Significance
Visual adaptation aftereffects have been termed “the psychologist’s microelectrode” for the insight they provide into the neural bases of human vision. Less research has explored such aftereffects in touch. We exploit the distinction in vision between “low-level” aftereffects, showing location- and orientation-specificity characteristics of the early retinotopic cortex, and “high-level” aftereffects, showing high generality to these characteristics, and presumably arising from higher processing stages. We demonstrate that distance aftereffects in passive touch share numerous characteristics with low-level visual aftereffects, including orientation and region specificity, lack of transfer contralaterally or across palm and dorsum, and encoding in skin-based space. These results provide experimental evidence that tactile distance is a basic somatosensory feature, computed at relatively early somatosensory processing stages.
Keywords: touch, tactile distance, adaptation, aftereffects, somatosensory processing
Abstract
The stage at which processing of tactile distance occurs is still debated. We addressed this issue by implementing an adaptation-aftereffect paradigm with passive touch. We demonstrated the presence of a strong aftereffect, induced by the simultaneous presentation of pairs of tactile stimuli. After adaptation to two different distances, one on each hand, participants systematically perceived a subsequent stimulus delivered to the hand adapted to the smaller distance as being larger. We further investigated the nature of the aftereffects, demonstrating that they are orientation- and skin-region–specific, occur even when just one hand is adapted, do not transfer either contralaterally or across the palm and dorsum, and are defined in a skin-centered, rather than an external, reference frame. These characteristics of tactile distance aftereffects are similar to those of low-level visual aftereffects, supporting the idea that distance perception arises at early stages of tactile processing.
The perception of tactile distance has been widely used for exploring somatosensory organization (1, 2). However, the level of somatosensory processing at which tactile distance is computed is unclear. Classic results of Weber showed that the distance between two touches is perceived as larger on more sensitive skin surfaces (1). Moreover, perceived tactile distance is larger for stimuli oriented across the width of the limbs than along the length of the limb (2, 3), mirroring anisotropies in the shape of tactile receptive fields (RFs) (4). These results suggest that encoding of distance may reflect lower-level asymmetries in factors such as peripheral receptor density, cortical magnification, and RF geometry. In contrast, some researchers have claimed that computing tactile distance requires that sensory signals be integrated and referred to higher-order representations of body size and shape (5, 6). This view is supported by evidence that tactile distance perception is modulated by illusions of altered body size (7–9) and tool use (10, 11). Together, these results suggest that perceived tactile distance is shaped by both lower-level aspects of somatosensory organization and higher-level mental body representations. Herein, we investigate the level of somatosensory processing at which tactile distances are calculated by probing the properties of tactile distance adaptation aftereffects.
Adaptation aftereffects have been widely used, especially in vision, because they provide information about how different stimulus dimensions are processed by populations of selective neurons (12). Some aftereffects, such as those for motion (13), direction (14), and tilt (15), show high selectivity for stimulus characteristics, such as orientation or retinal location (i.e., the adapting and test stimuli must have the same location and orientation). This finding suggests that these aftereffects arise from relatively low-level, retinotopic visual areas. In contrast, other aftereffects generalize across orientation, retinal location, and stimulus size, suggesting that they result from higher levels of processing, beyond the retinotopic visual cortex. These aftereffects include those for object squishiness (16) or shape (17), and those for facial identity (18), expression (19), or attractiveness (20). This distinction between lower-level and higher-level aftereffects thus provides an experimental probe to investigate whether a stimulus characteristic results from relatively earlier or later stages of perceptual processing.
Many tactile dimensions are also susceptible to adaptation, from basic attributes, such as frequency (21) and amplitude (22), to more complex properties, such as curvature (23) and size (24), and even the perceived extent of passive motion (25). Basic tactile properties, such as pressure, frequency, and location (21, 22, 26), have typically been studied using passive cutaneous stimulation. In contrast, curvature and size have predominantly been studied using dynamic, haptic paradigms, which involve proprioceptive and kinesthetic information, in addition to touch. For example, in a well-known haptic size-contrast illusion, participants repeatedly grasp spheres of different size in each hand. An aftereffect is experienced when spheres of identical size are held: the sphere placed in the hand previously adapted to the smaller sphere is perceived as larger than the other, and vice-versa (24).
Here, we explore how adaptation to a distance between two separate points, passively delivered on the hand dorsum, affects perception of subsequent distances, a tactile analog of the classic visual size aftereffects described by Blakemore et al. (27, 28). We report clear tactile distance aftereffects with passive touch (i.e., independent of proprioceptive and kinesthetic information). Critically, these aftereffects share several characteristics with lower-level visual aftereffects, including orientation and region specificity, lack of transfer—both contralaterally or across palm and dorsum—and encoding in skin-based space. These results suggest that tactile distance is a basic somatosensory feature, computed at relatively early stages of somatosensory processing.
Results
Exp. 1: Adaptation to Tactile Distances Induces Tactile Distance Aftereffects.
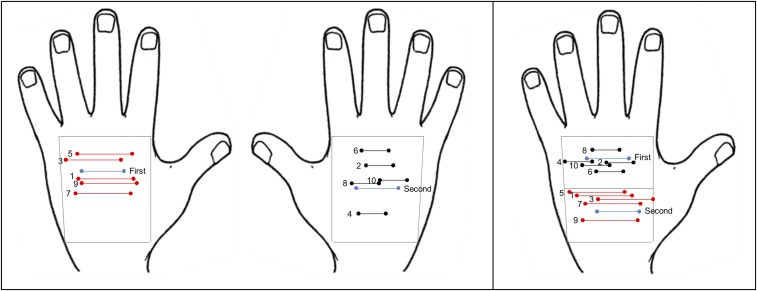
In Exp. 1 we examined if adaptation to two simultaneous touches a certain distance apart produces aftereffects on the perception of subsequent tactile distances. Stimuli were pairs of pointed wooden rods mounted in foam board and separated by 2, 3, or 4 cm, as in previous studies (3, 29). Each trial included an adaptation phase and a test phase. During adaptation, blindfolded participants were touched, alternately, on the dorsum of each hand, with two different stimuli, for 10 s, along the mediolateral hand axis (across hand width) (Fig. 1A and SI Materials and Methods). Across the adaptation period, the adapting stimuli were presented along the whole length and width of the participant’s hand, so that stimulation was never applied systematically to the exact same two points on the skin (Fig. S1). This should produce spatial summation of adaptation across the range of skin stimulated (30, 31). We varied the location of the adapting stimuli across presentations within each hand to ensure that we were inducing adaptation to the abstract property of distance (i.e., to a spatial relation between two tactile events), rather than adapting two exact locations on the skin. In one adaptation condition, the right hand (RH) was adapted to a 2-cm distance stimulus and the left hand (LH) to a 4-cm distance. This pattern was reversed in the other condition. After adaptation, two test stimuli were applied sequentially, one to each hand, from five possible pairs (RH/LH: 2/4, 2/3, 3/3, 3/2, and 4/2 cm) and participants made unspeeded judgments of whether the first or second stimulus felt larger (i.e., orthogonal to the RH/LH dimension).
Fig. 1.
Bimanual adaptation procedure and results. (A) During adaptation, blindfolded participants were touched in alternation on the dorsum of each hand with a different stimulus (either 2 or 4 cm) for 10 s (∼1 s each stimulus). In one condition, the 2-cm stimulus was presented on the right hand and the 4-cm stimulus on the left hand (RH < LH); in the other condition the opposite occurred (RH > LH). Stimuli during adaptation were oriented across the width of the hand (as illustrated in the figure) in Exps. 1 and 3, and along the length of the hand in Exp. 2. After adaptation, two stimuli were applied in sequence, one to each hand, from five possible pairs (2/4, 2/3, 3/3, 3/2, and 4/2 cm) and participants made unspeeded judgments of whether the first or second stimulus felt larger. Tactile distance aftereffects were found when adaptor and test stimuli were presented in the same orientation, (B) across the width (Exp. 1), and (C) along the length of the hand (Exp. 2), (D) but not when orientation varied across adaptation and test stimuli (Exp. 3). Curves are cumulative Gaussian functions. Error bars represent the SEM. Vertical lines represent PSEs.
Fig. S1.
(Left) Area of the hands where the adaptors and the test stimuli were presented (gray quadrilateral). This area was approximately delimited by the metacarpophalangeal joints, the wrist and the lateral sides of the hand. The adapting stimuli (red dipoles: 4-cm stimulus; black dipoles: 2-cm stimulus) were delivered in different random locations across this area, providing a minimum of 10 touches, 5 for each hand (numbers exemplify random stimulation during the adapting condition of a single trial). Following the adaptation phase, the test stimuli (3 cm on both hands in the example; light blue dipoles) were delivered in succession for 1s each. (Right) Area of the LH where the adaptors and the test stimuli were presented in Exp. 6 (gray quadrilateral). The dorsum area of the hand was arbitrarily subdivided in proximal and distal subregions. In this example, the 4- and 2-cm stimuli were delivered to proximal and distal sections, respectively.
Responses were then expressed as the proportion of RH stimuli perceived larger and modeled as a function of the ratio between the two stimuli (RH/LH) using cumulative Gaussian functions. The critical question concerns the point of subjective equality (PSE). An adaptation aftereffect should lead to a bias to judge distances as smaller on the hand that was adapted to the large distance. That is, we expected PSEs to be larger than 1 when the RH was adapted to the larger stimulus and the LH to the smaller one (indicating that distances at test presented on the RH were perceived as smaller than those on the LH) (SI Materials and Methods), and vice-versa in the opposite condition. All participants were naïve as to the purpose of the experiment, were paid or given course credits for their participation, and gave written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Department of Psychological Sciences Ethics Committee at Birkbeck, University of London.
Results are shown in Fig. 1B. There were clear opposite aftereffects, as shown by a significant difference in PSEs between conditions (t10 = 8.11, P < 0.0001, dz = 2.44). After being adapted to a small distance on the RH and a large distance on the LH, participants perceived the test stimulus on the RH as larger (mean PSE = 0.87; SD = 0.14). The opposite occurred after adaptation to a large distance on the RH and a small distance on the LH (mean PSE = 1.22; SD = 0.15). This effect was observed in all participants.
Exp. 2: Aftereffects also Occur in the Proximodistal Orientation.
Exp. 2 aimed to replicate this effect and to demonstrate that it was not specific to the mediolateral hand axis. Procedures were identical to Exp. 1 except that stimuli were rotated 90° to be aligned with the proximodistal hand axis (along hand length) (SI Materials and Methods). Results are shown in Fig. 1C. There were clear aftereffects, as demonstrated by a significant difference in PSEs (t10 = 5.27, P < 0.001, dz = 1.59). The mean PSE was 0.80 (SD = 0.16) in the RH small/LH large condition, and 1.13 (SD = 0.23) in the other condition. This effect was observed in 10 out of 11 participants.
Exp. 3: Aftereffects Are Orientation-Specific.
Many visual aftereffects are strongly orientation-specific, including those affecting perceived size (32). In contrast, aftereffects for facial identity (18) and attractiveness (20) occur when test stimuli are in a different orientation to the adapter, suggesting that face aftereffects take place at higher-level processing stages. In Exp. 3, we assessed the orientation specificity of tactile distance aftereffects by presenting adaptors oriented across the width of the hand and test stimuli oriented along the length of the hand (SI Materials and Methods). If, like face aftereffects, the aftereffects we have described arise from higher-level mechanisms in the somatosensory system, then adaptation should generalize across orientations, leading to different PSEs in the two adaptation conditions. If, in contrast, tactile distance aftereffects arise from lower-level mechanisms, we would expect no transfer between orientations.
There was no evidence for aftereffects, as shown in Fig. 1D (t11 = 0.30, P = 0.77, dz = 0.09). The mean PSE was 0.95 (SD = 0.19) in the RH small/LH large condition, and 0.97 (SD = 0.23) in the opposite condition.
An ANOVA between Exps. 2 and 3, which differed only in the orientation of the adapting stimuli, showed a significant main effect of adaptation condition (F1, 21 = 13.10, P = 0.002, ηp2 = 0.38) and an interaction between condition and experiment (F1, 21 = 10.02, P = 0.005, ηp2 = 0.32).
Exp. 4: Aftereffects Require Sustained Adaptation.
In addition to adaptation aftereffects, tactile perception can be influenced by preceding stimuli in a variety of ways. For example, memory for tactile forms drawn on the skin is impaired when stimuli are presented in rapid sequence, presumably because of aftersensations resulting from retention of tactile information in iconic memory (33). Similarly, preceding tactile stimuli can result in masking of subsequent stimuli (34) and misperception of location, as in the various “saltation” illusions (35). If the effects we have described reflect true adaptation aftereffects, they should only emerge following a sustained adaptation period. If, in contrast, they result from aftersensations of previous tactile stimuli stored in iconic (36) or working memory (37), they may emerge following presentation of a single prior stimulus.
In Exp. 4, we therefore used a procedure similar to Exp. 1 except that only a single adapting stimulus, lasting approximately 1 s, was applied to each hand on each trial. To avoid the possibility of progressive build-up of adaptation across repeated presentations of the same type, the two adaptation conditions were randomly ordered within blocks.
There was no evidence for aftereffects, as shown in Fig. S2 (t11 = 0.61, P = 0.55, dz = 0.18). The mean PSE for the two conditions was 0.97 (SD = 0.10) in the RH small/LH large condition, and 1.00 (SD = 0.14) in the opposite condition. An ANOVA between Exps. 1 and 4, which differed in the duration of the adaptation, showed a significant main effect of adaptation condition (F1, 21 = 38.80, p < 0.0001, ηp2 = 0.65), which critically was modulated by an interaction between condition and experiment (F1, 21 = 28.34, P < 0.0001, ηp2 = 0.57).
Fig. S2.
Results of Exps. 1 and 4. (A, Left) In Exp. 1, clear opposite aftereffects were found after a long adaptation phase. The dorsum of each hand was adapted with a different stimulus, either 2 or 4 cm, for 10 s, approximately (∼1 s each stimulus), in two different adaptation conditions. (Right) In Exp. 4, no aftereffects were found after a brief adaptation phase. The dorsum of each hand was adapted with a different stimulus, either 2 or 4 cm, only for 1 s each, and the two adaptation conditions were randomly ordered within blocks. (B) Mean PSEs for each condition of adaptation in the two experiments. (C) The difference in PSEs across the two adapting conditions in presented for each experiment. Error bars represent the SEM.
Exp. 5: Aftereffects Occur When Only One Hand Is Adapted.
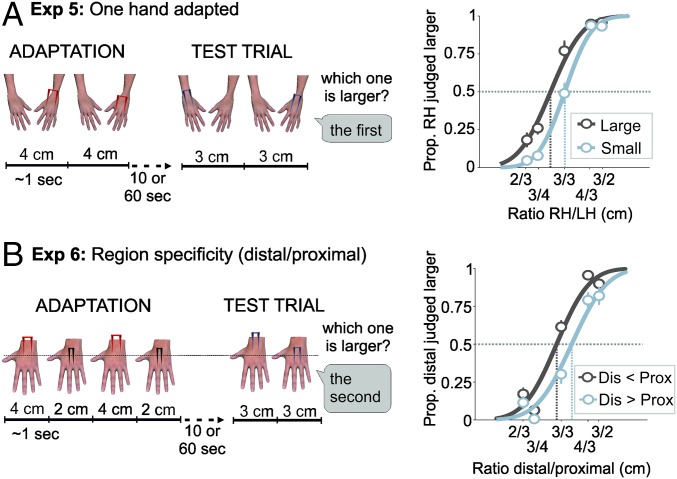
In the preceding experiments, adaptation involved a relative contrast in size between the two hands. In Exp. 5, we investigated whether aftereffects also occur in the absence of such contrast, following adaptation to a single distance. Methods were similar to Exp. 1, except that adapting stimuli (2 or 4 cm) were applied only to the LH (Fig. 2A, Left, and SI Materials and Methods). An aftereffect should lead, again, to different PSEs in the two adaptation conditions.
Fig. 2.
Procedure and results of Exps. 5 and 6. (A, Left) In the unimanual adaptation procedure of Exp. 5, only the LH dorsum was adapted in two separate conditions with either a large (4 cm) or a small (2 cm) across stimulus. In the test phase, two stimuli were delivered in alternation to each hand from five possible pairs (2/3, 3/4, 3/3, 4/3, and 3/2 cm). (Right) Clear aftereffects were found. (B, Left) Stimuli in Exp. 6 were delivered to the LH on two different regions: distal and proximal (Fig. S1). In one condition, the 2- and the 4-cm stimuli were delivered, respectively, to the distal and proximal surfaces of the LH dorsum (Dis < Prox, as illustrated in the figure); in the other condition, the opposite occurred (Dis > Prox). In the test phase, two stimuli were delivered in succession, one to the distal and one to the proximal part of the hand. (Right) Again, clear aftereffects were found.
Results are shown in Fig. 2A, Right. Clear aftereffects were observed, as shown by a significant difference in PSEs (t11 = 7.79, P < 0.0001, dz = 2.25). The mean PSE was 0.86 (SD = 0.10) following adaptation to a 4-cm stimulus, and 1.00 (SD = 0.08) following adaptation to a 2-cm stimulus. This effect was observed in all participants.
Exp. 6: Aftereffects Are Region-Specific.
In vision, lower-level aftereffects, such as direction, motion, and tilt, occur only when the adapting and test stimuli are at similar retinal locations (13–15). Conversely, higher-level aftereffects related to other properties, such as squishiness (16), or to faces (18), generalize across locations. In our previous experiments, we varied the location of the adapting stimuli across presentations within each hand to ensure that we were inducing adaptation to the abstract property of distance, rather than adapting two exact locations on the skin. That we nevertheless observed aftereffects implies some degree of spatial summation of adaptation across the range of skin stimulated (30, 31). However, because we have measured aftereffects by comparing the two hands, there must also be some level of skin specificity, because adaptation clearly produced different effects on each hand.
In Exp. 6 we investigated the level of location specificity of the effect by applying different adaptation to two adjacent regions of a single skin surface. Methods were similar to Exp. 1, except that, instead of being applied to the two hands, we divided the LH dorsum into proximal and distal halves, and applied different adapting stimuli (2 or 4 cm) to each region (Fig. 2B, Left). Each particular adaptor and test stimulus could fall at any point inside each region, so that stimulation was never applied systematically to the exact same two points (Fig. S1). As in Exp. 1, we aimed to induce spatial summation of adaptation to a particular distance across a continuous skin region (30, 31), although this time separately for the two stimulated regions, given that each was adapted to a different distance. If adaptation is spatially specific to the stimulated skin region, we would expect spatial summation of adaptation to occur within the local region of skin that had been adapted to a specific distance. That is, we would expect participants to perceive as larger the test stimulus applied on the region previously adapted to the smaller distance, compared with the other region adapted, and vice versa. In contrast, if the effect of adaptation arises in later areas whose organization does not preserve the somatotopic arrangement of the early somatosensory system, then the effects of adaptation of one region would generalize to the other. This should produce no differences across the two conditions of adaptation.
Results are shown in Fig. 2B, Right. There were clear aftereffects, as shown by a significant difference in PSE between conditions (t10 = 8.61, P < 0.0001, dz = 2.60). The mean PSE was 0.95 (SD = 0.08) in the distal small/proximal large adaptor, and 1.13 (SD = 0.09) in the opposite condition. This effect was observed in all participants.
Exp. 7: Aftereffects Do Not Transfer Contralaterally.
The presence of intermanual transfer for a specific aftereffect provides insight about the involvement of neurons in the somatosensory cortex with bilateral, rather than unilateral, RFs. For example, curvature aftereffects with dynamic finger exploration exhibit complete bilateral transfer between fingers of the two hands, and partial transfer is found for static finger adaptation (23). Conversely, no intermanual transfer at all is apparent for static stimulation on the hand (38). These results suggest that curvature information obtained dynamically is represented at a high level of sensory processing, whereas curvature information obtained statically is predominantly processed at a level that is connected to a single hand (23). In addition, lack of intermanual transfer has been demonstrated for size aftereffects through dynamic finger exploration of bars (39). Bilateral RF neurons process areas of the skin of homologous body parts (40), and are mostly found in structures beyond the primary somatosensory cortex (SI), in particular the secondary somatosensory cortex (SII) (41), although they also exist in SI (40). Exp. 7 investigated whether aftereffects transfer across hands by adapting one hand to a single distance and testing the effect of adaptation in the contralateral hand.
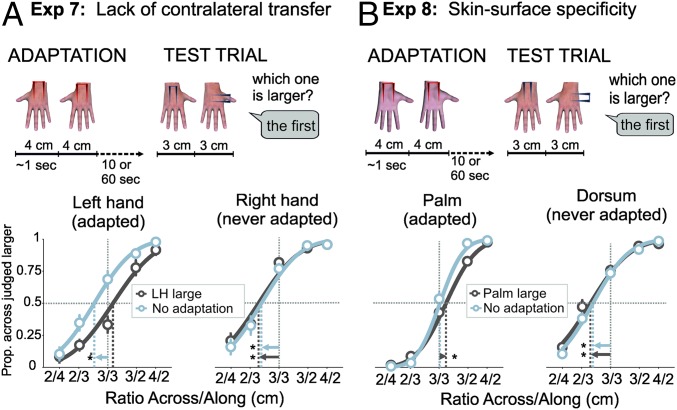
In the preceding experiments, our measure of adaptation involved a comparison between stimuli applied to the two hands. This experiment, conversely, required the test stimuli to be presented to the same hand, to isolate any effect of adaptation from the adapted to the nonadapted hand. To allow the presentation of the comparison stimuli on a single hand, we took advantage of the orientation specificity of the aftereffects (shown in Exp. 3), and used an established anisotropy in tactile distance perception on the hand dorsum (3, 29, 42), in which distances across the width of the hand are perceived as substantially larger than distances oriented along the length of the hand. We adapted the LH to a large adaptor (4 cm) in the across orientation. After adaptation, participants were touched twice on the dorsum of their hand (either the adapted LH or the nonadapted RH), once with the stimuli oriented along, and once oriented across the hand (Fig. 3A, Upper, and SI Materials and Methods), and responded which stimulus, the first or the second, was perceived as larger (i.e., orthogonal to the across/along dimension). To replicate the presence of the anisotropy, we also included a no-adaptation baseline condition.
Fig. 3.
Procedure and results of Exps. 7 and 8. (A, Upper) In Exp. 7 only the LH was adapted with a large (4 cm) across stimulus on the dorsum (LH large). In the no-adaptation condition, the test stimuli were presented without preceding adaptation. At test, participants were touched twice on the dorsum of their hand, once along and once across the hand, either on the nonadapted RH (as illustrated in the figure) or on the adapted LH. (Lower) Across distances were consistently perceived as larger than along ones (anisotropy effect) on the RH (Right) after adaptation to the LH (or no adaptation at all). Anisotropy was eliminated on the LH (Left) after preceding adaptation on that hand to a large across stimulus. (B, Upper) In Exp. 8, adaptation was on the left palm and test stimuli were either on the nonadapted left dorsum (as illustrated in the figure) or on the adapted left palm. (Lower) Anisotropy was always observed on the dorsum (which was never adapted). Anisotropy is known to be absent or largely reduced on the palm, and this was observed at baseline. Anisotropy was reversed for the adapting condition, indicating an adaptation aftereffect. Asterisks denote significant one-sample t test against 1.
In the baseline condition, participants should perceive across distances as larger than along distances, producing PSEs smaller than 1 (3). Adaptation to a large across-distance, however, should produce a bias to judge across distances as smaller on the adapted LH, reducing or eliminating the baseline anisotropy. The critical question is whether the presence of the adapting stimulus on the LH also modulates the anisotropy on the unadapted RH.
Results are shown in Fig. 3A, Lower. Clear anisotropies (i.e., PSEs smaller than 1) were apparent in the no-adaptation condition, for both the LH (mean PSE = 0.81; SD = 0.16, t test against 1: t11 = −3.55, P = 0.005, d = 1.51), and the RH (mean PSE = 0.76, SD = 0.17, t test against 1: t11 = −3.57, P = 0.004, d = 1.52), replicating the bias to perceive tactile distances as larger, when running across as opposed to along the hand. In the adaptation condition, this anisotropy was eliminated for test stimuli on the adapted LH (mean PSE = 1.10, SD = 0.23; t test against 1: t11 = 1.27, P = 0.23, d = 0.54), and significantly differed from the no-adaptation condition (t11 = 3.54, P = 0.005, dz = 1.02). Critically, after adaptation on the LH no effect was observed on the unadapted RH, as shown by a clear anisotropy (mean PSE = 0.73, SD = 0.14; t test against 1: t11 = −5.35, P = 0.0002, d = 2.28), which did not differ from the no-adaptation condition (t11 = 0.43, P = 0.68, dz = 0.12).
These effects were confirmed by an ANOVA, which revealed main effects of adaptation condition (F1, 11 = 8.18; P = 0.02; ηp2 = 0.43) and hand (F1, 11 = 7.18; P = 0.02; ηp2 = 0.40), with both effects driven by a significant interaction (F1, 11 = 8.70; P = 0.01; ηp2 = 0.44). Overall, the results of this study fail to show any evidence of bilateral transfer of tactile distance aftereffects.
Exp. 8: Aftereffects Are Skin-Surface–Specific.
The exact overlap between representations of the dorsal and palmar skin surfaces in the human somatosensory cortex is unknown. However, inputs from the dorsal and glabrous surfaces of the fingers are closely overlapping in the primate SI (43). Similarly, the dorsum and palm representations of the hand, although with less overlap, are closely represented in the monkey’s primary sensory cortex (43). Thus, depending on the actual overlap in humans, transfer of adaptation across analogous skin areas of the hand dorsum and palm might be possible.
The methods of Exp. 8 were similar to those of Exp. 7, except that adaptation (4 cm) was always applied to the palm of the LH, and test stimuli were applied to either the palm (congruent condition) or dorsum of the LH (incongruent condition) (Fig. 3B, Upper, and SI Materials and Methods). We also included a no-adaptation baseline condition for each skin surface. The critical question is whether the adapting stimulus on the palm modulates the anisotropy both on the palm and on the dorsum. Baseline anisotropy occurs mostly on the dorsum, but it is absent or largely reduced on the palm of the hand (3, 29, 42). Thus, adapting to a large across stimulus on the palm, should produce a bias at test to judge across distances as smaller than they are, producing an anisotropy in the opposite direction for the palm (across stimuli perceived as smaller). Furthermore, if transfer of adaptation across skin sites occurs, then we should expect a reduction or elimination of the anisotropy on the dorsum.
Results are shown in Fig. 3B, Lower. In the no-adaptation conditions anisotropy was, as expected, observed on the dorsum (mean PSE = 0.79, SD = 0.17, t test against 1: t18 = −5.58, P < 0.0001, d = 1.86) but not the palm (mean PSE = 1.01, SD = 0.12, t test against 1: t18 = 0.27, P = 0.79, d = 0.09). In the congruent condition, adaptation produced a reverse anisotropy (mean PSE = 1.11, SD = 0.14; t test against 1: t18 = 3.21, P = 0.005, d = 1.07), which differed significantly from the no-adaptation condition (t18 = 3.67, P = 0.002, dz = 0.84), indicating the presence of aftereffects. Critically, adaptation on the palm did not influence responses at test on the dorsum, as we found a clear anisotropy in the incongruent condition (mean PSE = 0.75, SD = 0.14; t test against 1: t18 = −7.27, P < 0.0001, d = 2.42), which did not differ from the no-adaptation anisotropy (t18 = 1.39, P = 0.18, dz = 0.32).
These effects were confirmed by an ANOVA showing a main effect of congruency (F1, 18 = 55.18; P < 0.0001; ηp2 = 0.75) and a clear interaction between adaptation condition and congruency (F1, 18 = 13.16; P = 0.002; ηp2 = 0.42). These results show that the orientation specificity of tactile distance aftereffects is confined to the specific region adapted, and does not transfer across the two sides of the hand, thus reinforcing the region specificity found in Exp. 6. The results also show that distance aftereffects can be induced over other regions of the body, beyond the hand dorsum.
Exp. 9: Aftereffects Are in the Hands, Not External Space.
In different contexts, we perceive the location of touch in either a skin-centered frame of reference (e.g., “I feel the fly land on my forearm”) or in an external frame of reference (e.g., “I feel the light switch off to the left”). Studies have shown that stimuli are rapidly transformed from skin-centered to external coordinates, within 180 ms of stimulus onset (44). In Exp. 9 we investigated whether the orientation specificity we described in Exp. 3 is defined in a skin-centered or in an external, more abstract reference frame (such as horizontal or vertical, in external space). Methods were identical to Exp. 7, except that test stimuli were always applied to the adapted left hand, which rested in the same position as during adaptation (canonical position) or rotated 90°. In the rotated condition, across and along stimuli were therefore reversed in external space compared with the canonical condition (Fig. S3, Upper, and SI Materials and Methods and SI Results). As in Exp. 7, we included both an adaptation (4 cm, across orientation), and a no-adaptation baseline condition. If tactile distance aftereffects are defined in a skin-centered reference frame, adaptation to a large across distance should counteract the standard anisotropy in the two postures. If, however, distance aftereffects are defined in an external frame of reference, the anisotropy should be reduced or eliminated in the canonical, but not the rotated condition.
Fig. S3.
Procedure and results of Exp. 9. (Upper) In Exp. 9, a large (4 cm) adapting stimulus was presented on the left hand dorsum. After adaptation, the LH was rotated 90° (as illustrated in the figure), or placed in the same posture as during adaptation. (Lower) Anisotropy was eliminated after adaptation to a large stimulus in the across orientation, regardless of whether the hand was rotated before test. Asterisks denote significant one-sample t tests against 1.
The anisotropy was eliminated in both postures (Fig. S3, Lower) (canonical posture: mean PSE = 0.96, SD = 0.21, t test against 1: t9 = −0.86, P = 0.42, d = 0.40; rotated posture: mean PSE = 0.99, SD = 0.31, t test against 1: t9 = −0.53, P = 0.61, d = 0.25). Importantly, the anisotropy differed from the no-adaptation conditions, both in the canonical (t9 = 3.84, P = 0.004, dz = 1.21; mean PSE = 0.80, SD = 0.15, t test against 1: t9 = −3.74, P = 0.005, d = 1.76) and rotated postures (t9 = 4.81, P = 0.001, dz = 1.52; mean PSE = 0.76, SD = 0.21, t test against 1: t9 = −2.99, P = 0.015, d = 1.41).
These effects were confirmed by an ANOVA with a main effect of adaptation (F1, 9 = 27.20; P = 0.0006; ηp2 = 0.75), but no main effect of posture (F1, 9 = 0.65; P = 0.44; ηp2 = 0.07), and no interaction (F1, 9 = 2.29; P = 0.16; ηp2 = 0.20). These results show that the orientation specificity of tactile distance aftereffects is defined in a skin-centered, rather than an external, frame of reference.
SI Materials and Methods
Participants.
One-hundred and four healthy volunteers participated in the nine experiments. According to a poor model fit criteria (R2 < 0.5 in at least one condition), data from six participants (one each in Exps. 1, 2, 6, and 8, and two in Exp. 9) were excluded from analyses. Fit to the remaining data were excellent with a mean across experiments of 0.94, SD = 0.08. The remaining sample was as follows: Exp. 1 (mean 29.9 y; SD = 6.6; n = 11; 4 female), Exp. 2 (mean 26.0 y; SD = 5.9; n = 11; 4 female), Exp. 3 (mean 26.9 y; SD = 6.5; n = 12; 8 female), Exp. 4 (mean 27.3 y; SD = 9.5; n = 12; 7 female), Exp. 5 (mean 24.5 y; SD = 5.9; n = 12; 9 female), Exp. 6 (mean 32.5 y; SD = 7.9; n = 11; 5 female), Exp. 7 (mean 32.7 y; SD = 9.2; n = 12; 8 female), Exp. 8 (mean 28.9 y; SD = 8.1; n = 19; 11 female), and Exp. 9 (mean 24.4 y; SD = 4.3; n = 10; 5 female). All but two participants were right-handed, as assessed by the Edinburgh Inventory (60), with a mean across experiments of 82.68 (SD = 35.68), and reported no abnormalities in tactile perception. All participants were naïve as to the purpose of the experiment, were paid or given course credits for their participation, and gave written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Department of Psychological Sciences Ethics Committee at Birkbeck, University of London.
Materials and General Procedure.
Stimuli were pairs of pointed wooden rods mounted on foamboard and separated by 2, 3, or 4 cm, similar to those used in previous studies (3, 29). The tip of each post was rounded off. Stimuli were delivered manually by the experimenter. Participants were blindfolded and sat with their palms resting on the table, and their hands with the digits oriented toward the experimenter, who sat in front of them. Participants were asked to keep their hands still, and the position was monitored by the experimenter. On each trial participants were exposed to an adaptation phase and a test phase.
Exp. 1: Basic aftereffects, across orientation.
Participants were exposed to an adaptation phase and a test phase on each trial, with the two rods of each stimulus oriented across the width of the hand. In one condition of adaptation, participants were touched, alternatingly, on the LH and RH dorsum, with the 2- and 4-cm stimuli, respectively. In the other condition, the reverse pattern was presented. The duration of each touch was ∼1 s, and was delivered alternating between the RH and the LH for 10 s, resulting in a minimum of 10 touches, 5 on each hand. Longer periods of adaptation (60 s, approximately) were delivered on the first trial of each block, to de-adapt from possible residual adaptation from the previous adaptation condition and adapt to the new condition, and after breaks (every 20 trials), to reinforce the adaptation when adaptation loss could have occurred, given to the possibility for participants to move their hands. These adapting times (60 s for the initial trial of each block and after breaks, and 10-s top-up before each test trial) were chosen based on previous studies (24, 27, 45).
Across the adaptation period, the adapting stimuli were presented along the whole length and width of the participant’s hand, so that stimulation was never applied systematically to the exact same two points on the skin (Fig. S1). Applying touches across different locations served to induce adaptation to the spatial relation between two tactile events and to avoid sensitization or pain from repeated stimulation of the same skin locations.
Following adaptation, two stimuli (in the across orientation) were delivered in succession, one to each hand, from a pool of five possible pairs (ratio RH/LH stimuli: 2/4, 2/3, 3/3, 3/2, and 4/2 cm). Immediately after the second touch, participants were asked to make untimed, two-alternative forced-choice verbal judgments of whether the first or the second stimulus was perceived as bigger. This response mode, orthogonal to the tested dimension (i.e., RH–LH), reduces the likelihood that any biases observed are because of response bias, rather than perceptual bias (3). The order of the pairs—and which stimulus was applied first—were randomized within blocks. Each pair of test stimuli was delivered 16 times for each condition of adaptation (8 times applying first the RH stimulus, 8 times the LH one), and the experiment was divided into four blocks of 40 trials (ABBA; counterbalanced starting condition).
Stimulation in both phases was accomplished manually by the experimenter. To minimize the intrinsic variability of a manual stimulation, the experimenter paced the adaptors presentation approximately every 1 s (self-paced, counting), and was instructed to try to impress pressure on each touch as constantly as possible, to avoid wavering, and to make sure that both the rods of the stimulus touched the skin simultaneously. If any error occurred in any given trial (e.g., nonsimultaneous presentation of the two rods of a test stimulus, or perceived changes in pressure across the two rods), this was repeated at the end of the block.
Exp. 2: Basic aftereffects, along orientation.
Methods in Exp. 2 were similar to Exp. 1, except that the two rods of both the adapting and the test stimuli were oriented along the length of the hand.
Exp. 3: Orientation specificity.
Methods in Exp. 3 were similar to Exp. 1, except that the orientations of the adapting and test stimuli were orthogonal to each other. Adapting stimuli were presented in the across orientation and test stimuli in the along orientation.
Exp. 4: Brief adaptation.
Methods in Exp. 4 were similar to Exp. 1, except that the duration of the adaptation phase was 2 s (i.e., one touch on each hand) rather than 10 s. The two adaptation conditions were randomly ordered within blocks to avoid the possibility of progressive build-up of adaptation across repeated presentations of the same type.
Exp. 5: One hand adapted.
Methods in Exp. 5 were similar to Exp. 1, except that only the LH was adapted to a stimulus in the across orientation (either 4-cm or 2-cm stimulus, in two separate conditions), whereas the right hand was never adapted. In the test phase, two stimuli were delivered in succession, one to each hand, from a pool of five possible pairs (ratio RH/LH stimuli: 2/3, 3/4, 3/3, 4/3, and 3/2 cm).
Exp. 6: Region specificity.
Methods in Exp. 6 were similar to Exp. 1, except that stimuli were delivered to the LH (across orientation) on two different regions: on the distal and on the proximal part of the hand. These two regions were identified and marked by the experimenter, who drew a line from the adjacent surfaces of the thumb and index metacarpals to the lateral part of the dorsum of the hand; the distal surface was delimited by the line and the metacarpophalangeal joints, and the proximal hand skin surface was delimited by that same line and the wrist (Fig. S1, Right). In one condition, the 2- and the 4-cm stimuli were delivered respectively to the distal and to the proximal surfaces of the LH; in the other condition, the opposite occurred. In the test phase, two stimuli were delivered in succession, one to the distal and one to the proximal part of the hand, from a pool of five possible pairs (ratio distal/proximal stimuli: 2/3, 3/4, 3/3, 4/3, and 3/2 cm). The design and the number of trials were identical to that of Exp. 1.
Exp. 7: Bilateral transfer.
In Exp. 7, participants were exposed to one condition of adaptation only: 4-cm across stimulus on the LH. In a second condition, no adaptation occurred before the presentation of the test stimuli (baseline condition). The five different test pairs of tactile stimuli (ratio across/along stimuli: 2/4, 2/3, 3/3, 3/2, and 4/2 cm) were applied in succession, one in the across and one in the along orientation. Each pair of test stimuli was delivered in separate trials either to the LH or RH. Each pair was delivered 12 times for each condition of adaptation (adaptation and no-adaptation), in four blocks of 60 trials each (ABBA design), for a total of 240 trials. The factor hand (right vs. left) was randomized across trials.
Exp. 8: Skin-surface specificity.
Methods in Exp. 8 were similar to Exp. 7, with the exception that stimulation could be on the palm or on the dorsum of the LH. During adaptation, the LH rested palm up. In one condition of adaptation, participants were adapted to the 4-cm across stimulus; in the baseline condition, no adaptation occurred. In the test phase, two test stimuli were delivered to the LH, one in the across orientation, and one in the along, and both were presented either on the palm, or on the dorsum (which was never adapted). In the dorsum test trials, after the adaptation phase, and before the delivery of the test stimuli, participants were asked to rotate the LH palm down, and lean it on the table. At the end of the trial, after the response was given, participants were asked to bring the hand back to the palm up position. In the palm test trials, participants were asked to first rotate the LH palm down, bring it back to the palm up position, and then lean it on the table. We chose this procedure to balance the amount of movement required in the dorsum and palm conditions. The factor hand-side (palm, dorsum) was randomized across trials. The design and number of trials are identical to that of Exp. 7.
Exp. 9: Skin-centered reference frame.
Methods in Exp. 9 were similar to Exp. 7, with the exception that in the test phase the LH could be oriented in the canonical position (as during adaptation), or rotated 90° to the right (digits toward the midsagittal plane). In the rotated trials, after the adaptation phase and before the delivery of the test stimuli, participants were asked to rotate the left hand 90° to the right and lean it on the table. At the end of the trial, participants were asked to bring the hand back to the canonical position. In the canonical trials, participants were asked to first rotate the left hand 90° to the right and bring it back to the canonical position, and then lean it on the table. The factor hand-posture (canonical, rotated) was randomized across trials. The design and number of trials are identical to that of Exp. 7.
Analyses.
The proportion of trials in which the test stimulus delivered to the RH (distal part or across stimuli) was judged as having the two rods farther apart was analyzed as a function of the ratio of the length of the RH and LH stimuli (the distal and proximal stimuli or the across and along stimuli), for each condition. Note that participants responded to the order of the stimulus that was felt as having the two rods farther apart (i.e., the first or the second), and responses were therefore orthogonal to the tested dimensions (e.g., right–left hand, across-along).
Cumulative Gaussian functions for each condition of adaptation were fitted to each participant’s data using maximum likelihood (Palamedes toolbox, MATLAB) (61). PSEs were determined as the point at which the psychometric function crossed 50%. We also computed interquartile ranges as a measure of the slope of the psychometric function (i.e., the distance between where the psychometric function crossed 25% and 75%). For statistical analysis, the ratios were transformed logarithmically, to produce a symmetrical distribution around the point of actual equality (x = 1). In Exps. 1–6, PSE values smaller than 1 indicated a tendency to perceive a distance delivered on the right hand (or the distal part in Exp. 6) as smaller than those delivered on the left hand (or the proximal part), whereas PSE values larger than 1 indicated the opposite. In Exps. 7–9, PSE values smaller than 1 indicated a tendency to perceive a distance delivered across as being smaller than those delivered along the hand, whereas PSE values larger than 1 indicated the opposite.
The PSEs of the two adaptation conditions were compared against each other using two-tailed, paired-sample t tests. In experiments where more than one factor was tested, a repeated-measures ANOVA was performed. In addition to these analyses, in Exps. 7–9, PSEs were also compared with value 1, to confirm anisotropy effects. For all t tests, two-tailed P values were used, with a level of significance set at α = 0.05. To quantify the magnitude of the effects we report, we provide partial η2 values for F-tests, Cohen’s d for one-sample t test, and dz for paired t tests (62).
SI Results
Exp. 4: Brief Adaptation.
In Exp. 4, we tested whether the aftereffects observed required a sustained adaptation period or could emerge following the presentation of a single prior stimulus on each hand. We used a procedure similar to Exp. 1, except that only a single adapting stimulus, lasting approximately 1 s, was applied to each hand on each trial.
We found no evidence for aftereffects, as shown in Fig. S2A, Right (t11 = 0.61, P = 0.55, dz = 0.18). An ANOVA between Exps. 1 (Fig. S2A, Left) and 4, which differed in the duration of the adaptation, showed a significant main effect of adaptation condition (F1, 21 = 38.80, P < 0.0001, ηp2 = 0.65), which was modulated by an interaction between condition and experiment (F1, 21 = 28.34, P < 0.0001, ηp2 = 0.57).
Exp. 9: Skin-Centered Reference Frame.
The results from Exp. 9 are shown in Fig. S3.
Discussion
Our results demonstrate definite tactile distance aftereffects with passive touch. After prolonged adaptation to a tactile distance, participants perceive subsequent smaller distances as being smaller than they actually are, and distances that are larger as being even wider than they are. Natural haptic experiences with objects involve the integration of several classes of signals, from cutaneous mechanoreceptors, proprioceptive afferents, and kinesthetic information. Previous studies of adaptation aftereffects for tactile size perception have used continuous tactile surfaces, usually with dynamic touch [e.g., haptic size (24) and curvature aftereffects (23)]. To get an estimate of the length of a given object in these previous studies, the brain might compute the extent of continuous tactile contact on the skin, their pattern, or other properties of the object, such as shape and edges. Aside from these, proprioceptive and kinesthetic information would also provide information about object size and shape. In this study, we limited information to passive cutaneous signals to focus on a basic form of tactile size perception, which concerns exclusively the computation of the distance between two distinct points touching the skin. Our results thus provide clear evidence that the spatial relationship between two tactile events is a tactile attribute susceptible to sensory adaptation.
Several aspects of our results suggest that the observed aftereffects arise from relatively early stages of somatosensory processing. First, the effects are orientation-specific; no aftereffect is found for test stimuli which are rotated compared with the adapting stimulus. Second, the effects are region-specific; adapting to one skin region produces aftereffects only on that specific region. Third, the effects are skin-surface–specific; no aftereffect is found if the test stimulus is on the dorsum when the adapting stimulus had been on the palm. Fourth, the effects show no contralateral transfer; no aftereffects are found for test stimuli on the contralateral hand. Finally, the effects occur in skin-space, rather than in external space, suggesting that the phenomenon is localized in the hand itself, rather than at a higher level phenomenal space (45). Together, these results suggest that tactile distance is computed at a relatively early stage of somatosensory processing.
The existence of these basic aftereffects with passive touch and their characteristics might indicate the presence of neurons tuned to specific ranges of distance at relatively early stages of somatosensory processing, akin to neurons with spatial frequency tuning in the visual cortex (46). As in vision, tactile RFs are hierarchically defined within the somatosensory pathway (40). Neurons in SI have relatively small RFs and show orientation-tuning (47, 48), comparable to neurons in V1 (49). Neurons in SII have larger, even bilateral RFs (40), and receive inputs directly from SI (50–52), which suggests that SII may underlie integration of information from multiple skin locations and putatively be sensitive to a gap between two simultaneous touches. This finding is supported by evidence showing that many SII neurons respond to stimuli administered on several finger pads (53), and are tuned for particular stimulus orientations across different pads (47, 54). SII neurons, thus, have the potential both to integrate information from different skin locations and to represent larger-scale spatial features of tactile stimuli (55). Indeed, it has been suggested that SII might be the first representation of a tactile field underpinning the spatial organization of tactile events (55). The properties of these neurons might provide the basis for the adaptation aftereffects we report.
Alternatively, the reported aftereffects could result from rapid modulation of the geometry of tactile RFs in the somatosensory cortex. Perceived tactile distance varies systematically across skin surfaces and these effects might correspond to properties of tactile RFs (2, 3). The underlying idea is that perceived tactile distance involves a process of counting the number of RF widths crossed by a stimulus (3). Because RFs are smaller on more sensitive skin surfaces, a given tactile distance will cross more RFs on a sensitive than a less-sensitive skin surface, and thus will be perceived as larger. Analogously, because RFs on the limbs are oval-shaped, elongated along the longitudinal limb axis (4, 56), tactile distances oriented along the mediolateral axis will cross more RFs than those oriented along the proximodistal axis, and thus will be perceived as larger. On the palm, where RFs are circular shaped (57), distance anisotropies depending on the orientation should be absent or largely reduced. In the present study, we induced a reduction of this anisotropy on the hand dorsum (Exps. 7 and 9) and induced an inverted anisotropy on the palm (Exp. 8), by adapting participants to a distance delivered across the hand. Adaptation to a distance along a specific axis may, therefore, selectively modulate that dimension of RFs, resulting in modulation of the anisotropy in RF geometry, and a corresponding change in perceptual anisotropy. Adaptation to a large across stimulus could have increased the size of SI RFs in the mediolateral axis, so that a subsequent stimulus would be perceived as smaller than it actually is. The selectivity of the adaptation to one RF axis is supported by the orientation specificity of the aftereffect (Exp. 3). Rapid modulation of RF size has been reported in contexts such as the visual enhancement of touch (58). Furthermore, it has been proposed that enhancement of touch from viewing the body during a tactile distance judgment improves discrimination performance by sharpening RFs organization in the SI, by either intracortical or thalamocortical inhibition (58, 59). This hypothesis is further supported by evidence demonstrating that changes in intracortical inhibition can affect the shape of SI RFs, even along a single axis (4).
Our study demonstrates that something as abstract as the distance between two distinct and isolated tactile events is a property of somatosensation susceptible to adaptation. Furthermore, the characteristics of the aftereffects provide strong evidence in favor of the idea that computation of tactile distance arises at early stages of somatosensory processing, before sensory signals are referred to higher-order representations of body size and shape.
Acknowledgments
This research was supported in part by European Research Council Grant ERC-2013-StG-336050 under the FP7 (to M.R.L.). G.V. was supported in part by a Fondo di Ateneo grant from the University of Milano-Bicocca, which fulfils in part E.C.’s doctoral research program, and by a Ricerca Corrente grant from the Istituto di Ricovero e Cura a Carattere Scientifico Istituto Auxologico Italiano, Milan, Italy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614979114/-/DCSupplemental.
References
- 1.Weber EH. De subtilitate tactus. In: Ross HE, Murray DJ, editors. E. H. Weber on the Tactile Senses. 2nd Ed. Academic; London: 1996. pp. 21–128. [Google Scholar]
- 2.Green BG. The perception of distance and location for dual tactile pressures. Percept Psychophys. 1982;31:315–323. doi: 10.3758/bf03202654. [DOI] [PubMed] [Google Scholar]
- 3.Longo MR, Haggard P. Weber’s illusion and body shape: Anisotropy of tactile size perception on the hand. J Exp Psychol Hum Percept Perform. 2011;37:720–726. doi: 10.1037/a0021921. [DOI] [PubMed] [Google Scholar]
- 4.Alloway KD, Rosenthal P, Burton H. Quantitative measurements of receptive field changes during antagonism of GABAergic transmission in primary somatosensory cortex of cats. Exp Brain Res. 1989;78:514–532. doi: 10.1007/BF00230239. [DOI] [PubMed] [Google Scholar]
- 5.Longo MR, Azañón E, Haggard P. More than skin deep: Body representation beyond primary somatosensory cortex. Neuropsychologia. 2010;48:655–668. doi: 10.1016/j.neuropsychologia.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Medina J, Coslett HB. From maps to form to space: Touch and the body schema. Neuropsychologia. 2010;48:645–654. doi: 10.1016/j.neuropsychologia.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor-Clarke M, Jacobsen P, Haggard P. Keeping the world a constant size: Object constancy in human touch. Nat Neurosci. 2004;7:219–220. doi: 10.1038/nn1199. [DOI] [PubMed] [Google Scholar]
- 8.de Vignemont F, Ehrsson HH, Haggard P. Bodily illusions modulate tactile perception. Curr Biol. 2005;15:1286–1290. doi: 10.1016/j.cub.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 9.Tajadura-Jiménez A, et al. Action sounds recalibrate perceived tactile distance. Curr Biol. 2012;22:R516–R517. doi: 10.1016/j.cub.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Canzoneri E, et al. Tool-use reshapes the boundaries of body and peripersonal space representations. Exp Brain Res. 2013;228:25–42. doi: 10.1007/s00221-013-3532-2. [DOI] [PubMed] [Google Scholar]
- 11.Miller LE, Longo MR, Saygin AP. Tool morphology constrains the effects of tool use on body representations. J Exp Psychol Hum Percept Perform. 2014;40:2143–2153. doi: 10.1037/a0037777. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SG, Kohn A. Moving sensory adaptation beyond suppressive effects in single neurons. Curr Biol. 2014;24:R1012–R1022. doi: 10.1016/j.cub.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapen T, Rolfs M, Cavanagh P. The reference frame of the motion aftereffect is retinotopic. J Vis. 2009;9:1–7. doi: 10.1167/9.5.16. [DOI] [PubMed] [Google Scholar]
- 14.Wenderoth P, Wiese M. Retinotopic encoding of the direction aftereffect. Vision Res. 2008;48:1949–1954. doi: 10.1016/j.visres.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Knapen T, Rolfs M, Wexler M, Cavanagh P. The reference frame of the tilt aftereffect. J Vis. 2010;10:1–13. doi: 10.1167/10.1.8. [DOI] [PubMed] [Google Scholar]
- 16.Arnold DH, Petrie K, Gallagher R, Yarrow K. An object-centered aftereffect of a latent material property: A squishiness visual aftereffect, not causality adaptation. J Vis. 2015;15:4. doi: 10.1167/15.9.4. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S. Attention-dependent brief adaptation to contour orientation: A high-level aftereffect for convexity? Vision Res. 2001;41:3883–3902. doi: 10.1016/s0042-6989(01)00249-8. [DOI] [PubMed] [Google Scholar]
- 18.Benton CP, Jennings SJ, Chatting DJ. Viewpoint dependence in adaptation to facial identity. Vision Res. 2006;46:3313–3325. doi: 10.1016/j.visres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Butler A, Oruc I, Fox CJ, Barton JJS. Factors contributing to the adaptation aftereffects of facial expression. Brain Res. 2008;1191:116–126. doi: 10.1016/j.brainres.2007.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodes G, Jeffery L, Watson TL, Clifford CWG, Nakayama K. Fitting the mind to the world: Face adaptation and attractiveness aftereffects. Psychol Sci. 2003;14:558–566. doi: 10.1046/j.0956-7976.2003.psci_1465.x. [DOI] [PubMed] [Google Scholar]
- 21.Tommerdahl M, et al. Human vibrotactile frequency discriminative capacity after adaptation to 25 Hz or 200 Hz stimulation. Brain Res. 2005;1057:1–9. doi: 10.1016/j.brainres.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Delemos KA, Hollins M. Adaptation-induced enhancement of vibrotactile amplitude discrimination: The role of adapting frequency. J Acoust Soc Am. 1996;99:508–516. doi: 10.1121/1.414509. [DOI] [PubMed] [Google Scholar]
- 23.van der Horst BJ, Willebrands WP, Kappers AML. Transfer of the curvature aftereffect in dynamic touch. Neuropsychologia. 2008;46:2966–2972. doi: 10.1016/j.neuropsychologia.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Uznadze D. The Psychology of Set. Consultants Bureau; New York: 1966. [Google Scholar]
- 25.Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC. Perception of movement extent depends on the extent of previous movements. Exp Brain Res. 2009;195:167–172. doi: 10.1007/s00221-009-1780-y. [DOI] [PubMed] [Google Scholar]
- 26.Tannan V, Whitsel BL, Tommerdahl MA. Vibrotactile adaptation enhances spatial localization. Brain Res. 2006;1102:109–116. doi: 10.1016/j.brainres.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 27.Blakemore C, Sutton P. Size adaptation: A new aftereffect. Science. 1969;166:245–247. doi: 10.1126/science.166.3902.245. [DOI] [PubMed] [Google Scholar]
- 28.Blakemore C, Campbell FW. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969;203:237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Cornu, Knight F, Longo MR, Bremner AJ. Categorical perception of tactile distance. Cognition. 2014;131:254–262. doi: 10.1016/j.cognition.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Gescheider GA, Güçlü B, Sexton JL, Karalunas S, Fontana A. Spatial summation in the tactile sensory system: Probability summation and neural integration. Somatosens Mot Res. 2005;22:255–268. doi: 10.1080/08990220500420236. [DOI] [PubMed] [Google Scholar]
- 31.Reid E, Harvie D, Miegel R, Spence C, Moseley GL. Spatial summation of pain in humans investigated using transcutaneous electrical stimulation. J Pain. 2015;16:11–18. doi: 10.1016/j.jpain.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Blakemore C, Nachmias J. The orientation specificity of two visual after-effects. J Physiol. 1971;213:157–174. doi: 10.1113/jphysiol.1971.sp009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heller MA. Tactile retention: Reading with the skin. Percept Psychophys. 1980;27:125–130. [Google Scholar]
- 34.Craig JC, Evans PM. Vibrotactile masking and the persistence of tactual features. Percept Psychophys. 1987;42:309–317. doi: 10.3758/bf03203085. [DOI] [PubMed] [Google Scholar]
- 35.Geldard FA, Sherrick CE. The cutaneous “rabbit”: A perceptual illusion. Science. 1972;178:178–179. doi: 10.1126/science.178.4057.178. [DOI] [PubMed] [Google Scholar]
- 36.Gallace A, Tan HZ, Haggard P, Spence C. Short term memory for tactile stimuli. Brain Res. 2008;1190:132–142. doi: 10.1016/j.brainres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Gilson EQ, Baddeley AD. Tactile short-term memory. Q J Exp Psychol. 1969;21:180–184. doi: 10.1080/14640746908400211. [DOI] [PubMed] [Google Scholar]
- 38.Vogels IM, Kappers AM, Koenderink JJ. Investigation into the origin of the haptic aftereffect of curved surfaces. Perception. 1997;26:101–117. doi: 10.1068/p260101. [DOI] [PubMed] [Google Scholar]
- 39.Walker JT, Shea KS. A tactual size aftereffect contingent on hand position. J Exp Psychol. 1974;103:668–674. doi: 10.1037/h0037136. [DOI] [PubMed] [Google Scholar]
- 40.Iwamura Y. Bilateral receptive field neurons and callosal connections in the somatosensory cortex. Philos Trans R Soc Lond B Biol Sci. 2000;355:267–273. doi: 10.1098/rstb.2000.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eickhoff SB, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30:6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longo MR, Ghosh A, Yahya T. Bilateral symmetry of distortions of tactile size perception. Perception. 2015;44:1251–1262. doi: 10.1177/0301006615594949. [DOI] [PubMed] [Google Scholar]
- 43.Pons TP, Wall JT, Garraghty PE, Cusick CG, Kaas JH. Consistent features of the representation of the hand in area 3b of macaque monkeys. Somatosens Res. 1987;4:309–331. doi: 10.3109/07367228709144612. [DOI] [PubMed] [Google Scholar]
- 44.Azañón E, Soto-Faraco S. Changing reference frames during the encoding of tactile events. Curr Biol. 2008;18:1044–1049. doi: 10.1016/j.cub.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 45.Cameron P, Wertheimer M. Kinesthetic aftereffects are in the hands, not in phenomenal space. Percept Mot Skills. 1965;20:1131–1132. doi: 10.2466/pms.1965.20.3c.1131. [DOI] [PubMed] [Google Scholar]
- 46.Henriksson L, Nurminen L, Hyvärinen A, Vanni S. Spatial frequency tuning in human retinotopic visual areas. J Vis. 2008;8:1–13. doi: 10.1167/8.10.5. [DOI] [PubMed] [Google Scholar]
- 47.Hsiao SS, Lane J, Fitzgerald P. Representation of orientation in the somatosensory system. Behav Brain Res. 2002;135:93–103. doi: 10.1016/s0166-4328(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 48.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 49.Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc London Ser B, Biol Sci. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- 50.Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- 51.Friedman DP, Jones EG, Burton H. Representation pattern in the second somatic sensory area of the monkey cerebral cortex. J Comp Neurol. 1980;192:21–41. doi: 10.1002/cne.901920103. [DOI] [PubMed] [Google Scholar]
- 52.Pons TP, Garraghty PE, Friedman DP, Mishkin M. Physiological evidence for serial processing in somatosensory cortex. Science. 1987;237:417–420. doi: 10.1126/science.3603028. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field (RF) properties of the macaque second somatosensory cortex: RF size, shape, and somatotopic organization. J Neurosci. 2006;26:6485–6495. doi: 10.1523/JNEUROSCI.5061-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: Representation of orientation on different finger pads. J Neurosci. 2006;26:6473–6484. doi: 10.1523/JNEUROSCI.5057-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serino A, Giovagnoli G, de Vignemont F, Haggard P. Spatial organisation in passive tactile perception: Is there a tactile field? Acta Psychol (Amst) 2008;128:355–360. doi: 10.1016/j.actpsy.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Brown PB, Fuchs JL, Tapper DN. Parametric studies of dorsal horn neurons responding to tactile stimulation. J Neurophysiol. 1975;38:19–25. doi: 10.1152/jn.1975.38.1.19. [DOI] [PubMed] [Google Scholar]
- 57.Powell TPS, Mountcastle VB. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: A correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull Johns Hopkins Hosp. 1959;105:133–162. [PubMed] [Google Scholar]
- 58.Haggard P, Christakou A, Serino A. Viewing the body modulates tactile receptive fields. Exp Brain Res. 2007;180:187–193. doi: 10.1007/s00221-007-0971-7. [DOI] [PubMed] [Google Scholar]
- 59.Cardini F, Longo MR, Haggard P. Vision of the body modulates somatosensory intracortical inhibition. Cereb Cortex. 2011;21:2014–2022. doi: 10.1093/cercor/bhq267. [DOI] [PubMed] [Google Scholar]
- 60.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 61.Prins N, Kingdom FAA. 2009 Palamedes: MATLAB routines for analyzing psychophysical data. Available at www.palamedestoolbox.org.
- 62.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]