Abstract
Objective
Sleep problems have been linked to increased risk of mortality in the general population. Limited evidence suggests similar relationships among people diagnosed with cancer. The aims of the present study were to investigate the type and rates of sleep problems in advanced cancer patients and examine whether sleep problems are associated with survival.
Methods
A prospective study of 292 patients with advanced cancers affecting the hepatobiliary and pancreatic systems were administered a battery of questionnaires measuring sociodemographic information, sleep and depression. Descriptive statistics, ANOVA, Chi-square, Kaplan-Meier survival, and Cox regression analyses were performed to test the aims.
Results
The majority of patients were male (64%) and the mean age was 62 years (SD = 11). Fifty-nine percent of patients reported poor sleep quality; 43% reported sleeping ≤6 h and 2% ≥10 h; 40% reported sleep latency of 30 min or greater; 80% reported poor sleep efficiency. Of the 292 patients, 58% reported clinically levels of depression and depressive symptoms were related to shorter sleep duration (p = 0.02). After adjusting for factors known to contribute to survival, a curvilinear relationship was observed between sleep duration and mortality: short and long sleep duration were associated with increased mortality [linear term: hazard ratio (HR) = 0.485, 95% confidence interval (CI) = 0.275–0.857; quadratic term: HR = 1.064, 95% CI = 1.015–1.115].
Conclusions
Consistent with findings in the general population, a curvilinear relationship between sleep duration and mortality was observed in advanced cancer patients. The high prevalence of sleep problems and link with mortality warrants routine screening and development of evidence-based treatments for sleep problems in the oncology setting.
Keywords: Sleep, Survival Cancer, Sleep duration, Hepatobiliary, PSQI
Introduction
Sleep problems are prevalent in cancer patients, with 45–80% reporting poor sleep quality [1,2], compared to 29–32% in the general population [3,4]. Cancer patients report various types of sleep problems [5]. On average, cancer patients report sleep duration of 4.8–7 h per night with sleep latency of 21–55 min [1,2,6], while sleep duration in the general population is between 6.5 and 7.5 h per night, and sleep latency on average 15–20 min [2,4]. Cancer patients may underreport sleep problems; actigraphy data from a sample of lung cancer patients indicated that they slept, on average, 4.4 h per night, despite their self-reports averaging 5.8 h per night [2]. Palesh and colleagues reported that 80% of cancer patients believe that their sleep problems are caused by the treatments and 60% believe their symptoms are temporary [7]. However, sleep problems have been reported to persist long after cessation of treatment of the cancer [8]. Sleep problems are also associated with depression, anxiety, and decreased quality of life [9,10]. Because depression is associated with lower survival in cancer patients [11] it is important to account for depression when examining the effect of poor sleep on survival in people with cancer.
Short sleep duration and insomnia predict mortality in both the general population and in the chronically ill [6,12,13]. A meta-analysis of general population studies found that, in comparison to reference groups (7–8 h of sleep per night), people with short and long sleep duration have increased risk of mortality [13]. Although limited, the link between sleep problems and mortality has also begun to be examined in cancer patients. Øthus and colleagues examined in a cohort of head and neck squamous cell carcinoma patients and found that one item from a questionnaire measuring quality of life, sleep disturbances, predicted survival [14]. Palesh and colleagues observed in a sample of women with advanced breast cancer that poor sleep efficiency, measured with actigraphy, was significantly associated with increased mortality after adjusting for disease and treatment specific factors [6].
There exists little knowledge about the type and prevalence of sleep problems experienced by patients with advanced cancers, and whether specific sleep problems are related to survival. Therefore, the aims of the proposed study were to (1) describe the type and prevalence of sleep problems in patients with advanced cancer; (2) examine the relationship between sleep and depressive symptoms; and (3) test the link between sleep problems and mortality after adjusting for sociodemographic, disease-related, psychological, and sleep factors shown to be previously linked to survival.
Methods
Design and Participants
Patients from two prospective studies conducted at a large tertiary medical center were included in this secondary data analysis. The Center evaluates and treats patients with advanced cancers related to the hepatobiliary-pancreatic system. The recruitment periods for the two studies were January 2008 to June 2012 (K07CA118576) and November 2012 to October 2013 (R01CA176809-03). Inclusion criteria and exclusion criteria were the same for both studies: (1) biopsy or radiographic-proven diagnosis of cancer affecting the hepatobiliary or pancreatic system; (2) age 21 years or older; (3) fluent in English, and (4) no evidence of thought disorder, hallucinations, or delusions. For the purpose of these analyses, organ-transplant patients were excluded due to their significantly longer survival time.
Instruments
Sociodemographic, Disease, and Treatment Specific Factors
Sociodemographic data included patients’ age, gender, body mass index (BMI), race, ethnicity, educational level, occupation, income, and health insurance status, and was reported on a questionnaire designed specifically for these studies. Disease-specific and treatment related information was gathered from the patients’ electronic medical records including diagnosis, presence or absence of cirrhosis, maximum tumor size, number of lesions, vascularity of lesions, and vascular invasion. Survival was measured from the time of diagnosis of cancer until death. Death was determined by records in the electronic medical record or the Social Security Death Index.
Sleep
The Pittsburgh Sleep Quality Index (PSQI) is an 18-item self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval [15]. The PSQI is composed of seven component scores describing sleep problems including: sleep duration, sleep disturbances, sleep latency (≥30 min indicates poor latency), daytime dysfunction, sleep efficiency (time asleep divided by time spent in bed × 100; <85% indicates poor efficiency), subjective sleep quality, sleep medication usage. The sum of component scores yields a global score of sleep quality (higher scores indicate worse sleep quality; scores greater than 5 indicate poor sleep quality). Adequate levels of internal consistency, test-retest reliability, and validity have been reported for the PSQI in cancer patients [16].
Depressive Symptoms
The Center for Epidemiologic Studies—Depression (CES-D) is a 20-item self-report questionnaire designed to assess depressive symptoms. The patient responds on a four-point scale by reporting weekly frequency of depressive symptoms (“rarely,” “some days,” “occasionally,” “most days”). A score of 16 or greater represents depressive symptoms in the clinical range. The CES-D has demonstrated adequate construct validity and reliability in cancer patients [17].
Procedure
Both studies were approved by the University of Pittsburgh’s Institutional Review Board. Patients were referred to the study team by their attending physician. If the patient agreed to speak to a member of the study team, the risks and benefits of the study were explained to the individual and written informed consent was obtained from the patient prior to them completing the questionnaires.
Data Analysis
All data were entered, verified, and analyzed with SPSS version 21 (IBM Corp, Armonk, NY) and R version 2.15.2 (CRAN, http://cran.r-project.org/). Besides survival time, all analyses were performed on baseline data. Descriptive statistics were performed to obtain measures of central tendency, distribution, and proportions for each variable. Predictor variables for the survival analyses were: gender (male, female), age, race/ethnicity (Caucasian, Black/African American, Other), Education (4-year college degree: yes or no), BMI (continuous variable), presence of vascular invasion (yes or no), diagnosis (hepatocellular carcinoma and cholangiocarcinoma; neuroendocrine tumor; appendix, gallbladder, stomach, or pancreatic cancers; other primary tumor metastasized to the liver), CES-D score (continuous variable), snoring (not in the past month, less than once per week, one to two times per week, three or more times per week), sleep latency (continuous variable, derived from the PSQI question “how long (in minutes) has it usually taken you to fall asleep each night?”), sleep efficiency (continuous variable, time spent asleep divided by time spent in bed), sleep disturbances (categorical, PSQI component score), and sleep duration (continuous variable). Unadjusted Cox regressions were used to test relationships between continuous predictor variables and survival, and Kaplan—Meier (Breslow/Wilcoxon) survival analyses were used for categorical variables. A final multivariable Cox regression model was developed to examine the potential curvilinear relationship between sleep duration (linear and quadratic terms both included in the model) and survival after adjusting for demographics (age, gender, education), disease-specific factors (diagnosis, vascular invasion), depression (CES-D score), and snoring. To further inspect the curvilinear relationship, a natural cubic splines technique was used to plot the natural log of the hazard across sleep duration. This allowed for estimation of the hazard function across a continuous variable within a multivariable Cox regression model; the package smoothHR was used to perform these analyses in R and has been used in previous studies [18].
Results
Sociodemographic and Disease Specific Factors
A total of 292 participants with cancer affecting the hepatobiliary-pancreatic system were included in the study, [105 (36%) female, mean age 62 years, standard deviation (SD) = 11]. Ninety-one percent of the sample was Caucasian, 8% Black/African American, and 1% was from ‘other’ or mixed ethnic/racial backgrounds. Fifty-one percent of the sample had a primary diagnosis of hepatocellular carcinoma or cholangiocarcinoma, 11% had neuroendocrine tumors, 4% had cancer of the appendix, gallbladder, stomach, or pancreas, and 34% had other primary cancers (e.g., colorectal, breast, ovarian) which had metastasized to the liver. Vascular invasion was present in 10.5% of patients. Mean survival time was 15.2 months (SD = 13.3). See Table 1 for sociodemographic and disease specific characteristics.
Table 1.
Sample characteristics.
| Age (years) | |
| Mean (SD) | 61.9 (10.9) |
| Gender (N, %) | |
| Male | 186 (63.7) |
| Female | 106 (36.3) |
| Race (N, %) | |
| Caucasian | 260 (90.9) |
| Black/African American | 23 (8.0) |
| Other | 3 (1.0) |
| Education Level (N,%) | |
| High School graduate or less | 211 (74.8) |
| Four-year college degree or more | 71 (25.2) |
| Diagnosis (N, %) | |
| HCC or CCC | 151 (51.4) |
| NET | 32 (11.0) |
| Appendix/gallbladder/stomach/pan creatic | 12 (4.1) |
| Other primary cancers with liver metastases | 97 (33.6) |
| Vascular invasion (N, %) | |
| No | 257 (89.5) |
| Yes | 30 (10.5) |
CCC, cholangiocarcinoma; HCC, hepatocellular carcinoma; NET, neuroendocrine tumor.
Type and Prevalence of Sleep Problems in Advanced Cancer Patients
Patients averaged 6.5 h (SD = 1.6) of sleep per night; a mean of 29.9 min (SD = 38.3 min) to fall asleep, with 39.9% of patients reporting 30 min or greater; and average sleep efficiency was 79.9% (SD = 17.9), with 53% of patients reporting sleep efficiency of less than 85%. Twenty-seven percent of patients rated their sleep quality during the past month as “fairly poor” or “very poor,” and 56.7% reported nighttime or early morning awakenings three or more times per week. Means and standard deviations of the component scores of the PSQI can be found in Table 2. Global PSQI scores indicated that 58.9% of patients had poor sleep. No association was found between diagnosis and sleep duration [F(3,292) = 2.26, p = 0.08].
Table 2.
Pittsburgh Sleep Quality Index related scores.
| Mean (SD) | |
|---|---|
| Sleep duration (h) | 6.5 (1.6) |
| Sleep latency (min) | 29.9 (38.3) |
| Sleep efficiency (time asleep/time in bed) | 79.9 (17.9) |
| Global PSQI score | 7.6 (4.4) |
| PSQI component score (0–3)* | |
| Sleep duration | 0.9 (1.1) |
| Sleep disturbance | 1.6 (0.7) |
| Sleep latency | 1.3 (1.0) |
| Daytime dysfunction | 0.9 (0.7) |
| Sleep efficiency | 1.1 (1.2) |
| Subjective sleep quality | 1.2 (0.8) |
| Sleep medication | 0.7 (1.2) |
PSQI, Pittsburgh Sleep Quality Index.
Higher scores indicate more significant problems.
Sleep Problems and Depression in Advanced Cancer Patients
One-hundred and fifty-two (54.1%) patients reported depressive symptoms in the clinical range of the CES-D (total score ≥16). Patients with clinically significant depressive symptoms had shorter sleep duration (mean = 6.3 h/night, SD = 1.8) in comparison to patients who did not report depressive symptoms in the clinical range (mean = 6.8 h/night, SD = 1.5), [F(1,279) = 5.55, p = 0.019].
Univariable analyses between sociodemographics, disease-specific factors, depression, sleep problems and survival
Unadjusted survival analyses are displayed in Table 3 and Table 4. Age, BMI, sleep latency, sleep efficiency, sleep disturbances, gender, race, and educational level were not significantly related to survival. Cox regression revealed that depressive symptoms were significantly related to survival [hazard ratio (HR) = 1.038, 95% confidence interval (CI) = 1.014–1.061]. Kaplan—Meier survival analyses, using the Breslow/Wilcoxon test, showed that diagnosis (χ2 = 9.352, p = 0.025), vascular invasion (χ2 = 28.974, p<0.001), and snoring (χ2 = 8.808, p = 0.032) were also significantly associated with survival. In addition, sleep duration had a curvilinear relationship with survival (linear term: HR = 0.611, 95% CI = 0.347–0.997; quadratic term: HR = 1.042; 95% CI = 1.000–1.085).
Table 3.
Univariable Cox regression survival analyses.
| β | p | HR | 95% CI | |
|---|---|---|---|---|
| Age | 0.002 | 0.817 | 1.002 | (0.984–1.021) |
| BMI | −0.026 | 0.436 | 0.974 | (0.912–1.040) |
| CES-D | 0.037 | 0.001 | 1.038 | (1.014–1.061) |
| Sleep latency | −0.002 | 0.609 | 0.998 | (0.992–1.005) |
| Sleep efficiency | −0.001 | 0.911 | 0.999 | (0.988–1.011) |
| Sleep duration (curvilinear) | ||||
| Sleep duration: Linear term | −0.493 | 0.049 | 0.611 | (0.374–0.997) |
| Sleep duration: Quadratic term | 0.041 | 0.050 | 1.042 | (1.000–1.085) |
BMI, body mass index; CES-D, Center for Epidemiological Studies Depression Scale; CI, confidence interval; HR, hazard ratio.
Table 4.
Univariable Breslow/Wilcoxon tests.
| Chi-Square | p | |
|---|---|---|
| Gender | 1.609 | 0.205 |
| Race | 1.428 | 0.839 |
| Education | 2.427 | 0.119 |
| Diagnosis | 9.352 | 0.025 |
| Vascular invasion | 28.974 | <0.001 |
| Snoring | 8.808 | 0.032 |
Adjusted Model of Sleep Duration and Survival
Table 5 displays the results for the final Cox regression model. After adjusting for gender, age, education, diagnosis, vascular invasion, depression, and snoring, a significant curvilinear relationship was observed between sleep duration and survival. Hazard-ratios (HRs) for sleep duration and its quadratic term were 0.485 (95% CI = 0.275–0.857), and 1.064 (95% CI = 1.015–1.115), indicating that mortality hazard was higher for patients with short or long sleep duration.
Table 5.
Adjusted multivariable Cox regression analyses.
| β | p | HR | 95% CI | |
|---|---|---|---|---|
| Gender: female vs male | −0.495 | 0.058 | 0.609 | 0.366–1.016 |
| Age | −0.009 | 0.498 | 0.991 | 0.966–1.017 |
| Education: high school or less vs high school or greater | 0.622 | 0.063 | 1.863 | 0.966–3.592 |
| Diagnosis | ||||
| Other primary cancer with liver metastases (ref) | ||||
| HCC or CCC | −0.017 | 0.949 | 0.983 | 0.573–1.684 |
| NET | −1.917 | 0.065 | 0.147 | 0.019–1.123 |
| Appendix/gallbladder/stomach/pancreatic | 0.718 | 0.277 | 2.050 | 0.562–7.474 |
| Vascular invasion: present vs absent | 0.745 | 0.009 | 2.106 | 1.209–3.668 |
| CES-D | 0.035 | 0.011 | 1.035 | 1.008–1.063 |
| Snoring | ||||
| Not during the past month (reference) | ||||
| Less than 1 time/week | 0.144 | 0.619 | 1.155 | 0.655–2.035 |
| 1–2 times/week | −1.032 | 0.049 | 0.356 | 0.128–0.994 |
| 3+ times/week | −0.977 | 0.015 | 0.376 | 0.171–0.827 |
| Sleep duration: linear term | −0.723 | 0.013 | 0.485 | 0.275–0.857 |
| Sleep duration: quadratic term | 0.062 | 0.009 | 1.064 | 1.015–1.115 |
CCC, cholangiocarcinoma; CES-D, Center for Epidemiological Studies Depression Scale; HCC, hepatocellular carcinoma; NET, neuroendocrine tumor.
Figure 1 displays the natural log of the HR (ie, B coefficient in the regression model) across sleep duration. The model uses a natural cubic splines technique with a reference value of 6.5 h per night (the sample mean), adjusting for all covariates included in the final Cox regression model.
Fig. 1.
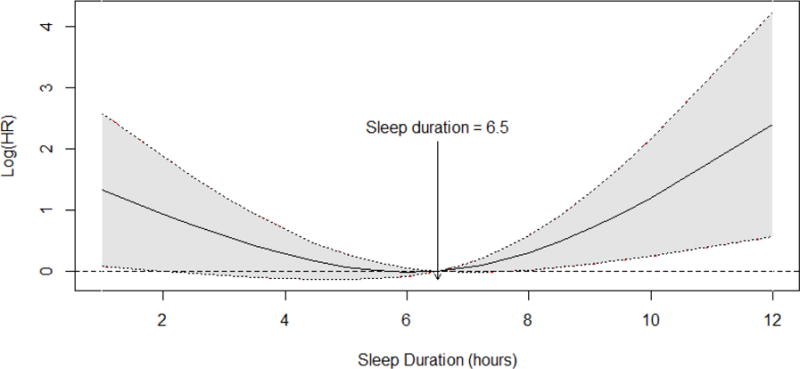
Smooth Log hazard ratio across sleep duration. The dotted lines represent the upper and lower bounds of the 95% confidence intervals
Discussion
Consistent with research in other cancer patient populations, we found that sleep problems were highly prevalent and that short sleep duration was associated with depression [1,2,5–8]. Furthermore, a significant relationship between sleep duration and survival, indicating that both short and long sleep duration were associated with increased mortality. This U-shaped relationship between sleep duration and mortality is well supported by research in the general population. However, this study is the first to identify such a relationship in cancer patients.
In comparison to other studies of sleep problems in cancer patients, our patients reported similar prevalence of sleep problems. Studies examining PSQI scores in cancer patients have found that between 45% and 80% report poor sleep quality [1,2]. The average sleep duration for our sample (6.5 h/night) was similar to estimates for the general population [3,4,12,13]. However, sleep efficiency for healthy individuals has been reported at 95% and sleep latency of 12 min [19], whereas our sample of advanced cancer patients reported 80% efficiency and sleep latency of 30 min.
Only two studies to date have examined a relationship between sleep problems and survival in cancer patients but this is the first to show that sleep duration is linked to mortality. Some researchers have questioned the validity of the U-shaped relationship between sleep and mortality. They suggest that the higher mortality may reflect death-imminent processes, or that it may not be related to any real long-term health risks [20,21]. Tamakoshi and Ohno attempted to remove the potential bias from death-imminent processes by removing subjects from the analyses who experience mortality within 2 years of the initial measurement of sleep duration [22]. They found that removing this group did not change the significant mortality hazard associated with of any indicator variables for sleep duration; however, for both analyses, the only sleep duration subgroups with significant associations with lower survival were long sleepers (8 h or more) and very short sleeping women (≤4 h). Ferrie and colleagues examined changes in sleep duration over a 5-to 6-year period on all-cause mortality and found that changes to sleeping shorter or longer than 7–8 h per night were linked to increased mortality [23].
One mechanism that may explain the link between sleep and survival is immune system dysregulation. Experimental sleep deprivation significantly reduces Natural Killer (NK) cell activity in healthy human subjects [24], and more recently, inflammation has been linked to cancer prognosis [25]. While immune system dysregulation may explain the association between short sleep duration and mortality, it is not clear whether the same biological mechanisms can explain the association between long sleep duration and mortality. Initial assumptions may be that patients with long sleep duration have lower survival because longer sleep duration may be associated with sleep fragmentation, which is associated with poorer health. Furthermore, prior studies have shown that sleep fragmentation is associated with larger, more aggressive tumors in animal models, but this has not been shown in humans [26].
Interestingly, we found that both sleep duration and depression independently predicted survival, warranting further research concerning the underlying biological mechanisms that may differentially link these factors to survival. Our study also found that snoring one to two times per week or three or more times per week was associated with lower risk of mortality; this was inconsistent with results from the general population, which have identified snoring as a mortality risk [27]. Self-reports of snoring were likely based on information patients had previously gathered from their spouse or intimate partner and therefore may be indicative of social support, a factor related to longer survival [28].
Limitations of this study include a heterogeneous sample, consisting of patients with different cancer types affecting the hepatobiliary-pancreatic system. However, we found no differences in the type or prevalence of sleep problems by diagnosis and the cancer diagnosis was also included in the adjusted survival analyses. In addition, sleep problems were self-reported. Although the PSQI is a valid and reliable measure of perceived sleep problems in both the general population and in cancer patients, patients may nonetheless report sleep duration estimates that differ from objective measures, such as actigraphy or polysomnography [2]. We also included only baseline measures of sleep and understanding the changes in sleep over time may provide a greater understanding of sleep across the continuum of cancer survivorship.
Future research should investigate whether similar relationships are found in patients with non-advanced cancers. In addition, the development of interventions is warranted to improve sleep and quality of life and to decrease psychological and physical morbidity in advanced cancer patients. Cognitive—behavioral interventions have been demonstrated to produce sustained improvements in sleep quality and duration over time without the potential adverse effects of medication [29]. If our findings are replicated, screening and treatment of sleep disorders in the oncology setting may be warranted to identify, and potentially mitigate a modifiable risk factor for mortality.
Supplementary Material
Highlights.
A high rate of sleep problems including short and long duration of sleep, poor sleep quality and efficiency were observed in advanced cancer patients
A curvilinear relationship between sleep and mortality was observed in advanced cancer patients
Sleep duration and depression independently predicted survival in advanced cancer patients
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
References
- 1.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. Journal of Pain and Symptom Management. 2002;24(5):471–80. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 2.Dean GE, Redeker NS, Wang YJ, et al. Sleep, mood, and quality of life in patients receiving treatment for lung cancer. Oncology Nursing Forum. 2013;40(5):441–51. doi: 10.1188/13.ONF.441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeitlhofer J, Schmeiser-rieder A, Tribl G, et al. Sleep and quality of life in the Austrian population. Acta Neurol Scand. 2000;102(4):249–57. doi: 10.1034/j.1600-0404.2000.102004249.x. [DOI] [PubMed] [Google Scholar]
- 4.Doi Y, Minowa M, Uchiyama M, Okawa M. Subjective sleep quality and sleep problems in the general Japanese adult population. Psychiatry Clin Neurosci. 2001;55(3):213–5. doi: 10.1046/j.1440-1819.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- 5.Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nature and Science of Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palesh O, Aldridge-gerry A, Zeitzer JM, et al. Actigraphy-Measured Sleep Disruption as a Predictor of Survival among Women with Advanced Breast Cancer. Sleep. 2014;37(5):837–42. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palesh O, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. Journal of Clinical Oncology. 2010;28(2):292–8. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savard J, Ivers H, Villa J, Caplette-gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. Journal of Clinical Oncology. 2011;29(26):3580–6. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 9.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues in Clinical Neuroscience. 2008;10(4):473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60(12):1364–71. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. Journal of Clinical Oncology. 2007;25(17):2397–405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- 12.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Archives of General Psychiatry. 2002;59(2):131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 13.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Østhus AA, Aarstad AK, Olofsson J, Aarstad HJ. Prediction of survival by pretreatment health-related quality-of-life scores in a prospective cohort of patients with head and neck squamous cell carcinoma. JAMA Otolaryngology Head and Neck Surgery. 2013;139(1):14–20. doi: 10.1001/jamaoto.2013.1056. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. Journal of Pain and Symptom Management. 2004;27(2):140–8. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46(5):437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 18.Meira-machado L, Cadarso-suárez C, Gude F, Araújo A. smoothHR: an R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med. 2013;2013:745742. doi: 10.1155/2013/745742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donoghue GM, Fox N, Heneghan C, Hurley DA. Objective and subjective assessment of sleep in chronic low back pain patients compared with healthy age and gender matched controls: a pilot study. BMC Musculoskeletal Disorders. 2009;10(1):122. doi: 10.1186/1471-2474-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamatakis KA, Punjabi NM. Long sleep duration: a risk to health or a marker of risk? Sleep Med Rev. 2007;11(5):337–9. doi: 10.1016/j.smrv.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bliwise DL, Young TB. The parable of parabola: what the U-shaped curve can and cannot tell us about sleep. Sleep. 2007;30(12):1614–5. doi: 10.1093/sleep/30.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27(1):51–4. [PubMed] [Google Scholar]
- 23.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30(12):1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosomatic Medicine. 1994;56(6):493–8. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Sharma R, Zucknick M, London R, Kacevska M, Liddle C, Clarke SJ. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clinical Colorectal Cancer. 2008;7(5):331–7. doi: 10.3816/CCC.2008.n.044. [DOI] [PubMed] [Google Scholar]
- 26.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Medicine Reviews. 2004;8(3):159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Rich J, Raviv A, Raviv N, Brietzke SE. An epidemiologic study of snoring and all-cause mortality. Otolaryngology Head and Neck Surgery. 2011;145(2):341–6. doi: 10.1177/0194599811402475. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258(21):3125–30. [PubMed] [Google Scholar]
- 29.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. Journal of Clinical Oncology. 2005;23(25):6083–96. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


