as 10 cm in the vertical and as large as 10 km in the horizontal. One simple kinematic explanation is that these layers result from the stretching of horizontal inhomogeneities by the vertical shear of horizontal currents. This produces an environment potentially favoring motile organisms that can maintain their position in this layer. Others argue that thin layers result from HAB species’ sensitivity to high shear, or to their chemotropism or simple avoidance of grazers.
Fig. 5.

Vertical distribution of temperature (°C), particulate total volume (relative units), and fractional cell concentration of dinoflagellates (percentage) off the “pertuls d’Antioche,” France. In this example, the HAB species Dinophysis acuminata was a significant component of the dinoflagellate assemblage at the pycnocline, but was absent elsewhere in the water column. (Modified from Gentien et al. (1995).)
A particular case of HABs in thin layers is that of mucilage-forming blooms. Diatom blooms, harmless in most systems, may at times and places, e.g., the Adriatic Sea, exude large quantities of polysaccharides that once established in the pycnocline, trap sinking organic matter, including materials that enhance mucilage persistence. Mucilage formation can be a form of bioengineering or manipulation of the physical environment, e.g., by the dinoflagellate Karenia mikimotoi, and create a microcosmic layer where complex microbial interactions take place.
At an even smaller scale, shear associated with turbulent mixing may alter growth, behavior, and in extreme cases induce mortality (e.g., Pollingher & Zemel 1981; Thomas & Gibson 1990; Berdalet 1992). Turbulence may cause physical damage of cells, colonies or filaments, and is known to effect growth and mortality of some dinoflagellate species.
A critical factor in bloom development is nutrient supply. This includes the major nutrients such as nitrogen, phosphorus and silicate, and a variety of micronutrients such as trace metals and vitamins. Where once inorganic nutrients (e.g., NO3, PO4) were the main target of study, it is now apparent that HAB cells can obtain their nutrition through the utilization of organic compounds obtained either in dissolved or particulate form (Taylor & Pollinger 1987, Berg et al. 1997). There is clear evidence that some HAB species can utilize dissolved organic P and N-compounds. For example, the brown tide organism Aureococcus anophagefferens has been shown to preferentially use organic nitrogen over nitrate (Berg et al. 1997). Recent ecogenomic studies revealed that the ability of A. anophagefferens to outcompete co-occurring phytoplankton in estuaries with elevated levels of dissolved organic matter and turbidity and low levels of dissolved inorganic nitrogen may be because it has more genes involved in light harvesting, organic carbon and nitrogen use, and trace metal utilization than competing phytoplankton (Gobler et al. 2011) These findings suggest that anthropogenic activities may have created a niche within coastal ecosystems that suits the unique ecological niche of A. anophagefferens.
In a related strategy, some species obtain organic nutrients by ingesting detritus, bacteria, other phytoplankton, or even grazers (e.g., Jeong et al. 2005a,b). A major breakthrough in HAB science occurred when the mixotrophic nature of the DSP-causing Dinophysis species was conclusively demonstrated in culture by Park et al. (2006), who showed that not only did Dinophysis consume the ciliate Myrionecta, but that it also acquired chloroplasts that had previously been stolen from its food, the cryptophyte Teleaulax. Now that culturing is possible, new knowledge of toxin production and growth are rapidly emerging (e.g., Tong et al. 2011; Fux et al. 2011).
A particular concern is the relationship between HABs and the growing eutrophication of coastal waters (Anderson et al. 2002; GEOHAB 2006). Historically, the conceptual understanding of HABs in eutrophic systems has been based on the simplistic notion that more nutrients yield higher algal biomass. However, it is now widely accepted that the composition and relative proportional availability of nutrient pools, the range of physiological responses by different phytoplankton, and the interactions of other dynamic factors such as physics and grazing are all important controlling responses to cultural eutrophication by HABs. The sources of nutrients that may stimulate blooms include sewage, atmospheric and groundwater inputs, and agricultural and aquaculture runoff. It has been estimated that the flux of phosphorus to the oceans has increased 3-fold compared to preindustrial, pre-agricultural levels, while the flux of nitrogen increased four fold into the Mississippi River and more than ten fold into the rivers entering the North Sea (Smil 2001). Alterations in the composition of nutrient loads have been correlated with shifts from diatom-dominated to flagellate-dominated assemblages.
There exist prominent examples where HABs increased with increasing pollution and eutrophication: the Inland Sea of Japan in the mid-1970’s (Okaichi 1997), and the northwestern Black Sea in the 1970s and 1980s (Bodeanu & Ruta 1998). These are especially informative datasets, as they show a decrease in HABs as a result of definitive actions that first reduced nutrient inputs. Another more current example of the effect of increasing inputs of algal nutrients from agricultural sources is in China, where the need to feed a rapidly growing population has led to major changes in coastal water quality. For example, nitrate concentrations at the mouth of the Changjiang River have increased four fold in 40 years, while phosphate concentrations have increased by 30%. The nitrate derives mostly from the mid and lower reaches of the river, one of the most intensely farmed agricultural areas in China. These non-point source nutrient inputs have led to significantly higher algal biomass, and a change in the phytoplankton community composition. Concurrently, HABs have increased dramatically in this area in both number and size. About 30–80 red tide events have been recorded each year from 2000 to 2005 in the East China Sea, with the scale of some blooms in excess of 10,000 km2 (Zhou et al. 2006).
In other parts of the world, the linkage between HABs and eutrophication is not readily apparent such as with HABs that occur in open coastal waters where the predominant sources of nutrients are natural (Anderson et al. 2008).
During the development of a HAB, population losses due to parasites and bacterial or viral infections can be significant (Salomon & Imai 2006). Bacteria can play an important role in controlling HABs and regulating their impacts, including their toxicity (Kodama et al 2006). Bates et al. (1995) showed that the toxicity of the diatom Pseudo-nitzschia was dramatically enhanced by the presence of bacteria in laboratory cultures. Jones et al. (2010) examining bacterial communities in Karenia brevis blooms using16S rRNA clone libraries found positive correlations between the HAB populations and certain bacterial groups. Ishida et al. (1997) demonstrated that a bacterium could be responsible for the decline of Karenia mikimotoi blooms in Japan. Bacteria may also interact with HABs in a positive manner by stimulating their growth. Cyanobacteria, in particular, establish mutually beneficial consortia by chemotactically attracting and supporting micro-organisms involved in nutrient cycling and the production of growth factors. The extent to which any of the above biological interactions occur in natural waters, and affect HAB dynamics and toxicity is not known, and represents an important line of inquiry which underpins our quest to understand the ecological basis for HAB toxin production.
MANAGEMENT
Anderson et al. (2001) review the different approaches adopted by countries and commercial enterprises worldwide to monitor and manage HABs in coastal waters. This is typically accomplished through the establishment of programs for toxin and cell detection (and quantitation) in water, aerosols, shellfish, fish, etc., development of bloom forecasting and early warning capabilities as well as medical intervention and therapeutic strategies, and to a growing extent, bloom prevention and mitigation strategies. There are, however, many challenges associated with these activities, due to the complexity and diversity of HAB phenomena. Resource managers and regulatory officials must deal with multiple toxins and multiple toxic algal species, multiple toxic fisheries resources, and large- and small-scale HAB events that occur intermittently. Many new technologies are emerging that can address these management challenges – more than can be adequately covered here. Instead, we highlight two broad areas where progress has been substantial – new approaches to HAB cell detection and bloom monitoring, and numerical models used for bloom forecasting and hindcasting.
Cell identification and enumeration
One novel approach that addresses the need for species-specific yet rapid and accurate cell identification and enumeration utilizes optical characters unique to the target organism Karenia brevis, the Florida red tide organism. This species, as well as some co- occurring Karenia species that are also toxic, produce a pigment called gyroxanthin- diester that is sufficiently unique to be a useful biomarker within the Gulf of Mexico region (Kirkpatrick et al. 2000; Richardson & Pinckney 2004). Instruments have been developed that quantify this pigment in water samples, and these have been mounted on research vessels (Kirkpatrick et al. 2003) and inside an autonomous underwater vehicle (AUV) called the BreveBuster. This approach has great potential for the monitoring of those HAB species that have this unique pigment, but for the majority of other HAB species, alternative approaches to automated detection are needed.
A more broadly applicable approach involves the development of species- or strain-specific molecular “probes” that can label HAB cells of interest so they can be detected visually, electronically, or chemically. This line of research has been a hallmark of the HAB field because of the need for species-specific measurements. Progress has been rapid and probes and assays of multiple types are already available for many of the HAB species. The most promising of these are short pieces of synthetic DNA (probes or primers) that bind to complementary portions of those molecules in the target HAB species. These targets, typically ribosomal RNA, can be visualized and/or quantified using a variety of techniques such as fluorescent in situ hybridization (FISH; Anderson et al. 2005a); sandwich hybridization assays (SHA; Scholin et al. 1996), and a variety of PCR-based assays (e.g., Penna & Magnani 1999; Bowers et al. 2006; Coyne et al. 2005). These developments have reached the stage where the new molecular counting methods are routinely employed in major research programs, as well as in some monitoring programs (e.g., Anderson et al. 2005a; Haywood et al. 2009; Rhodes et al. 2001).
These cell detection technologies open the door to an era where remote, subsurface, near real-time detection of specific HAB taxa can be envisioned. An exciting development in this regard is the advent of ocean observing systems (OOSs) - arrays of moored and mobile instruments that can collect and transmit data continuously from remote locations to shore-based scientists and managers. Just as networks of meteorological stations and numerical models of atmospheric dynamics greatly improved our ability to provide accurate forecasts of weather events, OOSs and their associated numerical models of ocean dynamics have the potential to document long-term patterns and changes in the sea, to detect infrequent events that previously went unobserved, and to make predictions or forecasts about these and other phenomena that directly affect human populations and marine ecosystems.
HABs are frequently cited as phenomena that can be better understood and managed using ocean observatories (e.g., ORION Executive Steering Committee 2005). However, HABs represent a biological component of coastal waters that challenges present technologies, in part because water samples need to be processed through filters or other concentrating devices and then manipulated for extraction and analysis of toxins or the cellular targets needed for species identification and enumeration. Technologies are available for many of these analyses, but they must be incorporated into an instrument that can be deployed underwater and that can perform the series of robotic functions needed for each analysis.
One instrument that provides these capabilities and that can be configured for use for HAB cell and toxin detection in ocean observing systems is the Environmental Sampling Processor (ESP; Goffredi et al. 2006; Scholin et al. 2009; Fig. 6). The ESP autonomously collects discrete water samples from the ocean subsurface, concentrates microorganisms (particulates), and automates application of molecular probes to identify specific microorganisms and their gene products. The instrument can be bundled with contextual sensors such as a CTD, fluorometer, transmissometer, and nutrient analyzer. Data from the external sensors along with results of the probe assays are uploaded periodically from the deployed instrument to a shore station for analysis and interpretation. Two-way communication allows for rescheduling of mission sampling profiles if desired. The ESP is now commercially available, and instruments will soon be deployed to augment HAB research and management programs.
Fig. 6.

The Environmental Sample Processor (ESP), a device developed for in situ automated detection of HAB species and toxins (Source: C. Scholin, Monterey Bay Aquarium Research Institute).
Conceptual, empirical, and numerical models
Technological advances have expanded our capabilities for research and monitoring of HABs, but the blooms will always be under sampled because of the large space and time scales over which they occur. As a result, models are being used to help extrapolate and interpret these sparse observations (Franks 1997; McGillicuddy et al. submitted). These include conceptual models (e.g., Anderson et al. 2005b), empirical models (e.g., Blauw et al. 2010; Raine et al. 2010), and complex numerical models (e.g., He et al. 2008). An example of an innovative and useful empirical model is that of Raine et al. (2010) who described a chain of observable events that lead to blooms of Dinophysis acuminata blooms in Bantry Bay, southwestern Ireland. Easterly winds tend to accelerate the coastal current in that area, delivering D. acuminata blooms from the continental shelf to the mouth of Bantry Bay. Subsequent southwest winds can then transport the populations into the bay. Raine et al. (2010) defined a single index that quantifies these patterns and used that to evaluate past outbreaks, and to predict new ones.
Numerical models with varying levels of sophistication have been developed. Some are purely three-dimensional physical models capable of resolving hydrography, and into which HAB cells are introduced as passive particles. This is the approach taken by Velo-Suarez et al. (2010) who used particle techniques to explain the disappearance of D. acuminata blooms in the Bay of Biscay, France. Although biological processes may have contributed to the decline of the bloom, a great deal was learned from this type of physical analysis. A similar approach is followed by a HAB forecasting system developed for K. brevis blooms in the Gulf of Mexico (Stumpf et al. 2009). HAB forecasts are made twice weekly during bloom events, using a combination of satellite derived image products, wind predictions, and a rule-based model derived from previous observations and research. Blooms are detected and defined using ocean color satellite images, and bloom transport is then predicted using hydrographic modeling with passive particle transport.
The next step in sophistication and complexity is to couple a detailed biological submodel to a hydrographic model. One example is a physical-biological model of Alexandrium bloom dynamics that has been developed for the Gulf of Maine region in the US (McGillicuddy et al. 2005; He et al. 2008). This model is based on a hydrographic submodel that can realistically simulate water motion over this large region driven by winds, tides, stratification, river run off, and large-scale forcing from the open ocean. A second submodel is then coupled to the hydrography, simulating the germination of Alexandrium cysts from seed beds in the region, and the subsequent growth of the population, regulated by temperature, salinity, sunlight and nutrients. The timing and rates of cyst germination and cell growth are parameterized from laboratory experiments on cultures of A. fundyense. A temperature-dependent mortality function incorporates a range of loss factors, including grazing and encystment. This model has demonstrated good skill at reproducing observations (Stock et al. 2005; He et al. 2008; Fig. 7) and has been heavily used for hindcasts (looking at past events to understand underlying mechanisms; He et al. 2008; Li et al. 2009). It is also being used to issue weekly nowcasts and forecasts (looking forward 3 or 4 days) and even seasonal or annual forecasts (McGillicuddy et al. submitted).
Fig. 7.

Comparison of observed (a) and modeled (b) surface Alexandrium fundyense cell concentrations during a bloom in the Gulf of Maine (Li et al. 2009). Open circles denote stations sampled. The model slightly underestimates cell concentrations, but does capture the major features of the extensive coastal bloom.
Despite its sophistication, there are many aspects of this modeling effort that need to be improved. Paramount among these is the simplicity of the mortality function, which lumps a number of different loss factors into a single parameterization. Another area for advancement is the need for data obtained on a real-time basis that can be assimilated into the model to improve accuracy, much as is done with meteorological sensor networks and weather forecasts. Major developments in this regard are instruments that can robotically sample water and detect HAB cells and their toxins, such as the ESP described above. A realistic vision for the future would be that of arrays of moored instruments capable of detecting HAB cells and their toxins (Figure 8) and transmitting this information to shore where the data can be assimilated into numerical models and used by managers to make decisions for harvesting closures or other mitigation strategies to reduce HAB impacts. In this regard, HAB sensors are viewed by many as an important component of the emerging ocean observing system infrastructure worldwide.
Fig. 8.

Locations for a hypothetical array of Environmental Sample Processors (ESPs) to provide an early warning of bloom delivery to coastal shellfish harvesting sites and provide cell abundance data for assimilation into the Alexandrium population dynamics model (modified from Anderson 2008).
HABS AND CLIMATE CHANGE
Humanity, traditionally focused on terrestrial plants and animals, has significantly undervalued invisible ocean plant production. All the microalgal cells in the world oceans could be packed in a plank, 386,000km long, 7cm thick and 30cm wide, stretched from the Earth to the Moon (Andersen 2005). The oceans are a core component of the global climate system because they store 93% of the world’s carbon, and marine phytoplankton accounts for 50% of global primary productivity (Longhurst et al. 1995). Such increased recognition of phytoplankton as a climate driver is reflected in the recent commercial interests in ocean fertilization to combat anthropogenic climate change (Strong et al. 2009). Whereas in the past two decades unexpected new algal bloom phenomena have often been attributed to eutrophication or ballast water introduction, increasingly novel algal bloom episodes are now circumstantially linked to climate change. A number of scattered publications started to address the topic of HABs and climate change, but they usually focused on single environmental factors (e.g. CO2, temperature increase, stratification), single biological properties (photosynthesis, calcification; nutrient uptake) or discussing selected “pet” species categories only. Complex factor interactions are rarely considered in climate simulation scenarios and ecophysiological experiments never cover the full range of genetic diversity and physiological plasticity of microalgal taxa. Prediction of the impact of global climate change on algal blooms thus is fraught with uncertainties (Hallegraeff 2010). Very few long-term records exist of algal blooms at any single locality, and as a rule we need at least 30 consecutive years before trends can realistically be detected. However we can learn from long-term data sets available from the Continuous Plankton Recorder surveys and short-term phytoplankton community responses to El Niño-Southern Oscillation and North Atlantic Oscillation episodes.
A re-examination of the phytoplankton fossil record using increasingly sophisticated geochemical tools is underway (Dale 2001). Undoubtedly, climate change of the magnitude that we will be experiencing in the next 100 years has happened before, albeit at a much slower pace and starting from a cooler baseline than present (IPCC 2008). Past episodes of climate change over long periods of geological and evolutionary history allowed organisms to adapt to their changing environment. The first photosynthetic cyanobacteria evolved 3.5 billion years ago at CO2 levels 1000× those of the present, followed by green algae 1000 My (500× present) and dinoflagellates 330–400 My (8× present) whereas more recently evolved diatoms and haptophytes operated under comparatively low CO2 environments (2–3 × present) (Beardall & Raven 2004).
Climate change confronts marine ecosystems with multifactorial stressors such as increased temperature, enhanced surface stratification, alteration of ocean currents, intensification or weakening of nutrient upwelling, stimulation of photosynthesis by elevated CO2, reduced calcification from ocean acidification, and changes in land runoff and micronutrient availability (Fig.9). Complex factor interactions are rarely covered by simulated ecophysiological experiments. Traditional experimental challenges last days to weeks and impose new growth conditions rather quickly, thus only allowing for limited acclimation (testing short-term physiological plasticity but without genetic changes). Our knowledge of the potential of marine microalgae to adapt remains very limited. Collins and Bell (2004) grew the freshwater microscopic alga Chlamydomonas reinhardtii over 1000 generations at almost 3x present atmospheric CO2 concentration. The cells acclimated to the change but did not show any genetic mutations that could be described as adaptation. Many more such experiments are needed.
Fig.9.

Summary diagram of known feedback mechanisms between physicochemical climate variables and biological properties of marine phytoplankton systems. Left: Greenhouse warming raises surface temperatures and causes a shoaling of mixed-layer depths, but can also have broader impacts on global currents, upwelling, and even the deep-ocean conveyor belt. Selected phytoplankton such as coccolithophorids produce dimethylsulfoxide (DMS), acting as cloud condensation nuclei, thereby reducing solar irradiation Middle: Increased atmospheric CO2 drives the biological pump, can alter phytoplankton species composition, and can alter ocean pH, influencing calcification of coccolithophorids but also nutrient availability. Right: Marine food- web structure, including top-down as well as bottom-up influences on phytoplankton species composition. Other anthropogenic influences in terms of eutrophication, shipping (ballast water introductions), and fishing are also indicated. Without exception, all perturbations will drive changes in phytoplankton (and HAB) species composition.
Laboratory studies should aim to mimic environmental conditions as closely as possible (Rost et al. 2008). An example is the problem of the potential impact of increased CO2 on the coccolithophorid Emiliania huxleyi. Initial concerns focused on reduced calcification (Riebesell et al. 2000), but increased CO2 at the same time stimulates photosynthesis (Iglesias-Rodriguez et al. 2008). Complex factor interactions between increased CO2, light and temperature on the calcification vs photosynthesis dynamics of Emiliania huxleyi have been elegantly demonstrated by Feng et al. (2008), while geographic strain variability of this “cosmopolitan” taxon has confounded the extensive literature on this nanoplankton species (Langer et al. 2009). Coastal plankton appears to tolerate pH changes predicted by the end of the century (Tor Nielsen et al. 2010)s but oceanic species are expected to be more vulnerable. Undoubtedly, there will be winners and losers from climate change, but one thing we can be certain about is that there will be local changes in species composition, abundance and timing of algal blooms.
The greatest problems for human society will be caused by being unprepared for significant range extensions of HAB species or the appearance of algal biotoxin problems in currently poorly monitored areas. For example, a range extension of the ciguatera- causative benthic dinoflagellate from coaral reef systems into warm-temperate sea grass beds might place, other previously unaffected coastal fisheries unexpectedly at risk. Likewise, polar expansion of domoic acid producing Pseudo-nitzschia australis could pose a novel threat to krill-feeding whales (Lefebvre et al. 2002).
Coupled climate-carbon models are increasingly revealing feedback mechanisms which were unpredicted from first principles. Phytoplankton play a key role in several global biogeochemical cycles and thereby exert important feedback effects on climate by influencing the partitioning of climate-relevant gases between the ocean and the atmosphere. The well-known Gaia hypothesis is based on species such as E. huxleyi and Phaeocystis producing dimethylsulfonium propionate, a precursor of dimethylsulfoxide (DMS), which in the atmosphere is oxidized into sulfate which forms condensation nuclei for clouds (Charleson et al. 1987). Woods & Barkmann’s (1993) “plankton multiplier” is an example of a positive feedback mechanism linking greenhouse warming to the biological pump. Enhanced greenhouse carbon dioxide induces ocean surface warming, diminishing winter convection and nutrient availability and thereby primary production, thus weakening the biological pump and further enhancing atmospheric CO2. Any reduction in net ocean CO2 uptake caused by shifts in ocean circulation or reduced phytoplankton growth in surface waters reducing the export of organic matter to the deep sea via the ‘biological pump’ could lead to an acceleration in the rate of atmospheric CO2 increase and global warming. Models have estimated that a 50% decrease in oceanic calcification from ocean acidification thus would reduce atmospheric CO2 by 10–40 ppm, equivalent to 5–20 years of industrial emissions. Another powerful mechanism for algal bloom formation occurs through “top-down control” of the marine foodweb (Turner & Granéli 2006). Overfishing removes top fish predators, stimulating small fish stocks which graze away zooplankton, thus relieving phytoplankton grazing pressure. The impact of past whaling in the Southern Ocean and its impact on iron cycling is actively being researched (Roman & McCarthy 2010). Differential impacts of climate change on individual zooplankton or fish grazers (uncoupling between trophic levels) thus can result in stimulation of HABs.
In conclusion, we can expect: (1) Range changes in both warm- and cold-water species, with some expansions and some contractions; (2) Species-specific changes in the abundance and seasonal window of growth of HAB taxa; (3) Earlier timing of peak production of some phytoplankton; (4) Secondary effects for marine food webs, notably when individual zooplankton and fish grazers are differentially impacted (“match- mismatch”) by climate change. Some species of harmful algae (e.g. toxic dinoflagellates benefitting from land runoff and/or water column stratification, tropical benthic dinoflagellates responding to increased water temperatures and coral reef disturbance) may become more prevalent, while others may diminish in areas currently impacted. Changes in phytoplankton communities provide a sensitive early warning for climate- driven perturbations to marine ecosystems. Phytoplankton monitoring should therefore play an integral role in planned ocean observation systems which are necessary if we wish to detect long-term change, define management options, forecast ocean-related risks to human health and safety, and shed light on the impact of climate variability on marine life and humans in general.
SUMMARY
This is an historic time in the HAB field. Impacts are growing worldwide, and society’s need for information on these phenomena is more pressing than ever (McGillicuddy 2010). Increased public health, tourism, fisheries and ecosystem impacts from HABs have led to heightened scientific and regulatory attention and an increased awareness of the value of ocean ecosystems to human society. HABs represent a biological component of coastal waters that challenges present technologies, in part because of the need for species- or toxin-specific detection capabilities. Advanced technologies are now available for identification and enumeration of HAB species and for extraction and analysis of seafood toxins. One vision for the future is that these functions will be incorporated into instruments that can be deployed along coastlines in networked arrays, performing analyses in situ and transmitting results to shore where the information is used by managers directly, while it is also assimilated into numerical models to improve forecasting accuracy. With this type of advance warning and prediction of bloom transport and landfall, impacts can be minimized and productive fisheries sustained, even in areas with recurrent HAB outbreaks. Another component of this vision is that the development and application of the advanced technologies of genomics, transcriptomics, proteomics, and metabolomics will continue to provide revolutionary shifts in our understanding of the ecology, evolution, and biogeography of HAB species. We will better understand the link between HABs and human activities – such as ballast water intoductions, or stimulation of blooms by anthropogenic nutrient inputs. Historically, the conceptual understanding of HABs in eutrophic systems has been based on the simplistic notion that more nutrients yield higher algal biomass. However, we now recognize that the composition and relative availability of nutrient pools, the range of physiological responses by different phytoplankton, and the interactions of other factors such as physics and grazing are all important aspects of the linkage between eutrophication and HABs. This knowledge will lead to informed policy decisions on point and non-point nutrient discharges that should lead to reductions in some of the high biomass HABs that are so characteristic of polluted coastal waters. We can also anticipate changes in phytoplankton communities as a sensitive, early warning indicator for climate-driven perturbations to marine ecosystems. With this will come range expansions by some HAB species, and contractions or even the disappearance of others. Many of these thoughts are speculative, but one thing is certain however - the global problem of HABs will challenge scientists and managers for many years to come.
SUMMARY POINTS.
There is a general scientific consensus that the public health, recreational and tourism, fisheries, aquaculture and ecosystem impacts from HABs have all increased over the last few decades. There are multiple reasons for this expansion. Although this increase is true on a global level, HAB impacts are highly variable and may even be decreasing at the local and regional scale in some areas.
Over the last 50 years hundreds of phycotoxin analogues have been found in plankton, seawater and seafood matrices, belonging to >20 structural groups. Nevertheless, the dramatic decrease in the rate of discovery of new classes of phycotoxins associated with human toxicity in the past decade suggests that major associated toxin syndromes may already be fully described.
Advances in understanding the origin, structural and functional diversity, and ecological and evolutionary significance of HAB toxins were initiated by the development of high-resolution analytical separation and detection technology (e.g. liquid chromatography coupled with tandem mass spectrometry), but additional breakthroughs have been contributed by molecular investigations of toxin biosynthetic pathways and their regulation.
Evidence does not support a general mechanism or primary role for the known phycotoxins as defensive compounds against plankton competitors or predators. Toxin production may still have important implications, however, for food web interactions and thus dynamics of HABs.
The exact chemical nature of allelopathic compounds remains to be determined, as does their ecological role, but they are distinct from the known phycotoxins and thus can cause broad-based trophodynamic effects.
Historically, the conceptual understanding of HABs in eutrophic systems has been based on the simplistic notion that more nutrients yield higher algal biomass. However, it is now widely accepted that the composition and relative proportional availability of nutrient pools, the range of physiological responses by different phytoplankton, and the interactions of other dynamic factors such as physics and grazing are all important controlling responses to the effects of eutrophication on HABs.
Technological advances have expanded our capabilities for research and monitoring of HABs, but the blooms will always be under-sampled because of the large spatial and temporal scales over which they occur. As a result, models are being used to help extrapolate and interpret these sparse observations.
FUTURE ISSUES.
New cell detection technologies are opening the door to the opportunity for remote, subsurface, near real-time detection of specific HAB taxa. One can envision networked arrays of multiple instruments moored at key locations in HAB transport pathways, bundled with other sensors to provide relevant oceanographic data on a near real-time basis.
The recent development and application of advanced technologies from the generically defined ‘omics sciences (genomics, transcriptomics, proteomics, metabolomics) coupled with bioinformatics platforms is providing deep and often revolutionary shifts in understanding the ecology and evolution of HAB species and bloom dynamics.
Acknowledgments
Support to DMA was provided by the National Institute of Environmental Health Sciences (1-P50-ES012742) and the National Science Foundation through the Woods Hole Center for Oceans and Human Health (OCE-0430724), and by NOAA Grants NA09NOS4260212 and NA09OAR4320129.
Contributor Information
Donald M. Anderson, Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA 02543 USA, Phone: (508) 289-2351
Allan D. Cembella, Alfred Wegener Institute for Polar and Marine Research, Am Handelshafen 12 27570 Bremerhaven Germany
Gustaaf M. Hallegraeff, Institute for Marine and Antarctic Studies, Private Bag 55, University of Tasmania, Hobart, Tasmania 7001, Australia
LITERATURE CITED
- Adolf JE, Place AR, Stoecker DK, Harding LV. Modulation of polyunsaturated fatty acids in mixotrophic Karlodinium veneficum (Dinophyceae) and its prey, Storeatula major (Cryptophyceae)l. J Phycol. 2007;43(6):1259–70. [Google Scholar]
- Alpermann TJ, Beszteri B, John U, Tillmann U, Cembella AD. Implications of life-history transitions on the population genetic structure of the toxigenic marine dinoflagellate Alexandrium tamarense. Mol Ecol. 2009;18(10):2122–33. doi: 10.1111/j.1365-294X.2009.04165.x. [DOI] [PubMed] [Google Scholar]
- Alpermann TJ, Tillmann U, Beszteri B, Cembella AD, John U. Phenotypic variation and genotypic diversity in a planktonic population of the toxigenic marine dinoflagellate Alexandrium tamarense (Dinophyceae) J Phycol. 2010;46(1):18–32. [Google Scholar]
- Andersen RA, editor. Algal culturing techniques. Elsevier; NY: 2005. p. 596. [Google Scholar]
- Anderson DM. Toxic algal blooms and red tides: a global perspective. In: Okaichi T, Anderson DM, Nemoto T, editors. Red Tides: Biology, Environmental Science and Toxicology. Elsevier; 1989. pp. 11–16. [Google Scholar]
- Anderson DM. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. 1998:29–48. See Ref. 10. [Google Scholar]
- Anderson DM. Harmful algal blooms and ocean observing systems: needs, present status and future potential. In: Tsukamoto K, Kawamura T, Takeuchi T, Beard TD Jr, Kaiser MJ, editors. Fisheries for Global Welfare and Environment. Terrapub; Tokyo: 2008. pp. 317–334. [Google Scholar]
- Anderson DM, Andersen P, Bricelj VM, Cullen JJ, Rensel JE. Monitoring and Management Strategies for Harmful Algal Blooms in Coastal Waters. Asia Pacific Economic Program, Singapore, and Intergovernmental Oceanographic Commission; Paris: 2001. p. 268. [Google Scholar]
- Anderson DM, Burkholder JM, Cochlan WP, Glibert PM, Gobler CJ, et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae. 2008;8:39–53. doi: 10.1016/j.hal.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Cembella AD, Hallegraeff GM, editors. The Physiological Ecology of Harmful Algal Blooms. Springer-Verlag; Heidelberg: 1998. p. 600. [Google Scholar]
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries. 2002;25(4b):704–26. [Google Scholar]
- Anderson DM, Kulis DM, Doucette GJ, Gallager JC, Balech E. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeast United States and Canada as determined by morphology, bioluminescence, toxin composition, and mating compatibility. Mar Biol. 1994;120:467–78. [Google Scholar]
- Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, Scholin CA. Identification and enumeration of Alexandrium spp from the Gulf of Maine using molecular probes. 2005a:2467–90. See Ref. 15. [Google Scholar]
- Anderson DM, Stock CA, Keafer BA, Bronzino Nelson A, Thompson B, et al. Alexandrium fundyense cyst dynamics in the Gulf of Maine. 2005b:2522–42. See Ref. 15. [Google Scholar]
- Anderson DM, Townsend DW, McGillicuddy DJ, Turner JT, editors. The Ecology and Oceanography of Toxic Alexandrium fundyense Blooms in the Gulf of Maine. Deep-Sea Res II. 2005c;52(19–21):2365–76. [Google Scholar]
- Armbrust EV. The life of diatoms in the world’s oceans. Nature. 2009;459:185–192. doi: 10.1038/nature08057. [DOI] [PubMed] [Google Scholar]
- Bates SS, Bird CJ, de Freitas ASW, Foxall R, Gilgan M, et al. Pennate diaton Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can J Fish Aquat Sci. 1989;46:1203–15. [Google Scholar]
- Bates SS, Douglas DJ, Doucette GJ, Léger C. Effects of reintroducing bacteria on domoic acid production by axenic cultures of the diatom Pseudonitzschia pungens f. multiseries. In: Lassus P, Arzul G, Erard E, Gentien P, Baut C Marcaillou-Le, editors. Harmful marine algal blooms. Lavoisier Sci. Publ; Paris: 1995. pp. 401–406. [Google Scholar]
- Beardall J, Raven JA. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia. 2004;43:26–41. [Google Scholar]
- Berdalet E. Effects of turbulence on the marine dinoflagellate Gymnodinium nelsoni. J Phycol. 1992;28:267–72. [Google Scholar]
- Berg GM, Glibert PM, Lomas MW, Burford MA. Organic nitrogen uptake and growth by the chrysophyte Aureococcus anophagefferens during a brown tide event. Mar Biol. 1997;129:377–87. [Google Scholar]
- Blauw AN, Los FJ, Huisman J, Peperzak L. Nuisance foam events and Phaeocystis globosa blooms in Dutch coastal waters analyzed with fuzzy logic. J Mar Sys. 2010;83:115–26. [Google Scholar]
- Bodeanu N, Ruta G. Development of the planktonic algae in the Romanian Black Sea sector in 1981–1996. In: Reguera B, Blanco J, Fernandez ML, Wyatt T, editors. Harmful Algae. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO; Paris, France: 1998. pp. 188–191. [Google Scholar]
- Bolch CJS, de Salas MF. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australasia. Harmful Algae. 2007;6:465–85. [Google Scholar]
- Bowers HA, C Tomas C, Tengs T, Kempton JW, Lewitus AJ, Oldach DW. Raphidophyceae [Chadefaud ex Silva] systematics and rapid identification: Sequence analyses and real-time PCR assays. J Phycol. 2006;2:1333–48. doi: 10.1111/j.1529-8817.2006.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteleyn G, Chepurnov VA, Leliaert F, Mann DG, Bates SS, et al. Pseudo- nitzschia pungens (Bacillariophyceae): A cosmopolitan diatom species? Harmful Algae. 2008;7:241–57. [Google Scholar]
- Cembella AD. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia. 2003;42(4):420–47. [Google Scholar]
- Cembella AD, Bauder AG, Lewis NI, Quilliam MA. Association of the gonyaulacoid dinoflagellate Alexandrium ostenfeldii with spirolide toxins in size- fractionated plankton. J Plankton Res. 2001;23:1413–19. [Google Scholar]
- Cembella AD, Sullivan JJ, Boyer GL, Taylor FJR, Anderson RJ. Variation in paralytic shellfish toxin composition within the Protogonyaulax tamarensis/catenella species complex: red tide dinoflagellates. Biochem System Ecol. 1987;15:171–86. [Google Scholar]
- Chan LL, Hodgkiss IJ, Lam PKS, Wan JMF, Chou H-N, et al. Use of 2-DE to differentiate morphospecies of Alexandrium minutum, a PSP toxin-producing agent of harmful algal blooms. Proteomics. 2005;5:1580–93. doi: 10.1002/pmic.200401020. [DOI] [PubMed] [Google Scholar]
- Chan LL, Sit WH, Lam PKS, Hsieh DPH, Hodgkiss IJ, et al. Identification and characterization of a ‘biomarker of toxicity’ from the proteome of the paralytic shellfish toxin-producing dinoflagellate, Alexandrium tamarense. Proteomics. 2006;6:654–66. doi: 10.1002/pmic.200401350. [DOI] [PubMed] [Google Scholar]
- Charleson RJ, Lovelock JE, Andreae MO, Warren SG. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature. 1987;326:655–61. [Google Scholar]
- Collins S, Bell G. Phenotypic consequences of 1000 generations of selection at elevated CO2 in a green alga. Nature. 2004;431:566–69. doi: 10.1038/nature02945. [DOI] [PubMed] [Google Scholar]
- Coyne KJ, Handy SM, Demir E, Whereat EB, Hutchins DA, et al. Improved quantitative real-time PCR assays for enumeration of harmful algal species in field samples using an exogenous DNA reference standard. Limnol Oceanogr Methods. 2005:381–91. [Google Scholar]
- Dale B. The sedimentary record of dinoflagellate cysts: looking back into the future of phytoplankton blooms. Sc Mar. 2001;65:257–72. [Google Scholar]
- Daugbjerg N, Hansen G, Larsen J, Moestrup Ø. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmored dinoflagellates. Phycologia. 2000;39:302–17. [Google Scholar]
- de Salas MF, Laza-Martínez A, Hallegraeff GM. Five new species of Karlodinium and one new Takayama (Kareniaceae, Dinophyceae) from open Southern Ocean waters. J Phycol. 2008;44:241–57. doi: 10.1111/j.1529-8817.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- Dorantes-Aranda JJ, Waite TD, Godrant A, Rose AL, Tovar CD, et al. Novel application of a fish gill cell line assay to assess ichthyotoxicity of harmful marine microalgae. Harmful Algae. 2011;10:366–73. [Google Scholar]
- Feng Y, Warner ME, Zhang Y, Sun J, Fu F-X, et al. Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae) Europ J Phycol. 2008;43:87–98. [Google Scholar]
- Franks PJS. Coupled physical-biological models for the study of harmful algal blooms. Ocean Res. 1997;19:153–60. [Google Scholar]
- Franks PJS, Anderson DM. Alongshore transport of a toxic phytoplankton bloom in a buoyancy current: Alexandrium tamarensis in the Gulf of Maine. Mar Biol. 1992;112:153–164. [Google Scholar]
- Fux E, Smith JL, Tong M, Guzmán L, Anderson DM. Toxin profiles of five geographical isolates of Dinophysis spp. from North and South America. Toxicon. 2011;57(2):275–87. doi: 10.1016/j.toxicon.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Garcés E, Montresor M, Lewis J, Rengefors K, Anderson DM, Barth H, editors. Phytoplankton Life Cycles and Their Impacts on the Ecology of Harmful Algal Blooms. Deep-Sea Res II. 2010;57(3–4):159–322. [Google Scholar]
- Gentien P, Donaghay P, Yamazaki H, Raine R, Reguera B, Osborn T. Harmful algal blooms in stratified environments. Oceanogr. 2005;18(2):172–83. [Google Scholar]
- Gentien P, Lunven M, Lazure P, Youenou A, Crassous MP. Motility and autotoxicity in Karenia mikimotoi (Dinophyceae) Phil Trans R Soc B. 2007;362:1937–46. doi: 10.1098/rstb.2007.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentien P, Lunven M, Lehaitre M, Duvent JL. In situ depth profiling of particle sizes. Deep-Sea Res. 1995;42(8):1297–312. [Google Scholar]
- Glibert P, editor. GEOHAB. Global Ecology and Oceanography of Harmful Algal Blooms, Harmful Algal Blooms in Eutrophic Systems. IOC and SCOR; Paris and Baltimore: 2006. p. 74. [Google Scholar]
- Glibert PM, Mayorga E, Seitzinger S. Prorocentrum minimum tracks anthropogenic nitrogen and phosphorus inputs on a global basis: Application of spatially explicit nutrient export models. Harmful Algae. 2008;8(1):33–38. [Google Scholar]
- Gobler CJ, Berry DL, Dyhrman ST, Wilhelm SW, Salamov A, et al. Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proc Natl Acad Sci. 2011;108(11):4352–57. doi: 10.1073/pnas.1016106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffredi SK, Jones W, Scholin C, Marin R, Hallam S, Vrjenhoek RC. Molecular detection of marine larvae. Mar Biotechnol. 2006;8:149–60. doi: 10.1007/s10126-005-5016-2. [DOI] [PubMed] [Google Scholar]
- Gordon BR, Leggat W. Symbiodinium—invertebrate symbioses and the role of metabolomics. Marine Drugs. 2010;8:2546–68. doi: 10.3390/md8102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granéli E, Turner J, editors. Ecology of Harmful Algae. Springer-Verlag Berling; Heidelberg: 2006. p. 413. (Ecological Studies Series 189). [Google Scholar]
- Gribble KE, Keafer BA, Quilliam MA, Cembella AD, Kulis DM. Distribution and toxicity of Alexandrium ostenfeldii (Dinophyceae) in the Gulf of Maine, USA. 2005:2745–63. See Ref. 15. [Google Scholar]
- Hackett JD, Scheetz TE, Yoon HS, Soares MB, Bonaldo MF, et al. Insights into a dinoflagellate genome through expressed sequence tag analysis. BMC Genomics. 2005;6:80. doi: 10.1186/1471-2164-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett JD, Wisecaver JH, Brosnahan ML, Kulis DM, Anderson DM, et al. Independent evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol Biol and Evol. doi: 10.1093/molbev/mss142. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- Hallegraeff GM. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. J Phycol. 2010;46:220–35. [Google Scholar]
- Hallegraeff GM, Anderson DM, Cembella AD, editors. Manual on Harmful Marine Microalgae. Vol. 11. UNESCO; Paris: 2003. p. 792. (UNESCO Monographs on Oceanographic Methodology). [Google Scholar]
- Haywood AJ, Scholin CA, Marin R, III, Petrik K, Pigg R, et al. Proceedings of the 6th International Conference on Molluscan Shellfish Safety. Blenheim, Marlborough, New Zealand: 2009. Detection of Karenia brevis in Florida coastal waters using sandwich hybridization assays in two formats; pp. 95–100. (Royal Society of New Zealand Miscellaneous Series No. 71). [Google Scholar]
- He R, McGillicuddy DJ, Keafer BA, Anderson DM. Historic 2005 toxic bloom of Alexandrium fundyense in the western Gulf of Maine: 2. Coupled Biophysical Numerical Modeling. J Geophys Res-Oceans. 2008;113:C07040. doi: 10.1029/2007JC004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller S, Krock B, Cembella A, Luckas B. Rapid detection of cyanobacterial toxins in precursor ion mode by liquid chromatography tandem mass spectrometry. J Mass Spectro. 2007;42:1238–50. doi: 10.1002/jms.1257. [DOI] [PubMed] [Google Scholar]
- Holligan PM. Dinoflagellate blooms associated with tidal fronts around the British Isles. In: Taylor DL, Seliger HH, editors. Toxic Dinoflagellate Blooms. Elsevier/North Holland: 1979. pp. 249–256. (Proc. Int’l. Conf., 2nd). [Google Scholar]
- Huntley M, Sykes P, Rohan S, Marin V. Chemically-mediated rejection of dinoflagellate prey by the copepods Calanus pacificus and Paracalanus parvus: mechanism, occurrence and significance. Mar Ecol Prog Ser. 1986;28:105–20. [Google Scholar]
- Iglesias-Rodriguez MD, Halloran PR, Rickaby RE, Hall IR, Colmenero-Hidalgo E, et al. Phytoplankton calcification in a high-CO2 world. Science. 2008;320:336–40. doi: 10.1126/science.1154122. [DOI] [PubMed] [Google Scholar]
- IPCC. Climate Change 2007 – Impacts, Adaptation and Vulnerability. Intergovernmental Panel on Climate Change; 2008. (Working Group II contribution to the Fourth Assessment Report of the IPCC). [Google Scholar]
- Ishida Y, Yoshinaga I, Kim M-C, Uchida A. Possibility of bacterial control of harmful algal blooms. In: Martins MT, Sato MIZ, Tiedje JM, Hagler LCN, Dobereiner J, Sanchez PS, editors. Progress in Microbial Ecology. SBM/ICOME; Brazil: 1997. pp. 495–500. [Google Scholar]
- Jaeckisch N, Singh R, Curtis B, Cembella A, John U. Genomic characterization of the spirolide-producing dinoflagellate Alexandrium ostenfeldii with special emphasis on PKS genes. In: Moestrup O, Doucette G, Enevoldsen H, Godhe A, Hallegraeff G, et al., editors. Proceedings of the 12th International Conference on Harmful Algae. ISSHA and IOC-UNESCO; Copenhagen: 2008. pp. 65–67. [Google Scholar]
- James KJ, Moroney C, Roden C, Satake M, Yasumoto T, et al. Ubiquitous ‘benign’ alga emerges as the cause of shellfish contamination responsible for the human toxic syndrome, azaspiracid poisoning. Toxicon. 2003;41:145–51. doi: 10.1016/s0041-0101(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Jenkinson I, Arzul G. Effect of the flagellates Gymnodinium mikimotoi, Heterosigma akashiwo and Pavlova lutheri on flow through fish gills. In: Reguera B, Blanco J, Fernandez ML, Wyatt T, Xunta de Galicia, editors. Proceedings of 8th International Conference on Harmful Algae. Pontevedro and Intergovernmental Oceanographic Commission of Unesco; Vigo, Spain: 1998. pp. 425–28. [Google Scholar]
- Jeong HJ, Kim JS, Kim JH, Kim ST, Seong KA. Feeding and grazing impact of the newly described heterotrophic dinoflagellate Stoeckeria algicida on the harmful alga Heterosigma akashiwo. Mar Ecol Prog Ser. 2005a;295:69–78. [Google Scholar]
- Jeong HJ, Yoo YD, Park JY, Song JY, Kim ST, et al. Feeding by phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. Aquat Microb Ecol. 2005b;40:133–50. [Google Scholar]
- John U, Beszteri B, Derelle E, van de Peer Y, Read B, et al. Novel Insights into evolution of protistan polyketide synthases through phylogenomic analysis. Protist. 2008;159(1):21–30. doi: 10.1016/j.protis.2007.08.001. [DOI] [PubMed] [Google Scholar]
- John U, Beszteri S, Gloeckner G, Singh R, Medlin LK, Cembella AD. Genomic characterisation of the ichthyotoxic prymnesiophyte Chrysochromulina polylepis, and the expression of polyketide synthases genes in synchronised cultures. Eur J Phycol. 2010;45(3):215–29. [Google Scholar]
- Jones KL, Mikulski CM, Barnhorst A, Doucette GJ. Comparative analysis of bacterioplankton assemblages from Karenia brevis bloom and nonbloom water on the west Florida shelf (Gulf of Mexico, USA) using 16S rRNA gene clone libraries. FEMS Microbiol Ecol. 2010;73:468–85. doi: 10.1111/j.1574-6941.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- Kamykowski D. Possible interactions between phytoplankton and semidiurnal internal tides. J Mar Res. 1974;32(1):67–89. [Google Scholar]
- Kellmann R, Mihali TK, Jeon YJ, Pickford R, Pomati F, Neilan BA. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. App Environ Microbiol. 2008;74:4044–53. doi: 10.1128/AEM.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick GJ, Millie DF, Moline MA, Schofield O. Optical discrimination of a phytoplankton species in natural mixed populations. Limnol Oceanogr. 2000;45(2):467–71. [Google Scholar]
- Kirkpatrick GJ, Orrico C, Moline MA, Oliver M, Schofield OM. Continuous hyperspectral absorption measurements of colored dissolved organic material in aquatic systems. Appl Opt. 2003;42(33):6564–68. doi: 10.1364/ao.42.006564. [DOI] [PubMed] [Google Scholar]
- Kodama M, Doucette GJ, Green DH. Relationships between bacteria and harmful algae. 2006:243–255. See Ref. 52. [Google Scholar]
- Kooistra WHCF, Sarno D, Balzano S, Gu H, Andersen RA, Zingone A. Global diversity and biogeography of Skeletonema species (Bacillariophyta) Protist. 2008;159:177–93. doi: 10.1016/j.protis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Krock B, Seguel CG, Valderrama K, Tillmann U. Pectenotoxins and yessotoxin from Arica Bay, North Chile as determined by tandem mass spectrometry. Toxicon. 2009;54(3):364–67. doi: 10.1016/j.toxicon.2009.04.013. [DOI] [PubMed] [Google Scholar]
- La Claire JW. Analysis of expressed sequence tags from the harmful alga, Prymnesium parvum (Prymnesiophyceae, Haptophyta) Mar Biotechnol. 2006;8:534–46. doi: 10.1007/s10126-005-5182-2. [DOI] [PubMed] [Google Scholar]
- Langer G, Nehrke G, Probert I, Ly J, Ziveri P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonic chemistry. Biogeosci Disc. 2009;6:4361–83. [Google Scholar]
- Lefebvre KA, Bargu S, Kieckhefer T, Silver MW. From sanddabs to blue whales: The pervasiveness of domoic acid. Toxicon. 2002;40:971–77. doi: 10.1016/s0041-0101(02)00093-4. [DOI] [PubMed] [Google Scholar]
- Legrand C, Rengefors K, Fistarol GO, Granéli E. Allelopathy in phytoplankton - biochemical, ecological and evolutionary aspects. Phycologia. 2003;42(4):406–19. [Google Scholar]
- Li Y, He R, McGillicuddy DJ, Jr, Anderson DM, Keafer BA. Investigation of the 2006 Alexandrium fundyense bloom in the Gulf of Maine: In situ observations and numerical modeling. Cont Shelf Res. 2009;29(17):2069–82. doi: 10.1016/j.csr.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidie KL, Ryan JC, Barbier M, Van Dolah FM. Gene expression in the Florida red tide dinoflagellate Karenia brevis: Analysis of an expressed sequence tag (EST) library and development of a DNA microarray. Mar Biotechnol. 2005;7:1–14. doi: 10.1007/s10126-004-4110-6. [DOI] [PubMed] [Google Scholar]
- Lilly EL, Halaynch KM, Anderson DM. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae) J Phycol. 2007;43:1329–38. [Google Scholar]
- Lilly EL, Kulis DM, Gentien P, Anderson DM. Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from the western Pacific: evidence from DNA and toxin analysis. J Plankton Res. 2002;24:443–52. [Google Scholar]
- Litaker RW, Vandersea MW, Faust MA, Kibler SR, Chinain M, et al. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae) Phycologia. 2009;48:344–90. [Google Scholar]
- Longhurst A, Sathyendranath S, Platt T, Caverhill C. An estimate of global primary production in the ocean from satellite radiometer data. J Plankton Res. 1995;17:1245–71. [Google Scholar]
- Ma H, Krock B, Tillmann U, Cembella A. Towards characterization of lytic compound(s) produced by Alexandrium tamarense. In: Ho K, et al., editors. Proceedings of the 13th International Conference on Harmful Algae. International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO; Hong Kong, China: 2010. pp. 142–46. [Google Scholar]
- Marshall JA, Nichols PD, Hamilton B, Lewis RJ, Hallegraeff GM. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): the synergistic role of reactive oxygen species and free fatty acids. Harmful Algae. 2003;2:273–81. [Google Scholar]
- Masseret E, Grzebyk D, Nagai S, Genovesi B, Lasserre B, et al. Unexpected genetic diversity among and within populations of the toxic dinoflagellate Alexandrium catenella as revealed by nuclear microsatellite markers. Appl Environment Microbiol. 2009;75:2037–45. doi: 10.1128/AEM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ., Jr . Models of harmful algal blooms: Conceptual, empirical, and numerical approaches. In: McGillicuddy D, editor. GEOHAB Modeling. 2010. pp. 105–07. J. Mar. Sys. 83(3–4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ, Jr, Anderson DM, Lynch DR, Townsend DW. Mechanisms regulating large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: Results from a physical-biological model. 2005:2698–14. See Ref. 15. [Google Scholar]
- McGillicuddy DJ, Jr, Townsend DW, He R, Keafer BA, Kleindinst JL, et al. Submitted. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Cont Shelf Res. doi: 10.4319/lo.2011.56.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe EA, Van Dolah FM. The toxic dinoflagellate Karenia brevis encodes novel type I-like polyketide synthases containing discrete catalytic domains. Protist. 2008;159:471–82. doi: 10.1016/j.protis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Montagna PA, Stockwell D, Street G. Effect of the Texas brown tide on Mulinia lateralis populations and feeding. J Shellfish Res. 1993;12(1):142. [Google Scholar]
- Mooney BD, Dorantes-Aranda JJ, Place AR, Hallegraeff GM. Ichthyotoxicity of gymnodinioid dinoflagellates (Kareniaceae): activity of PUFA and superoxide against sheepshead minnow larvae and rainbow trout gill cells. Mar Ecol Prog Ser. 2011:426. (in press) [Google Scholar]
- Nagai S, Nishitani G, Sakamoto S, Sugaya T, Lee CK, et al. Genetic structuring and transfer of marine dinoflagellate Cochlodinium polykrikoides in Japanese and Korean coastal waters revealed by microsatellites. Mol Evol. 2009;18:2337–52. doi: 10.1111/j.1365-294X.2009.04193.x. [DOI] [PubMed] [Google Scholar]
- Nosenko T, Lidie KL, Van Dolah FM, Lindquist E, Cheng J-F, et al. Chimeric plastid proteome in the Florida “Red Tide” dinoflagellate Karenia brevis. Mol Biol Evol. 2006;23:2026–38. doi: 10.1093/molbev/msl074. [DOI] [PubMed] [Google Scholar]
- Oda T, Ishimatsu A, Shimada S, Takeshita S, Muramatsu T. Oxygen-radical-mediated toxic effects of the red tide flagellate Chattonella marina on Vibrio alginolyticus. Mar Biol. 1992;112:505–09. [Google Scholar]
- Okaichi T. Red tides in the Seto Inland Sea. In: Okaichi T, Yanagi Y, editors. Sustainable Development in the Seto Inland Sea, Japan - From the Viewpoint of Fisheries. Terra Scientific Publishing Company; Tokyo, Japan: 1997. pp. 251–304. [Google Scholar]
- ORION Executive Steering Committee. Ocean Observatories Initiative Science Plan. Washington, DC: 2005. p. 102. [Google Scholar]
- Park MG, Kim S, Kang YG, Yih W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat Microb Ecol. 2006;45:101–06. [Google Scholar]
- Penna A, Magnani M. Identification of Alexandrium (Dinophyceae) species using PCR and rDNA-targeted probes. J Phycol. 1999;35:615–21. [Google Scholar]
- Prince EK, Myers TL, Kubanek J. Effects of harmful algal blooms on competitors: Allelopathic mechanisms of the red tide dinoflagellate Karenia brevis. Limnol Oceanogr. 2008;53(2):531–41. [Google Scholar]
- Pollinger U, Zemel E. In situ and experimental evidence of the influence of turbulence on cell division processes of Peridinium cinctum forma westii (Lemm.) Lefevre. British J Phycol. 1981;16:281–87. [Google Scholar]
- Quilliam MA. The role of chromatography in the hunt for red tide toxins. J Chromatogr A. 2003;100(1–2):527–48. doi: 10.1016/s0021-9673(03)00586-7. [DOI] [PubMed] [Google Scholar]
- Raine R, McDermott G, Silke J, Lyons K, Nolan G, Cusack C. A simple short range model for the prediction of harmful algal events in the bays of southwestern Ireland. J Mar Sys. 2010;83:150–57. [Google Scholar]
- Rensel JE. Severe blood hypoxia of Atlantic salmon (Salmo salar) exposed to the marine diatom Chaetoceros concavicornis. In: Smayda TJ, Shimizu Y, editors. Toxic Phytoplankton Blooms in the Sea. Amsterdam: Elsevier; 1993. pp. 625–30. [Google Scholar]
- Rhodes L, Scholin CA, Tyrrell J, V J, Adamson J, Todd K. The integration of DNA probes into New Zealand’s routine phytoplankton monitoring programs. In: Hallegraeff GM, Blackburn SI, Bolch CJ, Lewis RJ, editors. Harmful Algal Blooms 2001. Intergovernmental Oceanographic Commission of UNESCO; 2001. pp. 429–32. [Google Scholar]
- Rhodes L, Smith KF, Munday R, Selwood A, McNabb P, et al. Toxic dinoflagellates (Dinophyceae) from Rarotonga, Cook Islands. Toxicon. 2010;56(5):751–58. doi: 10.1016/j.toxicon.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Richardson TL, Pickney JL. Monitoring of the toxic dinoflagellate Karenia brevis using gyroxanthin-based detection methods. J Appl Phycol. 2004;16:315–28. [Google Scholar]
- Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FMM. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–67. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- Roman J, McCarthy JJ. The whale pump: Marine mammals enhance primary productivity in a coastal basin. PLoS ONE. 2010;5(10):e13255. doi: 10.1371/journal.pone.0013255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B, Zondervan I, Wolf-Gladrow D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar Ecol Prog Ser. 2008;373:227–37. [Google Scholar]
- Salomon PS, Imai I. Pathogens of harmful microalgae. 2006:271–82. See Ref. 52. [Google Scholar]
- Scholin CA, Anderson DM. Identification of species and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). I. RFLP analysis of SSU rRNA genes. J Phycol. 1994;30:744–54. [Google Scholar]
- Scholin CA, Buck KR, Britschgi T, Cangelosi G, Chavez FP. Identification of Pseudo-nitzschia australis (Bacillariophyceae) using rRNA-targeted probes in whole cell and sandwich hybridization formats. Phycologia. 1996;35:190–97. [Google Scholar]
- Scholin C, Doucette G, Jensen S, Roman B, Pargett D, et al. Remote detection of marine microbes, small invertebrates, harmful algae and biotoxins using the Environmental Sample Processor (ESP) Oceanogr. 2009;22:158–67. [Google Scholar]
- Scholin CA, Hallegraeff GM, Anderson DM. Molecular evolution of the Alexandrium tamarense “species complex”. (Dinophyceae) and their dispersal in the North American and West Pacific regions. Phycologia. 1995;34:472–85. [Google Scholar]
- Skovgaard A, Hansen PJ. Food uptake in the harmful alga Prymnesium parvum mediated by excreted toxins. Limnol Oceanogr. 2003;48(3):1161–66. [Google Scholar]
- Smayda TJ. Primary production and the global epidemic of phytoplankton blooms in the sea: a linkage? In: Cosper EM, Carpenter EJ, Bricelj M, editors. Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Brown Tide and Other Unusual Blooms. Springer-Verlag; New York: 1989. pp. 213–28. [Google Scholar]
- Smayda TJ. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. & Oceanogr. 1997;42:1137–53. [Google Scholar]
- Smetacek V. A watery arms race. Nature. 2003;411:745. doi: 10.1038/35081210. [DOI] [PubMed] [Google Scholar]
- Smil V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food. The MIT Press; Cambridge, Massachusetts: 2001. [Google Scholar]
- Steidinger KA, Wolny JL, Haywood AJ. Identification of Kareniaceae (Dinophyceae) in the Gulf of Mexico. In: Medlin LK, Doucette GJ, Villac MC, editors. Phytoplankton evolution, taxonomy and ecology. Vol. 133. Nova Hedwigia; 2008. pp. 269–84. [Google Scholar]
- Stock CA, McGillicuddy DJ, Solow AR, Anderson DM. Evaluating hypotheses for the initiation and development of Alexandrium fundyense blooms in the western Gulf of Maine using a coupled physical-biological model. 2005:2715–44. See Ref. 15. [Google Scholar]
- Strong AL, Cullen JJ, Chisholm SW. Ocean fertilization: science, policy, and commerce. Oceanogr. 2009;22:236–60. [Google Scholar]
- Stucken K, John U, Cembella A, Murillo AA, Soto-Liebe K, et al. The smallest known genomes of multicellular and toxic cyanobacteria: comparison, minimal gene sets for linked traits and the evolutionary implications. PLoS ONE. 2010;5(2):e9235, 15. doi: 10.1371/journal.pone.0009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf RP, Tomlinson MC, Calkins JA, Kirkpatrick B, Fisher K, et al. Skill assessment for an operational algal bloom forecast system. J Mar Sys. 2009;76:151–61. doi: 10.1016/j.jmarsys.2008.05.016. ISSN 0924–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Kokubo T, Fukuyo Y, Matsuoka K. Quantitative relationship among vegetative cells, planozygotes, and hypnozygotes of Alexandrium catenella (Dinophyceae) in its blooming season at Tanabe Bay, Central Japan. 7th Int’l. Conf. on Toxic Phytoplankton. Sendai, Japan (Abstr.) 1995 [Google Scholar]
- Taylor FJR, Pollingher U. Ecology of dinoflagellates. In: Taylor FJR, editor. The biology of dinoflagellates. Blackwell Scientific Publications; Oxford: 1987. pp. 398–501. (Botanical Monograph 21). [Google Scholar]
- Teegarden GJ, Cembella AD. Grazing of toxic dinoflagellates Alexandrium spp. by adult copepods of coastal Maine: Implications for the fate of paralytic shellfish toxins in marine food webs. J Exp Mar Biol Ecol. 1996;196:145–76. [Google Scholar]
- Thomas WH, Gibson CH. Effects of small-scale turbulence on microalgae. J Appl Phycol. 1990;2:71–77. [Google Scholar]
- Tillmann U. Interactions between planktonic microalgae and protozoan grazers. J Phycol. 2003;39(S1):56. doi: 10.1111/j.1550-7408.2004.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Tillmann U, Alpermann T, John U, Cembella A. Allelochemical interactions and short-term effects of the dinoflagellate Alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae. 2008;7(1):52–64. [Google Scholar]
- Tong M, Kulis DM, Smith JL, Fux E, Hess P, et al. The effects of growth stage and light intensity on the toxicity of Dinophysis acuminata in the northeast U.S. Harmful Algae. 2011;10(3):254–64. [Google Scholar]
- Tor Nielsen T, Jakobsen HH, Hansen PJ. High resilience of two coastal plankton communities to twenty-first century seawater acidification: Evidence from microcosm studies. Mar Biol Res. 2010;6:542–55. [Google Scholar]
- Turner JT. Harmful algae interactions with marine planktonic grazers. 2006:259–270. See Ref. 52. [Google Scholar]
- Turner JT, Graneli E. Top-down predation control on marine harmful algae. 2006:355–366. See Ref. 52. [Google Scholar]
- Uribe P, Fuentes D, Valdés J, Shmrayahu A, Zúñiga A, et al. Preparation and analysis of an expressed sequence tag library from the toxic dinoflagellate Alexandrium catenella. Mar Biotechnol. 2008;10:692–700. doi: 10.1007/s10126-008-9107-8. [DOI] [PubMed] [Google Scholar]
- Velo-Suarez L, Reguera B, Gonzales-Gil S, Lunven M, Lazure P, et al. Application of a 3D Lagrangian model to explain the decline of a Dinophysis acuminata bloom in the Bay of Biscay. J Mar Sys. 2010;83:242–52. [Google Scholar]
- Walsh JJ, Kirkpatrick G, editors. Ecology and Oceanography of Harmful Algal Blooms in Florida. Cont. Shelf Res. 2008;28(1):1–214. [Google Scholar]
- Woo SPS, Liu W, Au DWT, Anderson DM, Wu RSS. Antioxidant responses and lipid peroxidation in gills and erythrocytes of fish (Rhabdosarga sarba) upon exposure to Chattonella marina and hyydrogen peroxide: Implications on the cause of fish kills. J Exp Mar Biol & Ecol. 2006;336:230–41. [Google Scholar]
- Woods J, Barkmann W. The plankton multiplier—positive feedback in the greenhouse. J Plankton Res. 1993;15:1053–74. [Google Scholar]
- Work TM, Barr B, Beale AM, Fritz L, Quilliam MA, Wright JLC. Epidemiology of domoic acid poisoning in brown pelicans (Pelecanus occidentalis) and Brandt’s cormorants (Phalacrocorax penicillatus) in California. J. Zoo Wildlife Medicine. 1993;24:54–62. [Google Scholar]
- Yang I, John U, Beszteri S, Gloeckner G, Krock B, et al. Comparative gene expression in toxic versus non-toxic strains of the marine dinoflagellate Alexandrium minutum. BMC Genomics. 2010;11:248. doi: 10.1186/1471-2164-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Truman P, Boucher M, Keyzers RA, Northcote P, Jordan TW. The algal metabolite yessotoxin affects heterogeneous nuclear ribonucleoproteins in HepG2 cells. Proteomics. 2009;9:2529–42. doi: 10.1002/pmic.200800725. [DOI] [PubMed] [Google Scholar]
- Zhou M-J, Shen Z-L, Yu R-C. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont Shelf Res. 2008;28:1483–89. [Google Scholar]


