Abstract
Osteoblasts, the bone-forming cells of the remodeling unit, are essential for growth and maintenance of the skeleton. Clinical disorders of substrate availability (e.g., diabetes mellitus, anorexia nervosa, and aging) cause osteoblast dysfunction, ultimately leading to skeletal fragility and osteoporotic fractures. Conversely, anabolic treatments for osteoporosis enhance the work of the osteoblast by altering osteoblast metabolism. Emerging evidence supports glycolysis as the major metabolic pathway to meet ATP demand during osteoblast differentiation. Glut1 and Glut3 are the principal transporters of glucose in osteoblasts, although Glut4 has also been implicated. Wnt signaling induces osteoblast differentiation and activates glycolysis through mammalian target of rapamycin, whereas parathyroid hormone stimulates glycolysis through induction of insulin-like growth factor-I. Glutamine is an alternate fuel source for osteogenesis via the tricarboxylic acid cycle, and fatty acids can be metabolized to generate ATP via oxidative phosphorylation although temporal specificity has not been established. More studies with new model systems are needed to fully understand how the osteoblast utilizes fuel substrates in health and disease and how that impacts metabolic bone diseases.
Osteoblast differentiation is essential for bone formation and is dependent on metabolic pathways.
Essential Points
Metabolic programming is essential for adenosine triphosphate (ATP) generation during bone remodeling but is cell type and temporally specific
Oxidative phosphorylation and glycolysis are the two final common pathways for the generation of ATP needed to fuel osteoblast-mediated bone formation
Glucose is the preferred substrate for energy generation via glycolysis in both aerobic and anaerobic environments of the osteoblast and its progenitors
Wnt and insulin-like growth factor-1 signaling drive glycolysis during osteoblast differentiation
Fatty acids, citrate, intracellular proteins, glutamine, and lipids can be used to generate ATP for osteoblastic needs via the citric acid cycle, although much less is known about their utilization
Intermittent parathyroid hormone administration increases bone formation in part by enhancing glycolysis
Disorders associated with osteoporosis such as diabetes mellitus and anorexia nervosa are fundamentally diseases of substrate availability and hence are related to the metabolic flexibility of the osteoblast
Cellular metabolism has become a focus for basic investigations into the pathophysiology of chronic diseases. Importantly, novel therapeutic targets have emerged thanks to the rapid advances in our understanding of bioenergetics in cancer cells. Similarly, bone biologists have embarked on efforts to understand the dynamic processes of energy generation in bone cells. Remarkably, investigation of substrate utilization by bone cells can be traced back more than 50 years. The early studies, first with bone slices and then with isolated calvarial bone cells, established glucose as a major nutrient and lactate as a prominent end product (1–3). Subsequent work fine-tuned the culture conditions, particularly the inclusion of ascorbate and β-glycerophosphate, for supporting not only growth but also the collagen-producing and mineralizing activity of isolated osteoblasts, thus providing the foundation for studies of osteoblast bioenergetics in vitro (4, 5). Nichols and Neuman (6) extended those studies to hormonal regulation of metabolism in calvariae cultures ex vivo. However, not long after that, investigations into bone cell bioenergetics were placed on the “back burner.”
The current interest in osteoblast bioenergetics has been fueled by several recent developments. First, the emergence of anabolic agents to treat osteoporosis led investigators to explore the role of substrate utilization in the osteoblast during periods of greater work (i.e., during collagen synthesis) (7, 8). Second, studies in anorexia nervosa and subsequently in mouse models of calorie restriction demonstrated the necessity of substrate availability to maintain normal skeletal remodeling (9, 10). Third, work from several laboratories established that bone is an integral part of whole-body glucose homeostasis through elaboration of growth factors and bone-specific peptides such as osteocalcin (11). Some of these are mediated by insulin, which bridges the network between bone and metabolic homeostasis, in part by stimulating bone formation and enhancing bone resorption. Finally, technological advances for measuring cellular and mitochondrial bioenergetics facilitated studies of osteoblast metabolism in vitro. Nevertheless, there are still major hurdles to overcome in the quest to understand fuel choices and cellular metabolism in bone. In this review we focus on the bioenergetics of the osteoblast and its progenitors, but note that other cell types, including osteocytes and cells of the osteoclast lineage, are critical for the health of the bone remodeling sequence (Fig. 1). Each of these cells also has a distinct relationship to the function of the osteoblast and hence to its bioenergetics.
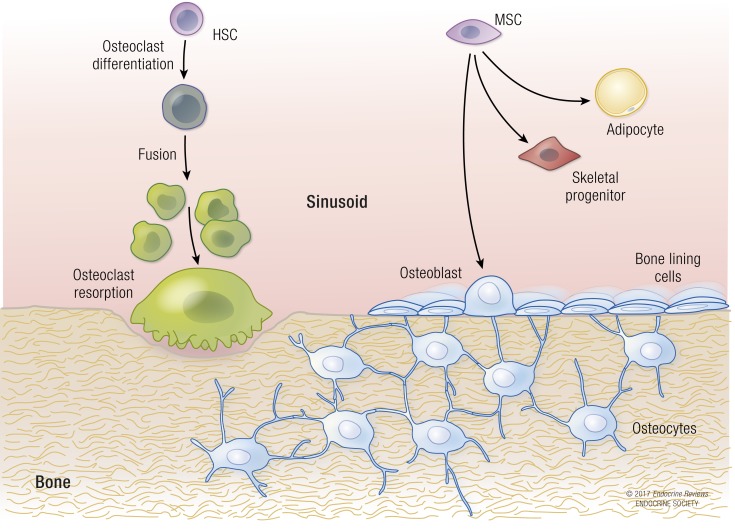
Figure 1.
The bone remodeling unit is composed of several distinct cell types that originate from either the hematopoietic or mesenchymal lineages. Osteocytes are the “command and control” cells that regulate both resorption by osteoclasts and formation by osteoblasts. Bone-lining cells and mesenchymal stromal cells can differentiate into osteoblasts or adipocytes. Osteoblast progenitor differentiation is very dependent on glycolytic pathways. HSC, hematopoietic stem cell; MSC, mesenchymal stromal cell.
Bone Remodeling and Energy Utilization
The bone remodeling unit is composed of multiple cell types of either osteoblast or osteoclast lineage. Whereas the former encompasses the mesenchymal progenitors that produce osteoblasts, which in turn give rise to bone lining cells or osteocytes, the latter lineage includes both mature osteoclasts and their macrophage precursors. Proper regulation and coordination of differentiation and function of both cell lineages play essential roles in the maintenance of the adult skeleton by coupling bone resorption to bone formation (Fig. 1) (12). The process of remodeling takes ∼120 days in humans from resorption to formation, and the entire skeleton is remodeled every 10 years. Maintenance of bone mass during remodeling is essential for calcium homeostasis and for the strength of the skeleton. Trabecular remodeling occurs over a much wider surface area than cortical remodeling, and the rate of remodeling is fourfold faster than in cortical bone. Cortical modeling by osteoblasts during growth takes place on the periosteal and endocortical surfaces of bone and occurs with little or no osteoclastic activity. Both modeling and remodeling require the synthesis of new collagen by osteoblasts and thus consume a significant amount of adenosine triphosphate (ATP).
Remodeling is initiated by hormonal, environmental, and nutritional factors. This requires the recruitment of progenitor cells and their subsequent differentiation to osteoblasts. Progenitor cells primarily use glycolysis as their main energy source within hypoxic endosteal niches as well as in the more favorable oxygen environments outside those protected areas (13) (see “Osteoblast Bioenergetics” section). However, substrate use (glucose, glutamine, fatty acids, as well as autophagy) during several distinct periods of differentiation has not been defined.
Multinucleated osteoclasts resorb bone during a finite period and this process is tightly coupled to bone formation to maintain skeletal mass. Several studies have shown that during the active process of resorption, glycolysis is the primary process to generate ATP through induction of lactate dehydrogenase (14, 15). Alternatively, osteoclast differentiation from circulating monocytic precursors, and the fusion process itself (i.e., the merging of several osteoclast progenitors into a multinucleated giant cell; see Fig. 1) is driven principally by oxidative phosphorylation and is associated with significant mitochondrial biogenesis. These data are consistent with earlier findings that osteoclasts have more mitochondria per surface area than virtually any other cell (16). Besides glucose, whether and to what extent other substrates such as fatty acids and amino acids contribute to oxidative phosphorylation during osteoclastogenesis remain unclear at present.
Osteocytes are the most abundant cells in bone. They can sense mechanical stress and possess canaliculi that serve as a network between the osteocyte and the surface cells, that is, endosteal osteoblasts and bone-lining cells, thereby providing a means of communicating signals to initiate or stop the remodeling sequence (Fig. 1) (17, 18). Osteocytes are derived from terminally differentiated osteoblasts entombed in the bone matrix, but there are virtually no data on osteocyte bioenergetics. However, it is assumed that these cells are metabolically less active than osteoblasts. More recently, immortalized cell models and better isolation techniques of primary osteocytes have led to a greater understanding of these cells, particularly in their capacity to resorb bone during extreme calcium deficiency. Bone-lining cells are relatives of osteocytes. These cells are of mesenchymal origin are flat and “fibroblastic-like” and exhibit markers characteristic of the osteocyte as well as stem cells. These cells can become osteoblasts with parathyroid hormone (PTH) treatment (19, 20), but they can also remain quiescent or retract to expose bone surfaces prior to osteoclast-mediated bone resorption. Little is known about their energy status or their ultimate fate during remodeling, but new isolation techniques should provide even greater insights into how skeletal progenitors are recruited during greater workload.
Osteoblast Bioenergetics
Glucose metabolism in the osteoblast
Glucose is a major energy and carbon source for mammalian cells. In most cell types, glucose is transported across the plasma membrane via multiple members of the solute carrier family 2, commonly known as Glut transporters (21). Glucose transport by the Glut proteins occurs along a concentration gradient and does not require energy. Intracellular glucose is phosphorylated by a hexokinase to glucose-6-phosphate (G6P), which can then be either converted to glycogen or further metabolized to produce energy and building blocks for biosynthesis (Fig. 2). In most cell types, most G6P enters the core glycolysis pathway to generate pyruvate that is then either further metabolized in the mitochondria or converted to lactate in the cytoplasm. In the mitochondria, complete oxidation of pyruvate through the tricarboxylic acid (TCA) cycle (also known as the Krebs cycle) is coupled with oxidative phosphorylation and extracts considerably more energy from glucose than does the cytoplasmic conversion of pyruvate to lactate (>30 vs 2 ATP per glucose molecule). However, the lactate pathway consumes glucose at a faster pace and produces energy without the need for oxygen. Beyond the core glycolysis pathway, several glycolytic intermediates can be metabolized through alternative mechanisms, including shunting of G6P through the pentose phosphate pathway that is critical for nucleotide and lipid synthesis, and conversion of fructose-6-P for protein glycosylation via the hexosamine biosynthetic pathway (22). Moreover, 3-P-glycerate can be used for de novo synthesis of serine and glycine, whereas glyceraldehyde-3-P is a precursor for glycerol that is the backbone of triglycerides and phospholipids (Fig. 2). Overall, glucose is metabolized through multiple pathways, but the relative prevalence of each metabolic fate likely varies according to the specific energy and biosynthesis requirement in the cell.
Figure 2.
Metabolic fates of glucose in mammalian cells. Major biochemical pathways are denoted in red, with the main product from each pathway shown in blue. Glucose is used to produce not only energy but also intermediate metabolites for biosynthesis. Several key enzymes are highlighted in green. Note that citrate can be exported from mitochondria and converted to acetyl-coA in the nucleus to exert epigenetic regulation on gene expression. Although the metabolic pathways are common among different cell types, their relative importance likely varies depending on the biological function of each cell. G3pdh, glycerol-3-phosphate dehydrogenase; G6pdh, G6P dehydrogenase; Gfat, glutamine–fructose-6-phosphate transaminase; Pdh, pyruvate dehydrogenase; Phgdh, phosphoglycerate dehydrogenase; Ldha, lactate dehydrogenase; OAA, oxaloacetate; PRPP, phosphoribosyl pyrophosphate; UDPGlcNAc, uridine diphosphate N-acetylglucosamine.
Glucose has been long known as a major nutrient for osteoblasts. Historical studies in the early 1960s demonstrated that bone explants as well as primary cultures of calvarial osteoblasts consumed glucose at a brisk rate (1–3). Recent work with radiolabeled glucose analogs has confirmed a significant uptake of glucose by bone in the mouse (23, 24). The Glut transporters appear to be mainly responsible for glucose uptake in osteoblast lineage cells. Expression studies detected both Glut1 and Glut3 in osteoblastic cell lines (25–27). More recently, Glut1 was shown to be a major transporter in primary osteoblast cultures and modulates the posttranslational modification of Runx2 by suppressing adenosine 5'-monophosphate kinase and blocking ubiquitination of Runx2 (23); selective deletion of Glut1 in osteoblast precursors suppressed osteoblast differentiation in vitro and in vivo (23). However, others have reported that Glut4 increases in neonatal calvarial osteoblast cultures in response to β-glycerophosphate and ascorbate, or insulin, even though genetic deletion of Glut4 does not cause an obvious skeletal phenotype (28). Future studies are necessary to address potential functional redundancy among different Glut transporters in osteoblasts in vivo.
Lactate is a major end product of glucose metabolism in osteoblasts regardless of oxygen conditions (29). The historical studies with either bone slices or primary calvarial osteoblasts reported that most glucose carbons were secreted as lactate even in the presence of abundant oxygen (1–3). The phenomenon of lactate production from glucose under replete oxygen conditions is akin to the Warburg effect, also known as aerobic glycolysis, as observed in many cancer cells (30). In keeping with the historical reports, more recent studies have confirmed aerobic glycolysis as a predominant mode of glucose metabolism in primary calvarial osteoblasts, even though oxidative phosphorylation increases during mineralization in response to ascorbic acid and β-glycerophosphate (31, 32). Importantly, stimulation of aerobic glycolysis through stabilization of Hif1α in preosteoblasts markedly increases osteoblast production and bone formation in the mouse; the phenotype is independent of the increase in angiogenesis but susceptible to glycolytic suppression (33). Thus, aerobic glycolysis is not only a prominent metabolic feature of osteoblasts, but it also is likely to be integral to the osteoblast phenotype.
The prominence of aerobic glycolysis is counterintuitive to the energy requirement of osteoblasts. In cancer cells, aerobic glycolysis has been proposed to support cell proliferation through rapid ATP production and provision of metabolic intermediates for active lipid and nucleotide synthesis (34, 35). Mature osteoblasts generally exhibit little proliferation in vivo, but they are biosynthetically active in producing large amounts of extracellular matrix proteins (36, 37). Aerobic glycolysis therefore may be necessary for providing metabolic intermediates to support the synthesis of matrix proteins, as glucose carbons were shown to contribute significantly to amino acids in collagen (38). Additionally, aerobic glycolysis in osteoblasts may be coupled with the active secretion of citrate that is structurally important for the formation of apatite nanocrystals in bone (39–43). The high citrate output by osteoblasts could mean an elevated mitochondrial citrate level that suppresses pyruvate entering the TCA cycle. Moreover, active removal of citrate from the mitochondria (cataplerosis) may increase the reliance of osteoblasts on glycolysis for energy production. Further studies are necessary to elucidate the full mechanism through which aerobic glycolysis promotes the osteoblast phenotype.
Glutamine metabolism in osteoblasts
Glutamine, normally present at a concentration of 500 to 750 μM in human plasma, is the most abundant amino acid in circulation (44). Besides direct contribution to protein synthesis as a building block, glutamine is an important energy source as well as an essential carbon and nitrogen donor for the synthesis of amino acids, nucleotides, glutathione, and hexosamine (45–47) (Fig. 3). Moreover, glutamine efflux in exchange for the import of leucine through the antiporter stimulates the master regulator of protein synthesis mammalian target of rapamycin complex (mTORC)1 (48). Although it can be synthesized in mammalian cells and therefore is considered nonessential, glutamine is long known to be consumed at a greater rate than the other nonessential amino acids in cancer cells (49, 50). In recent years, glutamine metabolism has been extensively studied in the context of cancer biology (51, 52).
Figure 3.
Glutamine plays multiple roles in mammalian cells. Major biochemical pathways are shown in red. Glutamine can serve as a major energy source through oxidative phosphorylation in mitochondria. This process requires the conversion of glutamine to α-ketoglutarate, which is controlled by several key enzymes highlighted in green. Alt, alanine transaminase; Ast, aspartate transaminase; Gdh, glutamate dehydrogenase; Gls, glutaminase.
Studies to date have begun to reveal important roles for glutamine metabolism in osteoblasts. Early studies have shown active uptake and metabolism of glutamine in explants of calvaria and long bones (53). More recent work indicated that glutamine is required for matrix mineralization in calvarial osteoblast cultures (54). Intriguingly, consumption of glutamine by bone marrow stromal cells, a population containing osteoblast precursors, declines with aging and may be linked with impaired osteoblast differentiation (55). Importantly, stable isotope tracing experiments have provided direct evidence that glutamine is converted to citrate through oxidation in the TCA cycle and therefore contributes to energy production in the mitochondria in osteoblast precursors (56). Additionally, the contribution of glutamine to glutathione production plays a critical role in the survival of implanted osteoblast precursors in a murine bone regeneration model (47). Thus, glutamine contributes to not only energy production but also redox homeostasis, and future studies are likely to reveal additional functions for glutamine in osteoblast-lineage cells.
Molecular regulation of osteoblast metabolism
Wnt signaling is a major mechanism for stimulating bone accrual in both mice and humans (57). Recent work has linked the bone anabolic function of Wnt with increased aerobic glycolysis in osteoblast-lineage cells (58). Specifically, Wnt3a, Wnt7b, and Wnt10b, which are known to promote osteoblast differentiation in the bone marrow stromal cell line ST2 cells, all stimulate glucose consumption and lactate production. In contrast, Wnt5a does not induce either osteoblastogenesis or glycolysis in those cells. Mechanistically, Wnt3a signals mainly through Lrp5 to activate mTORC2 and Akt that, in turn, acutely increase the protein abundance of a number of key glycolytic enzymes (e.g., hexokinase 2, phosphofructokinase 1, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3, and lactate dehydrogenase) without affecting their messenger RNA (mRNA) levels (Fig. 4). Wnt3a also upregulates pyruvate dehydrogenase kinase 1 (Pdk1), a negative regulator of pyruvate dehydrogenase activity, and therefore diminishes the flux of glucose-derived pyruvate entering the TCA cycle (59). Remarkably, the reprogramming of glucose metabolism by Wnt3a lowers nuclear acetyl-coA levels and suppresses histone acetylation, hence favoring osteoblast over adipocyte differentiation from the bipotential ST2 cells (59). Consistent with the studies in vitro, mice lacking the Wnt coreceptor Lrp5 or expressing a hyperactive mutant Lrp5 allele expressed a lower or higher level of glycolytic enzymes in bone, respectively, than did the normal control (58). Overall, the bone anabolic function of Wnt signaling appears to be coupled with stimulation of aerobic glycolysis.
“Osteocytes can sense mechanical stress and possess canaliculi that serve as a network between the osteocyte and the surface cells.”
Figure 4.
Wnt signaling stimulates aerobic glycolysis, glutamine catabolism, and fatty acid oxidation in osteoblast-lineage cells. Wnt-mTOR signaling acutely increases the protein but not mRNA levels of key enzymes involved in glucose and glutamine metabolism. Wnt also signals through β-catenin to increase the mRNA levels of genes important for fatty acid oxidation. Acad, acyl-coA dehydrogenase; Cpt1, carnitine palmitoyl transferase 1; Fz, frizzled; Gls, glutaminase; Hadha, hydroxyacyl–coenzyme A dehydrogenase/3-ketoacyl–coenzyme A thiolase/enoyl–coenzyme A hydratase (trifunctional protein), α subunit; Hk2, hexokinase 2; Ldha, lactate dehydrogenase A; Pdc, pyruvate dehydrogenase; Pfk1, phosphofructokinase 1.
Bone anabolism by Wnt has also been linked with increased glutamine metabolism. In ST2 cells, Wnt3a stimulates glutamine oxidation in the TCA cycle by increasing glutaminase downstream of mTORC1, resulting in not only more energy production from glutamine but also Gcn2 activation due to reduced intracellular glutamine levels (56) (Fig. 4). Gcn2 activation promotes the osteoblast phenotype as it induces Atf4-dependent transcription of genes responsible for amino acid uptake or synthesis. Importantly, pharmacological inhibition of glutaminase ameliorates the excessive bone formation caused by hyperactive Wnt signaling in mice (56). Future genetic studies are necessary to determine whether glutamine catabolism contributes to normal bone accrual in vivo.
Wnt signaling has been shown to stimulate fatty acid oxidation in osteoblasts. Wnt activation through either expression of a hyperactive mutant Lrp5 allele or stimulation with Wnt10b increased the expression of fatty acid metabolism genes as well as oleate oxidation in murine calvarial osteoblasts (60) (Fig. 4). Conversely, Lrp5-deficient calvarial osteoblasts expressed lower levels of fatty acid metabolism genes and exhibit less oxidation of oleate. Stimulation of fatty acid oxidation by Wnt appears to be mediated by β-catenin, as either Gsk3β inhibition with LiCl or β-catenin overexpression exhibited a similar effect. Surprisingly, however, deletion of Lrp6 in osteoblasts did not reduce fatty acid metabolism in that study, even though Lrp6 has been shown to activate β-catenin signaling more effectively than Lrp5 (61). Moreover, the contribution of fatty acid oxidation to bone formation in vivo remains to be determined.
Intermittent use of PTH is well known to stimulate bone formation in both mice and humans (8, 62). Interestingly, PTH has been long recognized to stimulate aerobic glycolysis in long bone or calvarium explants, as well as in isolated calvarial osteoblasts (63–66). PTH was also shown to induce glucose uptake in a rat osteoblast cell line (27). Recent studies in MC3T3-E1 cells have uncovered that PTH stimulates aerobic glycolysis via activation of insulin-like growth factor (IGF) signaling, which in turn activates the PI3K/mTORC2 cascade and upregulates metabolic enzymes such as hexokinase 2, lactate dehydrogenase, and Pdk1 (67). The importance of IGF-1 in this case is consistent with genetic studies demonstrating that deletion of either Igf1 or Igf1r in osteoblasts essentially abolishes the bone anabolic effect of PTH in mice (68–70). Interestingly, even though PTH reduces the flux of glucose-derived pyruvate into the TCA cycle, it increases the oxygen consumption rate (OCR), indicating that PTH likely increases the use of alternative substrates to fuel mitochondrial oxidative phosphorylation even though the alternative fuel source is not yet clear at present. Nonetheless, the Pdk1 inhibitor dichloroaceteate that reduces glycolysis diminished the bone anabolic effect of intermittent PTH in the mouse. Thus, activation of aerobic glycolysis represents an important mechanism underlying the bone anabolic function of PTH.
Consistent with their role in mediating the metabolic regulation by Wnt and PTH, mTORC1 and mTORC2 have been shown genetically to perform important functions in bone formation. mTORC1 and mTORC2, two distinct complexes containing the same serine/threonine protein kinase mTOR, are known to play critical roles in coordinating the nutritional and energetic status with a multitude of biosynthetic activities in the cell (71). Genetic deletion of Raptor, a unique and essential component of mTORC1, has been shown to impede the transition of preosteoblasts to mature osteoblasts, as indicated by reduced matrix synthesis and mineralization in murine calvarial cell cultures, as well as osteopenia in the mouse (72). Moreover, inducible deletion of Raptor partially corrected the hyperactivity of osteoblasts in response to Wnt7b overexpression in postnatal mice (73). Conversely, deletion of Tsc2, which leads to constitutive activation of mTORC1, markedly increased osteoblast number and bone formation (74). Alternatively, specific disruption of mTORC2 by genetic deletion of Rictor mainly impaired osteoblast activity, resulting in a severe reduction in cortical bone growth both under basal conditions and in response to experimental mechanical loading (75). Interestingly, deletion of Rictor did not reduce trabecular bone mass despite the impaired osteoblast activity, likely due to the concurrent decrease in bone resorption (75, 76). However, Rictor deficiency diminished the anabolic response to the antisclerostin therapy in both trabecular and cortical bone, confirming the role of mTORC2 in Wnt-induced bone anabolism and lending support to the hypothesis that antisclerostin antibodies enhance the work of the osteoblast (77). Overall, mTOR likely functions downstream of multiple anabolic signals to regulate bone formation, perhaps partly by modulating cellular metabolism. Consistent with this view, it is well known that organ-transplant patients receiving mTOR inhibitors as immunosuppressive agents exhibit a higher incidence of osteoporosis (78).
Metabolism of other energy substrates in osteoblasts
Mitochondrial respiration
As discussed previously, both glucose and glutamine can be used for energy production through oxidative phosphorylation in the mitochondrial matrix. This generates reduced coenzymes (reduced NAD and FADH2) leading to the transfer of electrons through the electron transport chain complexes (I to IV) in the inner mitochondrial membrane and finally to molecular oxygen, the terminal electron acceptor. This transfer of electrons leads to pumping of H+ into the intermembrane space, generating the electrochemical gradient. The transfer of H+ back into the mitochondrial matrix through complex V is coupled to ATP generation (79). Additionally, recent studies have implicated mitochondrial reactive oxygen species generated during oxidative phosphorylation in a variety of signaling processes that control differentiation, cellular senescence, and apoptosis (80–82). Mitochondria are dynamic and undergo constant flux through both fusion and fission (83). These dynamic processes are likely dependent on substrate availability and energy demand at a particular time point. In this section of the review, we highlight some of the major substrates that are catabolized in the mitochondria of osteoblasts.
“Mice lacking the Wnt co-receptor Lrp5 or expressing a hyperactive mutant Lrp5 allele expressed a lower or higher level of glycolytic enzymes in bone.”
Pyruvate
Glucose-derived pyruvate, once transported into the mitochondria through the mitochondrial pyruvate transporter (MPC), presents on the inner mitochondrial membrane and is processed through the Krebs (TCA) cycle for generating reduced NAD and FADH2 (Fig. 2). The genes (MPC1 and MPC2) encoding the MPC have recently been identified in humans (84, 85). Impaired pyruvate transport in humans leads to severe developmental defects (86). Knockout of MPC2 in mice leads to embryonic lethality because of a decrease in pyruvate oxidation (87). Recent studies suggest that thiazolidinediones can bind to and inhibit pyruvate transporters. Although thiazolidinediones such as rosiglitazone are used as antidiabetic drugs, these agents cause bone loss (88, 89). In calvarial osteoblasts, there is a significant increase in oxygen consumption in the presence of exogenous pyruvate, indicating an increase in ATP production through oxidative phosphorylation. However, experiments are required to determine how exogenous pyruvate specifically affects osteoblast metabolism. It is noteworthy that recent studies have shown that a moderate increase in pyruvate levels significantly enhances osteoclastogenesis (90). Thus, pyruvate metabolism could potentially affect both formation and resorption.
Fatty acids
Fatty acids constantly circulate and are also present in bone marrow sera, although the relative concentrations and degree of saturation may differ (91). A number of in vitro studies have demonstrated that long-chain saturated fatty acids such as palmitate can inhibit calvarial osteoblast differentiation (92, 93) whereas oleate, an unsaturated fatty acid (monounsaturated fatty acid), can mitigate the effects of palmitate. Supplementation with long-chain polyunsaturated fatty acids (n-3) in animal models has shown a potential benefit for skeletal health (94). Additionally, recent work by Riddle and colleagues (60) has shown that Wnt-LRP5 signaling enhances β-oxidation of fatty acids and is necessary for optimal osteoblast differentiation.
Fatty acids are degraded in the mitochondria after transfer through carnitine-mediated transport for the generation of ATP (Fig. 4). Most studies suggest that fatty acids are generated from stored triacyglycerides or fat depots in response to lipolysis where they are released into the circulation. Peroxisomes can also process some long-chain fatty acids and these increase in number during the differentiation of osteoblasts, suggesting greater use of fatty acids as substrates for energy generation (95). The role of palmitate as an energy source during Wnt-induced osteoblast differentiation has already been described, but the amount of fatty acids in bone cells that is used for ATP production is currently unknown. The direct effect of fatty acids on mitochondrial oxygen consumption and ATP production has also not been addressed to date.
Amino acids
Amino acids can be divided into both essential amino acids and nonessential amino acids and have been shown through protein intake studies to be important for osteoblast differentiation (96). Mechanistically, the osteoblastic transcriptional factor ATF4 has been shown to increase amino acid uptake and collagen synthesis; mutations in this pathway such as the Coffin–Lowry syndrome have a profound effect on the skeleton (97, 98). The branched chain amino acids valine, leucine, and isoleucine are used specifically by differentiated adipocytes (3T3L1) compared with predifferentiated cells. The role that these amino acids play in osteoblast differentiation is currently not known, particularly with regard to cellular bioenergetics (99). Glutamine metabolism as discussed in the earlier part of this section has also been shown to play a very important role in osteoblast metabolism. Aromatic amino acids such as arginine can increase osteogenic differentiation in preosteoblasts (100). Despite these lines of evidence, it remains challenging to distinguish an instructive vs adaptive role for bioenergetic programming in cell differentiation.
Measurements of Oxidative Phosphorylation and Glycolysis
Much of what has been learned during the last decade about bone cell bioenergetics has occurred because of technological advances in measuring cell metabolism. Although oxygen consumption rate in isolated mitochondria has traditionally been measured using the Clarke’s electrode, one of the factors that has been hard to overcome is the isolation of coupled mitochondria from calcified skeletal tissue. Recent advances in microplate measurements of oxygen consumption rates and extracellular acidification rates using the Agilent Seahorse extracellular flux analyzer has opened up new avenues for performing metabolic studies of osteoblast differentiation in vitro (31). The Seahorse XF24 or XF96 analyzer and the Oroboros Instruments platform can measure oxygen consumption rates in a polystyrene (24- or 96-multiwell format) plate in a small volume over a monolayer of cells. This can be adapted to study osteoblast differentiation using calvarial osteoblasts, MC3T3-E1, ST2, or primary bone marrow mesenchymal stromal cells (58). Not only can basal measurements be performed for extracellular acidification or oxygen consumption on Seahorse or Oroboros Instruments platforms, but different workflows can be adapted to inject various inhibitors and stressors in sequence through the cartridge ports to test various mitochondrial enzymes. Those data can be used to draw direct conclusions about various metabolic parameters and, most importantly, these parameters can then be used to calculate ATP production rates (101).
Notwithstanding the technical advances, there remain limitations to defining bone cell bioenergetics. First, the in vitro environment is artificial and may not represent in vivo conditions, particularly when considering hypoxic niches. Some progress has been made with calvarial organoid culture studies using two-photon microscopy and different fluorescent dyes to define acidification and mitochondrial oxygen consumption, but recapitulation in situ will remain a difficult task (A.A. Gerencser, personal communication). Second, virtually all of the studies to date have been performed after exogenous addition of substrates, but no studies have been done to examine intracellular substrate use that almost certainly must occur during processes such as autophagy. Third, and most importantly, cells are “substrate” smart and will use any fuel available during tissue culture; thus, it is important to approximate physiological concentrations for the various substrates (e.g., glucose concentration ∼5 mM) in metabolic studies in vitro. Even so, culture conditions may not represent the context specific nature of fuel economy in vivo.
Clinical Correlates
Anabolic treatments for osteoporosis
Intermittent administration of PTH1–34, or abaloparatide, a PTH-related protein analog, activates the PTH1 receptor and stimulates new bone formation, increases bone mass, and reduces fracture risk (7, 8). The mechanism of action after activation of the PTH1 receptor is the induction of osteoblastogenesis from marrow stromal cells and bone-lining cells (102). This effect (i.e., greater number of osteoblasts) is particularly striking in the first 6 to 12 months of intermittent PTH treatment. Alternatively, recent animal data regarding romosozumab, the newest antisclerostin antibody that has been shown to build bone mineral density and reduce fractures, in the long term does not increase osteoblast number but rather increases the work of the osteoblasts on the bone surface (103). This subtle but important difference in the mechanism for the anabolics must also be reflected in the choice of substrate utilization to power collagen synthesis and mineralization.
“Cells are “substrate” smart and will use any fuel available during tissue culture.”
As previously noted, several studies have shown that PTH stimulates aerobic glycolysis in osteoblast lineage cells (29). Interestingly, although PTH reduces glucose oxidation through mitochondria, it promotes overall oxidative phosphorylation, which has been shown to increase with osteoblast differentiation (31, 67). Besides glucose, mitochondrial respiration utilizes other substrates, including amino acids, cellular products from autophagy, lipophagy, and exogenous fatty acids. With respect to the latter, recent clinical and basic observations suggest there may be a ready source of that fuel from marrow adipocytes. Marrow adipose tissue is extensive and occupies >70% of the bone marrow in rodents and humans (104). Stored triglycerides may be lipolyzed in the “regulated” marrow adipose depots under the influence of β adrenergic agents and/or the sympathetic nervous system (105). One study found that PTH1 receptor anabolic signaling is enhanced by activation of the β2 adrenergic receptor, supporting some degree of synergy between these two receptors (106). PTH has also been shown to stimulate lipolysis in 3T3-L1 cells and PTH-related protein can induce peripheral thermogenesis (102, 107). These data suggest that PTH could use endogenous marrow to fuel osteoblasts by lipolyzing triglycerides to produce free fatty acids and glycerol. Indeed, Cohen et al. (108) demonstrated that PTH treatment reduced the size of the marrow adipocyte when administered intermittently to young premenopausal women. Fan et al. (109) showed that 2 weeks of intermittent PTH1–34 significantly reduced bone marrow adiposity in C57BL/6J mice. Interestingly, Fazeli et al. (110) noted that anorexic women, who have three times the amount of marrow adipose tissue than do normal women, and were treated with PTH1–34, had a much greater skeletal anabolic response than did postmenopausal women, suggesting but not proving that marrow adipocytes might be called upon to generate substrates when osteoblasts are stimulated to make collagen. Further studies are needed to determine whether marrow adipose tissue represents a necessary reserve of endogenous substrate for osteoblast work during states of poor nutrition or altered skeletal remodeling.
Anorexia nervosa
Calorie malnutrition results in significant changes in whole-body metabolism, as energy utilization is committed to maintenance of tissue homeostasis rather than anabolic activity. Anorexia nervosa is one such condition, and lack of fuel availability has a profound impact on skeletal remodeling. Bone formation is suppressed in anorexia nervosa, but interestingly bone resorption is increased during calorie deprivation in both mice and humans. Anorexic patients have very low bone mass and markedly enhanced skeletal fragility, as well as persistent difficulties with hypoglycemia and hypothermia (111). Paradoxically, although marrow adipose tissue is extensive, it is reversible, as is the loss of bone mass with weight restoration and/or estrogen replacement (112). Estrogen also reduces marrow adiposity during replacement in postmenopausal women, and this too is associated with a gain in bone mass. Notwithstanding, it is not clear how bioenergetic profiles in osteoblast progenitors change during severe nutritional stress, particularly because it is likely that adenosine 5′-monophosphate kinase is activated in osteoblasts, and this in turn stimulates autophagy (113). Also, it is not known whether the increase in marrow adipose tissue is a result of a lineage shift from osteogenesis toward adipogenesis due to changes in the marrow progenitor’s inherent metabolic program. Mouse models have provided some preliminary insights, although translation to human studies has been difficult. Moreover, endocrine changes during anorexia nervosa are multifaceted and include hypothalamic, pituitary, and target tissues. Alterations in energy metabolism of the osteoblast may include lack of nutrient substrates (i.e., particularly glucose and fatty acids), enhanced fatty acid synthesis (due to increased cortisol secretion), reduced IGF-1 leading to impaired glycolytic pathways, and increased metabolism of substrates that could lead to ketosis from high FGF21 levels.
Diabetes mellitus
Type 1 diabetes and type 2 diabetes are chronic diseases characterized by impaired glucose metabolism in liver, fat, and skeletal muscle, leading to varying degrees of fuel starvation in those tissues. In type 1 diabetes, when there is an absolute lack of insulin, osteoblast function is markedly impaired, and skeletal fragility leads to very high rates of fractures. However, as noted previously, it is unlikely that insulin is required for glucose transport in osteoblasts, and hence cells should still be able to activate glycolysis as a principal energy-driving mechanism. However, glucose and lipid toxicity could interfere with substrate utilization, particularly at very high extracellular glucose concentrations. In type 2 diabetes, insulin resistance is a hallmark, but osteoblast function is also impaired. Both type 2 diabetes and late-stage type 2 diabetes impair insulin-dependent actions of the liver, including production and release of IGF-1, which could in turn affect the bioenergetics and function of osteoblasts. However, exactly how fuel metabolism in osteoblasts is altered in this disease is not clear at present.
Conclusions
Proper metabolic programming is essential for the normal function of each cell in the bone remodeling unit. Moreover, whole-body energy homeostasis is closely tied to fuel utilization during the various phases of remodeling. Substrate utilization and ATP generation are both context and cell specific. To date, the best characterized metabolic profile in bone cells is the pattern of glucose utilization by osteoblast progenitors during differentiation and their response to signaling peptides such as the Wnts and the IGFs. Less clear is how bone-forming cells use fatty acids, citrate, and intracellular proteins and lipids during differentiation. In part this is due to the difficulty in recapitulating the marrow niche ex vivo. Furthermore, the artificial environment in which in vitro studies are conducted can provide valuable insights, but these cells may alter substrate utilization depending on availability, thereby making it difficult to extrapolate those findings to in vivo conditions. Intriguingly, diseases of fuel metabolism such as diabetes mellitus might offer better clues into the importance of specific metabolic programs for osteoblasts and whether alternative metabolic programming in osteoprogenitors can lead to shifts in lineage allocation. By the same logic, medications to treat diabetes and its comorbidities may affect bone health and osteoblast fuel metabolism. Additionally, more in-depth studies of anabolic agents may illustrate how different stages of osteoblast differentiation in vivo depend on available substrate. Clearly, a better understanding of the bioenergetics of the other important cell types, including osteoclasts, osteocytes, and adipocytes, is necessary for a holistic view of the metabolic regulation of the bone organ. More translational studies and better technology will be needed to clearly define the bioenergetics of bone cells, and importantly how that knowledge can be applied not only to the treatment of chronic metabolic bone diseases but also to the development of new drugs.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health Grants DK092750 (to C.J.R.), AR071739 (to C.J.R.), AR068095 (to A.R.G.), AR055923 (to F.L.), and AR060456 (to F.L.). Additional support was provided by the Maine Medical Center Research Institute Physiology Core National Institutes of Health Grant P30 GM103465.
Footnotes
- ATP
- adenosine triphosphate
- G6P
- glucose-6-phosphate
- MPC
- mitochondrial pyruvate transporter
- mRNA
- messenger RNA
- mTOR
- mammalian target of rapamycin
- mTORC
- mammalian target of rapamycin complex
- Pdk1
- pyruvate dehydrogenase kinase 1
- PTH
- parathyroid hormone
- TCA
- tricarboxylic acid.
References
- 1.Borle AB, Nichols N, Nichols G Jr. Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:1206–1210. [PubMed] [Google Scholar]
- 2.Cohn DV, Forscher BK. Aerobic metabolism of glucose by bone. J Biol Chem. 1962;237:615–618. [PubMed] [Google Scholar]
- 3.Peck WA, Birge SJ Jr, Fedak SA. Bone Cells: Biochemical and Biological Studies after Enzymatic Isolation. Science. 1964;146(3650):1476–1477. [DOI] [PubMed] [Google Scholar]
- 4.Peck WA, Birge SJ Jr, Brandt J. Collagen synthesis by isolated bone cells: stimulation by ascorbic acid in vitro. Biochim Biophys Acta. 1967;142(2):512–525. [DOI] [PubMed] [Google Scholar]
- 5.Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987;122(1):49–60. [DOI] [PubMed] [Google Scholar]
- 6.Nichols FC, Neuman WF. Lactic acid production in mouse calvaria in vitro with and without parathyroid hormone stimulation: lack of acetazolamide effects. Bone. 1987;8(2):105–109. [DOI] [PubMed] [Google Scholar]
- 7.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C; ACTIVE Study Investigators . Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016;316(7):722–733. [DOI] [PubMed] [Google Scholar]
- 8.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 9.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25(9):2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, Gleysteen S, Mickley D, Herzog D, Klibanski A. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91(8):2931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–311. [DOI] [PubMed] [Google Scholar]
- 13.Palomäki S, Pietilä M, Laitinen S, Pesälä J, Sormunen R, Lehenkari P, Koivunen P. HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells. 2013;31(9):1902–1909. [DOI] [PubMed] [Google Scholar]
- 14.Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, Ikeda K. Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res. 2013;28(11):2392–2399. [DOI] [PubMed]
- 15.Ahn H, Lee K, Kim JM, Kwon SH, Lee SH, Lee SY, Jeong D. Accelerated Lactate Dehydrogenase Activity Potentiates Osteoclastogenesis via NFATc1 Signaling. PLoS One. 2016;11(4):e0153886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemma S, Sboarina M, Porporato PE, Zini N, Sonveaux P, Di Pompo G, Baldini N, Avnet S. Energy metabolism in osteoclast formation and activity. Int J Biochem Cell Biol. 2016;79:168–180. [DOI] [PubMed] [Google Scholar]
- 17.Bonewald LF, Kneissel M, Johnson M. Preface: the osteocyte. Bone. 2013;54(2):181. [DOI] [PubMed] [Google Scholar]
- 18.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34(5):658–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS, Baek WY, Kim JE, Kronenberg HM. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27(10):2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matic I, Matthews BG, Wang X, Dyment NA, Worthley DL, Rowe DW, Grcevic D, Kalajzic I. Quiescent Bone Lining Cells Are a Major Source of Osteoblasts During Adulthood. Stem Cells. 2016;34(12):2930–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Augustin R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life. 2010;62(5):315–333. [DOI] [PubMed] [Google Scholar]
- 22.Bouché C, Serdy S, Kahn CR, Goldfine AB. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev. 2004;25(5):807–830. [DOI] [PubMed] [Google Scholar]
- 23.Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, Takarada T, Iezaki T, Pessin JE, Hinoi E, Karsenty G. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell. 2015;161(7):1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoch ML, Abou DS, Clemens TL, Thorek DL, Riddle RC. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 2016;4:16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas DM, Rogers SD, Ng KW, Best JD. Dexamethasone modulates insulin receptor expression and subcellular distribution of the glucose transporter GLUT 1 in UMR 106-01, a clonal osteogenic sarcoma cell line. J Mol Endocrinol. 1996;17(1):7–17. [DOI] [PubMed] [Google Scholar]
- 26.Thomas DM, Maher F, Rogers SD, Best JD. Expression and regulation by insulin of GLUT 3 in UMR 106-01, a clonal rat osteosarcoma cell line. Biochem Biophys Res Commun. 1996;218(3):789–793. [DOI] [PubMed] [Google Scholar]
- 27.Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011;348(1-2):33–42. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Frey JL, Wong GW, Faugere MC, Wolfgang MJ, Kim JK, Riddle RC, Clemens TL. Glucose Transporter-4 Facilitates Insulin-Stimulated Glucose Uptake in Osteoblasts. Endocrinology. 2016;157(11):4094–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esen E, Long F. Aerobic glycolysis in osteoblasts. Curr Osteoporos Rep. 2014;12(4):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. [DOI] [PubMed] [Google Scholar]
- 31.Guntur AR, Le PT, Farber CR, Rosen CJ. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014;155(5):1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279(4):C1220–C1229. [DOI] [PubMed] [Google Scholar]
- 33.Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, Long F. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci USA. 2014;111(23):8673–8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owen M, MacPherson S. Cell Population Kinetics of an Osteogenic Tissue. Ii. J Cell Biol. 1963;19:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13(1):27–38. [DOI] [PubMed] [Google Scholar]
- 38.Flanagan B, Nichols G Jr. Metabolic studies of bone in vitro. V. Glucose metabolism and collagen biosynthesis. J Biol Chem. 1964;239:1261–1265. [PubMed] [Google Scholar]
- 39.Dixon TF, Perkins HR. Citric acid and bone metabolism. Biochem J. 1952;52(2):260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costello LC, Franklin RB, Reynolds MA, Chellaiah M.. The Important Role of Osteoblasts and Citrate Production in Bone Formation: “Osteoblast Citration” as a New Concept for an Old Relationship. Open Bone J. 2012;4:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu YY, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci USA. 2010;107(52):22425–22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenny AD, Draskoczy PR, Goldhaber P. Citric acid production by resorbing bone in tissue culture. Am J Physiol. 1959;197:502–504. [DOI] [PubMed] [Google Scholar]
- 43.Taylor TG. The nature of bone citrate. Biochim Biophys Acta. 1960;39:148–149. [DOI] [PubMed] [Google Scholar]
- 44.Walsh NP, Blannin AK, Robson PJ, Gleeson M. Glutamine, exercise and immune function. Links and possible mechanisms. Sports Med. 1998;26(3):177–191. [DOI] [PubMed] [Google Scholar]
- 45.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35(10):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stegen S, van Gastel N, Eelen G, Ghesquière B, D’Anna F, Thienpont B, Goveia J, Torrekens S, Van Looveren R, Luyten FP, Maxwell PH, Wielockx B, Lambrechts D, Fendt SM, Carmeliet P, Carmeliet G. HIF-1α Promotes Glutamine-Mediated Redox Homeostasis and Glycogen-Dependent Bioenergetics to Support Postimplantation Bone Cell Survival. Cell Metab. 2016;23(2):265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coles NW, Johnstone RM. Glutamine metabolism in Ehrlich ascites-carcinoma cells. Biochem J. 1962;83:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254(8):2669–2676. [PubMed] [Google Scholar]
- 51.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104(49):19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Matés JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15(2):157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biltz RM, Letteri JM, Pellegrino ED, Palekar A, Pinkus LM. Glutamine metabolism in bone. Miner Electrolyte Metab. 1983;9(3):125–131. [PubMed] [Google Scholar]
- 54.Brown PM, Hutchison JD, Crockett JC. Absence of glutamine supplementation prevents differentiation of murine calvarial osteoblasts to a mineralizing phenotype. Calcif Tissue Int. 2011;89(6):472–482. [DOI] [PubMed] [Google Scholar]
- 55.Huang T, Liu R, Fu X, Yao D, Yang M, Liu Q, Lu WW, Wu C, Guan M. Aging Reduces an ERRalpha-Directed Mitochondrial Glutaminase Expression Suppressing Glutamine Anaplerosis and Osteogenic Differentiation of Mesenchymal Stem Cells. Stem Cells. 2017;35(2):411–424. [DOI] [PubMed] [Google Scholar]
- 56.Karner CM, Esen E, Okunade AL, Patterson BW, Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest. 2015;125(2):551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maupin KA, Droscha CJ, Williams BO. A comprehensive overview of skeletal phenotypes associatred with alterations in Wnt/β-catenin signaling in humans and mice. Bone Res. 2013;1(1):27–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17(5):745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karner CM, Esen E, Chen J, Hsu FF, Turk J, Long F. Wnt Protein Signaling Reduces Nuclear Acetyl-CoA Levels to Suppress Gene Expression during Osteoblast Differentiation. J Biol Chem. 2016;291(25):13028–13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, Wolfgang MJ, Clemens TL, Riddle RC. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35(11):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacDonald BT, Semenov MV, Huang H, He X. Dissecting molecular differences between Wnt coreceptors LRP5 and LRP6. PLoS One. 2011;6(8):e23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703. [DOI] [PubMed] [Google Scholar]
- 63.Borle AB, Nichols N, Nichols G Jr. Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960;235:1211–1214. [PubMed] [Google Scholar]
- 64.Neuman WF, Neuman MW, Brommage R. Aerobic glycolysis in bone: lactate production and gradients in calvaria. Am J Physiol. 1978;234(1):C41–C50. [DOI] [PubMed] [Google Scholar]
- 65.Rodan GA, Rodan SB, Marks SC Jr. Parathyroid hormone stimulation of adenylate cyclase activity and lactic acid accumulation in calvaria of osteopetrotic (ia) rats. Endocrinology. 1978;102(5):1501–1505. [DOI] [PubMed] [Google Scholar]
- 66.Felix R, Neuman WF, Fleisch H. Aerobic glycolysis in bone: lactic acid production by rat calvaria cells in culture. Am J Physiol. 1978;234(1):C51–C55. [DOI] [PubMed] [Google Scholar]
- 67.Esen E, Lee SY, Wice BM, Long F. PTH Promotes Bone Anabolism by Stimulating Aerobic Glycolysis Via IGF Signaling. J Bone Miner Res. 2015;30(11):1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17(9):1570–1578. [DOI] [PubMed] [Google Scholar]
- 69.Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001;142(10):4349–4356. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22(9):1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Long F. mTORC1 Signaling Promotes Osteoblast Differentiation from Preosteoblasts. PLoS One. 2015;10(6):e0130627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, Rüegg MA, Hall MN, Ma L, Long F. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10(1):e1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riddle RC, Frey JL, Tomlinson RE, Ferron M, Li Y, DiGirolamo DJ, Faugere MC, Hussain MA, Karsenty G, Clemens TL. Tsc2 is a molecular checkpoint controlling osteoblast development and glucose homeostasis. Mol Cell Biol. 2014;34(10):1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J, Holguin N, Shi Y, Silva MJ, Long F. mTORC2 signaling promotes skeletal growth and bone formation in mice. J Bone Miner Res. 2015;30(2):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu DM, Zhao L, Liu TT, Jiao PL, Zhao DD, Shih MS, Tao B, Sun LH, Zhao HY, Liu JM. Rictor/mTORC2 loss in osteoblasts impairs bone mass and strength. Bone. 2016;90:50–58. [DOI] [PubMed] [Google Scholar]
- 77.Sun W, Shi Y, Lee WC, Lee SY, Long F. Rictor is required for optimal bone accrual in response to anti-sclerostin therapy in the mouse. Bone. 2016;85:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab. 2005;90(4):2456–2465. [DOI] [PubMed] [Google Scholar]
- 79.Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans. 2005;33(Pt 5):897–904. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Marsboom G, Toth PT, Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One. 2013;8(10):e77077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14(4):537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H, Yoon Y. Mitochondrial fission and fusion. Biochem Soc Trans. 2016;44(6):1725–1735. [DOI] [PubMed] [Google Scholar]
- 84.Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–96. [DOI] [PubMed] [Google Scholar]
- 85.Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS, Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brivet M, Garcia-Cazorla A, Lyonnet S, Dumez Y, Nassogne MC, Slama A, Boutron A, Touati G, Legrand A, Saudubray JM. Impaired mitochondrial pyruvate importation in a patient and a fetus at risk. Mol Genet Metab. 2003;78(3):186–192. [DOI] [PubMed] [Google Scholar]
- 87.Vigueira PA, McCommis KS, Schweitzer GG, Remedi MS, Chambers KT, Fu X, McDonald WG, Cole SL, Colca JR, Kletzien RF, Burgess SC, Finck BN. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Reports. 2014;7(6):2042–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, Henry RR, McDonald WG, Colca JR, Simon MI, Ciaraldi TP, Murphy AN. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci USA. 2013;110(14):5422–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146(3):1226–1235. [DOI] [PubMed] [Google Scholar]
- 90.Fong JE, Le Nihouannen D, Tiedemann K, Sadvakassova G, Barralet JE, Komarova SV. Moderate excess of pyruvate augments osteoclastogenesis. Biol Open. 2013;2(4):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pino AM, Miranda M, Figueroa C, Rodríguez JP, Rosen CJ. Qualitative Aspects of Bone Marrow Adiposity in Osteoporosis. Front Endocrinol (Lausanne). 2016;7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeh LC, Ford JJ, Lee JC, Adamo ML. Palmitate attenuates osteoblast differentiation of fetal rat calvarial cells. Biochem Biophys Res Commun. 2014;450(1):777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gunaratnam K, Vidal C, Boadle R, Thekkedam C, Duque G. Mechanisms of palmitate-induced cell death in human osteoblasts. Biol Open. 2013;2(12):1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lau BY, Cohen DJ, Ward WE, Ma DW. Investigating the role of polyunsaturated fatty acids in bone development using animal models. Molecules. 2013;18(11):14203–14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qian G, Fan W, Ahlemeyer B, Karnati S, Baumgart-Vogt E. Peroxisomes in Different Skeletal Cell Types during Intramembranous and Endochondral Ossification and Their Regulation during Osteoblast Differentiation by Distinct Peroxisome Proliferator-Activated Receptors. PLoS One. 2015;10(12):e0143439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacDonell R, Hamrick MW, Isales CM. Protein/amino-acid modulation of bone cell function. Bonekey Rep. 2016;5:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117(3):387–398. [DOI] [PubMed] [Google Scholar]
- 98.Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, Parada LF, Karsenty G. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4(6):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP, Metallo CM. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chevalley T, Rizzoli R, Manen D, Caverzasio J, Bonjour JP. Arginine increases insulin-like growth factor-I production and collagen synthesis in osteoblast-like cells. Bone. 1998;23(2):103–109. [DOI] [PubMed] [Google Scholar]
- 101.Mookerjee SA, Goncalves RL, Gerencser AA, Nicholls DG, Brand MD. The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta 2015;1847(2):171–181. [DOI] [PubMed]
- 102.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CAF, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375(16):1532–1543. [DOI] [PubMed] [Google Scholar]
- 104.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK, Khoury B, Jepsen KJ, Pilch PF, Klibanski A, Rosen CJ, MacDougald OA. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanyu R, Wehbi VL, Hayata T, Moriya S, Feinstein TN, Ezura Y, Nagao M, Saita Y, Hemmi H, Notomi T, Nakamoto T, Schipani E, Takeda S, Kaneko K, Kurosawa H, Karsenty G, Kronenberg HM, Vilardaga JP, Noda M. Anabolic action of parathyroid hormone regulated by the β2-adrenergic receptor. Proc Natl Acad Sci USA. 2012;109(19):7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Larsson S, Jones HA, Göransson O, Degerman E, Holm C. Parathyroid hormone induces adipocyte lipolysis via PKA-mediated phosphorylation of hormone-sensitive lipase. Cell Signal. 2016;28(3):204–213. [DOI] [PubMed] [Google Scholar]
- 108.Cohen A, Stein EM, Recker RR, Lappe JM, Dempster DW, Zhou H, Cremers S, McMahon DJ, Nickolas TL, Müller R, Zwahlen A, Young P, Stubby J, Shane E. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, Baron R, Bronson RT, Horowitz MC, Wu JY, Bilezikian JP, Dempster DW, Rosen CJ, Lanske B. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017;25(3):661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, Finkelstein JS, Bouxsein ML, Klibanski A. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab. 2014;99(4):1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122(5):409–414. [DOI] [PubMed] [Google Scholar]
- 112.Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27(9):1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xi G, Rosen CJ, Clemmons DR. IGF-I and IGFBP-2 Stimulate AMPK Activation and Autophagy, Which Are Required for Osteoblast Differentiation. Endocrinology. 2016;157(1):268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]